Effect of Magnesite Addition and Mechanical Activation on the Synthesis of Fly Ash-Based Geopolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MA
2.3. Preparation of Geopolymers
2.4. Dissolution Test
2.5. Characterization Methods
3. Results and Discussion
3.1. Effect of MA on the (FA + Magnesite) Blends
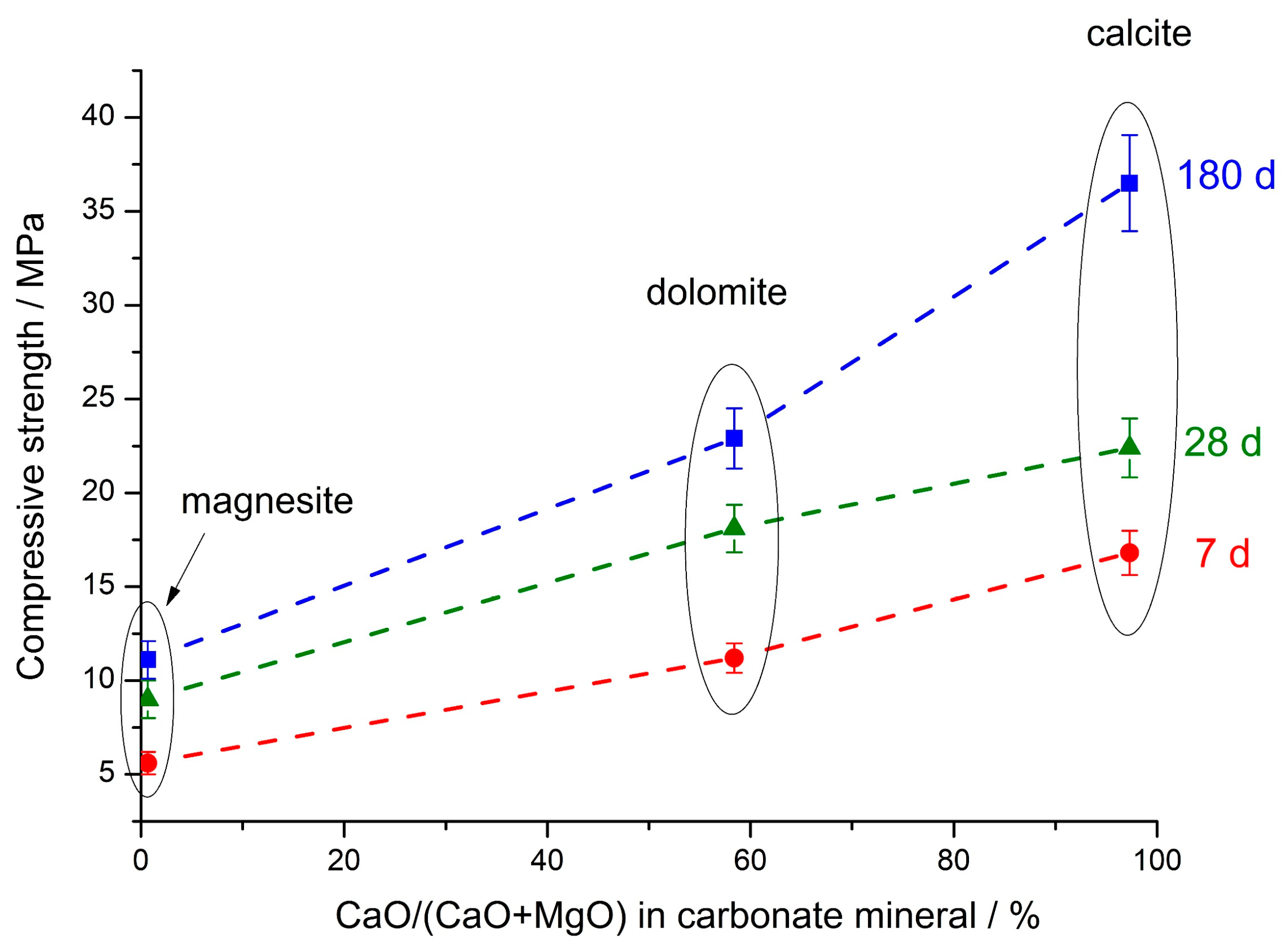
3.2. Mechanical Properties
