Mixed Valanced V3+,V2+ Phosphate Na7V4(PO4)6: A Structural Analogue of Mineral Yurmarinite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrothermal Synthesis and Scanning Electron Microscopy
2.2. X-ray Diffraction and X-ray Absorption Spectroscopy
3. Results
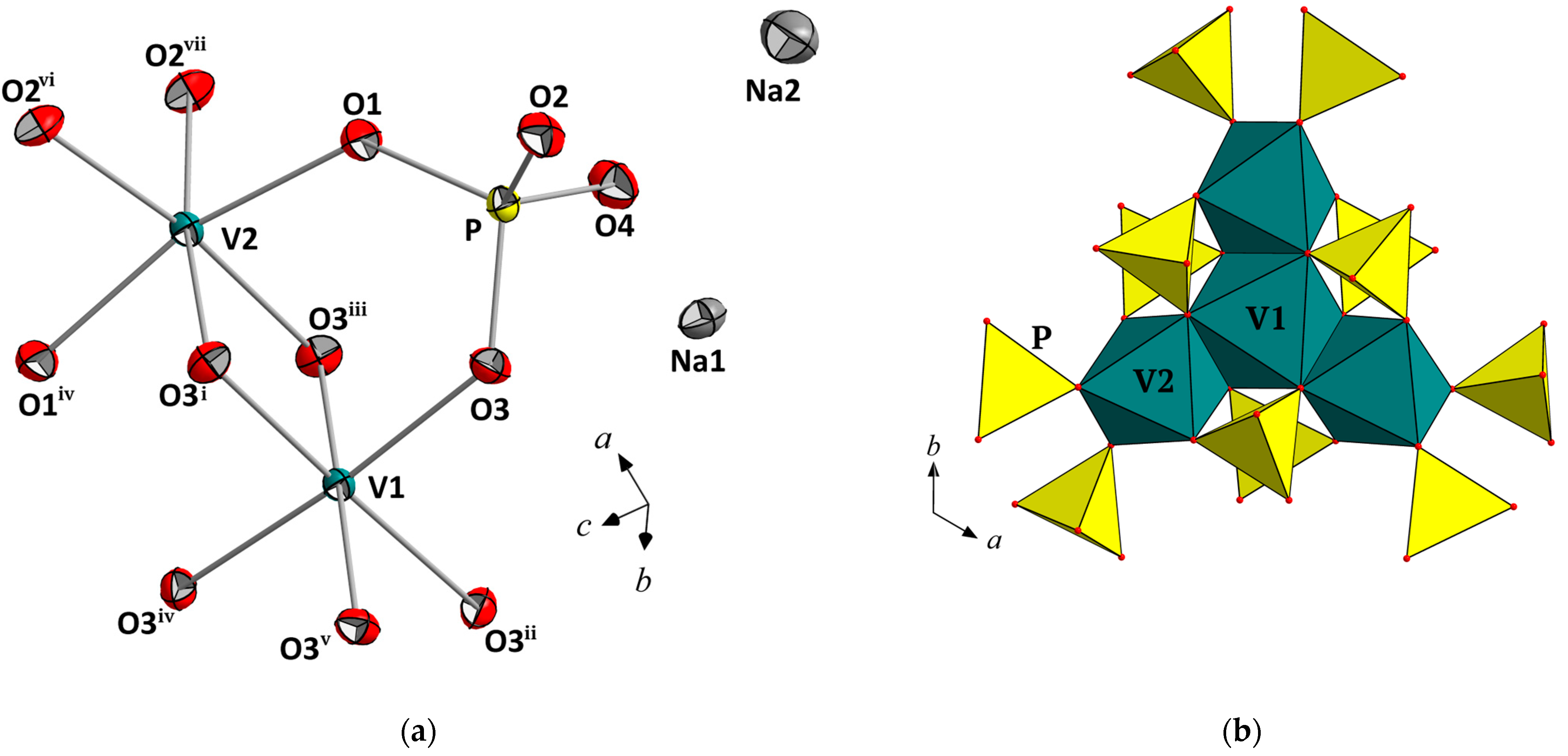
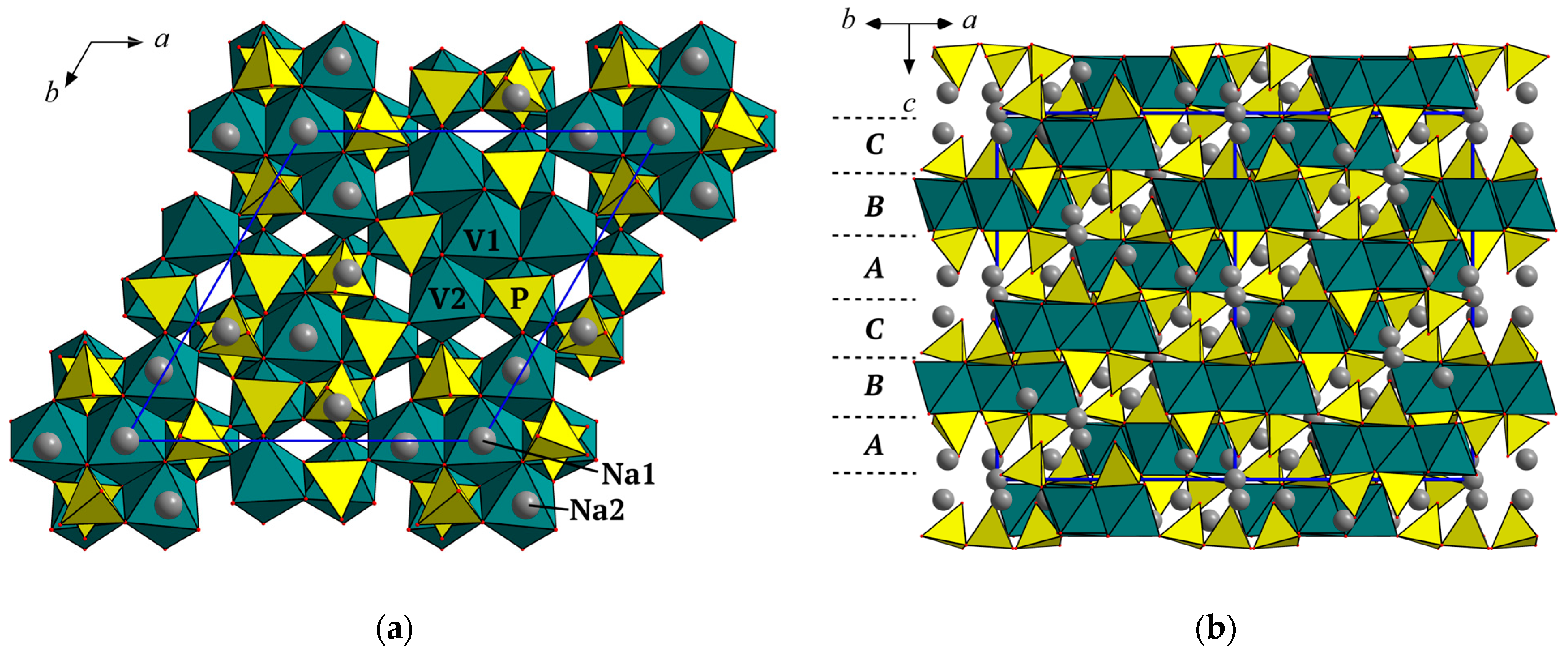
3.1. Crystal Structure Description in terms of Close-Packing
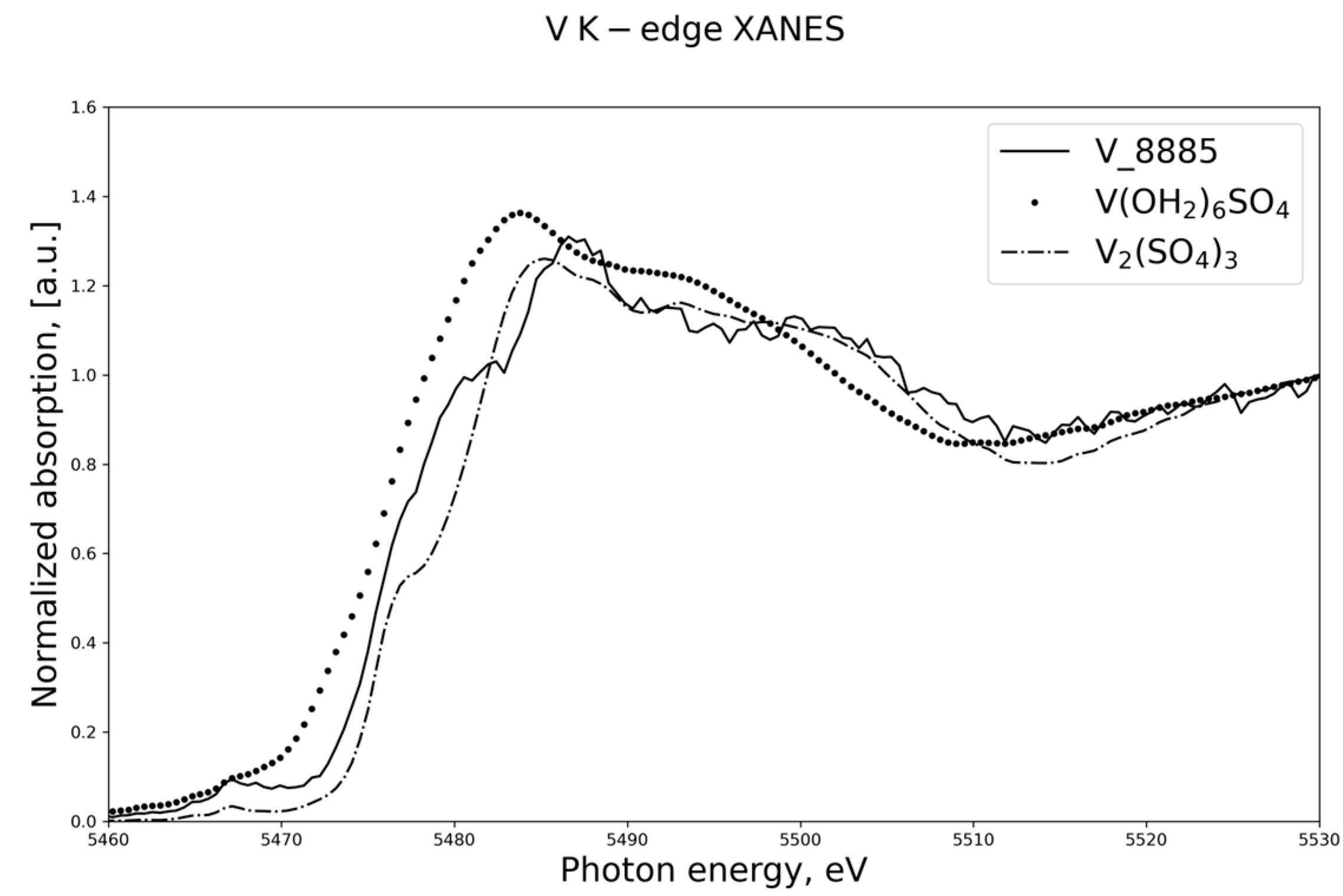
3.2. BVS Calculations and XANES Spectroscopy
3.3. The Comparative Crystal Chemistry of Na7V4(PO4)6 and the Database Survey of V2+-Containing Oxosalts
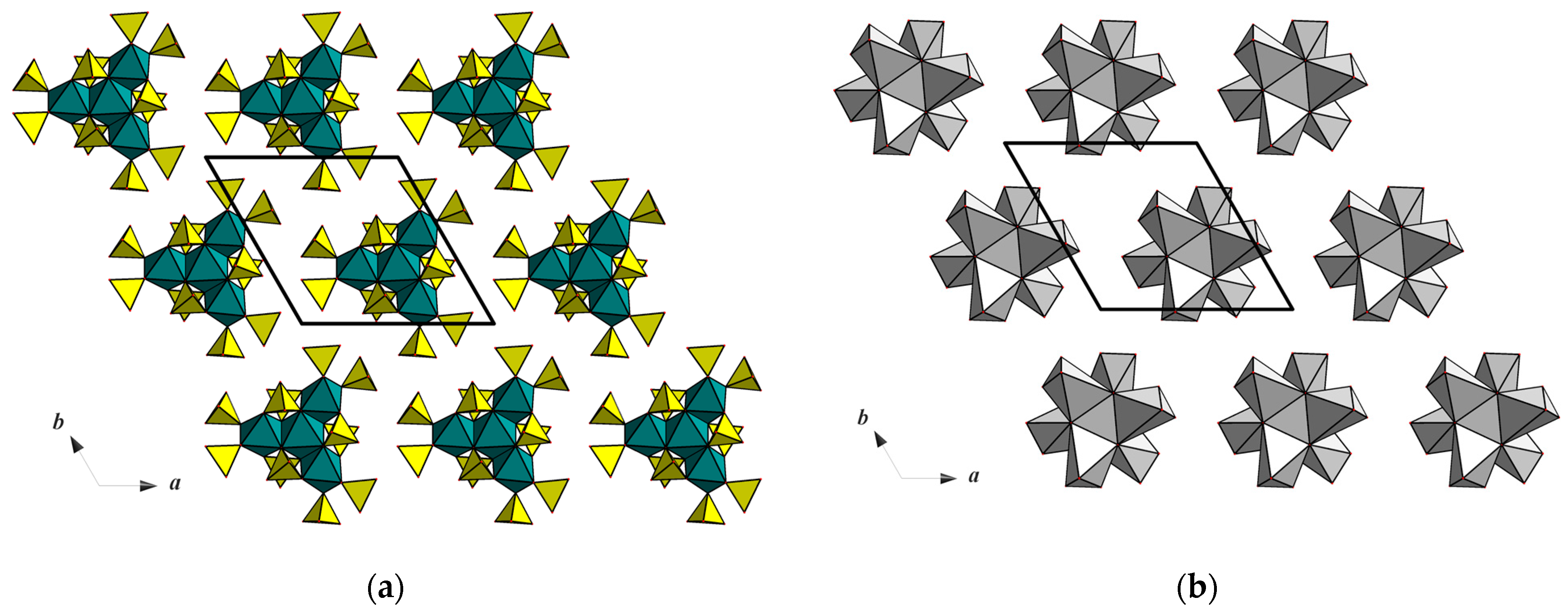
3.4. Topological Analysis of Ion Conductivity of Na7V4(PO4)6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Eleish, A.; Hystad, G.; Golden, J.J.; Downs, R.T.; Morrison, S.M.; Hummer, D.R.; Ralph, J.P.; Fox, P.; Hazen, R.M. Analysis and visualization of vanadium mineral diversity and distribution. Am. Miner. 2018, 103, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Schindler, M.; Hawthorne, F.C.; Baur, W.H. A crystal-chemical approach to the composition and occurrence of vanadium minerals. Can. Miner. 2000, 38, 1443–1456. [Google Scholar] [CrossRef] [Green Version]
- O’Loughlin, E.J.; Boyanov, M.I.; Kemner, K.M. Reduction of Vanadium(V) by Iron(II)-Bearing Minerals. Minerals 2021, 11, 316. [Google Scholar] [CrossRef]
- Cámara, F.; Bindi, L.; Pagano, A.; Pagano, R.; Gain, S.E.; Griffin, W.L. Dellagiustaite: A Novel Natural Spinel Containing V2+. Minerals 2019, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Pekov, I.V.; Zubkova, N.V.; Yapaskurt, V.O.; Belakovskiy, D.I.; Lykova, I.S.; Vigasina, M.F.; Sidorov, E.G.; Pushcharovsky, D.Y. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. I. Yurmarinite, Na7(Fe3+,Mg,Cu)4(AsO4)6. Miner. Mag. 2014, 78, 905–917. [Google Scholar] [CrossRef]
- Lii, K.-H. Na7Fe4(PO4)6: A mixed-valence iron phosphate containing a tetramer of edge-sharing FeO6 octahedra. J. Chem. Soc. Dalton Trans. 1996, 6, 819–822. [Google Scholar] [CrossRef]
- Masquelier, C.; d’Yvoire, F.; Collin, G. Crystal structure of Na7Fe4(AsO4)6 and α-Na3Al2(AsO4)3, two sodium ion conductors structurally related to II-Na3Fe2(AsO4)3. J. Solid State Chem. 1995, 118, 33–42. [Google Scholar] [CrossRef]
- Bourguiba, N.F.; Ouerfelli, N.; Zid, M.F. Crystal Structure, Charge-Distribution and Bond-Valence-Sum Investigations of a Novel Arsenate Na3.5Cr1.83(AsO4)3. J. Adv. Chem. 2014, 10, 3160–3170. [Google Scholar]
