Preparation and Evaluation of Ammonium Adipate Solutions as Inhibitors of Shale Rock Swelling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
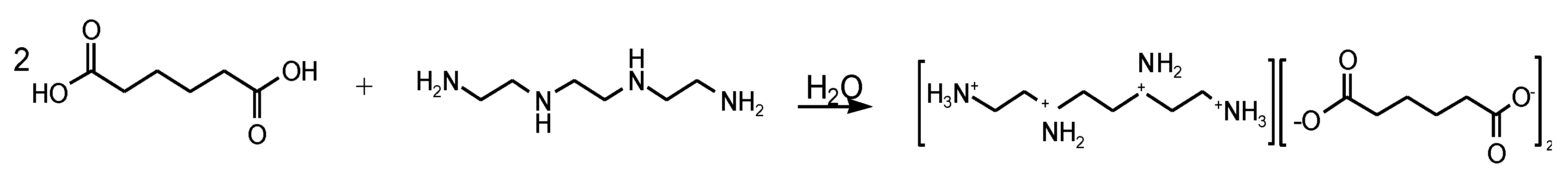
2.2. Synthesis of AASs
2.3. Inhibitive Ability Evaluation
2.4. Performance in Drilling Fluid
2.5. Laser Particle Size Distribution Analysis
2.6. TGA
2.7. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Effect of the Types of Inhibitors
3.2. Swelling Inhibition
3.3. Performance of AAS-8 in Water-Based Drilling Fluids
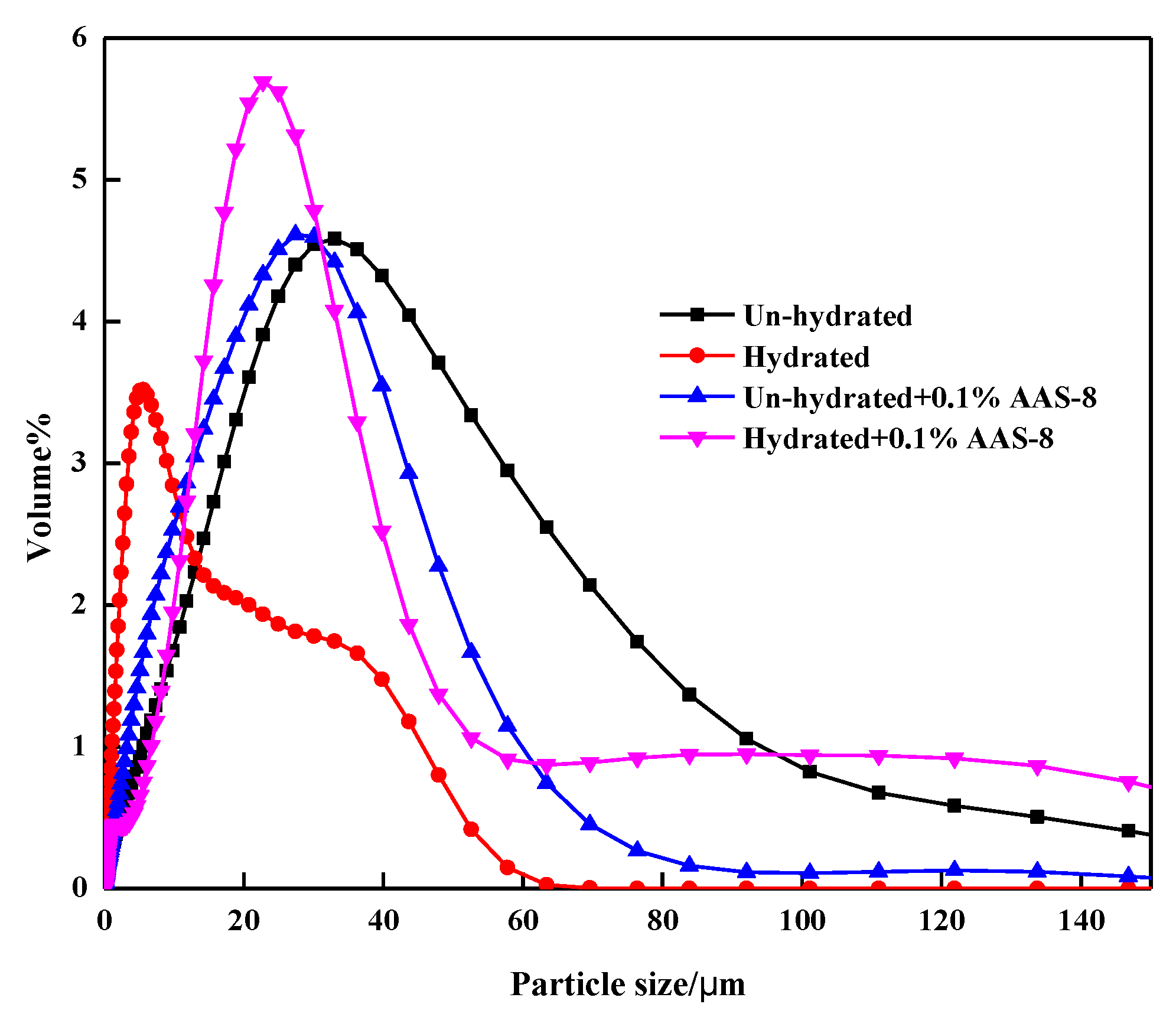
3.4. Laser Particle Size Distribution Analysis
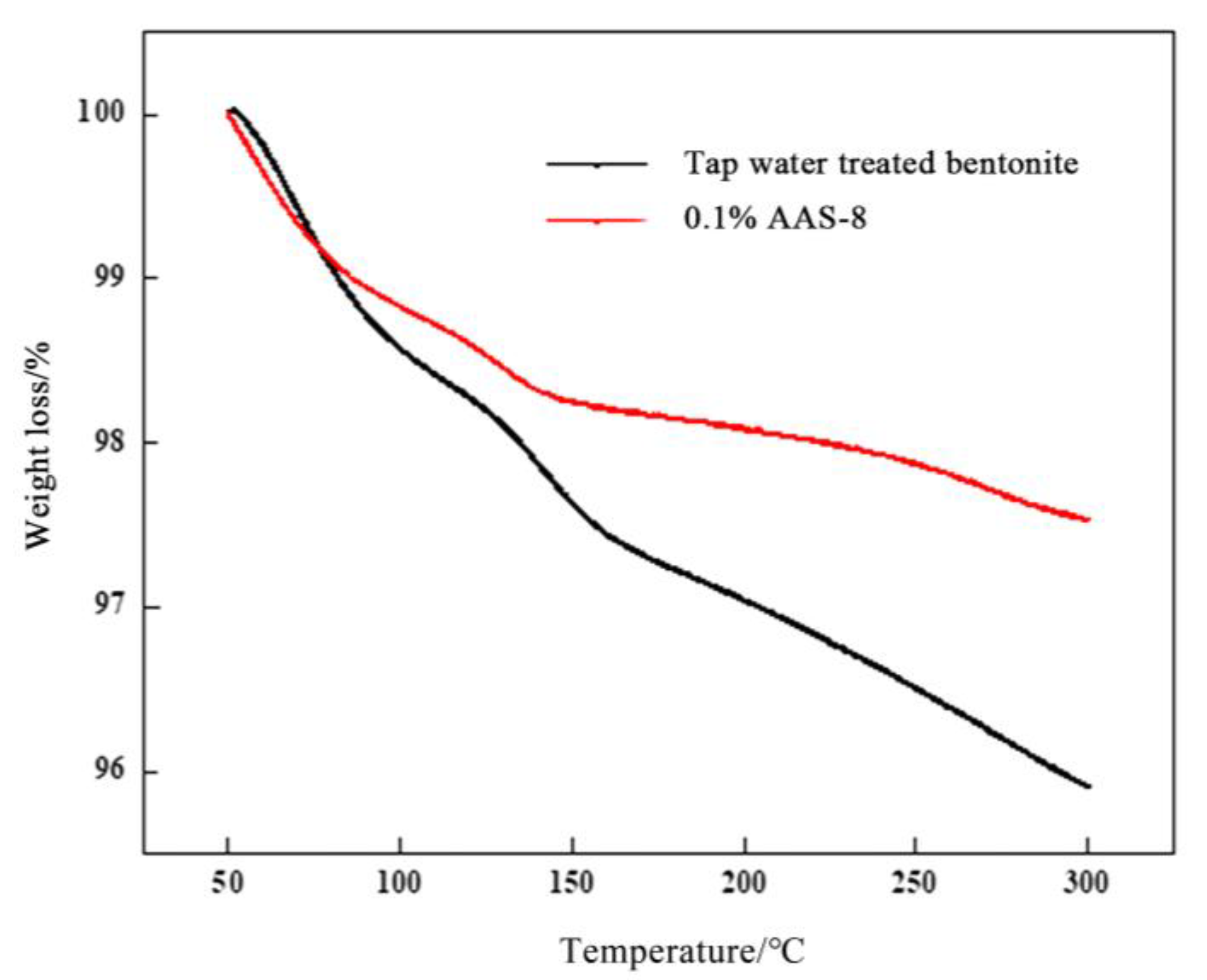
3.5. TGA
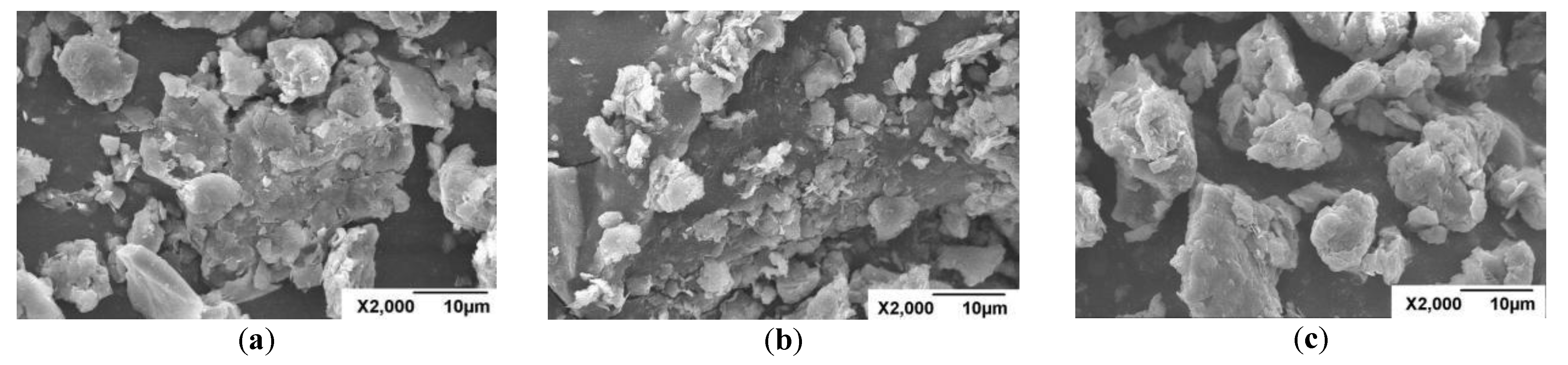
3.6. Scanning Electron Microscopy (SEM) Analysis
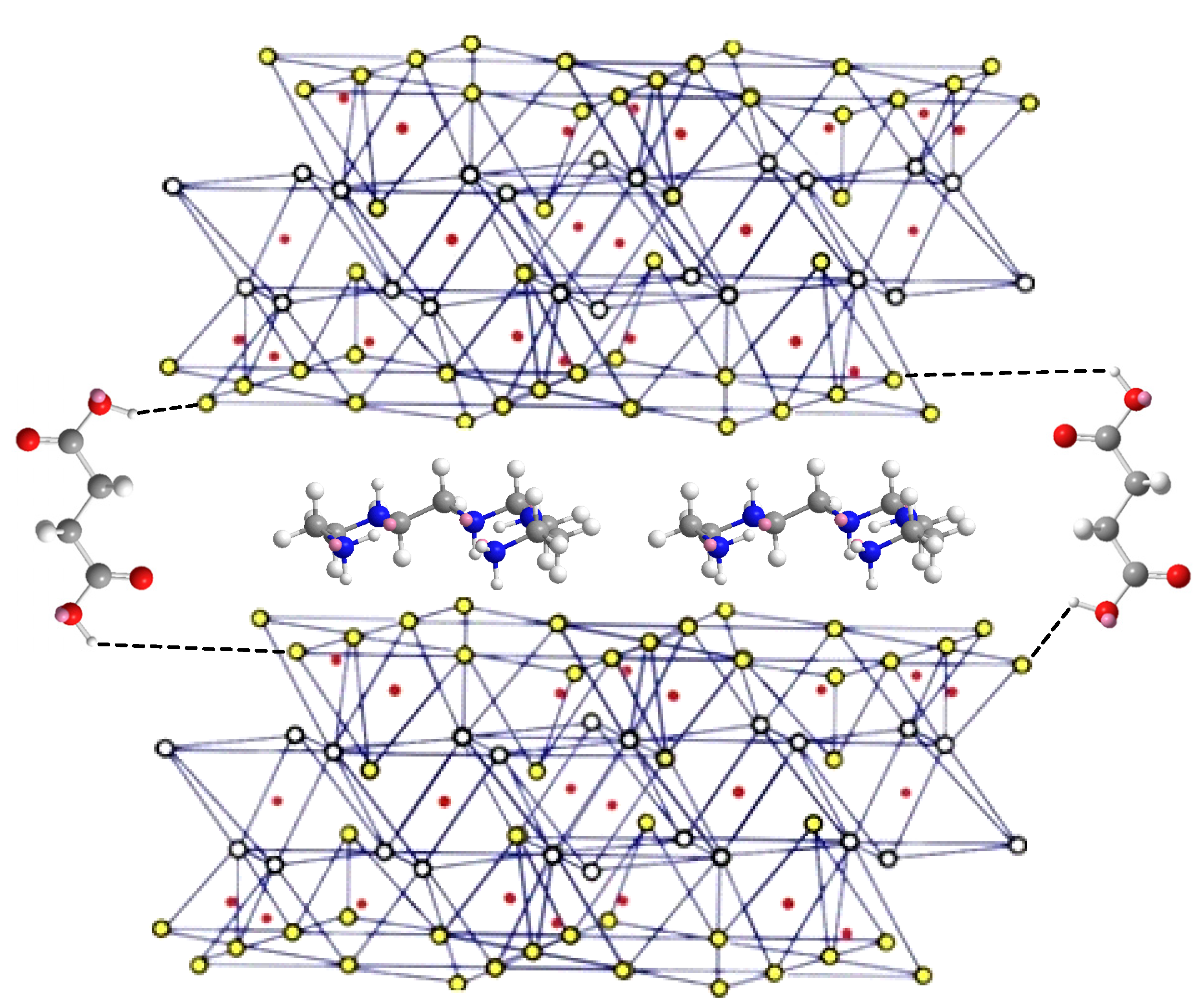
3.7. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bol, G.M. The effect of various polymers and salts on borehole and cutting stability in water-base shale drilling fluids. In Proceedings of the IADC/SPE Drilling Conference, Dallas, TX, USA, 10–12 February 1986. [Google Scholar]
