Variation in Iron Ore Sinter Mineralogy with Changes in Basicity
Abstract
1. Introduction
2. Experimental
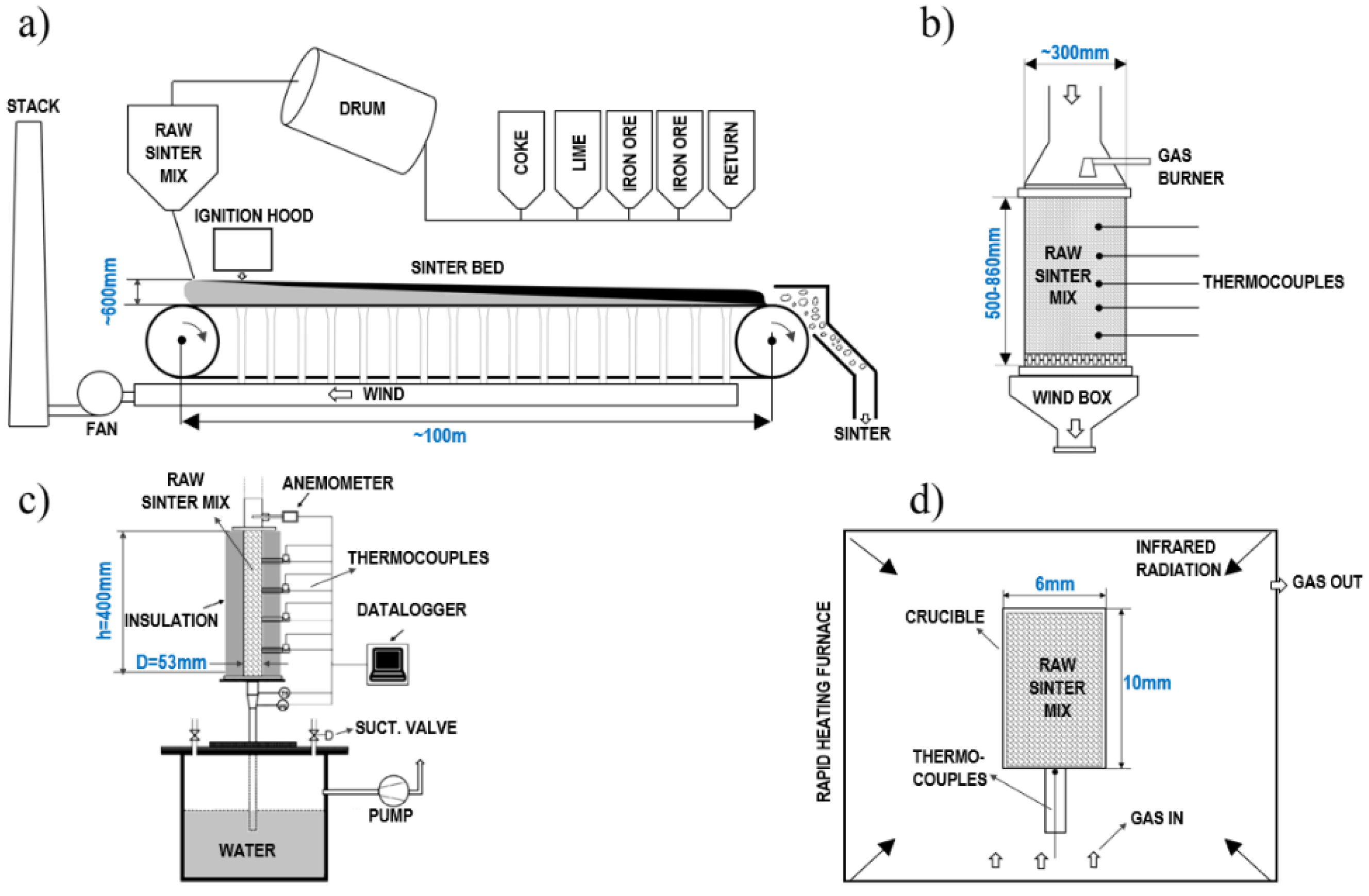
2.1. Industrial Scale—Sinter Strand (BlueScope Steel Australia)
- 1986–2009: 420 m2 strand area with 500 mm side plates.
- 2009–2021: 480 m2 strand area with 700 mm side plates.
- Pre 2011: Western Australian, South Australian, and Brazilian hematite dominated the blend.
- Post 2011: Western Australian goethite-rich ores dominated the ore blend and the steelworks moved to single blast furnace operations, resulting in the sinter plant being operated at significantly lower productivity (20–25 t/m2/day compared to previous 35–40 t/m2/day).
2.2. Pilot Scale—Pot Tests
2.2.1. CSIRO Pot Test (65–120 kg)
2.2.2. University of Newcastle Milli-Pot Test (1–2 kg)
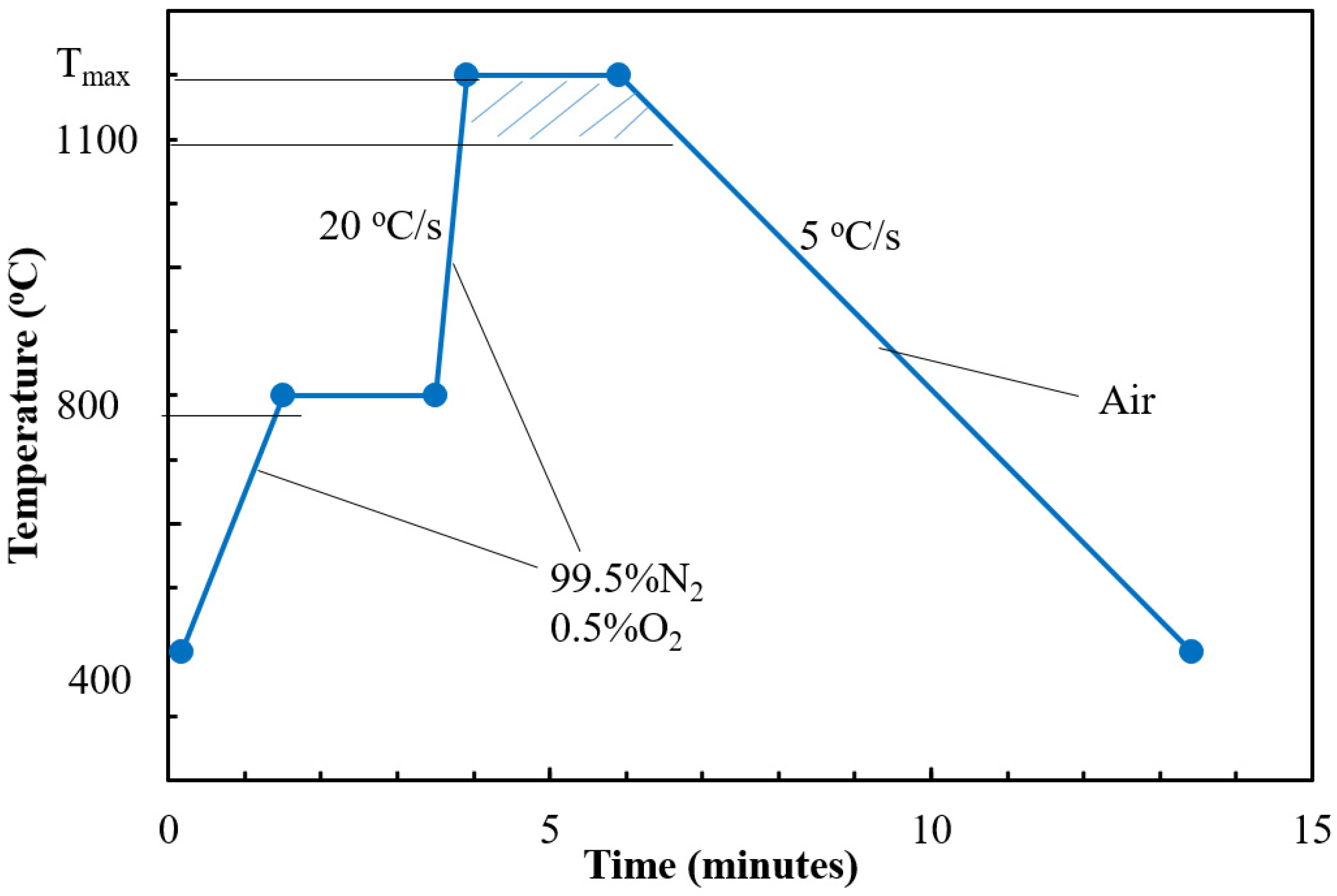
2.3. Laboratory Scale—Rapid Heating Furnace (University of Newcastle Australia)
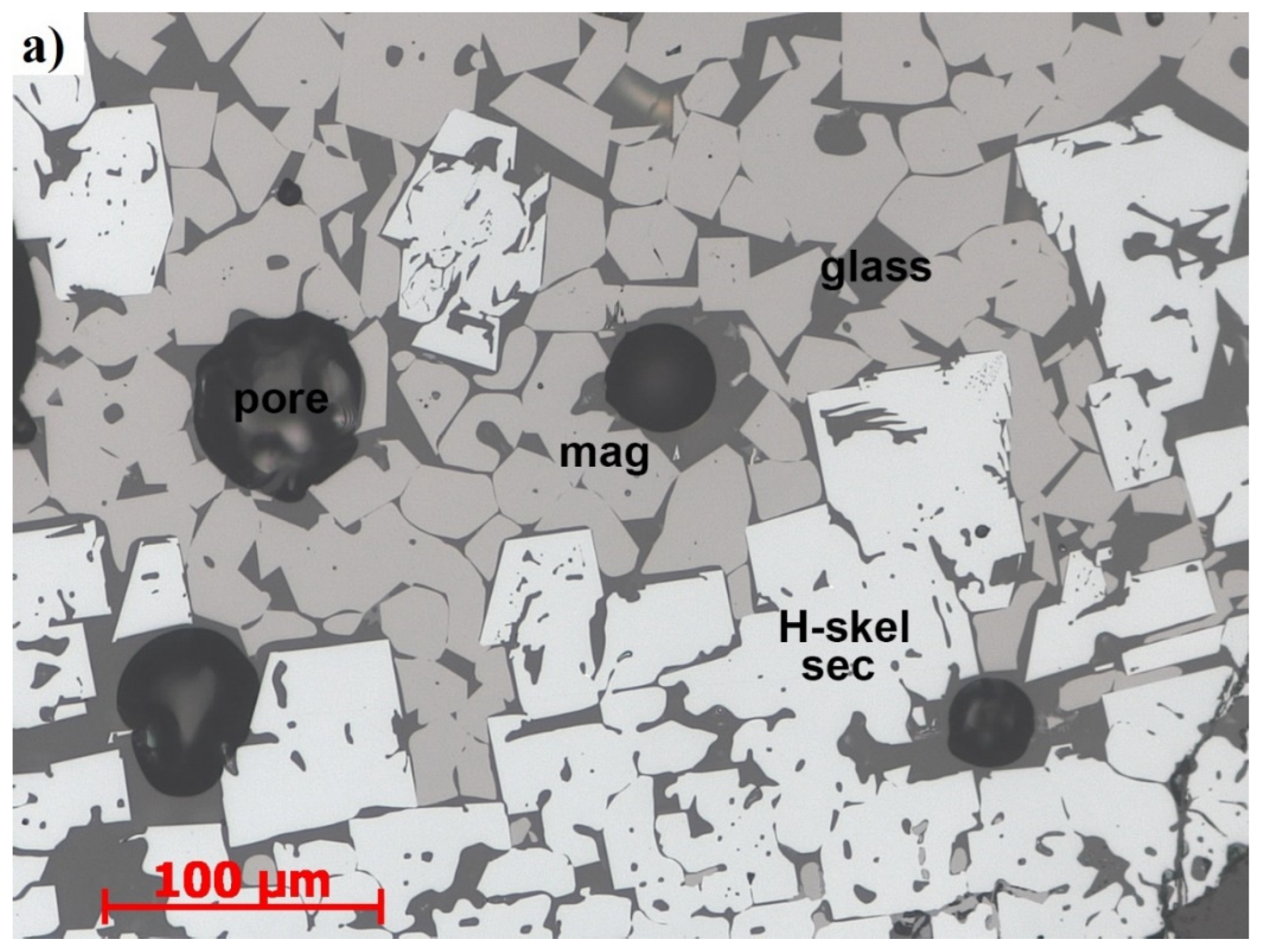
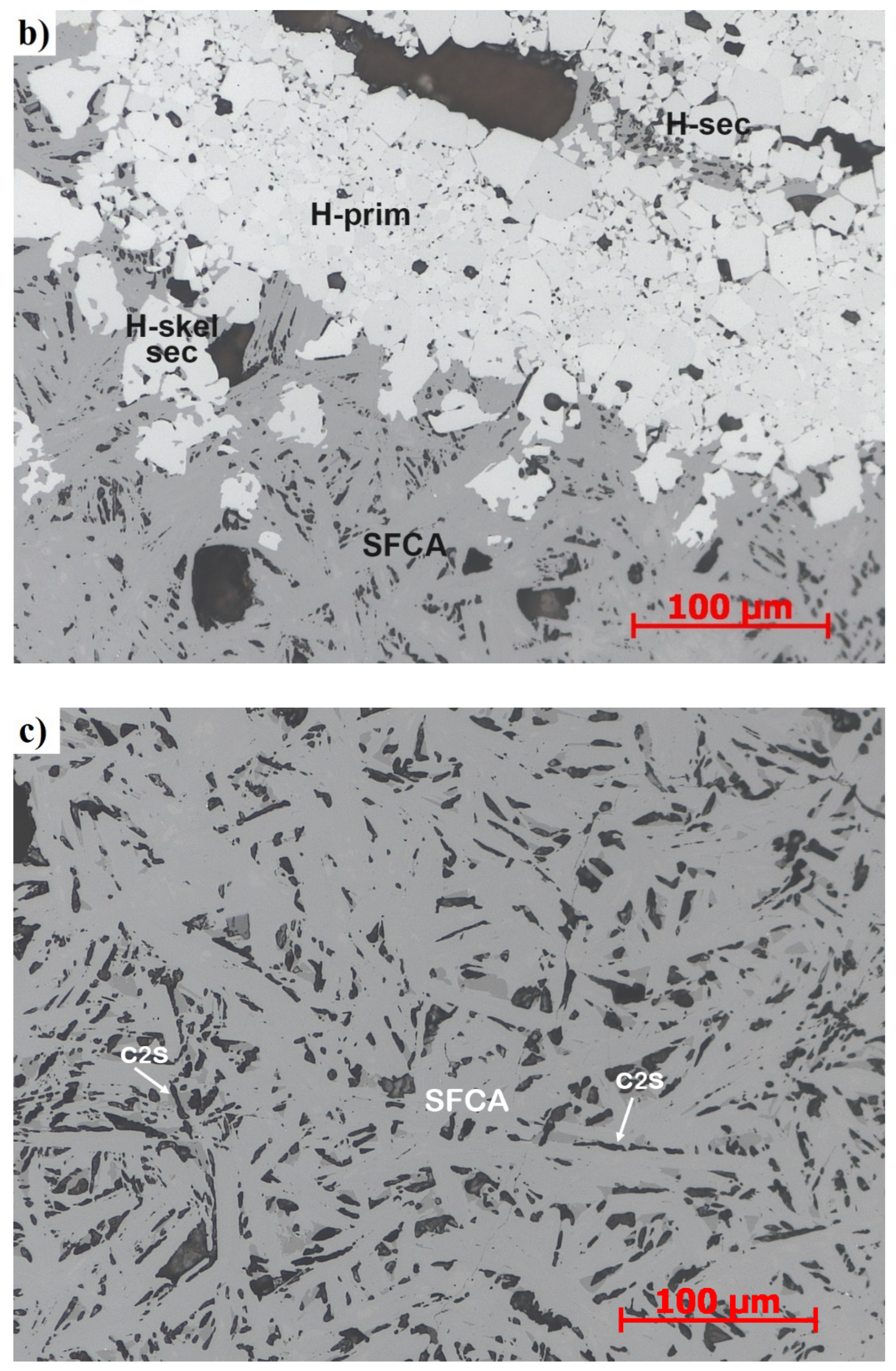
2.4. Sinter Mineralogy Determination
- Primary and Secondary hematite
- Magnetite:
