Zr-Rich Eudialyte from the Lovozero Peralkaline Massif, Kola Peninsula, Russia
Abstract
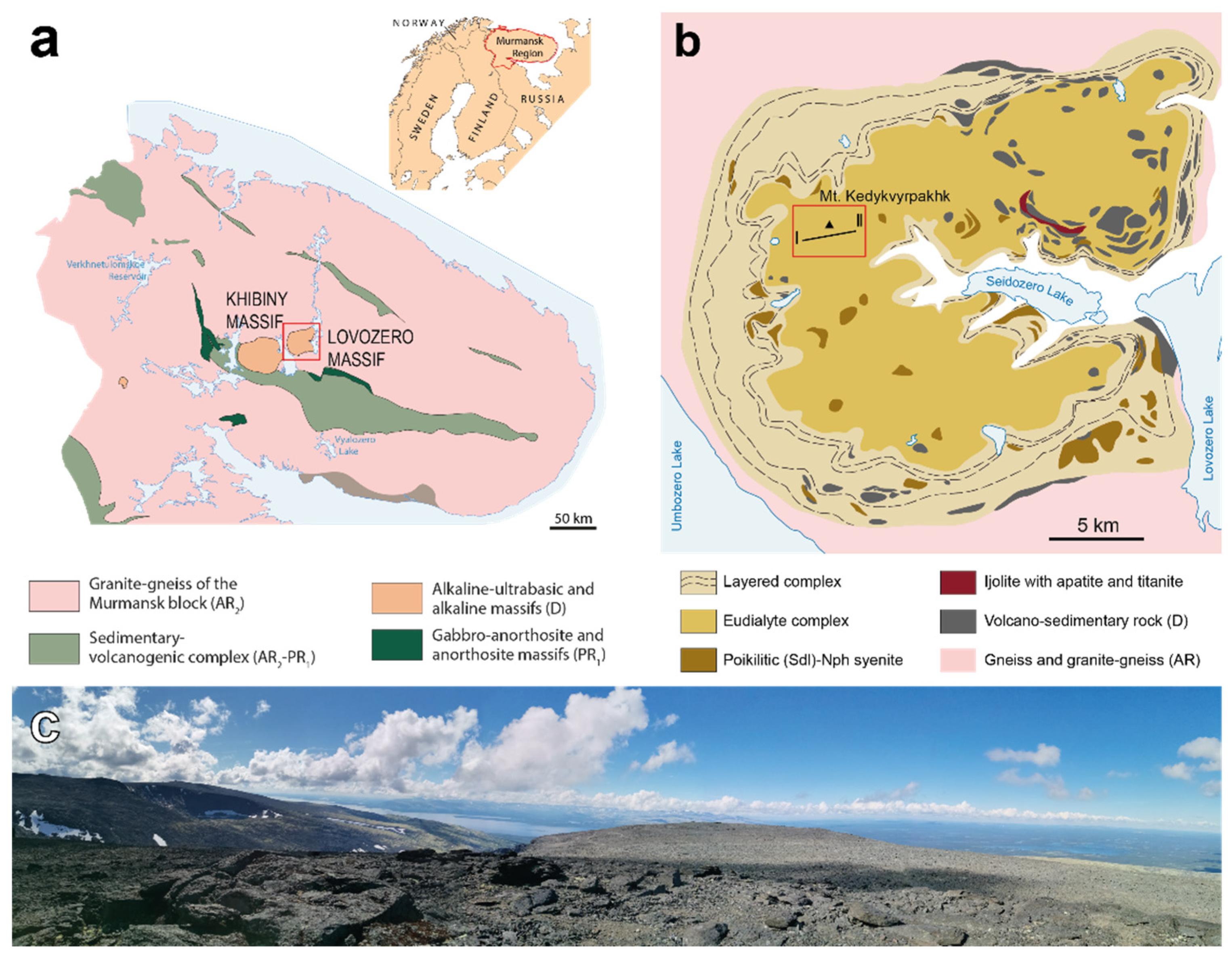
1. Introduction
2. Geological Setting
3. Materials and Methods
4. Results
4.1. Chemical Composition
4.2. Crystal Structure: General Scheme
4.3. Crystal Structure: LV-153/178
M4(Si0.54□0.46)Σ1.00Z1(Zr3.00)Si24O72X1,2ACl2.11X2A,B(H2O)3.36
M4(Si0.54□0.46)Σ1.00Z1(Zr3.00)Si24O68.34(OH)3.66X1,2ACl1.17X2A,B(H2O)1.42
4.4. Crystal Structure: LV-117/226
M4(Si0.85□0.15)Σ1.00Z1(Zr3.00)Si24O72X2CCl1.73X2A,B(H2O)6.12
M4(Si0.85□0.15)Σ1.00Z1(Zr3.00)Si24O68.70(OH)3.30X2CCl1.34X2A,B(H2O)4.66
5. Discussion
6. Conclusions
- (1)
- M4□ + M12Zr4+ ↔ M4Si4+ + M12Ca2+
- (2)
- M4Al3+ + M1Zr4+ ↔ M4Si4+ + M1REE3+
- (3)
- M3□ + M4Al3+ + M1Zr4+ ↔ M3Nb5+ + M4□ + M1Ca2+
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnsen, O.; Ferraris, G.; Gault, R.A.; Grice, J.D.; Kampf, A.R.; Pekov, I.V. The nomenclature of eudialyte-group minerals. Can. Mineral. 2003, 41, 785–794. [Google Scholar] [CrossRef]
- Kogarko, L.; Nielsen, T.F.D. Chemical Composition and Petrogenetic Implications of Eudialyte-Group Mineral in the Peralkaline Lovozero Complex, Kola Peninsula, Russia. Minerals 2020, 10, 1036. [Google Scholar] [CrossRef]
- Mikhailova, J.A.; Pakhomovsky, Y.A.; Panikorovskii, T.L.; Bazai, A.V.; Yakovenchuk, V.N. Eudialyte Group Minerals from the Lovozero Alkaline Massif, Russia: Occurrence, Chemical Composition, and Petrogenetic Significance. Minerals 2020, 10, 1070. [Google Scholar] [CrossRef]
- Mitchell, R.; Liferovich, R. Subsolidus deuteric/hydrothermal alteration of eudialyte in lujavrite from the Pilansberg alkaline complex, South Africa. Lithos 2006, 91, 352–372. [Google Scholar] [CrossRef]
- Atanasova, P.; Marks, M.A.W.; Heinig, T.; Krause, J.; Gutzmer, J.; Markl, G. Distinguishing Magmatic and Metamorphic Processes in Peralkaline Rocks of the Norra Kärr Complex (Southern Sweden) Using Textural and Compositional Variations of Clinopyroxene and Eudialyte-group Minerals. J. Petrol. 2017, 58, 361–384. [Google Scholar] [CrossRef]
- Schilling, J.; Marks, M.A.W.; Wenzel, T.; Markl, G. Reconstruction of magmatic to subsolidus processes in an agpaitic system using eudialyte textures and composition: A case study from Tamazeght, Morocco. Can. Mineral. 2009, 47, 351–365. [Google Scholar] [CrossRef]
- Johnsen, O.; Grice, J.D. The crystal chemistry of the eudialyte group. Can. Mineral. 1999, 37, 865–891. [Google Scholar]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Aksenov, S.M. Minerals of the Eudialyte Group: Crystal Chemistry, Properties, Genesis; Nizhegorodskiy State University: Nizhny Novgorod, Russia, 2012. (In Russian) [Google Scholar]
- The Official IMA-CNMNC List of Mineral Names Updated List of IMA-Approved Minerals. Available online: http://cnmnc.main.jp (accessed on 15 July 2021).
