Depositional Environment and Genesis of the Nabeba Banded Iron Formation (BIF) in the Ivindo Basement Complex, Republic of the Congo: Perspective from Whole-Rock and Magnetite Geochemistry
Abstract
1. Introduction
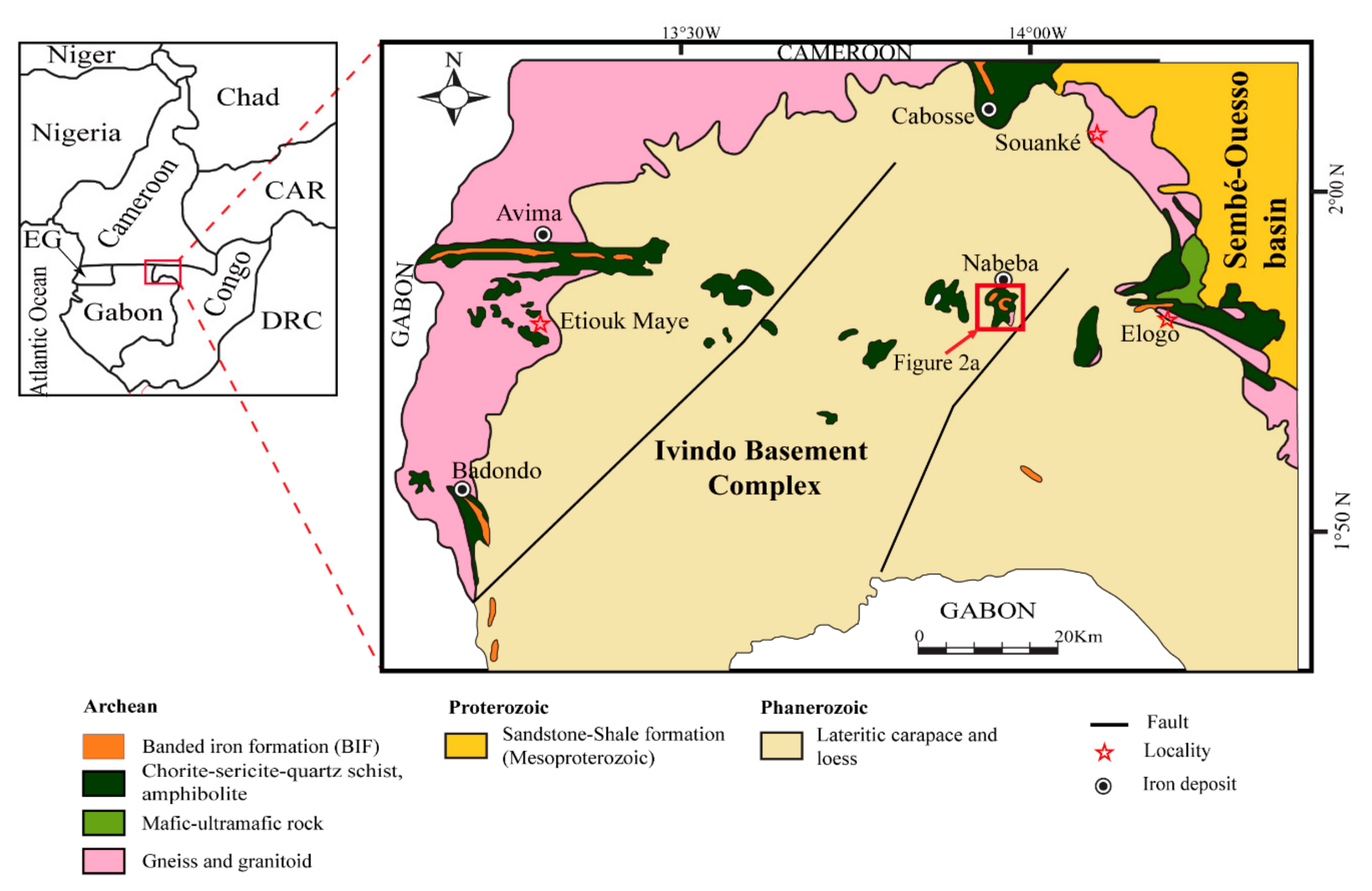
2. Regional Geology
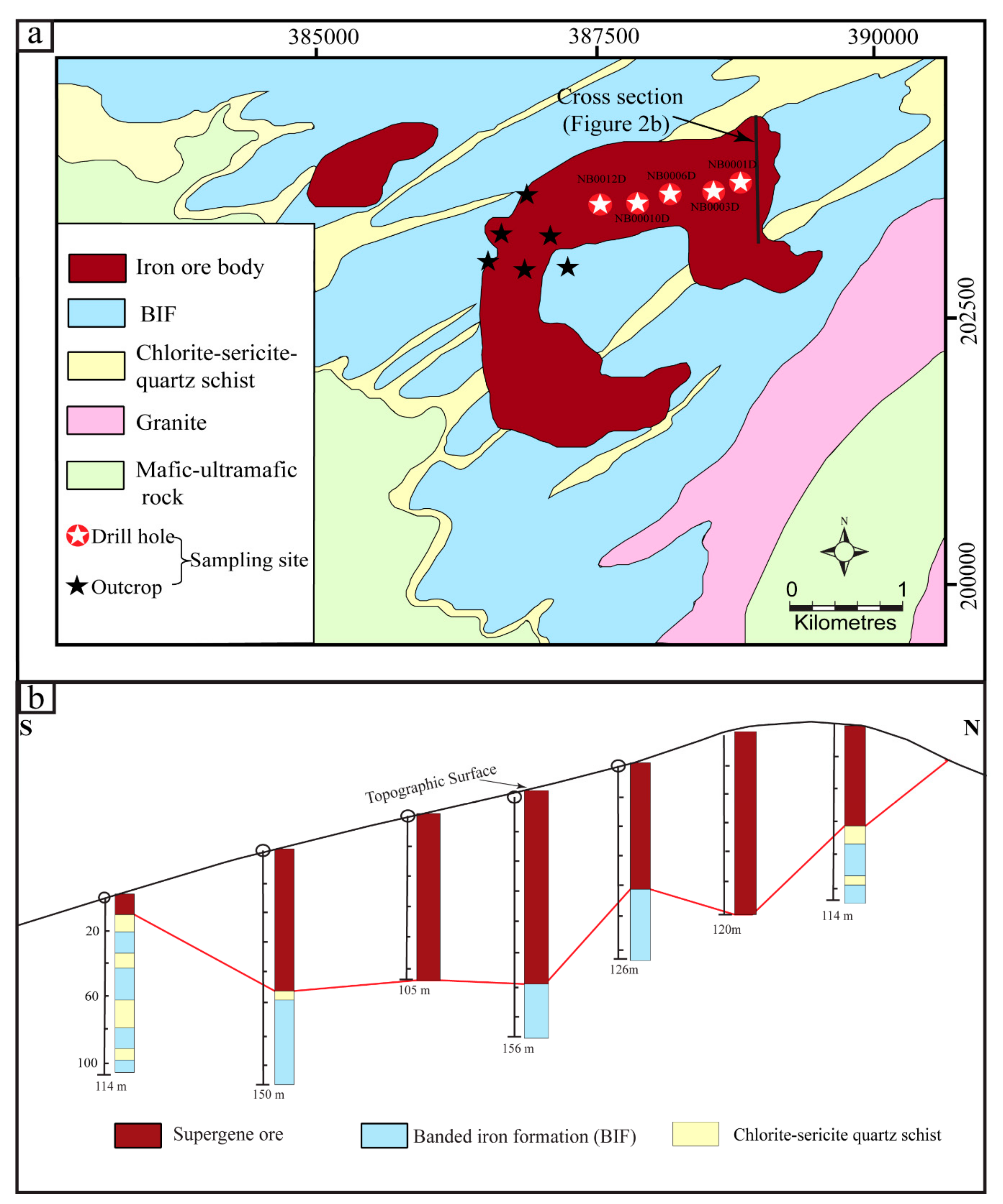
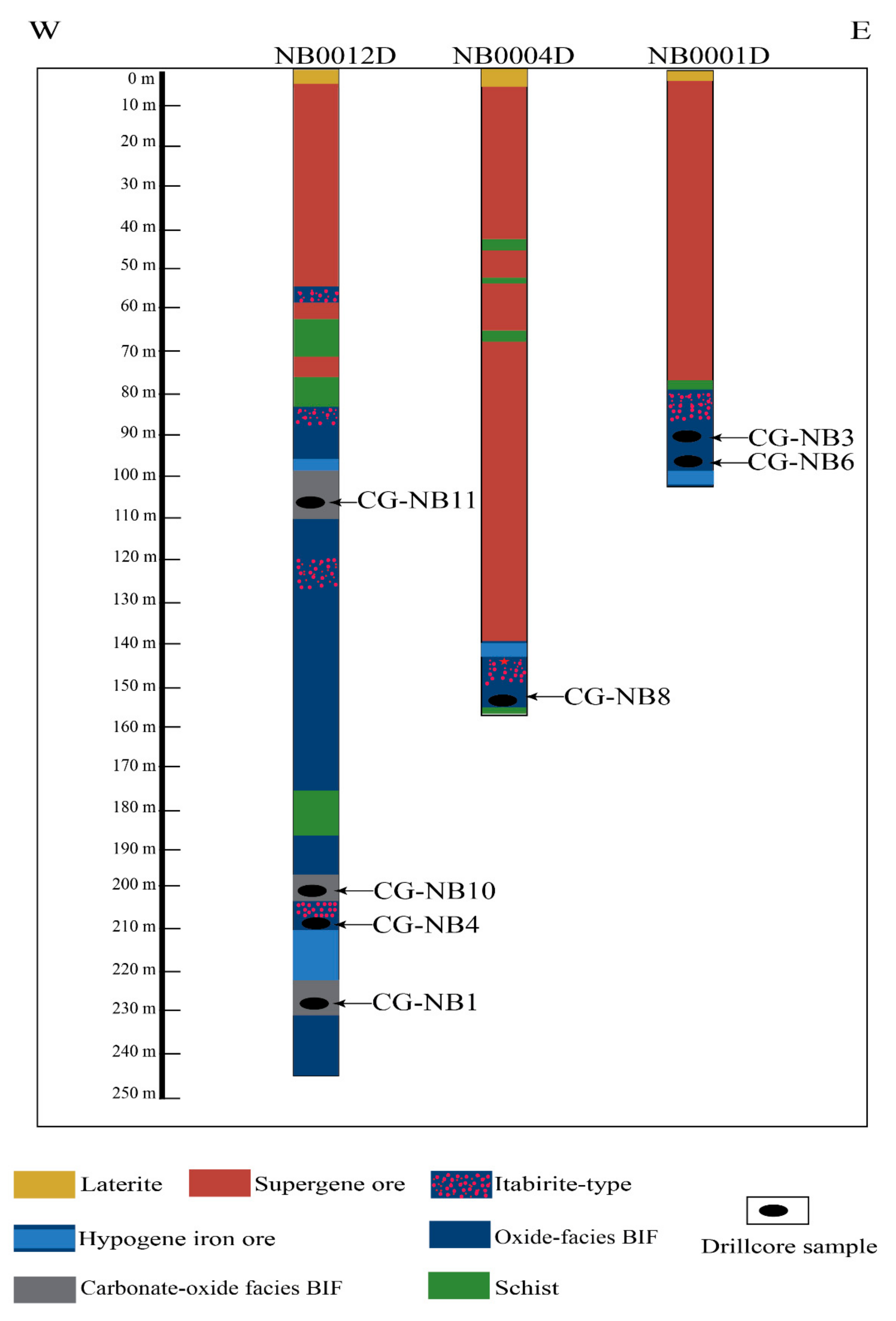
3. Deposit Geology
4. Sampling and Analytical Methods
5. Results
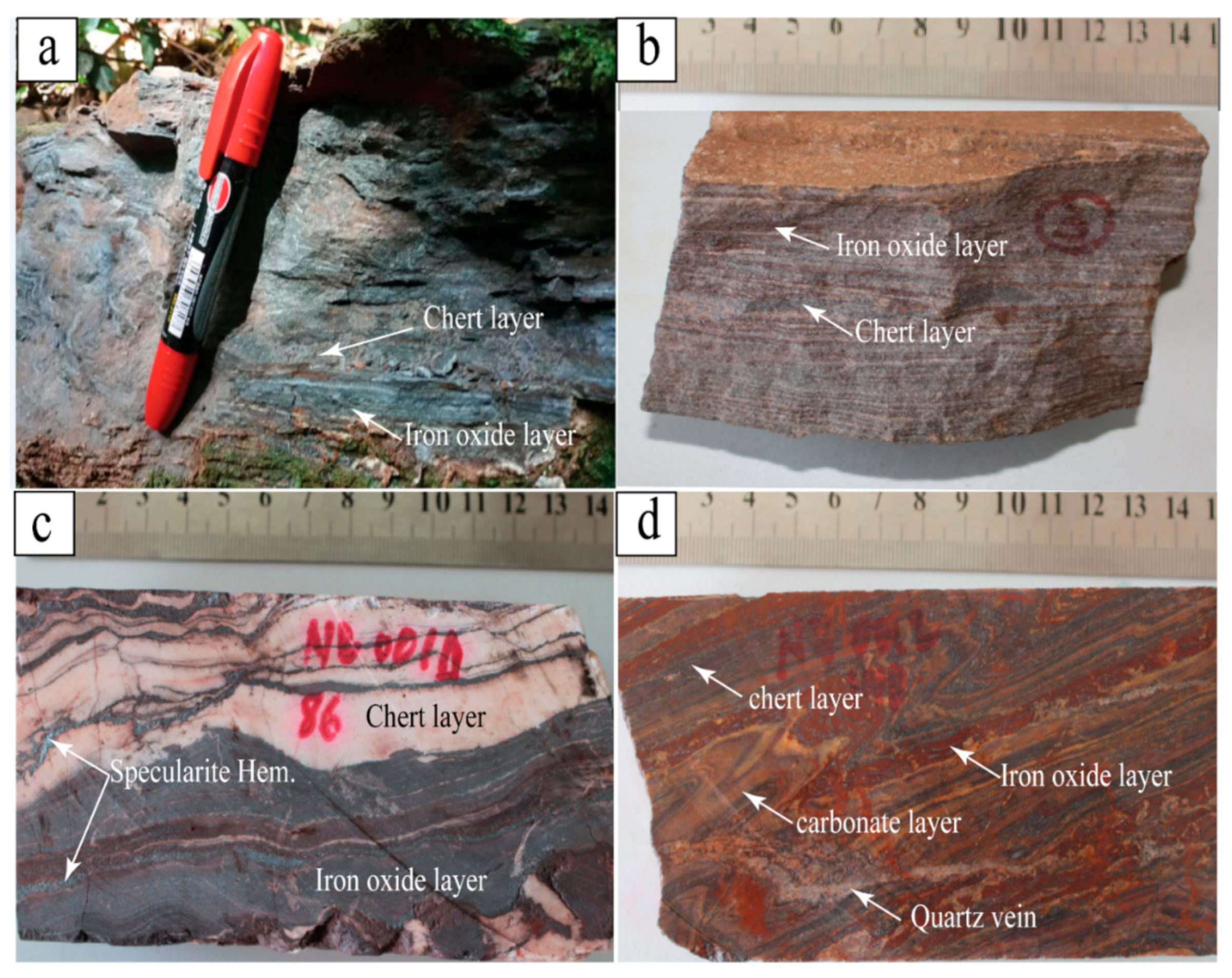
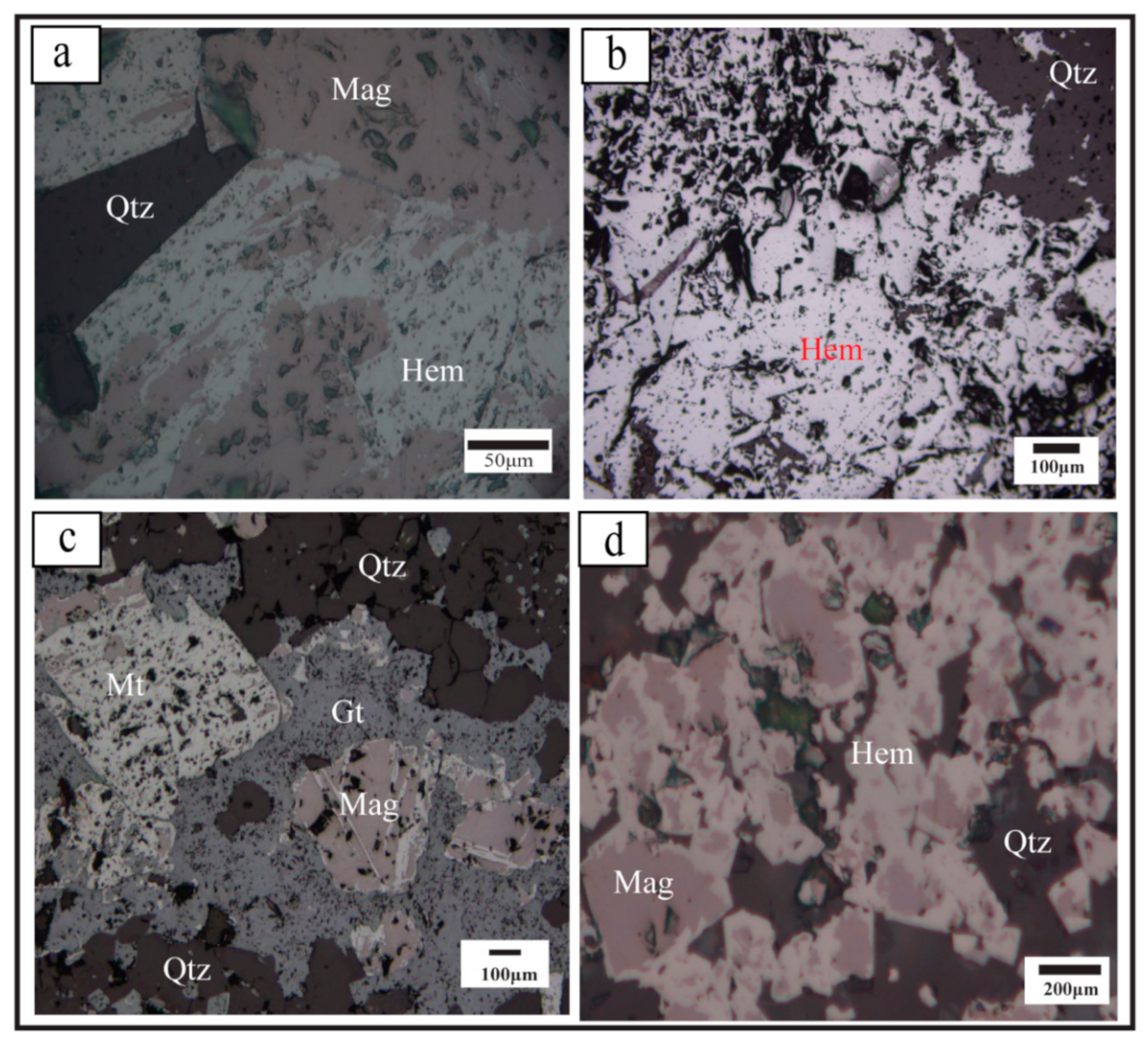
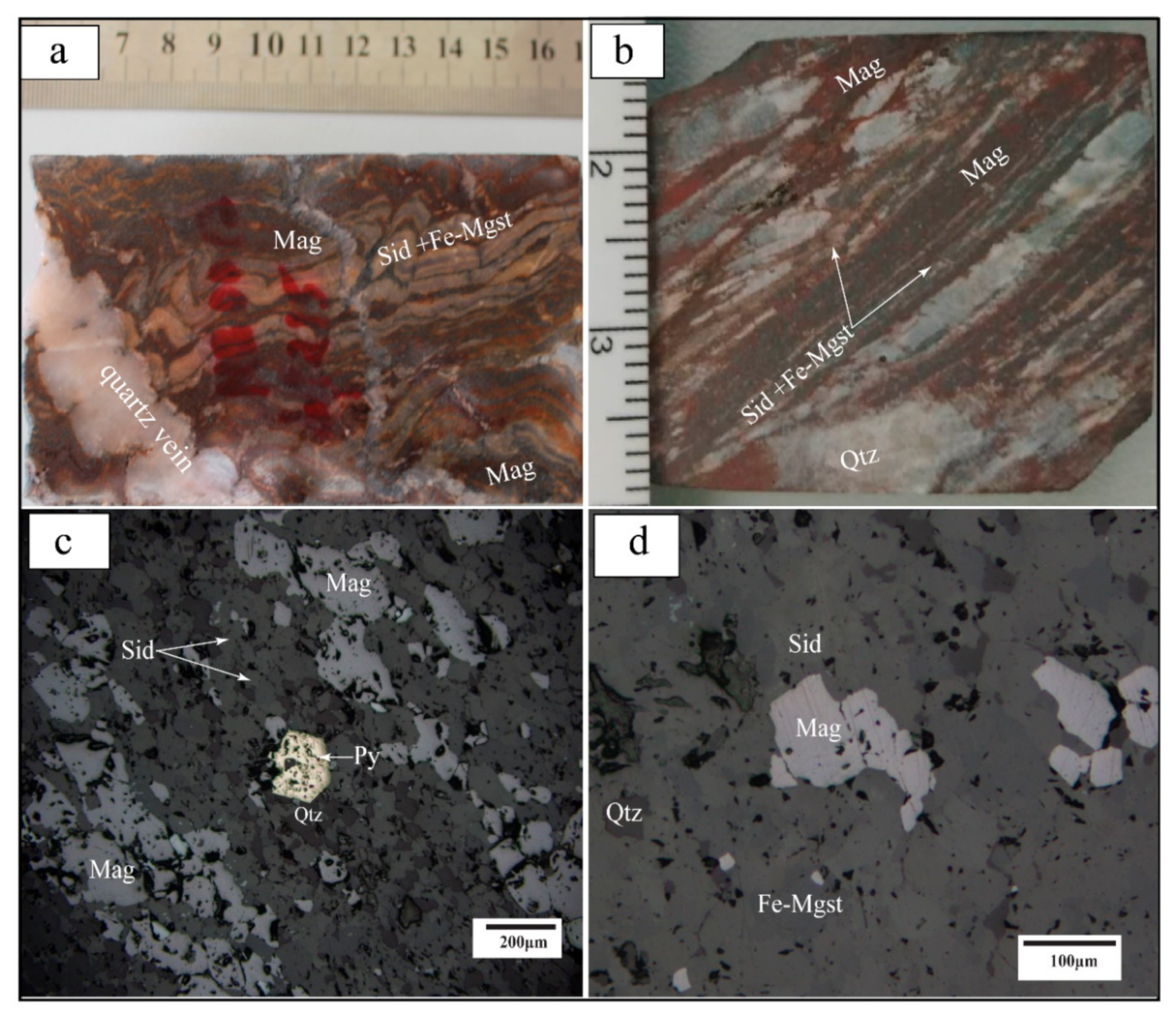
5.1. BIF Petrography
5.1.1. Oxide-Facies-BIF
5.1.2. Carbonate-Oxide Facies-BIF
5.2. Whole-Rock Geochemistry
5.2.1. Major Elements
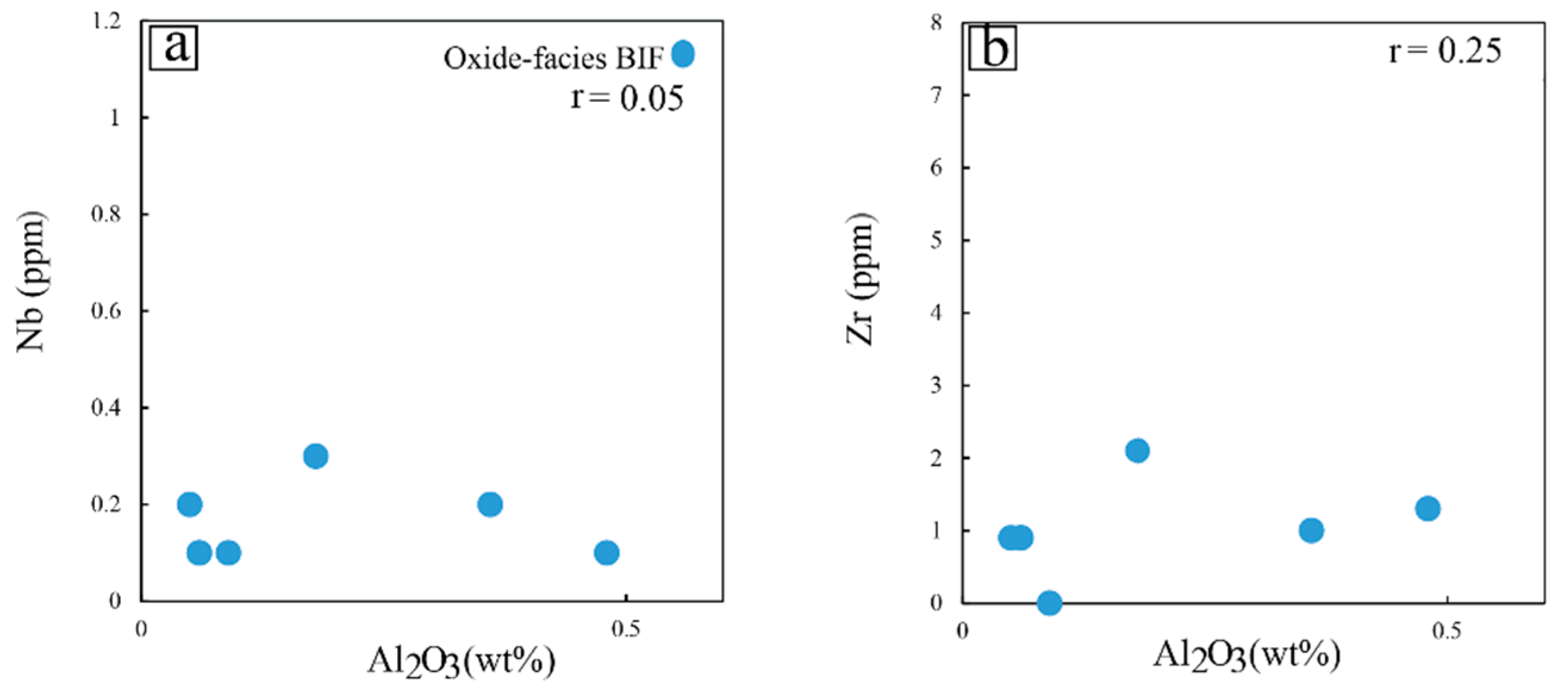
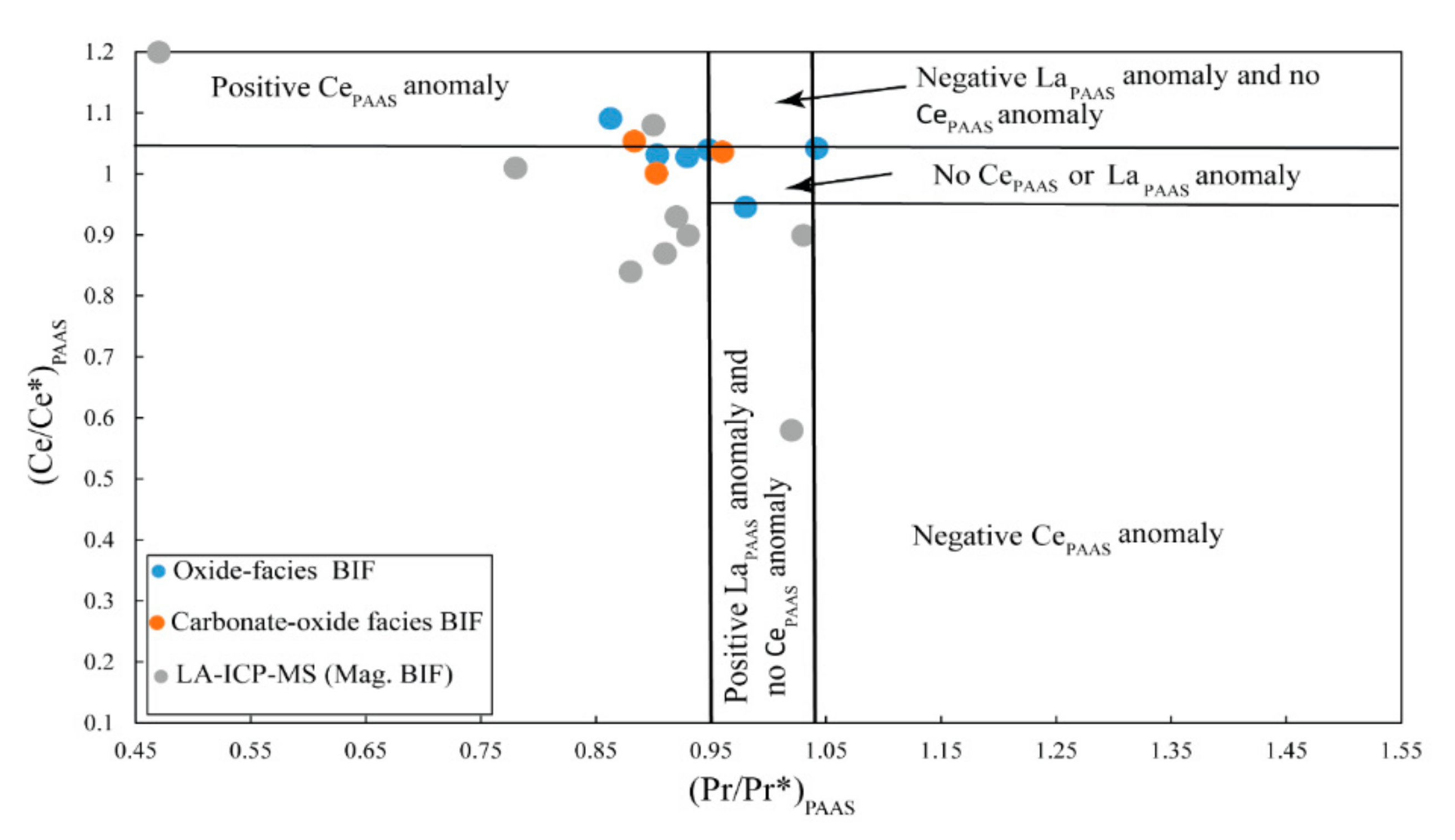
5.2.2. Trace Elements
| BIF Facies | Oxide | Carbonate-Oxide | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Name | CG-NB3 | CG-NB6 | CG-C2 | CG-C21 | CG-C23–1 | CG-C23–2 | CG-C25 | CG-NB1 | CG-NB10 | CG-C5 |
| Li | 0.4 | 0.3 | 0.6 | 0.2 | 0.2 | 0.2 | 0.2 | 2.4 | 2.1 | 0.2 |
| Be | 0.21 | 0.16 | 1.18 | 0.61 | 0.44 | 0.33 | 0.23 | 0.19 | 0.34 | 0.47 |
| V | 8 | 1 | 5 | 3 | 4 | 2 | 2 | 5 | 7 | 5 |
| Ni | 2.8 | 1.4 | 13.1 | 6.5 | 5.6 | 5.3 | 4.0 | 10.7 | 15.8 | 6.7 |
| Co | 0.3 | 0.2 | 2.5 | 2.0 | 2.0 | 2.0 | 1.0 | 5.7 | 3.4 | 2.3 |
| Cr | 52 | 39 | 25 | 22 | 23 | 19 | 15 | 39 | 50 | 12 |
| Sc | 0.8 | 0.2 | 0.4 | 0.6 | 0.3 | 0.2 | 0.2 | 0.4 | 0.9 | 0.3 |
| Rb | 1.4 | 0.4 | 0.2 | 0.3 | 2.0 | 1.5 | <0.1 | 1.0 | 0.5 | 1.0 |
| Ba | 13.3 | 10.6 | 2.7 | 4.4 | 59.7 | 41.1 | 2.1 | 42.0 | 51.9 | 22.9 |
| Sr | 2.9 | 3.2 | 0.5 | 0.2 | 0.7 | 0.6 | 0.2 | 5.1 | 20.4 | 0.7 |
| Th | 0.05 | 0.03 | 0.06 | 0.08 | 0.04 | 0.03 | 0.02 | 0.08 | 0.15 | 0.09 |
| Zr | 2.1 | 1.0 | 0.9 | 1.3 | 0.9 | 0.9 | <0.5 | 6.5 | 7.6 | 1.4 |
| Cu | <0.2 | 0.4 | 0.6 | 1.7 | 1.9 | 0.2 | 2.1 | 2.1 | 0.6 | 5.4 |