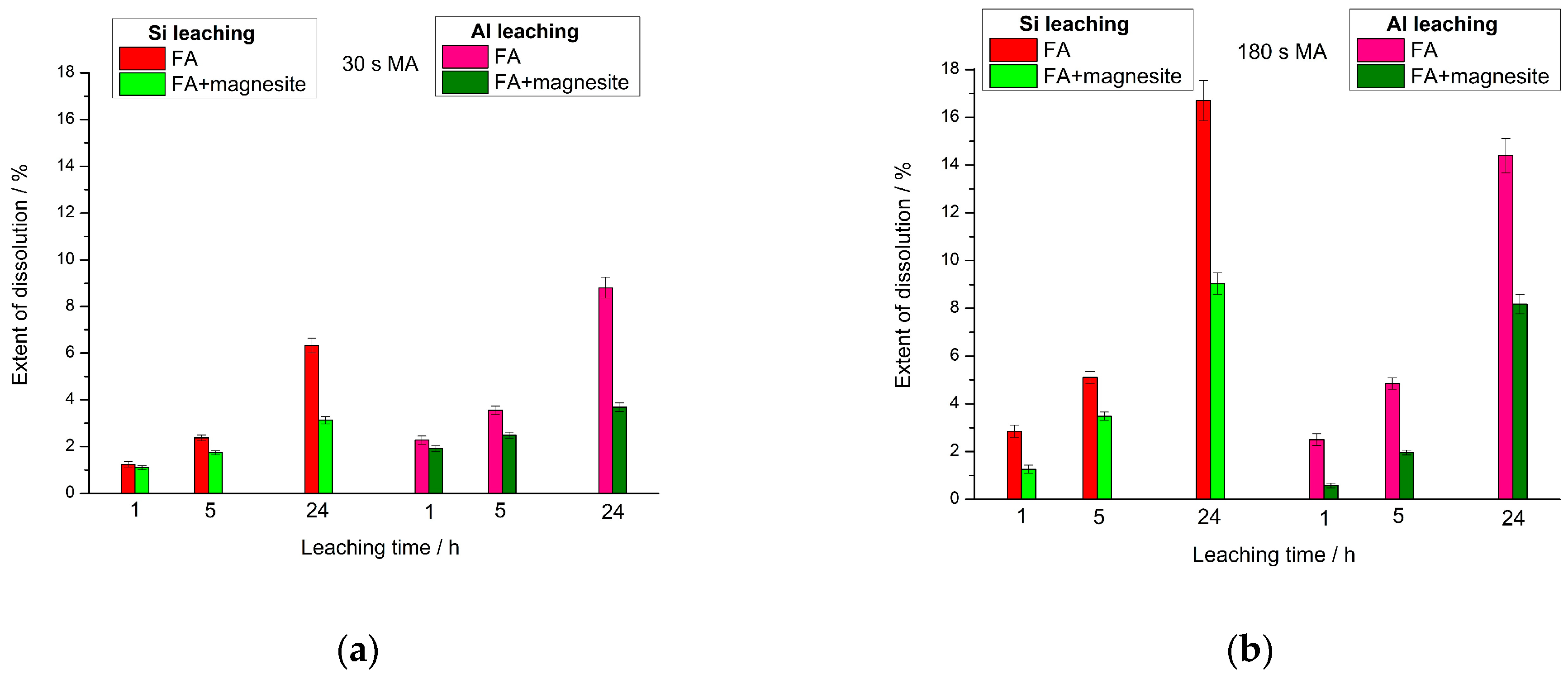
3.3. Dissolution Experiments
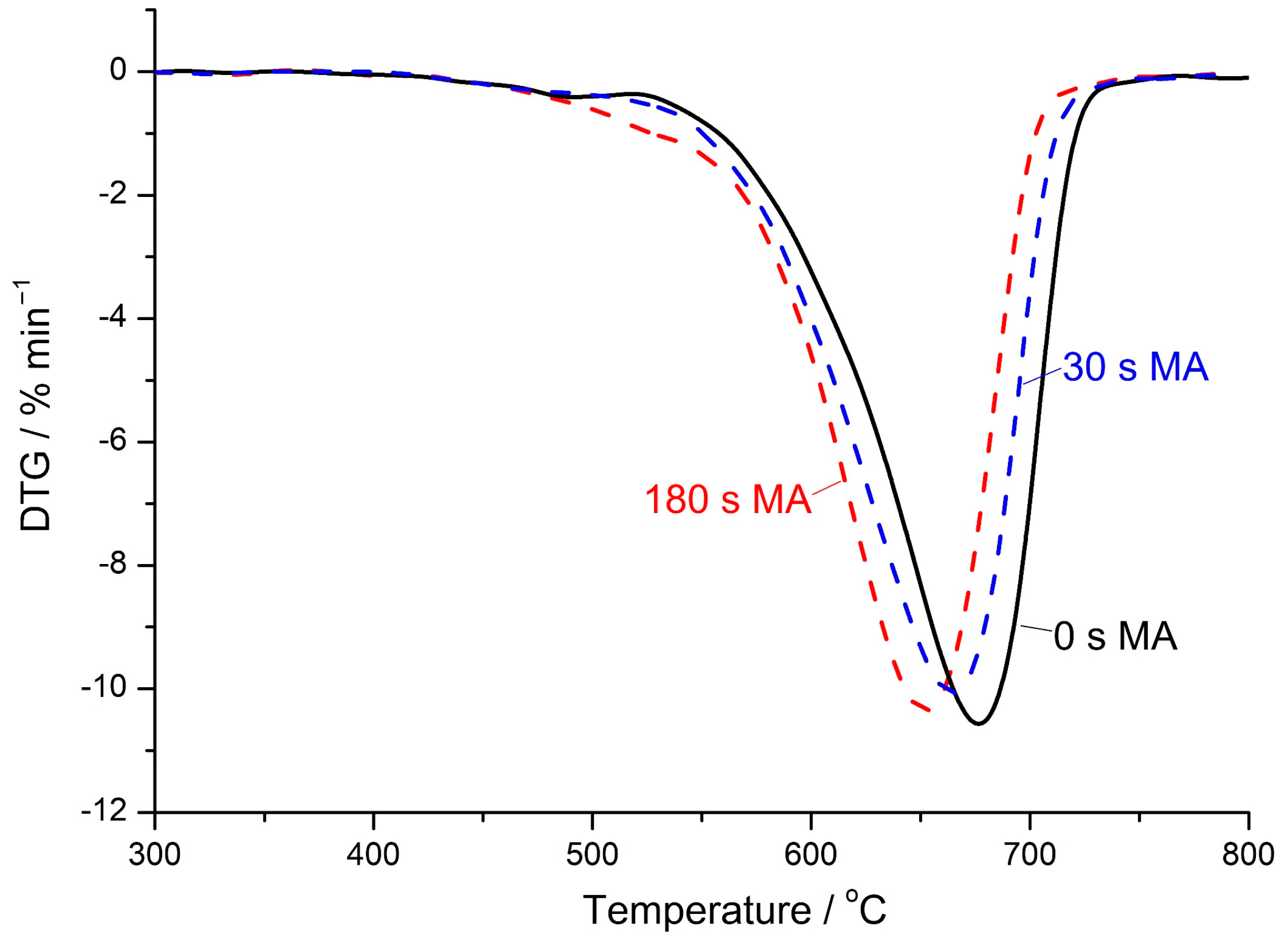
3.4. TG Analysis
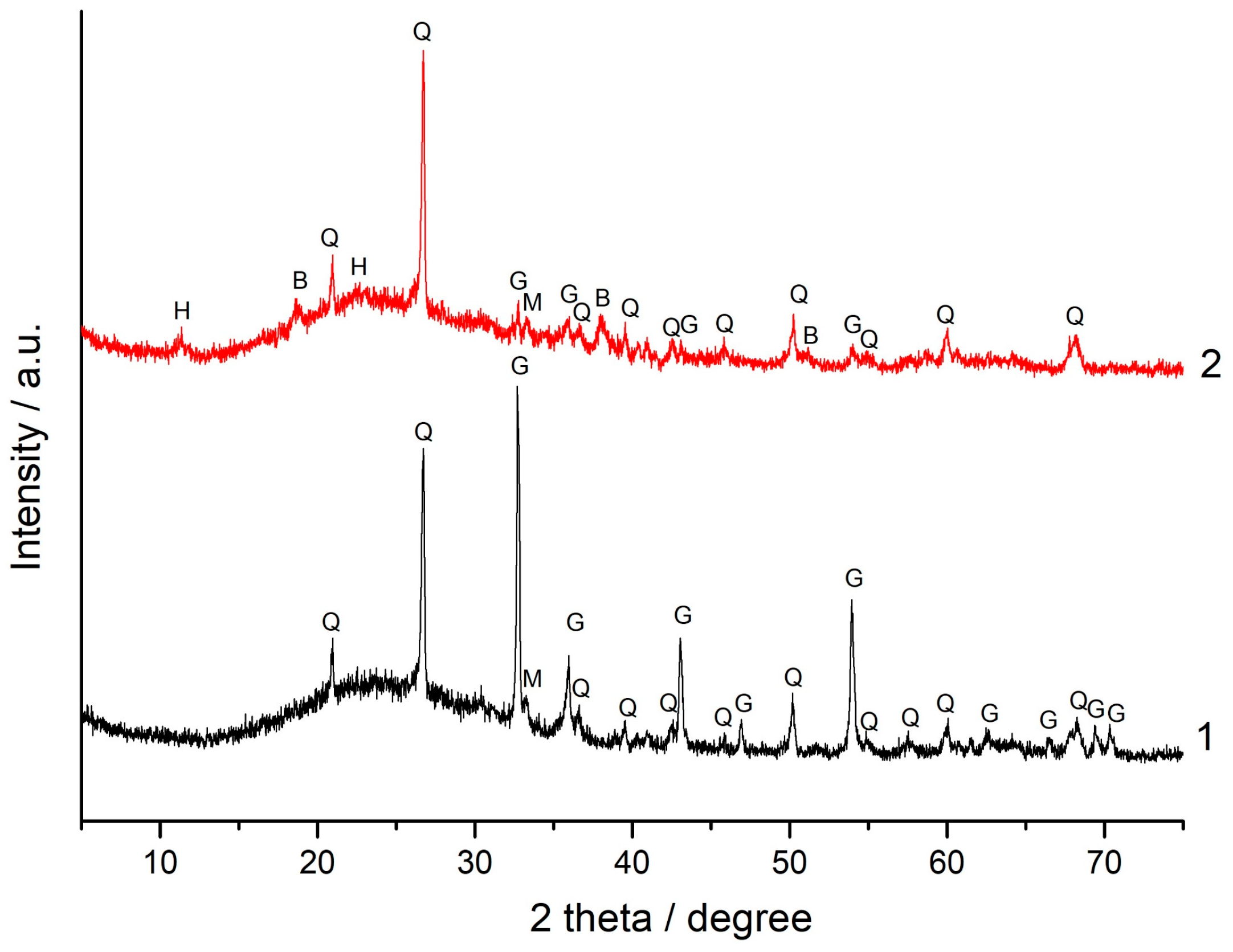
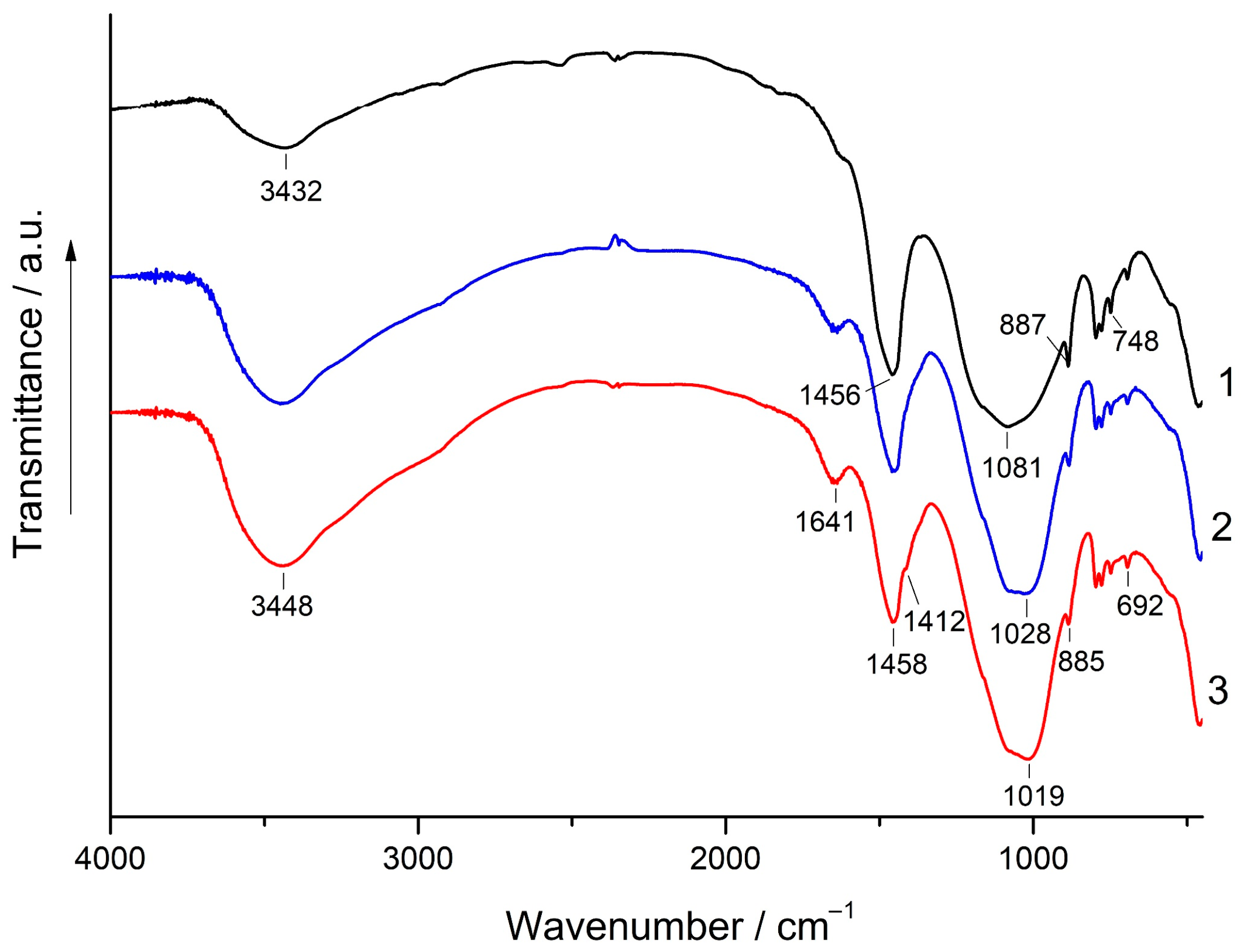
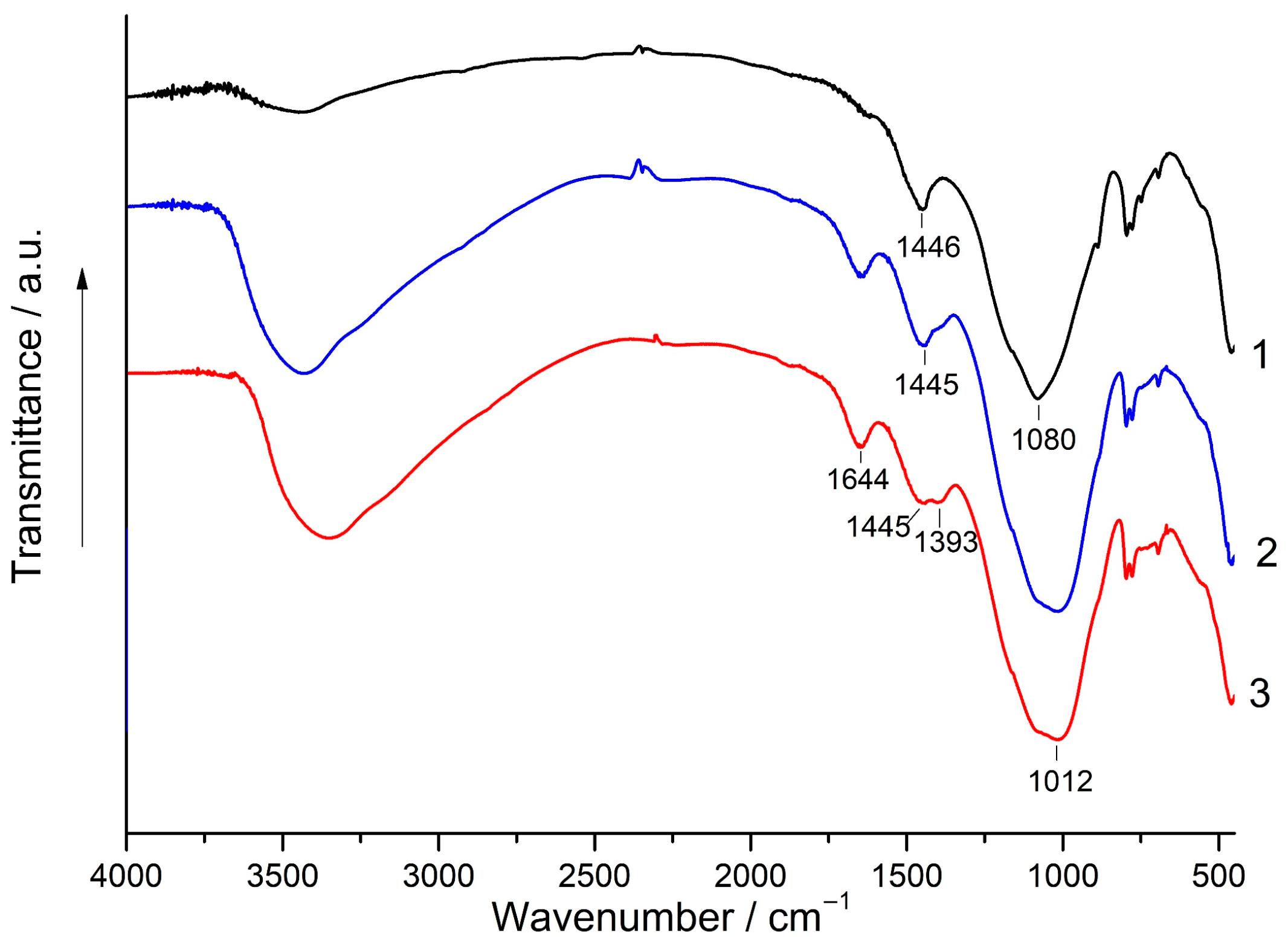
3.5. XRD and FT-IR Spectroscopy Analysis
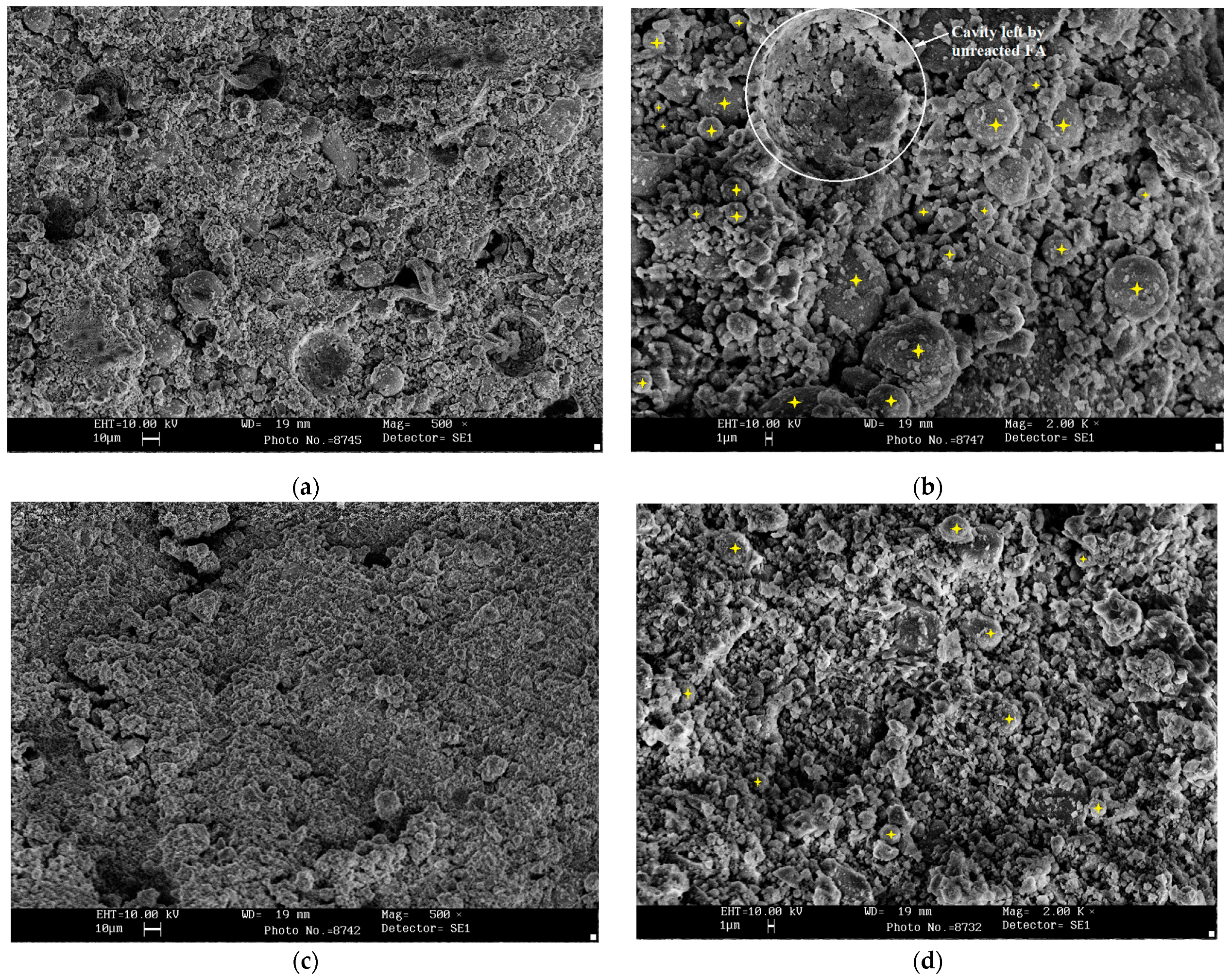
3.6. Microstructural Studies
4. Conclusions
- Experiments on leaching of the mechanically activated (FA + magnesite) blend with NaOH solution showed that the dissolution of silicon and aluminum occurred synchronously. This indicates that Si and Al were extracted from the FA into the alkaline solution in a linked form. The addition of magnesite to the FA led to a significant decrease in the concentration of both Si and Al in the solution. The reason for this decrease might be the formation of aluminosilicate gel, which was accelerated under the influence of magnesite. The increase in the MA time of the (FA + magnesite) blend from 30 to 180 s was accompanied by a marked increase in the degree of Si and Al transition into NaOH solution. This is explained by the increased reactivity of blend components with respect to sodium hydroxide solution.
