Mineralogy and Geochemistry (HFSE and REE) of the Present-Day Acid-Sulfate Types Alteration from the Active Hydrothermal System of Furnas Volcano, São Miguel Island, The Azores Archipelago
Abstract
1. Introduction
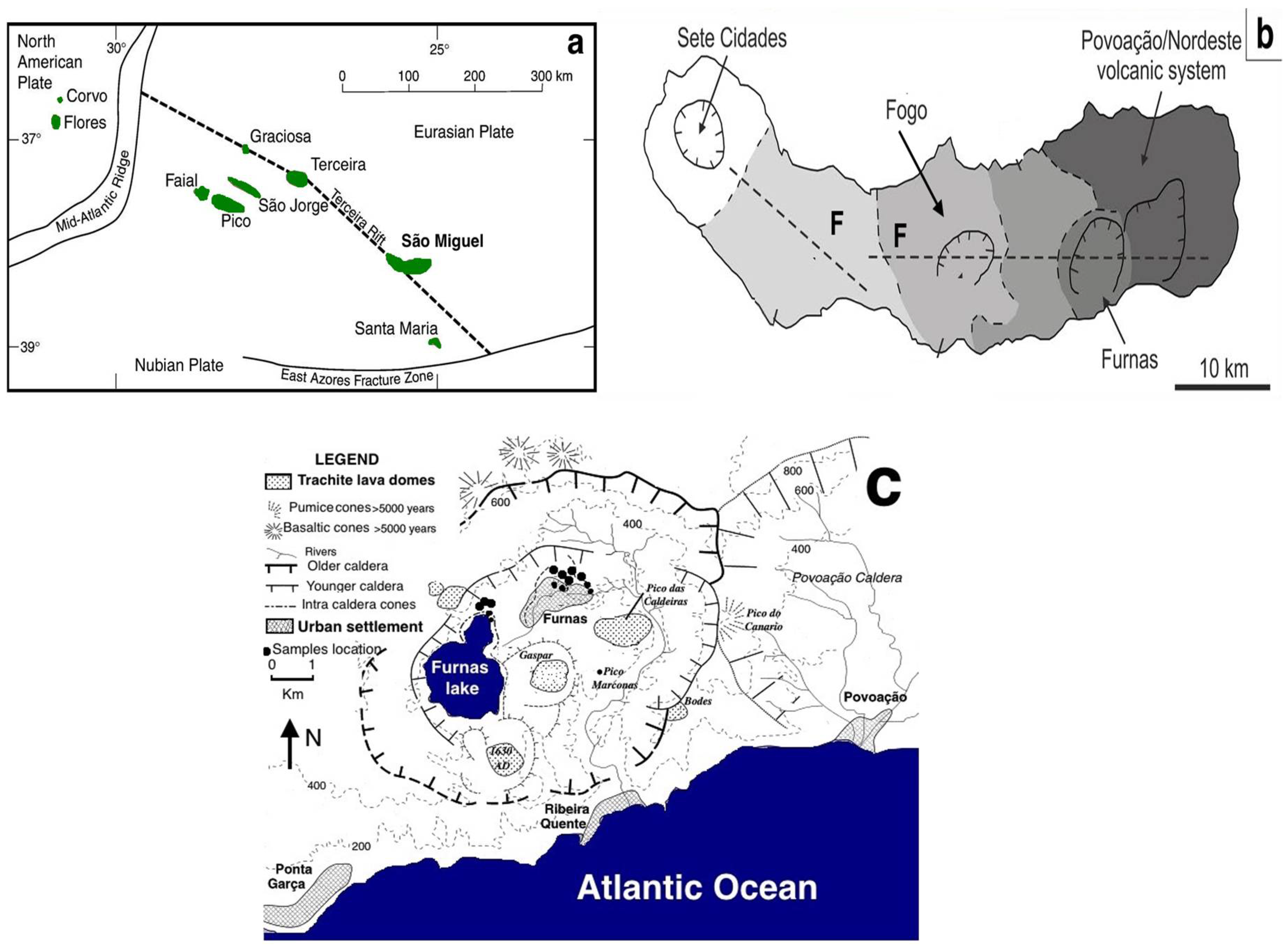
2. Geology
3. Materials and Methods
3.1. Sampling and Field Observation
3.2. Analytical Methods and Samples Preparations
4. Results