- Lal, M. Shale stability: Drilling fluid interaction and shale strength. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 21–23 April 1999. [Google Scholar]
- Steiger, R.P.; Leung, P.K. Qualitative determination of the mechanical properties of shales. SPE Drill. Eng. 1992, 7, 181. [Google Scholar] [CrossRef]
- Qiu, Z.S.; Zhong, H.Y.; Huang, W.A. Properties and mechanism of a new polyamine shale inhibitor. Acta Pet. Sin. 2011, 32, 678–682. [Google Scholar]
- Van Ort, E. On the physical and chemical stability of shales. J. Petrol. Sci. Eng. 2003, 38, 213–235. [Google Scholar] [CrossRef]
- Zhang, L.; Li, T.H.; Huang, L.; Ye, Z.Q.; Ye, Z.B.; Yan, X.; Li, L.L.; Deng, Q.; Chen, G.; Zhang, J.; et al. Preparation and application of melamine cross-linked poly ammonium as shale inhibitor. Chem. Cent. J. 2018, 12, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.L.; Yan, Q.B.; Guo, G.; Guo, Z.; Zhang, J.; Chen, G.; Deng, Q. Investigation of oleate graft copolyammonium as a clay swelling inhibitor for shale oil/gas exploration. Pet. Chem. 2018, 58, 255–259. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, W.M.; Zhang, L.; Li, T.H.; Cai, D.; Chen, G. Investigation of ammonium-lauric salt as shale swelling inhibitor and a mechanism study. Adsorpt. Sci. Technol. 2019, 37, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Du, W.C.; Slaný, M.; Wang, X.Y.; Chen, G.; Zhang, J. The inhibition property and mechanism of a novel low molecular weight zwitterionic copolymer for improving wellbore stability. Polymers 2020, 12, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.J.; Gao, L.; Du, W.C.; Hu, W.M.; Duan, W.G.; Gu, X.F.; Zhang, J.; Chen, G. Preparation and application of ferric chloride-lignin sulfonate as shale inhibitor in water-based drilling fluid. Molecules 2019, 24, 4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Yan, J.; Li, L.L.; Zhang, J.; Gu, X.F.; Song, H. Preparation and performance of amine-tartaric salt as potential clay swelling inhibitor. Appl. Clay Sci. 2017, 138, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.; Zhang, J.; Chen, G. Study on the effect of carboxy-amines small molecule shale inhibitor. Oilfield Chem. 2014, 31, 5–8. [Google Scholar]
- Zhang, J.; Cai, D.; Chen, G. Performance assessment of a polyamine swelling inhibitor for clays and shales. Nat. Gas Industry 2014, 34, 85–90. [Google Scholar]
- Chen, D.J.; Chen, F. Oil and Gas Fields Applied Chemistry; Petroleum Industry Press: Beijing, China, 2006. [Google Scholar]
- Ye, Z.Q.; Ye, Z.B.; Huang, L.; Bai, Y.; Li, L.L.; Zhang, J.; Qu, C.T.; Chen, G. Preparation and application of a new cross-linked polyammonium as shale inhibitor. J. Appl. Biomater. Funct. Mater. 2018, 16, 119–124. [Google Scholar]
- Zhang, R.J.; Wang, X.K.; Zhang, J.; Hu, W.M.; Duan, W.G.; Du, W.C.; Chen, G. Preparation and performance of ammonium-malic salts as shale swelling inhibitor and a mechanism study. Inorg. Nano-Met. Chem. 2020, 50, 1027–1031. [Google Scholar] [CrossRef]