- Bourguiba, N.F.; Guesmi, A.; Ouerfelli, N.; Mazza, D.; Zid, M.F.; Driss, A. Le Nouvel Arséniate Ag1.60Na1.40Al2(AsO4)3: Synthèse, Études Structurale Et Électrique, Simulation Des Chemins De Conduction Ionique. J. Soc. Chim. Tunis. 2012, 14, 201–211. [Google Scholar]
- Gulyaeva, O.A.; Solodovnikova, Z.A.; Solodovnikov, S.F.; Zolotova, E.S.; Mateyshina, Y.G.; Uvarov, N.F. Triple molybdates K3–xNa1 + xM4(MoO4)6 (M = Ni, Mg, Co) and K3+xLi1–xMg4(MoO4)6 isotypic with II-Na3Fe2(AsO4)3 and yurmarinite: Synthesis, potassium disorder, crystal chemistry and ionic conductivity. Acta Cryst. 2020, B76, 913–925. [Google Scholar]
- D‘Yvoire, F.; Bretey, E.; Collin, G. Crystal structure, non-stoichiometry and conductivity of II-Na3M2(AsO4)3 (M = Al, Ga, Cr, Fe). Solid State Ion. 1988, 28, 1259–1264. [Google Scholar] [CrossRef]
- Yakubovich, O.V. Microporous Vanadylphosphates—Perspective Materials for Technological Applications. In Minerals as Advanced Materials II; Krivovichev, S., Ed.; Springer: Berlin, Heidelberg, 2011; pp. 239–253. [Google Scholar]
- Yakubovich, O.V.; Steele, I.M.; Yakovleva, E.V.; Dimitrova, O.V. One-dimensional decavanadate chains in the crystal structure of Rb4[Na(H2O)6][HV10O28]·4H2O. Acta Cryst. 2015, C71, 465–473. [Google Scholar]
- Yakubovich, O.V.; Kiryukhina, G.V.; Dimitrova, O.V. Crystal chemistry of KCuMn3(VO4)3 in the context of detailed systematics of the alluaudite family. Crystallogr. Rep. 2016, 61, 566–575. [Google Scholar] [CrossRef]
- Shvanskaya, L.V.; Yakubovich, O.V.; Krikunova, P.V.; Kiriukhina, G.V.; Ivanova, A.G.; Volkov, A.S.; Dimitrova, O.V.; Borovikova, E.Y.; Volkova, O.S.; Vasiliev, A.N. Nonstoichiometric ellenbergerite-type phosphates: Hydrothermal synthesis, crystal chemistry, and magnetic behavior. Inorg. Chem. 2022, 61, 4879–4886. [Google Scholar] [CrossRef]
- Zatovsky, I.V. Nasicon-type Na3V2(PO4)3. Acta Cryst. 2010, E66, 112. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Rigaku, O.D. CrysAlis PRO; Rigaku Oxford Diffraction Ltd.: Yarnton, Oxfordshire, UK, 2018. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, K. Diamond; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- Chernyshov, A.A.; Veligzhanin, A.A.; Zubavichus, Y.V. Structural Materials Science end-station at the Kurchatov Synchrotron Radiation Source: Recent instrumentation upgrades and experimental results. Nucl. Instrum. Methods Phys. Res. 2009, A603, 95–98. [Google Scholar] [CrossRef]
