1. Introduction
Zinc hydrometallurgy, regardless of whether using traditional wet zinc smelting, high temperature, and high acid leaching or direct oxygen leaching, inevitably produces a huge amount of zinc-leaching residue (ZLR) [
1,
2]. ZLR contains a significant amount of valuable metals, such as zinc, silver, indium, lead, and copper [
3], which means the zinc-leaching slag has high economic and industrial value and places an emphasis on developing a method for their recovery [
4,
5]. Most metals in the zinc-leaching residue occur as sulfates, and except for lead sulfate, almost all of them leach into rainwater [
6,
7]. Heavy metal ions and sulfate ions can enter the surface via rainwater and pollute the environment [
8], which must be processed by high-temperature smelting. Heavy metals are difficult to decompose and readily accumulate in living organisms, potentially endangering the health of all organisms in the food chain [
9]. Zinc is an important component of heavy metal pollutants, as it pollutes both the soil and water [
10]. The massive discharge of zinc-containing liquids and solid wastes not only causes serious environmental pollution but negatively impacts the human body and is classified as a hazardous waste [
11,
12]. Therefore, the comprehensive recovery of valuable elements from zinc-leaching residues is important both from the environmental and resource conservation standpoints.
Due to extremely high levels of heavy metals and their harm to the environment, these residues usually undergo treatment using adsorption, stabilization, and vitrification to reduce their toxicity [
10,
13,
14,
15,
16]. Furthermore, other pyrometallurgical and hydrometallurgical methods, such as hot acid leaching [
17], alkaline leaching [
18], caustic leaching [
19], and transformation roasting [
20,
21], have been developed to dispose of ZLR. However, these processes suffer from high costs, elevated pollution levels, and immature technology, as well as low metal recovery rates. These studies rarely focus on the recovery of Ag present in the ZLR. The mineral processing industry currently uses flotation to recover valuable minerals from the associated gangue minerals based on surface physiochemical property differences [
22]. This technology is inexpensive, easy to operate, and has a high separation efficiency for fine particles [
23]. The recovery of valuable metals from ZLR ores by flotation allows for complete resource utilization and reduced environmental harm.
The purpose of this study was to explore a clean and effective method to recover valuable metals from ZLR and avoid potential environmental pollution risks. In this study, the recoveries of Zn and Ag from ZLR samples were conducted by water leaching followed by flotation. Metal sulfates readily separate from the ZLR by water leaching, leaving a detoxification residue. Water leaching optimizes the pulp environment during flotation. The influences of grinding time, (C4H9O)2PSSNH4 concentration, (NaPO3)6 concentration, pulp density, and flotation time on Ag and Zn recoveries were also analyzed. Furthermore, the toxicity characteristic leaching procedure (TCLP) was used to investigate the environmental activity of ZLR and flotation tailings for a short contact time. The findings of this study provided a basis for an environmentally friendly and comprehensive utilization of hazardous ZLR.
2. Materials and Methods
2.1. Minerals and Analysis
The highly acidic samples of zinc-leaching residue were obtained from a zinc hydrometallurgy smelter in Yunnan Province, China. The samples were dried, mixed, and reduced for subsequent research.
Table 1 shows the multielement chemical analysis of the samples and the levels of Zn, Ag, and Fe in the sample. Additionally, the residue also contained toxic heavy metals such as Pb, Cu, and Sn. The mineralogical characterizations of ZLR are described in the Results and Discussion. All aqueous solutions were prepared with distilled water and analytical-grade reagents used for flotation.
The chemical compositions in the ZLR samples were analyzed by atomic absorption spectrometry (AAS) using the flame technique (SUPER, TAS-990AFG). The levels of heavy metals in the leaching solution were analyzed by inductively coupled plasma (ICP-OES, Opima 5300DVtype, PerkinElmer Waltham, MA, USA). Scanning electron microscope (MLA) and mineral liberation analyzer (MLA) were used to determine the mineralogical characterization and elemental composition of the ore samples, respectively.
2.2. Water Leaching
Water leaching dissolved the metal sulfates formed and detoxified the residue. A 100-g sample of ZLR was weighed and stirred at room temperature using a predetermined leaching time (5-20min) and liquid-to-solid ratio (1:1 to 6:1). Upon reaction completion, the filtered residue was dried and subjected to chemical analyses, and the filtrate was analyzed by ICP. Finally, the filtered residue was returned to the hydrometallurgical zinc process and used for flotation. The metal-leaching rates helped evaluate the water leaching effects and was determined as follows:
where
γ is the precious metal (or iron) recovery rate;
C1 is the zinc, copper, or iron amount in the leaching liquid; and
C2 is the total zinc, copper, or iron amount in the roasted residue.
2.3. Flotation Experiments
The grinding process used the XMQ-φ240×90 conical ball mill produced by the Wuhan Prospecting Machinery Factory (Wuhan, China). Flotation tests were conducted in a hanging tank flotation machine with 1.5-L, 0.75-L, and 0.5-L cell volumes. The 1.5-L flotation cell was used for rougher flotation and cleaning, and the 0.75- and 0.5-L cells were used for flotation cleanings at an impeller speed of 2000 r·min−1. In each rougher flotation test, a certain amount of deionized water was added to the flotation cell. The regulator, collector, and frother of predetermined dosages were added and the slurry stirred for three minutes. Air was introduced at a rate of 0.3 L/h. After flotation, the concentrate, middling, and tailings were filtered, dried, and weighed, and grades of silver and zinc in the product were obtained through chemical analysis. According to the yield and grade of the product, the final calculation yielded the recovery rates of silver and zinc in the product. All of tests were repeated three times under the same conditions; the test results reported in this paper were the averages.
