Automated Optical Image Analysis of Iron Ore Sinter
Abstract
1. Introduction
2. Techniques for Characterising the Mineralogy of Iron Ore Sinter
2.1. Optical-Based Methods
2.2. X-ray Diffraction-Based Methods
2.3. Electron-Beam Based Methods
3. Sample Preparation, Imaging and Methodology
3.1. Sample Preparation
3.2. Sample Imaging
3.3. Optical Image Analysis Software
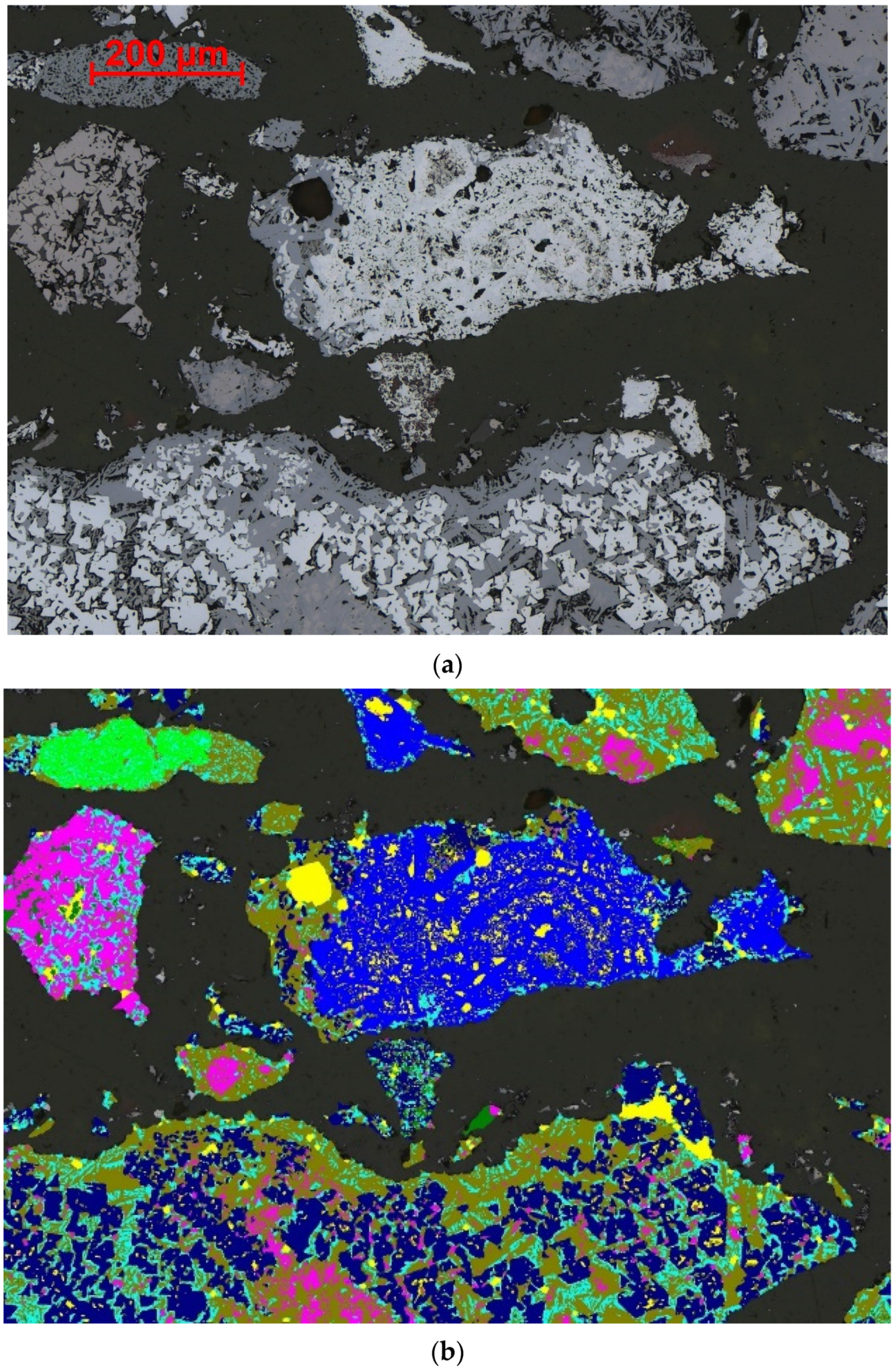
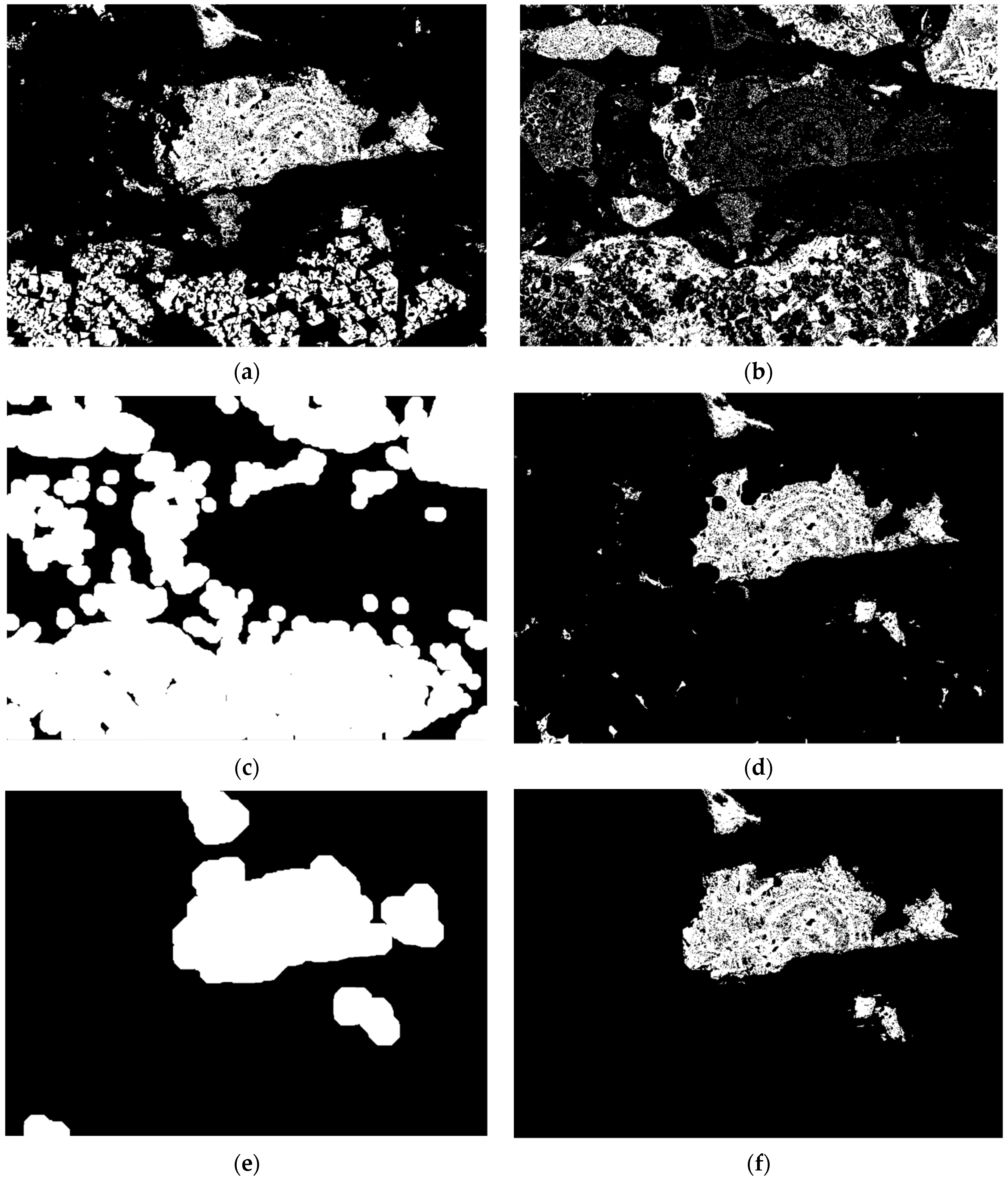
4. Automated Segmentation of Phases in Sinter
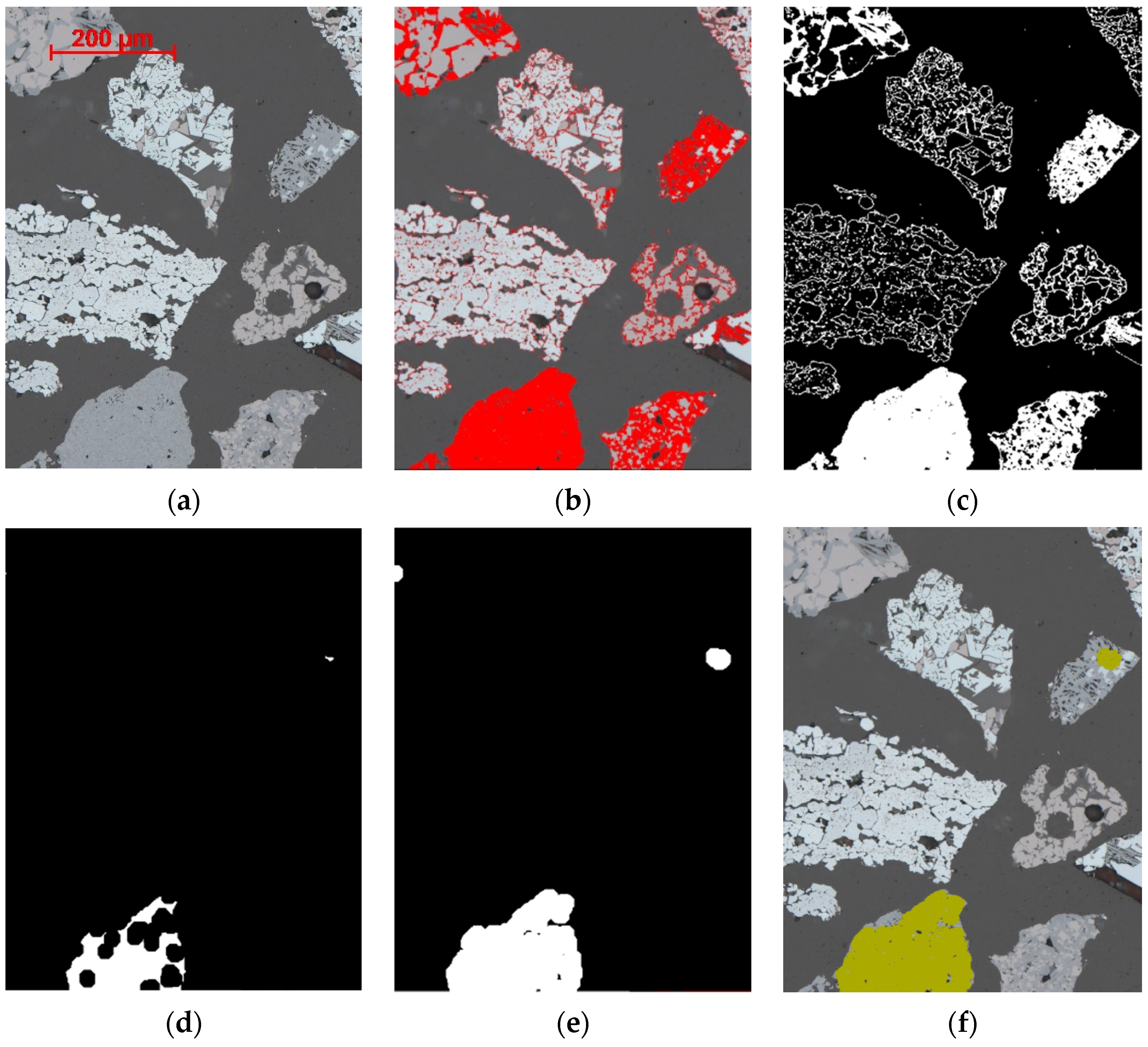
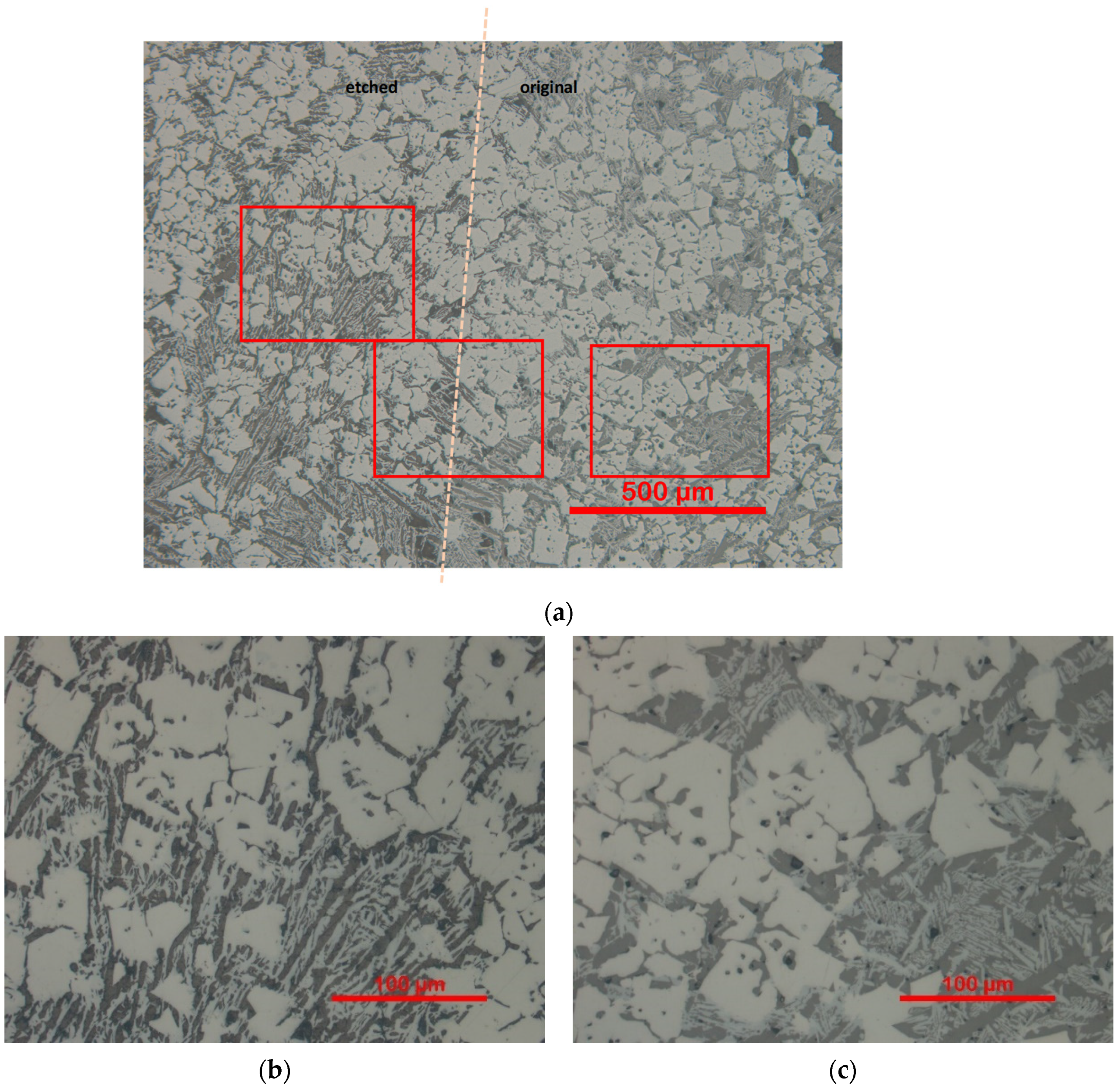
4.1. Segmentation of Primary and Secondary Hematite
4.2. Segmentation of SFCA Microtypes
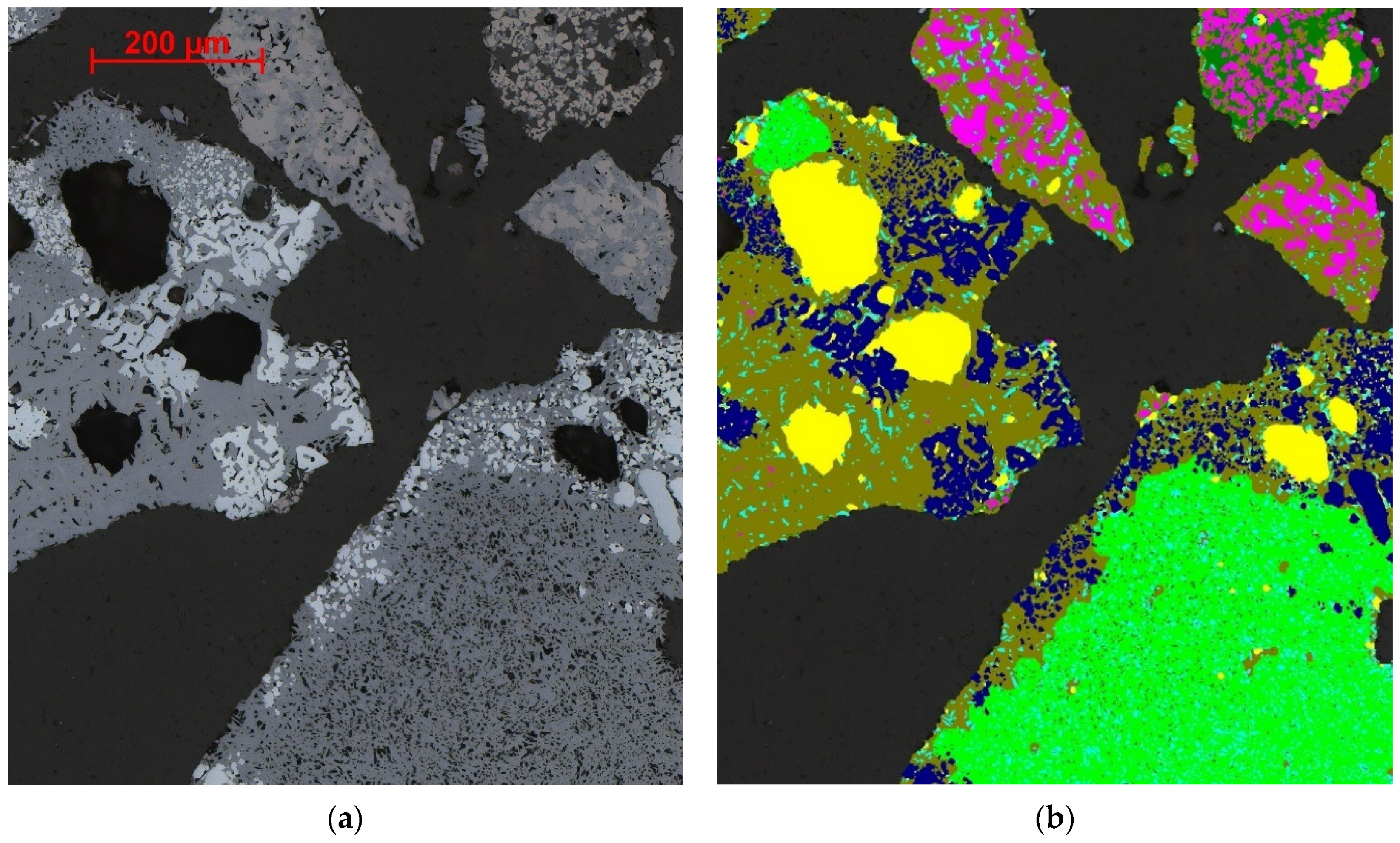
4.3. Segmentation of Magnetite, Glass, Larnite and Aluminosilicate
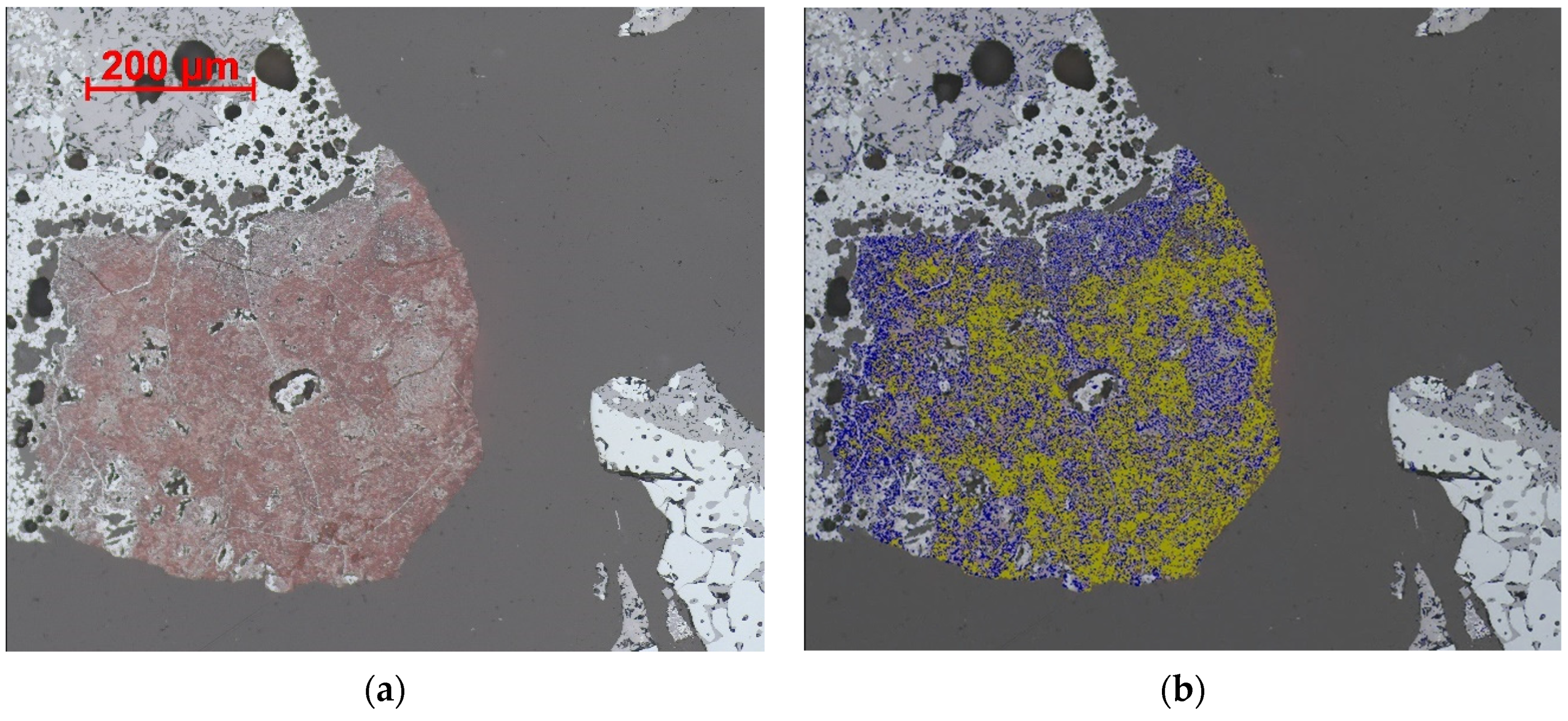
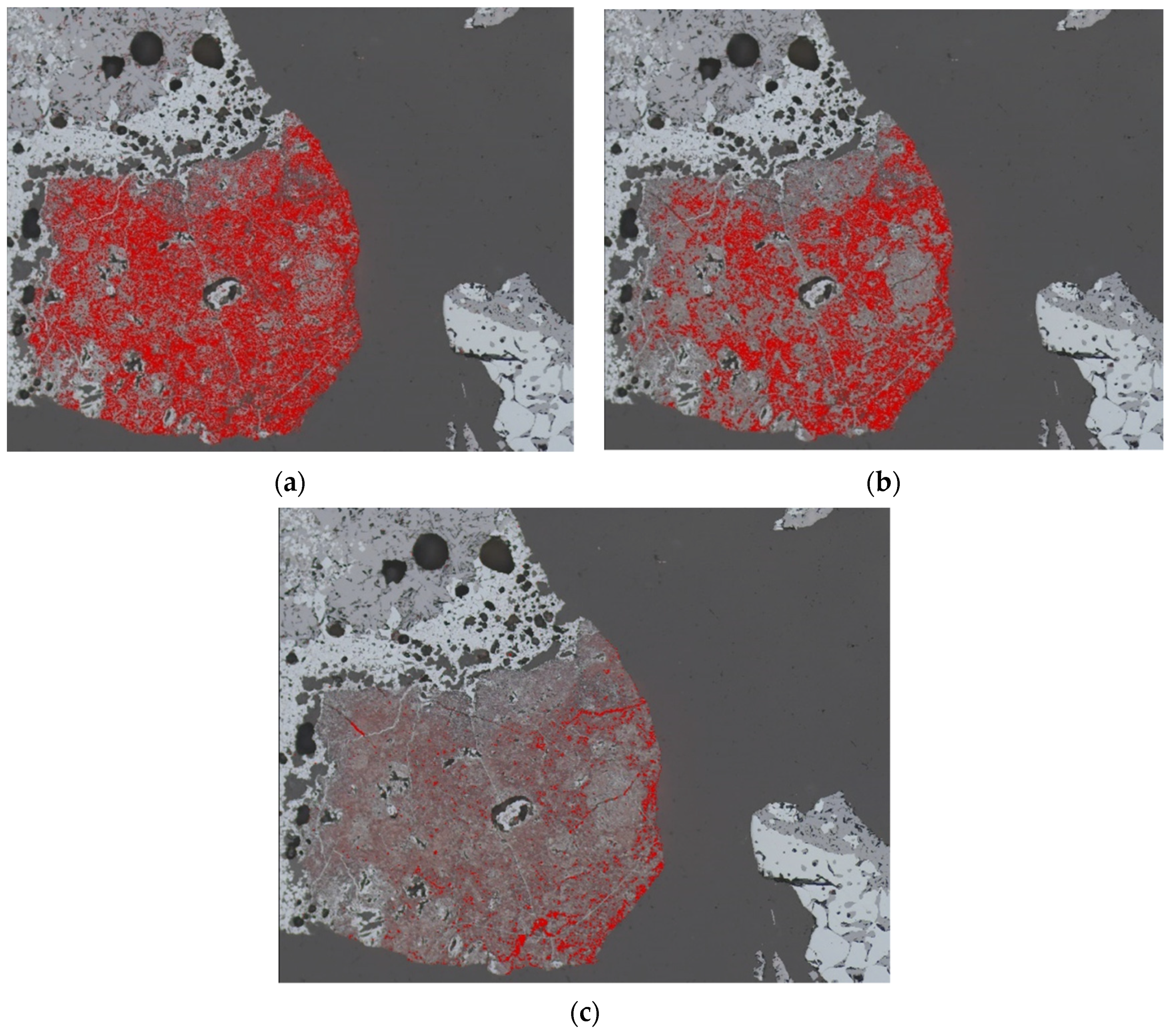
4.3.1. Magnetite
4.3.2. Glass
4.3.3. Larnite
4.3.4. Aluminosilicates and Quartz
5. Discussion
5.1. Comparison of OIA Results with PC
5.2. Comparison of OIA Results with QXRD
5.3. Comparison of OIA Results with Bulk Chemistry Determined by XRF
5.4. Sinter Microtextures and Mineral Associations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pownceby, M.I.; Clout, J.M.F. Importance of fine ore chemical composition and high temperature phase relations: Applications to iron ore sintering and pelletising. Miner. Process. Extr. Metall. 2003, 112, 44–51. [Google Scholar] [CrossRef]
- Harvey, T. Influence of Mineralogy and Pore Structure on the Reducibility and Strength of Iron Ore Sinter. Ph.D. Thesis, University of Newcastle, Callaghan, Australia, 2020; 400p. [Google Scholar]
- Wang, W.; Deng, M.; Xu, R.-S.; Xu, W.-B.; Ouyang, Z.-L.; Huang, X.-B.; Xue, Z.-L. Three-dimensional structure and micro-mechanical properties of iron ore sinter. J. Iron Steel Res. Int. 2017, 24, 1007–1015. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.-H.; Xu, R.-S.; Li, J.; Shen, W.-J.; Wang, S.-P. Research progress on multiscale structural characteristics and characterization methods of iron ore sinter. J. Iron Steel Res. Int. 2020, 27, 367–379. [Google Scholar] [CrossRef]
- Donskoi, E.; Manuel, J.R.; Lu, L.; Holmes, R.J.; Poliakov, A.; Raynlyn, T.D. Importance of textural information in mathematical modelling of iron ore fines sintering performance. Miner. Process. Extr. Metall. 2017, 127, 103–114. [Google Scholar] [CrossRef]
