Oxygen Isotopes in Carbonate and Phosphate of Modern Mammal Bioapatite: New Data and Critical Revision after about 25 Years from the First Recognitions
Abstract
:1. Introduction
2. Significance of the Measured
3. Methods and Materials
3.1. Materials
3.2. Calibration for Sample Analysis
3.3. Analytical Methods
3.3.1. Oxygen of the Carbonate
3.3.2. Oxygen of the Phosphate
- (1)
- The matrix effect apparently is not largely relevant for calibration; this makes the use of silver phosphate standards not strictly necessary.
- (2)
- Practically, the new phosphate BOKU cannot substitute the use of the standards listed above because calibration with only one standard is risky. Sample BOKU could be used only for “quality control”.
3.4. Analytical Uncertainty
4. Results and Discussion
4.1. Results
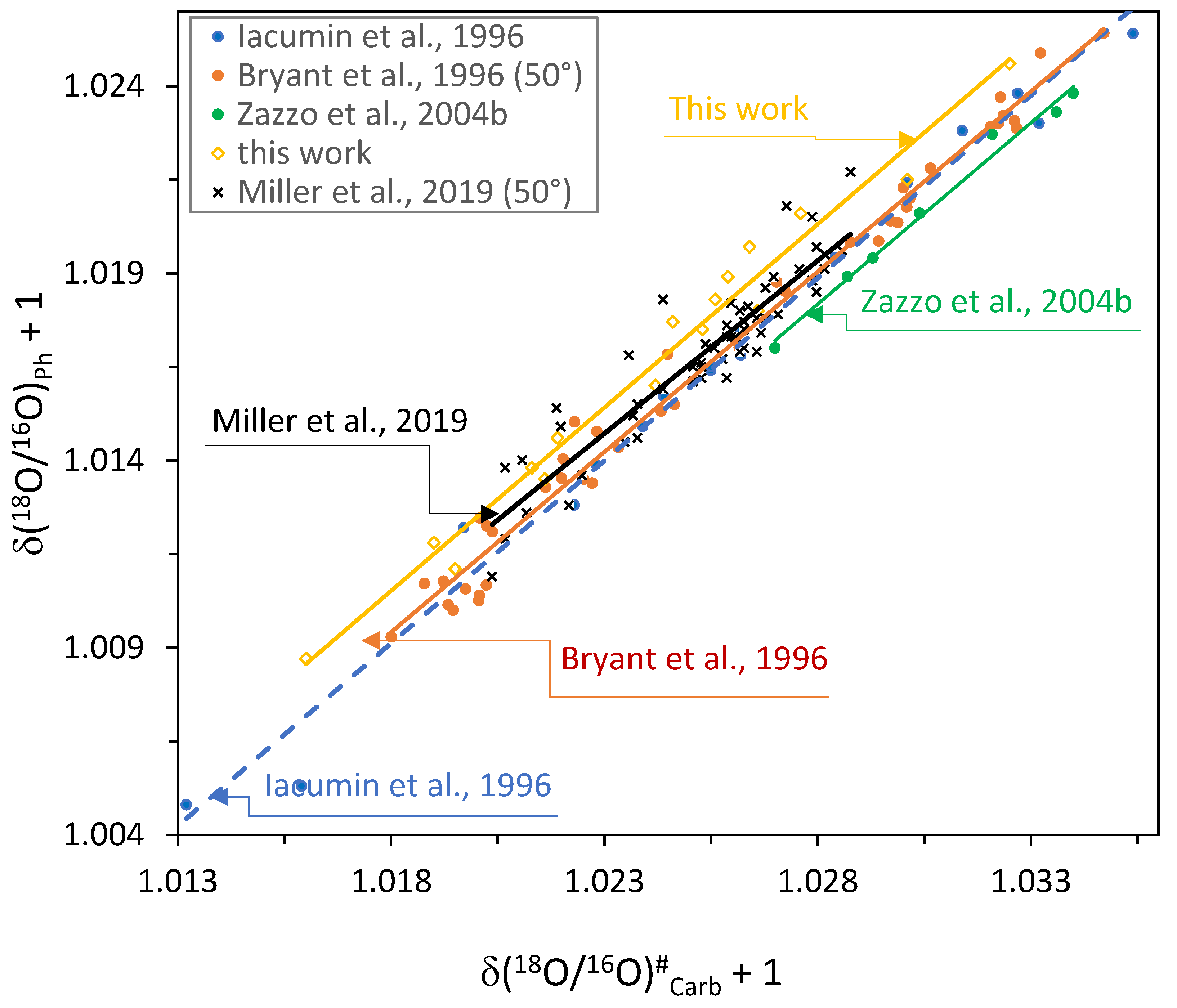
4.2. Comparison of + 1 on + 1 Regression Lines for Modern Samples
4.2.1. Data Used for Comparison
4.2.2. Data Obtained at Different Temperatures
4.2.3. The Regression Lines
4.2.4. The Role of Different Species and of Tooth (Enamel)/Bone Bioapatite on the Regressions
4.2.5. Final Considerations
- (a)
- Different standard materials used for calibration.
