1. Introduction
The exploration rights of the Interoceanmetal Joint Organization (IOM) are granted to the area located within the Clarion–Clipperton Zone (CCZ) in the Eastern Central Pacific Ocean. The Clarion–Clipperton Zone (CCZ) is the largest and most economically promising area of the occurrence of polymetallic nodules in the world.
All activities related to the exploration of minerals in the CCZ (the Area—the seabed and ocean floor beyond the limits of national jurisdiction) come under the Law of the Sea—United Nations Convention on the Law of the Sea, the Agreement relating to the implementation of Part XI of the Convention as well as Regulations on Prospecting and Exploration for Polymetallic Nodules in the Area—regulations established by the International Seabed Authority (ISA).
In addition to a geological survey and related environmental research, IOM develops the methods for the technology of mining and processing of the deep-sea polymetallic nodules (PMN). The IOM’s presently developed PMN processing technology schemes represent hydrometallurgical and pyrometallurgical methods of extracting metals from nodules. Other tested works in the past included processing of the PMN with sulfuric acid, using different reducing agents, HPAL (high-pressure acid leaching) technologies, carbonyl technology, and others.
Until now, the studies of optimizing PMN processing [
1] have been focused on three technologies (
Table 1):
- (1)
Hydro-pyrometallurgical technology, which is leaching at atmospheric pressure using SO2 as a reducing agent to obtain Ni and Co, obtaining Mn in an electric arc furnace and (NH)4SO2 as a byproduct (fertilizer). This technology has been studied on a laboratory scale and has a level 3–4 of knowledge in accordance with the European Commission Technology Readiness Level (EC TRL) standards.
- (2)
Pyro-hydrometallurgical technology, which is the processing of polymetallic nodules in electric furnaces to obtain ferroalloys such as SiMn and FeMn and hydrometallurgical processing with high pressure sulfuric acid to separate Ni, Zn, and Cu. This technology has been studied on a laboratory scale and has a level 3–4 of knowledge according to the EC TRL standards.
- (3)
Hydrometallurgical technology, which is leaching with sulfuric acid at high pressures using pyrite (HPAL technology) and the extraction of Ni, Co, Zn, and Cu with Pulp Resin technology, ion exchange resin, and subsequent extraction by roasting—oxide reduction from Mn. This technology has been studied on an expanded laboratory scale and has a level of knowledge up to 5–6 in accordance with the EC TRL standards.
It was decided by the IOM expert group that the HPAL technology would be prioritized for further studies.
As part of the collaboration between the IOM and the Canadian company CVMR, laboratory and extended laboratory tests on PMN from the IOM exploration area were carried out in the first half of 2018 using carbonyl technology to produce very high purity Ni and Fe powders that were highly valued in international markets. Tests carried out in vertical reactors showed the high efficiency of the Ni and Fe recovery at 95% and 87%, respectively. Cooperation with this organization will continue for the possible conclusion of a broader agreement. Bioprocessing, as an alternative and experimental method of metal extraction from PMN has been already proposed [
2,
3]. The idea of bioprocessing is to introduce organisms and microbes to process the bioreduction of manganese dioxide. Bioleaching is characterized by relatively low operating costs and by the fact it is environmentally friendly.
Polymetallic nodules (Mn nodules) are attributed a remarkable economic potential since they contain significant concentrations of manganese, copper, nickel, and cobalt. They are thought to be formed by hydrogenous non-biogenic processes and biogenic processes based on metabolic processes driven by microorganisms. In the micronodules, a dense accumulation of microorganisms/bacteria was seen. There were two morphotypes: (1) round-shaped cocci and (2) elongated rods. The cocci (diameter: ≈3.5 μm) were arranged in beadlike chains, while the rods (≈2 × 0.4 μm) were arranged either as palisades or in a linear row. Energy-dispersive X-ray spectroscopy analyses showed that the areas rich in microorganisms/bacteria were also rich in Mn, while in areas where there were no microorganisms, Si was dominant [
4].
Polymetallic nodules were first discovered during the Challenger Expedition [
5] and subsequently comprehensively studied and described. The first mining trials occurred in the late 1970s and early 1980s. After 2010, there was an increased interest in seabed minerals, which continues to the present day. As of 2021, ISA has entered into 19 contracts for exploration for polymetallic nodules in the Clarion–Clipperton Zone, Central Indian Ocean Basin, and Western Pacific Ocean.
Nodules appear to be unique to the marine environment and are found on the bottom of almost all oceans at depths from 100 m to 6000 m. Metals, e.g., manganese (Mn), cobalt (Co), and nickel (Ni), are of significant industrial value due to their economic importance. As metal resources are depleted worldwide, polymetallic nodules on the seabed are becoming increasingly important as a natural source of metals, especially Mn, Co, and Ni [
6].