| Zn | <2 | <2 | 21 | 10 | 8 | 9 | 3 | 107 | 175 | 11 |
| Ga | 0.51 | 0.25 | 0.54 | 0.32 | 0.78 | 0.62 | 0.21 | 0.65 | 0.74 | 0.46 |
| Nb | 0.3 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 1.1 | 0.3 | 0.1 |
| Cs | 0.10 | 0.03 | <0.01 | 0.04 | 0.06 | 0.03 | 0.01 | 0.03 | <0.01 | 0.01 |
| La | 1.6 | 1.4 | 1.4 | 1.6 | 1.8 | 1.7 | 1.5 | 1.5 | 1.4 | 2.4 |
| Ce | 3.8 | 3.4 | 3.0 | 4.0 | 4.2 | 3.9 | 3.1 | 3.5 | 3.1 | 4.4 |
| Pr | 0.45 | 0.40 | 0.37 | 0.36 | 0.48 | 0.40 | 0.32 | 0.39 | 0.34 | 0.42 |
| Nd | 2.1 | 1.5 | 1.7 | 1.6 | 2.1 | 1.9 | 1.4 | 1.9 | 1.4 | 1.7 |
| Sm | 0.44 | 0.35 | 0.51 | 0.43 | 0.55 | 0.40 | 0.41 | 0.40 | 0.35 | 0.41 |
| Eu | 0.21 | 0.14 | 0.23 | 0.29 | 0.35 | 0.24 | 0.18 | 0.25 | 0.15 | 0.21 |
| Gd | 0.57 | 0.35 | 0.63 | 0.54 | 1.06 | 0.68 | 0.58 | 0.58 | 0.39 | 0.50 |
| Tb | 0.10 | 0.06 | 0.11 | 0.10 | 0.13 | 0.11 | 0.10 | 0.10 | 0.07 | 0.09 |
| Dy | 0.61 | 0.36 | 0.68 | 0.61 | 0.69 | 0.71 | 0.63 | 0.62 | 0.47 | 0.55 |
| Y | 5.0 | 3.2 | 5.4 | 4.7 | 5.2 | 5.6 | 5.1 | 4.6 | 3.5 | 4.3 |
| Ho | 0.14 | 0.08 | 0.16 | 0.14 | 0.15 | 0.15 | 0.15 | 0.14 | 0.11 | 0.12 |
| Er | 0.39 | 0.25 | 0.49 | 0.41 | 0.45 | 0.46 | 0.43 | 0.40 | 0.36 | 0.35 |
| Tm | 0.06 | 0.04 | 0.08 | 0.06 | 0.07 | 0.07 | 0.06 | 0.06 | 0.06 | 0.05 |
| Yb | 0.38 | 0.26 | 0.59 | 0.39 | 0.45 | 0.45 | 0.40 | 0.39 | 0.41 | 0.32 |
| Lu | 0.06 | 0.04 | 0.10 | 0.06 | 0.08 | 0.08 | 0.06 | 0.06 | 0.07 | 0.05 |
| Hf | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | 0.2 | 0.2 | <0.2 |
| Ta | 0.15 | 0.06 | 0.09 | <0.05 | 0.08 | 0.08 | <0.05 | 0.05 | 0.05 | 0.05 |
| Pb | 1.0 | 1.4 | 0.9 | 0.9 | <0.5 | 0.5 | <0.5 | 2.6 | 0.7 | 0.6 |
| U | 0.09 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| SREE-Y | 15.52 | 11.58 | 14.96 | 14.88 | 17.31 | 16.39 | 13.99 | 14.49 | 11.82 | 15.52 |
