Abstract
Chalcopyrite is well known as being refractory to conventional leaching approaches at atmospheric pressure. The current study investigated a hybrid approach using aqueous ammonia as a pH modifier for glycine-based lixiviant systems to leach copper from chalcopyrite while maintaining surface refreshment using ceramic media at room temperature. The glycine–ammonia system exhibited significantly better copper extraction than the traditional glycine–NaOH system. A copper extraction of 91.5% was achieved after 72 h of leaching by using 0.71 M ammonia, at a Gly:Cu molar ratio of 4:1, by using a solid content of 1%, with a ceramic media: solid ratio of 3:5 and at ambient temperature. Adding ceramic beads during leaching led to a breakup of particles and a refreshment of particles’ surfaces that significantly improved the copper extraction. At a solid content of 10%, oxygen is essential for leaching; a copper extraction of 95.4% was achieved with oxygen injection, while only 33.4% copper extraction was obtained without introducing oxygen. A kinetic analysis indicates that the leaching rate is limited by combined liquid film diffusion and diffusion through the product layer. A conceptual flowsheet is proposed, where chalcopyrite concentrate can be extracted by a leaching–grinding process and copper can be recovered by a solvent extraction–electrowinning circuit.
1. Introduction
Chalcopyrite, as the most abundant source of sulfide copper in the Earth’s crust and one of the most refractory sulfide copper minerals, has been investigated intensively for copper extraction in past decades. Typically, copper from chalcopyrite is recovered by flotation followed by pyrometallurgical processes. However, the pyrometallurgical processes usually generate severe environmental pollutants such as furans, dioxins and highly acidic wastewater [], which has made the hydrometallurgical processing route more preferable []. Additionally, smelting poses a problem when the concentrate contains trace amounts of mercury, arsenic or volatile radionuclides that may be present as the daughter products of uranium in the ore (e.g., chalcopyrite from some iron oxide–copper–gold (IOCG) deposits, such as that found in South Australia).
Chalcopyrite is usually considered refractory under atmospheric pressure leaching conditions. Many lixiviants have been studied for chalcopyrite leaching, such as sulfuric acid, nitric acid, ferric chloride, ferric sulfate, cyanide, ammonia and bioleaching [,,]. Sulfuric acid with ferric ions is the most widely accepted lixiviant as it is the cheapest lixiviant for leaching many base metals from their ores. However, gangue minerals can be dissolved by acids, and iron can contaminate the leachate due to the simultaneous dissolution of copper and iron in acid media []. Additionally, the dissolution rate of chalcopyrite is generally slow under either acidic or alkaline conditions. In an acidic leaching system, it was speculated that the formation of a secondary product, e.g., a complex film of sulfide, polysulfide, elemental sulfide, ferric hydroxysulfate and jarosite, may passivate or form overlayers to impede the diffusion of reagents to the surface of chalcopyrite and/or the diffusion of ions away from the chalcopyrite surface []. However, a critical review on the passivation mechanism of chalcopyrite leaching conducted by O’Connor and Eksteen [] stated that the leaching of chalcopyrite may actually be inhibited by its semi-conductor behavior, not passivation layers. Depending on the pH and the leach system, there remains uncertainty on the contribution of the various kinetic-retarding factors for chalcopyrite leaching.
Leaching in alkaline conditions appears to have some advantages, considering the known challenges using acidic media. Aqueous ammonia solutions and its derivatives are the most widely accepted and industrially applied lixiviant for extracting base metals, such as copper, nickel and cobalt, due to its low cost, easy formation of water-soluble metal complexes and ease of regeneration by evaporation [], and is a process that has been commercialized at an early stage. Ammoniacal leaching processes allow for the selectivity leaching of copper against undesirable elements such as iron and calcium due to the alkaline nature of ammonia. In oxygenated ammonia solutions, the dissolution of chalcopyrite forms SO42− instead of S0, a soluble ammine complex with copper and iron rejected as iron oxides, and the overall oxidative reaction between ammonia and chalcopyrite is shown in Equation (1) [].
An early study suggested that the insolubility of iron was due to the layer comprising several species of iron oxide and oxydryoxide being a passivation layer []. Later, some researchers recognized that iron oxides are likely to play limited inhibiting roles due to the porous nature of iron oxides [,]. This is because it was observed that a high agitation speed can improve the leaching rate, while agitation has either no effect or reduces the leaching rate at acidic leaching environment. The leaching of chalcopyrite at alkaline conditions is not like that at acidic conditions, and the inhibition on the chalcopyrite surface in an alkaline solution is not fully understood []. In a batch system, there are many limitations, such as the loss of reagents through volatilization, oxidation or product formation, approaching their solubility limits in the leach liquor.
Recently, alkaline glycine solutions as the lixiviant for the leaching and recovery of base metals and precious metals were intensively studied [,,,,,,,,,,,,,,,,,]. Some of them studied glycine as a complexing agent for chalcopyrite leaching [,,]. Through an analysis using X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy, it was found that a loosely held porous layer that developed on the surface of chalcopyrite and consisted mainly iron oxyhydroxides has no apparent passivation behavior in an alkaline glycine leaching system []. A kinetic study conducted by Tanda, Eksteen, Oraby and O’Connor [] shows that after ultrafine grinding (100% passing 10 µm), a chalcopyrite mineral specimen comprising 67% chalcopyrite was leached by a 0.5 M glycine solution at pH 11.5, dissolved oxygen at 15 ppm and a temperature of 50 °C, with a copper extraction of about 90% after 96 h of leaching. This revealed that the leaching rate of chalcopyrite using glycine was markedly affected by the particle size and temperature, which means a high-energy intensity with commensurate costs. The anodic dissolution of chalcopyrite in alkaline glycine solutions may form sulfate or elemental sulfur, as described in Equations (2) and (3).