- Marks, M.A.W.; Markl, G. A global review on agpaitic rocks. Earth-Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Netschelyustov, G.N.; Rastsvetaeva, R.K. Alluaivite Na19(Ca,Mn)6(Ti,Nb)3Si26O74Cl·2H2O—A new titanosilicate of of eudialyte–like structure. Zap. RMO 1990, 119, 117–120. [Google Scholar]
- Chukanov, N.V.; Pekov, I.V.; Zadov, A.E.; Korovushkin, V.V.; Ekimenkova, I.A.; Rastsvetaeva, R.K. Ikranite (Na,H3O)15(Ca,Mn, REE)62Zr3 (_,Zr)(_,Si) Si24 O66(O,OH)6 Cl_ nH2O and raslakite Na15 Ca3 Fe3 (Na,Zr)3Zr3(Si,Nb)(Si25 O73)(OH,H2O)3(Cl, OH)—New eudialyte-group minerals from the Lovozero Massif. Zap. RMO 2003, 132, 22–33. (In Russian) [Google Scholar]
- Chukanov, N.V.; Aksenov, S.M.; Pekov, I.V.; Belakovskiy, D.I.; Vozchikova, S.A.; Britvin, S.N. Sergevanite, Na15(Ca3Mn3)(Na2Fe)Zr3Si26O72(OH)3·H2O, a new eudialyte-group mineral from the Lovozero alkaline massif, Kola Peninsula. Can. Mineral. 2020, 58, 421–436. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Nechelyustov, G.N.; Rastsvetaeva, R.K. Voronkovite, Na15(Na,Ca,Ce)3(Mn,Ca)3Fe3Zr3Si26O72(OH,O)4Cl · H2O, a new mineral species of the eudialyte group from the Lovozero alkaline pluton, Kola Peninsula, Russia. Geol. Ore Depos. 2009, 51, 750–756. [Google Scholar] [CrossRef]
- Borst, A.M.; Finch, A.A.; Friis, H.; Horsburgh, N.J.; Gamaletsos, P.N.; Goettlicher, J.; Steininger, R.; Geraki, K. Structural state of rare earth elements in eudialyte-group minerals. Mineral. Mag. 2020, 84, 19–34. [Google Scholar] [CrossRef]
- McLemore, V.; Reservation, M.I. Background and perspectives on the Pajarito mountain yttrium-zirconium deposit, Mescalero Apache Indian Reservation, Otero County, New Mexico. Am. Mineral. 1990, 72, 801–811. [Google Scholar]
- Kogarko, L.N. Ore-forming potential of alkaline magmas. Lithos 1990, 26, 167–175. [Google Scholar] [CrossRef]
- Sørrensen, H. Agpaitic nepheline syenites: A potential source of rare elements. Appl. Geochem. 1992, 7, 417–427. [Google Scholar] [CrossRef]
- Schilling, J.; Marks, M.A.W.; Wenzel, T.; Vennemann, T.; Horváth, L.; Tarassoff, P.; Jacob, D.E.; Markl, G. The magmatic to hydrothermal evolution of the intrusive Mont Saint-Hilaire Complex: Insights into the late-stage evolution of peralkaline rocks. J. Petrol. 2011, 52, 2147–2185. [Google Scholar] [CrossRef]
- Sjöqvist, A.; Cornell, D.; Andersen, T.; Erambert, M.; Ek, M.; Leijd, M. Three compositional varieties of rare-earth element ore: Eudialyte-group minerals from the Norra Kärr alkaline complex, Southern Sweden. Minerals 2013, 3, 94–120. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Müller, T.; Yurramendi, L. Rare Earths and the Balance Problem: How to Deal with Changing Markets? J. Sustain. Metall. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- Bogatyreva, E.V.; Khokhlova, O.V.; Muraveva, E.A.; Dolgov, A.V. Thermodynamic estimation of the probability of chemical reactions during alkaline decomposition of eudialyte concentrate. Tsvetnye Met. 2018, 46–56. [Google Scholar] [CrossRef]