| ΣREE | 10.52 | 8.38 | 9.56 | 10.18 | 12.11 | 10.79 | 8.89 | 9.89 | 8.32 | 11.22 |
| Y/Ho | 35.71 | 40.00 | 33.75 | 33.57 | 34.67 | 37.33 | 34.00 | 32.86 | 31.82 | 35.83 |
| (Ce/Ce*)SN | 1.03 | 1.04 | 0.96 | 1.22 | 1.04 | 1.09 | 1.03 | 1.05 | 1.04 | 1.00 |
| (Pr/Pr* )SN | 0.93 | 1.04 | 0.95 | 0.84 | 0.95 | 0.86 | 0.90 | 0.88 | 0.96 | 0.90 |
| (Eu/Eu*)SN | 1.86 | 1.77 | 1.80 | 2.60 | 2.43 | 2.12 | 1.65 | 2.32 | 1.77 | 2.03 |
| (La/Yb)SN | 0.24 | 0.31 | 0.14 | 0.24 | 0.23 | 0.22 | 0.22 | 0.22 | 0.20 | 0.43 |
| (Tb/Yb)SN | 0.90 | 0.79 | 0.64 | 0.88 | 0.99 | 0.84 | 0.86 | 0.88 | 0.59 | 0.97 |
| (Y/Y*)SN | 1.06 | 1.17 | 1.01 | 1.00 | 1.00 | 1.06 | 1.03 | 0.97 | 0.95 | 1.04 |
| (Sm/Yb)SN | 0.46 | 0.53 | 0.34 | 0.44 | 0.48 | 0.35 | 0.41 | 0.41 | 0.34 | 0.51 |
| (Eu/Sm)SN | 2.45 | 2.06 | 2.32 | 3.47 | 3.27 | 3.08 | 2.26 | 3.21 | 2.20 | 2.63 |
| (Pr/Yb)SN | 0.30 | 0.38 | 0.16 | 0.23 | 0.27 | 0.22 | 0.20 | 0.25 | 0.21 | 0.33 |
| (Tb/Yb)SN | 0.90 | 0.79 | 0.64 | 0.88 | 0.99 | 0.84 | 0.86 | 0.88 | 0.59 | 0.97 |
| (Gd/Gd*)SN | 1.15 | 1.10 | 1.14 | 1.09 | 1.65 | 1.29 | 1.18 | 1.19 | 1.09 | 1.11 |
| (Eu/Eu*)CN | 1.28 | 1.22 | 1.24 | 1.84 | 1.40 | 1.41 | 1.13 | 1.59 | 1.24 | 1.42 |
| (Sm/Yb)CN | 1.29 | 1.50 | 0.96 | 1.23 | 1.36 | 0.99 | 1.14 | 1.14 | 0.95 | 1.42 |
| (La/Yb)CN | 3.02 | 3.86 | 1.70 | 2.94 | 2.87 | 2.71 | 2.69 | 2.76 | 2.45 | 5.38 |
| (Tb/Yb)CN | 1.20 | 1.05 | 0.85 | 1.17 | 1.31 | 1.11 | 1.14 | 1.17 | 0.78 | 1.28 |
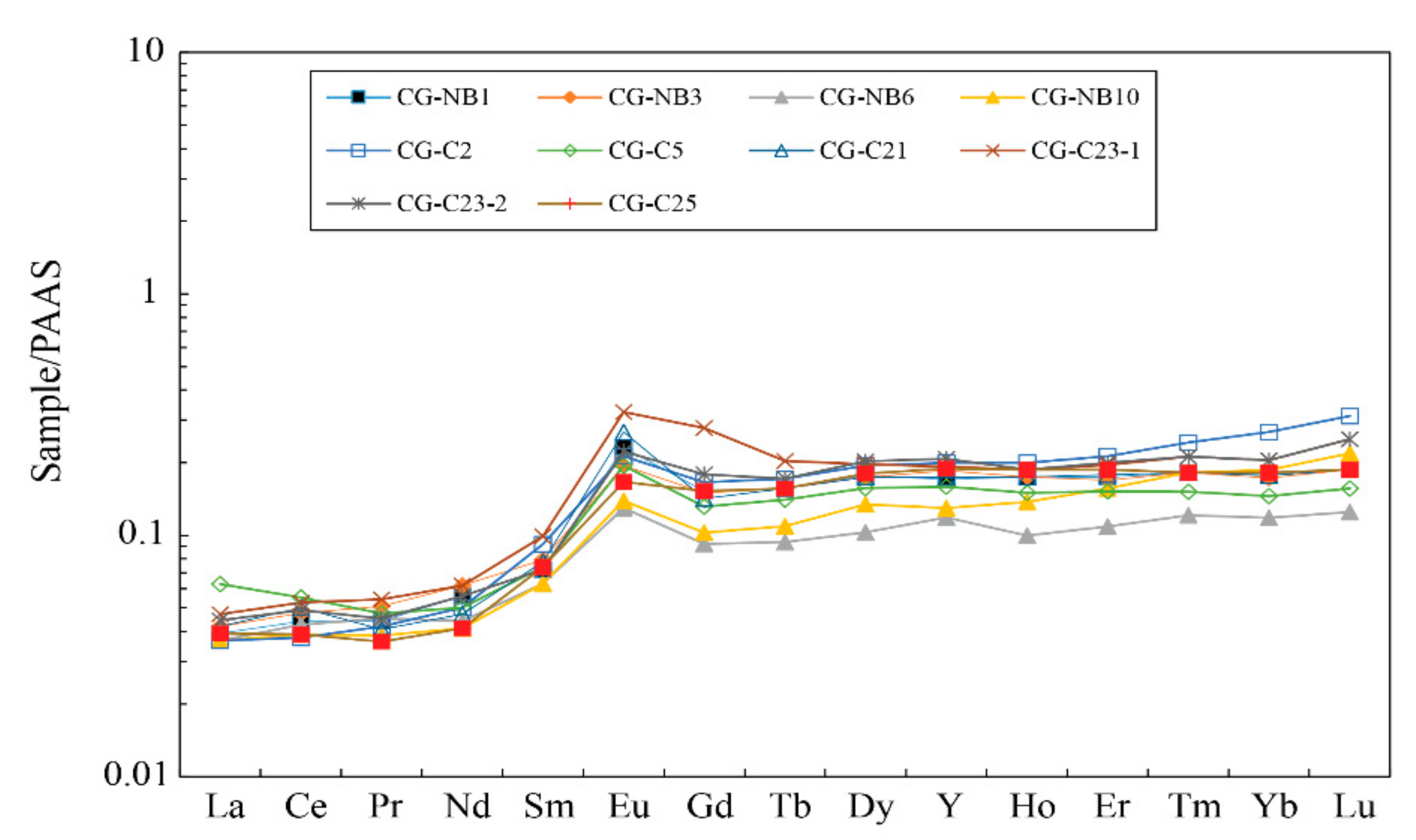
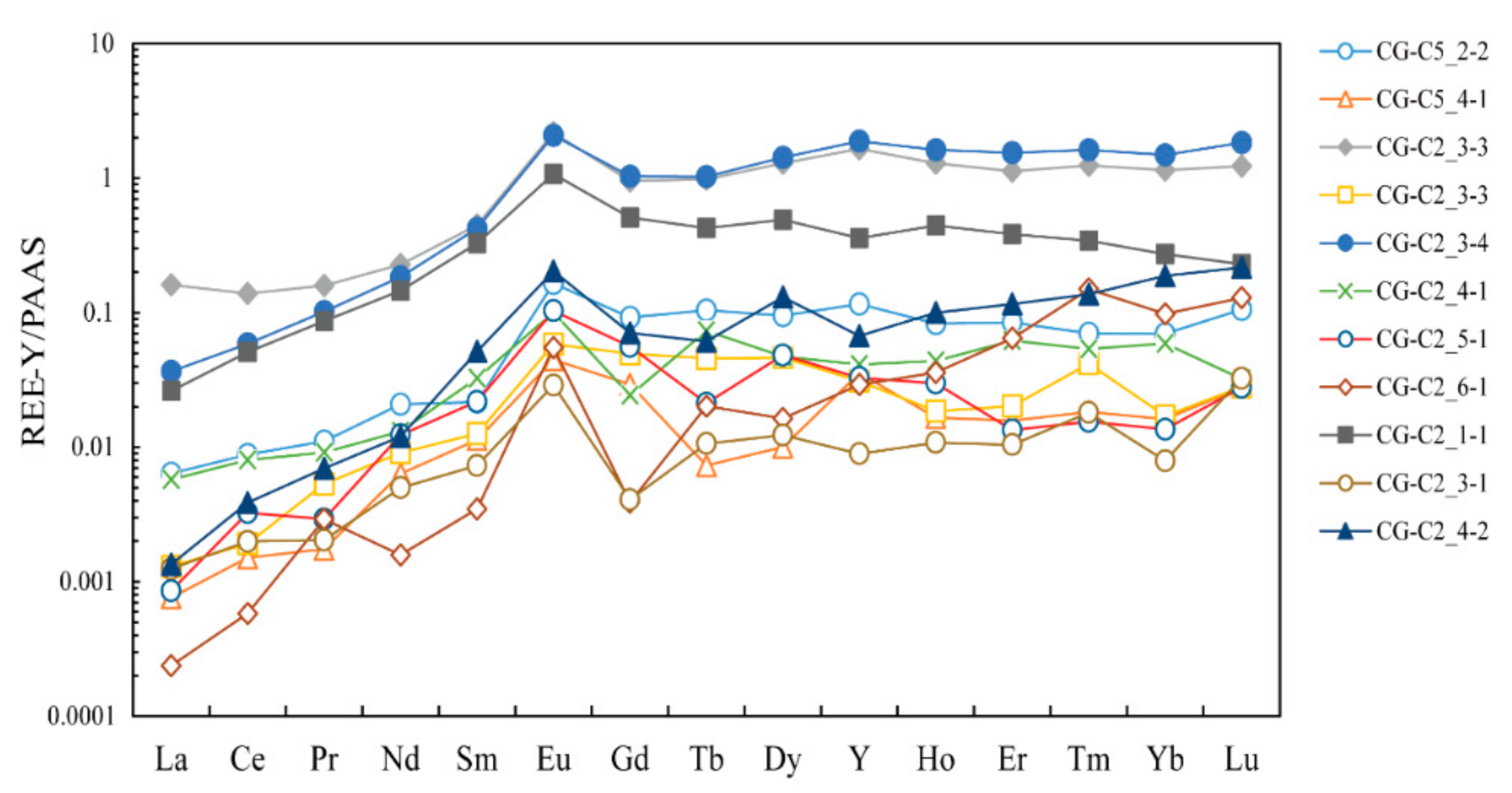
5.2.3. Rare Earth Elements
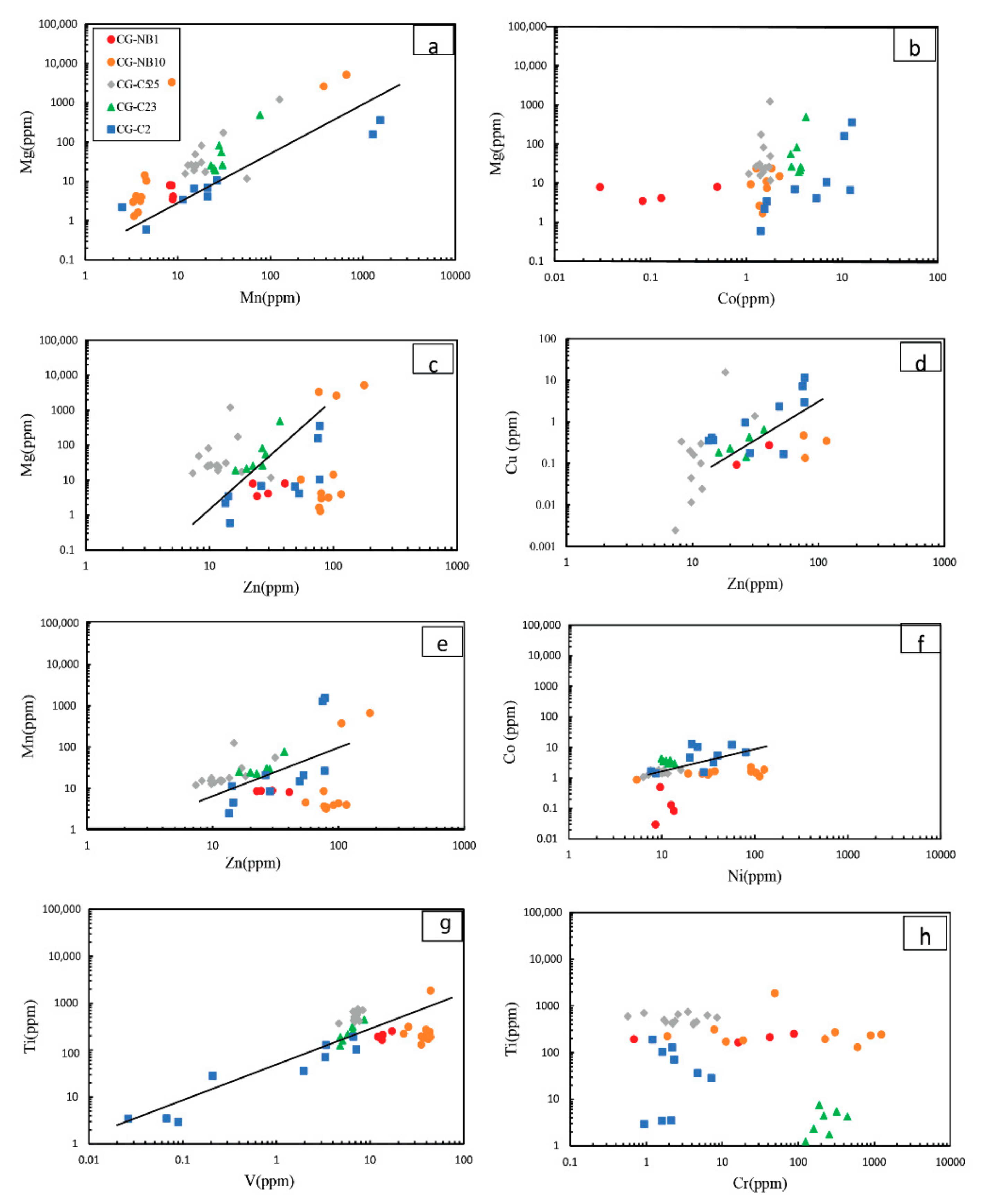
5.3. LA-ICP-MS Magnetite Chemical Compositions
5.3.1. Magnetite in the Oxide Facies-BIF
5.3.2. Magnetite in Carbonate-Oxide Facies-BIF
6. Discussion
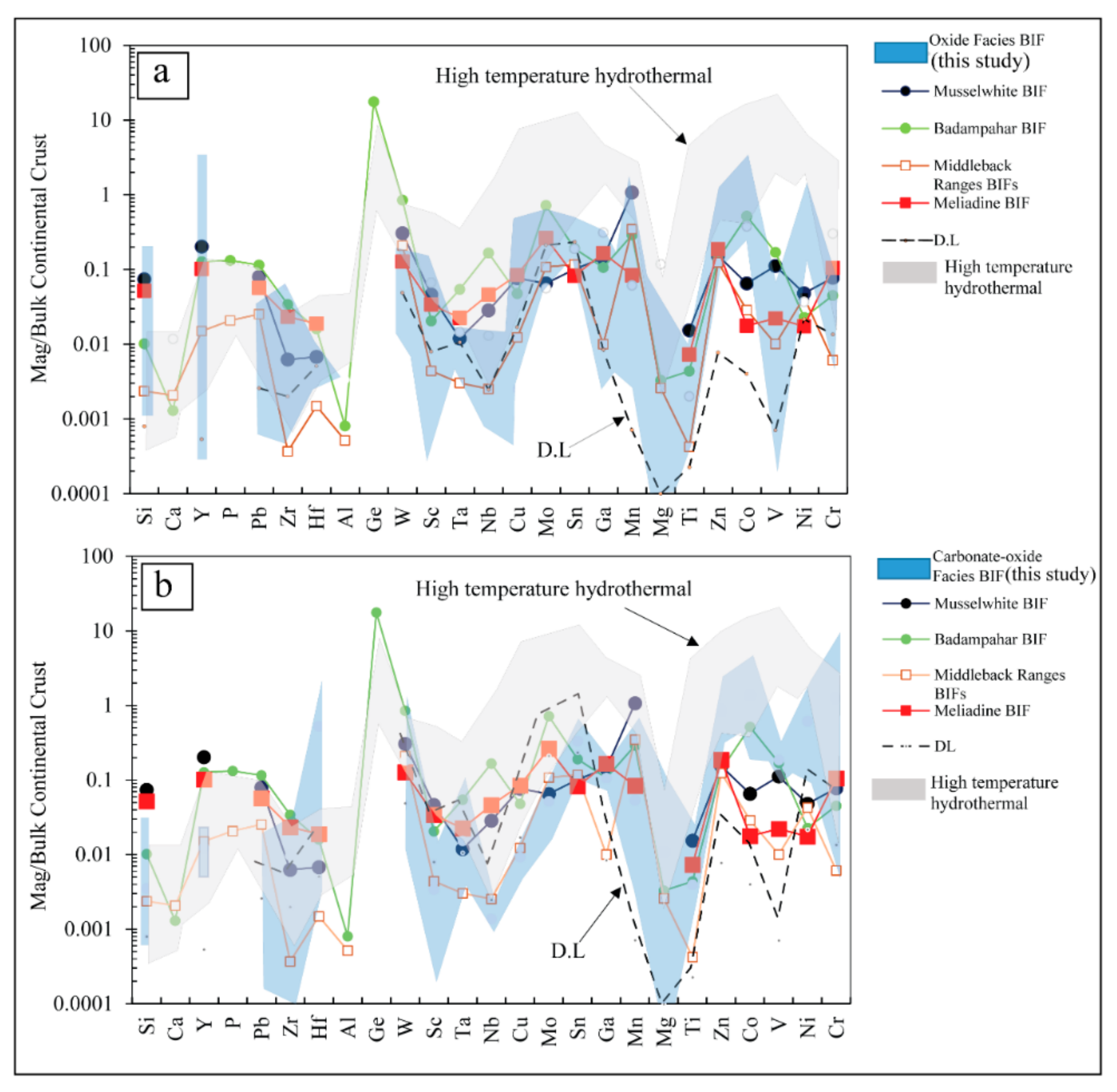
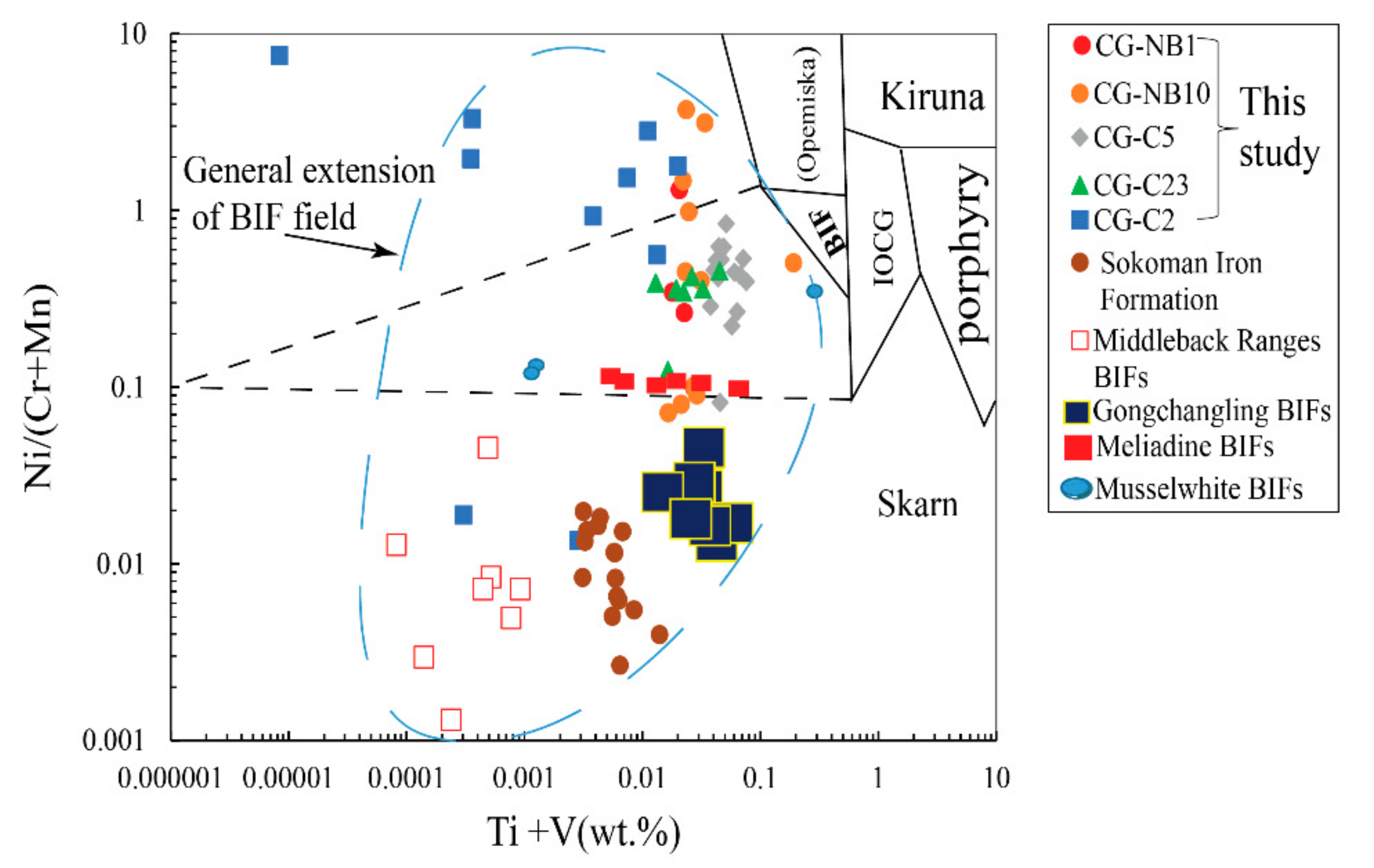
