Metallogenesis of Porphyry Copper Deposit Indicated by In Situ Zircon U-Pb-Hf-O and Apatite Sr Isotopes
Abstract
1. Introduction
2. Geological Background
2.1. Regional Geology
2.2. Deposit Geology
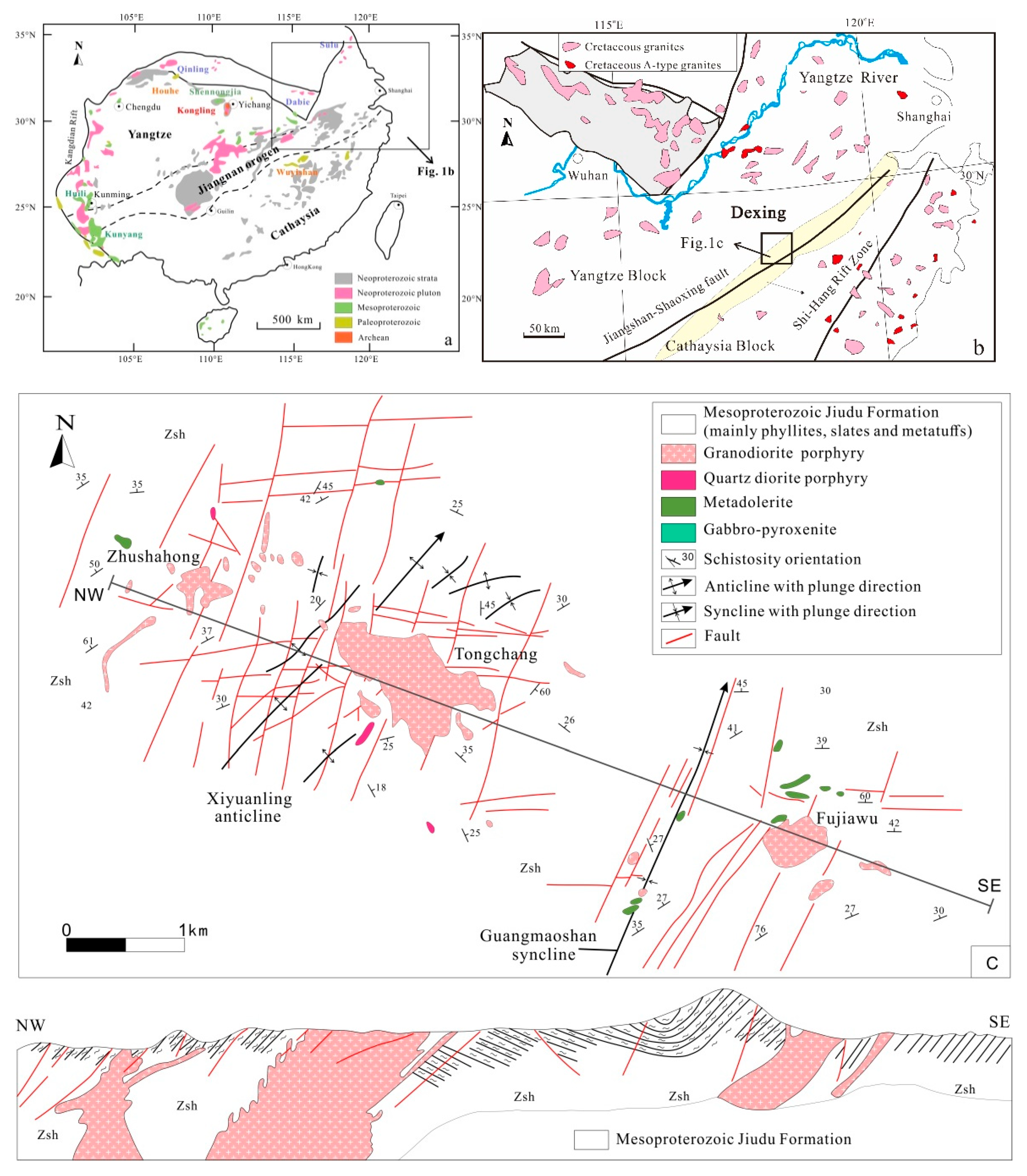
3. Petrography and Alteration
4. Sampling and Analytical Methods
4.1. Sampling
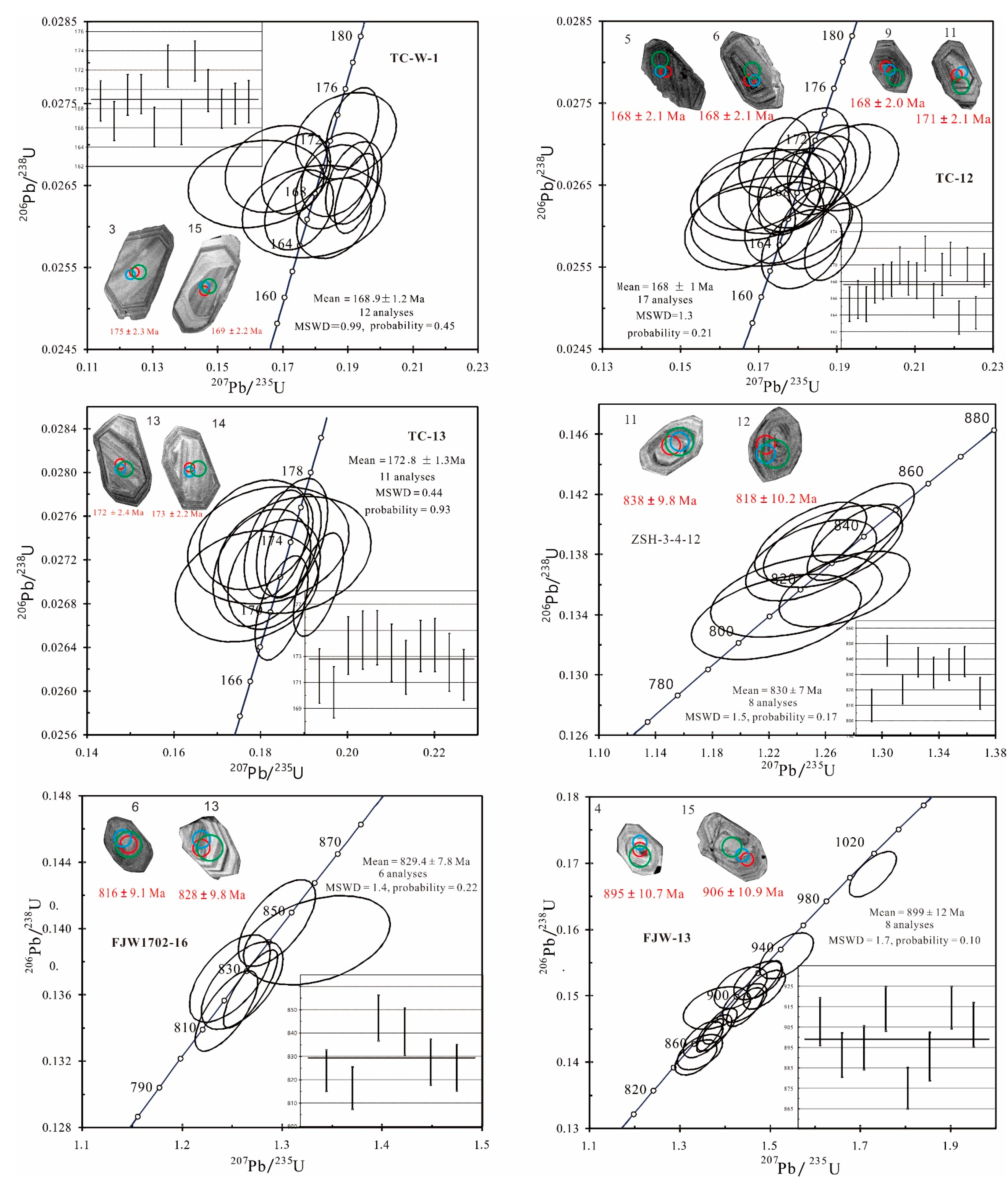
4.2. SHRIMP Zircon U-Pb Age Dating
4.3. Zircon Hf Isotopes
4.4. Zircon Oxygen Isotopes
4.5. Apatite Major and Trace Elements, Sr Isotopes
5. Results
5.1. Zircon SHRIMP U-Pb Age
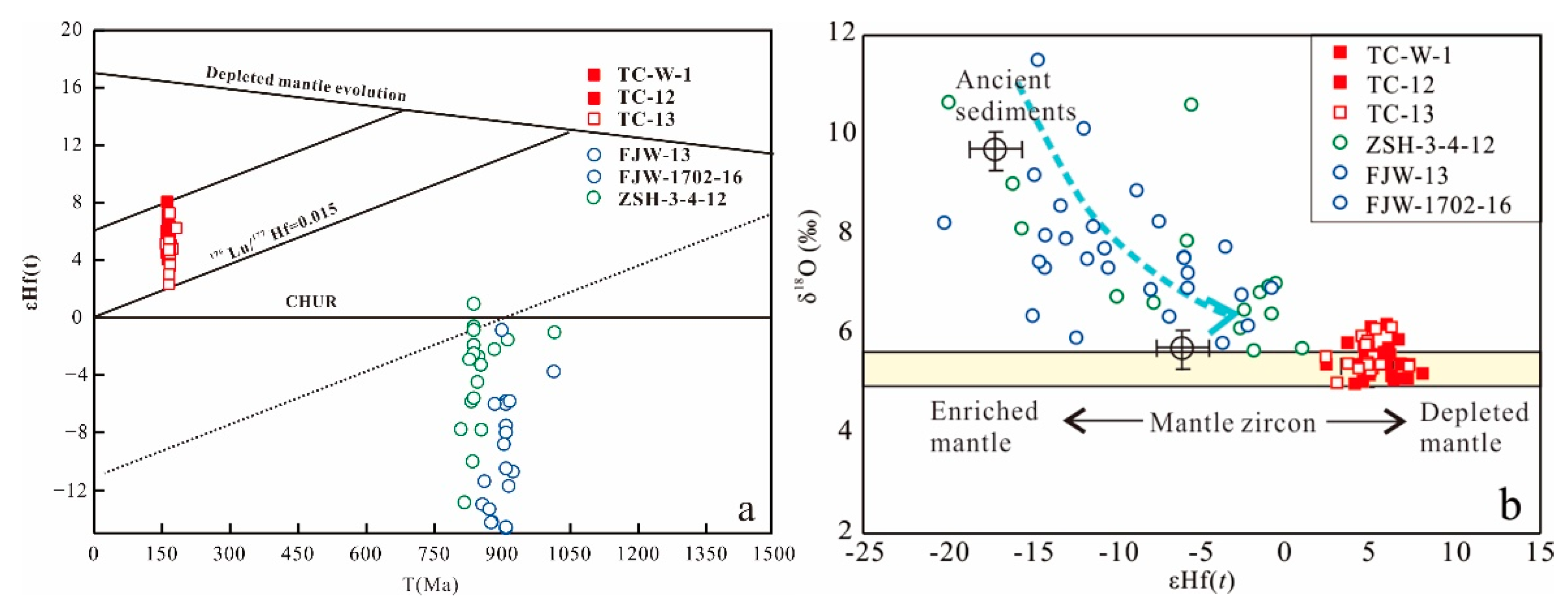
5.2. Zircon Hf isotope
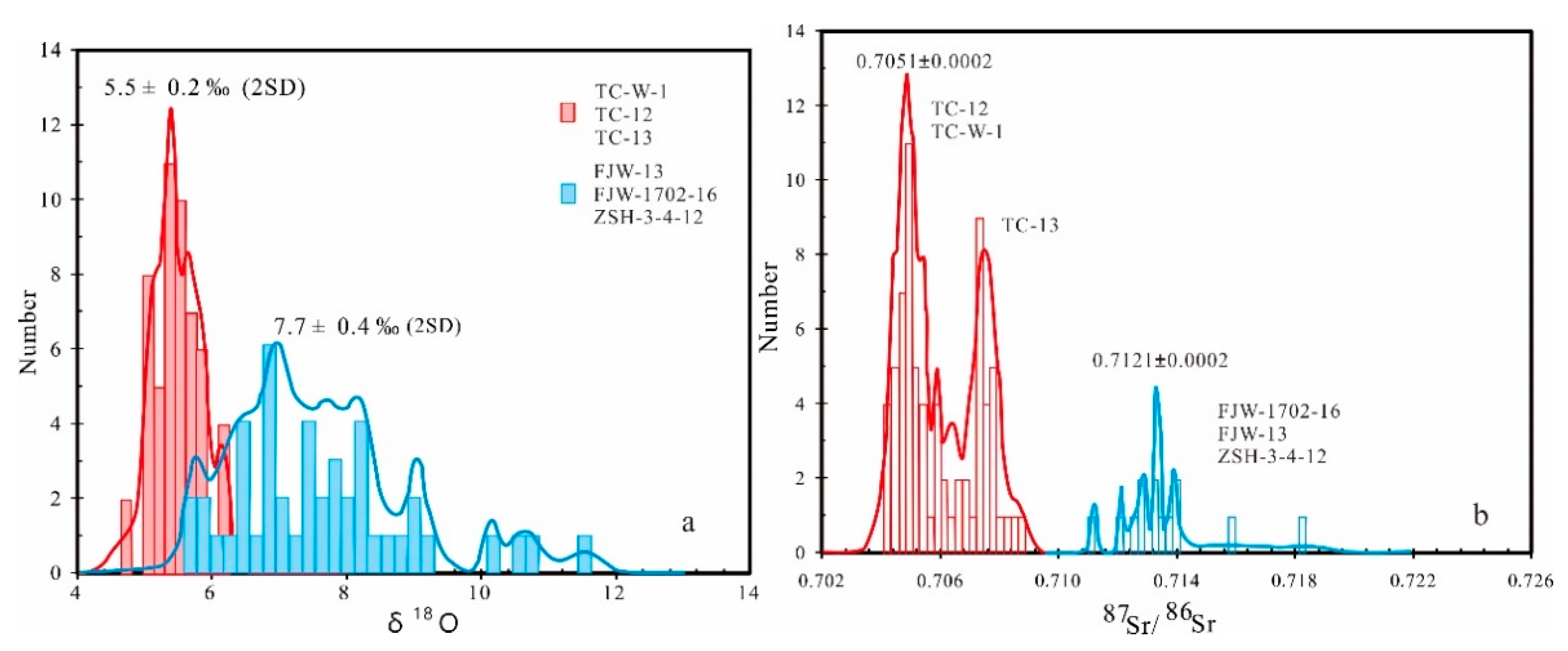
5.3. Zircon O Isotope
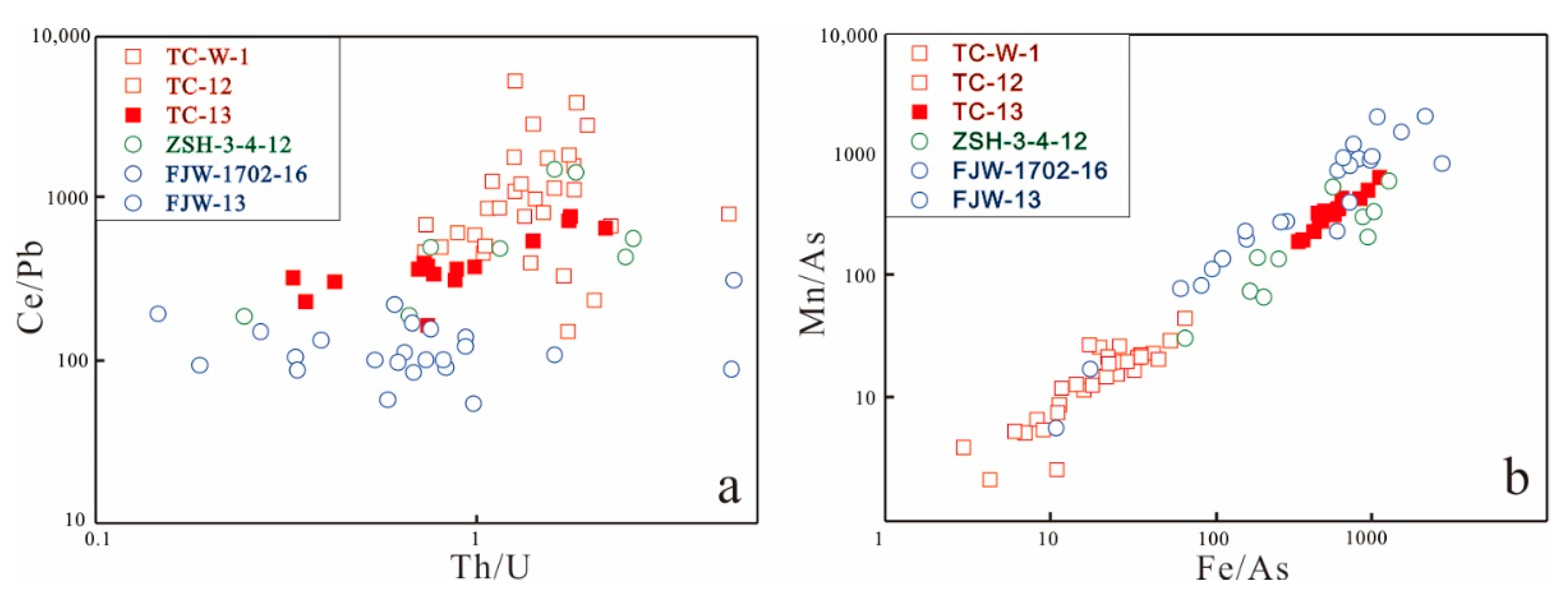
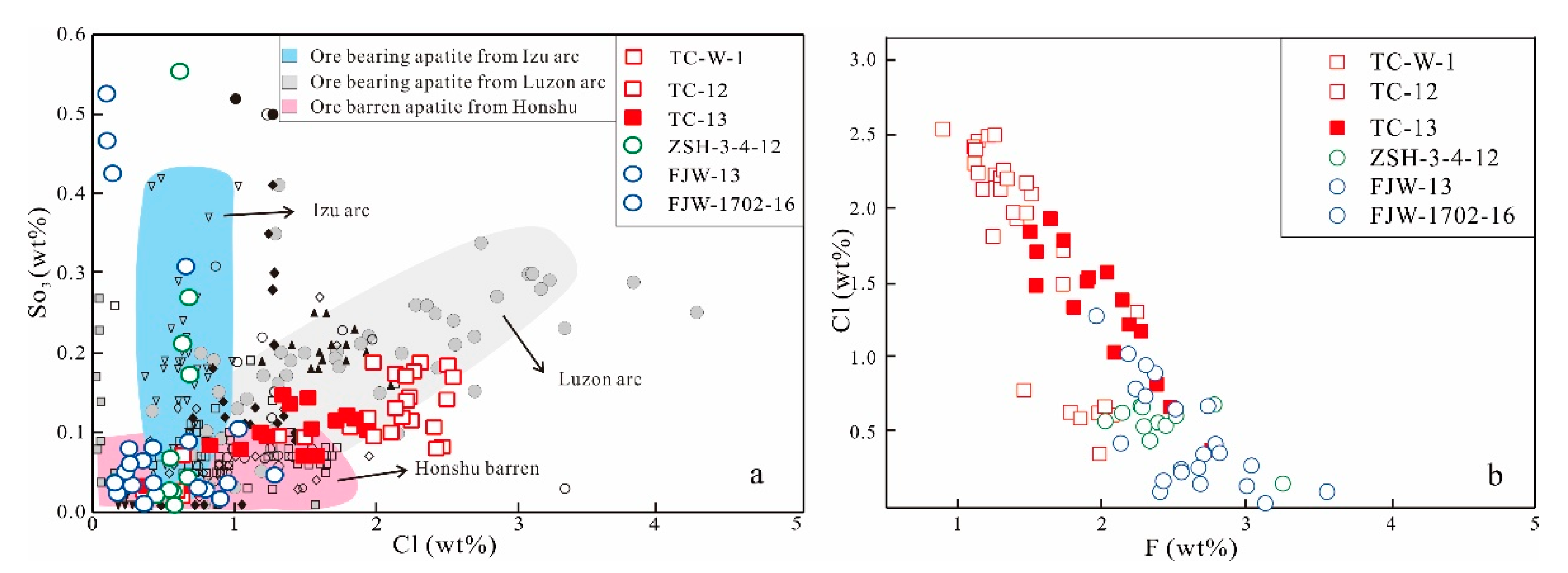
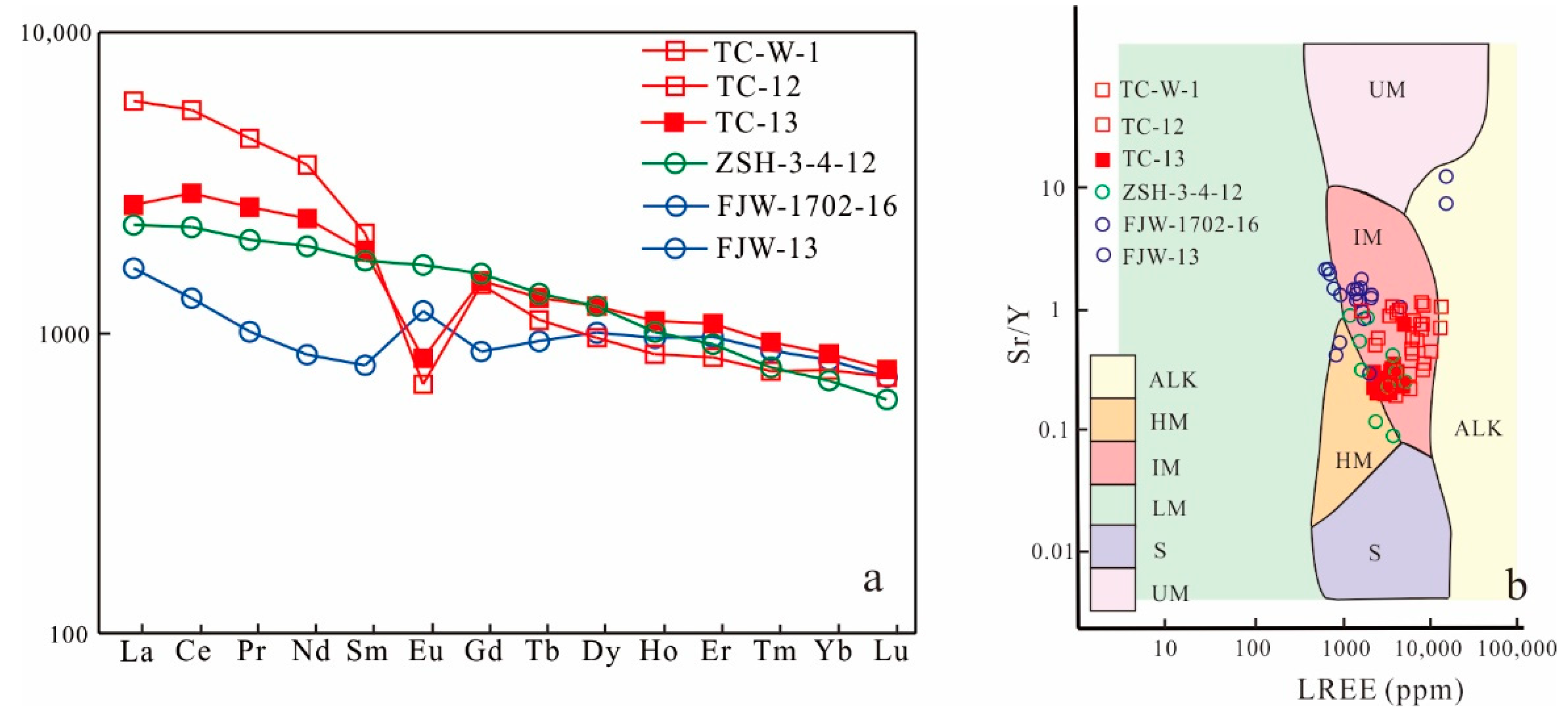
5.4. Apatite Elemental Compositions
5.5. Apatite Sr and Sr Isotope
6. Discussion
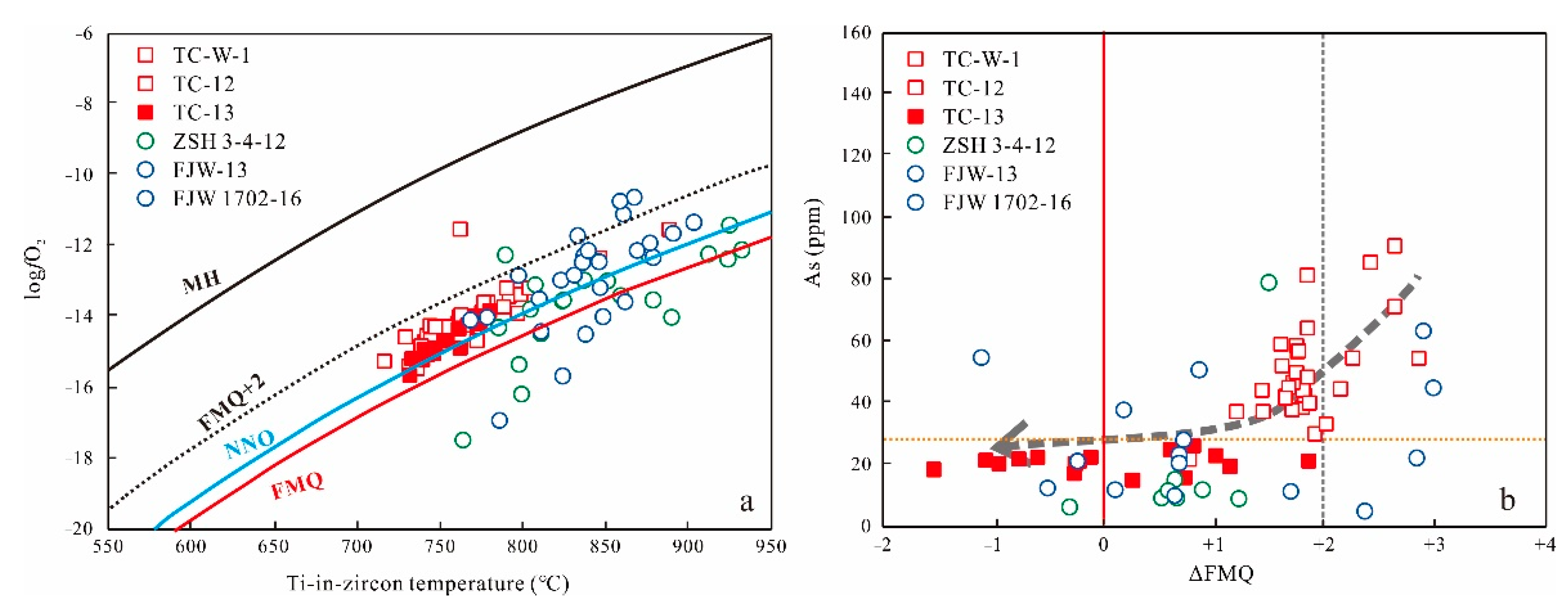
6.1. Different Oxygen Fugacity of Parental Magmas and Wall Rocks
6.1.1. Zircon Oxybarometer
6.1.2. Sulfur/Mn-in-Apatite Oxybarometer
6.2. Evolution of Ore-Forming Systems
6.3. Endowment in Cu Mineralization from Reworking of Neoproterozoic Strata
6.4. Metallogenesis of the Giant Dexing Cu-Deposit
7. Conclusions
- The porphyries of the giant Dexing Cu deposit formed in the Jurassic (172–168 Ma), whereas its host rock in the hanging wall was formed in the Neoproterozoic, based on the ages of the volcanics in the Shuangqiaoshan Group (900–830 Ma).
- The porphyries display relatively low zircon δ18O and apatite 87Sr/86Sr, but high zircon εHf, indicating a mantle source; however, the wall rocks yield characteristics transitional from magmatic to hydrothermal, implying alteration of ore-forming fluids.
- The apatites from the porphyries provide relatively high total REE and negative δEu, but high Cl and As contents. Combined, the porphyries underwent potassic–silica alteration, implying processes from magmatic to hydrothermal alteration, which is distinctly different from the Shuangqiaoshan Group rocks with hydrothermal fluid from the lower crust.
- The Dexing porphyries were derived from partial melting of the subducted Paleo-Pacific plate during the Jurassic rather than from that of the lower crust. High oxygen fugacity and high Cu and Cl contents are the key features for its metallogenesis.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooke, D.; Hollings, P.; Walsh, J. Giant porphyry deposits: Characteristics, distribution, and tectonic controls. Econ. Geol. 2005, 100, 801–818. [Google Scholar] [CrossRef]
- Sillitoe, R. Porphyry Copper Systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Sun, W.; Huang, R.; Li, H.; Hu, Y.; Zhang, C.; Sun, S.; Ling, M. Porphyry deposits and oxidized magmas. Ore Geol. Rev. 2015, 65, 97–131. [Google Scholar] [CrossRef]
- Ballard, J.; Palin, M.; Campbell, I. Relative oxidation states of magmas inferred from Ce(IV)/Ce(III) in zircon: Application to porphyry copper deposits of northern Chile. Contrib. Mineral. Petrol. 2002, 144, 347–364. [Google Scholar] [CrossRef]
- Liang, H.; Campbell, I.; Allen, C.; Sun, W.; Liu, C.; Yu, H.; Zhang, Y. Zircon Ce4+/Ce3+ ratios and ages for Yulong ore-bearing porphyries in eastern Tibet. Miner. Depos. 2006, 41, 152. [Google Scholar] [CrossRef]