- Wang, Y.L.; Yan, Q.B.; Du, W.C.; Gu, X.F.; Deng, Q. Synthesis of a cation glucoside and the study of swelling inhibition to montmorillonite. Fresenius Environ. Bull. 2020, 29, 9385–9392. [Google Scholar]
- Du, W.C.; Hu, Z.B.; Sun, Y.; Hu, W.M.; Zhang, J.; Zhang, M.; Song, S.X.; Chen, G. A green clay swelling inhibitor prepared from citric acid and a michanism study. Fresenius Environ. Bull. 2020, 29, 4905–4912. [Google Scholar]
- Du, W.C.; Wang, X.Y.; Chen, G.; Zhang, J.; Slaný, M. Synthesis, property and mechanism analysis of a novel polyhydroxy organic amine shale hydration inhibitor. Minerals 2020, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, L.L.; Chen, G.; Tang, Y.; Zhao, J.R.; Tang, D.Y. Synthesis and performance evaluation of quaternary ammonium salt as potential shale inhibitor. J. Chem. Soc. Pak. 2015, 37, 961–966. [Google Scholar]
- Song, Z.F.; Zhang, L.; Huang, L.; Song, Z.F.; Liu, X.X.; Zhang, J.; Du, W.C.; Chen, G. Preparation and application of a novel polyammonium as potent shale hydration inhibitor. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 57, 326–331. [Google Scholar] [CrossRef]