- (b)
- Difference in technical procedures.
- (c)
- Although the role of standard materials and procedures would need a separate approach, the effect of materials and procedures on the slope and elevation cannot be identified separately. As far as phosphate is concerned, there is no unequivocal answer because different authors frequently used different techniques and did not use international standards, or they did not always indicate the international standards to which the in-house standards used were referred. For instance, Iacumin et al. [21] determined oxygen of carbonate using in-house standards and Bryant et al. [20] used in-house standards for both carbonate and phosphate. Miller et al. [57] used in-house standards for determining the oxygen isotope ratio in precipitated Ag3PO4. (b) For phosphate, this point has been approached and discussed by several authors (e.g.,: [27,60] and references therein) to which we address the attention of the reader. For carbonate, point (b) has been briefly discussed above (Section 2 and Section 3.3.1).
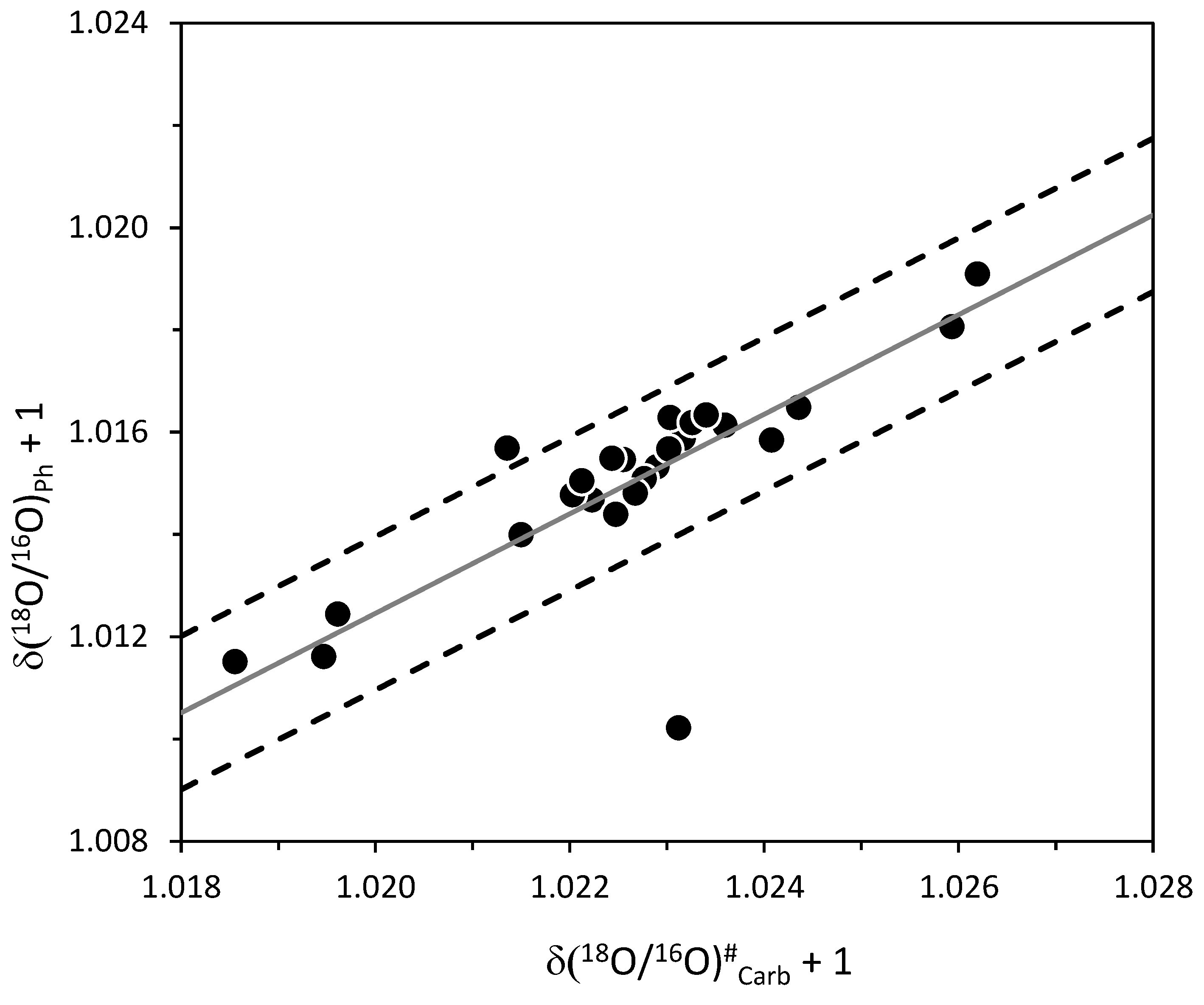
4.3. Identification of Potential Diagenetic Processes
5. Conclusions
- (1)
- We compared different on OLS regression lines of data obtained by several authors [20,21,56,57] on bioapatite of teeth (enamel) and bones of modern mammals (Ph = , and Carb = of bioapatite). The hypothesis that the slopes of the different regression lines are the same cannot be rejected (psame slope ≅ 0.9); on the contrary, the elevation varies significantly (psame elevation << 0.001). Thus, the data of the different authors considered do not belong to the same statistical population and they cannot be pooled to obtain a total or a common regression line. The new regression line we obtained using the procedure at Section 4.2.3 is the following: = 0.9787 + 0.0142, number of data couples = 16, standard error of regression = 0.00064.
- (2)
- Probably, the systematic difference in the elevation is prevalently due to different methods and standards used in the different laboratories.
- (3)
- The temperature of H3PO4 acid dissolution used for production for spectrometric analyses has some influence on the final isotopic results. Thus, it is better to perform analyses at the same temperature in all laboratories.
- (4)
- The scattering of the data around the values as calculated from the given values using the regression line reported above, is about 0.0015 (1.5‰) at the significance level of 0.05. In addition to other chemical and physical methods, the scattering of the data around the regression line may be used to select isotopic data which are not appropriate for palaeodiet and palaeclimatic reconstruction: values that fall outside the limiting array defined by the estimated values ± 1.5 ‰—where indicates the estimated value for a new sample i must be regarded with suspicion. This, of course, is valid only in the case the scientists use the same standard and analytical procedure as this paper.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. General
