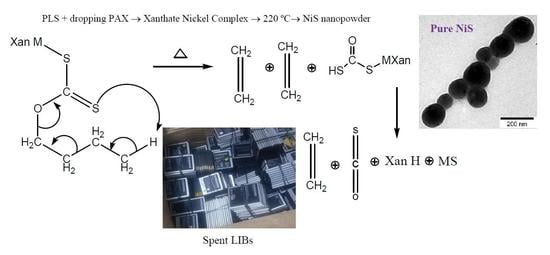
Hydrometallurgical Synthesis of Nickel Nano-Sulfides from Spent Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterization Methods
2.3. Preparation of Spent Batteries
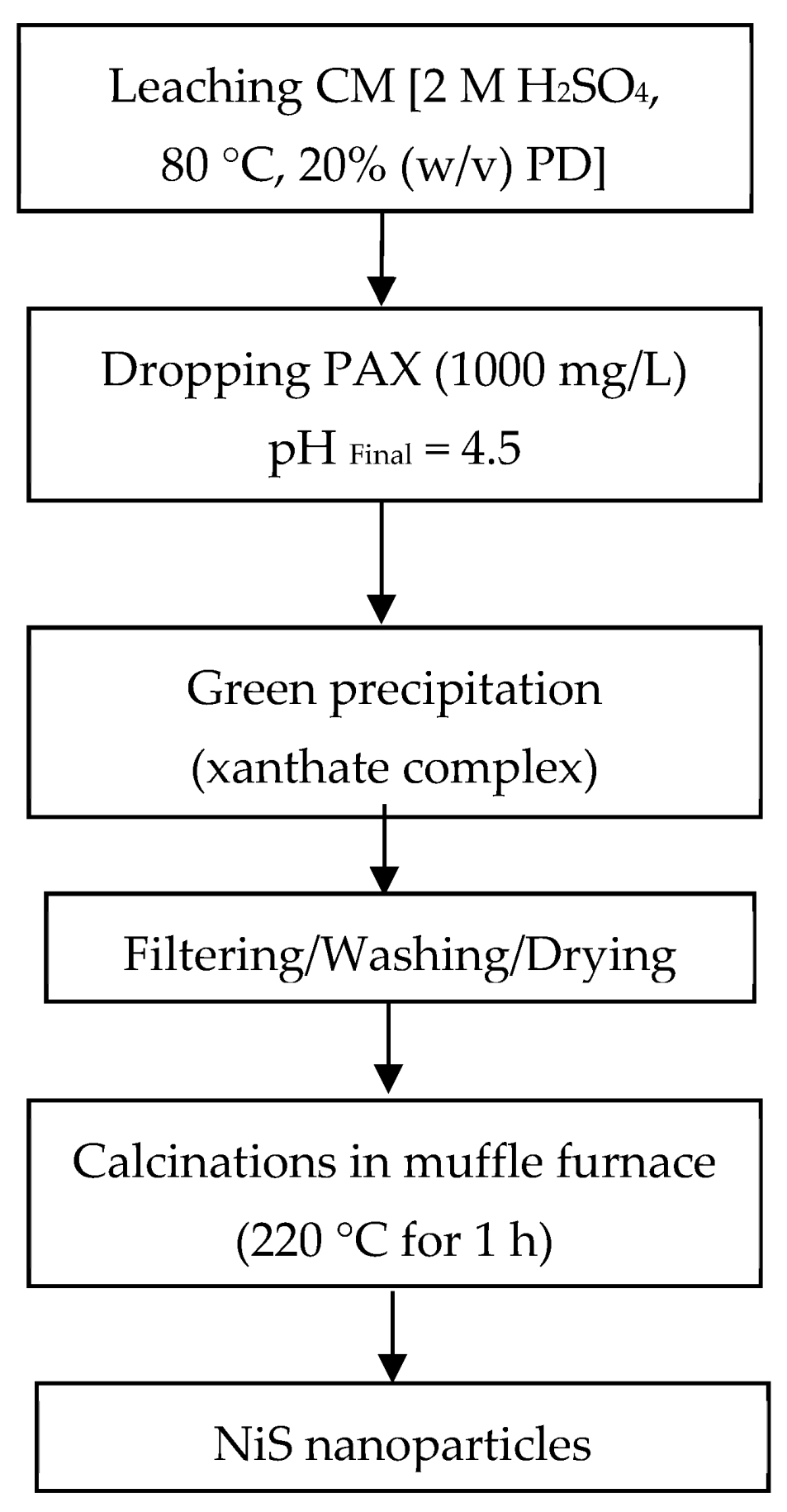
2.4. Leaching Process
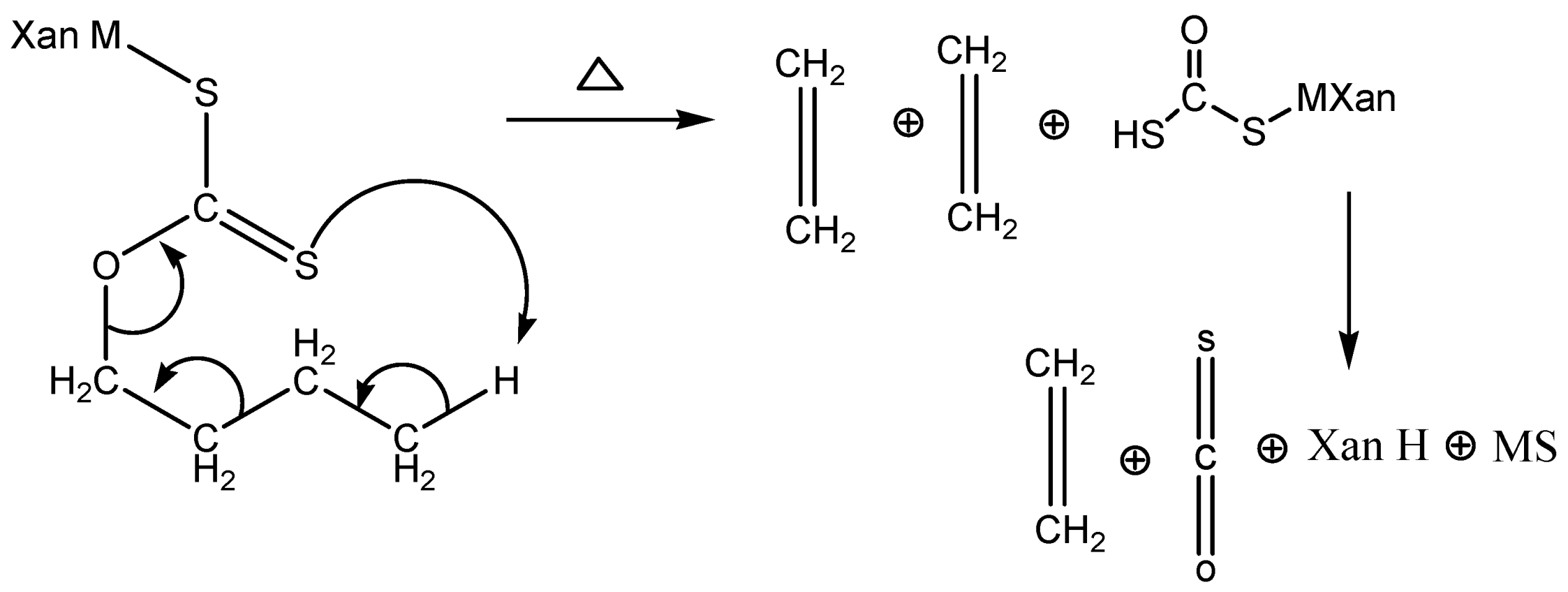
2.5. Fabrication of Nanoparticles
3. Results and Discussions
3.1. X-Ray Powder Diffraction (XRD)
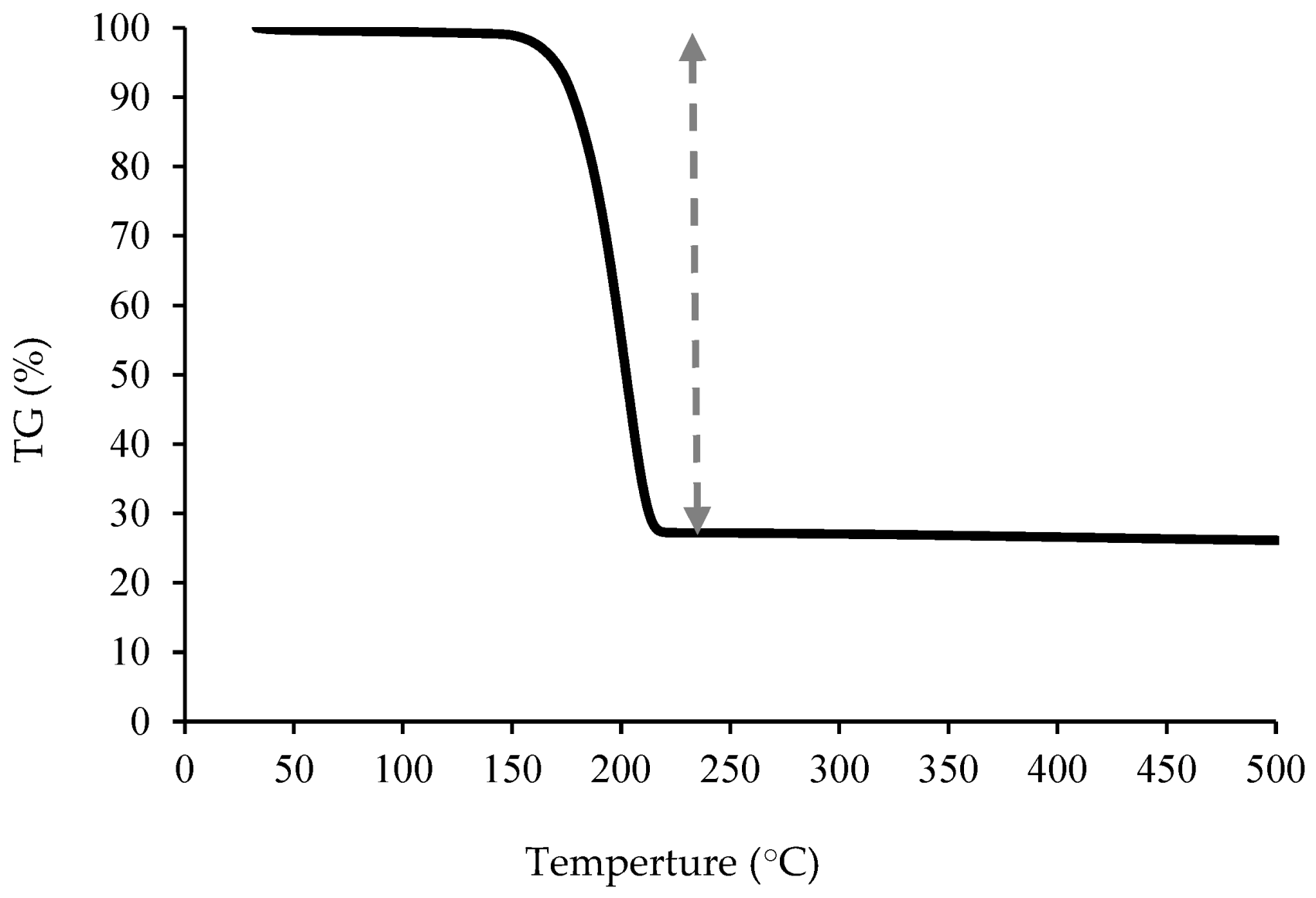
3.2. Thermogravimetry & Differential Thermal Analysis (TGA-DTA)
3.3. Fourier-Transform Infrared (FTIR) Spectroscopy
3.4. Scanning Electron Microscopy (SEM) Energy Dispersive X-Ray Spectrum (EDS)
3.5. Transmission Electron Microscopy (TEM)
3.6. Photon Correlation Spectroscopy (PCS)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciez, R.E.; Whitacre, J. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y. An overview of recycling and treatment of spent LiFePO4 batteries in China. Resour. Conserv. Recy. 2017, 127, 233–243. [Google Scholar] [CrossRef]
- Chen, L.; Tang, X.; Zhang, Y.; Li, L.; Zeng, Z.; Zhang, Y. Process for the recovery of cobalt oxalate from spent lithium-ion batteries. Hydrometallurgy 2011, 108, 80–86. [Google Scholar] [CrossRef]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.-M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
- Pranolo, Y.; Zhang, W.; Cheng, C. Recovery of metals from spent lithium-ion battery leach solutions with a mixed solvent extractant system. Hydrometallurgy 2010, 102, 37–42. [Google Scholar] [CrossRef]
- Lupi, C.; Pasquali, M. Electrolytic nickel recovery from lithium-ion batteries. Miner. Eng. 2003, 16, 537–542. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. Recycling of end-of-life lithium ion batteries, Part I: Commercial processes. J. Sustain. Met. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Flett, D.S. Cobalt-nickel separation in hydrometallurgy: A review. Chem. Sustain. Dev. 2004, 12, 81–91. [Google Scholar]