- Du, W.C.; Pu, X.L.; Sun, J.S.; Luo, X.; Zhang, Y.N.; Li, L. Synthesis and evaluation of a novel monomeric amine as sodium montmorillonite swelling inhibitor. Adsorpt. Sci. Technol. 2018, 36, 655–668. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.F.; Zhang, J.J.; Zhang, J.; Chen, G.; Ma, C.; Zhang, Z. Stabilization of montmorillonite by ammoniated lignosulfonates and its use in water-based drilling fluid. Sci. Adv. Mater. 2017, 9, 928–933. [Google Scholar] [CrossRef]
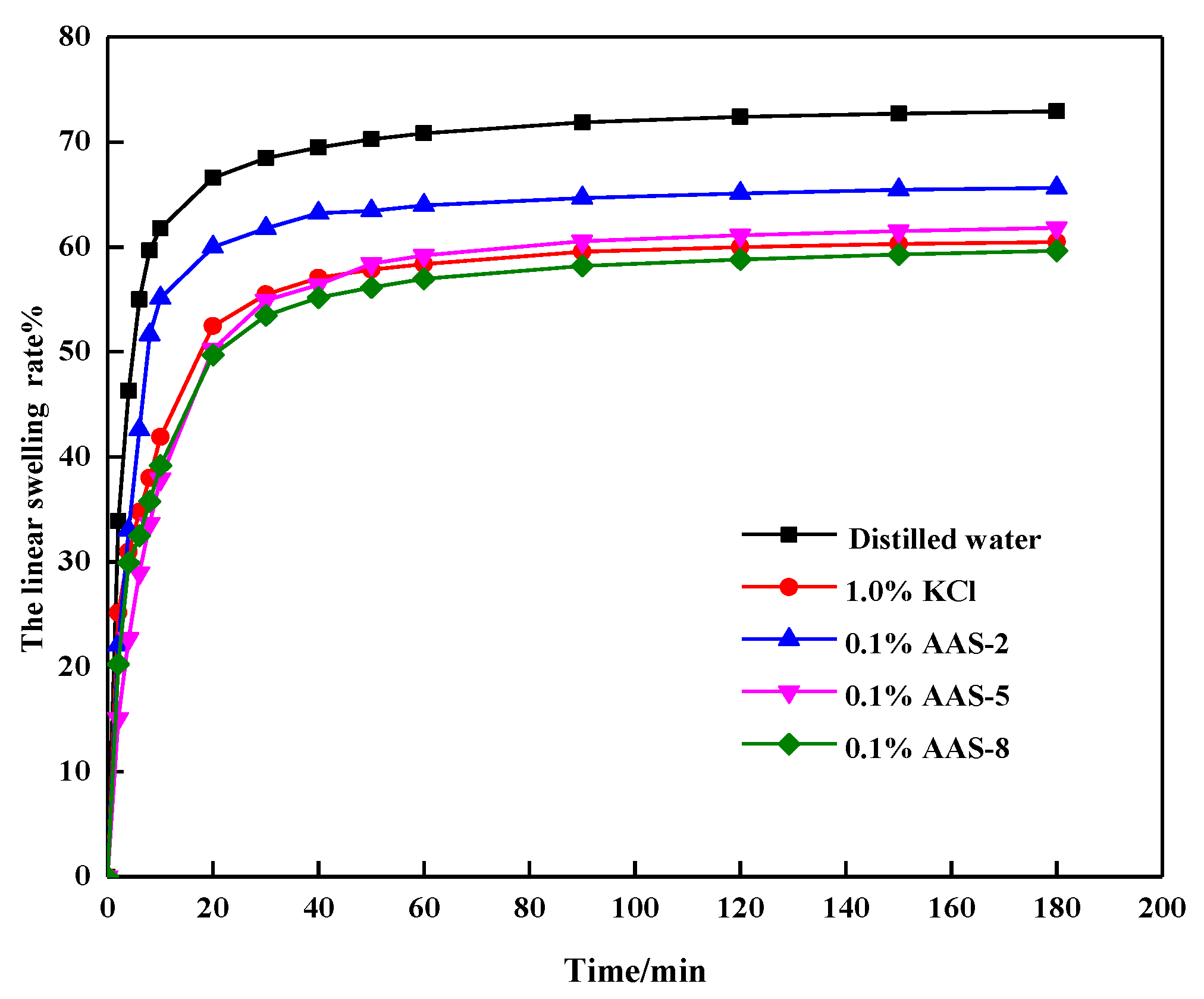
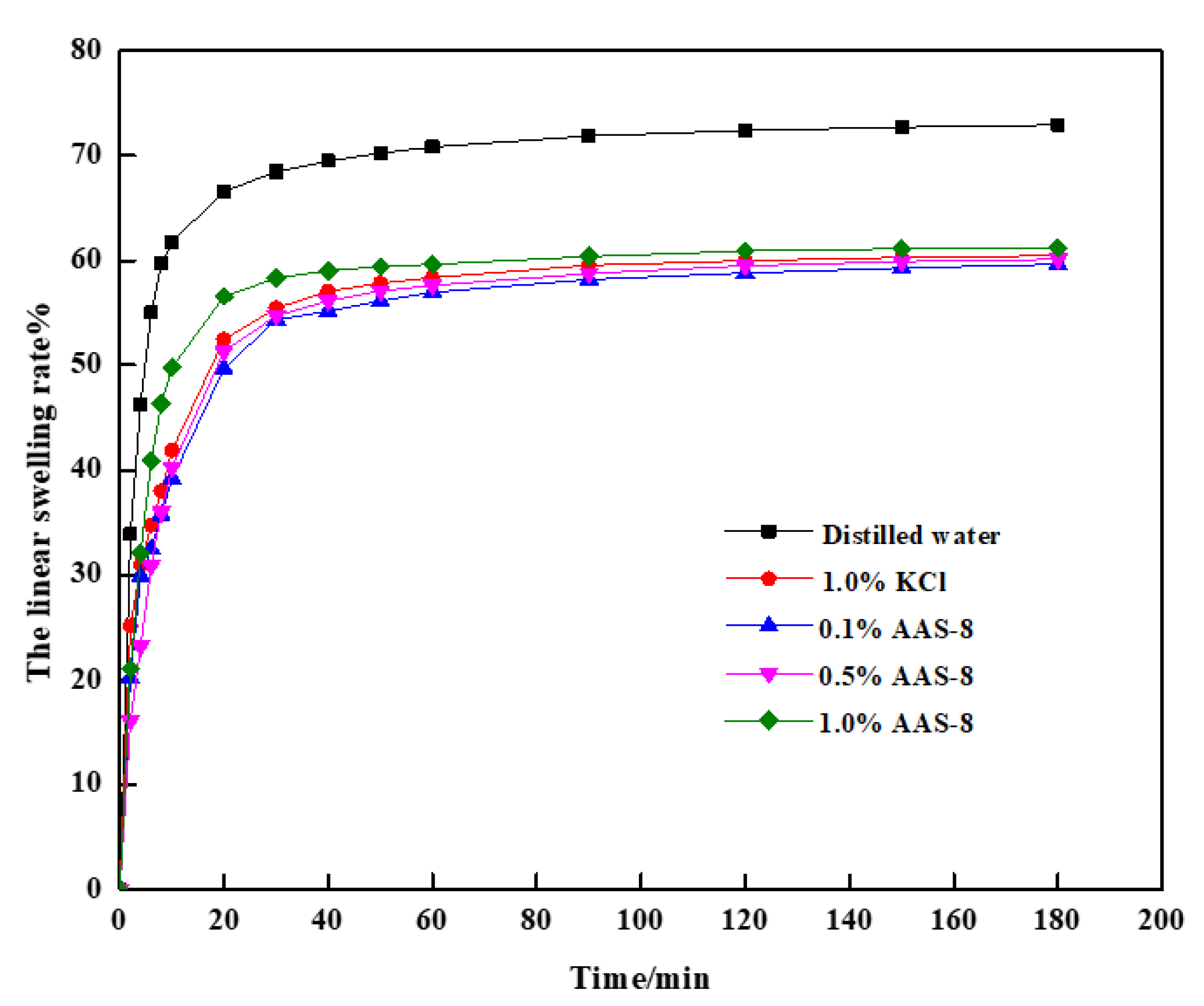
| Materials | Ratio of Acid Group to Amine Group | Names | Swelling Rate/% (180 min) | |
|---|---|---|---|---|
| Water | - | - | 72.92 | |
| KCl | 1.0% | - | 60.44 | |
| Adipic acid | Diethylenetriamine | 1:1 | AAS-1 | 63.63 |
| 1:2 | AAS-2 | 56.35 | ||
| 1:3 | AAS-3 | 65.63 | ||
| Triethylenetetramine | 1:1 | AAS-4 | 63.38 | |
| 1:2 | AAS-5 | 61.80 | ||