- Sinha, M.; Nistala, S.H.; Chandra, S.; Mankhand, T.R.; Ghose, A.K. Correlating mechanical properties of sinter phases with their chemistry and its effect on sinter quality. Ironmak. Steelmak. 2017, 44, 100–107. [Google Scholar] [CrossRef]
- Pirard, E.; Lebichot, S.; Krier, W. Particle texture analysis using polarized light imaging and grey level intercepts. Int. J. Miner. Process. 2007, 84, 299–309. [Google Scholar] [CrossRef]
- Donskoi, E.; Poliakov, A.; Manuel, J.R. Automated Optical Image Analysis of Natural and Sintered Iron Ore. In Iron Ore: Mineralogy, Processing and Environmental Sustainability; Lu, L., Ed.; Elsevier Inc.: Cambridge, UK, 2015; pp. 101–159. [Google Scholar]
- Donskoi, E.; Poliakov, A.; Manuel, J.R.; Peterson, M.; Hapugoda, S. Industrial Strength Optical Image Analysis System—Mineral4/Recognition4. In Proceedings of the Iron Ore 2013, Perth, Australia, 12–14 August 2013; pp. 227–241. [Google Scholar]
- Gomes, O.D.M.; Paciornik, S. Iron ore quantitative characterization through reflected light-scanning electron co-site microscopy. In Proceedings of the Ninth International Congress on Applied Mineralogy, Brisbane, Australia, 8–10 September 2008; pp. 699–702. [Google Scholar]
- Gomes, O.D.M.; Paciornik, S. RLM-SEM co-site microscopy applied to iron ore characterization. In Proceedings of the Annals of 2nd International Symposium on Iron Ore, São Luís, Brazil, 22–26 September 2008; pp. 218–224. [Google Scholar]
- Iglesias, J.C.A.; Augusto, K.S.; Da Fonseca Martins Gomes, O.; Domingues, A.L.A.; Vieira, M.B.; Casagrande, C.; Paciornik, S. Automatic characterization of iron ore by digital microscopy and image analysis. J. Mater. Res. Technol. 2018, 7, 376–380. [Google Scholar] [CrossRef]
- Bückner, B.; Mali, H. Extended Analyses of Iron Ore Sinter by Image Processing. Steel Res. Int. 2020, 91, 02000236. [Google Scholar] [CrossRef]
- Gottlieb, P.; Wilkie, G.; Sutherland, D.; Ho-Tun, E.; Suthers, S.; Perera, K.; Jenkins, B.; Spencer, S.; Butcher, A.; Rayner, J. Using quantitative electron microscopy for process mineralogy applications. JOM 2000, 52, 24–25. [Google Scholar] [CrossRef]