Appendix A.2. Transformation of Isotopic Value from Temperature T1 to Temperature T2
References
- LeGeros, R.Z. Apatites in biological systems. Prog. Cryst. Growth Charact. 1981, 4, 1–45. [Google Scholar] [CrossRef]
- Elliott, J.C. Calcium phosphate biominerals. Phosphates- Geochemical, geobiological, and materials importance. In Review in Mineralogy & Geochemistry; Mattew, J.K., Rakovan, J., Hughes, J.M., Eds.; Mineral Sociaety of America: Washington, DC, USA, 2002; Volume 48, pp. 427–454. [Google Scholar]
- Gómez-Morales, J.; Iafsco, M.; Delgado-López, J.M.; Sarda, S.; Drouet, C. Progress on the preparation of nanocrystalline apatites and surface characterization: Overview of fundamental and applied aspects. Prog. Cryst. Growth Charact. Mater 2013, 59, 1–46. [Google Scholar] [CrossRef]
- Del Sasso, G.; Asscher, Y.; Angelini, I.; Nodari, L.; Artioli, G. A universal curve of apatite crystallinity for the assessment of bone integrity and preservation. Sci. Rep. 2018, 8, 12025–12038. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.C.; van der Merwe, N.J. Isotopic evidence for early maize cultivation in New York state. Am. Antiq. 1977, 42, 238–242. [Google Scholar] [CrossRef]
- Ambrose, S.H.; Norr, L. Experimental evidence for the relationship of the carbon isotope ratio of whole diet and dietary protein to those of bone collagen and carbonate. In Prehistoric Human Bone; Lambert, J.B., Groupe, G., Eds.; Springer: New York, NY, USA, 1993; pp. 1–37. [Google Scholar]
- Iacumin, P.; Galli, E.; Cavalli, F.; Cecere, L. C4-consumers in Southern Europe: The case of Friuli, V.G. (NE-Italy) during early and middle Middle Ages. Am. J. Phys. Anthropol. 2014, 154, 561–574. [Google Scholar] [CrossRef] [PubMed]
- D’Angela, D.; Longinelli, A. Oxygen isotopes in living mammal’s bone phosphate: Further results. Chem. Geol. Isot. Geosci. Sect. 1990, 86, 75–82. [Google Scholar] [CrossRef]
- Pietsch, S.J.; Tütken, T. Oxygen isotope composition of North American bobcat (Lynx rufus) and puma (Puma concolor) bone phosphate: Implications for provenance and climate reconstruction. Isot. Environ. Health Stud. 2015. [Google Scholar] [CrossRef]
- Iacumin, P.; Di Matteo, A.; Usai, D.; Salvatori, S.; Venturelli, G. Stable isotope study on ancient population of Central Sudan: Insights on their diet and environment. Am. J. Phys. Antropol. 2016, 160, 498–518. [Google Scholar] [CrossRef]
- Guede, I.; Ortega, L.A.; Zuluaga, M.C.; Alonso-Olazabal, A.; Murelaga, X.; Pina, M.; Gutierrez, F.J.; Iacumin, P. Isotope analyses to explore diet and mobility in a medieval Muslim population at Tauste (NE Spain). PLoS ONE 2017, 12, e0176572. [Google Scholar] [CrossRef]