| 1:3 | AAS-6 | 68.72 | ||
| Tetraethylenepentamine | 1:1 | AAS-7 | 61.20 | |
| 1:2 | AAS-8 | 59.61 | ||
| 1:3 | AAS-9 | 60.28 | ||
| Drilling Fluid | AV mPa·s | PV mPa·s | YP /Pa | YP/PV Pa/mPa·s | ρ g/cm3 | FL /mL | μ |
|---|---|---|---|---|---|---|---|
| Base drilling fluid | 4.95 | 2.8 | 2.1973 | 0.7848 | 1.008 | 13.7 | 0.0524 |
| 0.1% AAS-8 | 7.10 | 3.4 | 3.7814 | 1.1122 | 1.016 | 16.2 | 0.0524 |
| 0.3% CMC | 4.90 | 2.8 | 2.1462 | 0.7665 | 1.018 | 11.0 | 0.0875 |
| 0.1% AAS-8 + 0.3% CMC | 8.40 | 3.8 | 4.7012 | 1.2372 | 1.020 | 12.3 | 0.0437 |
| 0.1% PAM | 8.00 | 4.2 | 3.8836 | 0.9247 | 1.022 | 12.0 | 0.0699 |
| 0.1% AAS-8 + 0.1% PAM | 11.70 | 5.0 | 6.8474 | 1.3695 | 1.020 | 19.0 | 0.0437 |
| 0.3% Modified starch | 6.00 | 4.0 | 2.0440 | 0.5110 | 1.022 | 7.5 | 0.0699 |
| 0.1% AAS-8 + 0.3% modified starch | 9.60 | 4.4 | 5.3144 | 1.2078 | 1.025 | 6.4 | 0.0699 |
| Content | Mean (µm) | Median (µm) |
|---|---|---|
| Unhydrated | 33.26 | 26.65 |
| Hydrated | 11.06 | 7.64 |
| Unhydrated + 0.1% AAS-8 | 28.58 | 24.48 |
| Hydrated + 0.1% AAS-8 | 27.99 | 22.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xian, S.; Chen, S.; Lian, Y.; Du, W.; Song, Z.; Chen, G. Preparation and Evaluation of Ammonium Adipate Solutions as Inhibitors of Shale Rock Swelling. Minerals 2021, 11, 1013. https://doi.org/10.3390/min11091013
Xian S, Chen S, Lian Y, Du W, Song Z, Chen G. Preparation and Evaluation of Ammonium Adipate Solutions as Inhibitors of Shale Rock Swelling. Minerals. 2021; 11(9):1013. https://doi.org/10.3390/min11091013
Chicago/Turabian StyleXian, Sirong, Shijun Chen, Yubo Lian, Weichao Du, Zhifei Song, and Gang Chen. 2021. "Preparation and Evaluation of Ammonium Adipate Solutions as Inhibitors of Shale Rock Swelling" Minerals 11, no. 9: 1013. https://doi.org/10.3390/min11091013
APA StyleXian, S., Chen, S., Lian, Y., Du, W., Song, Z., & Chen, G. (2021). Preparation and Evaluation of Ammonium Adipate Solutions as Inhibitors of Shale Rock Swelling. Minerals, 11(9), 1013. https://doi.org/10.3390/min11091013