- Maddren, J.; Ly, C.V.; Suthers, S.P.; Butcher, A.R.; Trudu, A.G.; Botha, P.W.S.K. A new approach to ore characterisation using automated quantitative mineral analysis. In Proceedings of the AusIMM, Iron Ore 2007, Perth, Australia, 20–22 August 2007; pp. 131–132. [Google Scholar]
- Hrstka, T.; Gottlieb, P.; Skala, R.; Breiter, K.; Motl, D. Automated mineralogy and petrology—Applications of TESCAN Integrated Mineral Analyzer (TIMA). J. Geosci. 2018, 63, 47–63. [Google Scholar] [CrossRef]
- König, U.; Gobbo, L.; Macchiarola, K. Using X-ray diffraction for grade control and minimising environmental impact in iron and steel industries. In Proceedings of the AusIMM, Iron Ore 2011, Perth, Australia, 11–13 July 2011; pp. 49–56. [Google Scholar]
- Honeyands, T.; Manuel, J.; Matthews, L.; O’Dea, D.; Pinson, D.; Leedham, J.; Zhang, G.; Li, H.; Monaghan, B.; Liu, X.; et al. Comparison of the mineralogy of iron ore sinters using a range of techniques. Minerals 2019, 9, 333. [Google Scholar] [CrossRef]
- Otsu, N. Threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62. [Google Scholar] [CrossRef]
- Donskoi, E.; Poliakov, A. Advances in Optical Image Analysis Textural Segmentation in Ironmaking. Appl. Sci. 2020, 10, 6242. [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Scarlett, N.V.Y.; Madsen, I.C. Quantification of phases with partial or no known crystal structures. Powder Diffr. 2006, 21, 278–284. [Google Scholar] [CrossRef]
- Figueroa, G.; Möller, K.; Buhot, M.; Gloy, G.; Haberlah, D. Advanced Discrimination of Hematite and Magnetite by Automated Mineralogy. In Proceedings of the 10th International Congress for Applied Mineralogy (ICAM), Trondheim, Norway, 1–5 August 2011; pp. 197–204. [Google Scholar]
- Keulen, N.; Malkki, S.N.; Graham, S. Automated quantitative mineralogy applied to metamorphic rocks. Minerals 2020, 10, 47. [Google Scholar] [CrossRef]
- Tonžetić, I.Ž. Quantitative analysis of iron ore using SEM-based technologies. In Iron Ore: Mineralogy, Processing and Environmental Sustainability; Lu, L., Ed.; Elsevier Inc.: Cambridge, UK, 2015; pp. 161–189. [Google Scholar]
- Nicol, S.; Chen, J.; Pownceby, M.; Webster, N. A review of the chemistry, structure and formation conditions of silico-ferrite of calcium and aluminium (‘SFCA’) phases. ISIJ Int. 2018, 58, 2157–2172. [Google Scholar] [CrossRef]