- Newville, M. Larch: An analysis package for XAFS and related spectroscopies. J. Phys. Conf. Ser. 2013, 430, 012007. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Cryst. 1985, B41, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Brown, I.D. Recent developments in the methods and applications of the bond valence model. Chem. Rev. 2009, 109, 6858–6919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles, D.S.; Guo, F.; Shan, X.; Kim, S.; Lebens-Higgins, Z.W.; Xu, W.; Su, D.; Piper, L.F.J.; Teng, X. Dual-stage K+ ion intercalation in V2O5-conductive polymer composites. J. Mater. Chem. 2021, A9, 15629–15636. [Google Scholar] [CrossRef]
- Dau, H.; Iuzzolino, L.; Dittmer, J. The tetra-manganese complex of photosystem II during its redox cycle—X-ray absorption results and mechanistic implications. Biochim. Biophys. Acta Bioenerg. 2001, 1503, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Chae, O.B.; Chae, S.; Ryu, J.H.; Oh, S.M. Amorphous Vanadium Titanates as a Negative Electrode for Lithium-ion Batteries. J. Electrochem. Sci. Technol. 2016, 7, 306–315. [Google Scholar] [CrossRef]
- Wang, X.; Hu, P.; Chen, L.; Yao, Y.; Kong, Q.; Cui, G.; Shi, S.; Chen, L. An α-CrPO4-type NaV3(PO4)3 anode for sodium-ion batteries with excellent cycling stability and the exploration of sodium storage behavior. J. Mater. Chem. 2017, A5, 3839–3847. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Vanadium geochemistry in the biogeosphere–speciation, solid-solution interactions, and ecotoxicity. Appl. Geochem. 2019, 102, 1–25. [Google Scholar] [CrossRef]
- Haskel, D.; Islam, Z.; Lang, J.; Kmety, C.; Srajer, G.; Pokhodnya, K.I.; Epstein, A.J.; Miller, J.S. Local structural order in the disordered vanadium tetracyanoethylene room-temperature molecule-based magnet. Phys. Rev. 2004, B70, 054422. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, B32, 751–767. [Google Scholar] [CrossRef]
- Montgomery, H.; Morosin, B.; Natt, J.J.; Witkowska, A.T.; Lingafelter, E.C. The crystal structure of Tutton’s salts. VI. Vanadium (II), iron (II) and cobalt (II) ammonium sulfate hexahydrates. Acta Cryst. 1967, 22, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Cotton, F.A.; Falvello, L.R.; Llusar, R.; Libby, E.; Murillo, C.A.; Schwotzer, W. Synthesis and characterization of four vanadium (II) compounds, including vanadium (II) sulfate hexahydrate and vanadium (II) saccharinates. Inorg. Chem. 1986, 25, 3423–3428. [Google Scholar] [CrossRef]
- Cotton, F.A.; Falvello, L.R.; Murillo, C.A.; Pascual, I.; Schultz, A.J.; Tomás, M. Neutron and X-ray Structural Characterization of the Hexaaquavanadium (II) Compound VSO4·7H2O. Inorg. Chem. 1994, 33, 5391–5395. [Google Scholar] [CrossRef]
- Ellis, B.L.; Ramesh, T.N.; Davis, L.J.M.; Goward, G.R.; Nazar, L.F. Structure and electrochemistry of two-electron redox couples in lithium metal fluorophosphates based on the tavorite structure. Chem. Mater. 2011, 23, 5138–5148. [Google Scholar] [CrossRef]