The mineralogy and processing options using pyrometallurgy or hydrometallurgy influence the economic viability of metal extraction from polymetallic nodules. The recovery of nickel and cobalt arises as one of the main issues in the processing of manganese nodules. General correlations show that higher nickel contents in nodules are associated with higher manganese contents, lower iron contents, and higher copper contents.
The hydrometallurgical processing of manganese nodules involves leaching with NH
3, HCl, or H
2SO
4 in the presence of other reagents [
7]. For example, the Cuprion process involves a reductive ammoniacal leach of a fine slurry of ground nodules in an agitated tank at low temperature using carbon monoxide gas produced from fuel oil. After countercurrent decantation in a series of thickeners, the Co is removed by sulfide precipitation, and Ni and Cu are separated using solvent extraction and recovered by electrolysis [
7].
Charewicz et al. [
8] achieved almost complete dissolution of all valuable metals from manganese nodules by chemical leaching using 4 mol dm
3 HCl at 30 °C. Lower acid concentrations yielded similar results at elevated temperatures. Kanungo and Jena [
9] observed the leaching of only Cu(II), Ni(II), and Co(II) in dilute HCl, without bringing much iron into solution, and there was a need for concentrated HCl and elevated temperature for the leaching of Co associated with Mn.
The reductive leaching tests for manganese nodules in the presence of nickel matte in hydrochloric acid produced elemental sulfur and gave leaching efficiencies of 90% Ni, 90% Cu, 100% Mn, and >80% Co in 1.5–2.0 mol dm
3 HCl at 95 °C after 80 min [
10].
The leaching of metals from nodules in weakly acidic solutions of pH 4.5 is also facilitated by the presence of a fungus—
Aspergillus niger, after activation of the sample in a high energy attrition mill for the surface charge characteristics of the particles. This process reduces the activated metal oxides/hydroxides and provides the complexing oxalate/citrate ligands to the biorecovery of more than 95% Cu, Ni, and Co after 15 days, when the nodules were activated for 10 min [
11].
Electrobioleaching in the presence of
Thiobacillus ferrooxidans and
Thiobacillus thiooxidans at pH 0.5 and 0.6 V (SCE—electrode served as the reference electrode, connected through a Luggin capillary) led to more than 90% dissolution of Mn, Fe, Cu, Ni, and Co [
12].
Most of the biotechnical processes for leaching of metals have been developed using aerobic microorganisms [
13,
14,
15]. However, highly oxidized metal compounds such as MnO, and Fe
2O, can be solubilized by reduction processes [
16]. Mn and Fe from MnO and Fe
2O can be recovered by the direct or indirect actions of heterotrophic microorganisms that thrive under microaerobic or anaerobic conditions [
17,
18,
19,
20,
21]. In the former case, the microorganisms can utilize MnO, as a final acceptor of electrons in the respiratory chain of their metabolism, instead of oxygen [
16]. That is, anaerobic heterotrophs such as iron-reducing bacteria, manganese-reducing bacteria, and sulfur-reducing bacteria donate electrons, which produce by the oxidation of organic substrates Fe
2O
3, MgO, MnO, SeO, and V
2O
5 and leach the reduced metal ions into the medium [
22,
23]. In the second case, the reduction process is associated with the formation of reducing compounds, which are products of their metabolism [
19,
20,
21].
Lee et al. (2010) [
24] reported that the anaerobic bioleaching technology of metals has not been commercialized yet, because it must be adapted for each type of metal. Moreover, there is a demand for a less expensive and more environmentally friendly anaerobic bioleaching process. Selecting more efficient bacterial cultures, optimizing operating parameters such as inoculum, incubation temperature, initial pH, mineral salts, carbon source and particle size, and/or performing leaching in columns or reactors should be carried out. The leaching efficiencies of Mn, Co, and Ni increased from 18%, 7%, and 10% to 77%, 70%, and 75%, respectively by the inoculation of the Mn-reducing bacteria in the enrichment culture broth. Metals could be efficiently recovered from the nodule in the ranges of pH from 5.0 to 6.5 and temperature from 30 to 45 °C by anaerobic bioleaching. External addition of mineral salts was not necessary for Mn, Co, and Ni leaching from the nodules. The optimum ratio of nodules to glucose was 0.1 (
w/
w). To obtain a leaching efficiency above 70%, the particle size of the nodules had to be less than 0.6 mm [
24].
Mehta et al. (2001) [
25] reported that the bioleaching of the metals from sea nodules was found to be more effective by fungal metabolites. The overall leaching of about 97% Cu, 98% Ni, 86% Co, 91% Mn, and 36% Fe was achieved after acid wash of the residue in presence of
A. niger after 30 days at an initial pH of 4.5, 35 °C, 5% pulp density, and low values of redox potential (380 mV). XRD (X-ray diffraction) identification of the leach residue showed the presence of some single and mixed oxidation phases of manganese and some unaltered iron phases (FeOOH, Fe
2O
3, Fe
3O
4, NiFe
2O
4, MnOOH, MnO
2, and MnOFe
2O
3) [
25].
