Abstract
In this study, a novel organic depressant maleyl 5-amino-1,3,4-thiadiazole-2-thiol (MATT) was synthesized and utilized as a galena depressant in the flotation separation of molybdenite and galena. The results of the flotation test indicated that MATT exhibited an excellent depression ability on galena but barely influenced the flotation of molybdenite in the pH range of 6.0–11.0. Zeta potential results suggested that MATT preferentially adsorbed on galena surface. UV-visible spectroscopy analysis indicated that the stoichiometric ratio of lead ion and reagent in the complex compound. XPS analysis demonstrated that the S (-SH) atom and N (1,3,4-thiadiazole group) atom of MATT coordinated with the Pb atom on galena surface.
1. Introduction
Molybdenum plays a vital role in steel, petroleum and chemical industries due to its excellent physical-chemical properties and mechanical performances [1]. Mo metal is mainly extracted from molybdenite, which is generally associated commonly with other sulfide ores like chalcopyrite, pyrite and galena [2,3,4] etc. Since the floatability of these minerals is very similar, it is difficult to obtain molybdenite of a high grade and few impurities. Especially, the presence of lead impurities in molybdenite concentrate not only degrades the quality of Mo metals, but also causes serious environmental contamination during the processing of Mo ores [5,6]. Generally, the content of lead in molybdenite concentrate is limited to be less than 0.05% [7]. Therefore, the removal of galena impurity from molybdenite is critical for the production of Mo metals of a high quality.
Froth flotation, as a physicochemical separation technique, is an efficient and environmentally-friendly approach to separate galena from molybdenite, based on the difference in surface properties [8,9]. In most cases, bulk flotation is adopted to recover molybdenite and galena from Mo-Pb raw ores, then molybdenite and galena is separated by preferential flotation [10]. It has been noted that both molybdenite and galena have a good floatability in the presence of a collector (non-polar oil or xanthate) or a frother (Z-200 or pine oil) [11]. Therefore, it is hard to separate galena from molybdenite efficiently without any depressant. At present, inorganic depressants have a wide application in industrial production, such as potassium dichromate, sulfite and sodium hydrosulfide [12,13,14,15,16]. Although these depressants mentioned above could selectively separate galena from molybdenite, these inorganic ones suffer from many disadvantages, which are highly toxic and could cause damage to human health.
In view of these disadvantages, many researchers have turned to research into some organic depressants. It has been reported that L-cysteine [17] could selectively depress the flotation of galena in Pb-Mo flotation. The results showed that the thiol and primary amine groups of L-cysteine could coordinate lead sites on galena surface. Similarly, Zhang et al. [18] found that poly(acrylamide-allylthiourea) (PAM-ATU) could separate galena from artificially mixed minerals successfully at pH around 10.5. Acetic acid-[(hydrazinylthioxomethyl)thio]-sodium (AHS) was synthesized and applied in the flotation of galena and molybdenite [7]. It was found that AHS chemisorbed on galena surface to form a five number ring with lead atoms on a galena surface.
Although a lot of efforts have been made to exploit eco-friendly and high-selective depressant of galena, it is still a difficult task to explore novel reagents to substitute traditional galena depressants. In this work, a novel depressant named MATT was synthesized and applied in the separation of molybdenite and galena in various conditions (diesel dosage, MATT dosage, and pH). Besides, its adsorption mechanism was studied through zeta potential, UV-Visible spectroscopy and X-ray photoelectron spectroscopy (XPS) analysis.
2. Materials and Methods
2.1. Minerals and Reagents
Pure galena and molybdenite minerals were collected from Guangdong Province, China. The ores were first selected by hand carefully, and then crushed, ground and sieved to obtain various size fractions. The fractions containing 38~74 μm particles were collected for flotation experiments and the others less than 5 μm for analysis tests. The XRD spectra of galena and molybdenite was presented in the Figure 1. The results of chemical analysis and XRD show that the purities of MoS2 and PbS are more than 95%.
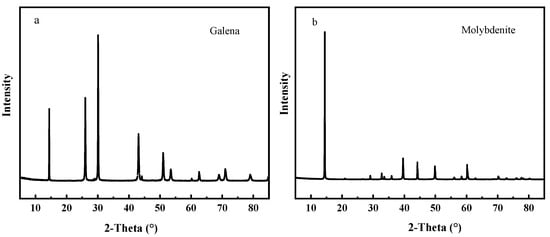
Figure 1.
XRD spectra of (a) galena and (b) molybdenite sample.
Sodium hydroxide (NaOH), hydrochloric acid (HCl) and potassium nitrate (KNO3) purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China) were used in the experiments. Diesel was obtained from Guangfu Chemical Co., Ltd. (Hengshui, China) and used as the collector. Diesel was added in the form of emulsification, which was prepared by a Cole Parmer ultrasonic processor (750 W and 20 kHz) at 60% amplitude for 5 min. Methyl isobutyl carbinol (MIBC) purchased from Xilong Chemical Co., Ltd. (Shantou, China) was used as the frother. Deionized water (18.2 MΩ·cm resistivity) was used in all experiments. The depressant MATT was synthesized in our lab according to the method provided in the reference [19].