- Armstrong, R.D.; Todd, M.; Atkinson, J.W.; Scott, K. Electroseparation of cobalt and nickel from a simulated wastewater. J. Appl. Electrochem. 1997, 27, 965–969. [Google Scholar] [CrossRef]
- Castillo, S.; Ansart, F.; Laberty-Robert, C.; Portal, J. Advances in the recovering of spent lithium battery compounds. J. Power Source 2002, 112, 247–254. [Google Scholar] [CrossRef]
- Miller, M.J.; Scheithauer, R.A. Method for Separation of Cobalt from Nickel. U.S. Patent 2,842,427, 8 July 1958. [Google Scholar]
- Liu, P.; Xiao, L.; Chen, Y.; Tang, Y.; Wu, J.; Chen, H. Recovering valuable metals from LiNixCoyMn1-x-yO2 cathode materials of spent lithium ion batteries via a combination of reduction roasting and stepwise leaching. J. Alloys Compd. 2019, 783, 743–752. [Google Scholar] [CrossRef]
- Chen, X.; Kang, D.; Cao, L.; Li, J.; Zhou, T.; Ma, H. Separation and recovery of valuable metals from spent lithium ion batteries: Simultaneous recovery of Li and Co in a single step. Sep. Purif. Technol. 2019, 210, 690–697. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Qu, L.; Feng, Y.; Li, J.; Liu, J.; Zhang, G.; Xie, W. Enhancement in leaching process of lithium and cobalt from spent lithium-ion batteries using benzenesulfonic acid system. Waste Manag. 2019, 88, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Zhang, Y.; Dong, P.; Wang, D.; Zhou, Z.; Duan, J.; Li, X.; Lin, Y.; Meng, Q.; Pai, K.V.; et al. An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. J. Environ. Chem. Eng. 2019, 7, 102854. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Chien, S.-K.; Jhang, S.-R.; Lin, Y.-C. Chemical leaching, precipitation and solvent extraction for sequential separation of valuable metals in cathode material of spent lithium ion batteries. J. Taiwan Inst. Chem. E 2019, 100, 151–159. [Google Scholar] [CrossRef]
- Wu, C.; Li, B.; Yuan, C.; Ni, S.; Li, L. Recycling valuable metals from spent lithium-ion batteries by ammonium sulfite-reduction ammonia leaching. Waste Manag. 2019, 93, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Luo, X.; Lou, X.; Guo, Y.; Su, R.; Guan, J.; Li, Y.; Yuan, H.; Dai, J.; Jiao, Z. Efficient sulfuric acid-Vitamin C leaching system: Towards enhanced extraction of cobalt from spent lithium-ion batteries. J. Mater. Cycles Waste 2019, 21, 942–949. [Google Scholar] [CrossRef]