- Stark, T.; Silin, I.; Wotruba, H. Mineral Processing of Eudialyte Ore from Norra Kärr. J. Sustain. Metall. 2017, 3, 32–38. [Google Scholar] [CrossRef]
- Davris, P.; Stopic, S.; Balomenos, E.; Panias, D.; Paspaliaris, I.; Friedrich, B. Leaching of rare earth elements from eudialyte concentrate by suppressing silica gel formation. Miner. Eng. 2017, 108, 115–122. [Google Scholar] [CrossRef]
- Coulson, I.M. Post-magmatic alteration in eudialyte from the North Qôroq centre, South Greenland. Mineral. Mag. 1997, 61, 99–109. [Google Scholar] [CrossRef][Green Version]
- Marks, M.A.W.; Eggenkamp, H.G.M.; Atanasova, P.; Mundel, F.; Kümmel, S.; Hagen, M.; Wenzel, T.; Markl, G. Review on the Compositional Variation of Eudialyte-Group Minerals in the Ilímaussaq Complex (South Greenland). Minerals 2020, 10, 1011. [Google Scholar] [CrossRef]
- Schønwandt, H.K.; Barnes, G.B.; Ulrich, T. A Description of the World-Class Rare Earth Element Deposit, Tanbreez, South Greenland. In Rare Earths Industry; Elsevier: New York, NY, USA, 2016; pp. 73–85. [Google Scholar]
- Bussen, I.V.; Sakharov, A.S. Petrology of the Lovozero Alkaline Massif; Nauka: Leningrad, Russia, 1972. (In Russian) [Google Scholar]
- Kramm, U.; Kogarko, L.N. Nd and Sr isotope signatures of the Khibina and Lovozero agpaitic centres, Kola Alkaline province, Russia. Lithos 1994, 32, 225–242. [Google Scholar] [CrossRef]
- Vlasov, K.A.; Kuzmenko, M.Z.; Eskova, E.M. The Lovozero Alkaline Massif; Izdatel’stvo Akademii Nauk: Moscow, Russia, 1959. (In Russian) [Google Scholar]
- Gerasimovsky, V.I.; Volkov, V.P.; Kogarko, L.N.; Polyakov, A.I.; Saprygina, T.V.; Balashov, Y.A. Geochemistry of Lovozero Alkaline Massif; Nauka: Leningrad, Russia, 1966. (In Russian) [Google Scholar]
- Kalinkin, M.M. New data on deep structure and tectonics of the Lovozero alkaline pluton. In Regional Geology, Metallogeny, and Geophysics; Kola Branch of the Academy of Sciences of the USSR: Apatity, Russia, 1974; pp. 55–58. (In Russian) [Google Scholar]
- Korchak, Y.A.; Men’shikov, Y.P.; Pakhomovskii, Y.A.; Yakovenchuk, V.N.; Ivanyuk, G.Y. Trap formation of the Kola peninsula. Petrology 2011, 19, 87–101. [Google Scholar] [CrossRef]
- Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Yakovenchuk, V.N. Eudialyte-group minerals in rocks of Lovozero layered complex at Mt. Karnasurt and Mt. Kedykvyrpakhk. Geol. Ore Depos. 2015, 57, 600–613. [Google Scholar] [CrossRef]
- Dolivo-Dobrovolsky, D.D. MINAL Version, Free Software 2016. Available online: http://www.dimadd.ru (accessed on 8 July 2013).
