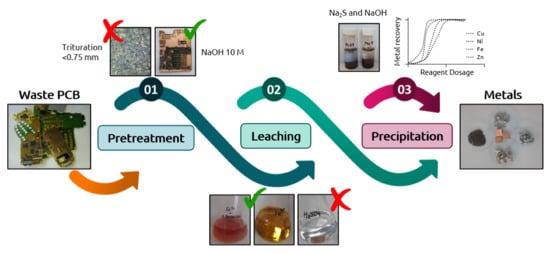
Metal Extraction and Recovery from Mobile Phone PCBs by a Combination of Bioleaching and Precipitation Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. PCB Samples
2.2. Microorganisms and Culture Media
2.3. PCB Sample Conditioning
2.4. Leaching Experiments
2.4.1. One-step Bioleaching Experiment
2.4.2. Two-Step Bioleaching Experiment
2.4.3. Leaching Experiments in Abiotic Medium
2.5. Chemical Precipitation Experiments for Metal Extraction
2.6. Analytical Methods
3. Results and Discussion
3.1. Effect of the Pre-Treatment
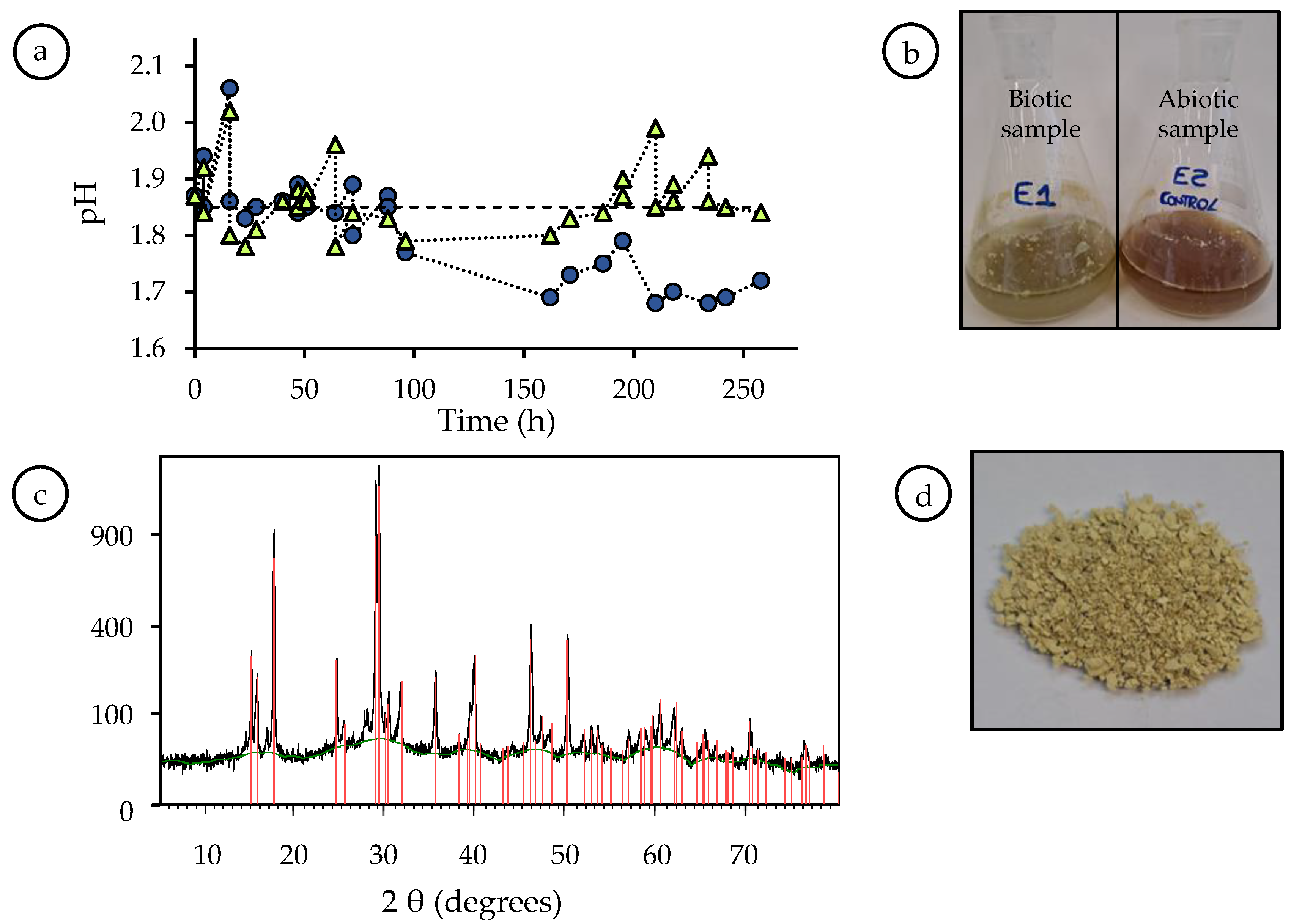
3.2. Effect of the Presence of A. Ferrooxidans Activity and the Leaching Agent
3.3. Effect of the Precipitating Agent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament, Council of the European Union. Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on Waste Electrical and Electronic Equipment (WEEE). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32012L0019 (accessed on 28 July 2021).