2. Materials and Methods
2.1. Polymetallic Nodules
The sedimentary cover in the CCZ is a mixture of carbonates, red brown clays, and siliceous sediments. Occurrences of nodules in the CCZ result from the complex processes present on regional and local scales for the past several millions of years. Polymetallic nodules of the CCZ have growth rates at 1 to 10 mm per 1 million years. They are composed of both nuclei and concentric layers of iron and manganese hydroxides and oxides. The nucleus can be composed of volcanoclastic debris, lithified sediment, bioclasts, or fragments of older nodules. The main manganese mineral components include todorokite, birnessite, and vernadite. The mineral composition, as well as the hydrogenetic and diagenetic processes, condition the nodule’s morphology. The nodules vary in size and range from micronodules to large pellets of more than 20 cm in diameter. Most nodules are between 4 and 8 cm in diameter. The selected averages of the nodule physical properties from the H22 exploration block (based on 205 samples collected) were: porosity 62%, specific nodule density 3.49 g/cm3, and natural water content 32%. Polymetallic nodules occur typically on the surface of the deep seabed. They are embedded in the semi-liquid surface layer and often are partly covered by a thin layer of unconsolidated sediments. Mn, Fe, Cu, Ni, and Co are the main metal elements present in nodules. These metals (with the exception of Fe) also represent the main economic potential of the prospective deposit.
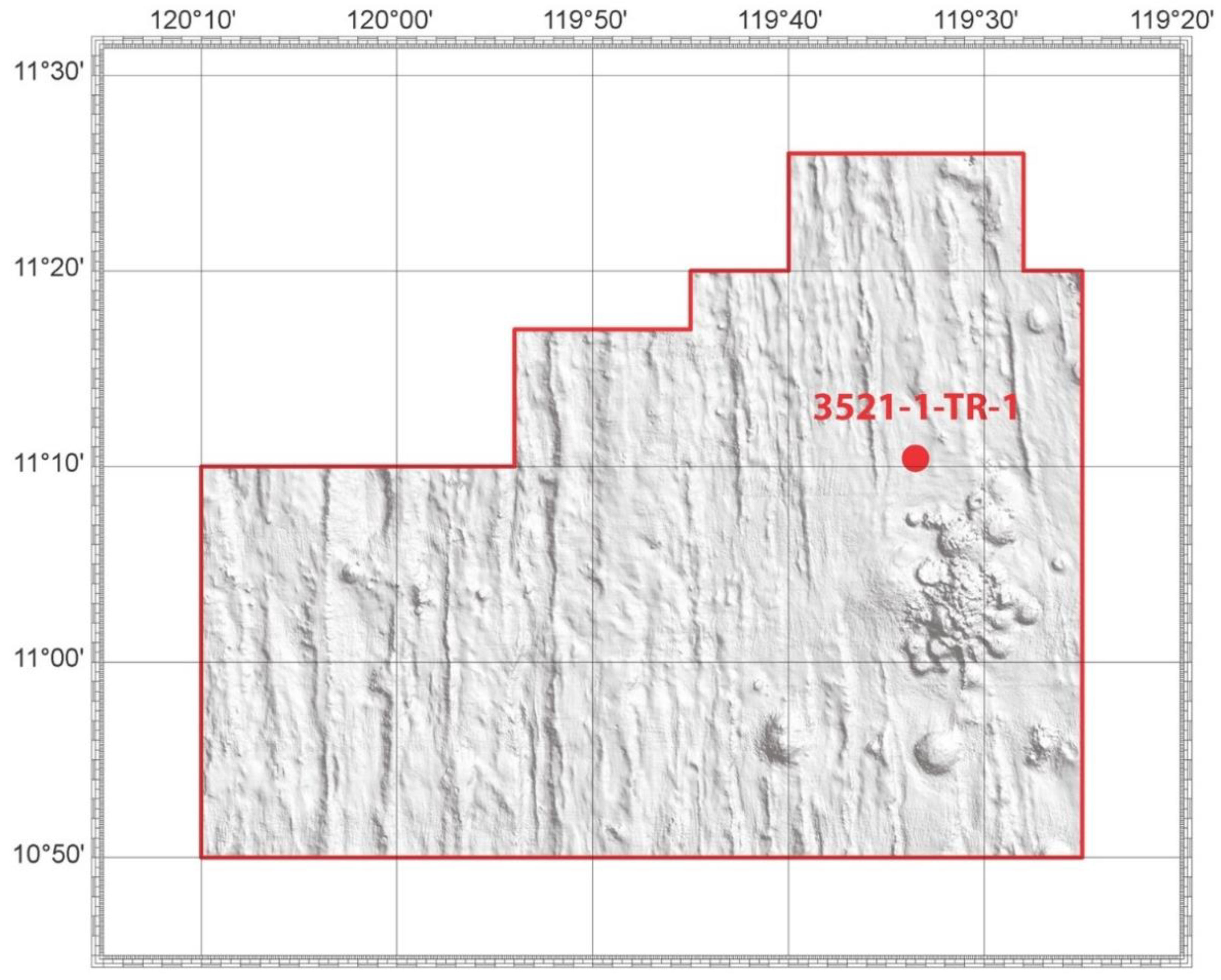
The polymetallic nodule sample (app. 2 kg) was collected by the Interoceanmetal Joint Organization from the Clarion–Cliperton Zone, in the eastern central Pacific Ocean, IOM exploration area, H22 exploration block (
Figure 1). A sample of the nodules lying on the seabed was collected by trawl during an IOM expedition in 2014. The depth of the sampling was from 4265 to 4291 m (sample No 3521-1-TR1).