- StatSoft. Inc. Statistica 8 2008. Available online: https://statistica.software.informer.com (accessed on 8 June 2009).
- Agilent Technologies CrysAlis CCD and CrysAlis RED; Oxford Diffr. Ltd.: Yarnton, UK, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Putz, H.; Brandernburg, K. Diamond‒Crystal and Molecular Structure Visualization 2014. Available online: https://www.crystalimpact.com/diamond/ (accessed on 4 September 2021).
- Golyshev, V.M.; Simonov, V.I.; Belov, N.V. About the crystal structure of eudialyte. Crystallography 1971, 16, 93–98. [Google Scholar]
- Giuseppetti, G.; Mazzi, F.; Tadini, C. The crystal structure of eudialyte. TMPM Tschermaks Mineral. und Petrogr. Mitteilungen 1971, 16, 105–127. [Google Scholar] [CrossRef]
- Ferraris, G.; Makovicky, E.; Merlino, S. Crystallography of Modular Materials; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Khomyakov, A.P.; Nechelyustov, G.N.; Rastvetaeva, R.K. Labyrinthite (Na,K,Sr)35 Ca12Fe3Zr6TiSi51O144 (O,OH,H2O)9Cl3, a new mineral with the modular eudialyte-like structure from Khibiny alkaline massif, Kola Peninsula. Zap. RMO 2006, 135, 38–48. (In Russian) [Google Scholar]
- Khomyakov, A.P.; Nechelyustov, G.N.; Arakcheeva, A.V. Rastsvetaevite, Na27K8Ca12Fe3Zr6Si4[Si3O9]4[Si9O27]4(O,OH,H2O)6Cl2, a new mineral with a modular eudialyte-like structure and crystal-chemical systematics of the eudialyte group. Zap. RMO 2006, 135, 49–65. (In Russian) [Google Scholar]
- Pfaff, K.; Wenzel, T.; Schilling, J.; Marks, M.A.W.; Markl, G. A fast and easy-to-use approach to cation site assignment for eudialyte-group minerals. Neues Jahrb. Mineral.-Abh. 2010, 187, 69–81. [Google Scholar] [CrossRef]
- Pekov, I.V. Lovozero Massif: History of Investigations, Pegmatites, Minerals; Ocean Pictures Ltd.: Moscow, Russia, 2000. (In Russian) [Google Scholar]
- Féménias, O.; Coussaert, N.; Brassinnes, S.; Demaiffe, D. Emplacement processes and cooling history of layered cyclic unit II-7 from the Lovozero alkaline massif (Kola Peninsula, Russia). Lithos 2005, 83, 371–393. [Google Scholar] [CrossRef]
- Hamilton, D.L. Nephelines as Crystallization Temperature Indicators. J. Geol. 1961, 69, 321–329. [Google Scholar] [CrossRef]
- Osipov, A.S.; Antonov, A.A.; Panikorovskii, T.L.; Zolotarev, A.A. Hydrated CO3-Bearing Analog of Manganoeudialyte from Alkali Pegmatites of the Konder Pluton, Khabarovsk Krai. Geol. Ore Depos. 2018, 60, 726–735. [Google Scholar] [CrossRef]