- Vakylabad, A.B.; Schaffie, M.; Naseri, A.; Ranjbar, M.; Manafi, Z. A procedure for processing of pregnant leach solution (PLS) produced from a chalcopyrite-ore bio-heap: CuO Nano-powder fabrication. Hydrometallurgy 2016, 163, 24–32. [Google Scholar] [CrossRef]
- Bai, Y.; Blake Hawley, W.; Jafta, C.J.; Muralidharan, N.; Polzin, B.J.; Belharouak, I. Sustainable recycling of cathode scraps via Cyrene-based separation. Sustain. Mater. Technol. 2020, 25, e00202. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Li, J.; Essehli, R.; Belharouak, I. Sustainable direct recycling of lithium-ion batteries via solvent recovery of electrode materials. ChemSusChem 2020, 13, 5664–5670. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.-K.; Passerini, S.; Stanley Whittingham, M.; Belharouak, I. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Walton, R.I. Subcritical solvothermal synthesis of condensed inorganic materials. Chem. Soc. Rev. 2002, 31, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Man, R.W.; Brown, A.R.; Wolf, M.O. Mechanism of Formation of Palladium Nanoparticles: Lewis Base Assisted, Low-Temperature Preparation of Monodisperse Nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 11350–11353. [Google Scholar] [CrossRef]
- Yu, H.; Gibbons, P.C.; Kelton, K.F.; Buhro, W.E. Heterogeneous seeded growth: A potentially general synthesis of monodisperse metallic nanoparticles. J. Am. Chem. Soc. 2001, 123, 9198–9199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wan, Z.; Yang, T.; Zhao, M.; Lv, X.; Wang, H.; Ren, X.; Mei, X. Preparation of Nickel Cobalt Sulfide Hollow Nanocolloids with Enhanced Electrochemical Property for Supercapacitors Application. Sci. Rep. UK 2016, 6, 25151. [Google Scholar] [CrossRef]
- RECHARGE-The-Batteries-Report-2018-April-18.pdf. Available online: https://www.storelio.com/files (accessed on 14 January 2021).