2.4. Toxicity Characteristic Leaching Procedure
The toxicity characteristic leaching procedure (TCLP) of heavy metals in the ZLR and tailings were conducted according to the Solid Waste-Extraction procedure for leaching toxicity—sulfuric acid and nitric acid method (HJ/T299-2007) implemented in 2007, China. Additional experiments determined the changes in pH.
The ZLR and tailings samples were crushed and sieved through a 9.5-mm sieve to remove oversized particles. A 50-g sample was weighed and heated at 105 °C for at least 12 h to stay dry. A sulfuric acid and nitric acid (mass ratio 2:1) solution was adjusted to a pH = 3.20 ± 0.05 and used as the leaching solution (liquid/solid ratio of 10:1). The flask was sealed and put into an oscillator; the flip oscillation process was kept for 18 h, and the filtrate was collected. The heavy metal-leaching toxicity was calculated after determining their concentrations once the separation concluded. All TCLP experiments were carried out in duplicate.
3. Results and Discussion
3.1. Technological Mineralogy
The process mineralogy of ZLR yielded the elemental composition, mineral composition, mineral symbiosis, mineral dissociation degree characteristics, and the occurrence of target elements; it also provided a reference to help formulate a mineral processing technology. According to mineralogical studies, the valuable minerals in the ore mainly included sphalerite, zinc sulfate, lead alum, hematite, chalcopyrite, and cassiterite; the gangue minerals mainly comprised diopside, quartz, anhydrite, gypsum, and biotite. Moreover, the independent silver minerals were native silver and argentite, and the independent zinc minerals were sphalerite and zinc sulfate.
Table 2 and
Table 3 show the forms of silver in ZLR and the relative amounts of various minerals. Native silver and argentite contained 37.09% and 12.52% of Ag, respectively, with 44.10% embedded in other minerals.
Table 4 summarizes the release rates of the primary ZLR minerals, as well as MLA and optical microscope analyses. The release degree of elemental silver, silverite and sphalerite was very low, which indicated the dissemination size of those minerals was extremely fine and a complex association and dissemination relationship between these minerals. Microscopic images of the target minerals in the ZLR sample are shown in