- Vaughan, G.B.M.; Gaubicher, J.; Le Mercier, T.; Angenault, J.; Quarton, M.; Chabre, Y. Crystal structure of the end product of electrochemical lithium intercalation in V2(SO4)3. Mater. Chem. 1999, 9, 2809–2812. [Google Scholar] [CrossRef]
- Glaum, R.; Gruehn, R. Beiträge zum thermischen Verhalten von wasserfreien Phosphaten. Synthese, Kristallstruktur und magnetisches Verhalten von V2PO5. Z. Kristallogr. Cryst. Mater. 1989, 186, 91–93. [Google Scholar]
- Pachoud, E.; Cumby, J.; Lithgow, C.T.; Attfield, J.P. Charge order and negative thermal expansion in V2OPO4. J. Am. Chem. Soc. 2018, 140, 636–641. [Google Scholar] [CrossRef] [Green Version]
- De Beaulieu, D.C.; Müller-Buschbaum, H. Gemischtvalente Oxovanadate. II. Synthese und Kristallstruktur von SrV10O15. Z. Anorg. Allg. Chem. 1981, 472, 33–37. [Google Scholar] [CrossRef]
- Matsuno, K.I.; Katsufuji, T.; Mori, S.; Moritomo, Y.; Machida, A.; Nishibori, E.; Takata, M.; Sakata, M.; Yamamoto, N.; Takagi, H. Orbital Order and Phase Transitions in Vanadium Spinels. J. Phys. Soc. Japan 2001, 70, 1456–1459. [Google Scholar] [CrossRef]
- Horibe, Y.; Shingu, M.; Kurushima, K.; Ishibashi, H.; Ikeda, N.; Kato, K.; Motome, Y.; Furukawa, N.; Mori, S.; Katsufuji, T. Spontaneous formation of vanadium “molecules” in a geometrically frustrated crystal: AlV2O4. Phys. Rev. Lett. 2006, 96, 086406. [Google Scholar] [CrossRef]
- Reuter, B.; Aust, R.; Colsmann, G.; Neuwald, C. Darstellung und Eigenschaften Vanadium (II)-haltiger und damit n-leitender Vanadium (III)-Spinelle. Z. Anorg. Allg. Chem. 1983, 500, 188–198. [Google Scholar] [CrossRef]
- Kalavathi, S.; Raju, S.V.; Williams, Q.; Sahu, P.C.; Sastry, V.S.; Sahu, H.K. Pressure-induced frustration in charge ordered spinel AlV2O4. J. Phys. Cond. Matter 2013, 25, 292201. [Google Scholar] [CrossRef] [PubMed]
- Kinomura, N.; Matsui, N.; Kumada, N.; Muto, F. Synthesis and crystal structure of NaV3P3O12: A stuffed structure of α-CrPO4. J. Solid State Chem. 1989, 79, 232–237. [Google Scholar] [CrossRef]
- Liu, G.; Greedan, J.E. Structure and Magnetism in AB10O15+x (A= Sr, Ba; B= V, Cr; x= 0, ∼1): Evidence for Geometric Frustration. J. Solid State Chem. 1996, 122, 416–427. [Google Scholar] [CrossRef]
- Urusov, V.S.; Serezhkin, V.N. Distortion of Vz+On coordination polyhedra and parameters of the bond valence model for VO bonds in inorganic crystals. Crystallogr. Rep. 2009, 54, 190–194. [Google Scholar] [CrossRef]