- Chen, X.; Liu, Q.; Bai, T.; Wang, W.; He, F.; Ye, M. Nickel and Cobalt Sulfide-Based Nanostructured Materials for Electrochemical Energy Storage Devices. Chem. Eng. J. 2020, 409, 127237. [Google Scholar] [CrossRef]
- Koohestani, B.; Khodadadi Darban, A.; Mokhtari, P.; Darezereshki, E.; Yilmaz, E.; Yilmaz, E. Influence of Hydrofluoric Acid Leaching and Roasting on Mineralogical Phase Transformation of Pyrite in Sulfidic Mine Tailings. Minerals 2020, 10, 513. [Google Scholar] [CrossRef]
- Akram, R.; Khan, M.D.; Zequine, C.; Zhao, C.; Gupta, R.K.; Akhtar, M.; Akhtar, J.; Malik, M.A.; Revaprasadu, N.; Bhatti, M.H. Cobalt sulfide nanoparticles: Synthesis, water splitting and supercapacitance studies. Mat. Sci. Semicon. Proc. 2020, 109, 104925. [Google Scholar] [CrossRef]
- Buchmaier, C.; Glänzer, M.; Torvisco, A.; Poelt, P.; Wewerka, K.; Kunert, B.; Gatterer, K.; Trimmel, G.; Rath, T. Nickel sulfide thin films and nanocrystals synthesized from nickel xanthate precursors. J. Mater. Sci. 2017, 52, 10898–10914. [Google Scholar] [CrossRef]
- Guan, B.; Li, Y.; Yin, B.; Liu, K.; Wang, D.; Zhang, H.; Cheng, C. Synthesis of hierarchical NiS microflowers for high performance asymmetric supercapacitor. Chem. Eng. J. 2017, 308, 1165–1173. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Lee, Y.-R. Binder-free electro-synthesis of highly ordered nickel oxide nanoparticles and its electrochemical performance. Electrochim. Acta 2018, 283, 1609–1617. [Google Scholar] [CrossRef]
- Sathiyaraj, E.; Thirumaran, S.; Ciattini, S.; Selvanayagm, S. Synthesis and characterization of Ni (II) complexes with functionalized dithiocarbamates: New single source precursors for nickel sulfide and nickel-iron sulfide nanoparticles. Inorg. Chim. Acta 2019, 498, 119162. [Google Scholar] [CrossRef]