Figure 1. These images show that sphalerite crystals were the main Ag carrier mineral and closely associated with gangue such as quartz, talc, and diopside (i.e., a secondary mineral). Native silver and argentite were irregular granular, foggy, and flocculent and distributed in sphalerite cavities or attached to the sphalerite edge.
3.2. Water Leaching
3.2.1. Effect of Leaching Conditions on Precious Metal Recoveries
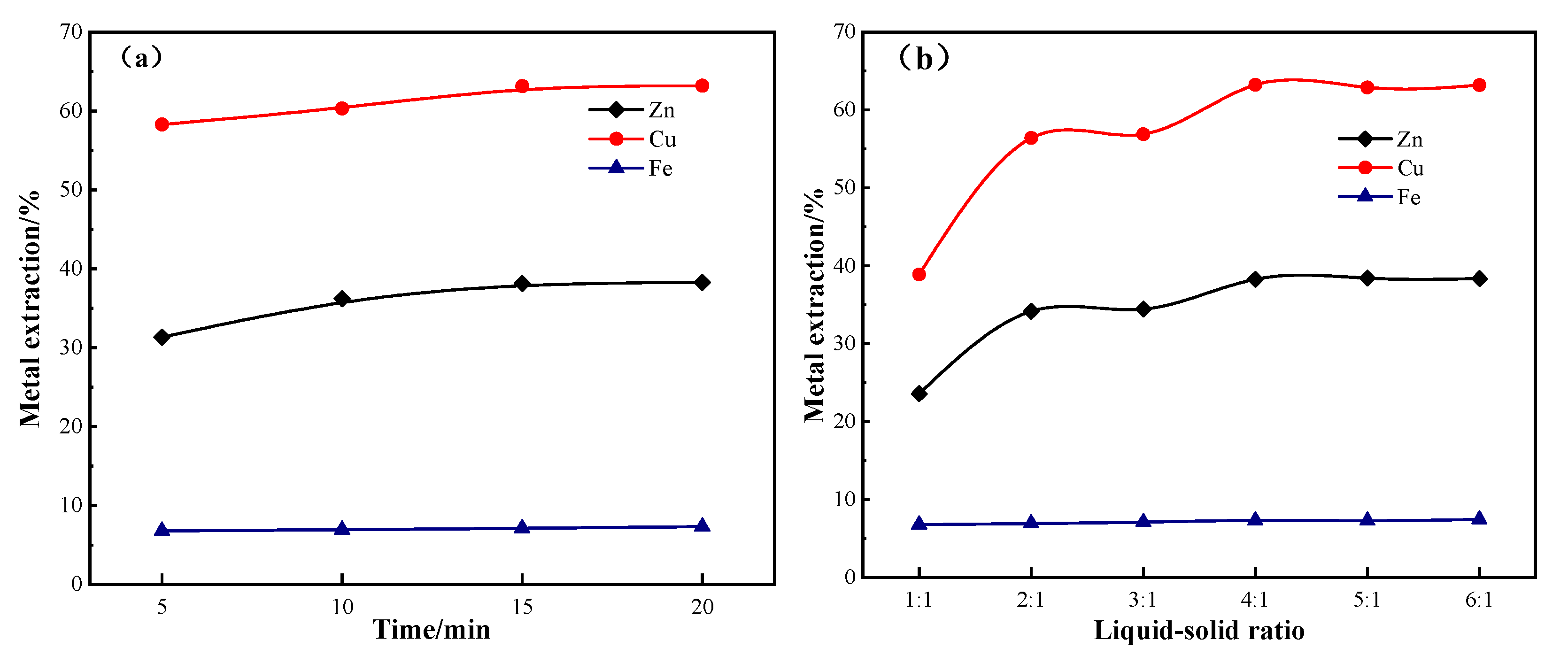
Water leaching of the ZLR improved the precious metal recovery rates in the flotation process and optimized the pulp environment. The experiments examined the influence of leaching time and liquid–solid ratio on the leaching rates of zinc, copper, iron, and silver enrichment (shown in
Figure 2). The leaching times and liquid-to-solid ratios (
Figure 2a,b) had a greater impact on the leaching rates of copper and zinc but less so for iron. It showed that, under the leaching time (15 min) and liquid–solid ratio (4:1), the recovery rate of zinc, copper, and iron was optimal. Under optimal conditions, the ionic concentrations in the leaching solution are shown in
Table 5. Most of the leaching solution containing zinc sulfate was recycled into the zinc hydrometallurgy process.
3.2.2. Chemical Composition and Phase Analysis of Water-Leached Residue
Table 6 shows the chemical composition of the sample after water leaching under the optimal conditions. Compared with the raw ZLR, the levels of Zn and other toxic heavy metals such as Cu and Fe decreased; however, the levels of Pb and Ag increased, and their amounts accounted for 2.9% and 822.4 g/t, respectively.
The filter residue phases were analyzed and showed the residue no longer contained the characteristic diffraction peaks of soluble sulfates (
Figure 3). After water leaching, the primary components remaining in the filter residue were CaSO
4, CaSO
4·2H
2O, and PbSO
4.
3.3. Flotation Experiments
3.3.1. Single-Factor Experiments