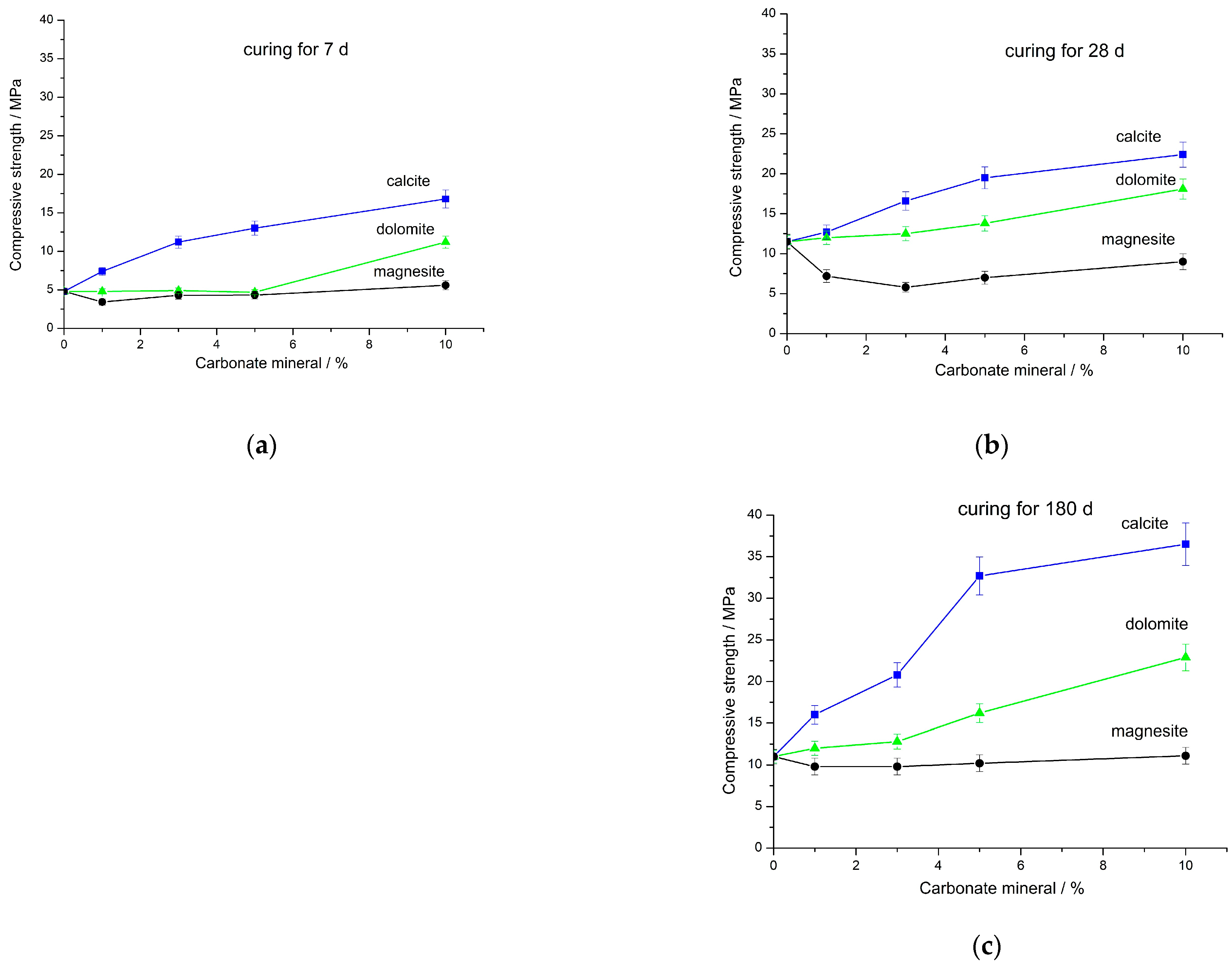
- In agreement with dissolution experiments, as well as with the results of FT-IR spectroscopy, TG analysis and SEM, increasing the MA time of the mixture of FA and magnesite from 30 to 180 s on average increased the strength of geopolymers by a factor of 8.0 ± 1.5, 3.0 ± 0.9, 1.5 ± 0.2, and 1.7 ± 0.5 after curing for 7, 28, 180, and 360 d, respectively. A further increase in the MA time to 400 s, despite the increase in the specific surface area, either did not change the strength, or increased it to a slight extent, which can be explained by the effect of “overgrinding”.
- For the studied calcium and magnesium carbonates, the strength of the corresponding composite geopolymers decreased in the series CaCO3 (calcite) > CaMg(CO3)2 (dolomite) > MgCO3 (magnesite). In contrast to calcite and dolomite, the addition of magnesite to the FA did not improve the strength of the composite geopolymer. The most probable reason for the lack of strength increase as a result of magnesite addition is magnesite’s chemical composition, in which magnesium dominates among the metals, while calcium is practically absent. A decrease in the strength of geopolymers based on the blends of FA with magnesite is also due to these blends’ higher water demand compared to the demands of blends of FA with calcite and dolomite.
- Although the addition of magnesite to FA did not improve the geopolymer performance, for most blends containing 1%–10% magnesite, their strength either did not decrease or it decreased to a minimal extent compared to geopolymers based on 100% FA. This can probably be explained by filler and dilution effects. Alkaline activation of the (FA + magnesite) blend according to FT-IR spectroscopy data apparently resulted in the formation of hydrotalcite in minute amounts, which was not determined by XRD in the geopolymers. To elucidate the influence of hydrotalcite on the properties of composite geopolymers, additional studies are needed.