2.2. Micro-Flotation Experiments
Micro-flotation tests were implemented using an XFG5-35 flotation machine (Jilin Prospecting Machinery Factory, Changchun, China) in a 40 mL cell. In each test, 2.0 g mineral sample and 30 mL deionized water was added into the cell, and pH was adjusted by using dilute hydrochloric acid and sodium hydroxide solution instead of commonly used pH regulator in industry in order to simplify the investigation. Subsequently, the depressant (MATT), the collector (kerosene) and the frother (MIBC) were introduced sequentially at each stage of 2 min conditioning before flotation. Finally, the flotation was performed for 3 min at an air flow rate of 0.2 L/min. The floated froth products and non-floated tailings were filtered, dried and weighted to a calculated floatability. Each test was performed three times and the average was reported. For micro-flotation tests of mixed minerals, 1.0 g galena and 1.0 g molybdenite was mixed and the flotation was performed as the same procedures mentioned above. The Pb and Mo grade of the concentrates was assayed using chemical assay method and the recovery of was calculated according the following equation:
where εc and mc represented the yield and grade of floated product, respectively. m0 represented the grade of pure mineral. Each test was repeated three times and the mean value was reported.
2.3. Zeta Potential Experiments
Zeta potential tests were carried out on a Malvern Zeta Sizer Nano Series (Malvern Instruments Ltd., Malvern, UK) to study the adsorption behaviors of MATT on galena and molybdenite; 0.1 g samples with particle size of less than 5 μm were dispersed in 100 mL of 0.01 M KNO3 solution. Prior to the adjustment of pH, 30 mL of 0.02 M MATT solution was added into the slurry. After that, dilute hydrochloric acid and sodium hydroxide solutions were used to adjust the slurry pH to a desired value. Finally, 1 mL suspension was taken out and introduced into a standard cell to measure its zeta potential. Diesel was not used in Zeta potential measurement. The results presented here were an average value of ten independent measurements, with a typical variation corresponding to ±2 mV.
2.4. XPS Measurements
The spectra of every samples were acquired by K-Alpha 1063 (Thermo Fisher Scientific Co., Waltham, MA, USA) using an Al Kα radiation (12 kV, 6 mA) in an analytical chamber with a pressure of 1.0 × 10–12 Pa. Galena samples were first treated in MATT solution (20 mg/L) at pH 9 for 20 min. Then the sample was filtered and dried in vacuum oven for 24 h. All binding energies were calibrated using the neutral C 1 s peak at 284.6 eV to eliminate the effect of surface charging. The XPS spectra was fitted and analyzed by Thermo Avantage software 5.5.
2.5. UV-Visible Spectroscopy of Complex
The UV-Visible spectroscopy was employed to identify the stoichiometric ratio of lead ion and reagent using a Cary 60 UV-Vis spectrophotometer (Agilent, Santa Clara, CA, USA). The Pb2+ solution was mixed with MATT solutions at pH 6 with different concentration, and then the solution was stirred for 20 min to make sure the reaction was completed. The mixture was stood for 24 h. After that, the supernatant was transferred to quartz utensil and the absorbance was measured within the range of 200 to 800 nm.
3. Results and Discussion
3.1. Flotation Test
3.1.1. Effect of Diesel Dosage
Figure 2 shows the effect of diesel dosage on the floatability of galena and molybdenite at natural pH around 6.4. The experimental results showed that molybdenite exhibited excellent natural floatability with or without diesel. The floatability of galena was not as good as molybdenite, only above 25% galena floated under natural conditions without diesel. The floatability of galena increased gradually as increasing the diesel dosage and a maximum recovery of around 75.0% was obtained at diesel dosage of 30 mg/L. These results illustrated that the presence of diesel made the effective flotation separation of molybdenite and galena become difficult without any depressant.
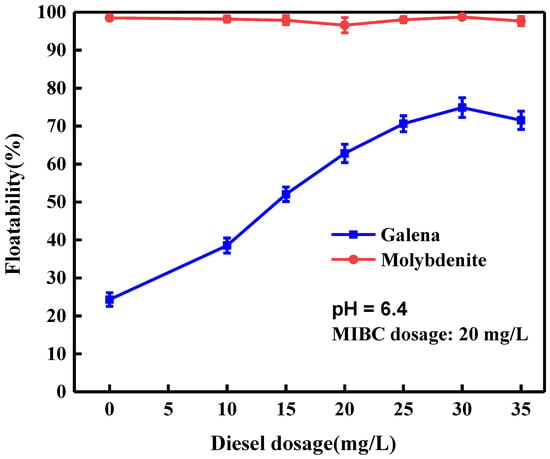
Figure 2.