- Donskoi, E.; Manuel, J.R.; Austin, P.; Poliakov, A.; Peterson, M.; Hapugoda, S. Comparative study of iron ore characterization using a scanning electron microscope and optical image analysis. Trans. Inst. Min. Metall. 2014, 122, 217–229. [Google Scholar]
- Sasaki, M.; Hida, Y. Considerations on the properties of sinter from the point of sintering reaction. Trans. Iron Steel Inst. Jpn. 1982, 68, 563–571. [Google Scholar] [CrossRef]
- Scarlett, N.V.Y.; Pownceby, M.I.; Madsen, I.C.; Christensen, A.N. Reaction sequences in the formation of SFCA and SFCA-I in iron ore sinter. Metall. Mater. Trans. B 2004, 35B, 929–936. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C.; Kimpton, J.A. Silico-ferrite of Calcium and Aluminium (SFCA) Iron Ore Sinter Bonding Phases: New Insights into Their Formation During Heating and Cooling. Metall. Mater. Trans. B 2012, 43, 1344–1357. [Google Scholar] [CrossRef]
- Lu, L.; Holmes, R.J.; Manuel, J.R. Effects of Alumina on Sintering Performance of Hematite Iron Ores. ISIJ Int. 2007, 47, 349–358. [Google Scholar] [CrossRef]
- Donskoi, E.; Raynlyn, T.D.; Poliakov, A. Image analysis estimation of iron ore particle segregation in epoxy blocks. Miner. Eng. 2018, 120, 102–109. [Google Scholar] [CrossRef]
- Donskoi, E.; Hapugoda, S.; Lu, L.; Poliakov, A.; Peterson, M.; Haileslassie, A. Advances in Optical Image Analysis of Iron Ore Sinter. In Proceedings of the AusIMM, Iron Ore 2015, Perth, Australia, 13–15 July 2015; pp. 543–548. [Google Scholar]
- Donskoi, E.; Poliakov, A.; Manuel, J.R.; Peterson, M.; Hapugoda, S. Novel developments in optical image analysis for iron ore, sinter and coke characterisation. Trans. Inst. Min. Metall. 2015, 124, 227–244. [Google Scholar] [CrossRef]
- Donskoi, E.; Suthers, S.P.; Fradd, S.B.; Young, J.M.; Campbell, J.J.; Raynlyn, T.D.; Clout, J.M.F. Utilization of optical image analysis and automatic texture classification for iron ore particle characterisation. Miner. Eng. 2007, 20, 461–471. [Google Scholar] [CrossRef]
- McAndrew, J.; Clout, J.M.F. The nature of SFCA and its importance as a bonding phase in iron ore sinter. In Proceedings of the 4th China-Australia Symposium on the Technology of Feed Preparation for Ironmaking, Dampier, Australia, June 1993; pp. 1–15. [Google Scholar]
