Abstract
The strong contamination of the interface is the main problem that results in low flotation efficiency of sphalerite in cyanide tailings. However, the consumption of cyanide and dissolved oxygen, as well as the concentration of ions including Zn2+ and SCN− in the leaching solution, decreased with the use of ceramic ball medium. The conclusions obtained from SEM–EDS indicated that the use of ceramic ball medium avoided the excessive surface oxidation caused by the galvanic couple actions between the iron ball medium and the sphalerite. XPS analysis also proved that the chemical environment on the surface of sphalerite was optimized by porcelain ball medium compared with iron ball medium, avoiding the formation of Fe–OOH and Fe–O hydrophilic substances, especially [Fe(CN)6]3−, thus increasing the adsorption of the collector on the surface of sphalerite. Therefore, grinding with ceramic ball medium exhibited excellent performance in terms of the cyanide process, which was approximately 5–10% higher than that obtained by grinding with iron ball media in the flotation test.
1. Introduction
Zinc mineral is one of the important mineral resources for human survival and development, which is widely used in metallurgy, chemical industry, military and biological materials [1,2,3]. As an important raw material for zinc extraction in industries, sphalerite has gradually attracted people’s attention. However, with the development of the economy, and the exploitation and utilization of minerals, the reserves of sphalerite are decreasing year after year. As a secondary resource, cyanide tailings contain a large amount of recoverable sphalerite. Flotation is the most common method for recovering sulfide minerals from ore and some tailings [4,5]. However, cyanide is an effective inhibitor of sphalerite flotation, which has a strong inhibitory effect on zinc sulfide during cyanide leaching and is a serious hindrance to the flotation recovery of the minerals [6,7]. At present, a large number of investigations have been conducted on the reaction mechanism between sphalerite and cyanide [6,8]. Qiu et al. [8] studied the inhibition mechanism of cyanide on sphalerite utilizing the density functional theory (DFT). The results showed that CN molecules were easily adsorbed on the surface of sphalerite (110), and Zn and S atoms on the mineral surface lost electrons, resulting in a decrease in the surface activity of sphalerite during flotation. However, Osathaphan et al. [9] indicated that part of Zn(CN)2 would be fixed on the surface of sphalerite when the CN/Zn ratio was less than 4. On the contrary, Prestidge et al. [10] found that the Zn-containing cyanide complex mainly existed in the cyanide pulp through XPS analysis. In summary, how to improve the recovery rate of sphalerite from cyanide tailings is faced with two main problems: (1) The mechanism of cyanide inhibiting sphalerite flotation in actual production is not clear; and (2) how to reduce the interface contamination of sphalerite during gold extraction by cyanidation, thereby increasing its surface hydrophobicity.
It is well known that grinding is a necessary process before cyanide leaching. During the grinding process, the mechanical force and mechanochemical interaction are established between minerals and the grinding medium [11,12]. As a result, changes in surface property with varying degrees occur on the surface and sub-surface layers of sulfide minerals. Many studies have reported that the surface properties and roughness of sulfide minerals during the grinding process are mainly related to the grinding medium and the grinding means; these properties play an important role in the wettability and flotation behavior of minerals [13,14,15]. Table 1 presents a summary of the current literature on the effects of different grinding media usage on mineral flotation and its main results. Ground by using ceramic media, the formation of metal-deficient sulfide surfaces enhances the hydrophobicity of sulfide minerals [14,16]. At the same time, in the case of grinding with iron ball media, the iron hydroxide produced by oxidation covering the sulfide minerals surface enhances minerals’ hydrophilicity [16,17]. On the contrary, Liao et al. [18] found that in iron media grinding environment, the collector produces dixanthogen at the surface of the pyrite and forms Fe–collector complexes. Therefore, the amount of adsorption of isobutyl xanthate at the surface of pyrite in iron media is higher than that of ceramic media. In addition, Cao et al. [19] studied cassiterite and found that compared with ceramic balls, steel balls are more conducive to the flotation of cassiterite. It can be seen that the use of grinding media is selective according to the different minerals. At present, there are still gaps in the research on the role of grinding in the cyanide leaching system and whether grinding media affects the interface properties and flotation performance of sphalerite during cyanide leaching.

Table 1.
Comparison of the effects of different grinding medium on mineral flotation.
Therefore, aiming at the problems in the existing studies: (1) The nature of the sphalerite interface in cyanide leaching is not clear; (2) the mechanism of cyanide inhibiting sphalerite flotation remains unclear; and (3) there is a lack of the correlation between surface adsorption, flotation behavior and the differences in grinding media in cyanidation system. This study provides insight into the influence of the grinding media on the mechanism of cyanide adsorption on the surface of sphalerite and flotation performance from a solution chemistry and surface chemistry perspective. SEM–EDS and contact angle were used to visualize the changes in sphalerite surface morphology and wettability. The contaminant composition of the sphalerite surface was investigated by using XPS. At the same time, the difference in grinding media was related to cyanide adsorption on the sphalerite surface, and flotation recovery was further compared by microflotation. The aim of this study is to provide theoretical guidance for the efficient recovery and utilization of sphalerite from cyanide tailings.