Floatability of galena and molybdenite as a function of diesel dosage.
3.1.2. Effect of the MATT Depressant Dosage
Figure 3 shows the effect of depressant dosage on the floatability of galena and molybdenite. Obviously, MATT barely influenced the floatability of molybdenite. Even at a MATT dosage of 35 mg/L, the floatability of molybdenite maintained around 98%. However, the presence of MATT significantly influenced the floatation performance of galena mineral particles. The floatability of galena reduced rapidly with the increase of the depressant dosage until the dosage reached 30 mg/L, at which, only about 3% galena was floated, indicating that MATT was an effective galena depressant in the flotation separation of galena and molybdenite.
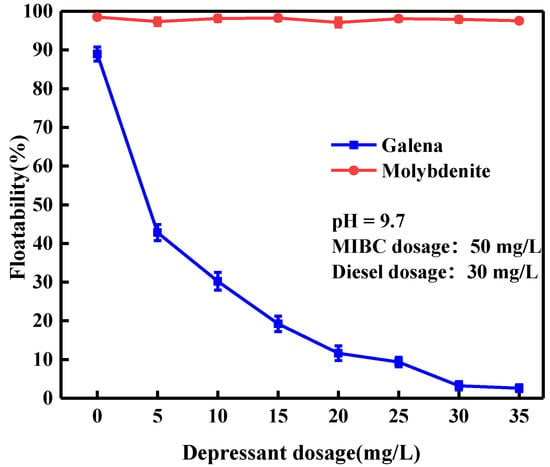
Figure 3.
Floatability of galena and molybdenite as a function of depressant dosage.
3.1.3. Effect of the Pulp pH
Figure 4 shows the effect of the pulp pH on the floatability of galena and molybdenite in the absence and presence of MATT. Molybdenite had a good floatability with or without MATT in the whole testing pH range of 6.0–11.0. The recovery of molybdenite was maintained at 98% under varied pH conditions. However, the pH significantly influenced the floatability of galena. As shown in Figure 4, galena exhibited a very low floatability when the pH value was greater than 10.0 without MATT due to the formation of hydroxyl species, which made galena surface hydrophilic [20,21]. The addition of MATT led to a very strong depressing effect on galena, only less than 2% galena was floated, whereas the floatability of molybdenite barely changed in the presence of MATT. In addition, it could be observed that galena was strongly depressed within a board pH range, which suggested that MATT could realize the selective separation of molybdenite and galena and have a good application prospect.
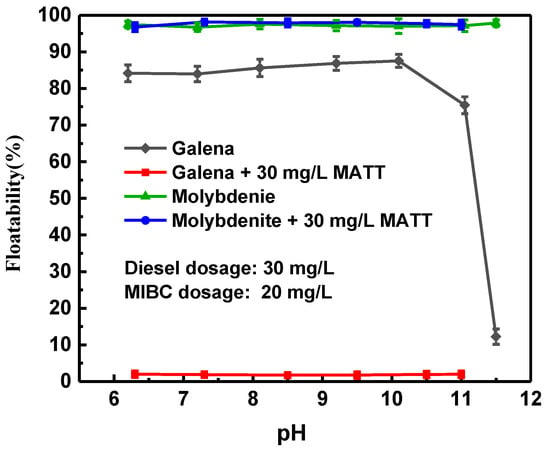
Figure 4.
Floatability of galena and molybdenite as a function of the pulp pH.
3.1.4. Artificially Mixed Minerals Flotation
The flotation tests of artificially mixed minerals were carried out to further examine the effect of MATT on the flotation separation of galena and molybdenite. Figure 5 shows the flotation separation results of artificially mixed minerals at pH 9.0. The recovery of PbS decreased with the increase of MATT dosage, and the recovery of galena reduced to around 8.6% when MATT dosage reached to 30 mg/L. For the case of molybdenite, the recovery of MoS2 still maintained at 88.9% as the increase of MATT dosage, which was in a good accordance with the results of single mineral flotation. The flotation separation results of artificially mixed minerals reconfirmed that MATT could selectively separate galena from molybdenite.
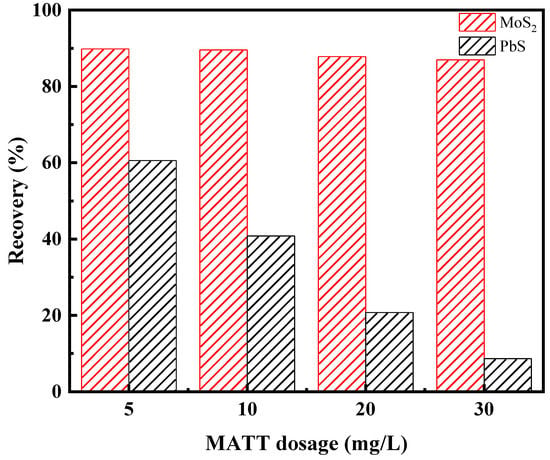
Figure 5.