- Eurostat. Waste Electrical and Electronic Equipment (WEEE) by Waste Management Operations. Available online: https://ec.europa.eu/eurostat/databrowser/view/ENV_WASELEE__custom_174344/default/table?lang=en (accessed on 28 July 2021).
- Ghosh, B.; Ghosh, M.K.; Parhi, P.; Mukherjee, P.S.; Mishra, B.K. Waste Printed Circuit Boards recycling: An extensive assessment of current status. J. Clean Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Evangelopoulos, P.; Persson, H.; Kantarelis, E.; Yang, W. Performance analysis and fate of bromine in a single screw reactor for pyrolysis of waste electrical and electronic equipment (WEEE). Process Saf. Environ. 2020, 143, 313–321. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Y.; Wu, Y.; Guo, F. Metal recovery from waste printed circuit boards: A review for current status and perspectives. Resour. Conserv. Recy. 2020, 157, 104787. [Google Scholar] [CrossRef]
- Palmieri, R.; Bonifazi, G.; Serranti, S. Recycling-oriented characterization of plastic frames and printed circuit boards from mobile phones by electronic and chemical imaging. Waste Manag. 2014, 34, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Yamane, L.H.; de Moraes, V.T.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of WEEE: Characterization of spent printed circuit boards from mobile phones and computers. Waste Manag. 2011, 31, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and the Council of the European Union. Directive 2002/95/EC of the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002L0095&from=SV (accessed on 28 July 2021).
- Birloaga, I.; Veglio, F. Study of multi-step hydrometallurgical methods to extract the valuable content of gold, silver and copper from waste printed circuit boards. J. Environ. Chem. Eng. 2016, 4, 20–29. [Google Scholar] [CrossRef]
- Diaz-Tena, E.; Barona, A.; Gallastegui, G.; Rodriguez, A.; Lopez de Lacalle, L.N.; Elias, A. Biomachining: Metal etching via microorganisms. Crit. Rev. Biotechnol. 2017, 37, 323–332. [Google Scholar] [CrossRef]
- Díaz-Martínez, M.E.; Argumedo-Delira, R.; Sánchez-Viveros, G.; Alarcón, A.; Mendoza-López, M.R. Microbial bioleaching of Ag, Au and Cu from printed circuit boards of mobile Phones. Curr. Microbiol. 2019, 76, 536–544. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Comparative assessment of metallurgical recovery of metals from electronic waste with special emphasis on bioleaching. Environ. Sci. Pollut. Res. Int. 2017, 24, 6989–7008. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, M.; Gurumurthy, K. Enhanced bioleaching of copper from circuit boards of computer waste by Acidithiobacillus ferrooxidans. Environ. Chem. Lett. 2019, 17, 1873–1879. [Google Scholar] [CrossRef]
- NASA. Technology Readiness Level. Available online: https://www.nasa.gov/directorates/heo/scan/engineering/technology/txt_accordion1.html (accessed on 28 July 2021).