2. Materials and Methods
2.1. Materials and Reagents
Sphalerite used in this study was obtained from the Lame mine, Nanning, China, China. Samples were crushed to −0.25 mm with a pulverizer (SJ1000-1, Nanchang, China) and then stored in a vacuum drying oven (DZF-1B, Shanghai, China). The chemical composition of the sample was analyzed by an inductively coupled plasma optical emission spectrometer (ICP-OES, Avio500, Waltham, MA, USA). The results of the chemical composition are shown in Table 2. As seen, the contents of Zn and S were 65.2% and 33.2%, respectively. The purity of sphalerite was as high as 97.17%. The X-ray diffraction (XRD, D 8, Saarbrucken, Germany) analysis (Figure 1) confirmed that sphalerite was the only detectable crystalline phase. All the reagents used in this paper were analytical reagents (AR). High-purity water was used for all the experiments. Sodium cyanide was provided by Shandong Zhaojin Group Co., Ltd.

Table 2.
Chemical analysis of sphalerite sample detected using ICP technique (wt%).
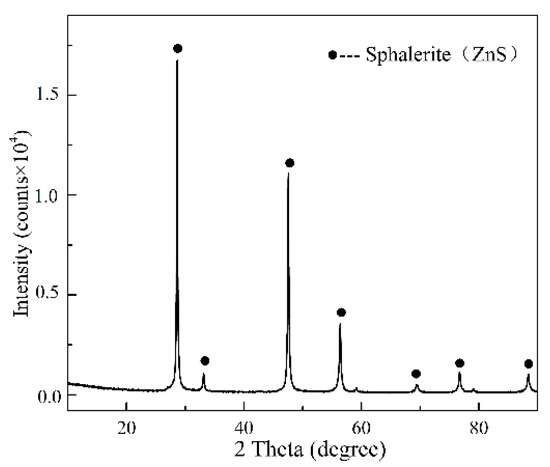
Figure 1.
X-ray diffraction pattern of sphalerite samples with particle size smaller than 75 µm.
2.2. Grinding Experiment
The grinding tests were carried out by using a planetary ball mill (QM3SP2, Nanjing, China). In this work, 100 mL zirconium jars were used. The milling process used high-purity water as the ball grinding agent with zirconium balls (Ceramic Ball Medium—CBM) and cast iron balls (Iron Ball Medium—IBM), under the conditions of 3 mm, 5 mm or 10 mm in diameter, a quality ratio of 3:2:1, a rotating speed of 400 r/min and a ball to powder ratio of 10:1. The 10 g samples combined with 10 mL of high-purity water were milled for 10 min by planetary ball mill and stored in a vacuum drying oven.
2.3. Cyanide Leaching Experiment
After grinding, the cyanide tests were carried out in a T09-1S magnetic stirrer at room temperature and a stirring speed of 900 r/min. For each leaching test, 200 mL of high-purity water were added to 5 g of the ground samples, pH was adjusted to 11.0–11.5 using 1 mol/L NaOH and mixed for 30 min, and then 1 g/L NaCN were added to the pulp. During the leaching, the pH values and NaCN concentration were kept at 11.0–11.5 and 1 g/L, respectively. In the specified time interval, 10 mL of pulp were filtered in a tube for testing. Each group was set up in three parallel samples.
2.4. Flotation Test
Flotation experiments of sphalerite were carried out in an XFG mechanical flotation machine (Changchun, Jilin province, China) with a 100 mL flotation cell. A sample of 5 g mixed with a certain amount of high-purity water was stirred for 1 min at a rotating speed of 1500 r/min and the injection of air at a rate of 0.1 Nm3/h. The desired pH value was adjusted to 8.5–9.0 using 1 M NaOH or 0.5 M H2SO4. Sodium butyl xanthate (SBX) and terpenic oil (20 g/t) were added to the cell, stirring for 3 min and 1 min, respectively. After scraping for 3 min, the concentrate and tailings were collected, filtered and dried to calculate the recovery of sphalerite. The flotation test was repeated three times under the same conditions, and the mean value was taken as the final result.
2.5. Characteristics of the Pulp
The inductively coupled plasma optical emission spectrometer (ICP-OES, Avio500) was used to detect the zinc concentration in the pulp. The concentrations of free CN− were titrated by the silver nitrate titration method. An amount of 1 mL filtrate was combined with 1 mL 25% (v/v) HNO3 and 1 mL 16% (m/v) NH4Fe(SO4)2∙12H2O to a final volume of 10 mL, and then the thiocyanate ion concentration was determined by spectrophotometry. The pH of the pulp was measured by a pH meter (PB–10, Gottingen, Germany). The DO concentration of the pulp was monitored by an oxygen meter (JPB-607A, Shanghai, China).
2.6. Characteristics of the Product
The phase composition of sphalerite was studied by X-ray diffraction (XRD, Saarbrucken, Germany) with Cu target Ka radiation at 40 kV and 40 mA. The morphology of the sample was analyzed by a Quanta250FEG SEM equipped with EDS, and the element composition was the average of five EDS test results in SEM images. The wettability of sphalerite was measured using a contact angle measuring instrument (JC2000C, Shanghai, China). The samples to be tested were pressed into smooth slices with a diameter of 1 cm. Then, the sample was placed on the measuring platform of the instrument, and high-purity water droplets were dripped on its surface using a micro-injector. Finally, balance contacts were analyzed with the Dropsnake plugin provided with the instrument. The average was measured from three different locations for each sample. X-ray photoelectron spectroscopy (XPS, Thermo EscaLab 250Xi, Waltham, MA, USA) was used to measure the bond energy and the surface species of the sphalerite with an aluminum target. Binding energies were corrected by adventitious carbon (C1s = 284.6 eV). The CasaXPS software was used to peak fit.