- Longinelli, A. Oxygen isotopes in mammal bone phosphate: A new tool for paleohydrological and paleoclimatological research? Geochim. Cosmochim. Acta 1984, 48, 385–390. [Google Scholar] [CrossRef]
- Luz, B.; Kolodny, Y.; Horowitz, M. Fractionation of oxygen isotopes beytween mammalian bone phosphate and environmental drinking water. Geochim. Cosmochim. Acta 1984, 48, 1689–1693. [Google Scholar] [CrossRef]
- Luz, B.; Kolodny, Y. Oxygen isotope variation in phosphate of biogenic apatite, IV. Mammal teeth and bones. Earth Planet. Sci. Lett. 1985, 75, 29–36. [Google Scholar] [CrossRef]
- Iacumin, P.; Venturelli, G. The δ18O of phosphate of ancient human biogenic apatite can really be used for quantitative palaeoclimate reconstructions? Eur. Sci. J. 2015, 11, 221–235. [Google Scholar]
- Pucéat, E.; Joachimski, M.M.; Bouilloux, A.; Monna, F.; Bonin, A.; Montreuil, S.; Morinière, P.; Hénard, S.; Mourin, J.; Dera, G. Revised phosphate-water fractionation equation reassessing paleotemperatures derived from biogenic apatite. Earth Planet. Sci. Lett. 2010, 298, 135–142. [Google Scholar] [CrossRef]
- Blake, E.; O’Neil, J.R.; Garcia, G.A. Oxygen isotope systematics of biologically mediated reactions of phosphates: I. Microbial degradation of organophosphorus compounds. Geochim. Cosmochim. Acta 1997, 61, 4411–4422. [Google Scholar] [CrossRef]
- Schoeninger, M.J.; Hallin, K.; Reeser, H.; Valley, J.W.; Fournelle, J. Isotopic alteration of mammalian tooth enamel. Intern. J. Osteoarchaeol. 2003, 13, 11–19. [Google Scholar] [CrossRef]
- Lee-Thorp, J.A. On isotopes and old bones. Archaeometry 2008, 50, 925–950. [Google Scholar] [CrossRef]
- Bryant, J.D.; Koch, P.L.; Froelich, P.N.; Showers, W.J.; Genna, B.J. Oxygen isotope partitioning between phosphate and carbonate in mammalia apatite. Geochim. Cosmochim. Acta 1996, 60, 5145–5148. [Google Scholar] [CrossRef]
- Iacumin, P.; Bocherens, H.; Mariotti, A.; Longinelli, A. Oxygen isotope analysis of co-existing carbonate and phosphate in biogenic apatite: A way to monitor diagenetic alteration of bone phosphate? Earth Planet Sci. Lett. 1996, 142, 1–6. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Winter, E.R.S.; Carlton, M.; Briscoe, H.V.A. The interchange of heavy oxygen between water and inorganic oxy-anions. J. Chem. Soc. 1940, 131–138. [Google Scholar] [CrossRef]
- Kolodny, Y.; Luz, B.; Navon, O. Oxygen isotope variations in phosphate of biogenic apatite. I. Fish bone apatite—Rechecking the rules of the game. Earth Planet. Sci. Lett. 1996, 64, 398–404. [Google Scholar] [CrossRef]
- Zazzo, A.; Lécuyer, C.; Mariotti, A. Experimentally-controlled carbon and oxygen isotope exchange between bioapatite and water under inorganic and microbially-mediated conditions. Geochim. Comochim. Acta 2004, 68, 1–12. [Google Scholar] [CrossRef]