The solid content for chalcopyrite leaching was generally low in the literature, normally ranging from 0.75% to 2%, using glycine or ammonia as the lixiviant [,,]. A study conducted by Khezri et al. [] revealed that the leachability of chalcopyrite is extremely low using alkaline glycine solutions, and a decrease in copper extraction was observed if the pulp density >1%. The decline in extraction could be attributed to the precipitation of copper as copper sulfide and crystallized copper glycinate when the copper concentration was too high. A study that focused on extracting copper from waste-printed circuit boards (WPCBs) using alkaline glycine solutions has also shown that Cu extraction was low when the solid content increased to 10% []. That is probably due to too high Cu concentration in the final leachate, which leads to cupric glycinate (bis-glycinato copper (II)) crystallization. Glycine forms a range of coordination complexes with metals with potential cis- and trans-isomers as well as hydrates that influence the solubility of the complexes and the transition of kinetically favored isomers to thermodynamically favored isomers depending on a range of conditions [,].
Although shown by numerous authors that chalcopyrite can be leached by alkaline glycine solutions, it appears to require ultra-fine grinding, elevated temperature and prolonged leaching periods to reach good copper recovery, which makes for unappealing process economics. To further improve a glycine-based leaching system for chalcopyrite by avoiding ultra-fine grinding process and heating, this study proposed the use of ammonia solutions as the pH modifier (as a pH modifier is required) for the alkaline glycine system to leach chalcopyrite at ambient temperature and pressure. The effects of operating conditions, such as pH, glycine additions (Gly:Cu molar ratio) and the addition of ceramic beads as stirred mill media, on the copper extraction from chalcopyrite were investigated and are discussed in this research. The leachability of chalcopyrite at 10% solid/liquid ratios were studied. A kinetic study was undertaken using the shrinking core model to investigate the leaching behaviors of chalcopyrite in an ammonia–glycine system.
2. Materials and Methods
2.1. Materials
The chalcopyrite samples used in this study were obtained from a museum-quality specimen in Perth, Western Australia. The P80 particle size of the head sample analyzed by Malvern Mastersizer 2000 is 96.15 µm. Quantitative XRD results (Table 1) show that the materials contain mostly chalcopyrite (64%). Table 2 shows the XRF analysis of the elemental contents of the chalcopyrite samples.

Table 1.
Mineralogy composition of chalcopyrite specimen.

Table 2.
Elemental composition of the chalcopyrite specimen.
2.2. Procedures
All tests were conducted at room temperature and at atmospheric pressure. The solid/liquid mass ratio for the initial tests was 1% (i.e., 5 g of ores in 495 g of deionized water). For exploratory tests, batch bottle rolls were performed, and the slurry was mildly agitated in a 2 L plastic bottle with a ~5 mm hole on the lid at a rotating speed of 100 rpm. The amount of glycine (99%, Sigma-Aldrich, St. Louis, MO, USA) added was correlated to the moles of Cu from the material. In this study, the chalcopyrite samples were leached at alkaline conditions, and ammonia solutions (25%, EMSURE®) were used as the pH modifier. Ceramic beads (97% ZrO2, ~800 µm) were added as the grinding/surface refreshing media. Leaching was monitored over a 72 h period with 10 mL samples taken at 2, 4, 6, 24, 48 and 72 h using a vacuum filter. The leached residue and leachate were separated by vacuum filtration, and the residues were rinsed with 200 mL of deionized water before drying in an oven at 60 °C overnight. The metal concentrations from solution samples were analyzed by Inductively Coupled Plasma (ICP) Mass Spectrometry, and the elements from the leached residues were determined by X-ray Fluorescence (XRF) Spectrometry, which are all carried out independently by Bureau Veritas, Perth. The metal extraction (E%) was calculated by Equation (4).
where mt and mf represent the mass of metal in the leachate at time t and in the final leachate at 72 h, respectively, and mr represents the mass of metal in the final leached residue.
For the leaching tests of the chalcopyrite specimen using a 10% solid ratio, a 1 L jacketed glass reactor fitted with a reflux condenser, an overhead stirrer and a Teflon-coated two-blade impeller was used. Oxygen can be injected into the reactor, and its flowrate was controlled by an oxygen flowmeter. Temperature was maintained by pumping heated water through the jacket, and the water was maintained at a constant temperature with an external water bath reservoir.
2.3. Kinetic Model
Leaching kinetics are of a significant aspect for industrial application since it determines the scale and design of reactors []. In this study, shrinking core model (SCM) as a widely applied kinetic model for the reaction of solids and minerals [] was used to describe the leaching kinetics of chalcopyrite. According to SCM, solid particles are considered to be spherical, non-porous and initially surrounded by fluid films, while mass transfer occurs between solid particles and solutions in the bulk. Leaching processes are generally controlled by (1) diffusion through fluid film, (2) diffusion through product layer, (3) and chemical reaction, where the step with the lowest speed is the controlling step. The integrated mathematical expressions of each step for SCM are shown in Equations (5)–(7).
where x is the metal extraction; t is the reaction time (h); and kl, kd and kr are the apparent rate constants of the different controlling steps.
3. Results
3.1. Thermodynamic Consideration
In the case of using ammonia to leach chalcopyrite, a number of studies have reported that tetra-ammine Cu (Cu(NH3)42+) is the dominant species of ammine–Cu complexes (Equation (1)) [,]. This is also supported by its highest cumulative formation constant (log K = 13.32) among ammine–Cu complexes as shown in Table 3. Cu(NH3)42+ present in the solution may also act as an additional oxidant, apart from oxygen, thereby generating Cu+ as di-ammine Cu (Cu(NH3)2+, log K = 10.86) which could, in turn, be oxidized back to Cu(NH3)42+ by oxygen [,]. On the other hand, when using glycine only to leach natural and secondary resources, various studies have shown that Cu glycinate (Cu(Gly)2, log K = 15.54) is the dominant species among Cu–glycine complexes under their experimental conditions, and a pH of 10–11 is optimal for Cu leaching [,,]. It should be noted that most of the ammonia that is added is not “free” like in a pure ammonia system, as a significant portion of the ammonia is used to form the ammonium glycinate salt.