3. Results and Discussion
3.1. Effect of Grinding Media on Physicochemical Properties of Sphalerite
3.1.1. Particle Size Distribution
Figure 2 shows the particle size distribution of sphalerite ground by different grinding media. The particle size of the sample obtained by media grinding with ceramic balls was 100% < 75 μm after 10 min of grinding, but a small part of coarse particle size (0.56%, >75 μm) was found in the samples when ground using iron ball medium. This may be due to the fact that the average density of ceramic ball medium is less than that of iron ball medium, resulting in the number of iron balls being less than that of ceramic under the condition that the ball–powder constant remains unchanged. It further leads to only a small part of the material being captured by the interface between the balls and jar, while the other fraction possesses the larger grain size and uneven particle distribution affected by the action of shear and kneading [20,21]. Gold concentrates are usually ground to −38 μm in industrial production. However, too fine particle size distribution will lead to the phenomenon of slurry slime. Further analysis showed that the sphalerite had good distribution in the particle size range of 45–20 μm (19.90%) with the use of ceramic ball medium. In industrial production [22], the grade and recovery of copper concentrates with a vertical mill in Dexing Copper Mine, Dexing, China increased by approximately 2% and 3%, respectively, after replacing the steel balls with ceramic balls in the regrinding stage. It was noteworthy that 3.3% of the coarse particle size (>75 μm) was found in the steel media product but not in the ceramic media. Therefore, the ceramic ball media increase the grinding efficiency to obtain finer particles. There is no significant difference in particle size distribution between ceramic ball media and iron ball media, which satisfies the condition of eliminating the variable of the particle size distribution of the flotation feed.

Figure 2.
Particle size distribution of sphalerite samples milled for 10 minutes under different grinding media.
3.1.2. Pulp Chemical Properties of Samples
The chemical properties of pulp, including pH, ion concentration, DO concentration, conductivity and redox potential, are affected by the grinding media [23,24]. Many studies have shown that the chemical properties of pulp are deeply controlled by the grinding environment, which further influences the flotation of sulfide minerals [24,25,26]. The pulp chemical properties of sphalerite under different grinding media are presented in Figure 3. It can be observed that the pH value and Zn2+ concentration of the pulp obtained by iron ball medium are relatively higher, and yet the concentration of dissolved oxygen is significantly lower than that of ceramic ball medium. The main reasons for this discrepancy are the local cell reaction between the sphalerite samples or the IBM itself, and the difference in electrostatic potential between the sphalerite and the IBM resulting in galvanic couple actions [27,28]. However, in the grinding process with ceramic ball, only the local cell reaction occurs on the surface of sphalerite. As a result, the redox reactions were more intense when the iron ball medium was used for grinding, which caused abundant Fe3+ and iron hydroxide to enter the pulp. Simultaneously, the rise of DO concentration corresponds to the production of OH− and a large amount of iron. As can be seen, the selection of grinding media can improve the pulp properties at the process of process source and thus provide a better environment for cyanidation.
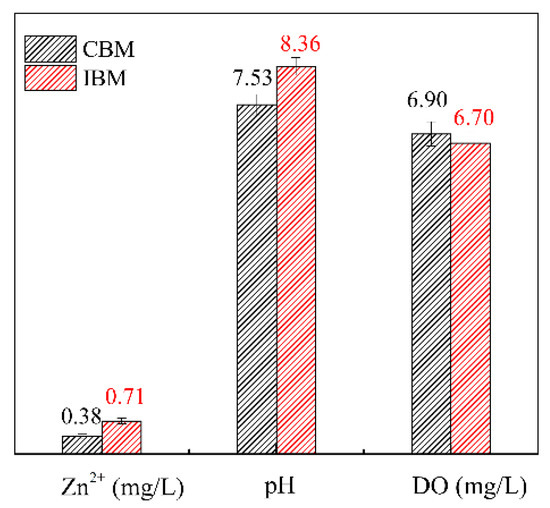
Figure 3.
Pulp chemical properties of samples milled for 10 minutes under different grinding media.
3.1.3. Surface Morphology of Samples
Sphalerite with different grinding media exhibited differences in the surface morphology, roughness and products. Figure 4 shows the SEM–EDS and contact angle (θ) analysis of sphalerite with different grinding media. The surfaces of the ground sphalerite present a bumpy and corroded appearance under the observation from SEM. The floccule of different sizes was attached to the surface of sphalerite, which was confirmed as iron–oxygen or zinc–oxygen substance by EDS (Figure 4a,b). In addition, it is not difficult to find that the surface of sphalerite was relatively smooth and even, and only a small amount of floccule was produced in the case of grinding by ceramic ball media (Figure 4a). This is because of the strong mechanochemical interaction between iron balls and sphalerite particles. As the electrostatic potential of iron ball (E = −0.255 V) is lower than that of sphalerite (E = 0.188V), the anodic oxidation and cathodic reduction reactions occur on the surface of sphalerite and iron ball surface. The main reactions are shown in Equations (1)–(9). The surface morphology and properties possess an impact on the hydrophobicity and hydrophilicity. According to the contact angle results (Figure 4a,b), the hydrophilicity of sphalerite samples prepared with CBM (40.6°) is lower than that of samples prepared with IBM (31.6°).