- Ravindhranath, K.; Ramamoorty, M. Nickel Based Nano Particles as Adsorbents in Water Purification Methods-A Review. Orient. J. Chem. 2017, 33, 1603. [Google Scholar] [CrossRef]
- Cao, L.; Tang, G.; Mei, J.; Liu, H. Construct hierarchical electrode with NixCo3-xS4 nanosheet coated on NiCo2O4 nanowire arrays grown on carbon fiber paper for high-performance asymmetric supercapacitors. J. Power Source 2017, 359, 262–269. [Google Scholar] [CrossRef]
- Mani, A.D.; Deepa, M.; Xanthopoulos, N.; Subrahmanyam, C. Novel one pot stoichiometric synthesis of nickel sulfide nanomaterials as counter electrodes for QDSSCs. Mater. Chem. Phys. 2014, 148, 395–402. [Google Scholar] [CrossRef]
- Ren, H.; Wang, J.; Cao, Y.; Luo, W.; Sun, Y. Nickel sulfide nanoparticle anchored reduced graphene oxide with improved lithium storage properties. Mater. Res. Bull. 2021, 133, 111047. [Google Scholar] [CrossRef]
- Chen, M.; Wang, R.; Qi, Y.; Han, Y.; Wang, R.; Fu, J.; Meng, F.; Yi, X.; Huang, J.; Shu, J. Cobalt and lithium leaching from waste lithium ion batteries by glycine. J. Power Source 2021, 482, 228942. [Google Scholar] [CrossRef]
- Liu, K.; Yang, S.; Lai, F.; Wang, H.; Huang, Y.; Zheng, F.; Wang, S.; Zhang, X.; Li, Q. Innovative Electrochemical Strategy to Recovery of Cathode and Efficient Lithium Leaching from Spent Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 4767–4776. [Google Scholar] [CrossRef]
- Fan, E.; Yang, J.; Huang, Y.; Lin, J.; Arshad, F.; Wu, F.; Li, L.; Chen, R. Leaching Mechanisms of Recycling Valuable Metals from Spent Lithium-Ion Batteries by a Malonic Acid-Based Leaching System. ACS Appl. Energy Mater. 2020, 3, 8532–8542. [Google Scholar] [CrossRef]