The chemical analyses of the Mn nodules were performed using a portable Vanta X-ray fluorescence spectrophotometer for rapid, accurate, laboratory-quality elemental analyses of the solid and liquid phases, and the concentrations of Co, Ni, Cu, Zn, and Mn in the leachates. Solid samples of the input of the Mn nodule (
Table 2) were measured by Vanta X-ray fluorescence spectrophotometer, and the bioleached sample No. 3 was confirmed by an accredited laboratory (EKOLAB Ltd., Košice, Slovakia).
XRF analyses were provided by the Vanta analyzer, which is the most advanced handheld X-ray fluorescence (XRF) spectrophotometer with laboratory-quality results in the field. It provides rapid accurate elemental analysis and alloy identification for high-throughput XRF testing. The preparation of the XRF measuring sample consisted of drying where necessary, followed by homogenization, crushing to 1–2 mm, and milling in an agate bowl mill to a powder below 0.075 mm. The sample was then placed into a plastic sample cup with a plastic support film. This ensured a flat surface for the X-ray analyzer and the sample to be supported over the X-ray beam. The measurements were carried out in 3 replicates, and the given concentrations are the average of all measurements for the sample.
Accredited laboratory analyses were provided by EKOLAB, according to the EPA Method 200.7: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry. The samples were mineralized in Aqua regia in a microwave digestion system. Analysis was performed using ICP-OES.
The average contents of Mn, Cu, Ni, Co, and Fe in the H22 exploration block were determined to be 31.0%, 1.3%, 1.3%, 0.2%, and 6.0% respectively (IOM data). Chemical analyses were performed onboard the research vessels during the IOM expeditions in period 2001–2014 and in accredited laboratories of Yuzhmorgeologiya (Gelendzhik, Russian Federation). The metal content in the samples collected was determined using a variety of methods, including atomic absorption spectroscopy and X-ray fluorescence.
The polymetallic nodule sample used in this test was pulverized using a bowl mill after being dried at room temperature. The particle size was 0.1–1 mm in diameter.
2.2. Prokaryotic Cultures Containing Mn Reducers
The first test of the Mn-reducing microorganism isolation was performed by the inoculation of the samples from different natural locations of the Fe/Mn weathering processes to 0.25 g Mn nodules in 250 mL of the HG medium (No. 1–No. 5, ekofertile™ medium, ekolive Ltd., Košice, Slovakia) and MD medium (No. 6–No. 9, mineral salt medium [
26]). The similar nature mud was added to the samples No. 1 and No. 6, and the mud from a different position was given were into No. 2 and No. 7. Moreover, the localization was similar for samples No. 3 and 8 or No. 4 and No. 9. The sampling sites of the bacteria are not stated because they are part of ekolive’s IP. The natural mud samples were subjected to metagenomic analysis provided by Bay Zoltán Nonprofit Ltd. for Applied Research, Division for Biotechnology BAY-BIO.
The metagenomic analysis included DNA extraction, PCR amplification, sequencing, and bioinformatic analysis.
Bacterial DNA was extracted from a 100–200 mg matrix per sample using the ZymoBIOMICS 96 MagBead DNA Kit (Zymo Research) according to the manufacturer’s protocol. The concentration of the genomic DNA was measured using a Qubit 3.0 Fluorometer with a Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific). Bacterial DNA was amplified with tagged 16S Amplicon PCR Forward Primer = (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG and 16S Amplicon PCR Reverse Primer = 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC) covering the V3–V4 region of the bacterial 16S rRNA gene [
27]. Polymerase chain reactions (PCR) and DNA purifications were performed according to Illumina’s demonstrated protocol (Part # 15044223 Rev. B). The PCR product libraries were quantified and qualified using High Sensitivity D1000 ScreenTape on a TapeStation 2200 instrument (Agilent). Equimolar concentrations of the libraries were pooled and sequenced on an Illumina MiSeq platform using MiSeq Reagent Kit v3 (600 cycles PE) (Illumina, San Diego, CA, USA).