The flotation separation results of artificially mixed minerals (diesel dosage 30 mg/L, MIBC 20 mg/L, pH = 9).
3.2. Zeta Potential Measurement
The zeta potentials of galena, MATT-treated galena, molybdenite and MATT-treated molybdenite are presented in Figure 6. The decrease of PbS zeta potential at pH less than 5 was ascribed to the formation of sulphoxy species due to oxidation of PbS, whereas lead hydroxyl species were identified as the most likely candidates accounting for the behavior under alkaline conditions according to previous reports [20].
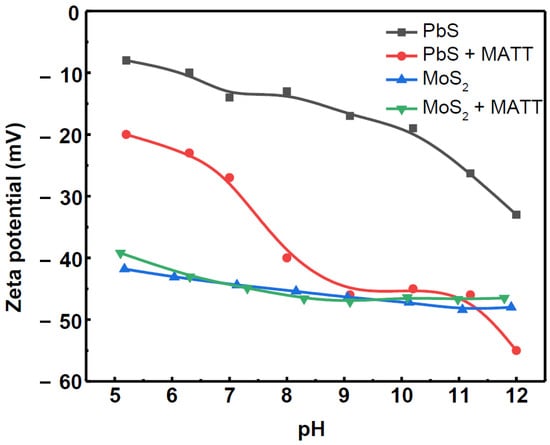
Figure 6.
The zeta potentials of galena and molybdenite as a function of pH.
After the treatment of MATT, zeta potential became more negative, which may be ascribed to the carboxyl acid (–COO–) and thiol (–SH) functional groups from the MATT molecule. Thiol group could be adsorbed on the metal site of galena surface, while carboxyl acid group (–COO–) formed negative molecular film, resulting in the more negative zeta potential than pure galena. The zeta potential of MATT-treated galena reached to a plateau at pH of about 9.0, indicating a maximum adsorption of MATT occurred within the pH range of 9.0–11.0. It should be noted that 1,3,4-thiadiazole group contributed to its adsorption onto the PbS surface.
However, it also could be observed from Figure 6 that MATT barely influenced the zeta potentials of molybdenite, which indicated that MATT was weakly adsorbed on molybdenite surface in the pH range of 5.0–12.0. These results could explain well that the floatability of molybdenite was almost not affected by the addition of MATT depressant as shown in Figure 3 and Figure 4.
3.3. UV-Visible Spectroscopy Analysis
Figure 7 shows the UV spectra of Pb2+ in the presence of various dosages of MATT at a designed desired concentration ratio. There was a maximum adsorption peak in 304 nm, which could be attributed to Pb-MATT complex. As the MATT concentration increased, the absorbance at 304 nm rose. Besides, it should be noted that these lines overlapped when the [Pb2+]/[MATT] ratio was 1:1, 1:2 and 1:4, indicating that the complexation reaction was saturated when the ratio was close to 1:1. A conclusion can be drawn from this, that the stoichiometric ratio of lead ion and reagent in this complex compound was 1:1, which was critical for estimating the adsorption mechanism of depressant MATT on the galena surface.
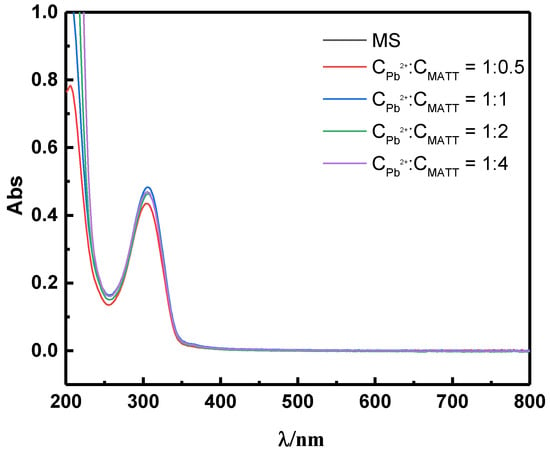
Figure 7.
UV spectra of MATT under various concentration ratios of Pb2+ ions and MATT.
3.4. XPS Analysis
The survey XPS spectra of galena treated at various conditions are shown in Figure 8. For galena treated by MATT, a new peak appeared at about 399.0 eV, which was the characteristic peak of N 1s. The surface atomic content of C, N, O, S and Pb atoms were presented in Table 1. The concentration of N atoms on the surface of galena treated with MATT increased by 2.98%. It could be inferred from those results that MATT was effectively adsorbed on the galena surface, because MATT was the unique source of N element, which was in agreement with the result of zeta potential.