- Refly, S.; Floweri, O.; Mayangsari, T.R.; Sumboja, A.; Santosa, S.P.; Ogi, T.; Iskandar, F. Regeneration of LiNi1/3Co1/3Mn1/3O2 Cathode Active Materials from End-of-Life Lithium-Ion Batteries through Ascorbic Acid Leaching and Oxalic Acid Coprecipitation Processes. ACS Sustain. Chem. Eng. 2020, 8, 16104–16114. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Farina, L.; Zanoni, R.; Altimari, P.; Cojocariu, I.; Rubino, A.; Navarra, M.A.; Panero, S.; Pagnanelli, S. Electrochemical synthesis of nanowire anodes from spent lithium ion batteries. Electrochim. Acta 2019, 319, 481–489. [Google Scholar] [CrossRef]
- Shin, S.-M.; Lee, D.-W.; Wang, J.-P. Fabrication of nickel nanosized powder from linio2 from spent lithium-ion battery. Metals 2018, 8, 79. [Google Scholar] [CrossRef]
- Ebin, B. Simple Preparation of Ni and NiO Nanoparticles Using Raffinate Solution Originated from Spent NiMH Battery Recycling. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2554–2563. [Google Scholar] [CrossRef]
- Tipman, N.R. Reactions of Potassium Ethyl Xanthate in Aqueous Solution; University of British Columbia: Vancouver, BC, Canada, 1970. [Google Scholar]
- Zhang, Y.-H.; Wu, L.-M.; Huang, P.-P.; Shen, Q.; Sun, Z.-X. Determination and application of the solubility product of metal xanthate in mineral flotation and heavy metal removal in wastewater treatment. Miner. Eng. 2018, 127, 67–73. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, F.; Song, S.; Tang, H.; Ding, S.; Sun, W.; Lei, S.; Xu, S. Recovering valuable metals from the leaching liquor of blended cathode material of spent lithium-ion battery. J. Environ. Chem. Eng. 2020, 8, 104358. [Google Scholar] [CrossRef]
- Aaltonen, M.; Peng, C.; Wilson, B.P.; Lundström, M. Leaching of metals from spent lithium-ion batteries. Recycling 2017, 2, 20. [Google Scholar] [CrossRef]
- Sasaki, Y. Extraction—Spectrophotometric determination of manganese (II) with xanthates. Anal. Chim. Acta 1982, 138, 419–424. [Google Scholar] [CrossRef]
- Pilipenko, A.; Ul’ko, N. Analytical Prverties of Xanthates (III). Zh. Analit. Khim 1955, 10, 299–304. [Google Scholar]