- Mumme, W.G.; Clout, J.M.F.; Gable, R.W. The crystal structure of SFCA-I, Ca3.18Fe3+14.30Fe2+0.55O25, a homologue of the aenigmatite structure type, and new crystal structure refinements of β-CFF, Ca2.99Fe3+14.30Fe2+0.55O25 and Mg-free SFCA, Ca2.45Fe3+9.04Fe2+0.16O20. Neues Jahrb. Für Mineral. Abh. 1998, 173, 93. [Google Scholar] [CrossRef]
- Ahsan, S.N.; Mukherjee, T.; Whiteman, J.A.; Whiteman, J.A. Structure of fluxed sinter. Ironmak. Steelmak. 1983, 10, 54–64. [Google Scholar]
- Mežibrický, R.; Fröhlichová, M. Silico-ferrite of Calcium and Aluminium Characterization by Crystal Morphology in Iron Ore Sinter Microstructure. ISIJ Int. 2016, 56, 1111–1113. [Google Scholar] [CrossRef]
- Pownceby, M.I.; Patrick, T.R.C. Stability of SFC (silico-ferrite of calcium): Solid solution limits, thermal stability and selected phase relationships within the Fe2O3-CaO-SiO2 (FCS) system. Eur. J. Mineral. 2000, 12, 455–468. [Google Scholar] [CrossRef]
- Donskoi, E.; Poliakov, A.; Manuel, J.R.; Raynlyn, T.D. Advances in optical image analysis and textural classification of iron ore fines. In Proceedings of the XXV International Mineral Processing Congress—IMPC2010, Brisbane, Australia, 6–10 September 2010; pp. 2823–2836. [Google Scholar]
- Donskoi, E.; Manuel, J.R.; Hapugoda, S.; Poliakov, A.; Raynlyn, T.; Austin, P.; Peterson, M. Automated optical image analysis of goethitic iron ores. Trans. Inst. Min. Metall. 2020. [Google Scholar] [CrossRef]
- Poliakov, A.; Donskoi, E. Automated relief-based discrimination of non-opaque minerals in optical image analysis. Miner. Eng. 2014, 55, 111–124. [Google Scholar] [CrossRef]
- Chan, C.J.; Kriven, W.M.; Young, J.F. Physical stabilization of the β → γ transformation in dicalcium silicate. J. Am. Ceram. Soc. 1992, 75, 1621–1627. [Google Scholar] [CrossRef]
- Patrick, T.R.C.; Lovel, R.R. Leaching dicalcium silicates from iron ore sinter to remove phosphorus and other contaminants. ISIJ Int. 2001, 41, 128–135. [Google Scholar] [CrossRef]
- Iglesias, J.C.Á.; Santos, R.B.M.; Paciornik, S. Deep learning discrimination of quartz and resin in optical microscopy images of minerals. Miner. Eng. 2019, 138, 79–85. [Google Scholar] [CrossRef]
- Honeyands, T.; Manuel, J.; Matthews, L.; O’Dea, D.; Pinson, D.; Leedham, J.; Monaghan, B.; Li, H.; Chen, J.; Hayes, P.; et al. Characterising the mineralogy of iron ore sinters—State-of-the-art in Australia. In Proceedings of the Iron Ore 2017, Perth, Australia, 24–26 July 2017; pp. 49–60. [Google Scholar]