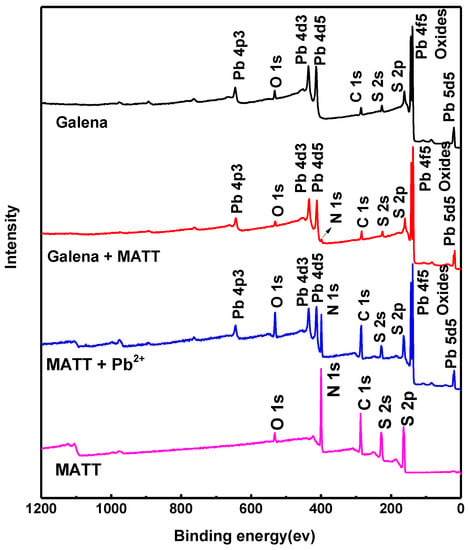
Figure 8.
The survey XPS spectra of galena, galena treated with MATT, MATT and MATT treated with Pb2+ ions.

Table 1.
Atomic concentrations of galena samples before and after treatment by MATT.
The C 1s spectra of galena and galena treated with MATT are shown in Figure 9. The peak located at 284.60 eV was represented as the referenced C 1s. For galena, the spectra of 285.46 eV, 286.47 eV, and 287.76 eV would be attributed to C–C, C–O, and C=O respectively [18]. The peak at 289.16 eV was assigned to the lead carbonate (PbCO3) [22,23,24], due to the oxidation on galena surface. As for the spectra of galena treated with MATT, a new peak increased at 285.50 eV (–C–N and –C–S group) [25,26] appeared, which reconfirmed that MATT could be adsorbed on the surface of galena.
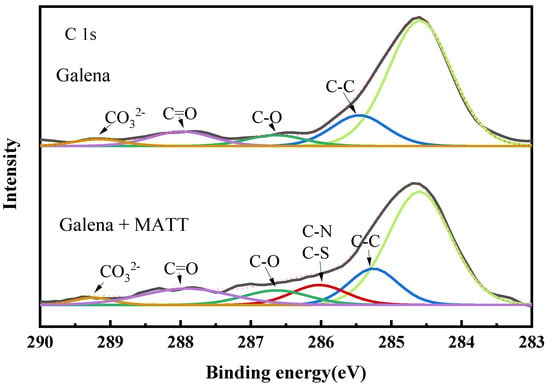
Figure 9.
C 1s spectra of galena, galena treated with MATT.
The S 2p spectra of galena, galena treated with MATT and MATT are shown in Figure 10. Obviously, the peaks located at 160.71 and 161.92 eV were the characteristic doublet peak of galena, which was consistent with those in previous reports [17]. The S 2p spectra of the depressant could be deconvoluted into three peaks at 161.82 eV, 163.68 eV and 164.26 eV, which represented C=S (tautomer form of –N=C–SH), –SH and C–S–C (from 1,3,4-thiadiazole group) respectively [27]. It should be noted that the peaks of the thiol group shifted to a higher binding energy significantly after the adsorption of MATT, while there was no obvious displacement that occurred to doublets of C=S and C–S–C, thus indicating that the sulfur atom from the thiol group might act as the reaction site when the depressant was adsorbed on the surface of galena.
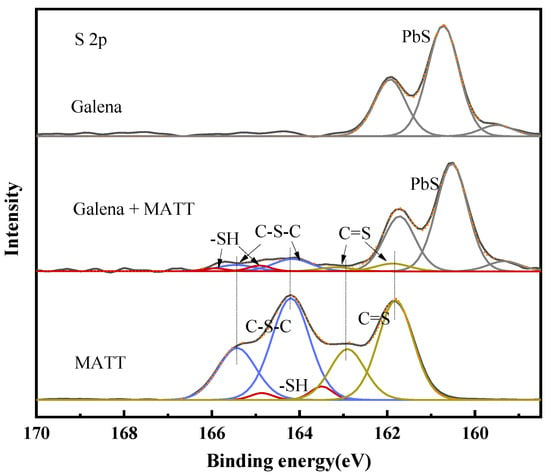
Figure 10.
S 2p spectra of galena, galena treated with MATT, and MATT.
Figure 11 shows the N 1s spectra of galena, galena treated with MATT and MATT treated with Pb2+ ions. As shown in Figure 11, the spectra of MATT could be fitted with two components at 399.42 and 400.48 eV, which might be associated with C–N–C, C=N–N=C, or C=N–N–C, respectively [28,29]. From the survey spectra of galena, there was no characteristic peak on the surface of galena. After the addition of MATT depressant, three new peaks increased at 398.54 eV, 399.12 eV and 399.69 eV, which were very similar to the spectra of MATT treated with Pb2+ ions. The peaks at 398.54 eV and 399.69 eV could be assigned to C–N and C=N [30,31], whereas the peak centered at 399.12 might be ascribed to the Pb–N bond caused by the adsorption, proving that the nitrogen atom might also participate in the chemisorption.