- Cotton, F.A.; Daniels, L.M.; Montero, M.L.; Murillo, C.A. Preparation of vanadium (II) compounds. Structures of a carboxylato and a 3-pyridinesulphonate compound. Polyhedron 1992, 11, 2767–2774. [Google Scholar] [CrossRef]
- Kranz, M.; Goldberg, D.E.; Ribner, A. Vanadium (II) Sulfate. Inorg. Synth. 1963, 7, 94–96. [Google Scholar]
- Li, W.; Niu, Z.; Zhu, X. Recovery of iron by jarosite crystallization and separation of vanadium by solvent extraction with extractant 7101 from titanium white waste liquid (TWWL). Water Sci. Technol. 2021, 83, 2025–2037. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, J.; Guo, Y.; Zhu, H.; Huang, X. Research progress on Na3V2(PO4)3 cathode material of sodium ion battery. Front. Chem. 2020, 8, 635. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, K.; Lv, X.; Shi, K.; Wang, Y.; Nian, Z.; Li, Y.; Wang, L.; Dai, L.; He, Z. Recent advances of NASICON-Na3V2(PO4)3 as cathode for sodium-ion batteries: Synthesis, modifications, and perspectives. J. Alloys Compd. 2021, 867, 159060. [Google Scholar] [CrossRef]
- Zheng, Q.; Yi, H.; Li, X.; Zhang, H. Progress and prospect for NASICON-type Na3V2(PO4)3 for electrochemical energy storage. J. Energy Chem. 2018, 27, 1597–1617. [Google Scholar] [CrossRef] [Green Version]
- Jian, Z.; Han, W.; Lu, X.; Yang, H.; Hu, Y.-S.; Zhou, J.; Zhou, Z.; Li, J.; Chen, W.; Chen, D.; et al. Superior electrochemical performance and storage mechanism of Na3V2(PO4)3 cathode for room-temperature sodium-ion batteries. Adv. Energy Mater. 2013, 3, 156–160. [Google Scholar] [CrossRef]
- Saravanan, K.; Mason, C.W.; Rudola, A.; Wong, K.H.; Balaya, P. The first report on excellent cycling stability and superior rate capability of Na3V2(PO4)3 for sodium ion batteries. Adv. Energy Mater. 2013, 3, 444–450. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; David, R.; Chotard, J.N.; Recham, N.; Masquelier, C. A high voltage cathode material for sodium batteries: Na3V(PO4)2. Inorg. Chem. 2018, 57, 8760–8768. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoon, G.; Kim, H.; Park, Y.U.; Kang, K. Na3V(PO4)2: A new layered-type cathode material with high water stability and power capability for Na-ion batteries. Chem. Mater. 2018, 30, 3683–3689. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.; Sheng, T.; Zheng, S.; Zhong, G.; Zheng, G.; Liang, Z.; Ortiz, G.F.; Zhao, W.; Mi, J.; et al. Novel 3.9 V Layered Na3V3(PO4)4 Cathode Material for Sodium Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 3603–3606. [Google Scholar] [CrossRef]