- Pakhomovsky, Y.A.; Panikorovskii, T.L.; Yakovenchuk, V.N.; Ivanyuk, G.Y.; Mikhailova, J.A.; Krivovichev, S.V.; Bocharov, V.N.; Kalashnikov, A.O. Selivanovaite, NaTi3(Ti,Na,Fe,Mn)4[(Si2O7)2O4(OH,H2O)4]·nH2O, a new rock-forming mineral from the eudialyte-rich malignite of the Lovozero alkaline massif (Kola Peninsula, Russia). Eur. J. Mineral. 2018, 30, 525–535. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Nechelyustov, G.N.; Rastsvetaeva, R.K.; Rozenberg, K.A. Davinciite, Na12K3Ca6Zr3(Si26O73OH)Cl2, a New K,Na-Ordered mineral of the eudialyte group from the Khibiny Alkaline Pluton, Kola Peninsula, Russia. Geol. Ore Depos. 2013, 55, 532–540. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Aksenov, S.M.; Rozenberg, K.A. Crystal structure and genesis of the hydrated analog of rastsvetaevite. Crystallogr. Rep. 2015, 60. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V. New Data on the Isomorphism in Eudialyte-Group Minerals. X: Crystal Structure of the Intermediate Member of the Raslakite–Sergevanite Series. Crystallogr. Rep. 2021, 66, 120–125. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V. New Data on the Isomorphism in Eudialyte-Group Minerals. 2. Crystal-Chemical Mechanisms of Blocky Isomorphism at the Key Sites. Minerals 2020, 10, 720. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Kabanova, N.A.; Chukanov, N.V.; Panikorovsky, T.L.; Blatov, V.A.; Krivovichev, S.V. The role of local heteropolyhedral substitutions on the stoichiometry, topology, and ion-migration paths in the eudialyte-type structures: A quantitative analysis. IUCrJ 2021. in print. [Google Scholar]
- Rastsvetaeva, R.K.; Chukanov, N.V. Classification of eudialyte-group minerals. Geol. Ore Depos. 2012, 54, 487–497. [Google Scholar] [CrossRef]








| Sample | 117/226 * Core | 153/178 Rim | 156/36 Core | 156/36 Rim | 169/143 Core | 169/143 Rim | 117/226 Core | 153/178 Rim |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 48.85 | 51.01 | 52.60 | 52.12 | 49.51 | 51.59 | 48.85 | 51.01 |
| TiO2 | 0.54 | 0.58 | 0.59 | 0.46 | 0.55 | 0.52 | 0.54 | 0.58 |
| Al2O3 | 0.24 | 0.16 | 0.35 | 0.23 | 0.16 | 0.07 | 0.24 | 0.16 |
| FeO | 1.51 | 2.23 | 2.96 | 2.87 | 1.97 | 2.00 | 1.51 | 2.23 |
| MnO | 3.11 | 2.19 | 2.42 | 2.68 | 3.09 | 3.08 | 3.11 | 2.19 |
| MgO | b.d | 0.07 | 0.10 | 0.08 | b.d | b.d | b.d | 0.07 |
| CaO | 6.21 | 7.99 | 7.33 | 8.17 | 7.90 | 8.32 | 6.21 | 7.99 |
| Na2O | 8.43 | 14.61 | 5.43 | 5.15 | 17.23 | 16.58 | 8.43 | 14.61 |
| K2O | 0.24 | 0.30 | 0.59 | 0.76 | 0.23 | 0.17 | 0.24 | 0.30 |
| SrO | 2.74 | 1.83 | 2.04 | 2.59 | 2.37 | 1.53 | 2.74 | 1.83 |
| Y2O3 | 0.41 | b.d | 0.61 | 0.66 | b.d | b.d | 0.41 | b.d |
| ZrO2 | 17.14 | 12.78 | 14.04 | 13.09 | 11.88 | 11.08 | 17.14 | 12.78 |
| Nb2O5 | 0.36 | 0.87 | 0.60 | 0.86 | 1.03 | 0.55 | 0.36 | 0.87 |
| BaO | 0.19 | b.d | 0.13 | 0.23 | 0.34 | 0.47 | 0.19 | b.d |