- Raven, M.D.; Birch, S.L. Summary of results of an International X-ray Diffraction Round Robin for Mineralogical Analysis of Iron Ores. In Proceedings of the Iron Ore 2017, Perth, Australia, 24–26 July 2017; pp. 599–604. [Google Scholar]
- Donskoi, E.; Manuel, J.R.; Clout, J.M.F.; Zhang, Y. Mathematical modelling and optimization of iron ore sinter properties. Isr. J. Chem. 2007, 47, 373–379. [Google Scholar] [CrossRef]
- Greco, A.; Jeulin, D.; Serra, J. The Use Of The Texture Analyser To Study Sinter Structure: Application To The Morphology Of Calcium Ferrites Encountered In Basic Sinters Of Rich Iron Ores. J. Microsc. 1979, 116, 199. [Google Scholar] [CrossRef]
- Murakami, T.; Kodaira, T.; Kasai, E. Reduction and disintegration behaviour of sinter under N2–CO–CO2–H2–H2O gas at 773 K. ISIJ Int. 2015, 55, 1181–1187. [Google Scholar] [CrossRef]
- Shigaki, I.; Sawada, M.; Gennai, N. Increase in low-temperature reduction due to hematite-alumina solid solution and degradation of iron ore sinter and columnar calcium ferrite. Trans. ISIJ 1986, 26, 503–511. [Google Scholar] [CrossRef]
- Clout, J.M.F.; Manuel, J.R. Fundamental investigations of differences in bonding mechanisms in iron ore sinter formed from magnetite concentrates and hematite ores. Powder Technol. 2003, 130, 393–399. [Google Scholar] [CrossRef]




| Phase | UN011 | UN 154 | UN016 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | OIA NVS | OIA VS | PC1 | PC2 | OIA | PC1 | PC2 | OIA | |
| Magnetite | 28.0 | 26.3 | 25.4 | 25.5 | 28.1 | 27.1 | 27.9 | 15.1 | 11.9 | 13.1 |
| Primary Hematite | 14.8 | 22.6 | 17.6 | 19.4 | 6.7 | 5.5 | 6.4 | 12.0 | 17.2 | 13.6 |
| Secondary Hematite | 24.9 | 19.8 | 19.7 | 15.5 | 17.6 | 19.3 | 16.5 | 19.2 | 15.4 | 16.7 |
| Total Hematite | 39.7 | 42.4 | 37.3 | 34.9 | 24.3 | 24.8 | 22.9 | 31.2 | 32.6 | 30.3 |
| Platy SFCA | 0.8 | 0.9 | 0.8 | 0.7 | 2.8 | 1.2 | 1.0 | 4.0 | 6.0 | 6.6 |
| SFCA | 17.3 | 18.5 | 20.0 | 23.0 | 29.9 | 33.4 | 32.8 | 38.3 | 36.6 | 36.2 |
| Total SFCA | 18.1 | 19.4 | 20.9 | 23.7 | 32.7 | 34.6 | 33.8 | 42.3 | 42.6 | 42.8 |
| Glass | 6.5 | 7.2 | 6.9 | 7.9 | 6.3 | 7.5 | 7.0 | 2.7 | 5.3 | 4.7 |
| Larnite | 6.7 | 3.7 | 9.2 | 7.2 | 6.8 | 5.4 | 7.5 | 4.9 | 3.8 | 8.5 |
| Quartz | 0.0 | 0.9 | 0.3 | 0.7 | 0.0 | 0.1 | 0.5 | 0.0 | 0.7 | 0.6 |