- Ke, L.; Dong, J.; Lin, B.; Yu, T.; Wang, H.; Zhang, S.; Deng, C. A NaV3(PO4)3@C hierarchical nanofiber in high alignment: Exploring a novel high-performance anode for aqueous rechargeable sodium batteries. Nanoscale 2017, 9, 4183–4190. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P. Analysis of voids in crystal structures: The methods of “dual” crystal chemistry. Acta Cryst. 2003, A59, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Blatov, V.A. Voronoi–dirichlet polyhedra in crystal chemistry: Theory and applications. Crystallogr. Rev. 2004, 10, 249–318. [Google Scholar] [CrossRef]
- Fedotov, S.S.; Kabanova, N.A.; Kabanov, A.A.; Blatov, V.A.; Khasanova, N.R.; Antipov, E.V. Crystallochemical tools in the search for cathode materials of rechargeable Na-ion batteries and analysis of their transport properties. Solid State Ion. 2018, 314, 129–140. [Google Scholar] [CrossRef]
- Meutzner, F.; Münchgesang, W.; Leisegang, T.; Schmid, R.; Zschornak, M.; Ureña de Vivanco, M.; Shevchenko, A.P.; Blatov, V.A.; Meyer, D.C. Identification of solid oxygen-containing Na-electrolytes: An assessment based on crystallographic and economic parameters. Cryst. Res. Technol. 2017, 52, 1600223. [Google Scholar] [CrossRef]

| Crystal Data | |
| Chemical formula | Na7V4P6O24 |
| Mr | 934.51 |
| Crystal system, space group | Trigonal, Rc:H |
| Temperature (K) | 110 |
| a, c (Å) | 13.3463 (19), 17.809 (4) |
| V (Å3) | 2747.2 (10) |
| Z | 6 |
| Radiation type | Mo Kα |
| µ (mm−1) | 2.81 |
| Crystal size (mm) | 0.08 × 0.08 × 0.04 |
| Data Collection 1 | |
| Diffractometer | Bruker D8 Quest |
| Absorption correction | Multi-scan |
| Tmin, Tmax | 0.453, 0.494 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 9263, 894, 781 |
| Rint | 0.045 |
| (sin θ/λ)max (Å−1) | 0.703 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.023, 0.059, 1.04 |
| No. of reflections | 894 |
| No. of parameters | 65 |
| Δρmax, Δρmin (e Å−3) | 0.46, −0.49 |
| V1 Octahedron | Na1 0ctahedron | ||
| V1—O3 × 6 | 2.0173 (13) | Na1—O4 × 6 | 2.3653 (14) |
| V2 Octahedron | Na2 [6+3] Polyhedron | ||
| V2—O1 × 2 | 1.9878 (13) | Na2—O4 | 2.2706 (16) |
| V2—O2 × 2 | 1.9942 (14) | Na2—O1 | 2.5290 (16) |
| V2—O3 × 2 | 2.0548 (13) | Na2—O4 | 2.5718 (17) |
| Na2—O2 | 2.5721 (16) | ||
| P Tetrahedron | Na2—O4 | 2.6202 (17) | |
| P—O4 | 1.5103 (14) | Na2—O2 | 2.6401 (17) |
| P—O2 | 1.5428 (15) | Na2—O1 | 2.8084 (17) |
| P—O1 | 1.5513 (14) | Na2—O3 | 2.8765 (16) |
| P—O3 | 1.5676 (14) | Na2—O1 | 3.0213 (14) |
| V1 | V2 | P | Na1 | Na22 | ||
|---|---|---|---|---|---|---|
| O1 | 0.510 ×2↓ | 1.189 | 0.134, 0.075, 0.049 | 1.96 | ||
| O2 | 0.501 ×2↓ | 1.215 | 0.123, 0.107 | 1.95 | ||