Radmehr et al. [,] have reported the Eh-pH diagram of the Cu–ammonia–H2O system at 25 °C. It reveals that at a pH of 10–11, Cu(NH3)2+ is only dominant in a reducing environment (Eh < 0 V vs. SHE), and above 0 V, Cu(NH3)42+ easily becomes dominant. Given that Cu(Gly)2 has a higher formation constant than Cu(NH3)42+ (Table 3), however, when glycine is added into the system, Cu may form a stronger complex with glycine than that with ammonia. It remains unclear if the ammonia forms an association (electron–donor–acceptor association) with glycine or other association and there is no clarity as to the exact nature of the ligand species that complexes the copper (i.e., whether the ammonia and glycine act independently or in some molecular association). Figure 1 plots the Eh-pH diagram of the Cu–glycine–ammonia–H2O system at 25 °C. It shows a wide pH-Eh range of Cu(Gly)2 but Cu(NH3)42+ does not occur under the present conditions. This implies that when the leaching of chalcopyrite reaches equilibrium, the majority of leached Cu may be present as Cu(Gly)2 instead of Cu(NH3)42+. The predominance of Cu(NH3)2+ indicates that ammonia may act not only as a pH modifier to buffer glycine but also as a synergistic lixiviant. With the increase in solution Eh, Cu(NH3)2+ can be further oxidized and be predominated as Cu(Gly)2 (Figure 1), if the species is assumed to act separately.
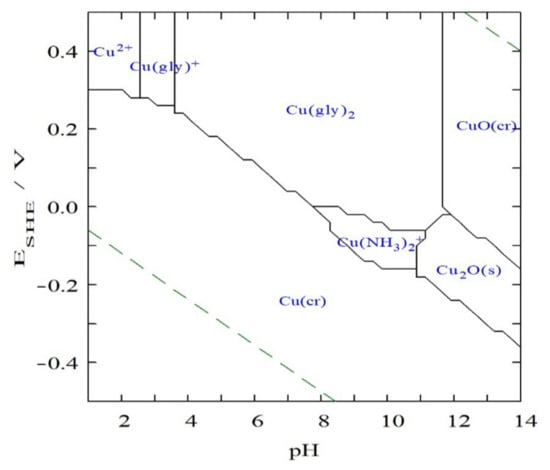
Figure 1.
Eh-pH diagram of the copper–glycine–ammonia–H2O system at 25 °C, copper concentration of 0.02 M, glycine concentration of 0.16 M and ammonia concentration of 0.5 M (made by Medusa-Hydra software, available at https://www.kth.se/che/medusa/, accessed on 30 August 2022).

Table 3.
Cumulative formation constants of selected complexes between metal and glycine or ammonia at 20–25 °C (M stands for Cu ion, and L stands for glycine or ammonia (ammine) ligand); data taken from [,].
Table 3.
Cumulative formation constants of selected complexes between metal and glycine or ammonia at 20–25 °C (M stands for Cu ion, and L stands for glycine or ammonia (ammine) ligand); data taken from [,].
| Complexing with Glycine | Complexing with Ammonia | |||||||
|---|---|---|---|---|---|---|---|---|
| logKML | logKML2 | logKML3 | logKML | logKML2 | logKML3 | logKML4 | logKML5 | |
| Cu+ | - | 10.0 | - | 5.93 | 10.86 | - | - | - |
| Cu2+ | 8.60 | 15.54 | 16.27 | 4.31 | 7.98 | 11.02 | 13.32 | 12.86 |
3.2. Effects of pH Modifier
Tanda, Eksteen, Oraby and O’Connor [] have reported that copper was poorly leached from chalcopyrite (28.5%) when using glycine-only solutions even at an elevated temperature of 50 °C, a high pH of 11.5 (adjusted and maintained by NaOH), a high glycine concentration of 1 M, a low solid percentage of 0.75% and a size fraction from 20 to 28 µm. In present study, the chalcopyrite leaching was performed in glycine solutions but with ammonia as the pH modifier and co-ligand. Two other leaching systems including glycine–NaOH and ammonia-only systems were conducted as comparisons. As indicated in Figure 2, glycine leaching using ammonia as a pH modifier enhanced the copper extraction significantly compared with using NaOH (91.5% vs. 30.6% at 72 h). By contrast, the leaching using ammonia only to maintain the same pH of 10.5 reached a copper extraction of only 65.4%.
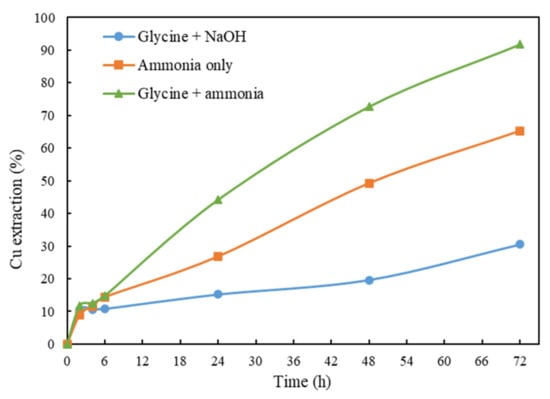
Figure 2.
Cu extraction after 72 h of leaching by glycine + NaOH (Gly:Cu = 4:1), ammonia only and glycine + ammonia (Gly:Cu = 4:1). Other conditions: ceramic beads: solid = 3:5, room temperature, rolling speed = 100 rpm, pH = 10.5, 1% solid ratio.
