The Evaluation of Carrageenan as a Novel and Environmentally Friendly Molybdenite Depressant
Abstract
:1. Introduction
2. Materials and Methods
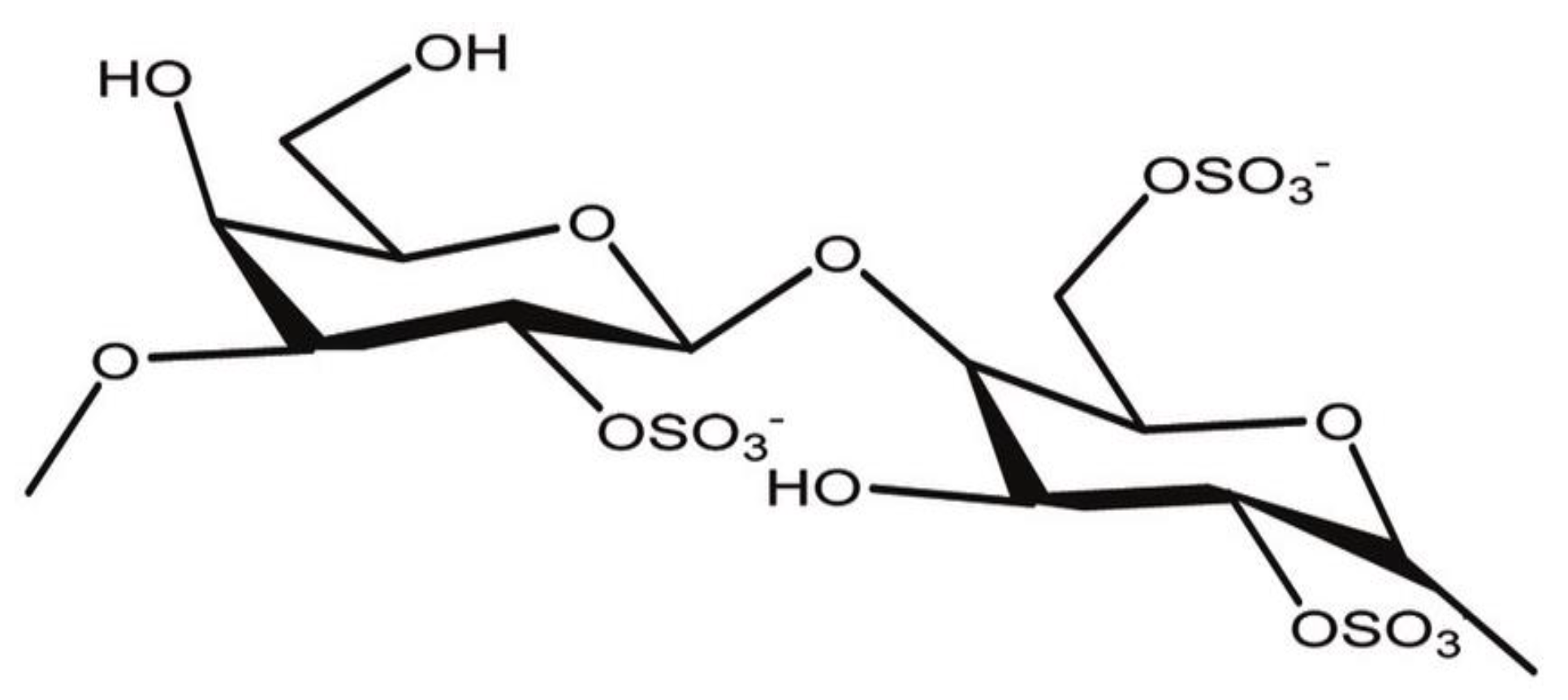
2.1. Minerals and Reagents
2.2. Microflotation Tests
2.3. Zeta Potential Experiments
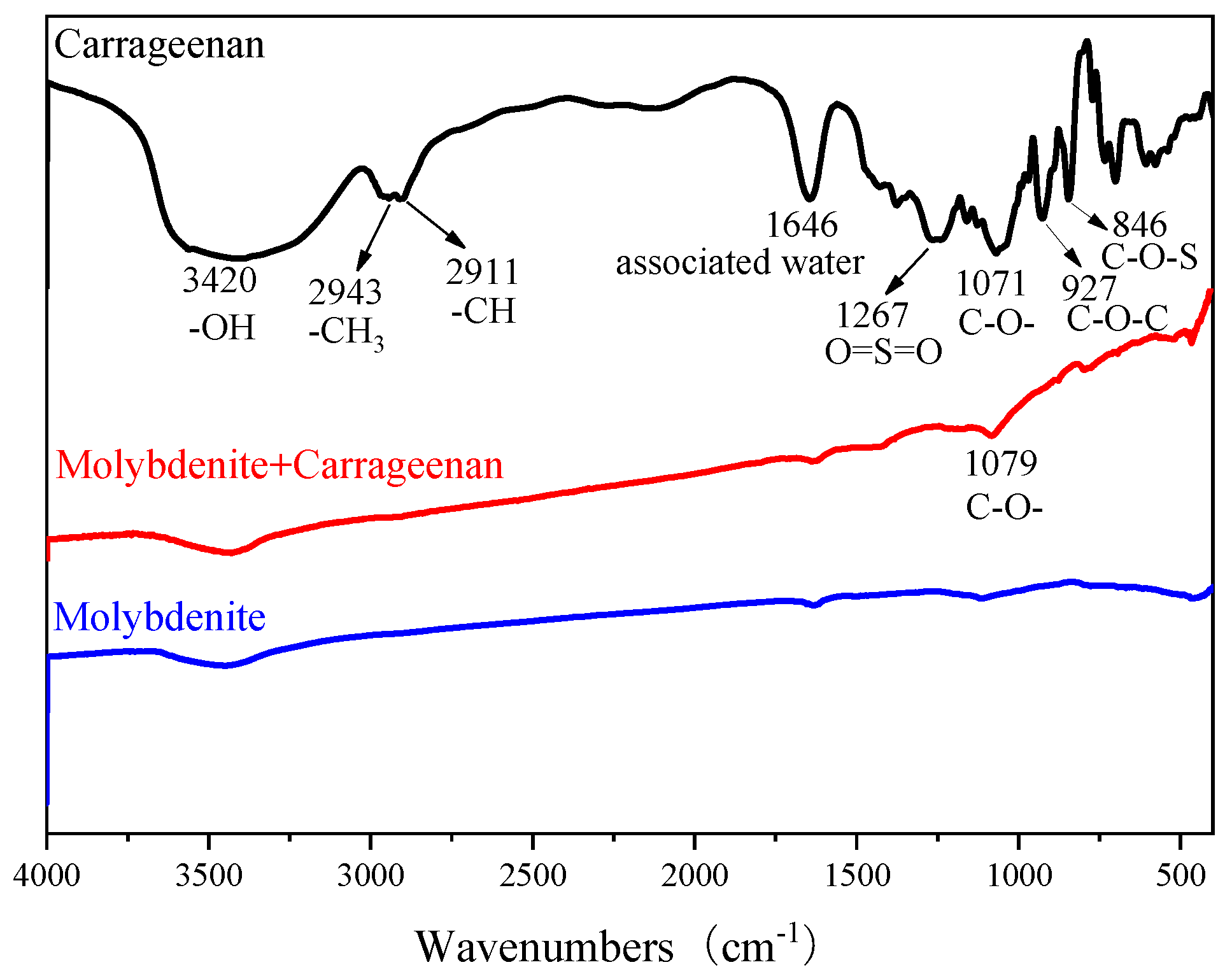
2.4. FTIR Measurement
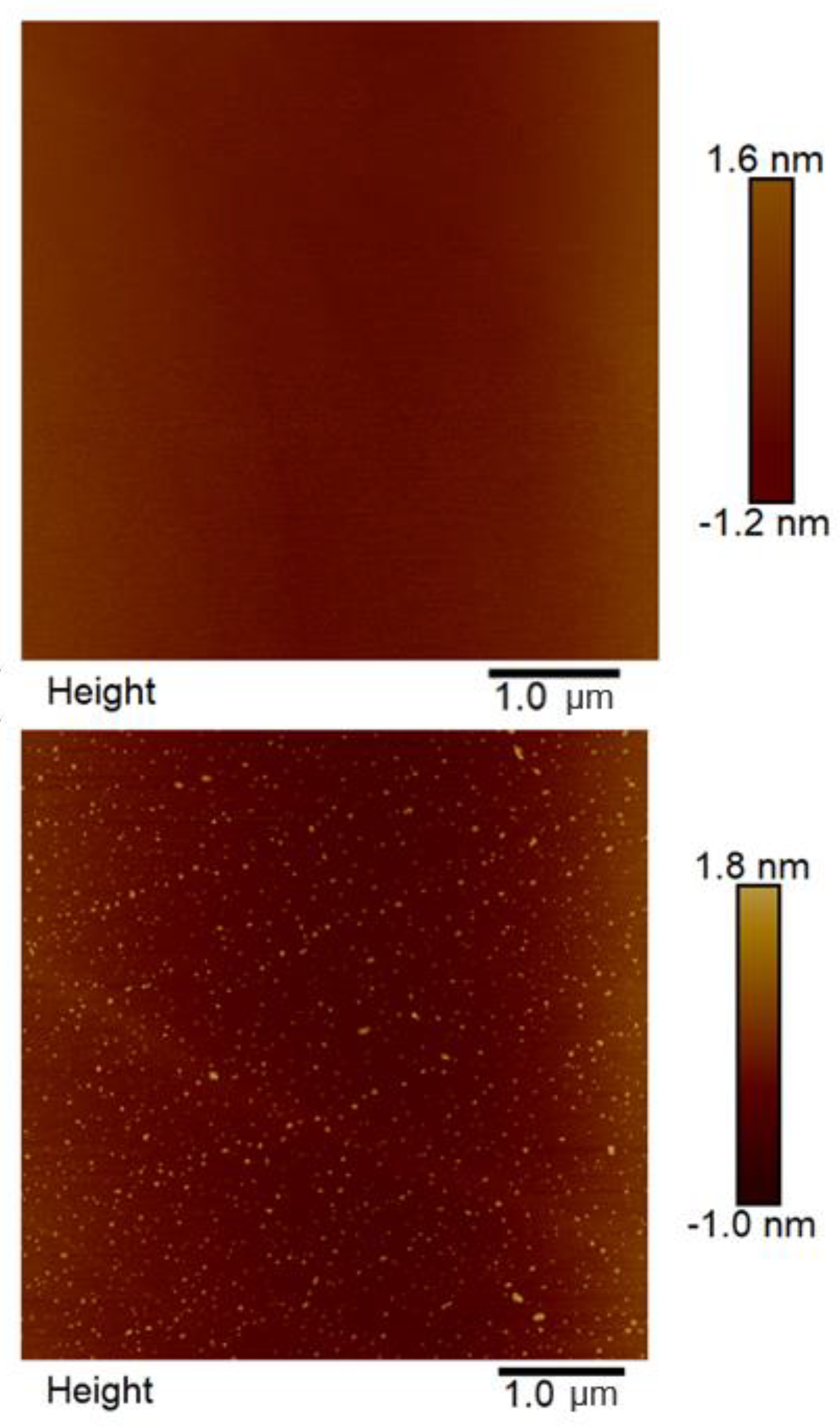
2.5. AFM Measurement
3. Results and Discussion
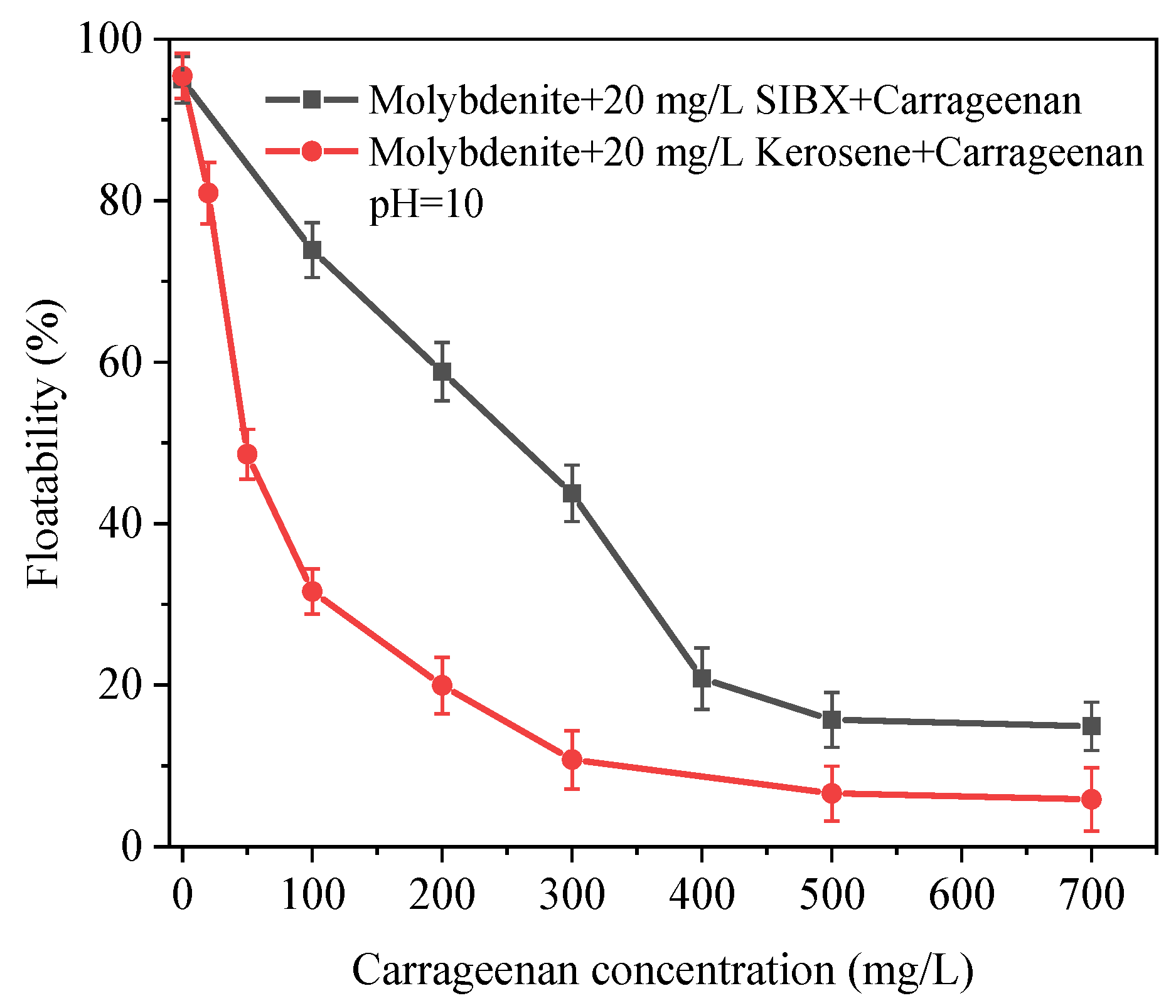
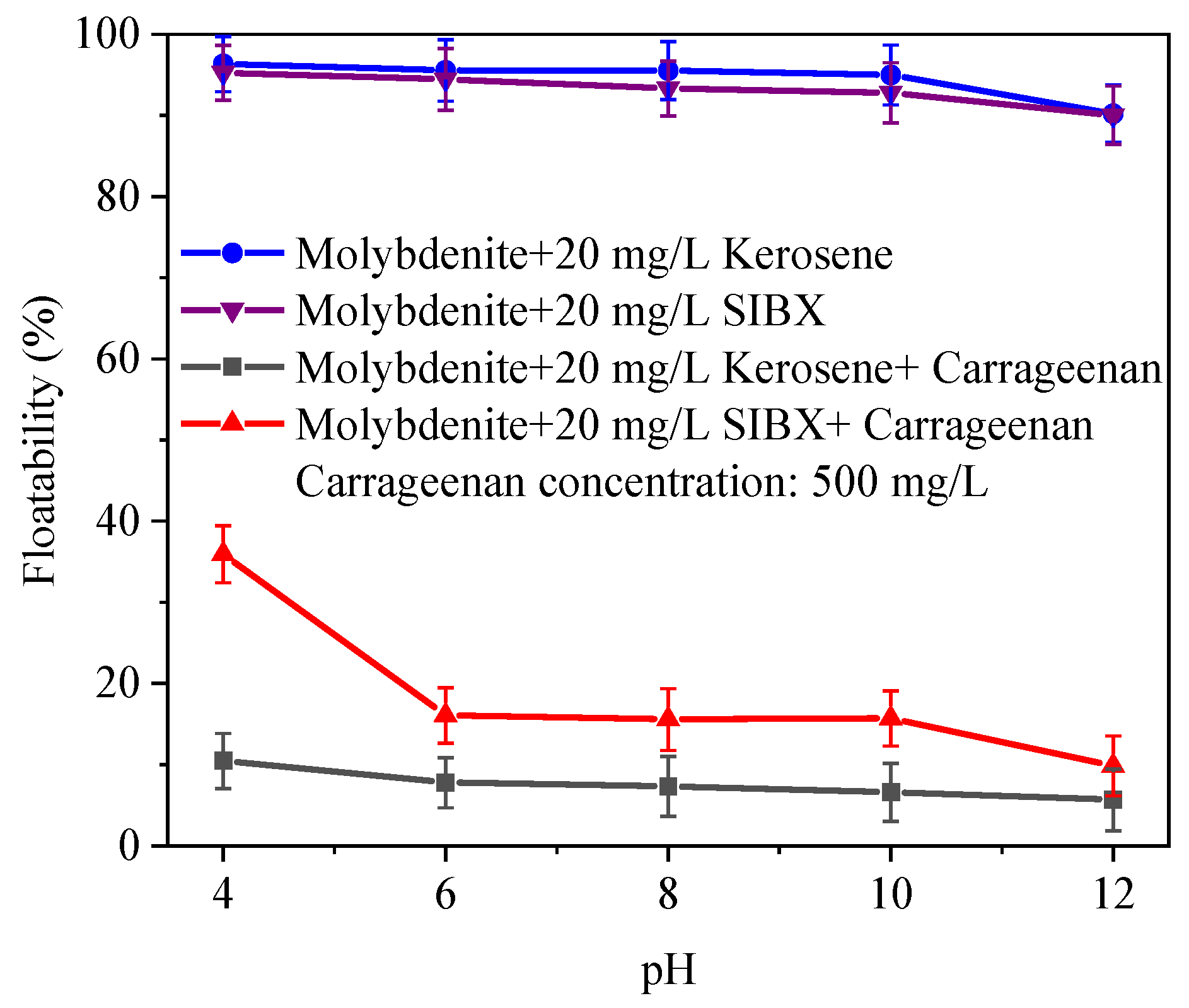
3.1. Microflotation Test
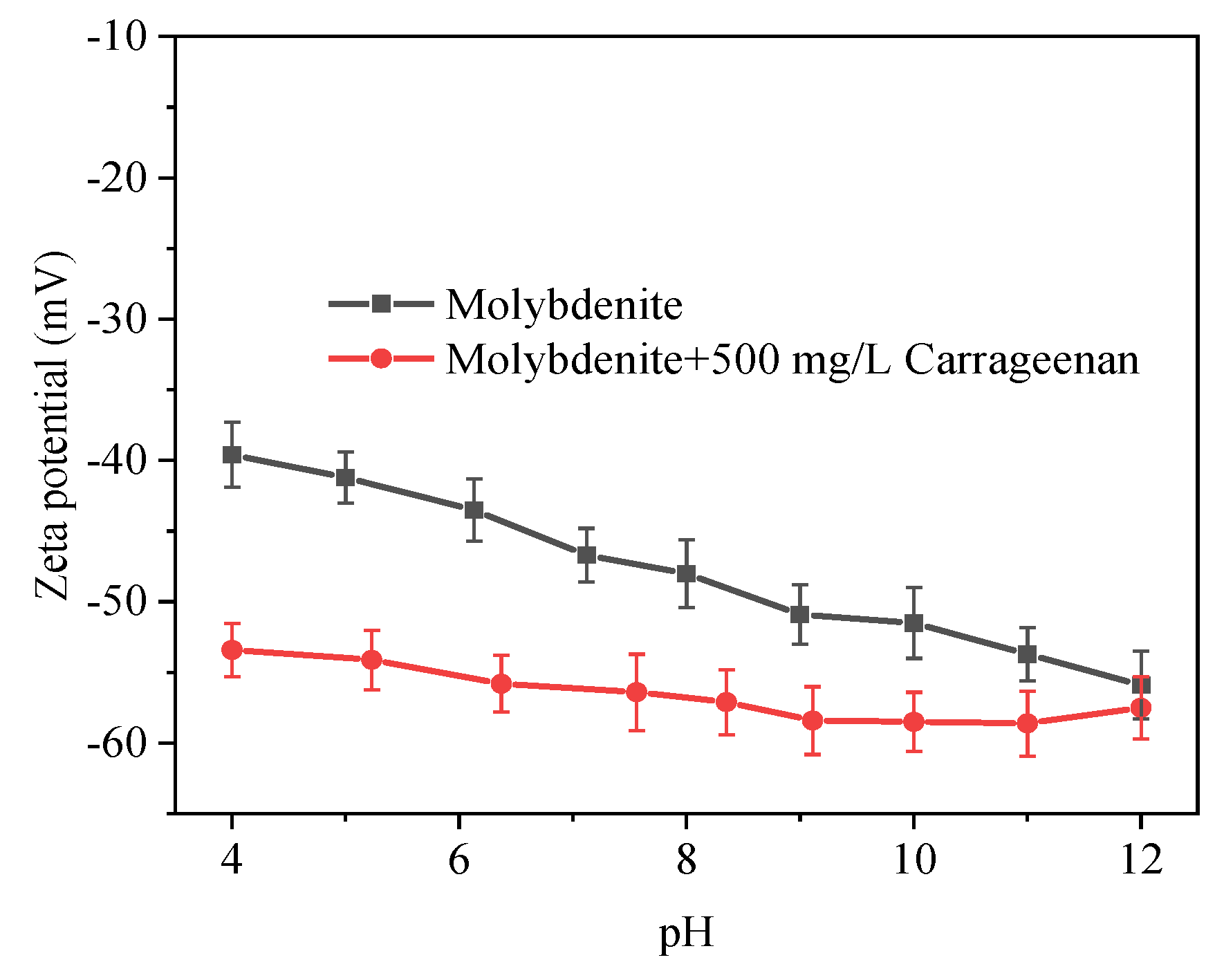
3.2. Zeta Potential Measurement
3.3. FTIR Measurement
3.4. AFM Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, X.; Chen, Y.; Liu, K.; Zeng, G.; Peng, Q.; Li, Z. Selective flotation separation of molybdenite and chalcopyrite by thermal pretreatment under air atmosphere. Colloids Surf. A 2019, 583, 123958. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Wu, M.; Xu, Z.; Tian, M.; Yin, Z.; Sun, W.; Zhang, C. Flotation separation of molybdenite from chalcopyrite using rhodanine-3-acetic acid as a novel and effective depressant. Miner. Eng. 2021, 162, 106747. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Yang, D.; Qin, X.; Gao, H.; Bai, X.; Wen, S. Application of a new depressant dithiocarbamate chitosan in separation of chalcopyrite and molybdenite. Colloids Surf. A 2022, 634, 127920. [Google Scholar] [CrossRef]
- Yan, H.; Yang, B.; Zeng, M.; Huang, P.; Teng, A. Selective flotation of Cu-Mo sulfides using xanthan gum as a novel depressant. Miner. Eng. 2020, 156, 106486. [Google Scholar] [CrossRef]
- Peng, H.; Wu, D.; Abdalla, M.; Luo, W.; Jiao, W.; Bie, X. Study of the Effect of Sodium Sulfide as a Selective Depressor in the Separation of Chalcopyrite and Molybdenite. Minerals 2017, 7, 51. [Google Scholar] [CrossRef]
- Yan, H.; Yang, B.; Zhu, H.; Huang, P.; Hu, Y. Selective flotation of Cu-Mo sulfides using dithiothreitol as an environmental-friendly depressant. Miner. Eng. 2021, 168, 106929. [Google Scholar] [CrossRef]
- Yang, B.; Yan, H.; Zeng, M.; Huang, P.; Jia, F.; Teng, A. A novel copper depressant for selective flotation of chalcopyrite and molybdenite. Miner. Eng. 2020, 151, 106309. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, S.; Xu, Z.; Zhang, C.; He, J.; Zou, J.; Chen, D.; Sun, W. Flotation separation of molybdenite from chalcopyrite using an environmentally-efficient depressant L-cysteine and its adsoption mechanism. Miner. Eng. 2020, 156, 106438. [Google Scholar] [CrossRef]
- Yuan, D.; Cadien, K.; Liu, Q.; Zeng, H. Flotation separation of Cu-Mo sulfides by O-Carboxymethyl chitosan. Miner. Eng. 2019, 134, 202–205. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Liu, R.; Chen, P.; Tian, M. Utilization of acetic acid-[(hydrazinylthioxomethyl)thio]-sodium as a novel selective depressant for chalcopyrite in the flotation separation of molybdenite. Sep. Purif. Technol. 2017, 179, 248–256. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Yamane, M.; Takida, E.; Kuroiwa, S.; Imaizumi, Y. Selective flotation of chalcopyrite and molybdenite using H2O2 oxidation method with the addition of ferrous sulfate. Miner. Eng. 2018, 122, 312–326. [Google Scholar] [CrossRef]
- Liao, R.; Feng, Q.; Wen, S.; Liu, J. Flotation separation of molybdenite from chalcopyrite using ferrate(VI) as selective depressant in the absence of a collector. Miner. Eng. 2020, 152, 106–369. [Google Scholar] [CrossRef]
- Kor, M.; Korczyk, P.M.; Addai-Mensah, J.; Krasowska, M.; Beattie, D.A. Carboxymethylcellulose adsorption on molybdenite: The effect of electrolyte composition on adsorption, bubble-surface collisions, and flotation. Langmuir 2014, 30, 11975–11984. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yan, H.; Zeng, M.; Zhu, H. Tiopronin as a novel copper depressant for the selective flotation separation of chalcopyrite and molybdenite. Sep. Purif. Technol. 2021, 266, 118576. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part I. Selective Coating Formation. Metals 2020, 10, 1667. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, L.; Cao, Y.; Yang, J.; Che, W.; Liu, J. The flotation separation of molybdenite from chalcopyrite using a polymer depressant and insights to its adsorption mechanism. Chem. Eng. J. 2020, 395, 125137. [Google Scholar] [CrossRef]
- Sinche-Gonzalez, M.; Fornasiero, D.; Zanin, M. Flotation of Chalcopyrite and Molybdenite in the Presence of Organics in Water. Minerals 2016, 6, 105. [Google Scholar] [CrossRef]