| O3 | 0.471 ×6↓ | 0.425 ×2↓ | 1.135 | 0.066 | 2.10 | |
| O4 | 1.328 | 0.189 ×6↓ | 0.230, 0.123, 0.111 | 1.98 | ||
| 2.83 | 2.87 | 4.87 | 1.13 | 1.02 |
| Na7V4(PO4)6 | Na7Fe4(PO4)6 | Yurmarinite, Na7(Fe3+, Mg, Cu)4(AsO4)6 | |
|---|---|---|---|
| a (b) | 13.3463(19) | 13.392(2) | 13.7444(2) |
| c | 17.809(4) | 17.858(3) | 18.3077(3) |
| V | 2747.2(10) | 2773.7(8) | 2995.14(8) |
| M2–O3 × 6 | 2.0173(13) | 2.063(2) | 1.9429(18) |
| <M2–O> | 2.02 | 2.06 | 1.94 |
| M3–O1 × 2 | 1.9877(13) | 1.974(2) | 1.991(2) |
| M3–O2 × 2 | 1.9942(14) | 1.997(2) | 2.0142(19) |
| M3–O3 × 2 | 2.0548(13) | 2.085(1) | 2.1170(19) |
| <M3–O> | 2.01 | 2.02 | 2.04 |
| Compound/ Mineral | Space Group, Z, ρ, mg/m3 | Unit-Cell Para-Meters, a, b, c, Å and Angles,° | R | Range of V–O Distances in Octahedra, Å | Synthesis Technique/Natural Genesis | Ref. |
|---|---|---|---|---|---|---|
| (NH4)2V(SO4)2·6H2O NH4,V-analogue of picromerite and Tutton’s salt | P21/a 2, 1.80 | a = 9.42(3) b = 12.76(3) c = 6.22(2) β =107.2(2) | 0.08 | 2.118–2.164 <2.15> | Modification of Kranz method to obtain VOSO4, its further cooling and electrolytic reduction in H2SO4 solution, then the addition of pyrogallol and recrystallization in N2 atmosphere. | [34] |
| V(H2O)6SO4 V-analogue of hexahydrite | C2/c 8, 1.91 | a = 10.081(3) b = 7.286(2) c = 24.445(7) β = 98.78(2) | 0.039 | 2.123–2.136 <2.13> 2.120–2.150 <2.13> | Modification of the Kranz method. Electrochemical reduction of VOSO4·2H2O in the solution of H2SO4 under N2 or Ag atmosphere followed by adding ethanol on cooling. | [35] |
| V(H2O)6SO4·H2O | P21/c 4, 1.86 | a = 14.130(3) b = 6.501(1) c = 11.017(2) β = 105.64(2) | 0.029 | 2.102–2.137 <2.12> 2.119–2.150 <2.13> | Recrystallization of VSO4·6H2O precursor in the solution of H2SO4 in N2 atmosphere with the addition of ethanol and cooling. | [36] |
| Li2VPO4F closely related to tavorite str. type | C2/c 4, 3.17 | a = 7.2255(1) b = 7.9450(1) c = 7.3075(1) β = 116.771(1) | 0.049 * | 2.119–2.139 <2.13> | Solid-state synthesis of LiV(PO4)F at 750 °C in an Ar atmosphere, followed by Li-intercalation with LiAlH4 in tetrahydrofuran under Ar. | [37] |
| Li2V2(SO4)3 derivative of NASICON str. type | C2/c 4, 3.01 | a =13.0582(5) b = 8.6526(4) c = 8.72447(4) β = 115.443(2) | 0.042 * | 2.073–2.230 <2.14> | Electrochemical Li-intercalation in V2(SO4)3 with n–butyllithium C4H9Li in a vacuum. | [38] |
| V2+,3+2OPO4 (high-T modif.) | I41/amd 4, 3.97 | a = 5.362(5) c = 12.378(9) | 0.044 | 2.047–2.074 <2.06> | Reduction of VPO4 by metallic V at 500 °C and 900 °C via chemical vapor transport in I2. | [39] |