- Kaksonen, A.H.; Lavonen, L.; Kuusenaho, M.; Kolli, A.; Närhi, H.; Vestola, E.; Puhakka, J.A.; Tuovinen, O.H. Bioleaching and recovery of metals from final slag waste of the copper smelting industry. Min. Eng. 2011, 24, 1113–1121. [Google Scholar] [CrossRef]
- Ye, M.; Yan, P.; Sun, S.; Han, D.; Xiao, X.; Zheng, L.; Huang, S.; Chen, Y.; Zhuang, S. Bioleaching combined brine leaching of heavy metals from lead-zinc mine tailings: Transformations during the leaching process. Chemosphere 2017, 168, 1115–1125. [Google Scholar] [CrossRef]
- Santaolalla, A.; Rojo, N.; Gutierrez, J.; Barona, A. Immobilization of Acidithiobacillus ferrooxidans on two hydrogels. Chem. Eng. Trans. 2020, 79, 7–12. [Google Scholar]
- Tabak, H.H.; Scharp, R.; Burckle, J.; Kawahara, F.K.; Govind, R. Advances in biotreatment of acid mine drainage and biorecovery of metals: 1. Metal precipitation for recovery and recycle. Biodegradation 2003, 14, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fu, X.; Liu, W.; Chen, L.; Zhang, D. Hydrometallurgical treatment of copper smelting dust by oxidation leaching and fractional precipitation technology. JOM 2017, 69, 1982–1986. [Google Scholar] [CrossRef]
- Bilgin, A.; Jaffé, P.R. Precipitation of copper (II) in a two-stage continuous treatment system using sulfate reducing bacteria. Waste Biomass Valorization 2019, 10, 2907–2914. [Google Scholar] [CrossRef]
- Wang, L.K.; Vaccari, D.A.; Li, Y.; Shammas, N.K. Chemical precipitation. In Physicochemical Treatment Processes; Wang, L.K., Hung, Y.T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2004; Volume 3, pp. 141–198. [Google Scholar]
- Zainuddin, N.A.; Mamat, T.A.R.; Maarof, H.I.; Puasa, S.W.; Yatim, S.R.M. Removal of nickel, zinc and copper from plating process industrial raw effluent via hydroxide precipitation versus sulphide precipitation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 551, 12122. [Google Scholar] [CrossRef] [Green Version]
- Kasper, A.C.; Berselli, G.B.T.; Freitas, B.D.; Tenorio, J.A.S.; Bernardes, A.M.; Veit, H.M. Printed wiring boards for mobile phones: Characterization and recycling of copper. Waste Manag. 2011, 31, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Le, H.-L.; Okumura, H.; Ishihara, K.N. MEMRECS—A sustainable view for metal recycling from waste printed circuit boards. J. Environ. Prot. Sci. 2013, 4, 803–810. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.B.; Tipre, D.R.; Dave, S.R. Chemical and biological processes for multi-metal extraction from waste printed circuit boards of computers and mobile phones. Waste Manag. Res. 2014, 32, 1134–1141. [Google Scholar] [CrossRef]
- Xiu, F.R.; Qi, Y.; Zhang, F.S. Leaching of Au, Ag, and Pd from waste printed circuit boards of mobile phone by iodide lixiviant after supercritical water pre-treatment. Waste Manag. 2015, 41, 134–141. [Google Scholar] [CrossRef]
- Isildar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manag. 2016, 57, 149–157. [Google Scholar] [CrossRef]
- LME (London Metal Exchange). LME COPPER. 2021. Available online: https://www.lme.com/en-GB/Metals/Non-ferrous/Copper#tabIndex=0 (accessed on 28 July 2021).
- Financial Times. Available online: https://www.ft.com/ (accessed on 28 July 2021).
- Hernández, P.C.; Taboada, M.E.; Herreros, O.O.; Graber, T.A.; Ghorbani, Y. Leaching of chalcopyrite in acidified nitrate using seawater-based media. Minerals 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Silverman, M.P.; Lundgren, D.G. Studies on the chemoautotrophic iron bacterium Ferroobacillus ferrooxidans: I. An improved medium and a harvesting procedure for securing high cell yields. J. Bacteriol. 1959, 77, 642–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhapure, N.N.; Dhakephalkar, P.K.; Dhakephalkar, A.P.; Tembhurkar, V.R.; Rajgure, A.V.; Deshmukh, A.M. Use of large pieces of printed circuit boards for bioleaching to avoid ‘precipitate contamination problem’ and to simplify overall metal recovery. MethodsX 2014, 1, 181–186. [Google Scholar] [CrossRef]