A mineralogical analysis of the ZLR showed a remarkably high liberation of wrapped silver and suggested that additional fine grinding was necessary.
Figure 4a shows the effects of grinding fineness on the flotation results. The flotation recovery rates gradually increased and then decreased with increasing the grinding time. The particle size of the ZLR may have been too fine; this created significant mud that affected the flotation of silver and zinc [
24]. The results of the pH testing (
Figure 4b) showed optimized recovery rates for zinc and silver that occurred at pH 3–5, which agreed with the other reports [
25]. The effect of the collector concentration on the flotation behavior of the ZLR is shown in
Figure 4c. In the presence of ammonium dibutyl dithiophosphate, the recovery increased sharply as the collector concentrations increased to 700 g/t, exceeding 80% at that concentration.
Figure 4d shows the flotation results at different pulp concentrations, with the optimal results at a 20% pulp concentration. This may be due to the fine nature of the mineral particles and that the slag needs dispersion.
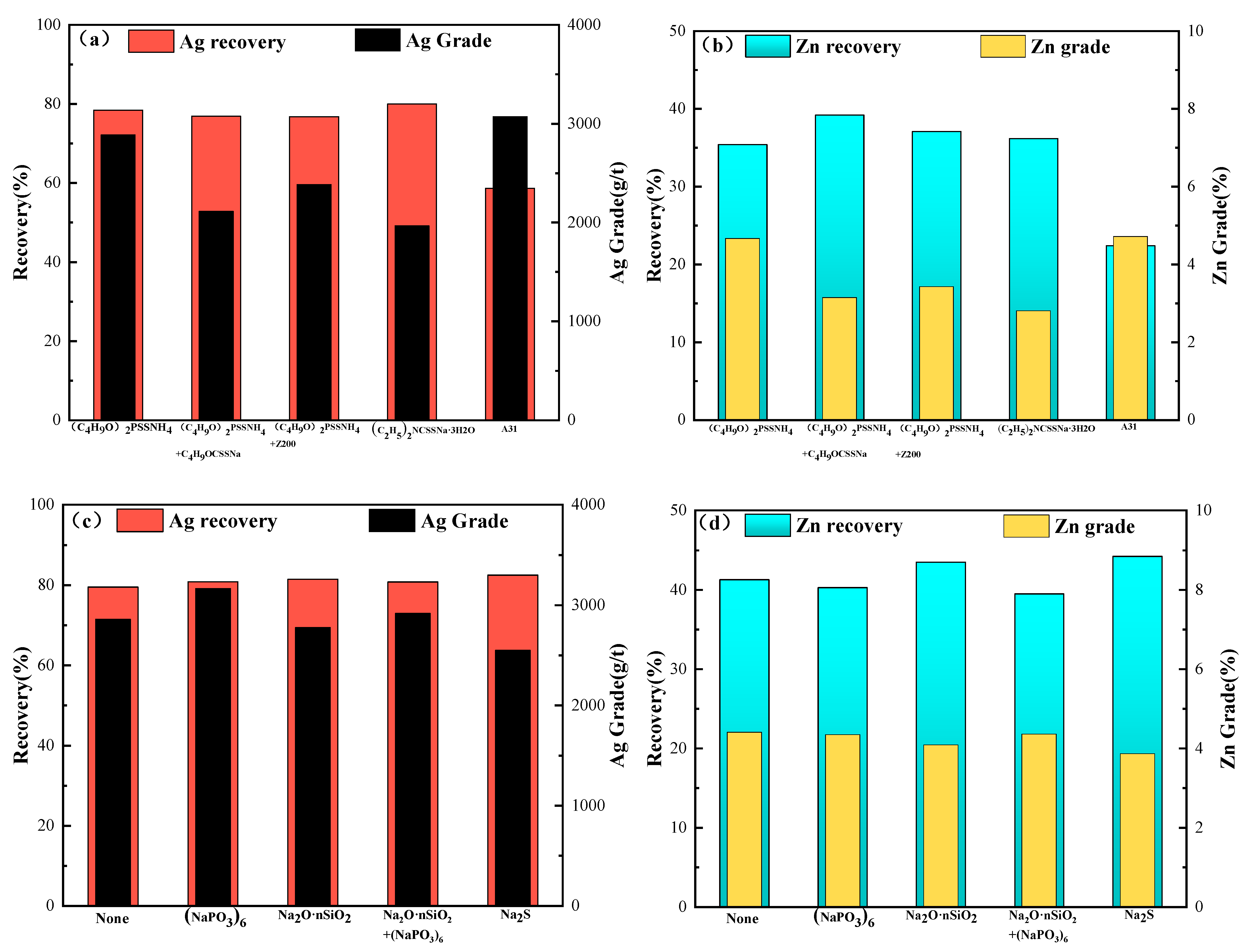
The effects of different collectors and regulators on the flotation were tested;
Figure 5a,b presents the residue grades and recoveries, respectively.
Figure 6a,b shows that using only butylamine black resulted in optimized recovery rates and grades for both silver and zinc. Adding the regulators sodium hexametaphosphate and sodium silicate both improved the flotation, which was observed as foaming during testing.
Figure 5 shows the recovery rates and grades for silver at different times and ultimately yielded three minutes as the optimized rough selection time. After a comprehensive study, the optimized experimental conditions were: grinding time = 5 min, pH 3–5, (C
4H
9O)
2PSSNH
4 concentration = 700 g/t, (NaPO
3)
6 concentration = 500 g/t, pulp density = 20%, and flotation time = 3 min.
3.3.2. Closed Circuit Flotation Experiments
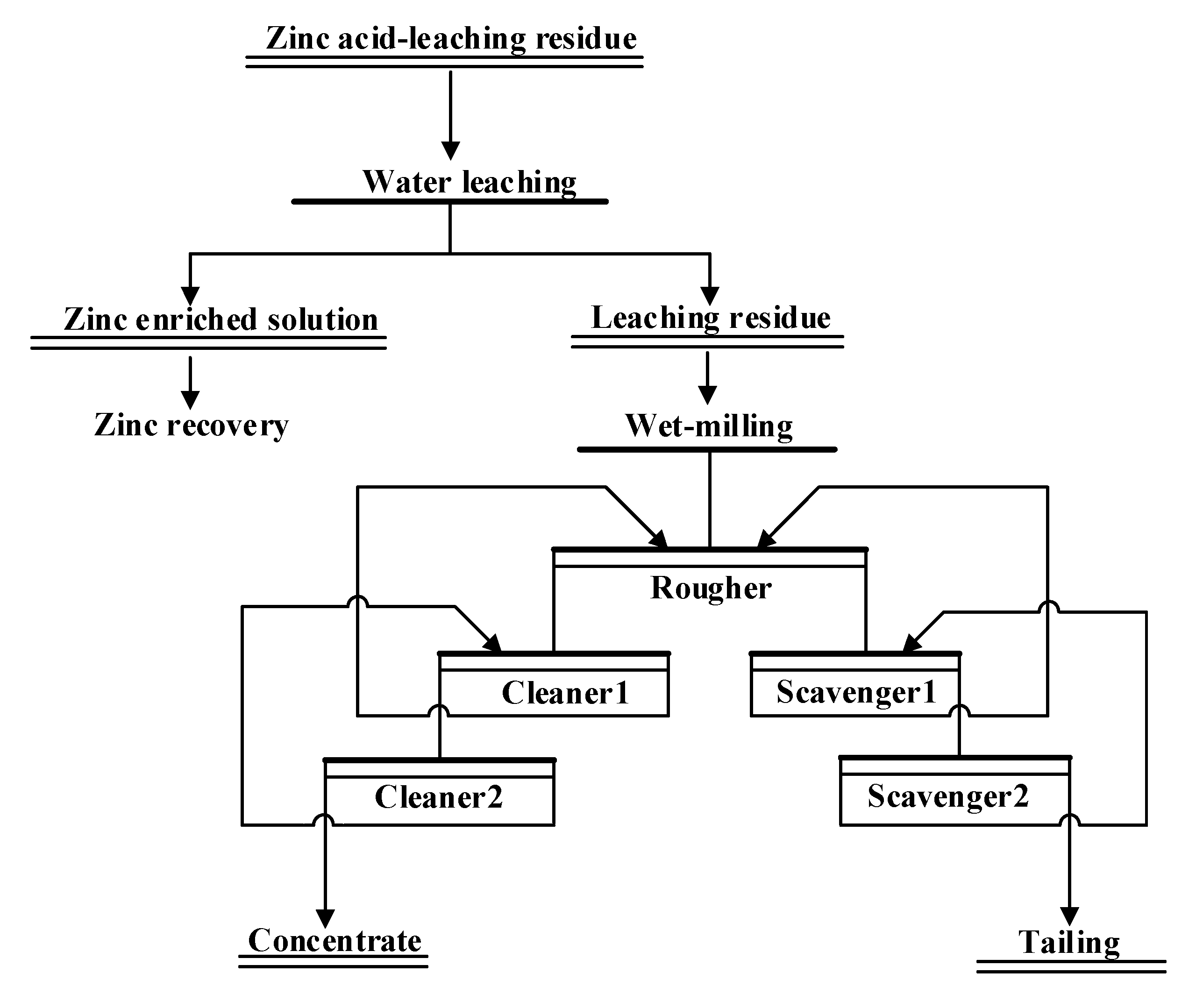
Based on the above experimental results, using the optimized test conditions, a closed-circuit flotation test of single-stage roughing, two-stage cleaning, and two-stage scavenging was performed.