- Mungall, J. Roasting the mantle: Slab melting and the genesis of major Au and Au-rich Cu deposits. Geology 2002, 30, 915–918. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, W.; Wang, J.; Zhang, L.; Sun, S.; Wu, K. Oxygen fugacity and porphyry mineralization: A zircon perspective of Dexing porphyry Cu deposit, China. Geochim. Cosmochim. Acta 2017, 206, 343–363. [Google Scholar] [CrossRef]
- Richards, J. Tectono-Magmatic Precursors for Porphyry Cu-(Mo-Au) Deposit Formation. Econ. Geol. 2003, 98, 1515–1533. [Google Scholar] [CrossRef]
- Richards, J. Porphyry copper deposit formation in arcs: What are the odds? Geosphere 2022, 18, 130–155. [Google Scholar] [CrossRef]
- Wang, Q.; Wyman, D.; Xu, J.; Jian, P.; Zhao, Z.; Li, C.; He, B. Early Cretaceous adakitic granites in the Northern Dabie Complex, central China: Implications for partial melting and delamination of thickened lower crust. Geochim. Cosmochim. Acta 2007, 71, 2609–2636. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Jian, P.; Bao, Z.; Zhao, Z.; Li, C.; Ma, J. Petrogenesis of adakitic porphyries in an extensional tectonic setting, dexing, South China: Implications for the genesis of porphyry copper mineralization. J. Petrol. 2006, 47, 119–144. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.; Jian, P.; Xu, J.; Bao, Z.; Ma, J. SHRIMP zircon geochronology and Nd-Sr isotopic geochetnistry of the Dexing granodiorite porphyries. Acta Petrol. Sin. 2004, 20, 315–324. [Google Scholar]
- Miles, A.; Graham, C.; Hawkesworth, C.; Gillespie, M.; Hinton, R.; Bromiley, G. Reply to comment by Marks et al. (2016) on ‘Apatite: A new redox proxy for silicic magmas?’. Geochim. Cosmochim. Acta 2016, 183, 271–273. [Google Scholar] [CrossRef]
- Ding, T.; Ma, D.; Lu, J.; Zhang, R. Apatite in granitoids related to polymetallic mineral deposits in southeastern Hunan Province, Shi–Hang zone, China: Implications for petrogenesis and metallogenesis. Ore Geol. Rev. 2015, 69, 104–117. [Google Scholar] [CrossRef]
- Pizarro, H.; Campos, E.; Bouzari, F.; Rousse, S.; Bissig, T.; Gregoire, M.; Riquelme, R. Porphyry indicator zircons (PIZs): Application to exploration of porphyry copper deposits. Ore Geol. Rev. 2020, 126, 103771. [Google Scholar] [CrossRef]
- Wang, F.; Liu, S.; Li, S.; Akhtar, S.; He, Y. Zircon U–Pb ages, Hf–O isotopes and trace elements of Mesozoic high Sr/Y porphyries from Ningzhen, eastern China: Constraints on their petrogenesis, tectonic implications and Cu mineralization. Lithos 2014, 200, 299–316. [Google Scholar] [CrossRef]
- Dilles, J.; Kent, A.; Wooden, J.; Tosdal, R.; Koleszar, A.; Lee, R.; Farmer, L. Zircon compositional evidence for sulfur-degassing from ore-forming arc magmas. Econ. Geol. 2015, 110, 241–251. [Google Scholar] [CrossRef]
- Lu, Y.; Smithies, R.; Wingate, M.; Evans, N.; McCuaig, T.; Champion, D.; Outhwaite, M. Zircon fingerprinting of magmatic-hydrothermal systems in the Archean Yilgarn craton. Perth: Geol. Surv. West. Aust. Rep. 2019, 22, 197. [Google Scholar]
- Loucks, R.; Fiorentini, M.; Henriquez, G. New magmatic oxybarometer using trace elements in zircon. J. Petrol. 2020, 61, egaa034. [Google Scholar] [CrossRef]