| V2+,3+2OPO4 (low-temperature modification) | C2/c 4, 3.98 | a = 7.56825(7) b = 7.60013(7) c = 7.21794(6) β =121.2751(5) | 0.074 * | 2.054–2.123 <2.09> 1.972–2.079 <2.03> | β-VOPO4 preparation at 600 °C in flowing O2;further solid-state synthesis at 1000 °C with metallic V in a vacuum. | [40] |
| SrV3+,2+10O15 ** | Ccmb 4, 5.20 | a = 9.915 b =11.574 c = 9.324 | 0.08 | 1.948–2.192 <2.01> 1.950–2.148 <2.05> 1.965–2.195 <2.04> | Solid-state synthesis at 1900 °C in H2 atmosphere | [41] |
| Al(V2+,3+)2O4 (frustrated spinel) | Rm 2, 4.65 | a = 5.7613(2) c = 28.6876(10) | 0.011 * | 2.052–2.063 <2.06> 2.007 | Solid-state synthesis with metallic Al at 1150 °C for 150 h in vacuum, phase transition at 427 °C | [42,43] |
| Al(V2+,3+)2O4 (inverse spinel) | Fdm 8, 4.66 | a = 8.1546(6) | No data | 2.040 | Solid-state synthesis with metallic V at 900 °C in a vacuum | [44,45] |
| Dellagiustaite, Al(V2+,V3+)2O4 (inverse spinel) | Fdm 8, 4.62 | a = 8.1950(1) | 0.014 | 2.045 | High redox conditions, probable crystallization from high-temperature melts of volcanic magma | [4] |
| NaV3+,2+3(PO4)3 | Imma 4, 3.42 | a = 10.488(2) b =13.213(3) c = 6.455(7) | 0.056 | 1.959–2.062 <1.99> 2.042–2.086 <2.06> | Step-wise solid-state synthesis at 900 °C in a vacuum | [46] |
| Na7V3+,2+4(PO4)6 V, P-analogue of yurmarinite | Rc 6, 3.39 | a =13.3463(19) c = 17.809(4) | 0.023 | 2.017 1.988–2.055 <2.01> | High-temperature hydrothermal synthesis at 450 °C with Na3C6H5O7 redox agent | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiriukhina, G.; Nesterova, V.; Yakubovich, O.; Volkov, A.; Dimitrova, O.; Trigub, A.; Lyssenko, K. Mixed Valanced V3+,V2+ Phosphate Na7V4(PO4)6: A Structural Analogue of Mineral Yurmarinite. Minerals 2022, 12, 1517. https://doi.org/10.3390/min12121517
Kiriukhina G, Nesterova V, Yakubovich O, Volkov A, Dimitrova O, Trigub A, Lyssenko K. Mixed Valanced V3+,V2+ Phosphate Na7V4(PO4)6: A Structural Analogue of Mineral Yurmarinite. Minerals. 2022; 12(12):1517. https://doi.org/10.3390/min12121517
Chicago/Turabian StyleKiriukhina, Galina, Valentina Nesterova, Olga Yakubovich, Anatoly Volkov, Olga Dimitrova, Alexander Trigub, and Konstantin Lyssenko. 2022. "Mixed Valanced V3+,V2+ Phosphate Na7V4(PO4)6: A Structural Analogue of Mineral Yurmarinite" Minerals 12, no. 12: 1517. https://doi.org/10.3390/min12121517
APA StyleKiriukhina, G., Nesterova, V., Yakubovich, O., Volkov, A., Dimitrova, O., Trigub, A., & Lyssenko, K. (2022). Mixed Valanced V3+,V2+ Phosphate Na7V4(PO4)6: A Structural Analogue of Mineral Yurmarinite. Minerals, 12(12), 1517. https://doi.org/10.3390/min12121517