| La2O3 | 0.30 | 0.24 | 0.26 | 0.35 | 0.28 | 0.41 | 0.30 | 0.24 |
| Ce2O3 | 0.54 | 0.83 | 0.59 | 0.84 | 0.84 | 1.20 | 0.54 | 0.83 |
| Nd2O3 | 0.39 | 0.35 | 0.30 | 0.32 | 0.35 | 0.47 | 0.39 | 0.35 |
| HfO2 | 0.22 | 0.00 | 0.65 | 0.31 | b.d | b.d | 0.22 | 0.00 |
| Cl | 1.56 | 1.44 | 1.41 | 1.53 | 1.56 | 0.74 | 1.56 | 1.44 |
| H2O † | 3.69 | 2.03 | 3.69 | 2.03 | ||||
| Sum | 97.69 | 99.51 | 93.00 | 93.29 | 99.27 | 98.76 | 97.69 | 99.51 |
| Formula based on Σ(Si + Al + Zr + Ti + Hf + Nb + Ta + W) normalized to 29 apfu | ‡ | § | ||||||
| Si4+ | 24.37 | 24.39 | 25.11 | 25.36 | 25.47 | 25.92 | 24.85 | 24.54 |
| Ti4+ | 0.20 | 0.22 | 0.21 | 0.17 | 0.21 | 0.20 | 0.21 | 0.21 |
| Al3+ | 0.14 | 0.09 | 0.20 | 0.13 | 0.10 | 0.04 | 0.15 | 0.09 |
| Fe2+ | 0.63 | 0.93 | 1.18 | 1.17 | 0.85 | 0.84 | 0.64 | 0.90 |
| Mn2+ | 1.31 | 0.92 | 0.98 | 1.10 | 1.34 | 1.31 | 1.34 | 0.89 |
| Mg2+ | - | 0.05 | 0.07 | 0.06 | - | - | - | 0.05 |
| Ca2+ | 3.32 | 4.26 | 3.75 | 4.26 | 4.35 | 4.48 | 3.39 | 4.12 |
| Na+ | 8.15 | 14.10 | 5.03 | 4.86 | 17.19 | 16.16 | 8.31 | 13.63 |
| K+ | 0.15 | 0.19 | 0.36 | 0.47 | 0.15 | 0.11 | 0.16 | 0.18 |
| Sr2+ | 0.79 | 0.53 | 0.56 | 0.73 | 0.71 | 0.44 | 0.81 | 0.51 |
| Y3+ | 0.11 | - | 0.16 | 0.17 | - | - | 0.11 | - |
| Zr4+ | 4.17 | 3.10 | 3.27 | 3.11 | 2.98 | 2.71 | 4.25 | 3.00 |
| Nb5+ | 0.08 | 0.20 | 0.13 | 0.19 | 0.24 | 0.12 | 0.08 | 0.19 |
| La3+ | 0.05 | 0.04 | 0.05 | 0.06 | 0.05 | 0.08 | 0.05 | 0.04 |
| Ce3+ | 0.10 | 0.15 | 0.10 | 0.15 | 0.16 | 0.22 | 0.10 | 0.15 |
| Nd3+ | 0.07 | 0.06 | 0.05 | 0.06 | 0.06 | 0.08 | 0.07 | 0.06 |
| Hf4+ | 0.03 | - | 0.09 | 0.04 | - | - | 0.03 | |
| H+ | 12.28 | 6.74 | - | - | - | - | 12.52 | 6.51 |
| Cl− | 1.32 | 1.22 | 1.14 | 1.26 | 1.36 | 0.63 | 1.34 | 1.17 |
| Sample | LV-153/178 | LV-117/226 |
|---|---|---|
| Temperature/K | 296.15 | 293(2) |
| Crystal system | trigonal | trigonal |
| Space group | Rm | R32 |
| a, Å | 14.2248(7) | 14.2081(4) |
| c, Å | 30.3453(15) | 30.3723(7) |
| Volume, Å3 | 5317.6(6) | 5309.8(3) |
| Z | 3 | 3 |
| ρcalc, g/cm3 | 2.876 | 2.897 |
| μ, mm−1 | 2.113 | 2.711 |
| F(000) | 4472.0 | 4495.0 |
| Crystal size, mm3 | 0.12 × 0.11 × 0.08 | 0.14 × 0.11 × 0.05 |
| Radiation | CuKα (λ = 1.54184) | MoKα (λ = 0.71073) |
| 2Θ range for data collection, ° | 7.746 to 143.09 | 5.734 to 54.978 |
| Index ranges | −17 ≤ h ≤ 13, −10 ≤ k ≤ 17, −36 ≤ l ≤ 36 | −13 ≤ h ≤ 12, −5 ≤ k ≤ 18, −39 ≤ l ≤ 29 |
| Reflections collected | 4917 | 4362 |
| Independent reflections | 1300 (Rint = 0.0292, Rsigma = 0.0259) | 2591 (Rint = 0.0186, Rsigma = 0.0313) |
| Data/restraints/parameters | 1300/0/152 | 2591/0/247 |