- Silico ferrites of calcium and aluminium (SFCA):
- Dicalcium silicate (C2S)
- Glass:
3. Results and Discussion
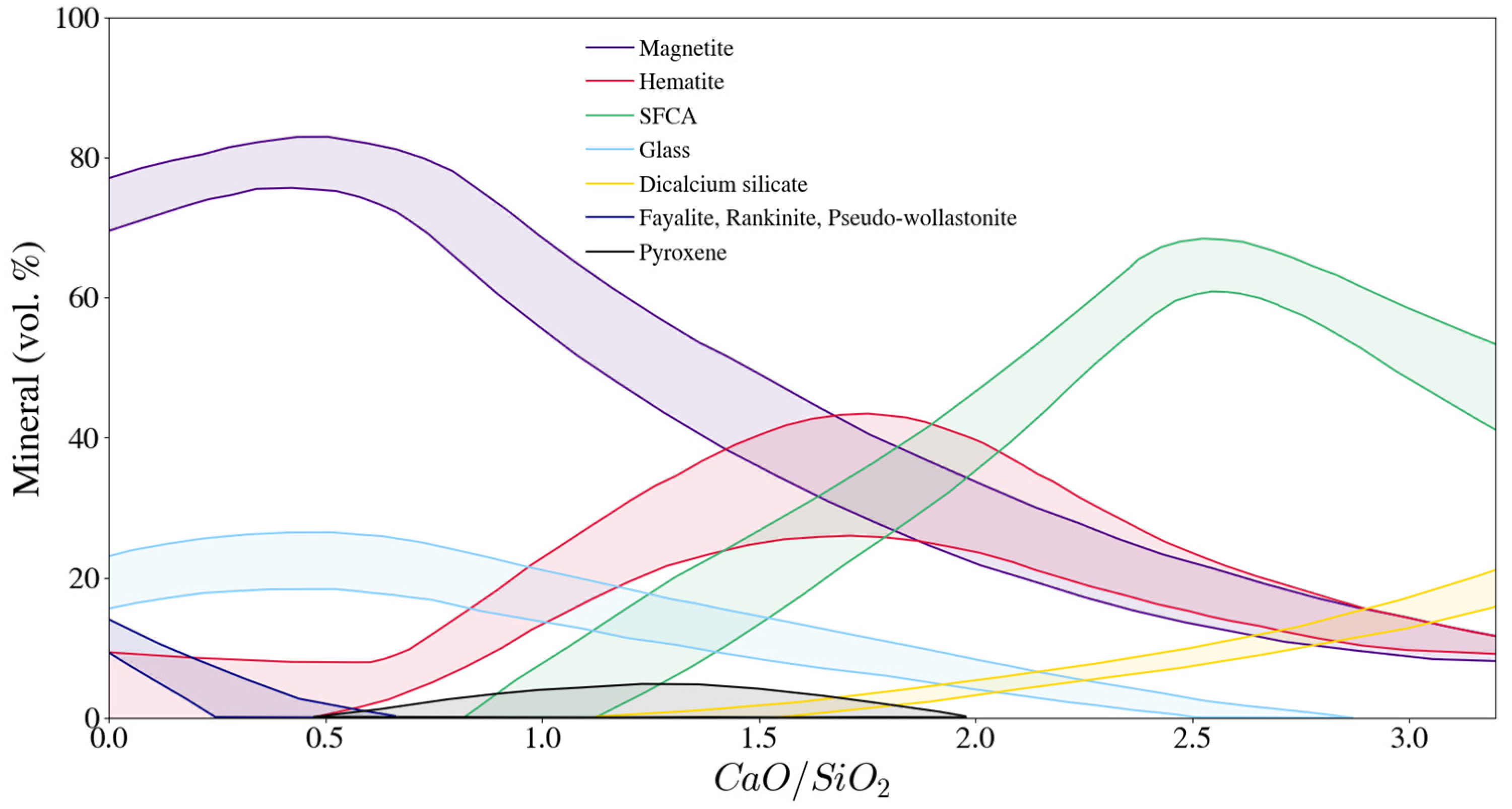
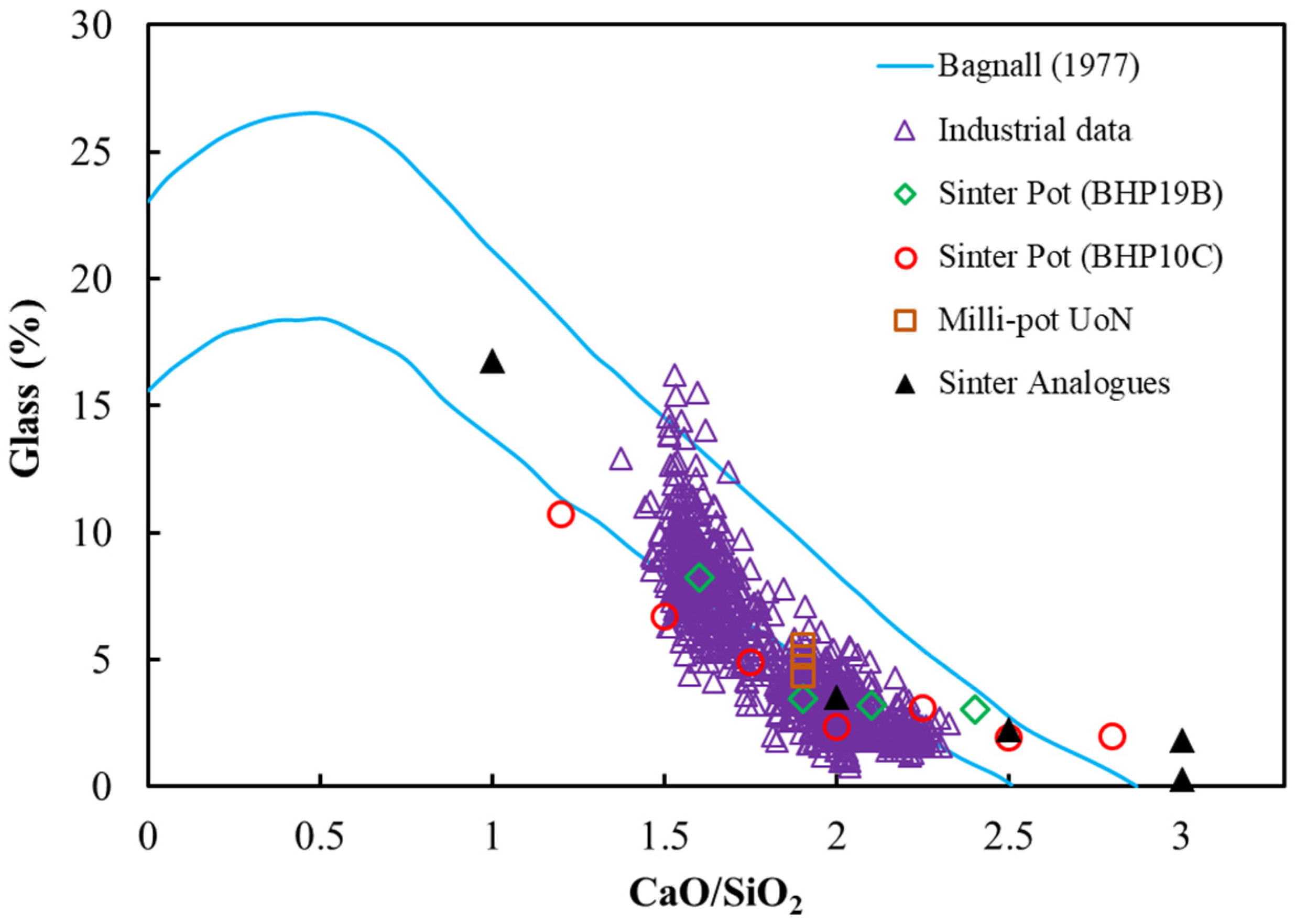
3.1. Comparison of Mineralogy of Sinters from Different Sources
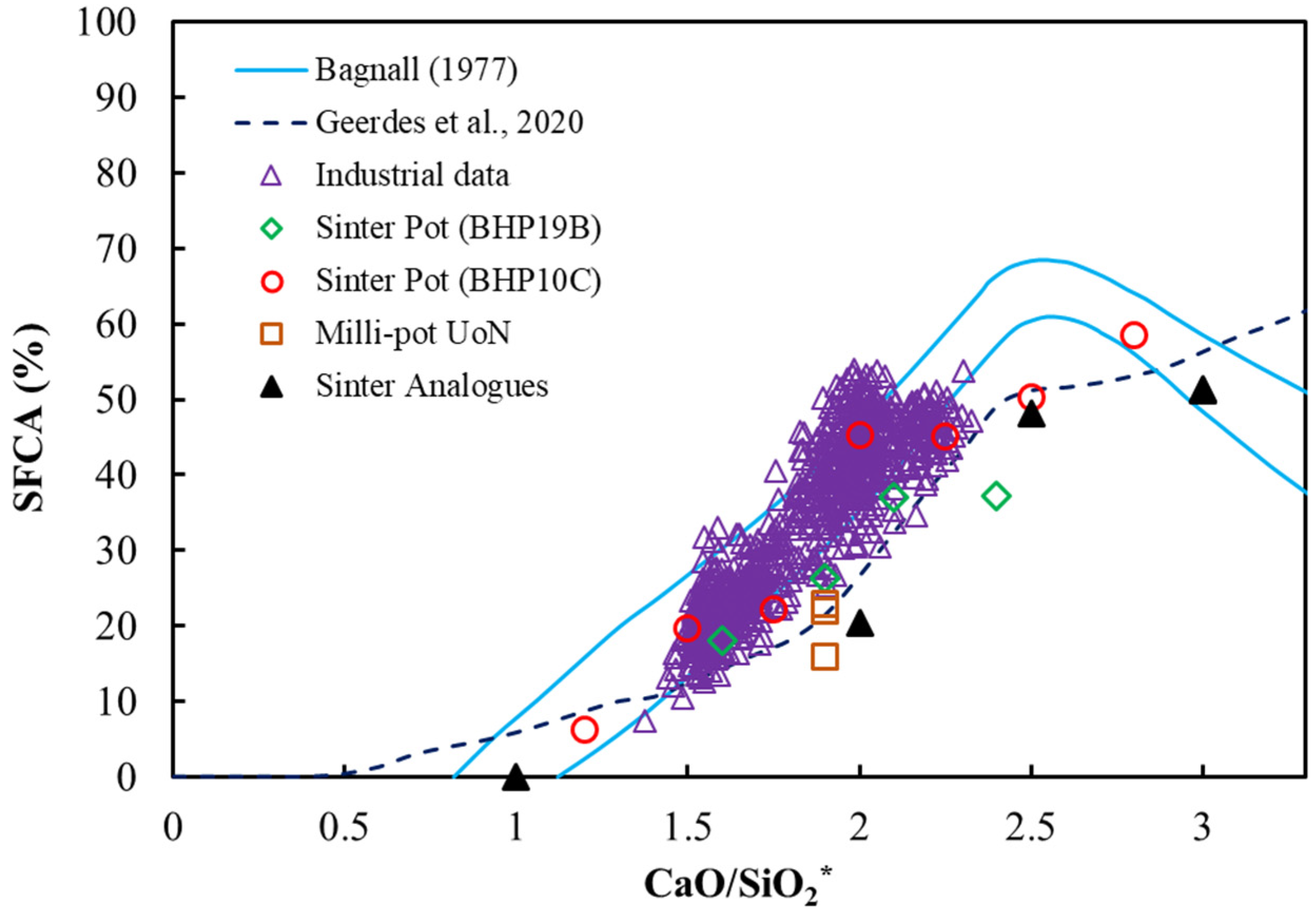
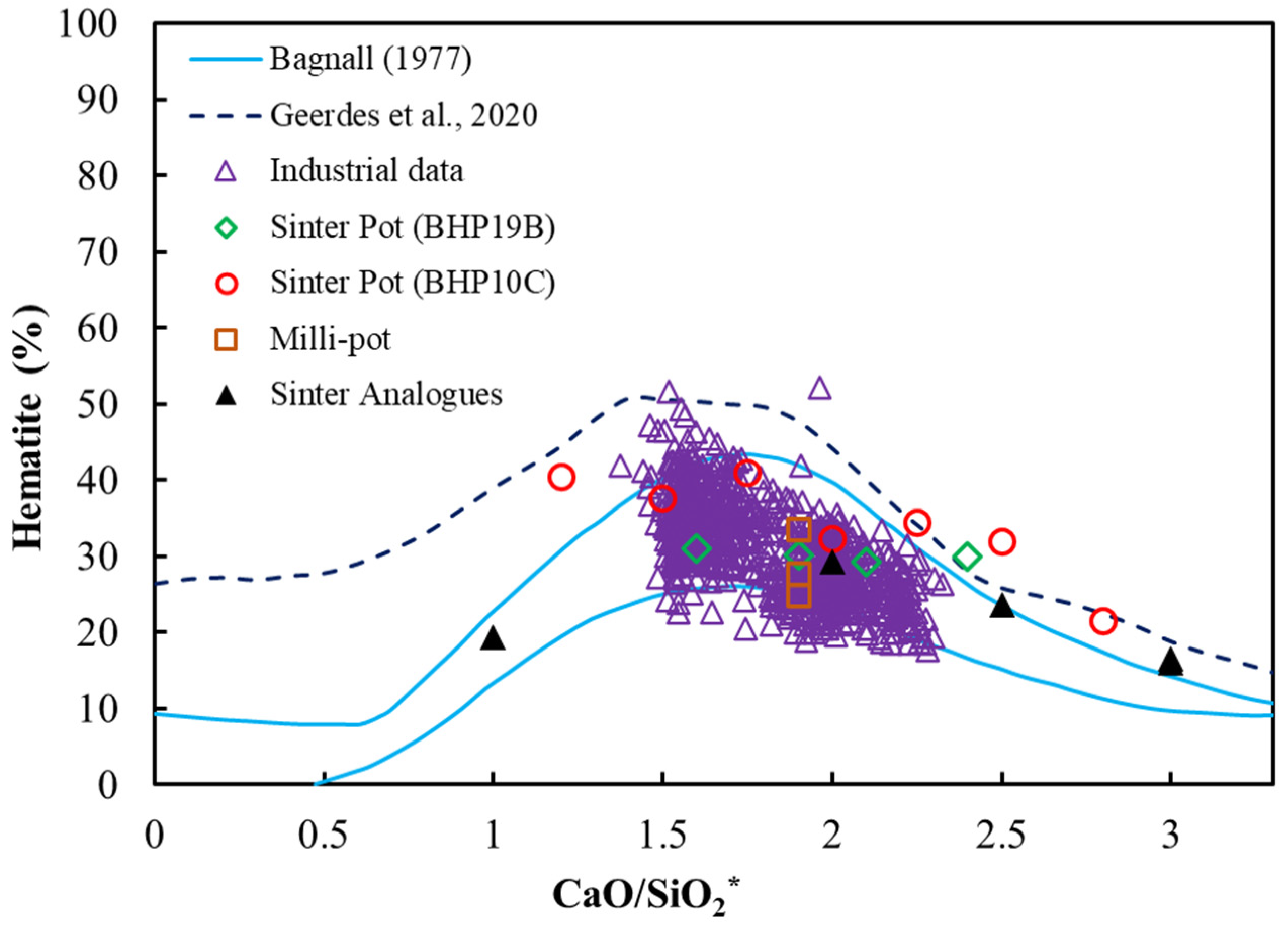
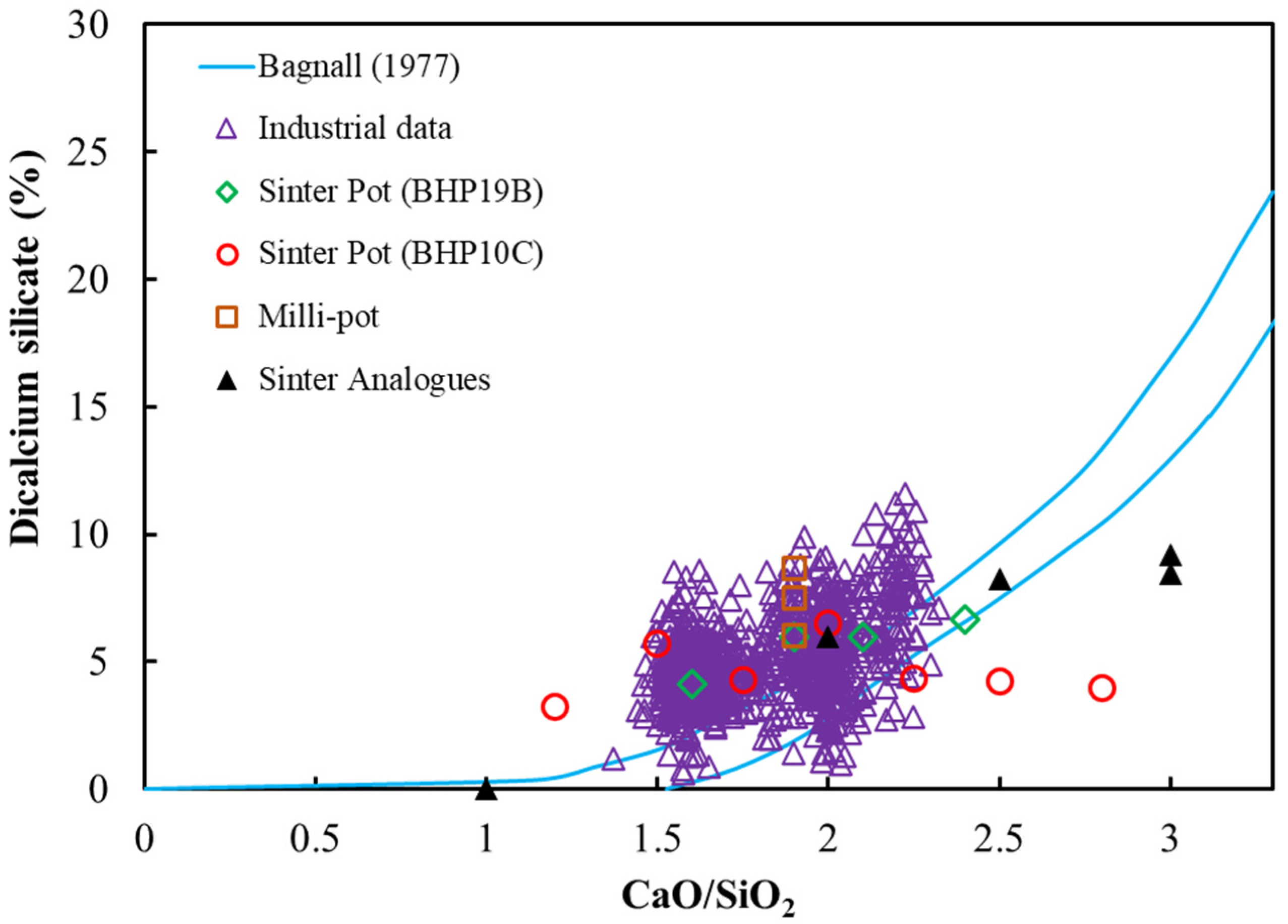
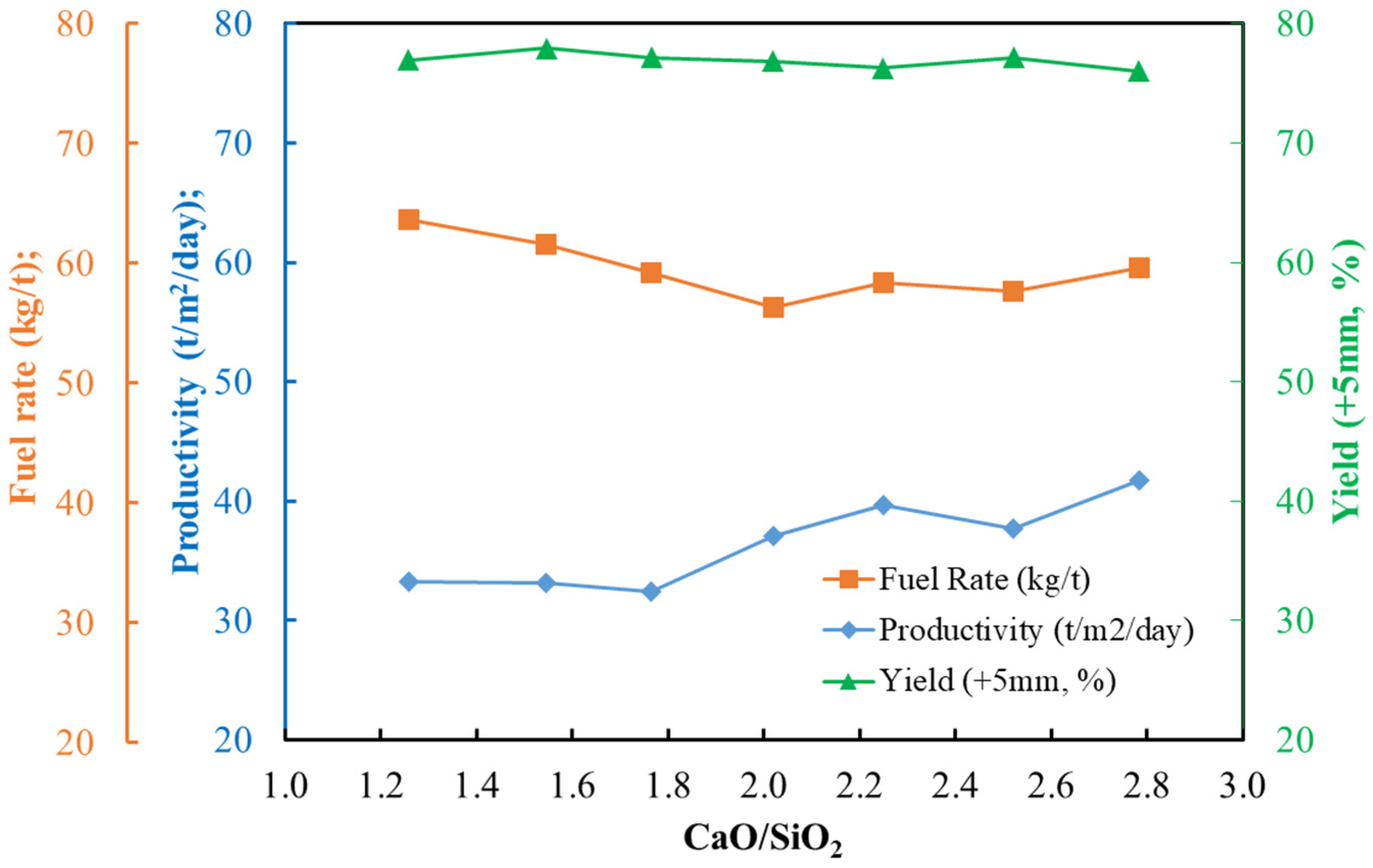
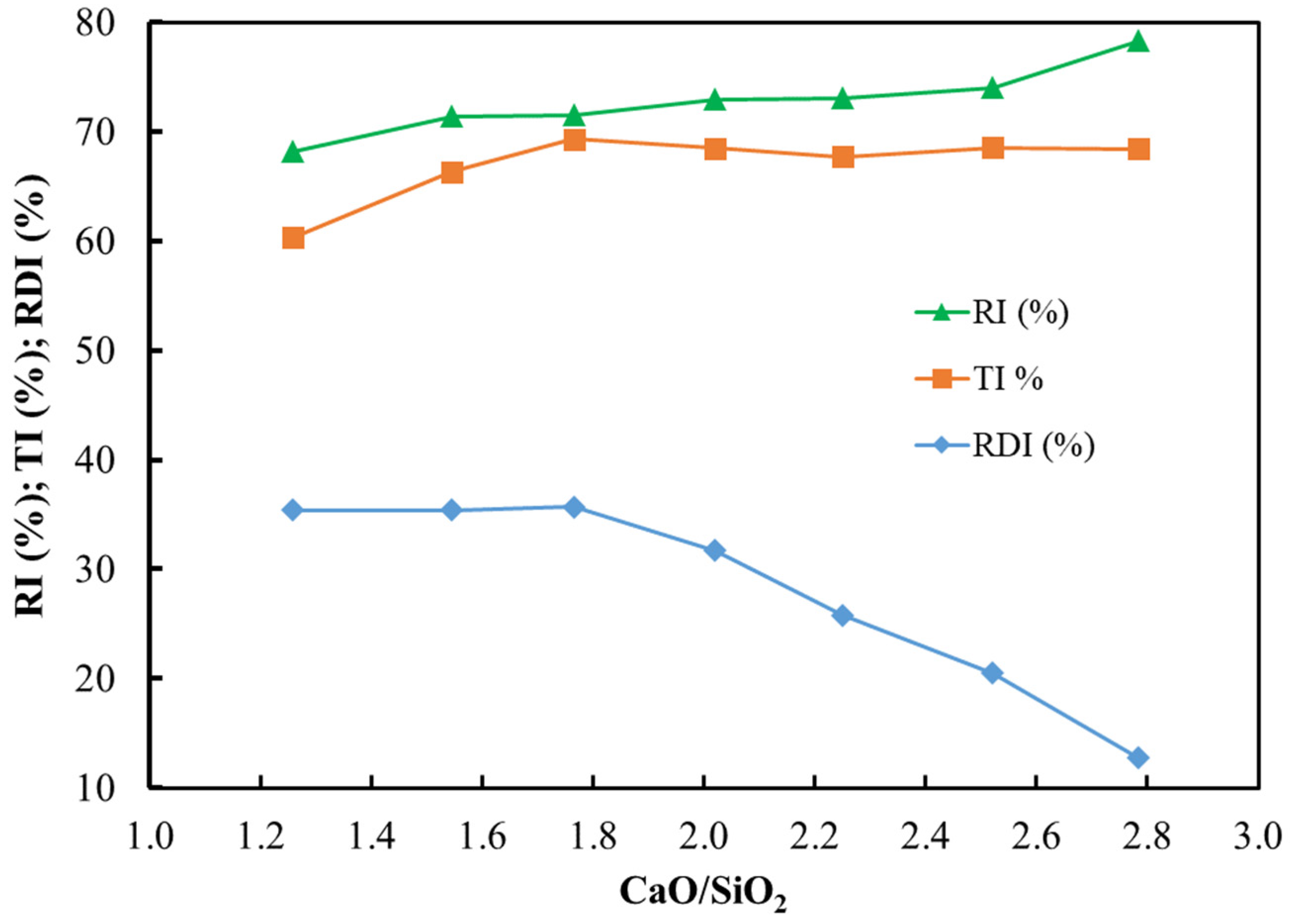
3.2. Basicity Effects on Pot Sinter Production and Quality
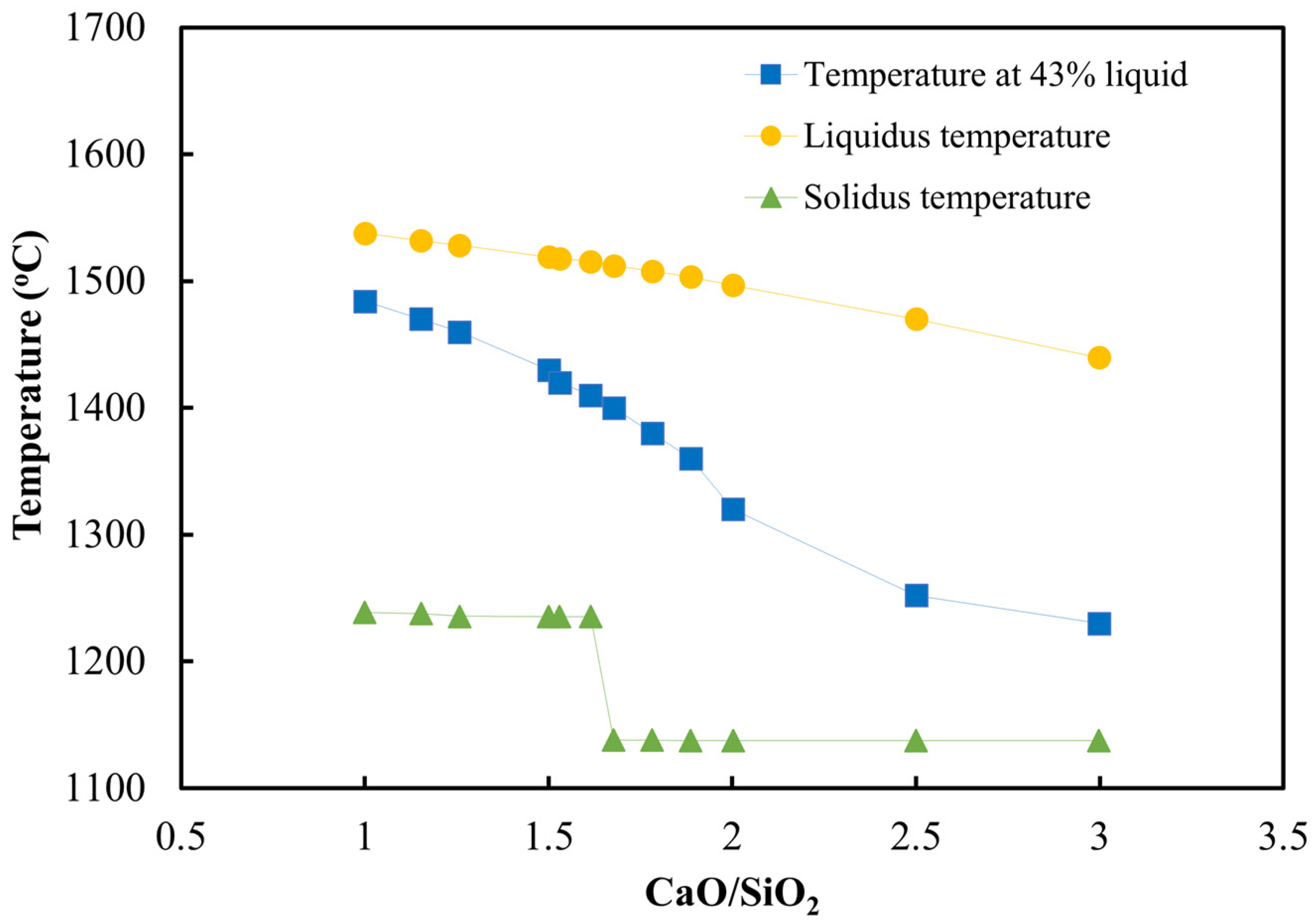
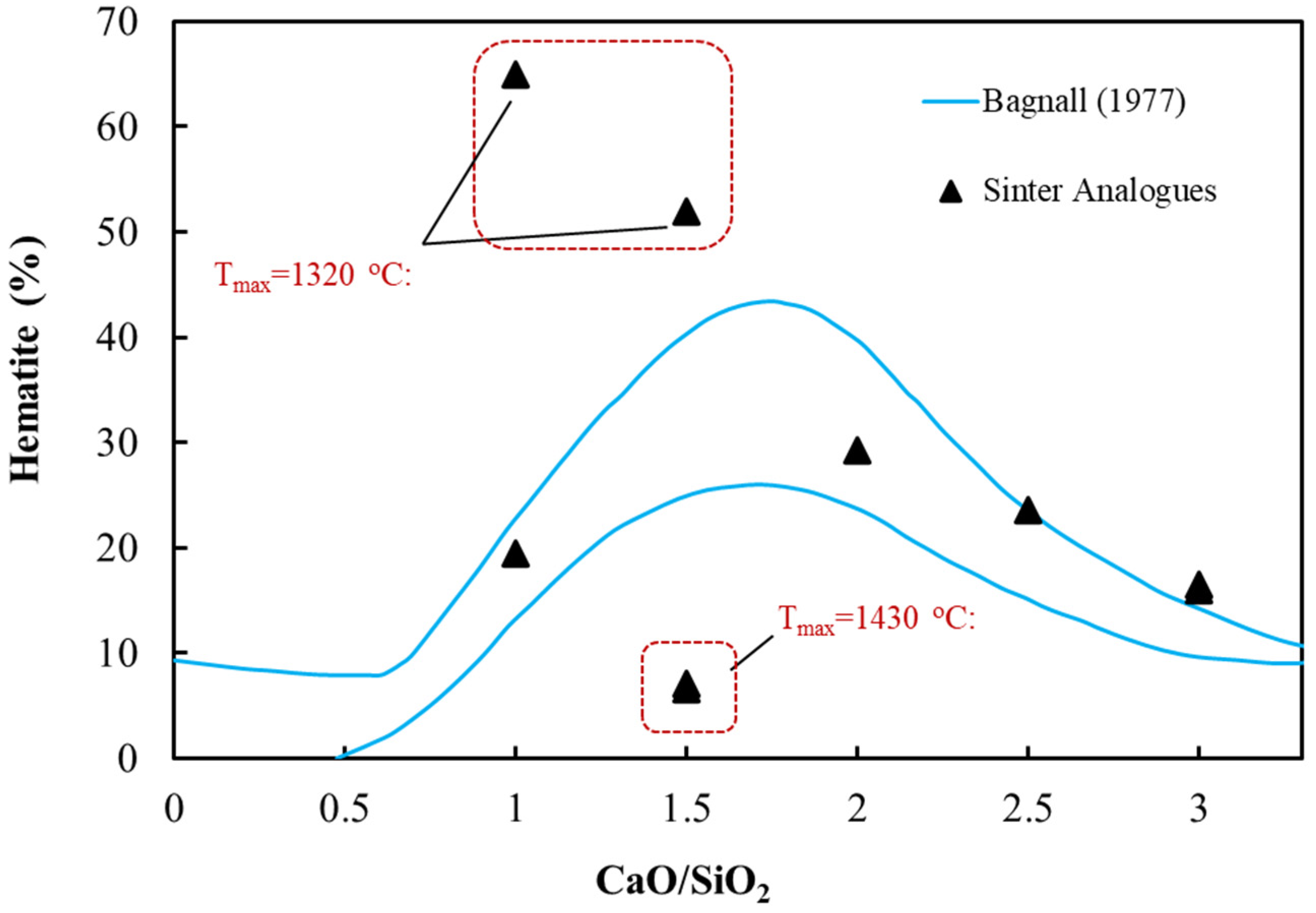
3.3. Analysis of Sintering Temperature using Analogue Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, D.D.; Cheng, S.S.; Wang, Y.S.; Jiang, X. The production and development of large blast furnaces in China during 2015. Ironmak. Steelmak. 2016, 44, 351–358. [Google Scholar] [CrossRef]
- Zhou, D.D.; Xu, K.; Xu, G.; Jiang, X. The production of large blast furnaces of China in 2017. Ironmak. Steelmak. 2018, 47, 316–321. [Google Scholar] [CrossRef]