- Konecke, B.; Fiege, A.; Simon, A.; Linsler, S.; Holtz, F. An experimental calibration of a sulfur-in-apatite oxybarometer for mafic systems. Geochim. Cosmochim. Acta 2019, 265, 242–258. [Google Scholar] [CrossRef]
- Sha, L.; Chappell, B. Apatite chemical composition, determined by electron microprobe and laser-ablation inductively coupled plasma mass spectrometry, as a probe into granite petrogenesis. Geochim. Cosmochim. Acta 1999, 63, 3861–3881. [Google Scholar] [CrossRef]
- Cao, M.; Li, G.; Qin, K.; Seitmuratova, E.; Liu, Y. Major and Trace Element Characteristics of Apatites in Granitoids from Central Kazakhstan: Implications for Petrogenesis and Mineralization. Resour. Geol. 2012, 62, 63–83. [Google Scholar] [CrossRef]
- Miles, A.; Graham, C.; Hawkesworth, C.; Gillespie, M.; Hinton, R.; Bromiley, G. Apatite: A new redox proxy for silicic magmas? Geochim. Cosmochim. Acta 2014, 132, 101–119. [Google Scholar] [CrossRef]
- Harlov, D. Apatite: A Fingerprint for Metasomatic Processes. Elements 2015, 11, 171–176. [Google Scholar] [CrossRef]
- Teiber, H.; Marks, M.; Wenzel, T.; Markl, G.; Arzamastsev, A. Compositional variation in apatite from various host rocks: Clues with regards to source composition and crystallization conditions. Neues Jahrb. Mineral. Abh. 2015, 192, 151–167. [Google Scholar] [CrossRef]
- Ishihara, S.; Moriyama, T. Apatite Composition of Representative Magnetite-series and Ilmenite-series Granitoids in Japan. Resour. Geol. 2016, 66, 55–62. [Google Scholar] [CrossRef]
- Mao, M.; Rukhlov, A.; Rowins, S.; Spence, J.; Coogan, L. Apatite trace element compositions: A robust new tool for mineral exploration. Econ. Geol. 2016, 111, 1187–1222. [Google Scholar] [CrossRef]
- Azadbakht, Z.; Lentz, D.; McFarlane, C. Apatite chemical compositions from Acadian-related granitoids of New Brunswick, Canada: Implications for petrogenesis and metallogenesis. Minerals 2018, 8, 598. [Google Scholar] [CrossRef]
- Duan, D.; Jiang, S. Using apatite to discriminate synchronous ore-associated and barren granitoid rocks: A case study from the Edong metallogenic district, South China. Lithos 2018, 310, 369–380. [Google Scholar] [CrossRef]
- Palma, G.; Barra, F.; Reich, M.; Valencia, V.; Simon, A.; Vervoort, J.; Romero, R. Halogens, trace element concentrations, and Sr-Nd isotopes in apatite from iron oxide-apatite (IOA) deposits in the Chilean iron belt: Evidence for magmatic and hydrothermal stages of mineralization. Geochim. Cosmochim. Acta 2019, 246, 515–540. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, L.; Li, H.; Liu, X. The synchronic Cenozoic subduction initiations in the west Pacific induced by the closure of the Neo-Tethys Ocean. Sci. Bull. 2020, 65, 2068–2071. [Google Scholar] [CrossRef]
- Rui, Z.; Zhang, L.; Wu, C.; Sun, X. Dexing porphyry copper deposits in Jiangxi, China. In Super Porphyry Copper & Gold Deposits: A Global Perspective, 2nd ed.; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2005; pp. 409–421. [Google Scholar]
- Zhu, X.; Huang, C.; Rui, Z.; Zhou, Y.; Zhu, X.; Hu, C.; Mei, Z. Dexing Porphyry Copper Ore Field; Geological Publishing House: Beijing, China, 1983; pp. 1–314. (In Chinese) [Google Scholar]