- O’Neil, J.R.; Roe, L.J.; Reinhard, E.; Blake, R.E. A rapid and precise method of oxygen isotope analysis of biogenic phosphate. Isr. J. Earth Sci. 1994, 43, 203–212. [Google Scholar]
- Vennemann, T.W.; Fricke, H.C.; Blake, R.E.; O’Neil, J.R.; Colmann, A. Oxygen isotope analysis of phosphates: A comparison of techniques for analysis of Ag3PO4. Chem. Geol. 2002, 185, 321–336. [Google Scholar] [CrossRef]
- McCrea, J.M. On the isotopic chemistry of carbonate and paleotemperature scale. J. Chem. Phys. 1950, 18, 849–857. [Google Scholar] [CrossRef]
- Keisch, B.; Kennedy, J.W.; Wahl, A.C. The exchange of oxygen between phosphoric acid and water. J. Chem. Soc. 1958, 80, 4778–4782. [Google Scholar] [CrossRef]
- Jameson, R.F. The composition of “strong” phosphoric acids. J. Chem. Soc., Part 1 1959, 1, 752–759. [Google Scholar] [CrossRef]
- Sarma, T.; Clayton, R.N. Measurement of 18O/16O ratios of total oxygen of carbonates. Geochim. Cosmochim. Acta 1965, 29, 1347–1353. [Google Scholar] [CrossRef]
- Wachter, E.A.; Hayes, J.M. Exchange of oxygen isotopes in carbon dioxide-phosphoric acid system. Chem. Geol. 1985, 52, 365–374. [Google Scholar]
- Swart, P.K.; Burns, S.J.; Leder, J.J. Fractionation of stable isotopes of oxygen and carbon in carbon dioxide during the reaction of calcite in acid as a function of temperature and technique. Chem. Geol. 1991, 86, 89–96. [Google Scholar] [CrossRef]
- Boettcher, M.E. 18O/16O and 13C/12C fractionation during the reaction of carbonates with phosphoric acid: Effect of cation substitution and reaction temperature. Isot. Environ. Health Stud. 1996, 32, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.L.; Tuross, N.; Fogel, M.L. The Effects of Sample Treatment and Diagenesis on the Isotopic Integrity of Carbonate in Biogenic Hydroxylapatite. J. Archaeol. Sci. 1997, 24, 417–429. [Google Scholar] [CrossRef]
- Garvie-Lok, S.J.; Varney, T.L.; Katzenberg, M.A. Preparation of bone carbonate for stable isotope analysis: The effect of treatment time and acid concentration. J. Archaeol. Sci. 2004, 31, 761–776. [Google Scholar] [CrossRef]
- Kim, S.-T.; Mucci, A.; Taylor, B.E. Phosphoric acid fractionation for calcite and aragonite between 25 and 75 °C: Revisited. Chem. Geol. 2007, 246, 135–146. [Google Scholar] [CrossRef]
- Wendeberg, M.; Richter, J.M.; Rothe, M.; Brand, W. δ18O anchoring to VPDB: Calcite digestion with 18O-adjusted ortho-phosphoric acid. Rapid Comm. Mass Spectrom. 2011, 25, 851–860. [Google Scholar] [CrossRef]
- Pellegrini, M.; Snoeck, C. Comparing bioapatite carbonate pre-treatment for isotopic mesurements: Part 2–Impact on carbon and oxygen isotope composition. Chem. Geol. 2016, 420, 88–96. [Google Scholar] [CrossRef]
- Passey, B.H.; Cerling, T.E.; Levin, N.E. Temperature dependence of oxygen isotope acid fractionation for modern and fossil tooth enamels. Rapid Comm. Mass Spectrom. 2007, 21, 2853–2859. [Google Scholar] [CrossRef]