6.1. Primary Geochemical Signature of the Nabeba BIF Magnetite
6.2. Characterization of the Nabeba BIF Depositional Environment
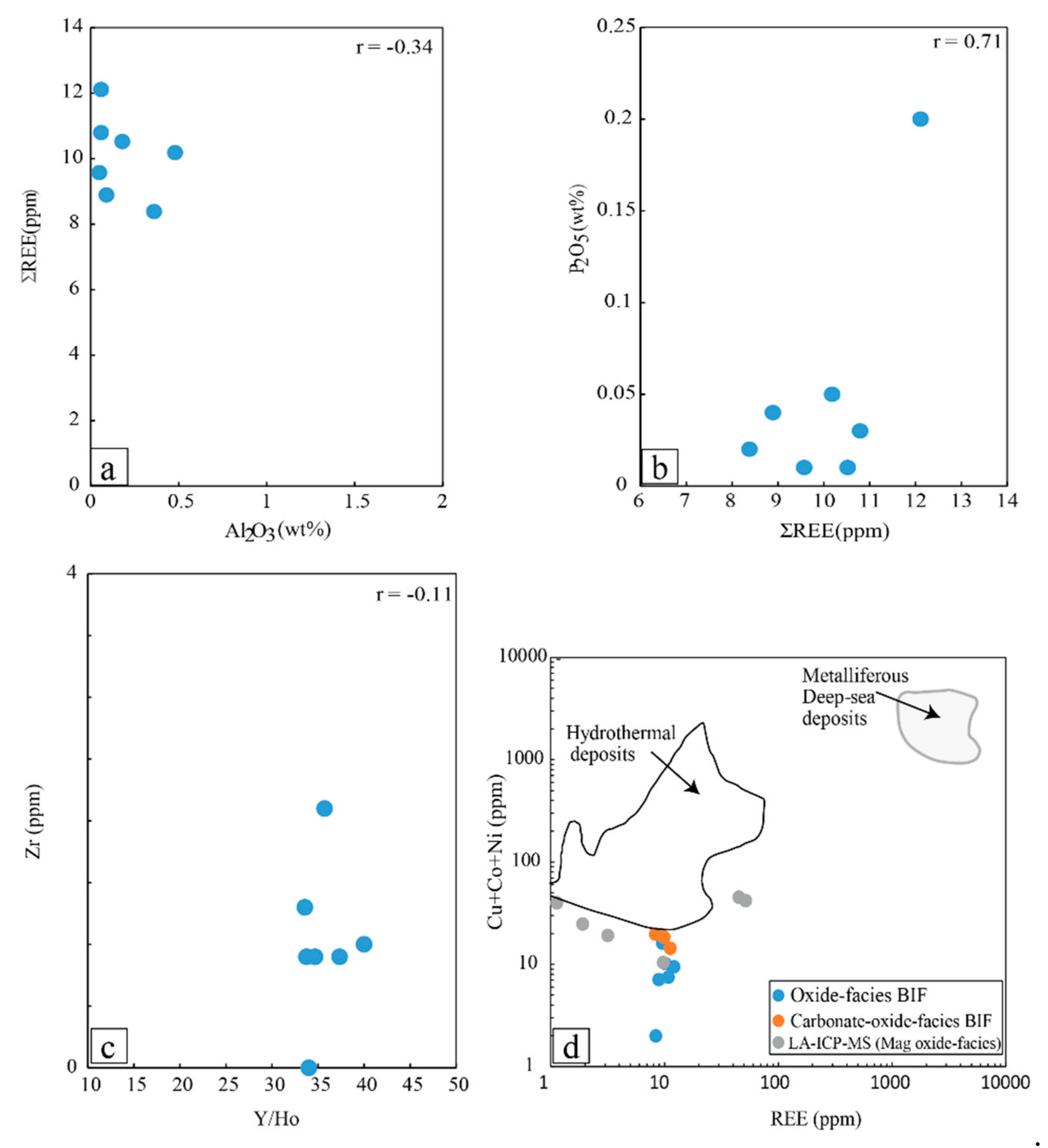
6.2.1. Purity of the Nabeba BIF Based on Amount of Detrital Input
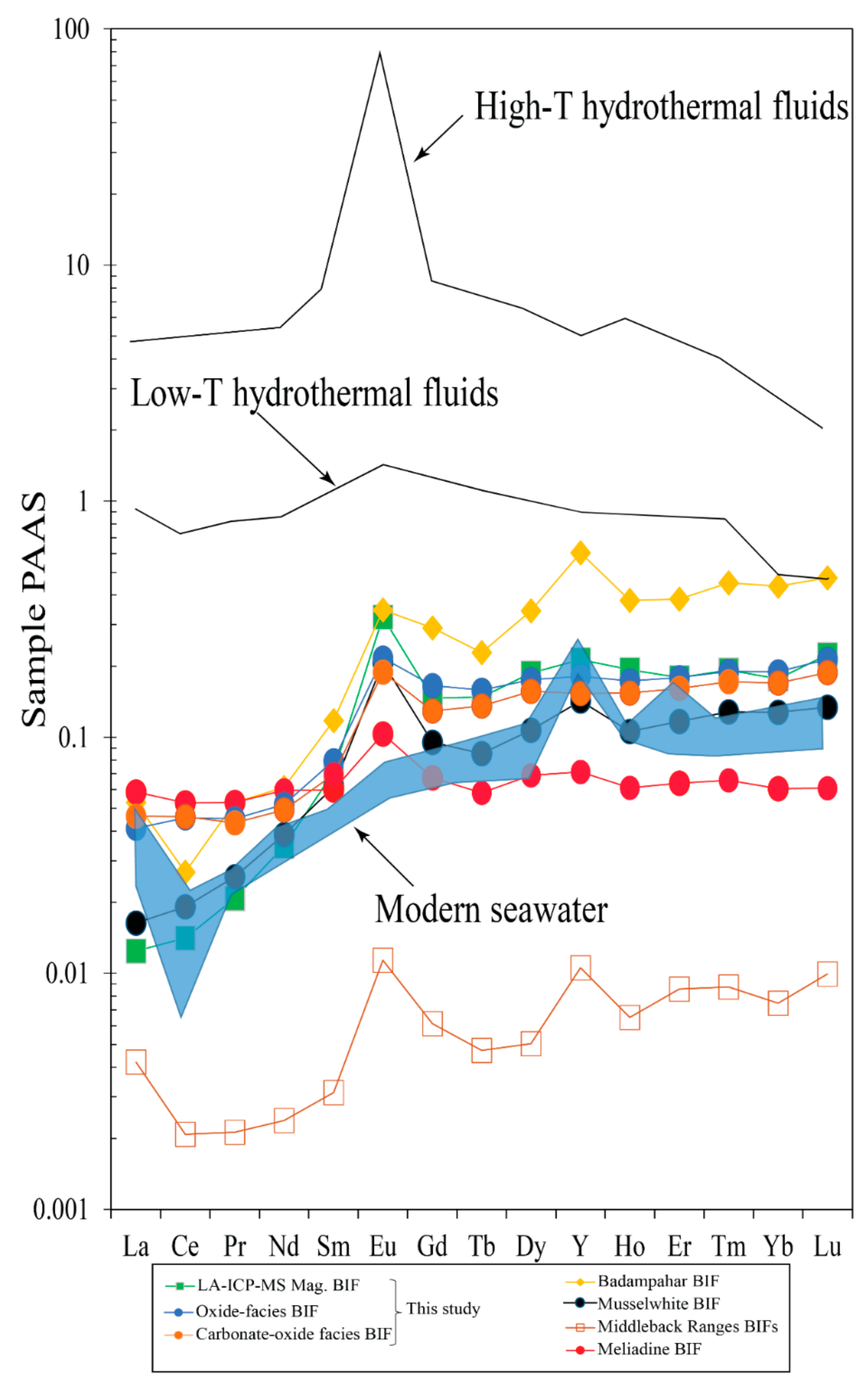
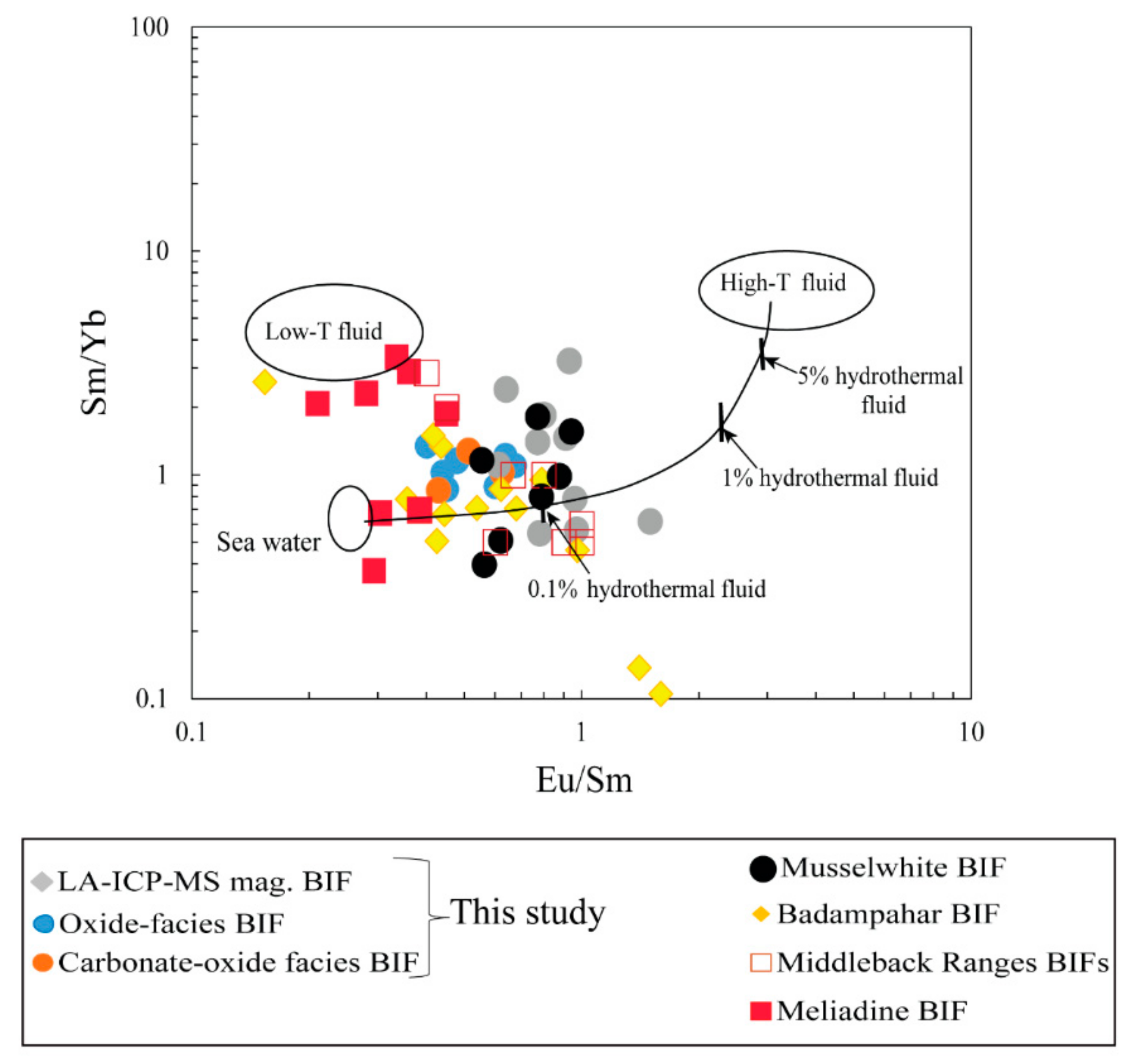
6.2.2. Fluid Mixing and T-fO2 Conditions of Nabeba BIF Precipitation
7. Conclusions
- The Nabeba BIF comprised two facies (oxide and carbonate-oxide) and displays alternating iron-rich and quartz-rich layers. The BIF consisted of magnetite, hematite, and quartz, together with minor siderite and magnesite. Geochemical analysis suggested that the Nabeba BIF had mainly Fe and Si, with minor CO3 and trace detrital material.
- Magnetite from both BIF facies had a wide range of trace element contents (e.g., Mn, Ti, Ni, Mg, Cr, V, and Zn), which suggested input of medium-high-temperature hydrothermal vent fluids. Redox and temperature conditions likely controlled the magnetite chemical compositions.
- Major and trace (including REE) element compositions suggested that Fe and Si were sourced from anoxic seawater mixed with medium-high-temperature (>250 °C) hydrothermal fluids. The Nabeba BIF was likely deposited in a reducing marine environment.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spier, C.A.; de Oliveira, S.M.B.; Rosiere, C.A. Geology and geochemistry of the Águas Claras and Pico Iron Mines, Quadrilátero Ferrífero, Minas Gerais, Brazil. Miner. Depos. 2003, 38, 751–774. [Google Scholar] [CrossRef]
- Gourcerol, B.; Thurston, P.; Kontak, D.; Côté-Mantha, O.; Biczok, J. Depositional setting of Algoma-type banded iron formation. Precamb. Res. 2016, 281, 47–79. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precamb. Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Beukes, N.J.; Gutzmer, J. Origin and paleoenvironmental significance of major iron formations at the Archean-Paleoproterozoic boundary. Rev. Econ. Geol. 2008, 15, 5–47. [Google Scholar]