- Hou, Z.; Pan, X.; Li, Q.; Yang, Z.; Song, Y. The giant Dexing porphyry Cu–Mo–Au deposit in east China: Product of melting of juvenile lower crust in an intracontinental setting. Miner. Depos. 2013, 48, 1019–1045. [Google Scholar] [CrossRef]
- Liu, X.; Fan, H.; Santosh, M.; Hu, F.; Yang, K.; Li, Q.; Liu, Y. Remelting of Neoproterozoic relict volcanic arcs in the Middle Jurassic: Implication for the formation of the Dexing porphyry copper deposit, Southeastern China. Lithos 2012, 150, 85–100. [Google Scholar] [CrossRef]
- Wang, G.; Ni, P.; Yao, J.; Wang, X.; Zhao, K.; Zhu, R.; Zhang, Y. The link between subduction-modified lithosphere and the giant Dexing porphyry copper deposit, South China: Constraints from high-Mg adakitic rocks. Ore Geol. Rev. 2015, 67, 109–126. [Google Scholar] [CrossRef]
- Zhang, H.; Ling, M.; Liu, Y.; Tu, X.; Wang, F.; Li, C.; Sun, W. High Oxygen Fugacity and Slab Melting Linked to Cu Mineralization: Evidence from Dexing Porphyry Copper Deposits, Southeastern China. J. Geol. 2013, 121, 289–305. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, Y.; Zhao, P.; Liao, S.; Jin, G. Origin of the Dexing Cu-bearing porphyries, SE China: Elemental and Sr–Nd–Pb–Hf isotopic constraints. Int. Geol. Rev. 2012, 54, 572–592. [Google Scholar] [CrossRef]
- Sun, W.; Ling, M.; Chung, S.; Ding, X.; Yang, X.; Liang, H.; Yin, Q. Geochemical constraints on adakites of different origins and copper mineralization. J. Geol. 2012, 120, 105–120. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J.; McCulloch, M.; Zhou, G.; Xing, F. Geochemical and Sm-Nd isotopic study of Neoproterozoic ophiolites from southeastern China: Petrogenesis and tectonic implications. Precambrian Res. 1997, 81, 129–144. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zhou, H.; Kinny, P. Grenvillian continental collision in south China: New SHRIMP U-Pb zircon results and implications for the configuration of Rodinia. Geology 2002, 30, 163–166. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, D.; Kennedy, A.; Li, Y.; Ding, J. SHRIMP U-Pb zircon geochronological and geochemical evidence for Neoproterozoic arc-magmatism along the western margin of the Yangtze Block, South China. Earth Planet. Sci. Lett. 2002, 196, 51–67. [Google Scholar] [CrossRef]
- Rui, Z.; Huang, C.; Qi, G.; Xu, J.; Zhang, H. Porphyry Copper (Molybdenum) Deposits of China; Geological Publishing House: Beijing, China, 1984. (In Chinese) [Google Scholar]
- Gilder, S.; Gill, J.; Coe, R.; Zhao, X.; Liu, Z.; Wang, G.; Wu, H. Isotopic and paleomagnetic constraints on the Mesozoic tectonic evolution of south China. Journal of Geophysical Research. Solid Earth 1996, 101, 16137–16154. [Google Scholar]
- Li, X.; Li, Z.; Ge, W.; Zhou, H.; Li, W.; Liu, Y.; Wingate, M. Neoproterozoic granitoids in South China: Crustal melting above a mantle plume at ca. 825 Ma? Precambrian Res. 2003, 122, 45–83. [Google Scholar] [CrossRef]
- Li, X.; Sasaki, M. Hydrothermal alteration and mineralization of middle jurassic dexing porphyry Cu-Mo deposit, southeast China. Resour. Geol. 2007, 57, 409–426. [Google Scholar] [CrossRef]