- Wang, L.P.; Chen, Y.J. Sequential precipitation of iron, copper, and zinc from wastewater for metal recovery. J. Environ. Eng. 2019, 145, 1–11. [Google Scholar] [CrossRef]
- Díaz-Tena, E.; Gallastegui, G.; Hipperdinger, M.; Donati, E.R.; Ramírez, M.; Rodríguez, A.; López de Lacalle, L.N.; Elías, A. New advances in copper biomachining by iron-oxidizing bacteria. Corros. Sci. 2016, 112, 385–392. [Google Scholar] [CrossRef]
- Zhu, N.; Xiang, Y.; Zhang, T.; Wu, P.; Dang, Z.; Li, P.; Wu, J. Bioleaching of metal concentrates of waste printed circuit boards by mixed culture of acidophilic bacteria. J. Hazard. Mater. 2011, 192, 614–619. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Extraction of metals from high grade waste printed circuit board by conventional and hybrid bioleaching using Acidithiobacillus ferrooxidans. Hydrometallurgy 2018, 177, 132–139. [Google Scholar] [CrossRef]
- Monneron-Enaud, B.; Wiche, O.; Schlömann, M. Biodismantling, a novel application of bioleaching in recycling of electronic wastes. Recycling 2020, 5, 22. [Google Scholar] [CrossRef]
- Sodha, A.B.; Tipre, D.R.; Dave, S.R. Optimisation of biohydrometallurgical batch reactor process for copper extraction and recovery from non-pulverized waste printed circuit boards. Hydrometallurgy 2020, 191, 105170. [Google Scholar] [CrossRef]
- Joshi, V.; Shah, N.; Wakte, P.; Dhakephalkar, P.; Dhakephalkar, A.; Khobragade, R.; Naphade, B.; Shaikh, S.; Deshmukh, A.; Adhapure, N. Comparative bioleaching of metals from pulverized and non-pulverized PCBs of cell phone charger: Advantages of non-pulverized PCBs. Environ. Sci. Pollut. Res. 2017, 24, 28277–28286. [Google Scholar] [CrossRef]
- Jiménez, L.; Arriaga, S.; Aizpuru, A. Assessing biofiltration repeatability: Statistical comparison of two identical toluene removal systems. Environ. Technol. 2015, 37, 681–693. [Google Scholar] [CrossRef]
- Zhang, S.; Forssberg, E. Mechanical separation-oriented characterization of electronic scrap. Resour. Conserv. Recycl. 1997, 21, 247–269. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Zhang, X.; Zhu, M.; Tan, W. Bioleaching of copper from waste printed circuit boards by bacteria-free cultural supernatant of iron–sulfur-oxidizing bacteria. Bioresour. Bioprocess. 2018, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Tena, E.; Gallastegui, G.; Hipperdinger, M.; Donati, E.R.; Rojo, N.; Santaolalla, A.; Ramirez, M.; Barona, A.; Elías, A. Simultaneous Culture and Biomachining of Copper in MAC Medium: A Comparison between Acidithiobacillus ferrooxidans and Sulfobacillus thermosulfidooxidans. ACS Sustain. Chem. Eng. 2018, 6, 17026–17034. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, U.; Hocheng, H. Hydrometallurgical recovery of metals from large printed circuit board pieces. Sci. Rep. 2015, 5, 14574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Yken, J.; Cheng, K.Y.; Boxall, N.J.; Nikoloski, A.N.; Moheimani, N.; Valix, M.; Sahajwalla, V.; Kaksonen, A.H. Potential of metals leaching from printed circuit boards with biological and chemical lixiviants. Hydrometallurgy 2020, 196, 105433. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Biometallurgical recovery of metals from waste printed circuit boards using pure and mixed strains of Acidithiobacillus ferrooxidans and Acidiphilium acidophilum. Process Saf. Environ. Prot. 2020, 143, 262–272. [Google Scholar] [CrossRef]
- Daoud, J.; Karamanev, D. Formation of jarosite during Fe2+ oxidation by Acidithiobacillus ferrooxidans. Miner. Eng. 2006, 19, 960–967. [Google Scholar] [CrossRef]
- Gramp, J.P.; Jones, F.S.; Bigham, J.M.; Tuovinen, O.H. Monovalent cation concentrations determine the types of Fe (III) hydroxysulfateprecipitates formed in bioleach solutions. Hydrometallurgy 2008, 94, 29–33. [Google Scholar] [CrossRef]
- Bao, P.; Xia, M.; Liu, A.; Wang, M.; Shen, L.; Yu, R.; Liu, Y.; Li, J.; Wu, X.; Fang, C.; et al. Extracellular polymeric substances (EPS) secreted by Purpureocillium lilacinum strain Y3 promote biosynthesis of jarosite. RSC Adv. 2018, 8, 22635–22642. [Google Scholar] [CrossRef] [Green Version]