- Darezereshki, E.; Bakhtiari, F.; Alizadeh, M.; Vakylabad, B.A.; Ranjbar, M. Direct thermal decomposition synthesis and characterization of hematite (α-Fe2O3) nanoparticles. Mat. Sci. Semicon. Proc. 2012, 15, 91–97. [Google Scholar] [CrossRef]
- Darezereshki, E.; Khodadadi Darban, A.; Abdollahy, M.; Jamshidi-Zanjani, A.; Vakylabad, B.A.; Mohammadnejad, S. The leachability study of iron-oxides from mine tailings in a hybrid of sulfate-chloride lixiviant. J. Environ. Chem. Eng. 2018, 6, 5167–5176. [Google Scholar] [CrossRef]
- Darezereshki, E.; Ranjbar, M.; Bakhtiari, F. One-step synthesis of maghemite (γ-Fe2O3) nano-particles by wet chemical method. J. Alloys Compd. 2010, 502, 257–260. [Google Scholar] [CrossRef]
- Pradhan, N.; Katz, B.; Efrima, S. Synthesis of high-quality metal sulfide nanoparticles from alkyl xanthate single precursors in alkylamine solvents. J. Phys. Chem. B 2003, 107, 13843–13854. [Google Scholar] [CrossRef]
- Efrima, S.; Pradhan, N. Xanthates and related compounds as versatile agents in colloid science. C. R. Chim. 2003, 6, 1035–1045. [Google Scholar] [CrossRef]
- Kristl, M.; Dojer, B.; Gyergyek, S.; Kristl, J. Synthesis of nickel and cobalt sulfide nanoparticles using a low cost sonochemical method. Heliyon 2017, 3, e00273. [Google Scholar] [CrossRef] [PubMed]

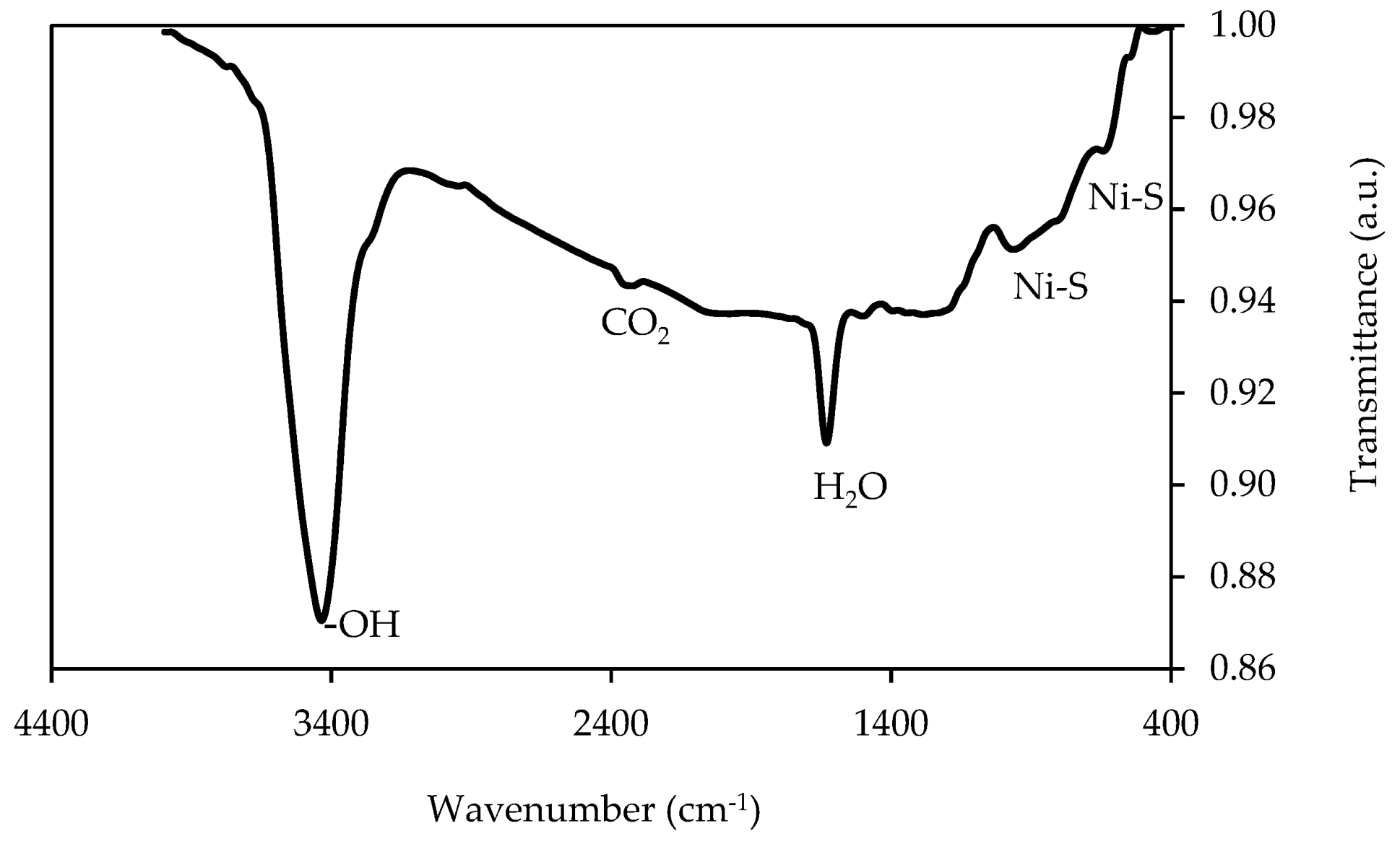
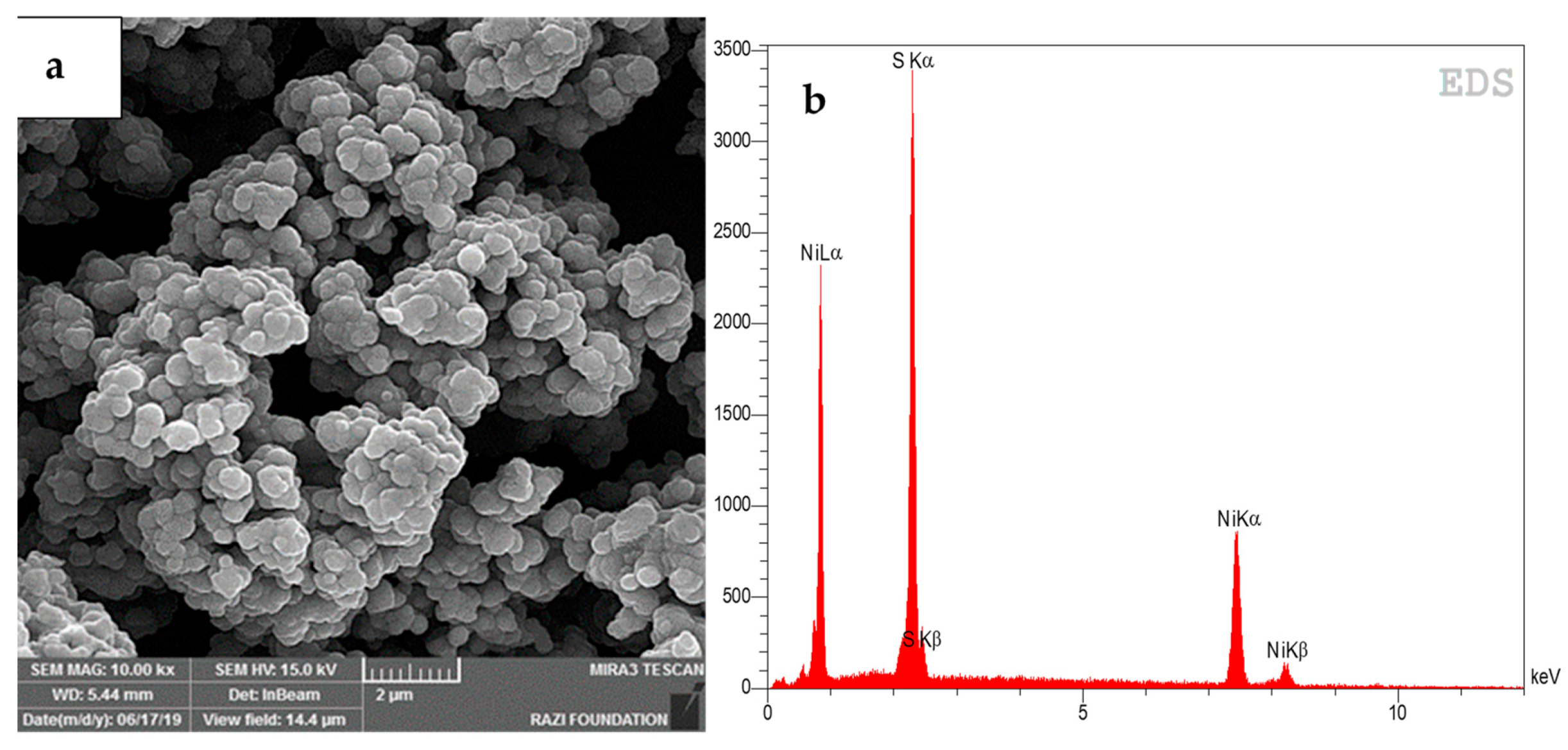
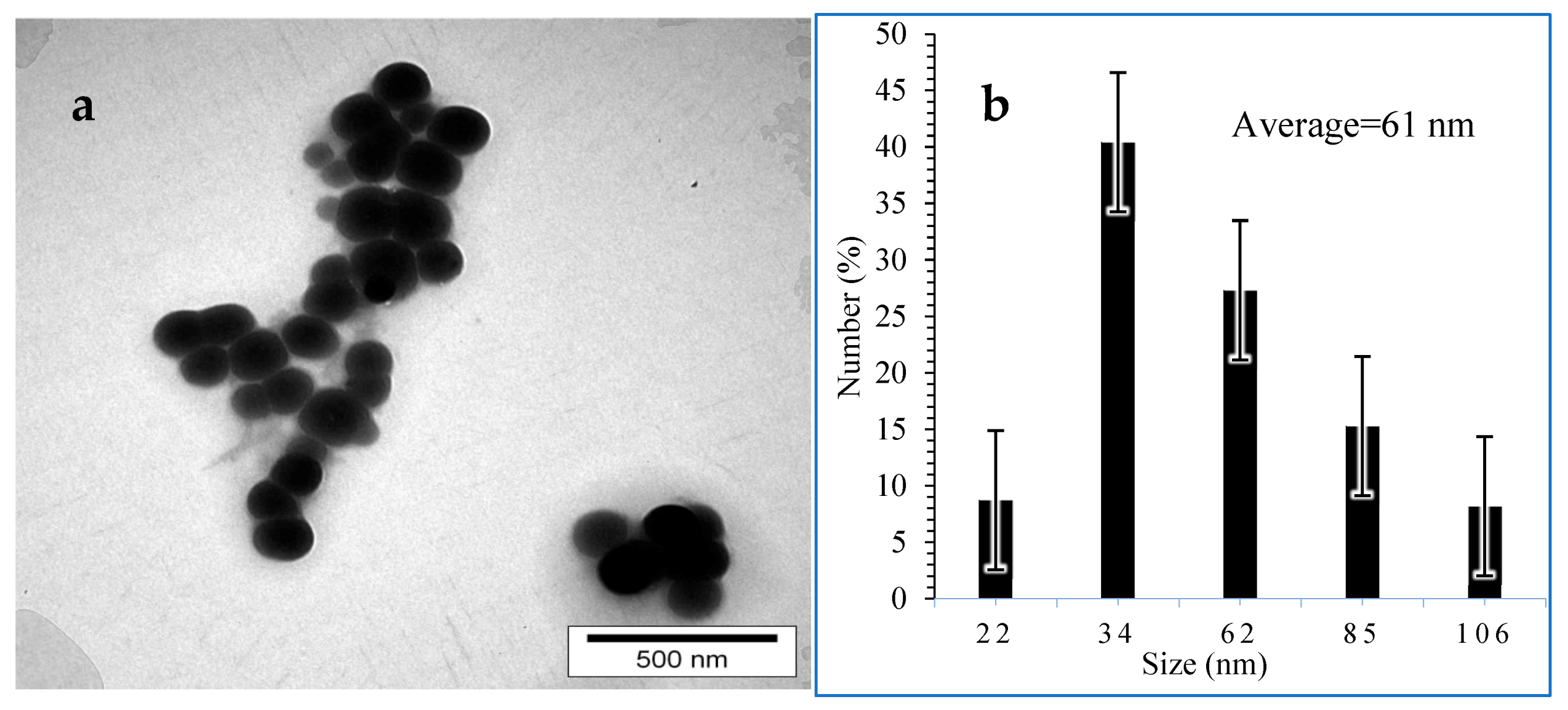
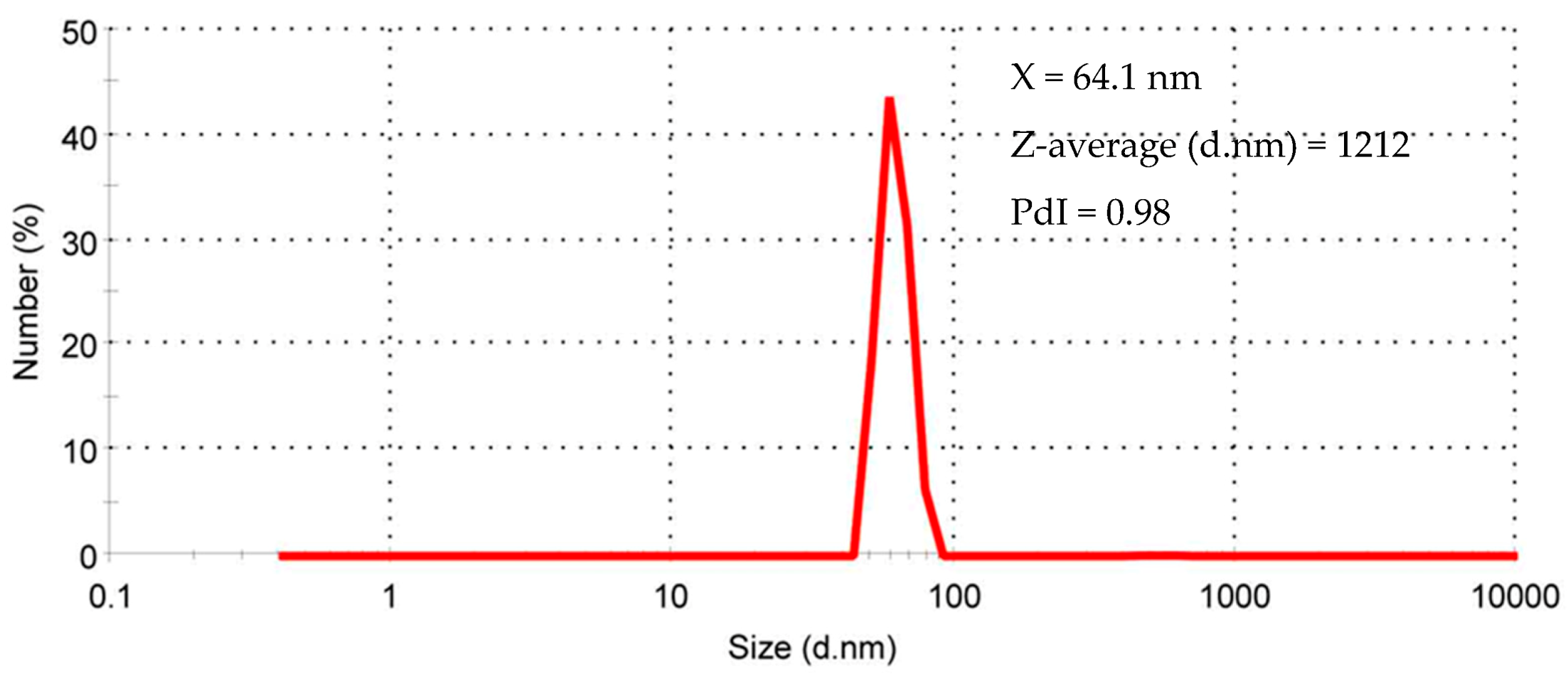
| Component | Co | Mn | Ni | Li |
|---|---|---|---|---|
| PLS (mg/L) | 3186.03 | 4155 | 443.1 | 793.7 |
| Feed CM (mg/L) | 199,127 | 27.7 | 26,105 | 46,690 |
| hkl | Pos. (°2Th.) | d-spacing (Å) | Crystallite Size (Å) | Microstrain (%) | Crystallite Size Only (Å) | Microstrain Only (%) |
|---|---|---|---|---|---|---|
| 100 | 30.4666 | 2.93168 | 261.8913 | 0.522286 | 157.3711 | 0.931453 |