Figure 7 shows the detailed flow chart, and
Table 7 gives the results. Each closed flotation experiment underwent five cycles. The weight of the concentrate and tailings and the grade and recovery of Zn and Ag in the concentrate and tailings remained constant during the last two cycles, which indicated an arrival of a balance between the metal quality and amount. The experimental results indicated the amount of silver in the concentrate was 9256.41 g/t, which corresponded to an 80.32% recovery, while the zinc grade was 12.26%, with a 42.88% recovery. In addition,
Table 8 shows the concentrate contained enriched copper, and the copper grade was 1.81%. The selective flotation of sphalerite, argentite, and elemental silver from the ZLR was achieved using C
4H
9OCSSNa as the collector in the absence of an activator.
The phases of the silver concentrate are shown in
Figure 8 and contain diffraction peaks characteristic of many valuable substances, such as Ag
2S, Ag, ZnS, and S, which indicated the enrichment of these substances during flotation.
3.4. Leaching Toxicity
The TCLP method simulated the process in which hazardous components enter the environment via wastewater due to acid rainfall after waste burial or long-term storage. Finally, the leaching toxicity of ZLR and flotation tailings were evaluated (
Table 9 and
Table 10) and showed that the ZLR concentrations for Cu, Zn, Cd, and Be were 127 mg/L, 1305 mg/L, 21.3 mg/L, and 0.039 mg/L, respectively, all of which far exceeded the established thresholds of 100, 100, 1, and 0.02 ppm, respectively [
26], and clearly indicated that ZLR poses a significant biohazard. However, after water-leaching flotation, the Cu, Zn, Cd, and Be concentrations decreased sharply to 2.6 mg/L, 45 mg/L, 0.94 mg/L, and 0.0078 mg/L, respectively, all well below the leaching toxicity limits. Moreover, the leaching concentrations of other impurities such as Pb, Cr, As, etc. were lower than the established thresholds. This was mainly due to toxic elements entering the leachate and flotation tail waters during precious metal recoveries by this water-leaching flotation process [
27,
28]. Therefore, the final flotation residue poses little environmental harm and can be safely stockpiled for future treatment or recycling.
4. Conclusions
This paper advances an alternate and environmentally benign method for the treatment of hazardous zinc-leaching residue waste. A combination of water-leaching and flotation recovers zinc and silver from ZLR and ultimately detoxifies the residual ZLR. The following conclusions were drawn:
(1) The hazardous ZLR primarily contained Zn, Pb, Cu, Fe, and Ag; sphalerite, zinc sulfate, lead alum, hematite, quartz, anhydrite, and gypsum were the main phases.
(2) Water leaching recovered a portion of the precious metals (Zn, Cu, and Fe) from the ZLR and increased the recovery rate of the subsequent flotation tests. The leaching solution was returned to the zinc-smelting process without pollution.
(3) Different parameters were optimized during the flotation tests, such as grinding time, pulp density, cumulative time, concentration of (C4H9OCSSNa), and flotation adjusters. These were obtained with one round of roughing, two rounds of scavenging, and two rounds of cleaning. These optimized parameters achieved a silver concentrate grade of 9256.41 g/t and a silver recovery grade of 80.32%.
(4) Moreover, this treatment lowered the heavy metal-leaching concentrations below the leaching toxicity limits and rendered the final tailings environmentally benign. This sustainable method provided a highly efficient, cost-effective, and environmentally friendly treatment of industrial ZLR waste.
Author Contributions
Y.D. and X.T. conceived and designed the experiments; X.X. and W.Z. performed the experiments and analyzed the date; Q.S. contributed reagents and materials; H.Y. and Y.D. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The first author gratefully acknowledges financial support provided by the National Natural Science Foundation of China (51764024 and 51764025).