- In this work, a planetary mill was used as the mechanical activator. Future research should aim to obtain similar results using other types of mills (e.g., vibratory and attrition mills), which are currently used in industry for large-scale production. A study on the effect of adding magnesite to other raw materials used to prepare alkali activated binders, such as BFS, is also of interest.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 2017, 8, 335–349. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. An Overview of geopolymers derived from industrial by-products. Constr. Build. Mater. 2016, 127, 183–198. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Hu, Y.; Zhou, J.L.; Tam, V.W.Y. Review on designs and properties of multifunctional alkali-activated materials (AAMs). Constr. Build. Mater. 2019, 200, 474–489. [Google Scholar] [CrossRef]
- Luukkonen, T.; Heponiemi, A.; Runtti, H.; Pesonen, J.; Yliniemi, J.; Lassi, U. Application of alkali-activated materials for water and wastewater treatment: A review. Rev. Environ. Sci. Biol. 2019, 18, 271–297. [Google Scholar] [CrossRef] [Green Version]
- Dogan-Saglamtimur, N.; Bilgil, A.; Ertürk, S.; Bozkurt, V.; Süzgeç, E.; Akan, A.G.; Nas, P.; Çetin, H.; Szechynska-Hebda, M.; Hebda, M. Eco-geopolymers: Physico-mechanical features, radiation absorption properties, and mathematical model. Polymers 2022, 14, 262. [Google Scholar] [CrossRef]
- Oglat, A.A.; Shalbi, S.M. An alternative radiation shielding material based on barium-sulphate (BaSO4)-modified fly ash geopolymers. Gels 2022, 8, 227. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C.; Spangenberg, J.; Mehrali, M. Hardening evolution of geopolymers from setting to equilibrium: A review. Cem. Concr. Compos. 2020, 114, 103729. [Google Scholar] [CrossRef]
- Mucsi, G.; Kumar, S.; Csoke, B.; Kumar, R.; Molnar, Z.; Racz, A.; Mádai, F.; Debreczeni, Á. Control of geopolymer properties by grinding of land filled fly ash. Int. J. Min. Proc. 2015, 143, 50–58. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.; Mehrotra, S.P. Towards sustainable solutions for fly ash through mechanical activation. Resourc. Conserv. Recyc. 2007, 52, 157–179. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Cristelo, N.; Tavares, P.; Lucas, E.; Miranda, T.; Oliveira, D. Quantitative and qualitative assessment of the amorphous phase of a Class F fly ash dissolved during alkali activation reactions—Effect of mechanical activation, solution concentration and temperature. Compos. Part. B Eng. 2016, 103, 1–14. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.; Alex, T.C.; Singla, R. Mapping of calorimetric response for the geopolymerisation of mechanically activated fly ash. J. Therm. Anal. Calorim. 2019, 136, 1117–1133. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Alex, T.C.; Bandopadhyay, A.; Mehrotra, S.P. Influence of reactivity of fly ash on geopolymerisation. Adv. Appl. Ceram. 2007, 106, 120–127. [Google Scholar] [CrossRef]
- Matsuoka, M.; Yokoyama, K.; Okura, K.; Murayama, N.; Ueda, M.; Naito, M. Synthesis of geopolymers from mechanically activated coal fly ash and improvement of their mechanical properties. Minerals 2019, 9, 791. [Google Scholar] [CrossRef] [Green Version]
- Temuujin, J.; Williams, R.P.; van Riessen, A. Effect of mechanical activation of fly ash on the properties of geopolymer cured at ambient temperature. J. Mater. Process Technol. 2009, 209, 5276–5280. [Google Scholar] [CrossRef]
- Chu, Y.-S.; Davaabal, B.; Kim, D.-S.; Seo, S.-K.; Kim, Y.; Ruescher, C.; Temuujin, J. Reactivity of fly ashes milled in different milling devices. Rev. Adv. Mater. Sci. 2019, 58, 179–188. [Google Scholar] [CrossRef]