- He, W.; Bao, Z.; Li, T. One-dimensional reactive transport models of alteration in the Tongchang porphyry copper deposit, Dexing district, Jiangxi Province, China. Econ. Geol. 1999, 94, 307–323. [Google Scholar] [CrossRef]
- Li, X.; Watanabe, Y.; Yi, X. Ages and Sources of Ore-Related Porphyries at Yongping Cu-Mo Deposit in Jiangxi Province, Southeast China. Resour. Geol. 2013, 63, 288–312. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, Y.; Zhao, P.; Liao, S.; Jin, G.; Liu, Z.; Jia, R. Shrimp U-Pb dating on hydrothermal zircons: Evidence for an Early Cretaceous epithermal event in the Middle Jurassic Dexing porphyry copper deposit, Southeast China. Econ. Geol. 2012, 107, 1507–1514. [Google Scholar] [CrossRef]
- Mao, J.; Xie, G.; Duan, C.; Pirajno, F.; Ishiyama, D.; Chen, Y. A tectono-genetic model for porphyry–skarn–stratabound Cu–Au–Mo–Fe and magnetite–apatite deposits along the Middle–Lower Yangtze River Valley, Eastern China. Ore Geol. Rev. 2011, 43, 294–314. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Qiu, J.; Gao, J. Geochemistry of the Meso- to Neoproterozoic basic–acid rocks from Hunan Province, South China: Implications for the evolution of the western Jiangnan orogen. Precambrian Res. 2004, 135, 79–103. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiao, W.; Zhao, G. Introduction to tectonics of China. Gondwana Res. 2013, 23, 1189–1206. [Google Scholar] [CrossRef]
- Yang, Z.; Cooke, D. Porphyry copper deposits in China. Soc. Econ. Geol. Spec. Publ. 2021, 22, 133–187. [Google Scholar]
- Pan, X.; Song, Y.; Wang, S.; Li, Z.; Yang, Z.; Hou, Z. Evolution of hydrothermal fluid of Dexing Tongchang copper-gold porphyry deposit. Acta Geol. Sin. 2009, 83, 1929–1950. [Google Scholar]
- Williams, I. U-Th-Pb geochronology by ion microprobe. Rev. Econ. Geol. 1998, 7, 1–35. [Google Scholar]
- Sláma, J.; Košler, C.; Crowley, J.; Gerdes, A.; Hanchar, J.; Horstwood, M.; Norberg, N. Plešovice zircon—A new natural reference material for U–Pb and Hf isotopic microanalysis. Chem. Geol. 2008, 249, 1–35. [Google Scholar] [CrossRef]
- Nasdala, L.; Hofmeister, W.; Norberg, N.; Martinson, J.; Corfu, F.; Dörr, W.; Reiners, P. Zircon M257-a homogeneous natural reference material for the ion microprobe U-Pb analysis of zircon. Geostand. Geoanalytical Res. 2008, 32, 247–265. [Google Scholar] [CrossRef]
- Ludwig, K. ISOPLOT 3.00: A Geochronological Toolkit for Microsoft Excel; BGC: Berkeley, CA, USA, 2003; pp. 1–39. [Google Scholar]
- Bao, Z.; Yuan, H.; Zong, C.; Liu, Y.; Chen, K.; Zhang, Y. Simultaneous determination of trace elements and lead isotopes in fused silicate rock powders using a boron nitride vessel and fsLA-(MC)-ICP-MS. J. Anal. At. Spectrom. 2016, 31, 1012–1022. [Google Scholar] [CrossRef]
- Yuan, H.; Gao, S.; Dai, M.; Zong, C.; Günther, D.; Fontaine, G.; Diwu, C. Simultaneous determinations of U–Pb age, Hf isotopes and trace element compositions of zircon by excimer laser-ablation quadrupole and multiple-collector ICP-MS. Chem. Geol. 2008, 247, 100–118. [Google Scholar] [CrossRef]
- Albarède, F.; Scherer, E.; Blichert-Toft, J.; Rosing, M.; Simionovici, A.; Bizzarro, M. γ-ray irradiation in the early Solar System and the conundrum of the 176Lu decay constant. Geochim. Cosmochim. Acta 2006, 70, 1261–1270. [Google Scholar] [CrossRef]