- Kail, H.; Inoue, K.; Harada, H.; Kawakita, H.; Ohto, K. Leaching behavior of heavy metals with hydrochloric acid from fly ash generated in municipal waste incineration plants. Trans. Nonferrous Met. Soc. China 2011, 21, 1422–1427. [Google Scholar]
- Hu, C.; Zhou, L. Failure analysis for micro-short circuit between two pins in printed circuit board assembly. In Proceedings of the 11th International Conference on Reliability, Maintainability and Safety (ICRMS), Hangzhou, China, 26–28 October 2016; pp. 1–4. [Google Scholar]
- Hong, Y.; Valix, M. Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria. J. Clean. Prod. 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Pohl, A. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Ye, M.; Li, G.; Yan, P.; Ren, J.; Zheng, L.; Han, D.; Sun, S.; Huang, S.; Zhong, Y. Removal of metals from lead-zinc mine tailings using bioleaching and followed by sulfide precipitation. Chemosphere 2017, 185, 1189–1196. [Google Scholar] [CrossRef]
- Wei, X.; Viadero, R.C. Synthesis of magnetite nanoparticles with ferric iron recovered from acid mine drainage: Implications for environmental engineering. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 280–286. [Google Scholar] [CrossRef]
- Prokkola, H.; Nurmesniemi, E.-T.; Lassi, U. Removal of Metals by Sulphide Precipitation Using Na2S and HS−-Solution. ChemEngineering 2020, 4, 51. [Google Scholar] [CrossRef]









| Metal | [7] | [23] | [24] | [25] | [26] | [27] | This Study | Price a,b |
|---|---|---|---|---|---|---|---|---|
| Cu | 345 | 378 | 389 | 360 | 408 | 230 | 435 ± 54 | 7.7 |
| Sn | nm | 25.5 | 24.9 | nm | 16.0 | nm | 32.9 ± 3.7 | 26.6 |
| Al | nm | 6.1 | 9.6 | 6.6 | nm | 10.3 | 19.6 ± 0.9 | 2.0 |
| Fe | 105.7 | 48.5 | 107.9 | 10.5 | 2.8 | 38.3 | 12.6 ± 0.2 | 0.2 c |
| Ni | 26.3 | 25.4 | 17.3 | 8.5 | 3.9 | 11.5 | 11.4 ± 1.1 | 14.5 |
| Pb | 18.7 | nm | 16.7 | 12.1 | 13.6 | 1.2 | 5.9 ± 1.4 | 1.8 |
| Zn | nm | 18.2 | 3.3 | 7.9 | 4.1 | 3.0 | 4.4 ± 1.1 | 2.4 |
| Pd | nm | 12.3 | 0.14 | 0.6 | <0.1 | nm | 0.6 ± 0.1 | 69,090.7 |
| Au | <0.01 | 0.9 | 1.6 | 0.1 | <0.1 | 0.32 | nm | 46,977.8 |
| Ag | 2.1 | 0.5 | 4.0 | 0.3 | 1.1 | nm | nm | 698.9 |
| Metal | Removal Efficiency (%) | |
|---|---|---|
| PCB Piece | Powder PCB | |
| Cu | 67.9 | 78.2 |
| Ni | 36.7 | 54.1 |
| Zn | 52.8 | 33.8 |
| Pb | 2.9 | 13.9 |
| Fe | S | K | Sn | Cu | Ba | Ni | Ag | Ti | Ta | Au | Si | Zn | Nb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biologically promoted jarosite | 39.8 | 10.1 | 4.23 | 0.63 | 0.61 | 0.34 | 0.10 | 0.09 | bdl | bdl | 0.04 | 0.05 | 0.02 | bdl |
| Non-biologically promoted jarosite | 39.0 | 10.8 | 4.06 | 0.67 | 0.53 | bdl | bdl | 0.08 | 0.05 | 0.07 | bdl | 0.03 | bdl | 0.02 |
| bdl = below detection limit | ||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santaolalla, A.; Lens, P.N.L.; Barona, A.; Rojo, N.; Ocio, A.; Gallastegui, G. Metal Extraction and Recovery from Mobile Phone PCBs by a Combination of Bioleaching and Precipitation Processes. Minerals 2021, 11, 1004. https://doi.org/10.3390/min11091004
Santaolalla A, Lens PNL, Barona A, Rojo N, Ocio A, Gallastegui G. Metal Extraction and Recovery from Mobile Phone PCBs by a Combination of Bioleaching and Precipitation Processes. Minerals. 2021; 11(9):1004. https://doi.org/10.3390/min11091004
Chicago/Turabian StyleSantaolalla, Arrate, Piet N. L. Lens, Astrid Barona, Naiara Rojo, Ainhoa Ocio, and Gorka Gallastegui. 2021. "Metal Extraction and Recovery from Mobile Phone PCBs by a Combination of Bioleaching and Precipitation Processes" Minerals 11, no. 9: 1004. https://doi.org/10.3390/min11091004
APA StyleSantaolalla, A., Lens, P. N. L., Barona, A., Rojo, N., Ocio, A., & Gallastegui, G. (2021). Metal Extraction and Recovery from Mobile Phone PCBs by a Combination of Bioleaching and Precipitation Processes. Minerals, 11(9), 1004. https://doi.org/10.3390/min11091004