- Dymek, R.F.; Klein, C. Chemistry, petrology and origin of banded iron-formation lithologies from the 3800 Ma Isua supracrustal belt, West Greenland. Precamb. Res. 1988, 39, 247–302. [Google Scholar] [CrossRef]
- Khalil, K.I.; El-Shazly, A.E.; Lehmann, B. Late Neoproterozoic banded iron formation (BIF) in the central Eastern Desert of Egypt: Mineralogical and geochemical implications for the origin of the Gebel El Hadid iron ore deposit. Ore Geol. Rev. 2015, 69, 380–399. [Google Scholar] [CrossRef]
- Simonson, B.M.; Chan, M.; Archer, A. Origin and evolution of large Precambrian iron formations. Spec. Papers Geol. Soc. Am. 2003, 370, 231–244. [Google Scholar]
- Gross, G.A. A classification of iron formations based on depositional environments. Canad. Mineral. 1980, 18, 215–222. [Google Scholar]
- James, H.L. Sedimentary facies of iron-formation. Econ. Geol. 1954, 49, 235–293. [Google Scholar] [CrossRef]
- Trendall, A. The significance of iron-formation in the Precambrian stratigraphic record. In Precambrian Sedimentary Environments: A Modern Approach to Ancient Depositional Systems; Wiley: Hoboken, NJ, USA, 2002; pp. 33–66. [Google Scholar]
- De Waele, B.; Lacorde, M.; Rivers, J. Banded iron formations and associated detrital iron deposits of the Western Congo Craton. In Proceedings of the SEG S015—World-Class Ore Deposits: Discovery to Recovery, Hobart, TAS, Australia, 27–30 September 2015. [Google Scholar]