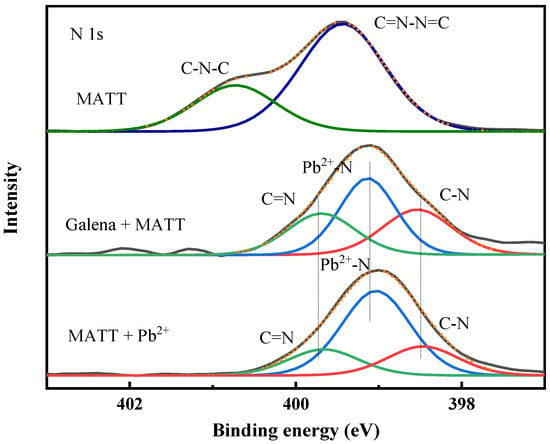
Figure 11.
N 1s spectra of galena, galena treated with MATT and MATT treated with Pb2+ ions.
The high resolution spectra of Pb 4f of galena treated and untreated with MATT is displayed in Figure 12. For the original galena sample, the Pb 4f spectra located at 137.50 eV and 142.50 eV were regarded as the characteristic peaks of galena and were coherent with the reported values in previous study [32], while the binding energy located at 138.45 eV and 143.45 eV were due to oxidation products of lead ions on galena surface. After the addition of MATT depressant, the binding energy of PbS was shifted to 137.29 eV and 142.18 eV, lower than that of original galena, while the spectra of Pb oxidizations were still at 138.42 eV and 143.42 eV. The decrease of Pb 4f binding energy demonstrated that the electronic density of lead atom was changed, which might be attributed to the combination of Pb sites and MATT.
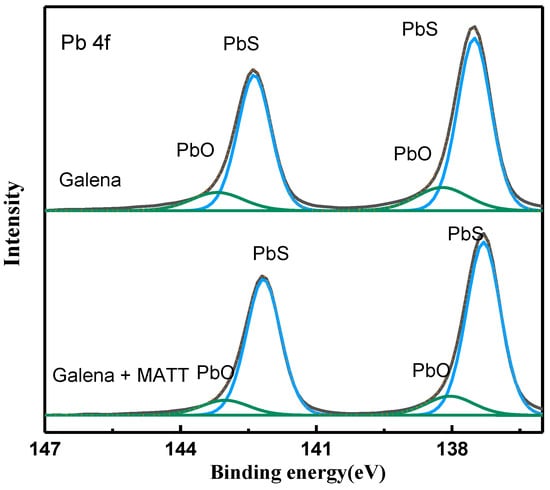
Figure 12.
Pb 4f spectra of galena with and without MATT treatment.
3.5. The Suggested Adsorption Model
These analysis results mentioned above approved that the sulfur atom (-SH), the nitrogen atom from 1,3,4-thiadiazole group, and the lead atom from galena surface played as reaction sites. Therefore, a potential adsorption mechanism might be drawn from former tests that four member rings could be formed on the galena surface through MATT and the lead atom. The potential adsorption model of MATT on the galena surface was proposed as shown in Figure 13, which was in agreement with the adsorption mechanism of CSC-1 (collector) [33] on the galena surface.
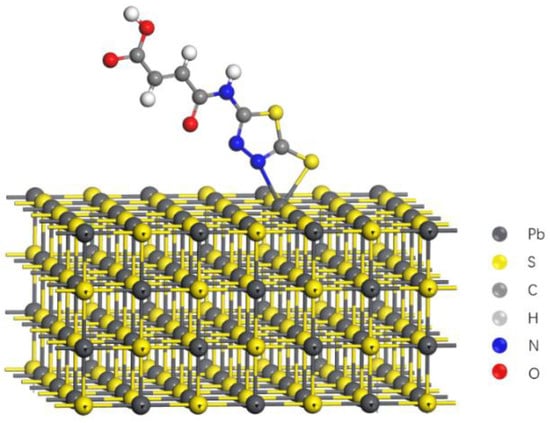
Figure 13.
The potential adsorption mechanism mode of MATT on galena surface.
4. Conclusions
In the present study, a novel galena depressant maleyl 5-amino-1,3,4-thiadiazole-2-thiol (MATT) was explored in the flotation separation of galena and molybdenite through flotation tests and various characterization techniques. The flotation experiments demonstrated that MATT could strongly depress the flotation of galena and realize the selective flotation separation of galena from molybdenite, which was more selective compared with traditional depressants. The proposed adsorption mechanism on the galena surface was investigated by zeta potential, UV-visible spectroscopy, and XPS methods. Zeta potential results revealed that the depression was due to the preferential adsorption of MATT on the galena surface other than molybdenite. UV-visible spectroscopy analysis further confirmed that the stoichiometric ratio of lead ions and MATT in this complex compound was 1:1. The XPS analysis indicated that MATT was complexed through the -SH and N atom from 1,3,4-thiadiazole group with Pb sites on galena surface (Figure 13). All these results shows that MATT might be a potential effective galena depressant in the flotation separation of Mo-Pb ores.