- Blichert-Toft, J.; Albarède, F. The Lu-Hf isotope geochemistry of chondrites and the evolution of the mantle-crust system. Earth Planet. Sci. Lett. 1997, 148, 243–258. [Google Scholar] [CrossRef]
- Griffin, W.; Pearson, N.; Belousova, E.; Jackson, S.; Van Achterbergh, E.; Reilly, S.; Shee, S. The Hf isotope composition of cratonic mantle: LAM-MC-ICPMS analysis of zircon megacrysts in kimberlites. Geochim. Cosmochim. Acta 2000, 64, 133–147. [Google Scholar] [CrossRef]
- Black, L.; Kamo, S.; Allen, C.; Davis, D.; Aleinikoff, J.; Valley, J.; Foudoulis, C. Improved 206Pb/238U microprobe geochronology by the monitoring of a trace-element-related matrix effect; SHRIMP, ID–TIMS, ELA–ICP–MS and oxygen isotope documentation for a series of zircon standards. Chem. Geol. 2004, 205, 115–140. [Google Scholar] [CrossRef]
- Liu, X.; Gao, S.; Diwu, C.; Yuan, H.; Hu, Z. Simultaneous in-situ determination of U-Pb age and trace elements in zircon by LA-ICP-MS in 20 mu m spot size. Chin. Sci. Bull. 2007, 52, 1257–1264, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Hu, Z.; Liu, Y.; Gao, S.; Xiao, S.; Zhao, L.; Günther, D.; Zong, K. A “wire” signal smoothing device for laser ablation inductively coupled plasma masss pectrometry analysis. Spectrochim. Acta Part B 2012, 78, 50–57. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, F.; Yang, J.; David, M.; Xie, L.; Chu, Z.; Huang, C. Sr and Nd isotopic compositions of apatite reference materials used in U-Th-Pb geochronology. Chem. Geol. 2014, 385, 35–55. [Google Scholar] [CrossRef]
- Simon, A.; Ripley, E. The role of magmatic sulfur in the formation of ore deposits. Rev. Mineral. Geochem. 2011, 73, 513–578. [Google Scholar] [CrossRef]
- Blundy, J.; Wood, B. Prediction of crystal–melt partition coefficients from elastic moduli. Nature 1994, 372, 452–454. [Google Scholar] [CrossRef]
- Parat, F.; Holtz, F.; Klügel, A. S-rich apatite-hosted glass inclusions in xenoliths from La Palma: Constraints on the volatile partitioning in evolved alkaline magmas. Contrib. Mineral. Petrol. 2011, 162, 463–478. [Google Scholar] [CrossRef]
- Parat, F.; Holtz, F.; Streck, M. Sulfur-bearing magmatic accessory minerals. Rev. Mineral. Geochem. 2011, 73, 285–314. [Google Scholar] [CrossRef]
- Webster, J.; Piccoli, P. Magmatic apatite: A powerful, yet deceptive, mineral. Elements 2015, 11, 177–182. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M. Compositions of the Apatite-Group Minerals: Substitution Mechanisms and Controlling Factors. Rev. Mineral. Geochem. 2002, 48, 13–49. [Google Scholar] [CrossRef]
- Jugo, P.; Wilke, M.; Botcharnikov, R. Sulfur K-edge XANES analysis of natural and synthetic basaltic glasses: Implications for S speciation and S content as function of oxygen fugacity. Geochim. Cosmochim. Acta 2010, 74, 5926–5938. [Google Scholar] [CrossRef]
- Imai, A. Variation of Cl and SO3 contents of microphenocrystic apatite in intermediate to silicic igneous rocks of Cenozoic Japanese island arcs: Implications for porphyry Cu metallogenesis in the western Pacific island arcs. Resour. Geol. 2004, 54, 357–372. [Google Scholar] [CrossRef]