Table 4 shows the reagent dosages used in each experiment. As the focus was on achieving the same operating pH, the alkalizing agent was adjusted to achieve the target pH.

Table 4.
Reagent dosages, final Cu concentration and final Cu extractions for different tests: T1 (Gly + NaOH), T2 (ammonia only) and T3 (Gly + ammonia).
3.3. Effects of Adding Ceramic Beads
Previous studies indicated that mechanical activation through grinding/ultrafine-grinding can significantly improve Cu extraction from chalcopyrite [,]. In this test, instead of grinding before leaching, grinding media (ceramic beads) were added during leaching. The effects of the mass ratio of ceramic beads to solid on Cu extraction were studied. From Figure 3, the final copper extraction is about 64.3% in the absence of ceramic beads, which is significantly lower than that of using ceramic beads at mass ratios of 1:5 (83.8%) and 3:5 (91.5%). The leaching kinetics at a ratio of 1:5 is seen to be faster than that at a ratio of 3:5; that is mainly attributed to the difference in time when ammonia was added for pH adjustment during the leaching. For all the tests, the addition of ammonia was similar at ~0.7 M NH3.
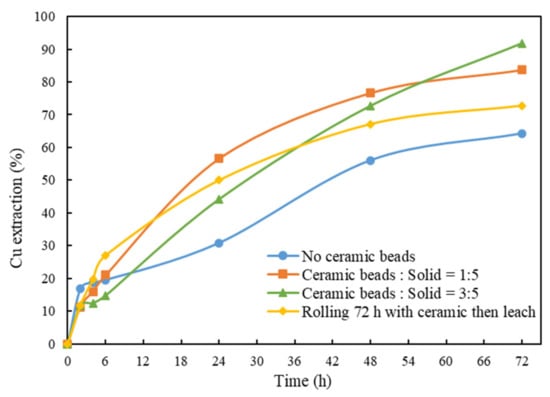
Figure 3.
Cu extraction after 72 h of leaching using different amounts of ceramic beads. Other conditions: solid/liquid ratio = 1%, pH = 10.5, room temperature and Gly:Cu = 4:1.
Figure 4 exhibits a change in particle size distribution (PSD) before and after leaching with ceramic beads added at a ratio of 3:5. A significant particle size reduction was observed, where P80 reduced from 96.2 µm to just 16.7 µm after leaching. Another PSD obtained from rolling the samples in water with the same ratio of ceramic beads for 72 h confirms that rolling the samples with ceramic beads reduced the particle size of the chalcopyrite sample. This sample was then leached by the glycine–ammonia system and a copper extraction of 72.7% was achieved. From these results, it appears that reducing the particle size increased the copper extraction by ~8%. Leaching with the presence of ceramic beads significantly increased the copper extraction by ~19%. This implies that rolling the chalcopyrite samples with the ceramic beads during leaching can not only reduce the particle size but also help refresh the surface of chalcopyrite.
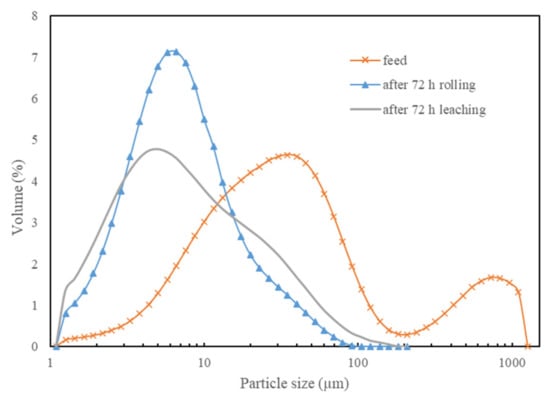
Figure 4.
PSD of the feed and the residue after leaching (ceramic beads: solid = 3:5) and rolling without leaching (ceramic beads: solid = 3:5).
It is noted that the PSD for rolling in water only leads to a narrower distribution compared to the situation with leaching. A new approach for identifying the rate-controlling step applied to the leaching of nickel from spent catalyzation is that in the case for where leaching took place, ferric hydroxide fines were loose particles (more particles in the 1–3 µm range) and again more particles were found in the 20–100 µm range, which probably reflects the larger particles due to the hydrous ferric hydroxide product layer that forms. After leaching, the product is predominantly hydrous ferric hydroxide, whereas the test performed only in water still had the chalcopyrite (no leaching, only that the particles shrunk).
3.4. Effects of Gly:Cu Molar Ratio
In this study, the amount of glycine added was correlated to the total moles of Cu from the material. The effects of Gly:Cu molar ratio on the leaching kinetics of chalcopyrite were investigated. The results shown in Figure 5 indicate that the Cu extraction increased with increasing Gly:Cu molar ratio from 2:1 to 4:1. A slight increase in final Cu extraction was obtained at Gly:Cu molar ratio of 8:1. However, as can be seen in Table 5, a higher glycine addition requires a higher dosage of ammonia to reach the target pH. Therefore, considering reagent consumptions, environmental and safety impacts, a Gly:Cu molar ratio of 4:1 was chosen for the following studies.
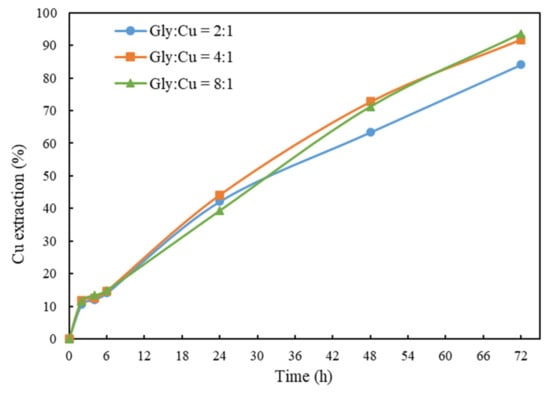
Figure 5.