Author Contributions
Conceptualization, X.Z.; Data curation, Y.H. and W.X.; Formal analysis, Y.H., Z.Z., X.Z. and B.Y.; Funding acquisition, X.Z. and Y.Z.; Investigation, Y.H., L.L. and W.X.; Project administration, X.Z.; Supervision, X.Z.; Validation, Z.Z. and S.L.; Writing—original draft, Y.H. and H.Z.; Writing—review and editing, H.Z., X.Z. and B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 51974030, the National Key R&D Program of China, grant number 2017YFE0133100 and the BGRIMM fund program, grant number 02-1903 and 02-1820.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Zuo, R.; Qi, S. Surfactant-free solvothermal synthesis and optical characterization of Bi2Fe4O9 in mixed H2O/EtOH solvent. Powder Technol. 2014, 254, 30–35. [Google Scholar] [CrossRef]
- Kholmogorov, A.G.; Kononova, O.N. Processing mineral raw materials in Siberia: Ores of molybdenum, tungsten, lead and gold. Hydrometallurgy 2005, 76, 37–54. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, C.Y. A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts. Hydrometallurgy 2009, 98, 1–9. [Google Scholar] [CrossRef]
- Yan, H.; Yang, B.; Zeng, M.; Huang, P.; Teng, A. Selective flotation of Cu-Mo sulfides using xanthan gum as a novel depressant. Miner. Eng. 2020, 156. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Shu, K.; Xu, Y.; Zhang, Z.; Chi, R.; Sun, W. Comparative studies of flotation and adsorption of Pb(II)/benzohydroxamic acid collector complexes on ilmenite and titanaugite. Powder Technol. 2019, 345, 35–42. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Yang, Y.; Sun, W.; Hu, Y. Effect of Pb2+ ions on ilmenite flotation and adsorption of benzohydroxamic acid as a collector. Appl. Surf. Sci. 2017, 425, 796–802. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Liu, R.; Jiang, W.; Zhang, C.; Guan, Q.; Zhang, C. Synthesis of acetic acid-[(hydrazinylthioxomethyl)thio]-sodium and its application on the flotation separation of molybdenite from galena. J. Ind. Eng. Chem. 2017, 52, 82–88. [Google Scholar] [CrossRef]
- Choi, J.; Lee, E.; Choi, S.Q.; Lee, S.; Han, Y.; Kim, H. Arsenic removal from contaminated soils for recycling via oil agglomerate flotation. Chem. Eng. J. 2016, 285, 207–217. [Google Scholar] [CrossRef]
- Yang, B.; Yan, H.; Zeng, M.; Huang, P.; Jia, F.; Teng, A. A novel copper depressant for selective flotation of chalcopyrite and molybdenite. Miner. Eng. 2020, 151. [Google Scholar] [CrossRef]
- Cao, Z.; Yue, Y.; Zhong, H.; Qiu, P.; Chen, P.; Wen, X.; Wang, S.; Liu, G. A novel approach for flotation recovery of molybdenite, galena and pyrite from a complex molybdenum-lead ore. Metall. Res. Technol. 2017, 114. [Google Scholar] [CrossRef]
- Qin, W.; Wu, J.; Jiao, F.; Zeng, J. Mechanism study on flotation separation of molybdenite from chalcocite using thioglycollic acid as depressant. Int. J. Min. Sci. Technol. 2017, 27, 1043–1049. [Google Scholar] [CrossRef]
- Chen, W.; Chen, F.; Zhang, Z.; Tian, X.; Bu, X.; Feng, Q. Investigations on the depressant effect of sodium alginate on galena flotation in different sulfide ore collector systems. Miner. Eng. 2021, 160. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, M.; Liu, R.; Sun, W. A review on the electrochemistry of galena flotation. Miner. Eng. 2020, 150. [Google Scholar] [CrossRef]
- Nikolaev, A.A.; Goryachev, B.E. Thermodynamic and flotation analysis of influence exerted by chromate ions on separation of galena and chaclopyrite in alkaline media. J. Min. Sci. 2007, 43, 670–679. [Google Scholar] [CrossRef]
- Goryachev, B.E.; Nikolaev, A.A. Thermodynamics of the interaction between chromate ions and a mineral complex of polymetallic ores. Galena. Russ. J. Non Ferr. Met. 2011, 52, 337–343. [Google Scholar] [CrossRef]