- De Waele, B.; Lacorde, M.; Cunningham, M.; Jupp, B. Understanding Geology and Structure: An Essential Part of Mineral Resource Estimation. ASEG Ext. Abstr. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Suh, C.; Cabral, A.; Shemang, E.; Mbinkar, L.; Mboudou, G. Two contrasting iron deposits in the Precambrian mineral belt of Cameroon, West Africa. Explor. Min. Geol. 2008, 17, 197–207. [Google Scholar] [CrossRef]
- Ganno, S.; Njiosseu Tanko, E.L.; Kouankap, N.G.D.; Djoukouo Soh, A.; Moudioh, C.; Ngnotue, T.; Nzenti, J.P. A mixed seawater and hydrothermal origin of superior-type banded iron formation (BIF)-hosted Kouambo iron deposit, Palaeoproterozoic Nyong series, Southwestern Cameroon: Constraints from petrography and geochemistry. Ore Geol. Rev. 2017, 80, 860–875. [Google Scholar]
- Soh Tamehe, L.; Nzepang, T.M.; Wei, C.T.; Ganno, S.; Ngnotue, T.; Kouankap, N.G.D.; Simon, S.J.; Zhang, J.J.; Nzenti, J.P. Geology and geochemical constrains on the origin and depositional setting of the Kpwa–Atog Boga banded iron formations (BIFs), northwestern Congo craton, southern Cameroon. Ore Geol. Rev. 2018, 95, 620–638. [Google Scholar] [CrossRef]
- Longley, R.; Kitto, P.; Thornett, S. Iron Ore. Exploration History of the Mbalam Iron Ore Project; Sundance Resources Ltd.: Perth, WA, Australia, 2013. [Google Scholar]
- Cunningham, M.; De Wael, B. Geological Mapping of Badondo and Iron Mineralisation Targets, Republic of Congo. Struct. Geol. Resour. 2012, 54, 54–56. [Google Scholar]
- Gatse Ebotehouna, C.; Xie, Y.; Adomako-Ansah, K.; Pei, L. Fluid inclusion and oxygen isotope characteristics of vein quartz associated with the nabeba iron deposit, Republic of Congo: Implications for the enrichment of hypogene ores. Minerals 2019, 9, 677. [Google Scholar] [CrossRef]
- Ministère des Mines et de L’Energie, Direction Générale des Mines. Carte Géologique de la Republique du Congo au 1:1000; Ministère des Mines et de L’Energie: Brazzaville, Republic of Congo, 1995.