- Wiedemann-Bidlack, F.B.; Colman, A.S.; Fogel, M.L. Phosphate oxygen isotope analysis on microsamples of bioapatite: Removal of organic contamination and minimization of sample size. Rapid Commun. Mass Spectrom. 2008, 22, 1807–1816. [Google Scholar] [CrossRef]
- Pestle, W.J.; Crowley, B.E.; Weirauch, M.T. Quantifying inter-laboratory variability in stable isotope analysis of ancient skeletal remains. PLoS ONE 2014, 9, e102844. [Google Scholar] [CrossRef]
- Crowley, B.E.; Wheatley, P.V. To bleach or not to bleach? Comparing treatment methods for isolating biogenic carbonate. Chem. Geol. 2014, 381, 234–242. [Google Scholar] [CrossRef]
- Snoeck, C.; Pellegrini, M. Comparing bioapatite carbonate pre-treatment for isotopic mesurements: Part 1—Impact on structure and chemical composition. Chem. Geol. 2015, 417, 394–403. [Google Scholar] [CrossRef]
- Demény, A.; Gugora, A.D.; Kesiar, D.; Lécuyer, C.; Fourel, F. Stable isotope analyses of the carbonate component of bones and teeth: The need of method standardization. J. Archaeol. Sci. 2019, 109, 104979. [Google Scholar] [CrossRef]
- Werner, R.A.; Brand, W.A. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Comm. Mass Spectrom. 2001, 15, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Gonfiantini, R.; Stichler, K.; Rozanski, K. Standards and intercomparison materials distributed by the International Atomic Energy Agency for stable isotope measurements. In References and intercomparison materials for stable isotopes of light elements. In Proceedings of the consultants meeting IAEA-TECDOC-825, Vienna, Austria, 1–3 December 1993. [Google Scholar]
- Halas, S.; Skrzypec, G.; Meier-Augenstein, W.; Pelc, A.; Kemp, H.F. Inter-laboratory calibration of new silver orthophosphate comparison materials for the stable oxygen isotope analysis of phosphates. Rapid Comm. Mass Spectrom. 2010, 25, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Watzinger, A.; Schott, K.; Hood-Nowotny, R.; Tamburini, F.; Arppe, L.; Cristini, D.; Knöller, K.; Skrzypek, G. New Ag3PO4 comparison material for stable oxygen isotope analysis. Rapid. Comm.Mass. Spectrom. 2021, 35, e9101. [Google Scholar] [CrossRef]
- Stephan, E. Oxygen isotope analysis of animal bone phosphate: Method refinement, influence of consolidants, and reconstruction of palaeotemperatures for Holocene sites. J. Archaeol. Sci. 2000, 27, 523–535. [Google Scholar] [CrossRef]
- Brand, W.; Coplen, T.B.; Aerts-Bijma, A.T.; Boelke, J.K.; Gehre, M.; Grilmann, H.; Groening, M.; Jsansen, H.G.; Meijr, H.A.J.; Mroczkowski, S.J.; et al. Comprehensive inter-laboratory calibration of reference materials for δ18O versus VSMOW using various on-line high-temperature techniques. Rapid Comm. Mass Spectrom. 2009, 23, 999–1019. [Google Scholar] [CrossRef]