- Geerdes, M.; Chaigneau, R.; Kurunov, I.; Lingiardi, O.; Ritketts, J. Modern Blast Furnace Ironmaking: An Introduction, 3rd ed.; Ios Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Loo, C.E.; Wong, D.J. Fundamental Factors Determining Laboratory Sintering Results. ISIJ Int. 2005, 45, 449–458. [Google Scholar] [CrossRef]
- Harvey, T.; Honeyands, T.; O’Dea, D.; Evans, G. Sinter Strength and Pore Structure Development using Analogue Tests. ISIJ Int. 2020, 60, 73–83. [Google Scholar] [CrossRef]
- Harvey, T.; Pownceby, M.I.; Chen, J.; Webster, N.A.S.; Nguyen, T.B.T.; Matthews, L.; O’Dea, D.; Honeyands, T. Effect of Temperature, Time, and Cooling Rate on the Mineralogy, Morphology, and Reducibility of Iron Ore Sinter Analogues. JOM 2020, 73, 345–355. [Google Scholar] [CrossRef]
- Higuchi, K.; Okazaki, J.; Nomura, S. Influence of Melting Characteristics of Iron Ores on Strength of Sintered Ores. ISIJ Int. 2020, 60, 674–681. [Google Scholar] [CrossRef]
- Honeyands, T.; Manuel, J.; Matthews, L.; O’Dea, D.; Pinson, D.; Leedham, J.; Zhang, G.; Li, H.; Monaghan, B.; Liu, X.; et al. Comparison of the Mineralogy of Iron Ore Sinters Using a Range of Techniques. Minerals 2019, 9, 333. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Shimomura, Y.; Sasaki, M.; Hida, Y.; Toda, H. Improvement of sinter quality based on the mineralogical properties of ores. Ironmak. Proc. 1983, 42, 17–29. [Google Scholar]
- Ji, Z.; Zhao, Y.; Gan, M.; Fan, X.; Chen, X.; Hu, L. Microstructure and Minerals Evolution of Iron Ore Sinter: Influence of SiO2 and Al2O3. Minerals 2019, 9, 449. [Google Scholar] [CrossRef]
- Mežibrický, R.; Csanádi, T.; Vojtko, M.; Fröhlichová, M.; Abart, R. Effect of alumina and silica content in the calcium aluminosilicoferrite Ca2 (Ca, Fe, Mg) 6 (Fe, Si, Al) 6O20 bonding phase on the strength of iron ore sinter. Mater. Chem. Phys. 2021, 257, 123733. [Google Scholar] [CrossRef]
- Scarlett, N.V.Y.; Pownceby, M.; Madsen, I.C.; Christensen, A.N. Reaction sequences in the formation of silico-ferrites of calcium and aluminum in iron ore sinter. Met. Mater. Trans. A 2004, 35, 929–936. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C. In situ X-ray diffraction investigation of the formation mechanisms of silico-ferrite of calcium and aluminium-I-type (SFCA-I-type) complex calcium ferrites. ISIJ Int. 2013, 53, 1334–1340. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C.; Kimpton, J.A. Silico-ferrite of Calcium and Aluminum (SFCA) Iron Ore Sinter Bonding Phases: New Insights into Their Formation During Heating and Cooling. Met. Mater. Trans. A 2012, 43, 1344–1357. [Google Scholar] [CrossRef]
- Bagnall, E.J. Influence of feed material properties on sinter for blast furnaces. Agglomeration 1977, 77, 2. [Google Scholar]
- Geerdes, M.; Chaigneau, R.; Lingiardi, O. Modern Blast Furnace Ironmaking: An Introduction; Ios Press: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Chen, J.; Cheng, S.; Shevchenko, M.; Hayes, P.C.; Jak, E. Investigation of the Thermodynamic Stability of C (A, F) 3 Solid Solution in the FeO-Fe2O3-CaO-Al2O3 System and SFCA Phase in the FeO-Fe2O3-CaO-SiO2-Al2O3 System. Metall. Mater. Trans. B 2021, 52, 517–527. [Google Scholar] [CrossRef]
- Harvey, T. Influence of Mineralogy and Pore Structure on the Reducibility and Strength of Iron Ore Sinter; University of Newcastle: Callaghan, Australia, 2020. [Google Scholar]
- Clout, J.; Manuel, J. Fundamental investigations of differences in bonding mechanisms in iron ore sinter formed from magnetite concentrates and hematite ores. Powder Technol. 2003, 130, 393–399. [Google Scholar] [CrossRef]
- Honeyands, T.; Manuel, J.; Matthews, L.; O’dea, D.; Pinson, D.; Leedham, J.; Monaghan, B.; Li, H.; Chen, J.; Hayes, P.; et al. Characterising the mineralogy of iron ore sinters—State-of-the-art in Australia. Proc. Iron Ore. 2017, 49–60. Available online: https://www.ausimm.com/publications/conference-proceedings/iron-ore-2017/characterising-the-mineralogy-of-iron-ore-sinters---state-of-the-art-in-australia/ (accessed on 21 August 2022).
- Lu, L. Iron Ore: Mineralogy, Processing and Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Singh, T.; Li, H.; Zhang, G.; Mitra, S.; Evans, G.; O’Dea, D.; Honeyands, T. Iron Ore Sintering in Milli-Pot: Comparison to Pilot Scale and Identification of Maximum Resistance to Air Flow. ISIJ Int. 2021, 61, 1469–1478. [Google Scholar] [CrossRef]