Figure 4.
The SEM images with EDS analysis and contact angle of sphalerite at different conditions (a) grinding with ceramic ball media, (b) grinding with iron ball media.
Anodic reaction:
- (1)
- iron ball
- (2)
- sphalerite
Cathodic reaction:
3.2. Chemical Properties of Cyaniding Pulp
Zinc sulfide minerals were dissolved in pulp because of the high alkali and cyanide environment, which led to the formation of the complex system in cyanide pulp. The main chemical reaction between sphalerite and cyanide is shown in Equations (10) and (11). As shown in Figure 5a, the increase in consumption of sodium cyanide gradually increased with leaching time. A large amount of iron was dissolved and transferred into the pulp and further reacted with cyanide, as listed in Equations (12)–(15). Furthermore, according to Equation (16), iron and zinc can form the complex Zn2(FeCN)6, which ultimately leads to higher consumption of NaCN by approximately 20.80% than that obtained by grinding with ceramic ball medium. The dissolution of sphalerite follows the generation of Zn2+ and SCN−. It can be seen that the concentration of Zn2+ and SCN− were obviously different in the grinding process by using different grinding media (Figure 5b,c). It is concluded that the galvanic coupling between iron ball medium and sphalerite sharply accelerates the dissolution of zinc ions [14]. Moreover, the data in Figure 5d displays that the concentration of dissolved oxygen has a dramatic decrease earlier and then rose gradually. The existence of iron in pulp accelerated the consumption of dissolved oxygen. As can be seen, the use of ceramic ball medium optimized the cyanide pulp environment and prevented the further dissolution of the sphalerite, thereby avoiding excessive adhesion of the resulting zinc hydroxide or zinc cyanide film to the surface of the sphalerite.
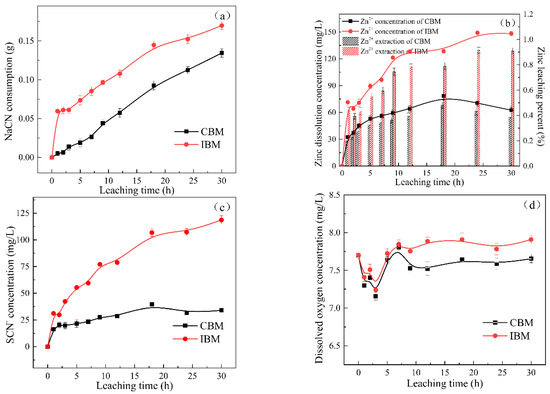
Figure 5.
Variation of (a) NaCN consumption, (b) Zn2+ dissolution concentration and extraction, (c) SCN− concentration and (d) dissolved oxygen concentration as a function of time in cyanidation system for sphalerite samples obtained by different grinding media.
3.3. Effect of Grinding Media on Surface Physicochemical Properties of Leaching Residues
3.3.1. SEM and Contact Angle Analysis
Mechanochemical reaction and cyanidation can promote the oxidative decomposition of sulfide minerals [13,29,30]. After grinding, sphalerite was immersed in an environment rich in oxygen, high alkali and NaCN for a long time, resulting in irregular patterns and corrosion on the sample surface (Figure 6). In addition, as indicated in EDS, a large number of oxygenated flocs could be observed in the IBM experimental group (Figure 6b). At the same time, compared with the experimental group of iron media before cyanidation, the content of Fe on the surface of sphalerite decreased. The decrease in iron concentration is related to the reaction of dissolved iron ion with cyanide to form ferricyanide complex. Furthermore, the use of IBM accelerated the consumption of the cyanide agents, which was consistent with Figure 4. This inevitably enhanced the etching reaction of the sphalerite, leading to a complex surface appearing on the sample. Although the existence of ferrocyanide or zinc cyanide complex was not proved from the EDS analysis, Equations (15) and (16) would be occurred according to thermodynamic calculations. The wettability of the mineral surface is one of the effective data to measure the floatability of minerals [31]. As previously studied [14,32], the use of ceramic media significantly improved the hydrophobicity of mineral surfaces, which was also confirmed in this work (Figure 5a). As found by the contact angle in Figure 6, this effect continues into the cyanidation process. Moreover, it is the fact that after cyanidation treatment, the contact angle of sphalerite in the IBM group decreased from 31.6° to 20.9°, while the contact angle in the CBM group decreased from 40.6° to 27.9°. Thus, this indicates that ceramic media improves the floatability of the cyanidation leach tailings to a certain extent.