| Dark Aluminosilicate | --- | --- | 0.1 | 0.1 | --- | --- | 0.4 | --- | --- | 0.1 |
| Other | 1.0 | 0.2 | --- | --- | 1.8 | 0.6 | --- | 3.7 | 3.1 | --- |
| Phase | Density Used | UN011 | UN154 | UN016 | ||||
|---|---|---|---|---|---|---|---|---|
| QXRD | OIA NVS | OIA VS | QXRD | OIA | QXRD | OIA | ||
| Magnetite | 4.9 | 29.2 | 28.8 | 29.2 | 27.5 | 32.7 | 15.1 | 15.6 |
| Primary Hematite | 5.0 | --- | 20.4 | 22.7 | --- | 7.7 | --- | 16.6 |
| Secondary Hematite | 4.8 | --- | 22.0 | 17.4 | --- | 19.0 | --- | 19.5 |
| Total Hematite | 37.7 | 42.4 | 40.1 | 30.0 | 26.7 | 36.1 | 36.0 | |
| SFCA-I (QXRD)/Platy SFCA (OIA) | 3.8 | 1.8 | 0.7 | 0.6 | 0.0 | 0.9 | 10.2 | 6.1 |
| SFCA | 3.7 | 16.0 | 17.2 | 19.9 | 22.3 | 29.1 | 19.6 | 32.6 |
| Total SFCA | 17.8 | 17.9 | 20.5 | 22.3 | 30.0 | 29.9 | 38.7 | |
| Glass | 2.5 | 9.3 | 4.0 | 4.6 | 14.0 | 4.4 | 12.4 | 2.8 |
| Larnite | 3.1 | 5.5 | 6.6 | 5.2 | 6.2 | 5.6 | 5.4 | 6.4 |
| Quartz | 2.5 | 0.4 | 0.2 | 0.4 | 0.0 | 0.3 | 0.6 | 0.4 |
| Dark Aluminosilicate | 3.0 | --- | 0.1 | 0.0 | --- | 0.3 | --- | 0.0 |
| Phase | UN011 | UN154 | UN016 | |||
|---|---|---|---|---|---|---|
| XRF | OIA VS | XRF | OIA | XRF | OIA | |
| Fe | 57.4 | 58.6 | 56.7 | 57.0 | 56.9 | 56.7 |
| SiO2 | 5.6 | 4.5 | 5.4 | 4.7 | 5.0 | 4.8 |
| Al2O3 | 1.8 | 1.8 | 1.9 | 2.2 | 1.7 | 2.1 |
| CaO | 9.2 | 7.6 | 10.0 | 8.9 | 9.8 | 9.7 |
| Particle Texture | UN011 (Plant, Basicity 1.66) | UN154 (Plant, Basicity 1.85) | UN016 (Pot, Basicity 1.99) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Particles | Area% | Wt% | No. of Particles | Area% | Wt% | No. of Particles | Area% | Wt% | |
| Primary Hematite Porous Mix | 662 | 14.1 | 14.5 | 55 | 3.49 | 3.83 | 195 | 8.41 | 8.94 |
| Primary Hematite LowPor. Mix | 115 | 0.33 | 0.39 | 26 | 0.78 | 0.96 | 61 | 1.99 | 2.52 |
| Secondary Hematite SFCA2 | 687 | 1.41 | 1.43 | 321 | 3.41 | 3.36 | 409 | 3.47 | 3.52 |
| Secondary Hematite Glass | 603 | 1.07 | 1.02 | 312 | 4.6 | 4.11 | 143 | 0.26 | 0.23 |
| Sec-Prime Hematite SFCA2 | 65 | 2.4 | 2.43 | 12 | 1.92 | 1.74 | 47 | 4.58 | 4.50 |
| Sec-Prime Hematite Mix | 345 | 12.6 | 12.4 | 49 | 3.55 | 3.39 | 94 | 5.59 | 5.58 |
| Magnetite SFCA2 | 904 | 6.4 | 6.54 | 444 | 7.45 | 7.95 | 171 | 1.51 | 1.69 |
| Magnetite Glass | 188 | 2.28 | 2.26 | 64 | 2.24 | 2.22 | 14 | 0.18 | 0.20 |