- Pickard, A. SHRIMP U–Pb zircon ages for the Palaeoproterozoic Kuruman Iron Formation, northern Cape Province, South Africa: Evidence for simultaneous BIF deposition on Kaapvaal and Pilbara cratons. Precambr. Res. 2003, 125, 275–315. [Google Scholar] [CrossRef]
- Huston, D.L.; Logan, G.A. Barite, BIFs and bugs: Evidence for the evolution of the Earth’s early hydrosphere. Earth Planet. Sci. Lett. 2004, 220, 41–55. [Google Scholar] [CrossRef]
- Bekker, A.; Slack, J.F.; Planavsky, N.; Krapez, B.; Hofmann, A.; Konhauser, K.O.; Rouxel, O.J. Iron formation: The sedimentary product of a complex interplay among mantle, tectonic, oceanic, and biospheric processes. Econ. Geol. 2010, 105, 467–508. [Google Scholar] [CrossRef]
- Planavsky, N.; Bekker, A.; Rouxel, O.J.; Kamber, B.; Hofmann, A.; Knudsen, A.; Lyons, T.W. Rare earth element and yttrium compositions of Archean and Paleoproterozoic Fe formations revisited: New perspectives on the significance and mechanisms of deposition. Geochim. Cosmochim. Acta 2010, 74, 6387–6405. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Planavsky, N.J.; Hardisty, D.S.; Robbins, L.J.; Warchola, T.J.; Haugaard, R.; Lalonde, S.V.; Partin, C.A.; Oonk, P.B.; Tsikos, H. Iron formations: A global record of Neoarchaean to Palaeoproterozoic environmental history. Earth Sci. Rev. 2017, 172, 140–177. [Google Scholar] [CrossRef]
- Alibert, C.; McCulloch, M.T. Rare earth element and neodymium isotopic compositions of the banded iron-formations and associated shales from Hamersley, Western Australia. Geochim. Cosmochim. Acta 1993, 57, 187–204. [Google Scholar] [CrossRef]
- Hansel, C.M.; Benner, S.G.; Neiss, J.; Dohnalkova, A.; Kukkadapu, R.K.; Fendorf, S. Secondary mineralization pathways induced by dissimilatory iron reduction of ferrihydrite under advective flow. Geochim. Cosmochim. Acta 2003, 67, 2977–2992. [Google Scholar] [CrossRef]
- Li, W.; Jin, X.; Gao, B.; Wang, C.; Zhang, L. Analysis of ultra-low level rare earth elements in magnetite samples from banded iron formations using HR-ICP-MS after chemical separation. Anal. Methods 2014, 6, 6125–6132. [Google Scholar] [CrossRef]
- Chung, D.; Zhou, M.-F.; Gao, J.-F.; Chen, W.T. In-situ LA–ICP-MS trace elemental analyses of magnetite: The late Palaeoproterozoic Sokoman Iron Formation in the Labrador Trough, Canada. Ore Geol. Rev. 2015, 65, 917–928. [Google Scholar] [CrossRef]
- Gourcerol, B.; Thurston, P.; Kontak, D.; Côté-Mantha, O. Interpretations and implications of LA ICP-MS analysis of chert for the origin of geochemical signatures in banded iron formations (BIFs) from the Meadowbank gold deposit, Western Churchill Province, Nunavut. Chem. Geol. 2015, 410, 89–107. [Google Scholar] [CrossRef]
- Gourcerol, B.; Kontak, D.J.; Thurston, P.C.; Duparc, Q. Do magnetite layers in algoma-type Banded Iron Formations (BIF) preserve their primary geochemical signature? A case study of samples from three archean BIF-hosted gold deposits. Canad. Mineral. 2016, 54, 605–624. [Google Scholar] [CrossRef]
- Ghosh, R.; Baidya, T.K. Mesoarchean BIF and iron ores of the Badampahar greenstone belt, Iron Ore Group, East Indian Shield. J. Asian Earth Sci. 2017, 150, 25–44. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, X.; Tang, H.; Luan, Y. In situ LA-ICP-MS trace element analysis of magnetite from the late Neoarchean Gongchangling BIFs, NE China: Constraints on the genesis of high-grade iron ore. Geol. J. 2018, 53, 8–20. [Google Scholar] [CrossRef]
- Keyser, W.; Ciobanu, C.L.; Cook, N.J.; Johnson, G.; Feltus, H.; Johnson, S.; Dmitrijeva, M.; Ehrig, K.; Nguyen, P.T. Petrography and trace element signatures of iron-oxides in deposits from the Middleback Ranges, South Australia: From banded iron formation to ore. Ore Geol. Rev. 2018, 93, 337–360. [Google Scholar] [CrossRef]
- Meloux, J.; Bigot, M.; Viland, J. Plan Minéral de la République Populaire du Congo; BRGM: Orléans, France, 1986.
- Schlüter, T. Geological Atlas of Africa. With Notes on Stratigraphy, Tectonics, Economic Geology, Geohazards, Geosites and Geoscientific Education of Each Country; Springer-Verlag: Berlin, Germany, 2008; p. 307. [Google Scholar]
- Dadet, P. Notice Explicative de la Carte Géologique de la République du Congo Brazzaville au 1/500 000: Zone Comprise entre les Parallèles 2 et 5 Sud; Éditions BRGM: Orléans, France, 1969.
- Desthieux, F. Notice Explicative de la Carte Géologique de la République du Congo au 1/1.000.000; Ministère des Mines et de L’énergie. Direction Générale des Mines: Brazzaville, République du Congo, 1993; p. 27.
- Feybesse, J.; Johan, V.; Triboulet, C.; Guerrot, C.; Mayaga-Mikolo, F.; Bouchot, V.; N’dong, J.E. The West Central African belt: A model of 2.5–2.0 Ga accretion and two-phase orogenic evolution. Precambr. Res. 1998, 87, 161–216. [Google Scholar] [CrossRef]
- Milesi, J.; Toteu, S.; Deschamps, Y.; Feybesse, J.; Lerouge, C.; Cocherie, A.; Penaye, J.; Tchameni, R.; Moloto-A-Kenguemba, G.; Kampunzu, H. An overview of the geology and major ore deposits of Central Africa: Explanatory note for the 1: 4,000,000 map. Geology and major ore deposits of Central Africa. J. African Earth Sci. 2006, 44, 571–595. [Google Scholar] [CrossRef]
- Dorling, S. Structural Geologogy of the Mbalam Iron Project; Unpublished Technical Report; CSA Global: Perth, WA, Australia, 2011. [Google Scholar]
- Gao, J.-F.; Zhou, M.-F.; Lightfoot, P.C.; Wang, C.Y.; Qi, L.; Sun, M. Sulfide saturation and magma emplacement in the formation of the Permian Huangshandong Ni-Cu sulfide deposit, Xinjiang, northwestern China. Econ. Geol. 2013, 108, 1833–1848. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Gao, S.; Günther, D.; Xu, J.; Gao, C.; Chen, H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Compositionandevolution; Blackwell Scientific Publications: Hoboken, NY, USA, 1985; pp. 1–328. [Google Scholar]
- Bau, M.; Koschinsky, A.; Dulski, P.; Hein, J.R. Comparison of the partitioning behaviours of yttrium, rare earth elements, and titanium between hydrogenetic marine ferromanganese crusts and seawater. Geochim. Cosmochim. Acta 1996, 60, 1709–1725. [Google Scholar] [CrossRef]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Ganno, S.; Ngnotue, T.; Djibril Kouankap, N.G.D.; Nzenti, J.P.; Notsa Fokeng, M. Petrology and geochemistry of the banded iron-formations from Ntem complex greenstones belt, Elom area, Southern Cameroon: Implications for the origin and depositional environment. Chem Erde. 2015, 75, 375–387. [Google Scholar]
- Rudnick, R.; Gao, S. Composition of the continental crust. Treatise Geochem. 2003, 3, 1–64. [Google Scholar]
- Sun, S.-S.; McDonough, W.F. Chemical and Isotopic Systematics of Oceanic Basalts: Implications for Mantle Composition and Processes; Special Publications; Geological Society: London, UK, 1989; Volume 42, pp. 313–345. [Google Scholar]
- McLennan, S.M. Rare earth elements in sedimentary rocks: Influence of provenance and sedimentary processes. Geochem. Mineral. Rare Earth Elements Rev. Mineral. 2018, 21, 169–200. [Google Scholar]
- Rao, T.G.; Naqvi, S. Geochemistry, depositional environment and tectonic setting of the BIF’s of the late Archaean Chitradurga schist belt, India. Chem. Geol. 1995, 121, 217–243. [Google Scholar] [CrossRef]
- Kato, Y.; Kawakami, T.; Kano, T.; Kunugiza, K.; Swamy, N. Rare-earth element geochemistry of banded iron formations and associated amphibolite from the Sargur belts, south India. J. Southeast. Asian Earth Sci. 1996, 14, 161–164. [Google Scholar] [CrossRef]
- Klein, C. Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and origins. Am. Mineral. 2005, 90, 1473–1499. [Google Scholar] [CrossRef]
- Ohmoto, H. Nonredox transformations of magnetite-hematite in hydrothermal systems. Econ. Geol. 2003, 98, 157–161. [Google Scholar] [CrossRef]
- Posth, N.R.; Köhler, I.; Swanner, E.D.; Schröder, C.; Wellmann, E.; Binder, B.; Konhauser, K.O.; Neumann, U.; Berthold, C.; Nowak, M. Simulating Precambrian banded iron formation diagenesis. Chem. Geol. 2013, 362, 66–73. [Google Scholar] [CrossRef]
- Thurston, P.; Kamber, B.; Whitehouse, M. Archean cherts in banded iron formation: Insight into Neoarchean ocean chemistry and depositional processes. Precambr. Res. 2012, 214, 227–257. [Google Scholar] [CrossRef]
- Ayres, D. Genesis of iron-bearing minerals in banded iron formation mesobands in the Dales Gorge Member, Hamersley Group, Western Australia. Econ. Geol. 1972, 67, 1214–1233. [Google Scholar] [CrossRef]
- Ewers, W.; Morris, R. Studies of the Dales Gorge member of the Brockman iron formation, Western Australia. Econ. Geol. 1981, 76, 1929–1953. [Google Scholar] [CrossRef]
- Ahn, J.H.; Buseck, P.R. Hematite nanospheres of possible colloidal origin from a Precambrian banded iron formation. Science 1990, 250, 111–113. [Google Scholar] [CrossRef]
- Morris, R. Genetic modelling for banded iron-formation of the Hamersley Group, Pilbara Craton, Western Australia. Precambr. Res. 1993, 60, 243–286. [Google Scholar] [CrossRef]
- Smith, A.J.; Beukes, N.J.; Gutzmer, J. The composition and depositional environments of Mesoarchean iron formations of the West Rand Group of the Witwatersrand Supergroup, South Africa. Econ. Geol. 2013, 108, 111–134. [Google Scholar] [CrossRef]
- Bau, M.; Alexander, B.W. Distribution of high field strength elements (Y, Zr, REE, Hf, Ta, Th, U) in adjacent magnetite and chert bands and in reference standards FeR-3 and FeR-4 from the Temagami iron-formation, Canada, and the redox level of the Neoarchean ocean. Precambr. Res. 2009, 174, 337–346. [Google Scholar] [CrossRef]
- Ghosh, R.; Baidya, T.K. Using BIF magnetite of the Badampahar greenstone belt, Iron Ore Group, East Indian Shield to reconstruct the water chemistry of a 3.3–3.1 Ga sea during iron oxyhydroxides precipitation. Precambr. Res. 2017, 301, 102–112. [Google Scholar] [CrossRef]
- Dare, S.A.; Barnes, S.-J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner.Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Dupuis, C.; Beaudoin, G. Discriminant diagrams for iron oxide trace element fingerprinting of mineral deposit types. Miner. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Wang, M.; Wang, W.; Liu, K.; Michalak, P.P.; Wei, K.; Hu, M. In–situ LA–ICP–MS trace elemental analyzes of magnetite: The Tieshan skarn Fe–Cu deposit, Eastern China. Chem Erde. 2017, 77, 169–181. [Google Scholar] [CrossRef]
- McLennan, S.; Hemming, S.; McDaniel, D.; Hanson, G. Geochemical approaches to sedimentation, provenance, and tectonics. Spec. Papers Geol. Soc. Am. 1993, 284, 21. [Google Scholar]
- Bau, M.; Möller, P. Rare earth element systematics of the chemically precipitated component in Early Precambrian iron formations and the evolution of the terrestrial atmosphere-hydrosphere-lithosphere system. Geochim. Cosmochim. Acta 1993, 57, 2239–2249. [Google Scholar] [CrossRef]
- Bau, M. Effects of syn-and post-depositional processes on the rare-earth element distribution in Precambrian iron-formations. Eur. J. Mineral. 1993, 5, 257–267. [Google Scholar] [CrossRef]
- Arora, M.; Govil, P.; Charan, S.; Raj, B.U.; Manikyamba, C.; Chatterjee, A.; Naqvi, S. Geochemistry and origin of Archean banded iron-formation from the Bababudan Schist Belt, India. Econ. Geol. 1995, 90, 2040–2057. [Google Scholar] [CrossRef]