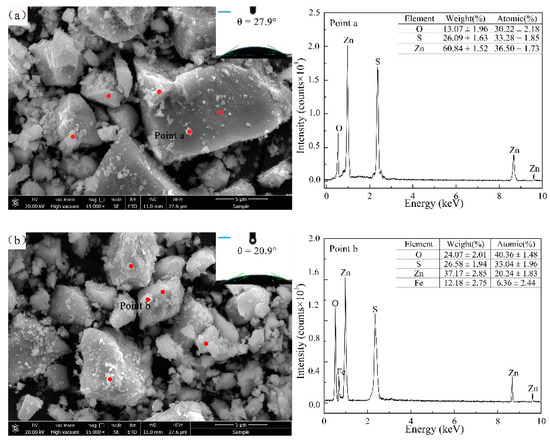
Figure 6.
The SEM images with EDS analysis and contact angle of sphalerite at different conditions (a) cyanide sphalerite after grinding with ceramic ball media, (b) cyanide sphalerite after grinding with iron ball media.
3.3.2. XPS Analysis
In this work, XPS analysis was conducted to investigate further and characterize the composition variation of the sphalerite after cyanide leaching under different grinding media. By comparing the survey spectra in Figure 7(a1,a2), a peak related to Fe 2p can be observed in the sphalerite samples ground by using iron ball medium, indicating that Fe was brought into the system. This is consistent with the analysis results obtained from SEM–EDS. As seen in Figure 7(b1), the Fe 2p3/2 spectrum was divided into two peaks positioned at ~716.98 eV, ~711.99 eV, which were assigned to Fe–S and Fe–OOH, respectively [33,34]. The two Fe 2p1/2 peaks located at around ~726.52 eV and ~720.78 eV were ascribed to Fe–OOH and Fe, respectively. There were four peaks that occurred at ~724.94 eV, ~721.70 eV, ~715.72 eV and ~710.88 eV in the case of cyanide leaching after grinding by iron ball medium (Figure 7(b2)), which were associated with Fe–OOH, Fe, Fe–S and Fe–O, respectively [15,34,35]. In addition, a minor peak at ~708.33 eV could be assigned to [Fe(CN)6]3− [36], which indicated the iron ball medium and cyanide leaching have significant effects on the interface reaction of sphalerite.
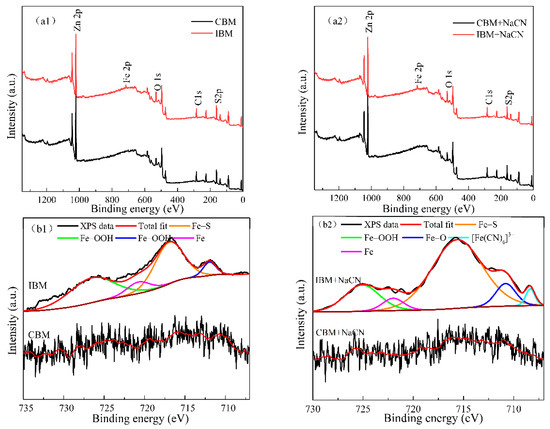
Figure 7.
XPS results on the surface of cyanide leaching residue as a function of different grinding media in 0.1% NaCN at T = 30 h: (a1,a2) full range, (b1,b2) Fe 2p, (c1,c2) O 1s, (d1,d2) S 2p, (e1,e2) Zn 2p.
Figure 7(c1,c2) depicts the O spectra, fitted with the 1s peaks. The data obtained from the O 1s spectra, for the samples ground by ceramic ball medium without cyanidation, the peak located at the binding energy of ~531.70 eV (Figure 7(c1)) was attributed to Zn–O on the sphalerite surface [37]. This occurs as a concomitant so as to increase the binding energy of O 1s peak (located at ~531.72 eV, as shown in Figure 7(c2), due to the presence of cyanide). Obviously, when using iron ball medium, the peaks located at a binding energy of ~531.97 eV and ~530.04 eV can be assigned to Zn–O and Fe–O bond on the sphalerite surface, while the peaks at ~531.70 eV and ~529.57 eV in the case of the cyanide leached products corresponded to Zn–O and Fe–O, respectively [2,35]. Consequently, both ceramic and iron grinding media trigger the surface oxidation of sphalerite. The presence of contamination with iron ball medium is corroborated by the formation of Fe–containing oxidative species on the sphalerite surface.
The S 2p XPS spectrum of sphalerite ground by using different media is presented in Figure 7(d1). The S 2p3/2 peak of the sphalerite ground by ceramic ball was divided into two peaks located at ~161.81 eV and ~163.02 eV, which were assigned to Zn–S and S22−, respectively [34,37,38]. However, ground by iron ball, the binding energy of S 2p3/2 peak decreased by 0.1eV (~161.71 eV, ~162.92 eV), which was due to the galvanic couple action between the iron ball and the mineral, resulting in the loss of charge on the sphalerite surface. Furthermore, cyanidation has little impact on the binding energy of sphalerite with grinding (Figure 7(d2)). The S 2p3/2 peaks were related to Zn–S (~161.73 eV, 161.51 eV) and S22− (~162.93 eV, 162.71 eV). Thus, there is a variation in the binding energy of S 2p3/2 on the sphalerite surface owing to cyanidation behavior. S2− can react with cyanogen to generate thiocyanate, but the SCN− peak (~162.0 eV) [39] was not detected due to the detection limit or the non–attachment of the thiocyanate complex.