Cu extraction after 72 h of leaching at different Gly:Cu molar ratios. Other conditions: solid/liquid ratio = 1%, pH = 10.5, room temperature and ceramic beads: solid = 3:5.

Table 5.
Reagent dosages and final Cu extraction at different Gly:Cu molar ratios.
3.5. Effects of pH
The effects of pH on Cu extraction were studied, and the results are summarized in Figure 6. The reagent dosages for the tests are listed in Table 6. When pH is 9, the extraction of copper from chalcopyrite is low at 25.1%. This is mainly because very less ammonia was used for pH adjustment at lower pH. The Cu extraction increases remarkably as pH increased to 10.5 or 11.5. It can be observed from the graph that the leaching kinetics at 11.5 are faster than those at 10.5, but their final Cu extractions are similar, at about 92%. This is mainly because more ammonia was used to buffer the solution to pH 11.5, which boosted the initial leaching rate of chalcopyrite.
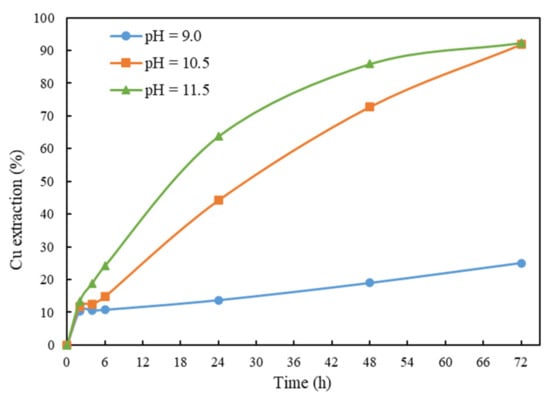
Figure 6.
Cu extraction after 72 h of leaching at solution pH. Other conditions: solid/liquid ratio = 1%, Gly:Cu = 4:1, room temperature and ceramic beads: solid = 3:5.

Table 6.
Reagent dosages and final Cu extractions at different pH.
3.6. Effects of Adding Lime
Lime (Ca(OH2)) is usually added during leaching for pH adjustment. Its addition may form a surface coating on the surface of chalcopyrite []. However, S in the form of sulfate in the leachate can be removed by adding lime due to the formation of gypsum that reduces the accumulation of S in the system. The effects of adding lime in the glycine–ammonia system were investigated. For comparison purposes, the dosage of ammonia was maintained and lime was added at the beginning of the tests using a Ca:S molar ratio of 1:1. The initial solution pH of the test was slightly higher than that of the one without lime at pH 10.7. From the results shown in Figure 7, both tests achieved the same final Cu extraction, at 91.5%. It is observed from the elemental assay of the final leachates shown in Table 7 that adding lime led to about a 50% lower S concentration in the final leachate. The results indicates that the addition of lime has no negative effect on the Cu extraction and can reduce the S concentration in the leachate.
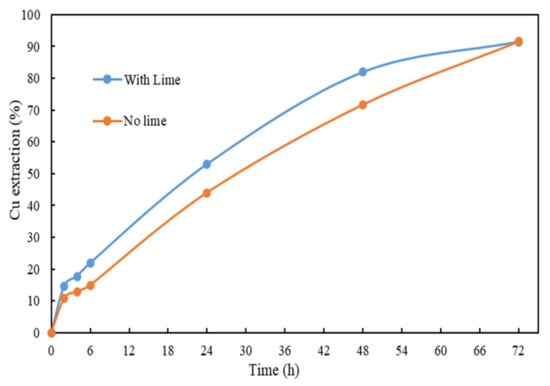
Figure 7.
Cu extraction after 72 h of leaching using glycine–ammonia system with/without adding lime. Other conditions: solid/liquid ratio = 1%, room temperature and ceramic beads: solid = 3:5.

Table 7.
Elemental assay of final leachate.
3.7. Leachability at 10% Solid
The current study investigated the leachability of chalcopyrite at 10% solid content using the optimal conditions obtained from the previous sections. From Figure 8, it can be seen that the Cu extraction is low, at 35.6%, when the test was conducted without injecting O2. When the test was carried out in a stirred reactor with O2 injection at a flowrate of 0.1 L/min, the Cu extraction increased to 95.4%. Table 8 shows the concentrations of different elements present in the final leachate. It can be seen that the impurity concentrations such as Fe, Ni, Ca, Pb and Zn are quite low compared to Cu and S. Gold was present in the materials; from the fire assay, Au extraction reached to about 60%, indicating that this system may also be suitable for precious metal leaching. These tests confirm that oxygen played an important role in the glycine–ammonia leaching system.
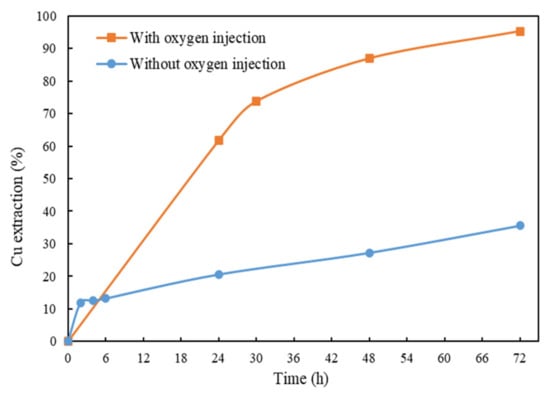
Figure 8.
Cu extraction after 72 h of leaching at 10% solid/liquid ratio. Other conditions: Gly:Cu = 4:1, pH = 10.5, room temperature and ceramic beads: solid = 3:5.

Table 8.
Elemental assay of final leachate at 10% solid content.