On average ca. 400,000 raw sequencing reads per sample were generated, which were demultiplexed, adapter-trimmed, and quality-filtered using MiSeq Control Software (Illumina, San Diego, CA, USA). Classification was performed using the Illumina 16S Metagenomics workflow based on the DADA2 formatted RefSeq RDP 16S v3 database [
28,
29].
2.3. Enrichment of Mn Reducers
Enrichment of the heterotrophic Mn-reducing microorganisms was obtained by acclimation of the isolated Mn-reducing bacteria in the Mn nodule according to the method described by Lee et al. [
26]. Therefore, the enrichment of the heterotrophic Mn-reducing microorganisms from the first test No. 1 and No. 6 was acclimated by serial transfer five times, kept in bottles, and transferred into a fresh medium every 5 d, utilizing 25 mL of inoculum per 250 mL of the mineral salt medium (MD) supplemented with 10 g/L sucrose (a labeled numerical sample (+) was enriched with 20 g/L sucrose) and 10 g/L nodule. The composition of the MD medium was as follows (g/L): NaHCO
3, 2.5; NH
4CI, 1.5; KH
2PO
4, 0.6; KCl, 0.1; citric acid, 4; and MgSO
4 × 7H
2O, 1 [
24]. Ten g/L sucrose was supplied to the medium as the carbon source, and the pH of the medium was adjusted to 5.5 ± 0.5 with 25% NH
4OH.
2.4. Bioleaching of the Polymetallic Nodules
The bioleaching experiments were conducted in a similar MD medium in sealed flasks No. 1–4 containing 0.25 g, 2.5 g, 25 g, and 50 g of the Mn-nodule sample and a total medium volume of 240 mL/10 mL bacterial inoculum, which was used for intermittent leaching with 150 mL of leachate collection of the five-times transfers after 6 days of bioleaching. Flask No. 5 contained 2.5 g Mn nodules and 240 mL taper water/10 g sucrose with the pH adjusted by 25% NH4OH and 1M H2SO4. The bottles were charged with 10 mL of the enrichment culture broth of Mn-reducing microorganisms. The bottles were sealed tightly with butyl rubber stoppers and incubated at 38 °C ± 0.5 °C and pH 5.5 ± 0.5.