Data Availability Statement
Data available on request due to restrictions privacyl. The data provided in this study can be obtained at the request of the corresponding author. As the data needs further research, the data is currently not publicly available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Min, X.-B.; Xie, X.-D.; Chai, L.-Y.; Liang, Y.-J.; Li, M.; Ke, Y. Environmental availability and ecological risk assessment of heavy metals in zinc leaching residue. Trans. Nonferrous Met. Soc. China 2013, 23, 208–218. [Google Scholar] [CrossRef]
- Ruşen, A.; Sunkar, A.; Topkaya, Y. Zinc and lead extraction from Çinkur leach residues by using hydrometallurgical method. Hydrometall. 2008, 93, 45–50. [Google Scholar] [CrossRef]
- Altundoǧan, H.S.; Erdem, M.; Orhan, R.; Özer, A.; Tümen, F. Heavy Metal Pollution Potential of Zinc Leach Residues Dis-carded in inkur Plant. Turk. J. Eng. Environ. Sci. 2014, 22, 167–177. [Google Scholar]
- Turan, M.D.; Altundoğan, H.S.; Tümen, F. Recovery of zinc and lead from zinc plant residue. Hydrometall. 2004, 75, 169–176. [Google Scholar] [CrossRef]
- Ju, S.; Zhang, Y.; Zhang, Y.; Xue, P.; Wang, Y. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy. J. Hazard. Mater. 2011, 192, 554–558. [Google Scholar] [CrossRef]
- Erdem, M.; Özverdi, A. Environmental risk assessment and stabilization/solidification of zinc extraction residue: II. Stabilization/solidification. Hydrometallurgy 2010, 105, 270–276. [Google Scholar] [CrossRef]
- Li, C.; Zhong, H.; Wang, S.; Xue, J. Leaching Behavior and Risk Assessment of Heavy Metals in a Landfill of Electrolytic Man-ganese Residue in Western Hunan, China. Hum. Ecol. Risk Assess. Int. J. 2014, 20, 1249–1263. [Google Scholar] [CrossRef]
- Zeng, F.; Wei, W.; Li, M.; Huang, R.; Yang, F.; Duan, Y. Heavy Metal Contamination in Rice-Producing Soils of Hunan Province, China and Potential Health Risks. Int. J. Environ. Res. Public Health 2015, 12, 15584–15593. [Google Scholar] [CrossRef]
- Cao, D.-Q.; Wang, X.; Wang, Q.-H.; Fang, X.-M.; Jin, J.-Y.; Hao, X.-D.; Iritani, E.; Katagiri, N. Removal of heavy metal ions by ultrafiltration with recovery of extracellular polymer substances from excess sludge. J. Membr. Sci. 2020, 606, 118103. [Google Scholar] [CrossRef]
- Çoruh, S.; Ergun, O.N. Use of fly ash, phosphogypsum and red mud as a liner material for the disposal of hazardous zinc leach residue waste. J. Hazard. Mater. 2010, 173, 468–473. [Google Scholar] [CrossRef]
- Stylianou, M.A.; Hadjiconstantinou, M.P.; Inglezakis, V.J.; Moustakas, K.G.; Loizidou, M.D. Use of natural clinoptilolite for the removal of lead, copper and zinc in fixed bed column. J. Hazard. Mater. 2007, 143, 575–581. [Google Scholar] [CrossRef]
- Çoruh, S. The removal of zinc ions by natural and conditioned clinoptilolites. Desalination 2007, 225, 41–57. [Google Scholar] [CrossRef]
- Demır, G.; Coruh, S.; Ergun, O.N. Leaching behavior and immobilization of heavy metals in zinc leach residue before and after thermal treatment. Environ. Prog. 2008, 27, 479–486. [Google Scholar] [CrossRef]
- Kul, M.; Topkaya, Y. Recovery of germanium and other valuable metals from zinc plant residues. Hydrometallugry 2008, 92, 87–94. [Google Scholar] [CrossRef]
- Vahidi, E.; Rashchi, F.; Moradkhani, D. Recovery of zinc from an industrial zinc leach residue by solvent extraction using D2EHPA. Miner. Eng. 2009, 22, 204–206. [Google Scholar] [CrossRef]
- Li, Y.-C.; Min, X.-B.; Chai, L.-Y.; Shi, M.-Q.; Tang, C.-J.; Wang, Q.-W.; Liang, Y.-J.; Lei, J.; Liyang, W.-J. Co-treatment of gypsum sludge and Pb/Zn smelting slag for the solidification of sludge containing arsenic and heavy metals. J. Environ. Manag. 2016, 181, 756–761. [Google Scholar] [CrossRef]
- Elgersma, F.; Witkamp, G.J.; Rosmalen, G.M.V. Simultaneous dissolution of zinc ferrite and precipitation of ammonium jar-osite. Hydrometallurgy 1993, 34, 23–47. [Google Scholar] [CrossRef]
- Dutra, A.; Paiva, P.; Tavares, L. Alkaline leaching of zinc from electric arc furnace steel dust. Miner. Eng. 2006, 19, 478–485. [Google Scholar] [CrossRef]