- Basta, F.F.; Maurice, A.E.; Fontboté, L.; Favarger, P.-Y. Petrology and geochemistry of the banded iron formation (BIF) of Wadi Karim and Um Anab, Eastern Desert, Egypt: Implications for the origin of Neoproterozoic BIF. Precambr. Res. 2011, 187, 277–292. [Google Scholar] [CrossRef]
- Bolhar, R.; Kamber, B.S.; Moorbath, S.; Fedo, C.M.; Whitehouse, M.J. Characterisation of early Archaean chemical sediments by trace element signatures. Earth Planet. Sci. Lett. 2004, 222, 43–60. [Google Scholar] [CrossRef]
- Cox, G.M.; Halverson, G.P.; Minarik, W.G.; Le Heron, D.P.; Macdonald, F.A.; Bellefroid, E.J.; Strauss, J.V. Neoproterozoic iron formation: An evaluation of its temporal, environmental and tectonic significance. Chem. Geol. 2013, 362, 232–249. [Google Scholar] [CrossRef]
- Gurvich, E.G. Metalliferous Sediments of the World Ocean: Fundamental Theory of Deep-Sea Hydrothermal Sedimentation; Springer: Berlin, Germany, 2006. [Google Scholar]
- Klein, C.; Beukes, N.J. Geochemistry and sedimentology of a facies transition from limestone to iron-formation deposition in the early Proterozoic Transvaal Supergroup, South Africa. Econ. Geol. 1989, 84, 1733–1774. [Google Scholar] [CrossRef]
- Douville, E.; Bienvenu, P.; Charlou, J.L.; Donval, J.P.; Fouquet, Y.; Appriou, P.; Gamo, T. Yttrium and rare earth elements in fluids from various deep-sea hydrothermal systems. Geochim. Cosmochim. Acta 1999, 63, 627–643. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Comparing yttrium and rare earths in hydrothermal fluids from the Mid-Atlantic Ridge: Implications for Y and REE behaviour during near-vent mixing and for the Y/Ho ratio of Proterozoic seawater. Chem. Geol. 1999, 155, 77–90. [Google Scholar] [CrossRef]
- Yang, X.-Q.; Zhang, Z.-H.; Duan, S.-G.; Zhao, X.-M. Petrological and geochemical features of the Jingtieshan banded iron formation (BIF): A unique type of BIF from the Northern Qilian Orogenic Belt, NW China. J. Asian Earth Sci. 2015, 113, 1218–1234. [Google Scholar] [CrossRef]
- Alexander, B.W.; Bau, M.; Andersson, P.; Dulski, P. Continentally-derived solutes in shallow Archean seawater: Rare earth element and Nd isotope evidence in iron formation from the 2.9 Ga Pongola Supergroup, South Africa. Geochim. Cosmochim. Acta 2008, 72, 378–394. [Google Scholar] [CrossRef]
- Bhattacharya, H.; Chakraborty, I.; Ghosh, K.K. Geochemistry of some banded iron-formations of the Archean supracrustals, Jharkhand-Orissa region, India. J. Earth Syst Sci. 2007, 116, 245–259. [Google Scholar] [CrossRef]
- Bordage, A.; Balan, E.; de Villiers, J.P.; Cromarty, R.; Juhin, A.; Carvallo, C.; Calas, G.; Raju, P.S.; Glatzel, P. V oxidation state in Fe–Ti oxides by high-energy resolution fluorescence-detected X-ray absorption spectroscopy. Phys. Chem. Miner. 2011, 38, 449–458. [Google Scholar] [CrossRef]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014, 61, 1–32. [Google Scholar] [CrossRef]
- Toplis, M.J.; Corgne, A. An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contrib. Mineral. Petrol. 2002, 144, 22–37. [Google Scholar] [CrossRef]
- Michard, A.; Albarede, F.; Michard, G.; Minster, J.; Charlou, J. Rare-earth elements and uranium in high-temperature solutions from East Pacific Rise hydrothermal vent field (13 N). Nature 1983, 303, 795. [Google Scholar] [CrossRef]
- Alibo, D.S.; Nozaki, Y. Rare earth elements in seawater: Particle association, shale-normalization, and Ce oxidation. Geochim. Cosmochim. Acta 1999, 63, 363–372. [Google Scholar] [CrossRef]

| Sample Type | N° | Sample Name | Location | Coordinates | Depth (m) | BIF Facies | Texture | Major Mineral Assemblage | |
|---|---|---|---|---|---|---|---|---|---|
| East | North | ||||||||
| BIF (Drill Core) | 1 | CG-NB 1 | NBDD012 | 387521 | 203459 | 224 | Carbonate-oxide | Fine banded, brown, grey, and black alternating bands | Magnetite + siderite + quartz |
| 2 | CG-NB 2 | NBDD001D | 388900 | 203208 | 86 | Oxide | Banded, alternation of white, brown, and grey | Magnetite + quartz | |
| 3 | CG-NB 3 | NBDD001D | 388900 | 203208 | 90 | Oxide | Fine banded, alternation of white, brown, and grey | Magnetite + quartz + hematite | |
| 4 | CG-NB 4 | NBDD012D | 387521 | 203459 | 215 | Oxide | Banded, cut by quartz veins | Magnetite + quartz | |
| 5 | CG-NB 6 | NBDD001D | 388900 | 203208 | 96 | Oxide | Fine banded, grey, brown, and white fold bands | Magnetite + quartz + hematite | |
| 6 | CG-NB 7 | NBDD004D | 388300 | 203588 | 156 | Oxide | Banded, brown, and white | Magnetite + quartz + hematite | |
| 7 | CG-NB 8 | NBDD004 | 388300 | 203588 | 185 | Oxide | Banded, brown, and white, cut by quartz veins | Magnetite + quartz | |
| 8 | CG-NB 9 | NBDD0012 | 387521 | 203459 | 220 | Oxide | Folded banded, brownish, cut by quartz veins | Magnetite + quartz | |
| 9 | CG-NB10 | NBDD0012 | 387521 | 203459 | 208 | Carbonate-oxide | Fines banded, gray to brown, crosscutting veins | Magnetite + quartz + siderite | |
| 10 | CG-NB11 | NBDD0012 | 387521 | 203459 | 98 | Carbonate-oxide | Fines banded, gray to brown, and white alternating bands | Magnetite + quartz + siderite + ferroan-magnesite | |
| BIF (Outcrop) | 11 | CG-C2 | Outcrop | 386688 | 202204 | Oxide | Fine banded, grey, brown, and white alternating bands | Magnetite + quartz + hematite + martite | |
| 12 | CG-C3 | Outcrop | 386221 | 202029 | Oxide | Brown and white alternating bands | Magnetite + quartz + hematite + martite | ||
| 13 | CG-C4 | Outcrop | 386711 | 202232 | Carbonate-oxide | Banded, brown, and grey | Magnetite + quartz + hematite + ferroan-dolomite | ||
| 14 | CG-C6 | Outcrop | 387214 | 202232 | Carbonate-oxide | Fine banded, brown, grey, and white rhythms | Magnetite + quartz + hematite + ferroan-dolomite | ||
| 15 | CG-C12 | Outcrop | 387176 | 202145 | Oxide | Banded, grey to brown slightly weathered, foliated, dominated by hematite bands | Magnetite + hematite + martite + quartz | ||
| 16 | CG-C14 | Outcrop | 386588 | 202279 | Carbonate-oxide | Banded, light grey to grey, slightly weathered, presence of sugary quartz in the chert bands | Magnetite + quartz + hematite + ferroan-dolomite | ||
| 17 | CG-C21 | Outcrop | 386520 | 202354 | Oxide | Fine banded, brown, grey, and white rhythms | Magnetite + quartz + hematite + marite | ||
| 18 | CG-C23 | Outcrop | 386547 | 202390 | Oxide | Fine banded, grey slightly weathered, foliated, dominated by hematite-goethite bands | Magnetite + quartz + hematite | ||
| 19 | CG-C25 | Outcrop | 386720 | 202287 | Oxide | Banded, grey slightly weathered, foliated, dominated by hematite-goethite bands, presence of quartz veins cutting the bands | Magnetite + martite + quartz | ||
| Oxide Facies-BIF | Carbonate-Oxide Facies-BIF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Name | CG-NB3 | CG-NB6 | CG-C2 | CG-C21 | CG-C23–1 | CG-C23–2 | CG-C25 | CG-NB1 | CG-NB10 | CG-C6 |
| SiO2 | 57.40 | 68.37 | 43.74 | 48.05 | 46.43 | 45.47 | 46.25 | 41.55 | 37.23 | 40.26 |
| TiO2 | 0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 | <0.01 |
| Al2O3 | 0.36 | 0.05 | 0.06 | 0.09 | 0.11 | 0.07 | 0.03 | 0.18 | 0.48 | 0.06 |
| TFe2O3 | 41.30 | 30.71 | 53.55 | 48.27 | 48.95 | 50.41 | 48.92 | 44.86 | 44.24 | 55.63 |
| Cr2O3 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | <0.01 |
| MnO | 0.01 | 0.01 | 0.01 | 0.01 | 0.11 | 0.12 | <0.01 | 0.44 | 0.30 | 0.15 |
| MgO | 0.02 | 0.02 | 0.03 | 0.03 | 0.08 | 0.08 | 0.03 | 1.57 | 1.99 | 0.18 |
| CaO | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 | 0.11 | 0.18 | 0.01 |
| Na2O | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| K2O | 0.02 | <0.01 | <0.01 | <0.01 | 0.02 | 0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| P2O5 | 0.02 | 0.01 | 0.20 | 0.04 | 0.02 | 0.02 | 0.03 | 0.01 | 0.05 | 0.03 |
| SO3 | <0.01 | <0.01 | <0.01 | 0.02 | 0.02 | 0.02 | 0.04 | 0.22 | 0.01 | 0.07 |
| LOI | 0.14 | 0.26 | 1.72 | 2.72 | 3.52 | 3.18 | 3.94 | 11.17 | 14.95 | 3.00 |
| Total | 99.15 | 99.18 | 97.60 | 96.53 | 95.76 | 96.22 | 95.34 | 88.94 | 84.50 | 96.39 |
| Fe2O3/Al2O3 | 114.72 | 614.20 | 892.50 | 536.33 | 445.00 | 720.14 | 1630.67 | 249.22 | 245.78 | 927.17 |
| SiO2/Al2O3 | 159.44 | 1367.40 | 729.00 | 533.89 | 422.09 | 649.57 | 1541.67 | 230.83 | 77.56 | 671.00 |
| SiO2 | Al2O3 | TFe2O3 | MnO | MgO | CaO | K2O | P2O5 | LOI | |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 1 | - | - | - | - | - | - | - | - |
| Al2O3 | −0.73630401 | 1 | - | - | - | - | - | - | - |
| TFe2O3 | 0.17460635 | −0.67069794 | 1 | - | - | - | - | - | - |
| MnO | −0.69788362 | 0.54176046 | −0.55797574 | 1 | - | - | - | - | - |
| MgO | −0.79756851 | 0.88406025 | −0.7198373 | 0.86809874 | 1 | - | - | - | - |
| CaO | −0.74643832 | 0.93913917 | −0.77845814 | 0.7593399 | 0.97445819 | 1 | - | - | - |
| K2O | 1 | 1 | −1 | −1 | not | not | 1 | - | - |
| P2O5 | −0.27770787 | 0.52483232 | −0.11711327 | −0.27348272 | 0.18820647 | 0.33746412 | not | 1 | - |
| LOI | −0.76954675 | 0.90910882 | −0.76268074 | 0.8279835 | 0.99066466 | 0.99334296 | 1 | 0.24325744 | 1 |
| SiO2 | Al2O3 | TFe2O3 | MnO | MgO | CaO | P2O5 | LOI 1000 | |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 1 | - | - | - | - | - | - | - |
| Al2O3 | −0.83856602 | 1 | - | - | - | - | - | - |
| TFe2O3 | 0.27337997 | −0.75329361 | 1 | - | - | - | - | - |
| MnO | 0.27174874 | 0.29641895 | −0.85141724 | 1 | - | - | - | - |
| MgO | −0.43677971 | 0.85635354 | −0.98470727 | 0.74702181 | 1 | - | - | - |
| CaO | −0.60562169 | 0.94137984 | −0.93100424 | 0.6012302 | 0.98035734 | 1 | - | - |
| P2O5 | −0.9740111 | 0.69337525 | −0.0484031 | −0.48266298 | 0.22167576 | 0.40964402 | 1 | - |
| LOI 1000 | −0.5167765 | 0.89976557 | −0.96478375 | 0.68346967 | 0.99585639 | 0.99423114 | 0.30943453 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatsé Ebotehouna, C.; Xie, Y.; Adomako-Ansah, K.; Gourcerol, B.; Qu, Y. Depositional Environment and Genesis of the Nabeba Banded Iron Formation (BIF) in the Ivindo Basement Complex, Republic of the Congo: Perspective from Whole-Rock and Magnetite Geochemistry. Minerals 2021, 11, 579. https://doi.org/10.3390/min11060579
Gatsé Ebotehouna C, Xie Y, Adomako-Ansah K, Gourcerol B, Qu Y. Depositional Environment and Genesis of the Nabeba Banded Iron Formation (BIF) in the Ivindo Basement Complex, Republic of the Congo: Perspective from Whole-Rock and Magnetite Geochemistry. Minerals. 2021; 11(6):579. https://doi.org/10.3390/min11060579
Chicago/Turabian StyleGatsé Ebotehouna, Chesther, Yuling Xie, Kofi Adomako-Ansah, Blandine Gourcerol, and Yunwei Qu. 2021. "Depositional Environment and Genesis of the Nabeba Banded Iron Formation (BIF) in the Ivindo Basement Complex, Republic of the Congo: Perspective from Whole-Rock and Magnetite Geochemistry" Minerals 11, no. 6: 579. https://doi.org/10.3390/min11060579
APA StyleGatsé Ebotehouna, C., Xie, Y., Adomako-Ansah, K., Gourcerol, B., & Qu, Y. (2021). Depositional Environment and Genesis of the Nabeba Banded Iron Formation (BIF) in the Ivindo Basement Complex, Republic of the Congo: Perspective from Whole-Rock and Magnetite Geochemistry. Minerals, 11(6), 579. https://doi.org/10.3390/min11060579