Figure 7(e1,e2) shows that the Zn 2p spectrum was fitted by two spin–orbit split peaks with an intensity ratio of 2:1. Zn 2p1/2 has the same properties as Zn 2p3/2 because the Zn 2p1/2 and Zn 2p3/2 doublets are single and symmetric [3,40]. The binding energy of the peaks in Figure 7(e1) (~1021.71 eV, ~1044.7 eV) of the sphalerite sample after grinding by iron ball medium was lower than that of the sample after grinding by ceramic ball medium (~1021.78 eV, ~1044.82 eV), which was caused by the Zn–S bond [37]. Similarly, the sphalerite samples under cyanide leaching after grinding can be explained. The product Zn(CN)2 affecting the flotation of sphalerite has not been found on the surface of sphalerite. Therefore, these changes reveal that grinding and cyanidation have little effect on the Zn product at the interface of sphalerite.
3.4. Flotation Performance
The flotation tests were carried out to verify the impact of the grinding media; the data are shown in Figure 8. During cyanidation, the flotation recovery of the sphalerite ground by using IBM and CBM increased from 13.65% and 18.81% to 51.56% and 61.67%, respectively, with an increase in the dosage of SBX from 5 to 80 mg/L (Figure 8a). As an increase in the CuSO4 (activator) dosage from 0 to 150 mg/L, the flotation recovery of sphalerite ground by IBM and CBM increased from 50.76% and 60.47% to 77.04% and 82.09%, respectively (Figure 8b). It can be seen that the flotation recovery of sphalerite ground by CBM increased by approximately 5%–10% compared with IBM. Most importantly, the use of CBM can extremely reduce the amount of CuSO4.
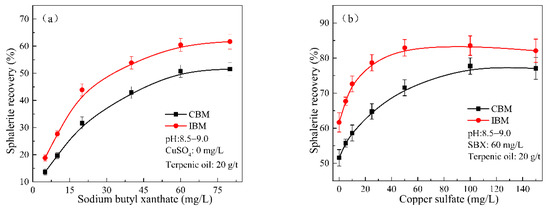
Figure 8.
Effect of (a) SBX and (b) copper sulfate concentration on recovery of cyanide leaching residues as a function of grinding media in the presence of 20 g/t terpenic oil.
As reported in previous studies, the different grinding media also affect the interfacial properties and flotation performance of other minerals (e.g., chalcopyrite, pyrite, etc.). The experiment conditions and results are shown in Table 3. Domestic and international studies have found that the corrosion products of iron media, whether forming Fe(OH)3 or FeOOH, will have a significant impact on the floatability of sulfide minerals. For the cyanidation process, these ground products have a negative impact on both the consumption of cyanide agents and the flotation properties of the minerals, which has been confirmed by this study. In addition, Zhao et al. [30] suggested that samples ground with iron media in cyanidation systems resulted in more reactions involving the positive charges on their surfaces. In this study (Figure 7), the interface of sphalerite after cyanidation is mainly adsorbed by oxides of Fe and Zn as well as negative ions such as SCN− and [Fe(CN)6]3−. However, the more negative charges adsorbed the surface of the sample, the less favorable to the triggering of collector adsorption. Based on the above, ceramic ball, as a grinding medium, is used to regrind sphalerite in the cyanidation process in this work, which not only improves the flotation recovery of sphalerite in cyanide tailings but also reduces the consumption of flotation reagents.

Table 3.
Comparison of flotation quota using different of grinding media.
4. Conclusions
In this study, the effects of ceramic ball media and iron ball media on the properties of sphalerite pulp, surface adsorption and the presence of surface species in the cyanidation system were studied by ICP–OES, SEM–EDS, contact angle and XPS. It provides a significant theoretical basis for understanding the adsorption mechanism of cyanide on sphalerite surface under different grinding media. Simultaneously, the influence of the two grinding media is verified in conjunction with flotation tests. The results suggested that the use of ceramic ball media avoided the peroxidation caused by galvanic couple actions between sphalerite and iron ball. Additionally, the XPS surface analysis technique revealed the formation of [Fe(CN)6]3− on the sphalerite surface after cyanidation with the iron ball media group, companying the decrease of the contact angle from 27.9° to 20.9°, resulting in the increment of consumption of SBX and CuSO4 in the flotation recovery. Consequently, the sphalerite recovery was approximately 5%–10% higher by grinding with ceramic ball media than that with iron ball media. This study provides an important surface chemical basis for an in–depth understanding of the mechanism of cyanide adsorption on the surface of sphalerite obtained by different grinding media, which will play a great positive role in increasing the possibility of comprehensive utilization of cyanide tailings resources.
Author Contributions
Conceptualization, Q.Z. (Qianfei Zhao) and L.T.; methodology, Q.Z. (Qianfei Zhao); formal analysis, Q.Z. (Qianfei Zhao); investigation, Q.Z. (Qianfei Zhao), R.J. and Q.Z. (Qin Zhang); resources, P.M.; Writing—original draft, Q.Z. (Qianfei Zhao); visualization, H.Y. and L.T.; funding acquisition, L.T. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China, grant number 2018YFC1902002 and 2018YFC1902001.