- Loo, C.E.; Tame, N.; Penny, G.C. Effect of Iron Ores and Sintering Conditions on Flame Front Properties. ISIJ Int. 2012, 52, 967–976. [Google Scholar] [CrossRef]
- Hsieh, L.-H.; Whiteman, J.A. Sintering conditions for simulating the formation of mineral phases in industrial iron ore sinter. ISIJ Int. 1989, 29, 24–32. [Google Scholar] [CrossRef]
- Nicol, S.; Jak, E.; Hayes, P.C. Microstructure evolution during controlled solidification of “Fe2O3”-CaO-SiO2 liquids in air. Metall. Mater. Trans. B 2019, 50, 2706–2722. [Google Scholar] [CrossRef]
- Liu, D.; Evans, G.; Loo, C.E. Iron ore sinter structure development under realistic thermal conditions. Chem. Eng. Res. Des. 2018, 130, 129–137. [Google Scholar] [CrossRef]
- Loo, C.E.; Heikkinen, J. Structural Transformation of Beds during Iron Ore Sintering. ISIJ Int. 2012, 52, 2158–2167. [Google Scholar] [CrossRef]
- Umadevi, T.; Brahmacharyulu, A.; Karthik, P.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Recycling of steel plant mill scale via iron ore sintering plant. Ironmak. Steelmak. 2012, 39, 222–227. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honeyands, T.; Nguyen, T.B.T.; Pinson, D.; Connolly, P.R.J.; Pownceby, M.I.; Manuel, J.; Matthews, L.; Leedham, J.; Singh, T.; O’Dea, D.P. Variation in Iron Ore Sinter Mineralogy with Changes in Basicity. Minerals 2022, 12, 1249. https://doi.org/10.3390/min12101249
Honeyands T, Nguyen TBT, Pinson D, Connolly PRJ, Pownceby MI, Manuel J, Matthews L, Leedham J, Singh T, O’Dea DP. Variation in Iron Ore Sinter Mineralogy with Changes in Basicity. Minerals. 2022; 12(10):1249. https://doi.org/10.3390/min12101249
Chicago/Turabian StyleHoneyands, Tom, Thi Bang Tuyen Nguyen, David Pinson, Paul R. J. Connolly, Mark I. Pownceby, James Manuel, Leanne Matthews, John Leedham, Tejbir Singh, and Damien P. O’Dea. 2022. "Variation in Iron Ore Sinter Mineralogy with Changes in Basicity" Minerals 12, no. 10: 1249. https://doi.org/10.3390/min12101249
APA StyleHoneyands, T., Nguyen, T. B. T., Pinson, D., Connolly, P. R. J., Pownceby, M. I., Manuel, J., Matthews, L., Leedham, J., Singh, T., & O’Dea, D. P. (2022). Variation in Iron Ore Sinter Mineralogy with Changes in Basicity. Minerals, 12(10), 1249. https://doi.org/10.3390/min12101249