- Jafari, A.; Farahani, M.; Sedighi, M.; Rabiee, N.; Savoji, H. Carrageenans for tissue engineering and regenerative medicine applications: A review. Carbohydr. Polym. 2022, 281, 119045. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Ou, L.; Mai, Q. Flotation separation of chalcopyrite from talc using a new depressant carrageenan. Colloids Surf. A 2020, 603, 125274. [Google Scholar] [CrossRef]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Miner. Eng. 2007, 20, 609–616. [Google Scholar] [CrossRef]
- Liu, X.; An, F.; Wang, Y.; Huang, Q.; Teng, H.; Song, H. Preparation of Carboxymethylated Carrageenan Gelswith High Water Holding Capacity and Soft Gelling Property. Sci. Technol. Food Ind. 2018, 39, 58–63. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Hu, Y.; Chen, J.; Zhu, Y.; Lu, L.; Xiong, W.; Luo, S. The Evaluation of Carrageenan as a Novel and Environmentally Friendly Molybdenite Depressant. Minerals 2022, 12, 1234. https://doi.org/10.3390/min12101234
Zhao Z, Hu Y, Chen J, Zhu Y, Lu L, Xiong W, Luo S. The Evaluation of Carrageenan as a Novel and Environmentally Friendly Molybdenite Depressant. Minerals. 2022; 12(10):1234. https://doi.org/10.3390/min12101234
Chicago/Turabian StyleZhao, Zhiqiang, Yangjia Hu, Jianhua Chen, Yangge Zhu, Liang Lu, Wei Xiong, and Sigang Luo. 2022. "The Evaluation of Carrageenan as a Novel and Environmentally Friendly Molybdenite Depressant" Minerals 12, no. 10: 1234. https://doi.org/10.3390/min12101234
APA StyleZhao, Z., Hu, Y., Chen, J., Zhu, Y., Lu, L., Xiong, W., & Luo, S. (2022). The Evaluation of Carrageenan as a Novel and Environmentally Friendly Molybdenite Depressant. Minerals, 12(10), 1234. https://doi.org/10.3390/min12101234