- Xia, D.; Picklesi, C. Microwave caustic leaching of electric arc furnace dust. Miner. Eng. 2000, 13, 79–94. [Google Scholar] [CrossRef]
- Holloway, P.C.; Etsell, T.H.; Murland, A.L. Roasting of La Oroya Zinc Ferrite with Na2CO3. Met. Mater. Trans. A 2007, 38, 781–791. [Google Scholar] [CrossRef]
- Holloway, P.C.; Etsell, T.H. Recovery of zinc, gallium and indium from La Oroya zinc ferrite using Na2CO3 roasting. Miner. Process. Extr. Met. 2008, 117, 137–146. [Google Scholar] [CrossRef]
- Zhang, L.; Khoso, S.A.; Tian, M.; Sun, W. Indium pre-enrichment from a Canadian sulphide ore via flotation technique. Miner. Eng. 2020, 156, 106481. [Google Scholar] [CrossRef]
- Gulden, S.; Riedele, C.; Kopf, M.-H.; Nirschl, H. Potential of flotation as alternative separation process in biotechnology with focus on cost and energy efficiency. Chem. Eng. Sci. 2020, 218, 115117. [Google Scholar] [CrossRef]
- Zhu, X.N.; Ni, Y.; Wang, D.Z.; Zhang, T.; Qu, S.J.; Qiao, F.M.; Ren, Y.G.; Nie, C.C.; Lyu, X.J.; Qiu, J.; et al. Effect of dissociation size on flotation behavior of waste printed circuit boards. J. Clean. Prod. 2020, 265, 21840. [Google Scholar] [CrossRef]
- Foroutan, A.; Abadi, M.A.Z.H.; Kianinia, Y.; Ghadiri, M. Critical importance of pH and collector type on the flotation of sphalerite and galena from a low-grade lead–zinc ore. Sci. Rep. 2021, 11, 3103. [Google Scholar] [CrossRef] [PubMed]
- GB5085.3-2007. Hazardous Wastes Distinction Standard-Leaching Toxicity Distinction; China Environmental Science Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Zou, D.; Li, H.; Deng, Y.; Chen, J.; Bai, Y. Recovery of lanthanum and cerium from rare earth polishing powder wastes utilizing acid baking-water leaching-precipitation process. Sep. Purif. Technol. 2020, 261, 118244. [Google Scholar] [CrossRef]
- Jiang, G.M.; Bing, P.E.N.G.; Liang, Y.J.; Chai, L.Y.; Wang, Q.W.; Li, Q.Z.; Ming, H.U. Recovery of valuable metals from zinc leaching residue by sulfate roasting and water leaching. Trans. Nonferrous Met. Soc. China 2017, 27, 1180–1187. [Google Scholar] [CrossRef]
Figure 1.
SEM images of ZLR and the dissemination characteristic of the main minerals.
Figure 1.
SEM images of ZLR and the dissemination characteristic of the main minerals.
Figure 2.
Effects of leaching parameters on the valuable metal recovery: (a) time and (b) liquid–solid ratio.
Figure 2.
Effects of leaching parameters on the valuable metal recovery: (a) time and (b) liquid–solid ratio.
Figure 3.
XRD pattern of the residue after water leaching.
Figure 3.
XRD pattern of the residue after water leaching.
Figure 4.
Effects of the flotation parameters on recovery: (a) grinding time, (b) pH, (c) proportion of (C4H9O)2PSSNH4, and (d) pulp density.
Figure 4.
Effects of the flotation parameters on recovery: (a) grinding time, (b) pH, (c) proportion of (C4H9O)2PSSNH4, and (d) pulp density.
Figure 5.
Recoveries and grades of Ag in rough concentrates as a function of the proportion of the cumulative time.
Figure 5.
Recoveries and grades of Ag in rough concentrates as a function of the proportion of the cumulative time.
Figure 6.
Effects of the flotation reagent selection on the recovery: (a) collector on Ag, (b) collector on Zn, (c) adjustor on Ag, and (d) adjustor on Zn.
Figure 6.
Effects of the flotation reagent selection on the recovery: (a) collector on Ag, (b) collector on Zn, (c) adjustor on Ag, and (d) adjustor on Zn.
Figure 7.
Flow chart for zinc and silver recoveries from the zinc acid-leaching residue.
Figure 7.
Flow chart for zinc and silver recoveries from the zinc acid-leaching residue.
Figure 8.
XRD pattern of the silver concentrate after flotation.
Figure 8.
XRD pattern of the silver concentrate after flotation.
Table 1.
Main chemical compositions of ZLR (mass fraction, %).
Table 1.
Main chemical compositions of ZLR (mass fraction, %).
| Elements | Cu | Pb | Zn | Fe | Ag* | S | In* | Sn | SiO2 |
|---|
| Content (wt.%) | 0.27 | 1.99 | 3.06 | 5.21 | 720.9 | 12.11 | 82.6 | 0.40 | 29.00 |
Table 2.
Relative amounts (%) of the major ZLR minerals.
Table 2.