- Nikolaev, A.A.; Goryachev, B.E. Thermodynamics of the interaction of chromate ions with the mineral complex of polymetallic ores. Chalcopyrite. Russ. J. Non Ferr. Met. 2013, 54, 417–424. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, L.; He, J.; Wu, H.; Fang, S.; Khoso, S.A.; Hu, Y.; Sun, W. Evaluation of l-cysteine as an eco-friendly depressant for the selective separation of MoS2 from PbS by flotation. J. Mol. Liq. 2019, 282, 177–186. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, L.; Zeng, H.; Hu, Z.; Zhu, Y.; Han, L. A macromolecular depressant for galena and its flotation behavior in the separation from molybdenite. Miner. Eng. 2020, 157. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.H.; Yu, S.; Ji, H.; Lai, Y.S.; Peng, S.X. Synthesis and biological evaluation of novel thalidomide analogues as potential anticancer drugs. Chin. Chem. Lett. 2008, 19, 928–930. [Google Scholar] [CrossRef]
- Qin, W.; Wang, X.; Ma, L.; Jiao, F.; Liu, R.; Yang, C.; Gao, K. Electrochemical characteristics and collectorless flotation behavior of galena: With and without the presence of pyrite. Miner. Eng. 2015, 74, 99–104. [Google Scholar] [CrossRef]
- Goryachev, B.E.; Nikolaev, A.A. Galena oxidation mechanism. J. Min. Sci. 2012, 48, 354–362. [Google Scholar] [CrossRef]
- Jia, K.; Feng, Q.; Zhang, G.; Shi, Q.; Chang, Z. Understanding the roles of Na 2 S and Pb(II)in the flotation of hemimorphite. Miner. Eng. 2017, 111, 167–173. [Google Scholar] [CrossRef]
- Pawel, N.; Kari, L.; Zhang, G. Oxidation og galena surface—An XPS study of the formation of sulfoxy species. Appl. Surf. Sci. 2000, 157, 101–111. [Google Scholar] [CrossRef]
- Jia, K.; Feng, Q.; Zhang, G.; Ji, W.; Zhang, W.; Yang, B. The role of S(II) and Pb(II) in xanthate flotation of smithsonite: Surface properties and mechanism. Appl. Surf. Sci. 2018, 442, 92–100. [Google Scholar] [CrossRef]
- Ikumapayi, F.; Makitalo, M.; Johansson, B.; Rao, K.H. Recycling of process water in sulphide flotation: Effect of calcium and sulphate ions on flotation of galena. Miner. Eng. 2012, 39, 77–88. [Google Scholar] [CrossRef]
- Dodero, G.; Michiel, D.; Cavallri, O.; Rao, K.H. L-Cysteine chemisorption on gold: A XPS and STM study. Coll. Surf. A Physicochem. Eng. Asp. 2000, 175, 121–128. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, G.; Liu, J.; Yang, X.; Zhang, Z. Thiadiazole-thione surfactants: Preparation, flotation performance and adsorption mechanism to malachite. J. Ind. Eng. Chem. 2018, 67, 99–108. [Google Scholar] [CrossRef]
- Liu, G.; Xiao, J.; Liu, J.; Qu, X.; Liu, Q.; Zeng, H.; Yang, X.; Xie, L.; Zhong, H.; Liu, Q.; et al. In situ probing the self-assembly of 3-hexyl-4-amino-1,2,4-triazole-5-thione on chalcopyrite surfaces. Coll. Surf. A Physicochem. Eng. Asp. 2016, 511, 285–293. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Zhong, H.; Yang, X. The role of HABTC’s hydroxamate and dithiocarbamate groups in chalcopyrite flotation. J. Ind. Eng. Chem. 2017, 52, 359–368. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Y.; Qu, X.; Xiao, J.; Yang, X.; Xu, Z. Understanding the hydrophobic mechanism of 3-hexyl-4-amino-1, 2,4-triazole-5-thione to malachite by ToF-SIMS, XPS, FTIR, contact angle, zeta potential and micro-flotation. Coll. Surf. A Physicochem. Eng. Asp. 2016, 503, 34–42. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, X.; Huang, K.; Wang, S.; Cao, Z.; Zhong, H. Synthesis, flotation performance and adsorption mechanism of 3-(ethylamino)-N-phenyl-3-thioxopropanamide onto galena/sphalerite surfaces. J. Ind. Eng. Chem. 2019, 77, 416–425. [Google Scholar] [CrossRef]
- Wang, X.; Qin, W.; Jiao, F.; Liu, R.; Wang, D. Inhibition of galena flotation by humic acid: Identification of the adsorption site for humic acid on moderately oxidized galena surface. Miner. Eng. 2019, 137, 102–107. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, Z.; Mulenga, H.; Sun, W.; Cao, J.; Gao, Z. Synthesis of a novel collector based on selective nitrogen coordination for improved separation of galena and sphalerite against pyrite. Chem. Eng. Sci. 2020, 226. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).