- Kornexl, B.E.; Werner, R.E.; Gehre, M. Standardization for oxygen isotope ratio measurement—Still an unsolved problem. Rapid Comm. Mass. Spectrom. 1999, 13, 1248–1251. [Google Scholar] [CrossRef]
- Fourel, F.; Martineau, F.; Lecuyer, C.; Kupta, H.-J.; Lange, L.; Ojeimi, C.; Seed, M. 18O/16O ratio measurements of inorganic and organic materials by elemental analysis-pyrolysis-isotope ratio mass spectrometry continuous-flow techniques. Rapid Commun. Mass Spectrom. 2011, 25, 2691–2696. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods; Iowa State University Press: Ames, IA, USA, 1968. [Google Scholar]
- Taylor, J.R. An Introduction to Error Analysis, 2nd ed.; Univ Sci. Books: Sausalito, CA, USA, 1997. [Google Scholar]
- Zazzo, A.; Lécuyer, C.; Sheppard, S.M.F.; Grandjean, P.; Mariotti, A. Diagenesis and reconstruction of paleoenvironments: A method to restore original δ18O values of carbonate and phosphate from fossil tooth enamel. Geochim. Cosmochim. Acta 2004, 68, 2245–2258. [Google Scholar] [CrossRef]
- Miller, H.; Chenery, C.; Lamb, A.L.; Sloane, H.; Carden, R.F.; Atici, L.; Sykes, N. The relationship between the phosphate and structural carbonate fractionation of fallow deer bioapatite in tooth enamel. Rapid Comm. Mass Spectrom. 2019, 33, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice Hall: London, UK, 2010. [Google Scholar]
- Ivanov, K. The development of the concepts of homeothermy and thermoregulation. J. Therm. Biol. 2006, 31, 24–29. [Google Scholar] [CrossRef]
- Chenery, C.A.; Pashley, V.; Lamb, A.L.; Sloane, H.J.; Evans, J.A. The oxygen isotope relationship between the phosphate and structural carbonate fractions of human bioapatite. Rapid Commun. Mass Spectrom. 2012, 26, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.F. Effect of temperature on the oxygen isotope composition for carbon dioxide (δCO218O) prepared from carbonate minerals by reaction with phosphoric acid: An example of the rhombohedral CaCO3- MgCO3 group minerals. Geochim. Cosmochim. Acta 2011, 74, 6406–6421. [Google Scholar] [CrossRef]
| Provenance | Species | VSMOW | + 1 | 103× VSMOW | + 1 |
|---|---|---|---|---|---|
| Spanish Sahara | Canis aureus 1 | 21.5 | 1.0215 | 30.1 | 1.0301 |
| Southern Spain | Vulpes vulpes 1 | 18.3 | 1.0183 | 25.6 | 1.0256 |
| Southern Spain | Vulpes vulpes 1 | 20.6 | 1.0206 | 27.6 | 1.0276 |
| Marocco | Vulpes zerda 1 | 24.6 | 1.0246 | 32.5 | 1.0325 |
| Southern Spain | Vulpes vulpes 1 | 19.7 | 1.0197 | 26.4 | 1.0264 |
| Central Spain | Vulpes vulpes 1 | 17.7 | 1.0177 | 24.6 | 1.0246 |
| Central Italy | Vulpes vulpes 1 | 18.9 | 1.0189 | 25.9 | 1.0259 |
| Central Italy | Vulpes vulpes 1 | 18.0 | 1.0180 | 26.6 | 1.0266 |
| Siberia | Vulpes lagopus 2 | 8.7 | 1.0087 | 16.0 | 1.0160 |
| Siberia | Alces alces 2 | 17.5 | 1.0175 | 25.3 | 1.0253 |
| Siberia | Alces alces 2 | 16.0 | 1.0160 | 24.2 | 1.0242 |
| Siberia | Alces alces 2 | 13.8 | 1.0138 | 21.3 | 1.0213 |