4. Discussion
The pulp density of the Mn nodule determines the available surface area for the bioleaching process. It is of particular interest to operate bioleaching processes at high solid concentrations due to process economics. However, in the BIOX process for biooxidation of gold bearing ores or concentrates, there are certain practical limitations to increasing pulp densities, and the operating pulp density is often limited to a threshold level of 20% solids by weight in industrial stirred-tank bio-oxidation practice [
30].
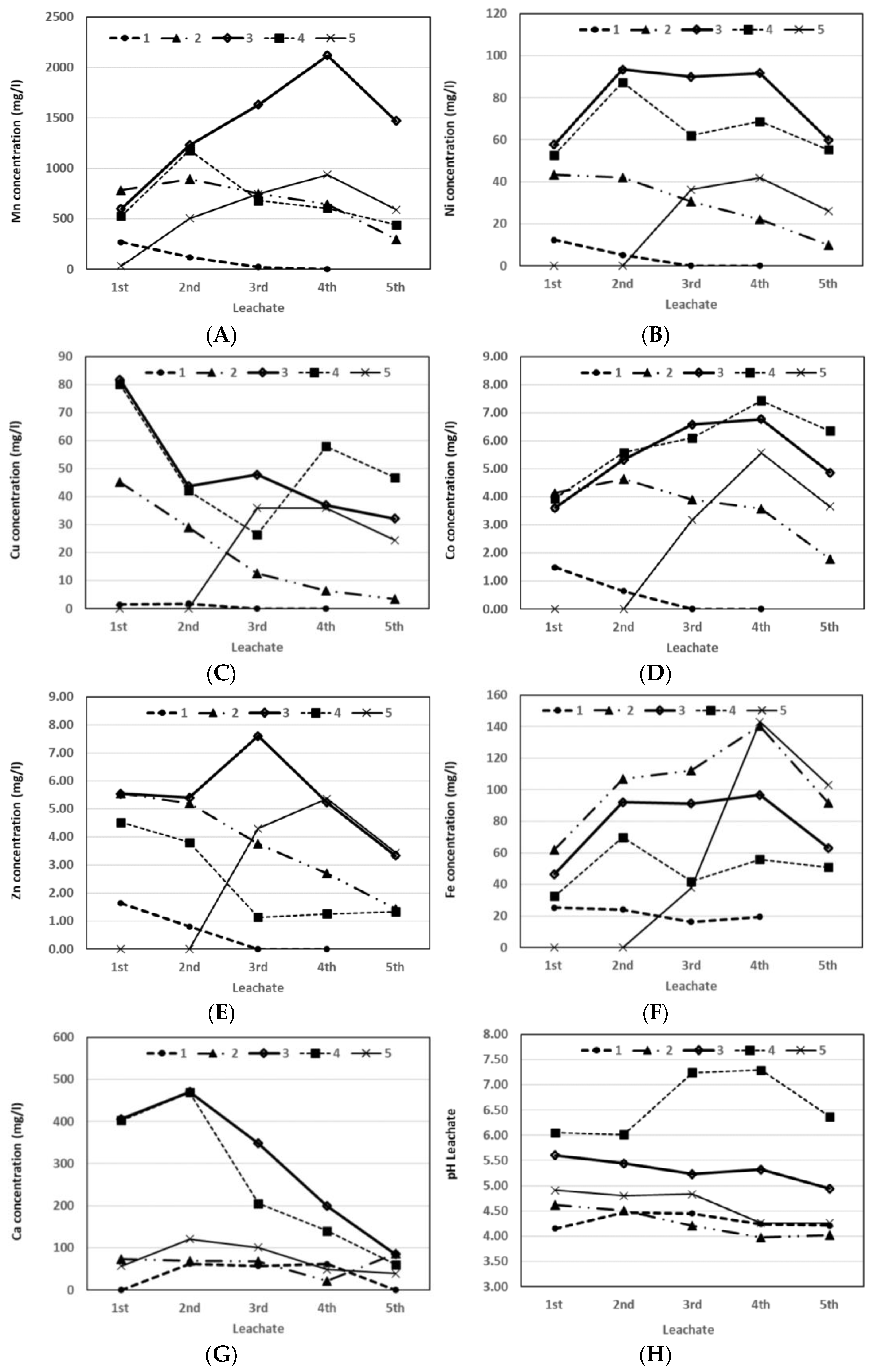
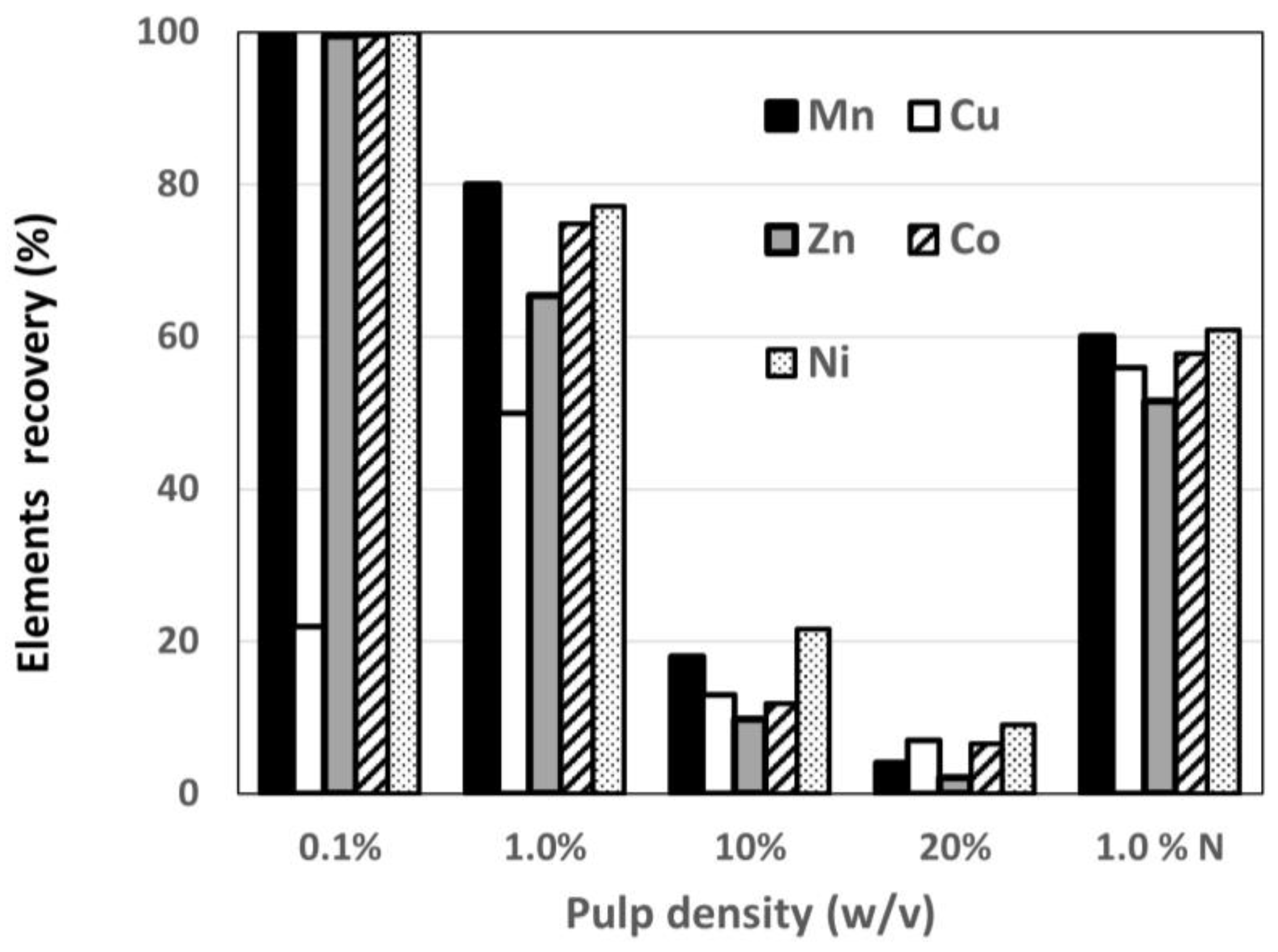
The laboratory experiments demonstrated two possibilities for extracting the useful elements from the polymetallic nodules at 0.1% pulp density during bioleaching with one media change and two leachate collections or at 10% pulp density during bioleaching with at least 25 media changes and leachate collections (
Figure 4 and
Figure 5). In the first possibility, 100% of the Mn, Ni, Co, and Zn was recovered and only 22% of the Cu. This effect indicated that the Cu extraction commenced later than that of other metals, and the heterotrophic leaching inhibited the Cu extraction. This inhibition can be used for selective elements recovery with two media changes after 10 days of bioleaching.
The low Cu concentration in the leachates with the presence of heterotrophic bacteria is likely due to the sequestration with oxalic acids, because X-ray analysis confirmed the formation of a secondary solid phase, weddellite (CaC
2O
4 × 2H
2O), in the bioleached soil sample. Weddellite formation is attributed to the destruction of Ca minerals and the formation of oxalic acid during bacterial metabolism [
31].
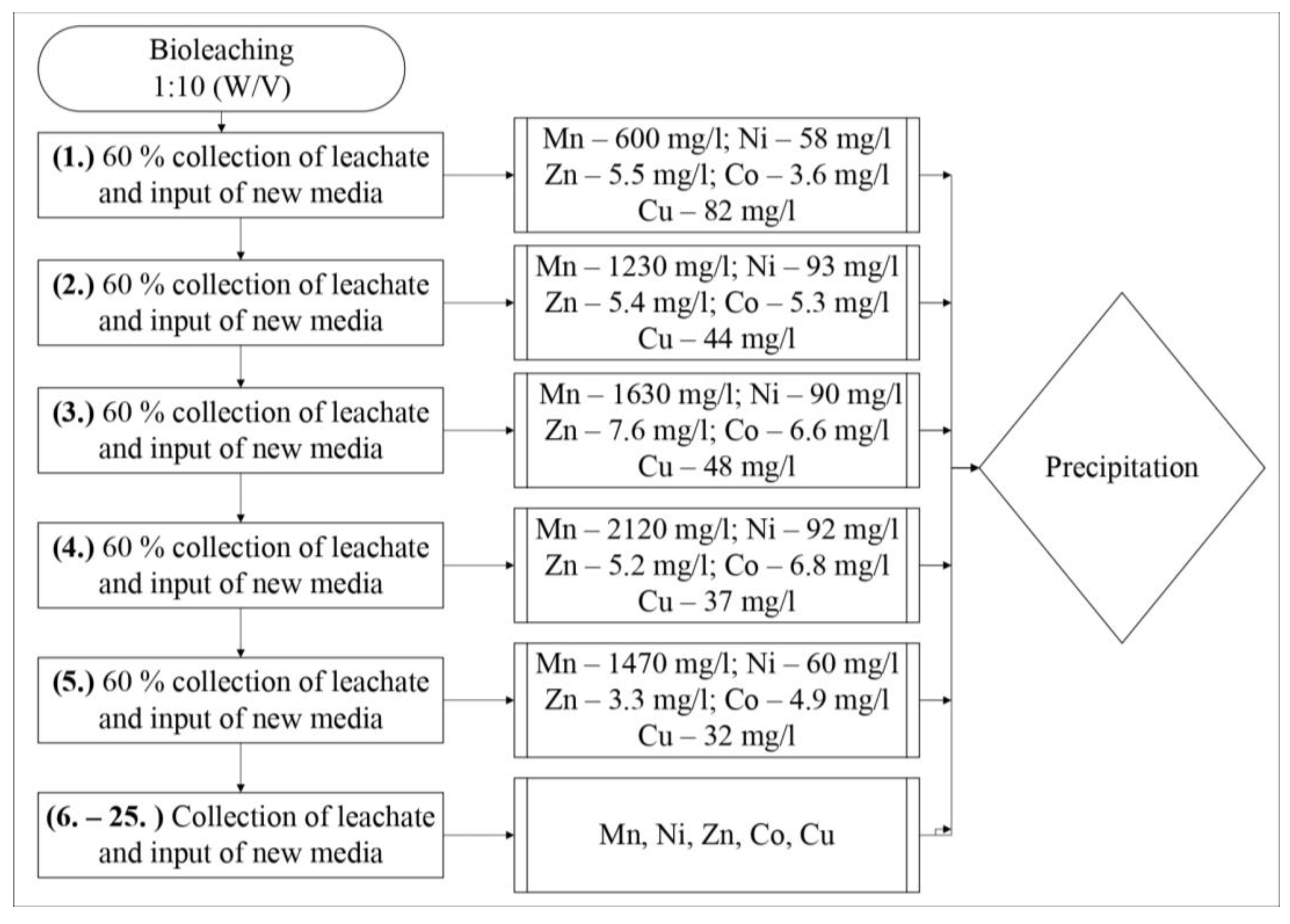