| Goodness-of-fit on F2 | 1.088 | 1.066 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0375, wR2 = 0.0903 | R1 = 0.0344, wR2 = 0.0829 |
| Final R indexes [all data] | R1 = 0.0418, wR2 = 0.0918 | R1 = 0.0374, wR2 = 0.0847 |
| Largest diff. peak/hole/e Å−3 | 0.85/−0.80 | 1.40/−0.71 |
| Flack parameter | n.d. | 0.50(3) |
| Site | Occupancy | x/a | y/b | z/c | Uani |
|---|---|---|---|---|---|
| M1 | Ca0.91Ce0.09 | ⅔ | 0.59499(7) | ⅚ | 0.0156(4) |
| M2A | Fe0.66□0.34 | ½ | ½ | ½ | 0.080(2) |
| M2B | □0.97Zr0.03 | 0.432(2) | 0.568(2) | 0.5022(7) | 0.080(2) |
| M3 | □0.67Nb0.33 | ⅔ | ⅓ | 0.45507(15) | 0.0419(15) |
| M4 | □0.73Si0.27 | ⅔ | ⅓ | 0.4118(3) | 0.011(4) |
| Z1 | Zr | ⅓ | ⅙ | ⅔ | 0.0124(2) |
| N1A | Na0.75□0.25 | 0.5568(2) | 0.4432(2) | 0.67991(13) | 0.0259(9) |
| N1B | □0.75Na0.25 | 0.5863(7) | 0.4137(7) | 0.6637(4) | 0.032(3) |
| N4 | Na0.91Sr0.09 | 0.4592(2) | 0.22959(12) | 0.54912(9) | 0.0473(12) |
| N5 | Na0.83□0.17 | ⅔ | ⅓ | 0.5957(3) | 0.061(3) |
| Si1 | Si | 0.73712(7) | 0.47424(13) | 0.75170(5) | 0.0135(4) |
| Si3 | Si | 0.79207(7) | 0.58413(14) | 0.42439(5) | 0.0179(4) |
| Si5 | Si | 0.39705(9) | 0.38977(9) | 0.59703(3) | 0.0132(3) |
| O1 | O | 0.60481(17) | 0.39519(17) | 0.75584(15) | 0.0215(10) |
| O2 | O | 0.78059(19) | 0.5612(4) | 0.79000(13) | 0.0258(11) |
| O3 | O | 0.7617(2) | 0.5234(4) | 0.70273(14) | 0.0277(11) |
| O4 | O | 0.48607(19) | 0.51393(19) | 0.80471(13) | 0.0196(9) |
| O5 | O | 0.3882(3) | 0.4339(3) | 0.72670(10) | 0.0301(8) |
| O6 | O | 0.4890(2) | 0.5110(2) | 0.61392(14) | 0.0238(10) |
| O7 | O | 0.4074(3) | 0.3036(3) | 0.62716(11) | 0.0293(8) |
| O8 | O | 0.4108(3) | 0.3742(3) | 0.54553(9) | 0.0215(7) |
| O9 | O0.64□0.36 | ⅔ | ⅓ | 0.5454(6) | 0.051(4) |
| O10 | O | 0.7271(2) | 0.4543(5) | 0.4257(3) | 0.0605(19) |
| O11 | □0.67(H2O)0.33 | 0.3920(11) | 0.6080(11) | 0.5055(8) | 0.017(5) |
| X1 | □0.77Cl0.33 | 0.5932(5) | 0.4068(5) | 0.3447(4) | 0.122(6) |
| X2A | □0.94Cl0.06 | ⅔ | ⅓ | 0.501(2) | 0.051(4) |
| X2B | □0.84(H2O)0.16 | ⅔ | ⅓ | 0.605(6) | 0.050 |
| X2C | □0.73(H2O)0.37 | ⅔ | ⅓ | 0.3593(10) | 0.045(7) |
| Site | Occupancy | x/a | y/b | z/c | Uani |
|---|---|---|---|---|---|
| M1A | Ca0.61Zr0.39 | 0.58616(14) | ⅔ | ⅙ | 0.0160(5) |
| M1B | Ca0.88Ce0.12 | 0.06533(14) | ⅔ | ⅙ | 0.0158(6) |
| M2A | Mn0.53□0.47 | 0.8287(4) | ⅔ | ⅙ | 0.085(3) |
| M2B | □0.89Fe0.11 | 0.7684(12) | 0.5478(8) | 0.1694(3) | 0.026(3) |
| M3 | □0.67Ti0.33 | ⅔ | ⅓ | 0.2143(2) | 0.0283(15) |
| M4 | □0.58Si0.42 | ⅔ | ⅓ | 0.25530(17) | 0.0097(19) |
| Z1 | Zr | ⅔ | 0.83849(8) | ⅓ | 0.01012(19) |
| N1 | Na0.91Sr0.09 | 0.1037(3) | 0.8933(3) | 0.21659(10) | 0.0626(14) |
| N3 | Na0.75□0.25 | 0.8861(6) | 0.7787(4) | 0.34644(12) | 0.0271(9) |