Relative amounts (%) of the major ZLR minerals.
| Mineral | Chemical Formula | Content | Mineral | Chemical Formula | Content |
|---|
| Argentite | Ag2S | 0.015 | Magnetite | Fe3O4 | 0.31 |
| Elemental silver | Ag | 0.037 | Pyrite | FeS2 | 0.1 |
| Sphalerite | (Zn, Fe)S | 2.27 | Pyrrhotite | Fe1−xS | 0.04 |
| Zinc sulfate | Zn[SO4]·6H2O | 6.54 | Arsenopyrite | FeAsS | 0.08 |
| Chalcopyrite | CuFeS2 | 0.27 | Diopside | CaMg[Si2O6] | 18.28 |
| Copper blue | CuS | 0.13 | Quartz | SiO2 | 19.89 |
| Copper sulfate | Cu[SO4]·5H2O | 0.38 | Anhydrite | Ca[SO4] | 18.05 |
| Cassiterite | SnO2 | 0.49 | Gypsum | Ca(SO4) 2H2O | 11.13 |
| Galena | PbS | 0.002 | Biotite | K{(Mg, Fe)3[AlSi3O10](OH)2} | 2.84 |
| Lead alum | (Pb, Sr)SO4 | 3.8 | Potassium feldspar | (K, Na)[AlSi3O8] | 2.08 |
| Lead oxide | PbO | 0.01 | Pearl mica | CaAl4Si2O10(OH)2 | 1.63 |
| Elemental sulfur | S | 3.36 | Talc | Mg3Si4O10(OH)2 | 1.54 |
| Hematite | Fe2O3 | 6.32 | Pyrolusite | MnO2 | 0.2 |
| Calcite | Ca[CO3] | 0.04 | Dolomite | CaMg[CO3]2 | 0.1 |
| Rutile | TiO2 | 0.01 | Barite | Ba[SO4] | 0.06 |
Table 3.
The physical phase composition of silver in the acid-leaching residue.
Table 3.
The physical phase composition of silver in the acid-leaching residue.
| Phase Composition | Ag | Ag2O | Ag2SO4 | Ag2S | Wrapped Ag |
|---|
| Ag content(g/t) | 278.20 | 30.95 | 93.90 | 13.60 | 330.80 |
| Phase occupation ratio (%) | 37.09 | 4.13 | 1.81 | 12.52 | 44.10 |
Table 4.
Liberation degrees (%) of the primary ZLR minerals.
Table 4.
Liberation degrees (%) of the primary ZLR minerals.
| Mineral | Liberation Degree | Mineral | Liberation Degree |
|---|
| Elemental silver | 30.68 | Copper blue | 82.83 |
| Argentite | 9.72 | Cassiterite | 85.9 |
| Sphalerite | 52.93 | Lead alum | 70.31 |
| Chalcopyrite | 81.88 | Hematite | 72.59 |
Table 5.
Ion concentrations in the leaching solution.
Table 5.
Ion concentrations in the leaching solution.
| Elements | Pb (mg/L) | Zn (g/L) | Ag (mg/L) | Fe (g/L) | Cu (g/L) | In (mg/L) |
|---|
| Content | 5.12 | 3.30 | 0.15 | 1.34 | 0.24 | 4.15 |
Table 6.
Chemical composition of the water-leached residue (mass fraction, %).
Table 6.
Chemical composition of the water-leached residue (mass fraction, %).
| Elements | Cu | Pb | Zn | Fe | Ag * |
|---|
| Content (wt.%) | 0.103 | 2.90 | 1.90 | 4.86 | 822.4 |
Table 7.
Iron and zinc distribution in magnetic and nonmagnetic products.
Table 7.
Iron and zinc distribution in magnetic and nonmagnetic products.
| Products | Production Ratio | Ag Grade (g/t) | Zinc Grade | Ag Distribution (%) | Zinc Distribution (%) |
|---|
| Concentrate | 6.28 | 9256.41 | 12.26 | 80.32 | 42.88 |
| Tailing | 93.72 | 145.2 | 1.45 | 19.68 | 57.12 |
| Leaching residue | 100 | 822.4 | 1.90 | 100 | 100 |
Table 8.
Chemical composition of the concentrate.
Table 8.
Chemical composition of the concentrate.
| Elements | Ag (g/t) | Zn | Cu | Fe | Pb |
|---|
| Content (wt.%) | 9256.41 | 12.26 | 1.81 | 6.31 | 1.98 |
Table 9.
Results of solid waste corrosion identification.
Table 9.
Results of solid waste corrosion identification.
| Sample | pH |
|---|
| ZLR | 1.45 |
| Tailing | 6.03 |
Table 10.
TCLP test results of the ZLR and water-leaching residues (mg/L).
Table 10.
TCLP test results of the ZLR and water-leaching residues (mg/L).
| Element | ZLR | Tailings | Regulatory Threshold (China) | Regulatory Threshold (USEPA) |
|---|
| Cu | 127 | 2.6 | 100 | — |
| Zn | 1305 | 45 | 100 | — |
| Cd | 21.3 | 0.94 | 1 | 1 |
| Pb | 3.6 | 0.3 | 5 | 5 |
| Cr | 0.11 | 0.05L | 5 | 5 |
| Hg | 0.00015 | 0.00037 | 0.1 | 0.2 |
| Be | 0.039 | 0.0078 | 0.02 | — |
| Ba | 0.1L | 3.2 | 100 | — |
| Ni | 0.17 | 0.04L | 5 | — |
| Se | 0.0004 | 0.0008 | 1 | 1 |
| As | 4.76 | 0.785 | 5 | 5 |
| Fluoride | 0.66 | 0.42 | 100 | — |
| cyanide | 0.004L | 0.005 | 5 | — |
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).