In the second possibility, in total, 18% Mn, 22% Ni, 12% Co, 10% Zn, and 13% Cu were recovered by bioleaching. However, the washing process removed the dissolved elements, and the percent removal increased to 55% Mn, 36% Ni, 15% Co, 27% Zn, and 38% Cu. The combination of the discontinuous repeated bioleaching and washing can decompose the nodule particles 100% recovery of the useful elements.
The mineral layer from which the elements are washed not only dictates the amount of sorption (with respect to the total content) but also influences the subsequent release of the elements; a greater proportion of adsorbed elements generally results in lower recovery rates of these elements from the sample after leaching [
32].
In these ways, different concentrations of elements can be obtained into leachates for subsequent precipitation processes. The highest concentrations of 2120 mg/L of Mn, 135 mg/L Cu, 94 mg/L Ni, and 8 mg/L Zn were at the 10% pulp density. In this heterotrophic bioleaching, the bacteria tolerated up to 2000 mg/L Mn after the fourth extraction of leachate. This means there was a gradual adaptation of the mixture to the bacteria during discontinuous bioleaching or the selection of bacterial species, which needs to be studied in the future. The synergistic bacterial effects of different factors are known to influence bioleaching.
The isolation of Mn-tolerant strains from Odisha, India was reported by Sanket et al. (2017) [
33] in which samples were collected from six different sites. The fifteen isolates were studied for Mn tolerance out of which five strains showed better tolerance such as 50 mg/L, 15 mg/L, 40 mg/L, 50 mg/L, and 30 mg/L respectively in comparison with others. These five isolates were characterized morphologically, microscopically, and biochemically and were identified as
Bacillus sp.,
Micrococcus sp.,
Pseudomonas fluorescens,
Bacillus sp., and