| N4 | □0.75Na0.25 | 0.9126(18) | 0.8343(12) | 0.3328(4) | 0.031(3) |
| Si1 | Si | 0.91643(12) | 0.4573(2) | 0.24247(5) | 0.0134(3) |
| Si2 | Si | 0.93912(18) | 0.67238(17) | 0.26343(8) | 0.0114(4) |
| Si3 | Si | 0.72728(16) | 0.67626(17) | 0.26376(8) | 0.0103(4) |
| Si4 | Si | 0.47422(12) | 0.7423(2) | 0.24804(4) | 0.0131(3) |
| O1 | O | 0.9596(5) | 0.3915(4) | 0.2738(2) | 0.0207(14) |
| O2 | O | 0.7865(4) | 0.3809(8) | 0.2397(3) | 0.060(2) |
| O3 | O | 0.9713(3) | 0.4878(6) | 0.19551(12) | 0.0184(8) |
| O4 | O | 0.9436(7) | 0.5613(6) | 0.2732(2) | 0.0319(17) |
| O5 | O | 0.8184(5) | 0.6455(3) | 0.28026(13) | 0.0195(8) |
| O6 | O | 0.0253(5) | 0.7666(6) | 0.2940(2) | 0.0282(15) |
| O7 | O | 0.7393(5) | 0.7057(5) | 0.2127(2) | 0.0186(13) |
| O8 | O | 0.7403(6) | 0.7732(5) | 0.2943(2) | 0.0227(14) |
| O9 | O | 0.5600(4) | 0.7863(7) | 0.20933(13) | 0.0292(12) |
| O10 | O | 0.3993(6) | 0.6091(6) | 0.24431(14) | 0.0214(9) |
| O11 | O | 0.5248(4) | 0.7687(8) | 0.29659(14) | 0.0302(12) |
| O12 | O | 0.9561(5) | 0.7014(5) | 0.2124(2) | 0.0214(14) |
| O13 | □0.67(H2O)0.33 | 0.729(4) | 0.457(3) | 0.1714(9) | 0.030(10) |
| O14 | □0.58(H2O)0.42 | ⅔ | ⅓ | 0.3078(4) | 0.013(5) |
| X1A | □0.67(H2O)0.33 | 0 | 0 | 0.1633(9) | 0.108(17) |
| X1B | □0.72(H2O)0.28 | 0 | 0 | 0.3096(14) | 0.10(3) |
| X2A | □0.22(H2O)0.78 | 0 | 0 | 0.2650(5) | 0.048(5) |
| X2B | □0.23(H2O)0.77 | 0 | 0 | 0.2089(2) | 0.008(2) |
| X2C | □0.69Cl0.31 | 0.5099(9) | 0.2708(19) | 0.3238(4) | 0.108(7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panikorovskii, T.L.; Mikhailova, J.A.; Pakhomovsky, Y.A.; Bazai, A.V.; Aksenov, S.M.; Kalashnikov, A.O.; Krivovichev, S.V. Zr-Rich Eudialyte from the Lovozero Peralkaline Massif, Kola Peninsula, Russia. Minerals 2021, 11, 982. https://doi.org/10.3390/min11090982
Panikorovskii TL, Mikhailova JA, Pakhomovsky YA, Bazai AV, Aksenov SM, Kalashnikov AO, Krivovichev SV. Zr-Rich Eudialyte from the Lovozero Peralkaline Massif, Kola Peninsula, Russia. Minerals. 2021; 11(9):982. https://doi.org/10.3390/min11090982
Chicago/Turabian StylePanikorovskii, Taras L., Julia A. Mikhailova, Yakov A. Pakhomovsky, Ayya V. Bazai, Sergey M. Aksenov, Andrey O. Kalashnikov, and Sergey V. Krivovichev. 2021. "Zr-Rich Eudialyte from the Lovozero Peralkaline Massif, Kola Peninsula, Russia" Minerals 11, no. 9: 982. https://doi.org/10.3390/min11090982
APA StylePanikorovskii, T. L., Mikhailova, J. A., Pakhomovsky, Y. A., Bazai, A. V., Aksenov, S. M., Kalashnikov, A. O., & Krivovichev, S. V. (2021). Zr-Rich Eudialyte from the Lovozero Peralkaline Massif, Kola Peninsula, Russia. Minerals, 11(9), 982. https://doi.org/10.3390/min11090982