Data Availability Statement
Data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aikawa, K.; Ito, M.; Segawa, T.; Jeon, S.; Park, I.; Tabelin, C.B.; Hiroyoshi, N. Depression of Lead-Activated Sphalerite by Pyrite via Galvanic Interactions: Implications to the Selective Flotation of Complex Sulfide Ores. Miner. Eng. 2020, 152, 106367. [Google Scholar] [CrossRef]
- Trejo-Ramos, A.I.; González-Chan, I.J.; Oliva, A.I. Physical Properties of Chemically Deposited ZnS Thin Films: Role of the Solubility Curves and Species Distribution Diagrams. Mater. Sci. Semicond. Process. 2020, 118, 105207. [Google Scholar] [CrossRef]
- Wang, H.; Wen, S.; Han, G.; Feng, Q. Effect of Copper Ions on Surface Properties of ZnSO4-Depressed Sphalerite and Its Response to Flotation. Sep. Purif. Technol. 2019, 228, 1–9. [Google Scholar] [CrossRef]
- Gül, A.; Yüce, A.E.; Sirkeci, A.A.; Özer, M. Use of Non-Toxic Depressants in the Selective Flotation of Copper-Lead-Zinc Ores. Can. Metall. Q. 2008, 47, 111–118. [Google Scholar] [CrossRef]
- Kydros, K.A.; Gallios, G.P.; Matis, K.A. Modification of Pyrite and Sphalerite Flotation by Dextrin. Sep. Sci. Technol. 1994, 29, 2263–2275. [Google Scholar] [CrossRef]
- Lv, C.; Ding, J.; Qian, P.; Li, Q.; Ye, S.; Chen, Y. Comprehensive Recovery of Metals from Cyanidation Tailing. Miner. Eng. 2015, 70, 141–147. [Google Scholar] [CrossRef]
- Nanda, S.; Kumar, S.; Mandre, N.R. Flotation Behavior of a Complex Lead-Zinc Ore Using Individual Collectors and Its Blends for Lead Sulfide. J. Dispers. Sci. Technol. 2022, 1–8. [Google Scholar] [CrossRef]
- Qiu, T.; Nie, Q.; He, Y.; Yuan, Q. Density Functional Theory Study of Cyanide Adsorption on the Sphalerite (110) Surface. Appl. Surf. Sci. 2019, 465, 678–685. [Google Scholar] [CrossRef]
- Osathaphan, K.; Boonpitak, T.; Laopirojana, T.; Sharma, V.K. Removal of Cyanide and Zinc-Cyanide Complex by an Ion-Exchange Process. Water Air Soil Pollut. 2008, 194, 179–183. [Google Scholar] [CrossRef]
- Prestidge, C.A.; Skinner, W.M.; Ralston, J.; Smart, R.S.C. Copper(II) Activation and Cyanide Deactivation of Zinc Sulphide under Mildly Alkaline Conditions. Appl. Surf. Sci. 1997, 108, 333–344. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Ning, J.; Shi, J. Effects and Mechanisms of Grinding Media on the Flotation Behavior of Scheelite. ACS Omega 2020, 5, 32076–32083. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, J.; Wang, J.; Zhang, X.; Wang, H. Effect of Different Grinding Methods on Floatation of Sphalerite. Nonferrous Met. (Miner. Process. Sect.) 2018, 35–51. [Google Scholar]
- Xia, L.; Hart, B.R. Correlation between the Hydrogen Peroxide Formed during Grinding and the Oxidized Species Present on the Surface of Sphalerite. Miner. Eng. 2019, 130, 165–170. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Gao, P.; Li, Y. Effects of Grinding Media on Grinding Products and Flotation Performance of Chalcopyrite. Miner. Eng. 2020, 145, 106070. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Gao, P.; Li, Y.; Sun, Y. Effects of Particle Size and Ferric Hydroxo Complex Produced by Different Grinding Media on the Flotation Kinetics of Pyrite. Powder Technol. 2020, 360, 1028–1036. [Google Scholar] [CrossRef]
- Rabieh, A.; Eksteen, J.J.; Albijanic, B. The Effect of Grinding Chemistry on Cyanide Leaching of Gold in the Presence of Pyrrhotite. Hydrometallurgy 2017, 173, 115–124. [Google Scholar] [CrossRef]
- Mu, Y.; Cheng, Y.; Peng, Y. The Interaction of Grinding Media and Collector in Pyrite Flotation at Alkaline PH. Miner. Eng. 2020, 152, 106344. [Google Scholar] [CrossRef]
- Liao, N.; Wu, C.; Xu, J.; Feng, B.; Wu, J.; Gong, Y. Effect of Grinding Media on Grinding-Flotation Behavior of Chalcopyrite and Pyrite. Front. Mater. 2020, 7, 176. [Google Scholar] [CrossRef]
- Cao, Y.; Tong, X.; Xie, X.; Song, Q.; Zhang, W.; Du, Y.; Zhang, S. Effects of Grinding Media on the Flotation Performance of Cassiterite. Miner. Eng. 2021, 168, 106919. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Q.; Yang, H.; Shi, D.; Qian, J. Photocatalytic Antibacterial Properties of Copper Doped TiO2 Prepared by High-Energy Ball Milling. Ceram. Int. 2020, 46, 16716–16724. [Google Scholar] [CrossRef]