- Kumar, S.; Mucsi, G.; Kristály, F.; Pekker, P. Mechanical activation of fly ash and its influence on micro and nano-structural behaviour of resulting geopolymers. Adv. Powder Technol. 2017, 28, 805–813. [Google Scholar] [CrossRef]
- Marjanovic, N.; Komljenovic, M.; Bascarevic, Z.; Nikolic, V. Improving reactivity of fly ash and properties of ensuing geopolymers through mechanical activation. Constr. Build. Mater. 2014, 57, 151–162. [Google Scholar] [CrossRef]
- Kato, K.; Xin, Y.; Hitomi, T.; Shirai, T. Surface modification of fly ash by mechano-chemical treatment. Ceram. Int. 2019, 45, 849–853. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Garcia-Lodeiro, I.; Maltseva, O.; Palomo, A. Mechanical-chemical activation of coal fly ashes: An effective way for recycling and make cementitious materials. Front. Mater. 2019, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; van Deventer, J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Rakhimova, N. Calcium and/or magnesium carbonate and carbonate-bearing rocks in the development of alkali-activated cements–a review. Constr. Build. Mater. 2022, 325, 126742. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Microscopy and microanalysis of inorganic polymer cements. 2: The gel binder. J. Mater. Sci. 2009, 44, 620–631. [Google Scholar] [CrossRef]
- Yip, C.K.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S. Carbonate mineral addition to metakaolin-based geopolymers. Cem. Concr. Compos. 2008, 30, 979–985. [Google Scholar] [CrossRef]
- Cwirzen, A.; Provis, J.L.; Penttala, V.; Habermehl-Cwirzen, K. The effect of limestone on sodium hydroxide-activated metakaolin-based geopolymers. Constr. Build. Mater. 2014, 66, 53–62. [Google Scholar] [CrossRef]
- Ortega-Zavala, D.E.; Santana-Carrillo, J.L.; Burciaga-Díaz, O.; Escalante-García, J.I. An initial study on alkali activated limestone binders. Cem. Concr. Res. 2019, 120, 267–278. [Google Scholar] [CrossRef]
- Firdous, R.; Hirsch, T.; Klimm, D.; Lothenbach, B.; Stephan, D. Reaction of calcium carbonate minerals in sodium silicate solution and its role in alkali-activated systems. Miner. Eng. 2021, 165, 106849. [Google Scholar] [CrossRef]
- Cousture, A.; Renault, N.; Gallias, J.L.; Ndiaye, K. Study of a binder based on alkaline activated limestone. Constr. Build. Mater. 2021, 311, 125323. [Google Scholar] [CrossRef]
- Ben Haha, M.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and drying shrinkage of reactive MgO modified alkali-activated slag paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Shen, W.G.; Wang, Y.H.; Zhang, T.; Zhou, M.K.; Li, J.S.; Cui, X.Y. Magnesia modification of alkali-activated slag fly ash cement. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 121–125. [Google Scholar] [CrossRef]
- Sreenivasan, H.; Kinnunen, P.; Heikkinen, E.P.; Illikainen, M. Thermally treated phlogopite as magnesium-rich precursor for alkali activation purpose. Miner. Eng. 2017, 113, 47–54. [Google Scholar] [CrossRef]
- Kalinkin, A.M.; Gurevich, B.I.; Myshenkov, M.S.; Chislov, M.V.; Kalinkina, E.V.; Zvereva, I.A.; Cherkezova-Zheleva, Z.; Paneva, D.; Petkova, V. Synthesis of fly ash-based geopolymers: Effect of calcite addition and mechanical activation. Minerals 2020, 10, 827. [Google Scholar] [CrossRef]
- Kalinkin, A.M.; Gurevich, B.I.; Kalinkina, E.V.; Chislov, M.V. and Zvereva, I.A. Geopolymers Based on Mechanically Activated Fly Ash Blended with Dolomite. Minerals 2021, 11, 700. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 89th ed.; Taylor and Francis Group: Oxfordshire, UK; CRC Press: Boca Raton, FL, USA, 2008; p. 2736. [Google Scholar]