3.8. Kinetic Rate Equation Analysis
The leaching rate controlling steps of chalcopyrite in alkaline glycine solution in the presence of ammonia can be analyzed by the shrinking core model (SCM). The experimental data were analyzed based on the SCM equations (Equations (5)–(7)). Table 9 shows the correlation coefficient values (R2) for different models and variables. Upon comparison of the correlation coefficient values for the fitted rate controlling models, it is noted that it is difficult to determine which steps control the leaching rate of chalcopyrite, given that the R2 values for all models under different experimental conditions are quite similar. If more than a single step controls the leaching rate, the shrinking core rate equations can be combined to estimate the contribution of individual rate controlling steps to the overall leaching kinetics []. The combined rate controlling model is illustrated by Equation (8). It was proposed by [] that a constrained multi-linear regression using the least square technique to calculate the values of kl, kd and kr can be used to estimate the contribution of each model on limiting the leaching process. It can identify if there is more than one mechanism at play in the rate of leaching (by obtaining more than one non-zero coefficient). This technique avoids comparing the correlation coefficient from experimental data and rate model equation testing [,]. The constrained least square technique is expressed in Equations (9) and (10).
Min φ subject to kl, kd and kr > 0

Table 9.
Correlation coefficient values of shrinking core kinetic models for chalcopyrite.
The results of kl, kd and kr shown in Table 10 were calculated using multi-paradigm programming language software (MATLAB). It can be illustrated from the results that the leaching rate of chalcopyrite using a glycine–ammonia lixiviant system is controlled by diffusion through the film and diffusion through the product layer. Figure 9 compares the experimental Cu extraction and the calculated Cu extractions based on the calculated kl, kd and kr. From the curve, it shows a good agreement between the experimental results and the calculated results.

Table 10.
Data showing rate-controlling model for chalcopyrite leaching at different Gly:Cu molar ratios using the least square technique of constrained multi-linear analysis.
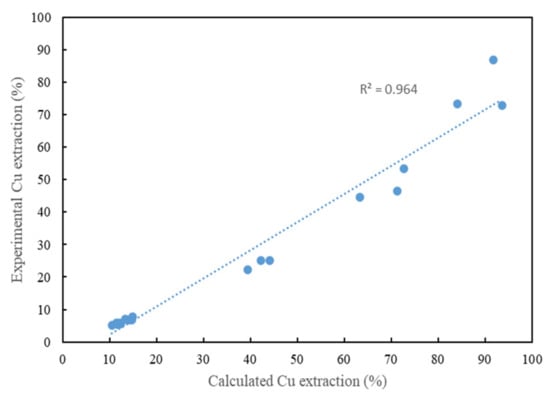
Figure 9.
Comparison of experimental Cu extraction and calculated Cu extraction based on the calculated reaction rate values (at different Gly:Cu molar ratios).
3.9. Conceptual Flowsheet
A conceptual flowsheet of the glycine–ammonia lixiviant system was proposed. Figure 10 shows the block diagram of this leaching process where chalcopyrite concentrate is leached by leaching-grinding processes using a glycine–ammonia lixiviant system in the presence of lime and oxygen/air, followed by solid/liquid separation and the recovery of copper by a solvent extraction-electrowinning circuit (SX-EW). Simultaneous leaching and grinding for the whole leaching period may not be suitable for the real process plant, as grinding is an energy-intensive process. Instead, separate grinding process can be integrated with the leaching process to further reduce the particle size and to polish the surface of chalcopyrite. Copper can be recovered using SX, where its feasibility for Cu recovery from glycine-based solutions has been demonstrated by [,,].
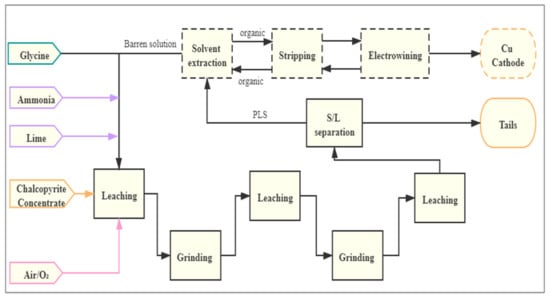
Figure 10.
Block diagram of the conceptual process for chalcopyrite leaching using a glycine–ammonia lixiviant system.
4. Conclusions
A novel method for leaching chalcopyrite using glycine with ammonia as a pH modifier was proposed. The following can be concluded:
- The leached Cu is mainly complexed with glycine as Cu(Gly)2 in a glycine–ammonia lixiviant system according to the Eh-pH diagram and the cumulative formation constant log K.
- The glycine–ammonia system shows much higher Cu extraction (91.5%) than the glycine–NaOH and ammonia-only systems.
- The addition of ceramic beads significantly improved the Cu extraction by reducing particle size and by refreshing the particle surface.
- Higher Cu extraction was achieved at a higher pH, but a higher dosage of ammonia solution was required.
- Adding lime can reduce sulfur accumulation from the final leachate without decreasing the amount of Cu extraction.
- High solid content leaching required oxygen, where a Cu extraction of 95.4% was achieved at solid content of 10% with O2 injected at a flowrate of 0.1 L/min.
- A kinetic rate equation analysis shows that the leaching rate of the glycine–ammonia lixiviant system is controlled by diffusion through film and diffusion through product layer.
- A conceptual flowsheet for the glycine–ammonia lixiviant system was proposed, which consists of leaching-grinding process for Cu extraction and the SX-EW circuit for Cu recovery.