Alteromonas sp. Although the Mn concentration in the ore sample was low, the tolerance level of the isolates was high, i.e., up to 50 mg/L [
33].
The collected mud sample used as inoculum contained 50% genus Lactobacillus, and the tolerance level was high, i.e., up to 2000 mg/L Mn during the Mn nodules’ bioleaching.
The presence of metals such as Ag, Cu, Fe, and Zn usually has an inhibitory effect on the bacterial performance, but the ability of the bacteria to improve their performance after long-term adaptation has been demonstrated [
34,
35].
Verification of semi-operational procedures would allow assessment of the economic aspects of the process and selection of the best method with a suitable pulp density for the decomposition and ecological recovery of beneficial elements from polymetallic nodules.
5. Conclusions
The initial bioleaching experiment demonstrated the feasibility of processing polymetallic nodules. An enrichment of heterotrophic Mn-reducing microorganisms was used for the extraction of metals. The bioleaching was conducted for different pulp density solutions. The efficiency of the polymetallic nodules’ bioleaching increased when the pulp density was reduced from 20% to 0.1% (w/v); at the same time, there was an almost 100% increase in the dissolution of Mn, Fe, Co, Ni, and Zn—except Cu. However, there are certain practical limitations on the pulp density, which is often limited to a threshold of 10–20% solids by weight in industrial tanks. The bioleached sample with a pulp density of 10% showed the maximum Mn concentration in the leachate (2120 mg/L), but at the same time, the recovery of Mn, Cu, Zn, Co, and Ni fell below 20%. To achieve total Mn, Cu, Zn, Co, and Ni recovery from the Mn nodules, an intermittent bioleaching with about 25 changes of 60% of the medium was required.
The developed bioleaching has promising potential as an environmentally friendly alternative to dangerous acidic chemical leaching.