- Patten, C.; Barnes, S.; Mathez, E. Partition coefficients of chalcophile elements between sulfide and silicate melts and the early crystallization history of sulfide liquid: LA-ICP-MS analysis of MORB sulfide droplets. Chem. Geol. 2013, 358, 170–188. [Google Scholar] [CrossRef]
- Holland, H. Granites, solutions, and base metal deposits. Econ. Geol. 1972, 67, 281–301. [Google Scholar] [CrossRef]
- Dingwell, D.; Scarfe, C.; Cronin, D. The effect of fluorine on viscosities in the system Na2O-Al2O3-SiO2: Implications for phonolites, trachytes and rhyolites. Am. Mineral. 1985, 70, 80–87. [Google Scholar]
- Sun, W.; Ding, X.; Hu, Y.; Li, X. The golden transformation of the Cretaceous plate subduction in the west Pacific. Earth Planet. Sci. Lett. 2007, 262, 533–542. [Google Scholar] [CrossRef]
- Brenan, J. Kinetics of fluorine, chlorine and hydroxyl exchange in fluorapatite. Chem. Geol. 1993, 110, 195–210. [Google Scholar] [CrossRef]
- O’Sullivan, G.; Chew, D.; Kenny, G.; Henrichs, I.; Mulligan, D. The trace element composition of apatite and its application to detrital provenance studies. Earth-Sci. Rev. 2020, 201, 103044. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, G.; Zhou, J.; Liu, Y.; Hu, J. Geochronology and Hf isotopes of zircon from volcanic rocks of the Shuangqiaoshan Group, South China: Implications for the Neoproterozoic tectonic evolution of the eastern Jiangnan orogen. Gondwana Res. 2008, 14, 355–367. [Google Scholar] [CrossRef]
- Smythe, D.; Brenan, J. Cerium oxidation state in silicate melts: Combined fO2, temperature and compositional effects. Geochim. Cosmochim. Acta 2015, 170, 173–187. [Google Scholar] [CrossRef]
- Wang, G.; Ni, P.; Li, L.; Wang, X.; Zhu, A.; Zhang, Y.; Li, B. Petrogenesis of the Middle Jurassic andesitic dikes in the giant Dexing porphyry copper ore field, South China: Implications for mineralization. J. Asian Earth Sci. 2020, 196, 104375. [Google Scholar] [CrossRef]

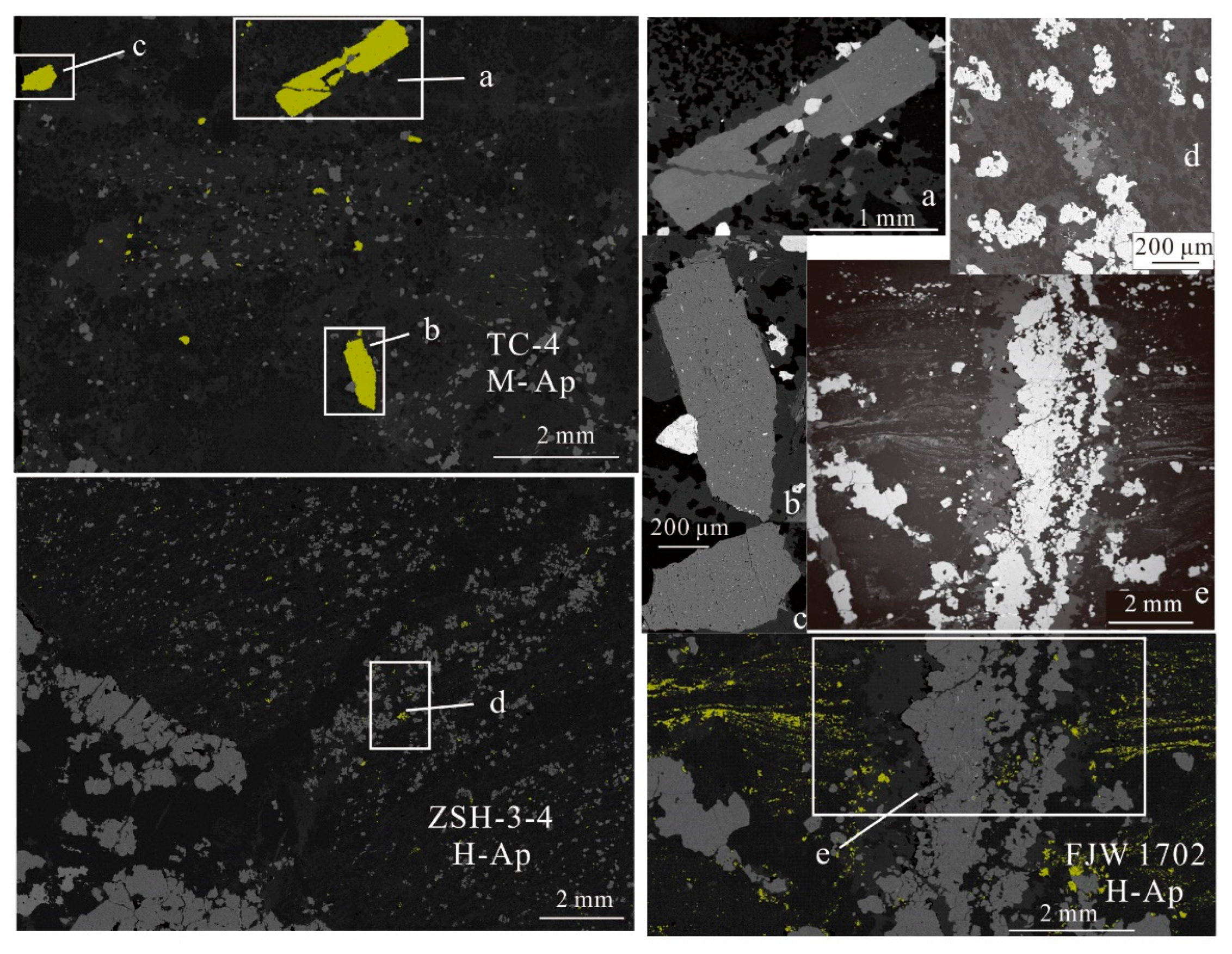
| Number | Sample Location | Rock Types | Minerals’ Assemblages | Zircon Features | Apatite Features |
|---|---|---|---|---|---|
| TC-W-1, TC-12, TC-13 | Tongchang open pit | Mineralized porphyry | Pla, Qtz, Kfs, Bi, Amp; Mag, Spe, Ap, Tit, Zr, Goe, Ill, Chl, and Ru; with potassic to siliceous veins | Length~100–400 μm; width~100–300 μm, zoning crystallization, light yellow, magmatic zircons | Length~200–1000 μm; width~50–200 μm crystallization, flesh-red or light brown, scare inclusions, magmatic-hydrothermal apatite |
| ZSH-3-4-12, FJW1702-16, FJW-13 | Zhushahong drilling hole | Mineralized siltstone or phyllite | Ser, Pla, Chl, Qtz; Ap, Zr, Ru, Ilm, Mag, and clay minerals; weak propylitic to phyllic alteration | Length~100–400 μm; width~100–300 μm, zoning crystallization, light yellow, magmatic zircons | ~50–200 μm irregular enriched inclusions colorless to gray-white hydrothermal apatite |
| Sample Name | 206Pb/238U | 1SE | δ18O (‰) (2SD) | 176Hf/177Hf (2SD) | εHf(t)(2SD) |
|---|---|---|---|---|---|
| TC-W-1 | 168.9 | 1.2 | 5.4 ± 0.2‰ | 0.282828 ± 0.000044 | 5.2 ± 1.5 |
| TC-12 | 168 | 1.0 | 5.6 ± 0.2‰ | 0.282816 ± 0.000043 | 5.6 ± 1.6 |