Author Contributions
Conceptualization E.O.; methodology, E.O. and Z.D.; validation, E.O., Z.D., H.L. and J.E.; formal analysis, Z.D., E.O. and H.L.; investigation, Z.D.; resources, E.O. and J.E.; data curation, Z.D.; writing—original draft preparation, Z.D. and H.L.; writing—review and editing, E.O., Z.D. and J.E.; visualization, Z.D. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial assistance from Curtin University.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, F.; Wang, S. Chapter 7—Bioleaching of electronic waste using extreme acidophiles. In Electronic Waste Management and Treatment Technology; Prasad, M.N.V., Vithanage, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 153–174. [Google Scholar]
- Baba, A.A.; Ghosh, M.K.; Pradhan, S.R.; Rao, D.S.; Baral, A.; Adekola, F.A. Characterization and kinetic study on ammonia leaching of complex copper ore. Trans. Nonferrous Met. Soc. China 2014, 24, 1587–1595. [Google Scholar] [CrossRef]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 2014, 146, 96–110. [Google Scholar] [CrossRef]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate–chloride and sulfate–nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Beckstead, L.W.; Miller, J.D. Ammonia, oxidation leaching of chalcopyrite —Surface deposit effects. Metall. Trans. B 1977, 8, 31–38. [Google Scholar] [CrossRef]
- O’Connor, G.M.; Eksteen, J.J. A critical review of the passivation and semiconductor mechanisms of chalcopyrite leaching. Miner. Eng. 2020, 154, 106401. [Google Scholar] [CrossRef]
- Meng, X.; Han, K.N. The principles and applications of ammonia leaching of metals—A review. Miner. Process. Extr. Metall. Rev. 1996, 16, 23–61. [Google Scholar] [CrossRef]
- Reilly, I.G.; Scott, D.S. The leaching of a chalcopyrite concentrate in ammonia. Can. J. Chem. Eng. 1977, 55, 527–533. [Google Scholar] [CrossRef]
- Cattarin, S. Interfacial Reactivity and Oscillating Behavior of Chalcopyrite Cathodes during H2O2 Reduction. J. Electrochem. Soc. 1990, 137, 3484. [Google Scholar] [CrossRef]
- Warren, G.W.; Wadsworth, M.E. The electrochemical oxidation of chalcopyrite in ammoniacal solutions. Metall. Trans. B 1984, 15, 289–297. [Google Scholar] [CrossRef]
- Deng, Z.; Oraby, E.A.; Eksteen, J.J. Sulfide precipitation of copper from alkaline glycine-cyanide solutions: Precipitate characterisation. Miner. Eng. 2020, 145, 106102. [Google Scholar] [CrossRef]
- Eksteen, J.J.; Oraby, E.A. The leaching and adsorption of gold using low concentration amino acids and hydrogen peroxide: Effect of catalytic ions, sulphide minerals and amino acid type. Miner. Eng. 2015, 70, 36–42. [Google Scholar] [CrossRef]
- Eksteen, J.J.; Oraby, E.A.; Tanda, B.C. A conceptual process for copper extraction from chalcopyrite in alkaline glycinate solutions. Miner. Eng. 2017, 108, 53–66. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Extraction of copper and the co-leaching behaviour of other metals from waste printed circuit boards using alkaline glycine solutions. Resour. Conserv. Recycl. 2020, 154, 104624. [Google Scholar] [CrossRef]
- Oraby, E.A. Gold leaching in Thiosulfate Solutions and Its Environmental Effects Compared with Cyanide. Ph.D. Thesis, Curtin University, Perth, Australia, 2009. [Google Scholar]
- Oraby, E.A.; Eksteen, J.J. The selective leaching of copper from a gold– copper concentrate in glycine solutions. Hydrometallurgy 2014, 150, 14–19. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. Gold leaching in cyanide-starved copper solutions in the presence of glycine. Hydrometallurgy 2015, 156, 81–88. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. The leaching of gold, silver and their alloys in alkaline glycine–peroxide solutions and their adsorption on carbon. Hydrometallurgy 2015, 152, 199–203. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J.; Tanda, B.C. Gold and copper leaching from gold-copper ores and concentrates using a synergistic lixiviant mixture of glycine and cyanide. Hydrometallurgy 2017, 169, 339–345. [Google Scholar] [CrossRef]
- Oraby, E.A.; Li, H.; Eksteen, J.J. An Alkaline Glycine-Based Leach Process of Base and Precious Metals from Powdered Waste Printed Circuit Boards. Waste Biomass Valorization 2019, 11, 3897–3909. [Google Scholar] [CrossRef]
- Tanda, B.C.; Oraby, E.A.; Eksteen, J.J. Recovery of copper from alkaline glycine leach solution using solvent extraction. Sep. Purif. Technol. 2017, 187, 389–396. [Google Scholar] [CrossRef]
- Deng, Z.; Oraby, E.A.; Eksteen, J.J. The sulfide precipitation behaviour of Cu and Au from their aqueous alkaline glycinate and cyanide complexes. Sep. Purif. Technol. 2019, 218, 181–190. [Google Scholar] [CrossRef]
- Deng, Z.; Oraby, E.A.; Eksteen, J.J. Cu adsorption behaviours onto chelating resins from glycine-cyanide solutions: Isotherms, kinetics and regeneration studies. Sep. Purif. Technology 2020, 236, 116280. [Google Scholar] [CrossRef]
- Deng, Z.; Oraby, E.A.; Eksteen, J.J. Gold recovery from cyanide-starved glycine solutions in the presence of Cu using a molecularly imprinted resin (IXOS-AuC). Hydrometallurgy 2020, 196, 105425. [Google Scholar] [CrossRef]