- Schumann, W. Handbook of Rocks, Minerals, and Gemstones; Houghton Mifflin Company: New York, NY, USA, 1993; p. 380. [Google Scholar]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process 2000, 59, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH solution on the synthesis of fly ash geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Yemelyanova, V.; Dosumova, B.; Dzhatkanbaeva, U.; Shakiyev, E.; Kurokava, H.; Kairbekov, Z.; Muhitova, D.; Shakiyeva, T.; Myltykbaeva, Z. The microspheric catalysts of sodium sulphite low-temperature oxidation by oxygen in water solutions. Chem. Bull. Kaz. Nat. Univ. 2013, 71, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Van Deventer, J.S.J. Use of infrared spectroscopy to study geopolymerization of heterogeneous amorphous aluminosilicates. Langmuir 2003, 19, 8726–8734. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Díez, S.; Vázquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef] [PubMed]
- White, W.B. The carbonate minerals. In The Infrared Spectra of Minerals; Farmer, W.C., Ed.; Mineralogical Society: London, UK, 1974; pp. 227–284. [Google Scholar]
- Kloprogge, J.T.; Frost, R.L. Fourier Transform Infrared and Raman spectroscopic study of the local structure of Mg, Ni and Co—hydrotalcites. J. Solid State Chem. 1999, 146, 506–515. [Google Scholar] [CrossRef]
- Ryskin, Y.I. The vibrations of protons in minerals: Hydroxyl, water and ammonium. In The Infrared Spectra of Minerals; Farmer, W.C., Ed.; Mineralogical Society: London, UK, 1974; pp. 137–181. [Google Scholar]
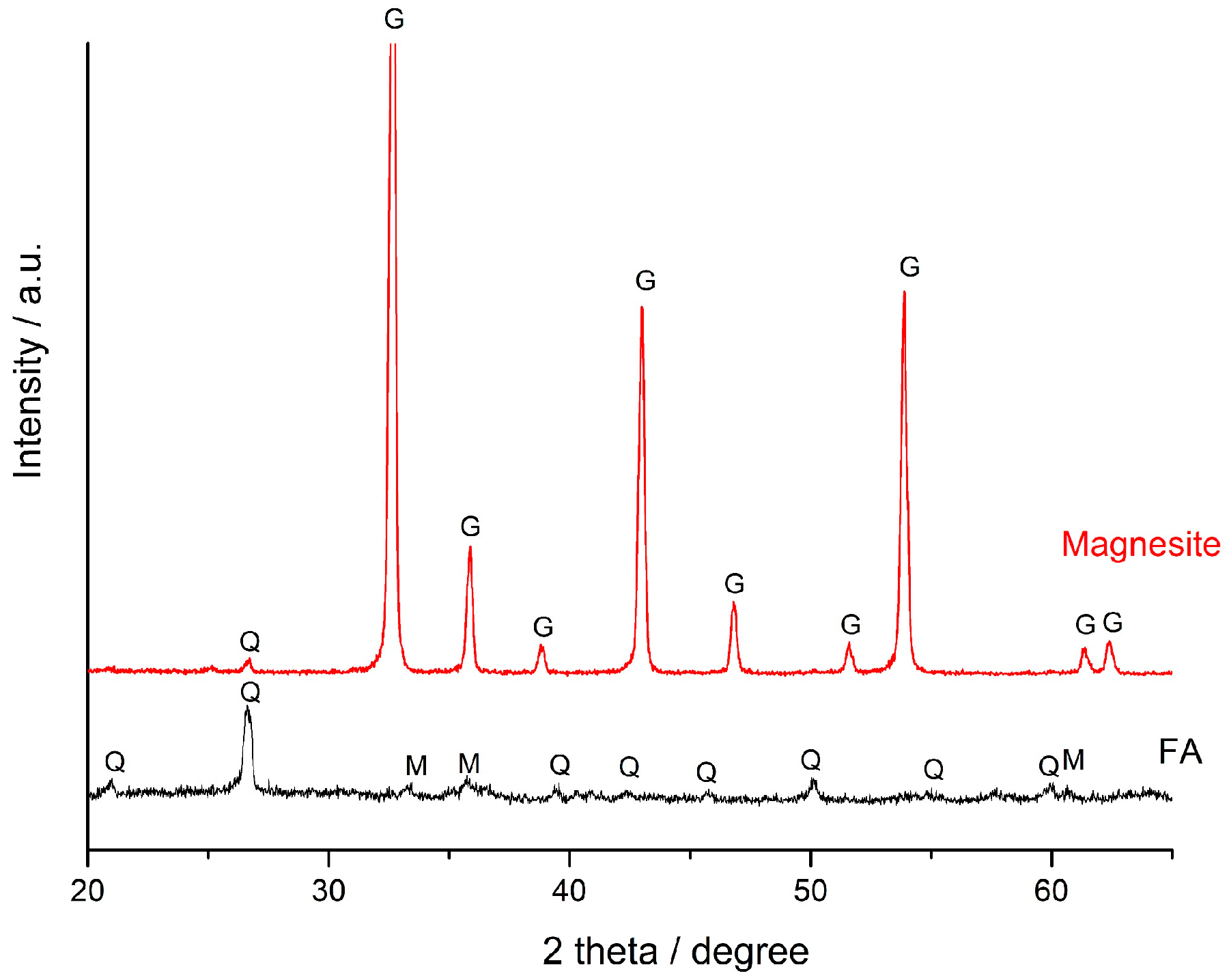


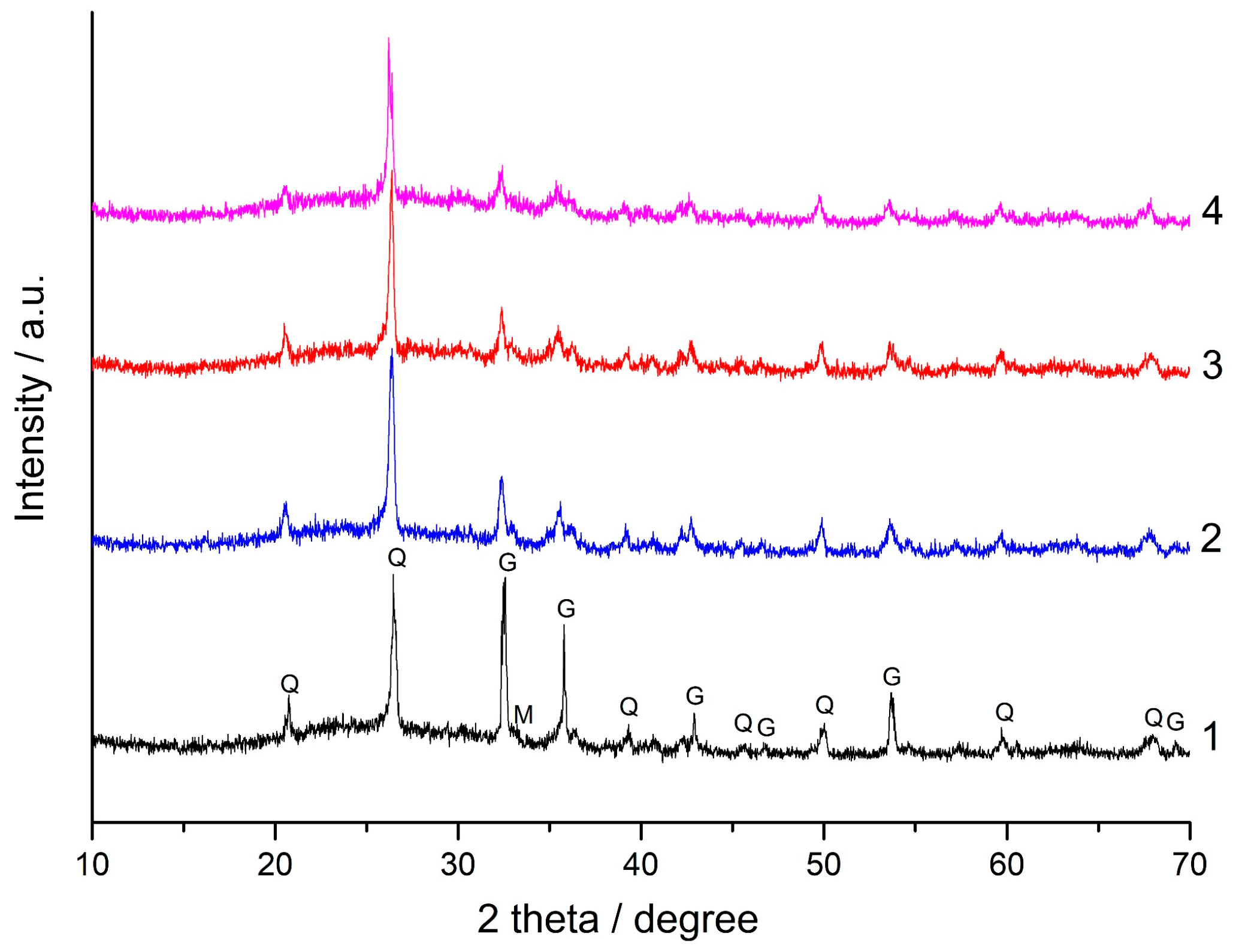



| SiO2 | Al2O3 | Fe2O3 | FeO | CaO | MgO | SO3 | Na2O | K2O | C | P2O5 | TiO2 | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 56.26 | 18.39 | 8.58 | 0.69 | 2.14 | 2.60 | 0.18 | 4.04 | 1.32 | 0.88 | 0.32 | 1.13 | 2.28 |
| Magnesite | 1.50 | n/a | 0.36 | n/a | 0.32 | 46.20 | 0.16 | 0.07 | 0.03 | n/a | n/a | 0.02 | 50.47 |
| FA (wt.%) | Magnesite (wt.%) | w/s Ratio (30 s MA) | w/s Ratio (180 s MA) | w/s Ratio (400 s MA) |
|---|---|---|---|---|
| 100 | 0 | 0.23 | 0.25 | 0.28 |
| 99 | 1 | 0.28 | 0.31 | 0.31 |
| 97 | 3 | 0.31 | 0.31 | 0.31 |
| 95 | 5 | 0.31 | 0.31 | 0.31 |
| 90 | 10 | 0.30 | 0.31 | 0.31 |
| 80 | 20 | 0.30 | 0.30 | 0.31 |
| Raw Material | MA Time, s | Dissolution Time, h | SiO2, mg·L−1 | Al2O3, mg·L−1 |
|---|---|---|---|---|
| FA | 30 | 1 | 1.75 | 1.08 |
| 30 | 5 | 3.34 | 1.68 | |
| 30 | 24 | 8.90 | 4.16 | |
| 180 | 1 | 3.98 | 1.18 | |
| 180 | 5 | 7.12 | 2.29 | |
| 180 | 24 | 23.53 | 6.82 | |
| 90% FA + 10% magnesite | 30 | 1 | 1.41 | 0.813 |
| 30 | 5 | 2.22 | 1.06 | |
| 30 | 24 | 3.96 | 1.57 | |
| 180 | 1 | 1.60 | 0.246 | |
| 180 | 5 | 4.41 | 0.832 | |
| 180 | 24 | 11.44 | 3.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinkin, A.M.; Kalinkina, E.V.; Ivanova, A.G.; Kruglyak, E.A. Effect of Magnesite Addition and Mechanical Activation on the Synthesis of Fly Ash-Based Geopolymers. Minerals 2022, 12, 1367. https://doi.org/10.3390/min12111367
Kalinkin AM, Kalinkina EV, Ivanova AG, Kruglyak EA. Effect of Magnesite Addition and Mechanical Activation on the Synthesis of Fly Ash-Based Geopolymers. Minerals. 2022; 12(11):1367. https://doi.org/10.3390/min12111367
Chicago/Turabian StyleKalinkin, Alexander M., Elena V. Kalinkina, Alla G. Ivanova, and Ekaterina A. Kruglyak. 2022. "Effect of Magnesite Addition and Mechanical Activation on the Synthesis of Fly Ash-Based Geopolymers" Minerals 12, no. 11: 1367. https://doi.org/10.3390/min12111367
APA StyleKalinkin, A. M., Kalinkina, E. V., Ivanova, A. G., & Kruglyak, E. A. (2022). Effect of Magnesite Addition and Mechanical Activation on the Synthesis of Fly Ash-Based Geopolymers. Minerals, 12(11), 1367. https://doi.org/10.3390/min12111367