- Xie, W.; Polikarpov, E.; Choi, J.P.; Bowden, M.E.; Sun, K.; Cui, J. Effect of Ball Milling and Heat Treatment Process on MnBi Powders Magnetic Properties. J. Alloys Compd. 2016, 680, 1–5. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Kawatra, S.K. Effects of Grinding Media on Grinding Products and Flotation Performance of Sulfide Ores. Miner. Process. Extr. Metall. Rev. 2020, 42, 172–183. [Google Scholar] [CrossRef]
- Bruckard, W.J.; Sparrow, G.J.; Woodcock, J.T. A Review of the Effects of the Grinding Environment on the Flotation of Copper Sulphides. Int. J. Miner. Process. 2011, 100, 1–13. [Google Scholar] [CrossRef]
- Wei, Y.; Sandenbergh, R.F. Effects of Grinding Environment on the Flotation of Rosh Pinah Complex Pb/Zn Ore. Miner. Eng. 2007, 20, 264–272. [Google Scholar] [CrossRef][Green Version]
- Peng, Y.; Grano, S.; Fornasiero, D.; Ralston, J. Control of Grinding Conditions in the Flotation of Galena and Its Separation from Pyrite. Int. J. Miner. Process. 2003, 70, 67–82. [Google Scholar] [CrossRef]
- Xia, L.; Hart, B.; Chen, Z.; Furlotte, M.; Gingras, G.; Laflamme, P. A ToF-SIMS Investigation on Correlation between Grinding Environments and Sphalerite Surface Chemistry: Implications for Mineral Selectivity in Flotation. Surf. Interface Anal. 2017, 49, 1397–1403. [Google Scholar] [CrossRef]
- Pozzo, R.L.; Iwasaki, I. Effect of Pyrite and Pyrrhotite on the Corrosive Wear of Grinding Media. Miner. Metall. Process. 1987, 4, 166–171. [Google Scholar] [CrossRef]
- Pozzo, R.L.; Malicsi, A.S.; Iwasaki, I. Pyrite-Pyrrhotite-Grinding Media Contact and Its Effect on Flotation. Miner. Metall. Process. 1990, 7, 16–21. [Google Scholar] [CrossRef]
- Rabieh, A.; Albijanic, B.; Eksteen, J.J. A Review of the Effects of Grinding Media and Chemical Conditions on the Flotation of Pyrite in Refractory Gold Operations. Miner. Eng. 2016, 94, 21–28. [Google Scholar] [CrossRef]
- Yang, J.; Shuai, Z.; Zhou, W.; Ma, S. Grinding Optimization of Cassiterite-Polymetallic Sulfide Ore. Minerals 2019, 9, 134. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y. The Effect of Grinding Media and Environment on the Surface Properties and Flotation Behaviour of Sulfide Minerals. Miner. Process. Extr. Metall. Rev. 1990, 7, 49–79. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, H.; Tong, L.; Jin, R.; Ma, P. Understanding the Effect of Grinding Media on the Adsorption Mechanism of Cyanide to Chalcopyrite Surface by ToF–SIMS, XPS, Contact Angle, Zeta Potential and Flotation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128799. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Moimane, T.; Plackowski, C.; Peng, Y. The Critical Degree of Mineral Surface Oxidation in Copper Sulphide Flotation. Miner. Eng. 2020, 145, 106075. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y.; Bradshaw, D. Effect of Regrinding Conditions on Pyrite Flotation in the Presence of Copper Ions. Int. J. Miner. Process. 2013, 125, 129–136. [Google Scholar] [CrossRef]
- Fernández Macía, L.; Petrova, M.; Hubin, A. ORP-EIS to Study the Time Evolution of the [Fe(CN)6]3−/[Fe(CN)6]4− Reaction Due to Adsorption at the Electrochemical Interface. J. Electroanal. Chem. 2015, 737, 46–53. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, S.; Ma, X.; Yang, J.; Zhong, H. Synthesis of Thioxopropanamide Surfactants for Studying the Flotation Performance and Adsorption Mechanism on Chalcopyrite. Appl. Surf. Sci. 2020, 505, 144539. [Google Scholar] [CrossRef]
- Khmeleva, T.N.; Georgiev, T.V.; Jasieniak, M.; Skinner, W.M.; Beattie, D.A. XPS and ToF-SIMS Study of a Chalcopyritepyrite-Sphalerite Mixture Treated with Xanthate and Sodium Bisulphite. Surf. Interface Anal. 2005, 37, 699–709. [Google Scholar] [CrossRef]
- Er, U.; Icli, K.C.; Ozenbas, M. Spin-Coated Copper(I) Thiocyanate as a Hole Transport Layer for Perovskite Solar Cells. J. Solid State Electrochem. 2020, 24, 293–304. [Google Scholar] [CrossRef]
- Smart, R.S.C.; Skinner, W.M.; Gerson, A.R. XPS of Sulphide Mineral Surfaces: Metal-Deficient, Polysulphides, Defects and Elemental Sulphur. Surf. Interface Anal. 1999, 28, 101–105. [Google Scholar] [CrossRef]
- Corin, K.C.; Song, Z.G.; Wiese, J.G.; O’Connor, C.T. Effect of Using Different Grinding Media on the Flotation of a Base Metal Sulphide Ore. Miner. Eng. 2018, 126, 24–27. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).