- Tauetsile, P.J.; Oraby, E.A.; Eksteen, J.J. Adsorption behaviour of copper and gold glycinates in alkaline media onto activated carbon. Part 1: Isotherms. Hydrometallurgy 2018, 178, 202–208. [Google Scholar] [CrossRef]
- Tauetsile, P.J.; Oraby, E.A.; Eksteen, J.J. Adsorption behaviour of copper and gold Glycinates in alkaline media onto activated carbon. Part 2: Kinetics. Hydrometallurgy 2018, 178, 195–201. [Google Scholar] [CrossRef]
- Tauetsile, P.J.; Oraby, E.A.; Eksteen, J.J. Activated carbon adsorption of gold from cyanide-starved glycine solutions containing copper. Part 1: Isotherms. Sep. Purif. Technol. 2019, 211, 594–601. [Google Scholar] [CrossRef]
- Tauetsile, P.J.; Oraby, E.A.; Eksteen, J.J. Activated carbon adsorption of gold from cyanide-starved glycine solutions containing copper. Part 2: Kinetics. Sep. Purif. Technol. 2019, 211, 290–297. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A.; O’Connor, G.M. The kinetics of chalcopyrite leaching in alkaline glycine/glycinate solutions. Miner. Eng. 2019, 135, 118–128. [Google Scholar] [CrossRef]
- O’Connor, G.M.; Lepkova, K.; Eksteen, J.J.; Oraby, E.A. Electrochemical behaviour and surface analysis of chalcopyrite in alkaline glycine solutions. Hydrometallurgy 2018, 182, 32–43. [Google Scholar] [CrossRef]
- Moyo, T.; Petersen, J. New Mining and Metallurgy Findings from University of Cape Town Reported (Study of the dissolution of chalcopyrite in solutions of different ammonium salts). Chem. Chem. 2016, 116, 509–516. [Google Scholar]
- Khezri, M.; Rezai, B.; Akbar Abdollahzadeh, A.; Molaeinasab, M.; Wilson, B.P.; Lundström, M. Glycine leaching of Sarcheshmeh chalcopyrite concentrate at high pulp densities in a stirred tank reactor. Miner. Eng. 2020, 157, 106555. [Google Scholar] [CrossRef]
- Delf, B.W.; Gillard, R.D.; O’Brien, P. ChemInform Abstract: The isomers of α-amino acids with copper(ii). Part 5. the cis and trans s isomers of bis(glycinato)Copper(II), and their novel thermal isomerization. J. Chem. Soc. Dalton Trans. 1979, 10, 1301–1305. [Google Scholar] [CrossRef]
- Hobart, D.B.; Berg, M.A.G.; Merola, J.S. Bis-glycinato complexes of palladium(II): Synthesis, structural determination, and hydrogen bonding interactions. Inorg. Chim. Acta 2014, 423, 21–30. [Google Scholar] [CrossRef]
- Li, M.; Zheng, S.; Liu, B.; Du, H.; Dreisinger, D.B.; Tafaghodi, L.; Zhang, Y. The leaching kinetics of cadmium from hazardous Cu-Cd zinc plant residues. Waste Manag. 2017, 65, 128–138. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Kong, D.; Zhou, Q.; Chen, X.; Hui, S. Fluid particle group reaction model and experimental verification. Adv. Powder Technol. 2013, 24, 200–206. [Google Scholar] [CrossRef]
- Moyo, T.; Petersen, J.; Nicol, M. The electrochemistry and kinetics of the oxidative dissolution of chalcopyrite in ammoniacal solutions. Part II–Cathodic reactions. Hydrometallurgy 2019, 184, 67–74. [Google Scholar] [CrossRef]
- Nabizadeh, A.; Aghazadeh, V. Dissolution study of chalcopyrite concentrate in oxidative ammonia/ammonium carbonate solutions at moderate temperature and ambient pressure. Hydrometallurgy 2015, 152, 61–68. [Google Scholar] [CrossRef]
- Moyo, T.; Petersen, J.; Nicol, M. The electrochemistry and kinetics of the oxidative dissolution of chalcopyrite in ammoniacal solutions: Part I–Anodic Reactions. Hydrometallurgy 2018, 182, 97–103. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. An investigation into the leaching behaviour of copper oxide minerals in aqueous alkaline glycine solutions. Hydrometallurgy 2017, 167, 153–162. [Google Scholar] [CrossRef]
- Radmehr, V.; Koleini, S.; Khalesi, M.; Tavakoli Mohammadi, R. Ammonia leaching in the copper industry: A review. In Proceedings of the XXVI International Mineral Processing Congress (IMPC) Proceedings, New Delhi, India, 24–28 September 2012; pp. 02512–02523. [Google Scholar]
- Radmehr, V.; Koleini, S.M.J.; Khalesi, M.R.; Mohammadi, M.R.T. Ammonia Leaching: A new approach of copper industry in hydrometallurgical processes. J. Inst. Eng. Ser. D 2013, 94, 95–104. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill Inc.: New York, NY, USA, 1999. [Google Scholar]
- Kiss, T.; Sovago, I.; Gergely, A. Critical survey of stability constants of complexes of glycine. Pure Appl. Chem. 1991, 63, 597–638. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Wang, B.; Qian, G.; Li, Z.; Zhu, Y. New insights into chalcopyrite leaching enhanced by mechanical activation. Hydrometallurgy 2019, 189, 105131. [Google Scholar] [CrossRef]
- Levenspiel, O.A. Chemical Reaction Engineering; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Nazemi, M.K.; Rashchi, F.; Mostoufi, N. A new approach for identifying the rate controlling step applied to the leaching of nickel from spent catalyst. Int. J. Miner. Process. 2011, 100, 21–26. [Google Scholar] [CrossRef]
- Tanda, B.C.; Oraby, E.A.; Eksteen, J.J. Kinetics of malachite leaching in alkaline glycine solutions. Miner. Process. Extr. Metall. 2021, 130, 16–24. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Recovery of copper and the deportment of other base metals from alkaline glycine leachates derived from waste printed circuit boards (WPCBs). Hydrometallurgy 2021, 199, 105540. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).