| TC-13 | 172.8 | 1.3 | 5.5 ± 0.2‰ | 0.282840 ± 0.000044 | 4.6 ± 1.5 |
| ZSH-3-4-12 | 830 | 7 | 7.4 ± 0.5‰ | 0.282471 ± 0.000044 | –6.0 ± 1.6 |
| FJW1702-16 | 829 | 8 | 8.1 ± 0.4‰ | 0.282262 ± 0.000046 | –8.6 ± 1.7 |
| FJW-13 | 899 | 12 | 7.4 ± 0.4‰ | 0.282222 ± 0.000049 | –10.9 ± 1.7 |
| Sample Name | TC-W-1 | TC-12 | TC-13 | FJW1702-16 | FJW-3 | ZSH-3-4-12 |
|---|---|---|---|---|---|---|
| CaO | 54.09 | 53.81 | 54.11 | 54.73 | 54.80 | 54.54 |
| P2O5 | 41.67 | 41.38 | 42.2 | 42.36 | 42.39 | 42.35 |
| F | 1.38 | 1.55 | 1.99 | 2.42 | 2.61 | 2.42 |
| Cl | 1.84 | 1.71 | 1.34 | 0.67 | 0.35 | 0.55 |
| FeO | 0.13 | 0.1 | 0.33 | 0.14 | 0.19 | 0.34 |
| MnO | 0.13 | 0.11 | 0.23 | 0.24 | 0.14 | 0.21 |
| TiO2 | 0.02 | 0.02 | 0.03 | 0.04 | 0.04 | 0.04 |
| SiO2 | 0.16 | 0.18 | 0.01 | 0 | 0.05 | 0.04 |
| MgO | 0.01 | 0.02 | 0.07 | 0.1 | 0.06 | 0.07 |
| Na2O | 0.09 | 0.08 | 0.11 | 0.09 | 0.08 | 0.08 |
| Al2O3 | 0.08 | 0.02 | 0.02 | 0.09 | 0.11 | 0.03 |
| SrO | 0.02 | 0.02 | 0.01 | 0.11 | 0.04 | 0.04 |
| SO3 | 0.11 | 0.1 | 0.1 | 0.05 | 0.16 | 0.13 |
| Total (%) | 99.73 | 99.09 | 100.55 | 101.04 | 101.01 | 100.85 |
| Sample Name | TC-W-1 | TC-12 | TC-13 | FJW1702-16 | FJW-3 | ZSH-3-4-12 |
|---|---|---|---|---|---|---|
| As | 55.5 | 77.4 | 6.9 | 13.9 | 4.3 | 15.6 |
| Fe | 1230 | 1045 | 3517 | 1268 | 2089 | 3962 |
| Mn | 845 | 811 | 1665 | 1041 | 1567 | 1419 |
| Sr | 354 | 464 | 473 | 602 | 1824 | 423 |
| Rb | / | 1.1 | 0.9 | 2.9 | 2.4 | 2.9 |
| Th | 46.9 | 78.6 | 36.2 | 6.5 | 21.7 | 37.0 |
| U | 33.9 | 56.2 | 35.1 | 5.7 | 24.3 | 31.2 |
| ΣREE | 7932 | 8195 | 5218 | 2028 | 2729 | 4432 |
| Sr/Y | 0.74 | 0.46 | 0.29 | 1.31 | 1.33 | 0.42 |
| LREE | 7638 | 7558 | 4429 | 1732 | 2047 | 3708 |
| HREE | 295 | 636 | 789 | 297 | 683 | 724 |
| LREE/HREE | 34.9 | 15.4 | 6.5 | 6.4 | 3.1 | 6.1 |
| Th/U | 1.37 | 1.64 | 0.96 | 0.92 | 1.06 | 1.14 |
| Ce/Pb | 1424 | 808 | 418 | 156 | 107 | 594 |
| Fe/As | 27.0 | 18.7 | 529 | 212 | 840 | 494 |
| Mn/As | 18.9 | 15.1 | 361 | 170 | 1002 | 254 |
| logfO2 | −11.6 | −11.5 | −13.4 | −12.0 | −13.2 | −12.9 |
| ∆FMQ | 1.84 | 1.92 | 0.04 | 1.26 | 0.23 | 0.58 |
| 87Sr/86Sr | 0.7047 | 0.7050 | 0.7077 | 0.7124 | 0.7078 | 0.7172 |
| 2SD | 0.0007 | 0.0004 | 0.0004 | 0.0003 | 0.0004 | 0.0016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; An, F.; Ling, M.; Feng, X.; Sun, W. Metallogenesis of Porphyry Copper Deposit Indicated by In Situ Zircon U-Pb-Hf-O and Apatite Sr Isotopes. Minerals 2022, 12, 1464. https://doi.org/10.3390/min12111464
Zhang H, An F, Ling M, Feng X, Sun W. Metallogenesis of Porphyry Copper Deposit Indicated by In Situ Zircon U-Pb-Hf-O and Apatite Sr Isotopes. Minerals. 2022; 12(11):1464. https://doi.org/10.3390/min12111464
Chicago/Turabian StyleZhang, Hong, Fang An, Mingxing Ling, Xiaolin Feng, and Weidong Sun. 2022. "Metallogenesis of Porphyry Copper Deposit Indicated by In Situ Zircon U-Pb-Hf-O and Apatite Sr Isotopes" Minerals 12, no. 11: 1464. https://doi.org/10.3390/min12111464
APA StyleZhang, H., An, F., Ling, M., Feng, X., & Sun, W. (2022). Metallogenesis of Porphyry Copper Deposit Indicated by In Situ Zircon U-Pb-Hf-O and Apatite Sr Isotopes. Minerals, 12(11), 1464. https://doi.org/10.3390/min12111464