| Magnetite Mix | 459 | 3.24 | 3.32 | 104 | 1.57 | 1.7 | 61 | 2.02 | 2.28 |
| Magnetite | 561 | 1.01 | 1.11 | 96 | 3.00 | 3.39 | 82 | 0.45 | 0.50 |
| Magnetite-Hematite Mix | 211 | 6.18 | 6.33 | 60 | 1.59 | 1.65 | 17 | 1.17 | 1.18 |
| Hematite-Magnetite Mix | 291 | 4.56 | 4.61 | 100 | 2.02 | 2.07 | 40 | 0.81 | 0.82 |
| SFCApl Mix | 19 | 0.09 | 0.08 | 11 | 0.35 | 0.33 | 74 | 1.7 | 1.63 |
| SFCA Magnetite | 580 | 4.07 | 4.11 | 656 | 12.7 | 12.9 | 217 | 5.08 | 5.24 |
| SFCA Hematite | 99 | 0.67 | 0.66 | 67 | 0.41 | 0.39 | 137 | 3.3 | 3.26 |
| SFCA HematiteMagnetite | 342 | 2.62 | 2.48 | 356 | 6.41 | 6.2 | 290 | 10.1 | 9.89 |
| SFCA Mix | 255 | 0.86 | 0.8 | 284 | 1.93 | 1.81 | 200 | 2.48 | 2.44 |
| SFCA2 | 757 | 0.45 | 0.41 | 943 | 1.19 | 1.12 | 779 | 4.71 | 4.52 |
| SFCA2-Magnetite Hematite | 66 | 2.41 | 2.47 | 35 | 1.82 | 1.87 | 16 | 1.15 | 1.12 |
| SFCA2-Magnetite Mix | 524 | 6.42 | 6.47 | 361 | 8.83 | 8.93 | 191 | 6.48 | 6.56 |
| Magnetite-SFCA2 Mix | 663 | 8.09 | 8.26 | 274 | 8.06 | 8.17 | 134 | 1.54 | 1.63 |
| SFCA2-Hematite Mix | 426 | 4.27 | 4.16 | 203 | 6.69 | 6.53 | 344 | 18.2 | 18 |
| Hematite-SFCA2-Magnetite Mix | 328 | 1.69 | 1.70 | 104 | 1.74 | 1.74 | 200 | 6.04 | 6.03 |
| HematiteMagnetiteGlass Mix | 54 | 0.99 | 0.95 | 42 | 2.36 | 2.35 | 5 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donskoi, E.; Hapugoda, S.; Manuel, J.R.; Poliakov, A.; Peterson, M.J.; Mali, H.; Bückner, B.; Honeyands, T.; Pownceby, M.I. Automated Optical Image Analysis of Iron Ore Sinter. Minerals 2021, 11, 562. https://doi.org/10.3390/min11060562
Donskoi E, Hapugoda S, Manuel JR, Poliakov A, Peterson MJ, Mali H, Bückner B, Honeyands T, Pownceby MI. Automated Optical Image Analysis of Iron Ore Sinter. Minerals. 2021; 11(6):562. https://doi.org/10.3390/min11060562
Chicago/Turabian StyleDonskoi, Eugene, Sarath Hapugoda, James Robert Manuel, Andrei Poliakov, Michael John Peterson, Heinrich Mali, Birgit Bückner, Tom Honeyands, and Mark Ian Pownceby. 2021. "Automated Optical Image Analysis of Iron Ore Sinter" Minerals 11, no. 6: 562. https://doi.org/10.3390/min11060562
APA StyleDonskoi, E., Hapugoda, S., Manuel, J. R., Poliakov, A., Peterson, M. J., Mali, H., Bückner, B., Honeyands, T., & Pownceby, M. I. (2021). Automated Optical Image Analysis of Iron Ore Sinter. Minerals, 11(6), 562. https://doi.org/10.3390/min11060562