| 101 | 34.9458 | 2.56549 | 349.4471 | 0.357726 | 205.2646 | 0.624922 |
| 102 | 46.1364 | 1.96592 | 362.6012 | 0.264356 | 212.9156 | 0.461666 |
| 110 | 53.7784 | 1.70320 | 284.0213 | 0.280356 | 170.4897 | 0.499501 |
| 103 | 61.0836 | 1.51584 | 390.6485 | 0.190465 | 228.5735 | 0.331587 |
| 201 | 65.4320 | 1.42523 | 303.3146 | 0.221017 | 181.4979 | 0.392629 |
| 004 | 71.0005 | 1.32649 | 252.9233 | 0.240170 | 153.4484 | 0.432225 |
| 202 | 73.2689 | 1.29092 | 320.6339 | 0.190644 | 191.1957 | 0.33759 |
| Average crystallite size (Å) | 315.68515 | 187.5945625 | ||||
| Composition | Concentration of the Elements (%) |
|---|---|
| Ni | 63.98 |
| S | 34.95 |
| Co | 0.32 |
| Cu | 0.21 |
| Cd | 0.11 |
| Na | 0.02 |
| Li | 0.12 |
| Mn | 0.11 |
| Si | 0.04 |
| Cl | 0.10 |
| Al | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darezereshki, E.; Vakylabad, A.B.; Hassanzadeh, A.; Niedoba, T.; Surowiak, A.; Koohestani, B. Hydrometallurgical Synthesis of Nickel Nano-Sulfides from Spent Lithium-Ion Batteries. Minerals 2021, 11, 419. https://doi.org/10.3390/min11040419
Darezereshki E, Vakylabad AB, Hassanzadeh A, Niedoba T, Surowiak A, Koohestani B. Hydrometallurgical Synthesis of Nickel Nano-Sulfides from Spent Lithium-Ion Batteries. Minerals. 2021; 11(4):419. https://doi.org/10.3390/min11040419
Chicago/Turabian StyleDarezereshki, Esmaeel, Ali Behrad Vakylabad, Ahmad Hassanzadeh, Tomasz Niedoba, Agnieszka Surowiak, and Babak Koohestani. 2021. "Hydrometallurgical Synthesis of Nickel Nano-Sulfides from Spent Lithium-Ion Batteries" Minerals 11, no. 4: 419. https://doi.org/10.3390/min11040419
APA StyleDarezereshki, E., Vakylabad, A. B., Hassanzadeh, A., Niedoba, T., Surowiak, A., & Koohestani, B. (2021). Hydrometallurgical Synthesis of Nickel Nano-Sulfides from Spent Lithium-Ion Batteries. Minerals, 11(4), 419. https://doi.org/10.3390/min11040419