| Siberia | Alces alces 2 | 11.1 | 1.0111 | 19.5 | 1.0195 |
| Siberia | Alces alces 2 | 14.6 | 1.0146 | 21.9 | 1.0219 |
| Siberia | Alces alces 2 | 11.8 | 1.0118 | 19.0 | 1.0190 |
| Siberia | Alces alces 2 | 13.5 | 1.0135 | 21.6 | 1.0216 |
| Iacumin et al. (1996) [20] | Bryant et al. (1996) [21] | Zazzo et al. (2004b) [56] | Miller et al. (2019) [57] | This Work | |
|---|---|---|---|---|---|
| s(Carb), s(Ph) u(Carb), u(Ph) | 0.2‰, 0.2‰ nd | 0.1‰, 0.1‰ nd | 0.2‰, 0.2‰ nd | 0.08‰, 0.23‰ (?) nd | 0.15‰, 0.20‰ 0.25‰, 0.35‰ |
| Number of data | 17 | 42 | 7 | 55 | 16 |
| Interval of X | 1.0132–1.0354 | 1.0180–1.0347 | 1.0270–1.0340 | 1.0204–1.0288 | 1.0160–1.0325 |
| Interval of Y | 1.0048–1.0254 | 1.0093–1.0254 | 1.0170–1.0238 | 1.0109–1.0217 | 1.0087–1.0246 |
| Normality test for X and Y | |||||
| W, A for X | 0.830, 0.797 | 0.002, < 0.001 | 0.733, 0.787 | 0.003, 0.001 | 0.984, 0.850 |
| W, A for Y | 0.344, 0.428 | 0.008, 0.007 | 0.607, 0.604 | 0.271, 0.101 | 0.995, 0.928 |
| Regression line OLS, Y = B X + A | |||||
| A ± s(A) | 0.0164 ± 0.0314 | 0.0297 ± 0.0189 | 0.0230 ± 0.0502 | 0.0708 ± 0.0541 | 0.0142 ± 0.0399 |
| B ± s(B) | 0.9751 ± 0.0306 | 0.9625 ± 0.0184 | 0.9681 ± 0.0487 | 0.9227 ± 0.0527 | 0.9787 ± 0.0390 |
| s(yx) | 0.00073 | 0.00061 | 0.00031 | 0.00085 | 0.00064 |
| R2 | 0.985 | 0.986 | 0.987 | 0.853 | 0.978 |
| p(A = 0) | 0.61 | 0.12 | 0.46 | 0.20 | 0.73 |
| Normality test for residuals | |||||
| Shapiro-Wilk test | 0.26 | 0.82 | 0.26 | 0.001 | 0.33 |
| Anderson-Darling test | 0.12 | 0.74 | 0.27 | < 0.001 | 0.45 |
| Autocorrelation Durbin-Watson test | 0.99 | 0.03 | 0.74 | 0.73 | 0.43 |
| Homoscedasticity Breusch-Pagan test | 0.13 | 0.03 | 0.35 | 0.17 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacumin, P.; Rossi, M.; Selmo, E.; Venturelli, G. Oxygen Isotopes in Carbonate and Phosphate of Modern Mammal Bioapatite: New Data and Critical Revision after about 25 Years from the First Recognitions. Minerals 2022, 12, 1204. https://doi.org/10.3390/min12101204
Iacumin P, Rossi M, Selmo E, Venturelli G. Oxygen Isotopes in Carbonate and Phosphate of Modern Mammal Bioapatite: New Data and Critical Revision after about 25 Years from the First Recognitions. Minerals. 2022; 12(10):1204. https://doi.org/10.3390/min12101204
Chicago/Turabian StyleIacumin, Paola, Mattia Rossi, Enricomaria Selmo, and Giampiero Venturelli. 2022. "Oxygen Isotopes in Carbonate and Phosphate of Modern Mammal Bioapatite: New Data and Critical Revision after about 25 Years from the First Recognitions" Minerals 12, no. 10: 1204. https://doi.org/10.3390/min12101204
APA StyleIacumin, P., Rossi, M., Selmo, E., & Venturelli, G. (2022). Oxygen Isotopes in Carbonate and Phosphate of Modern Mammal Bioapatite: New Data and Critical Revision after about 25 Years from the First Recognitions. Minerals, 12(10), 1204. https://doi.org/10.3390/min12101204