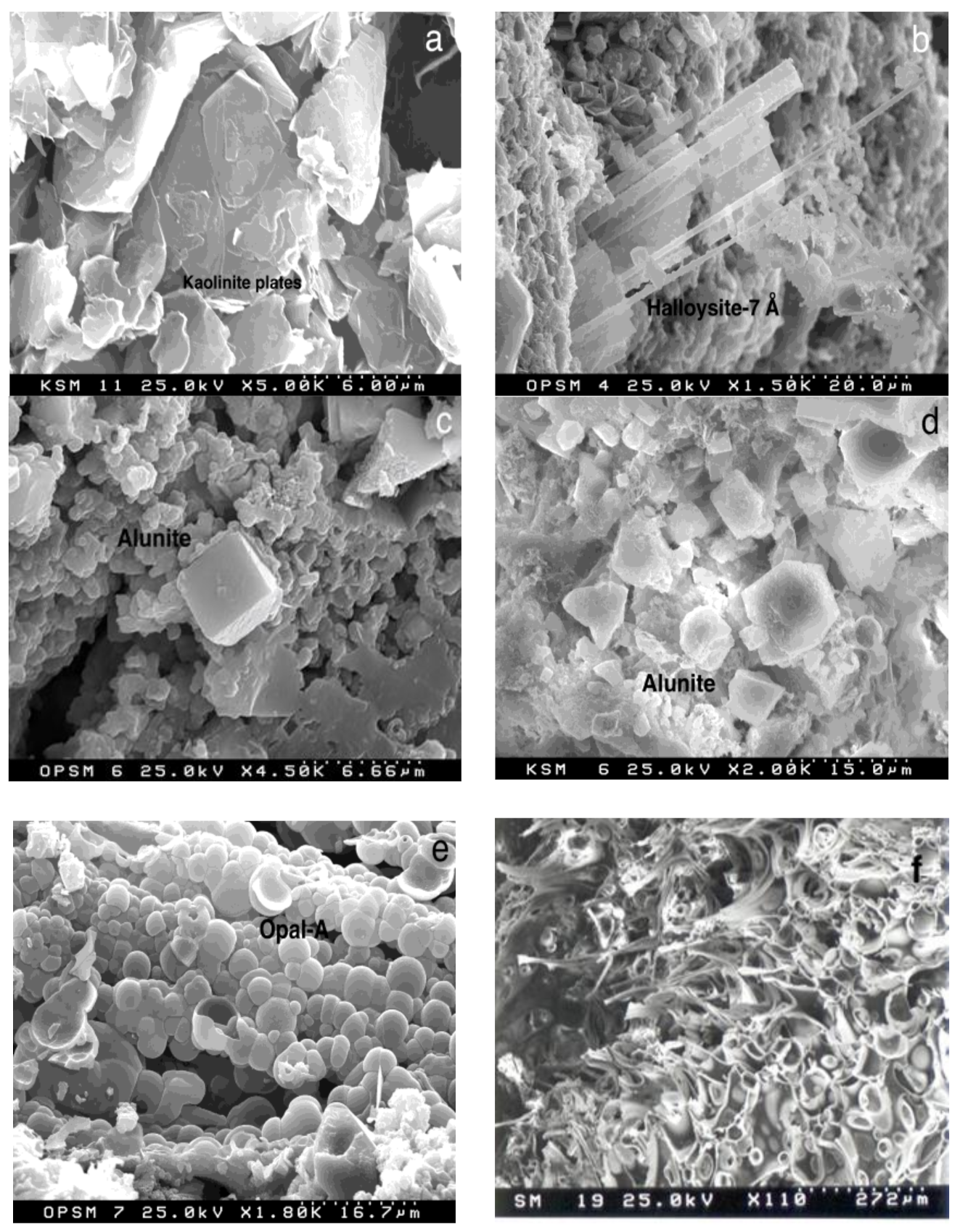
4.1. X-ray Diffraction
4.2. Scanning Electron Microscopy and Electron Microprobe Analysis
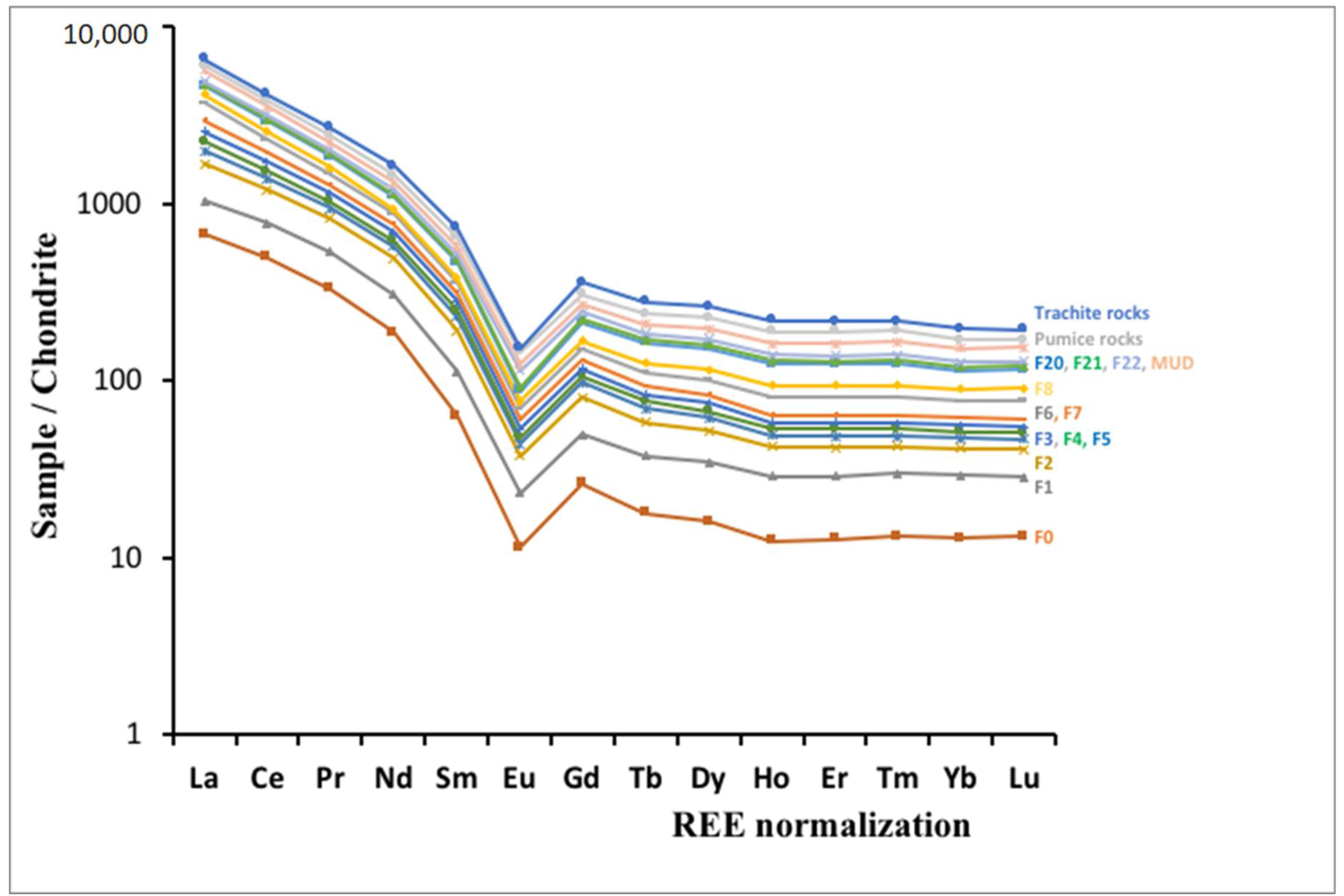
4.3. HFSE and REE Geochemistry
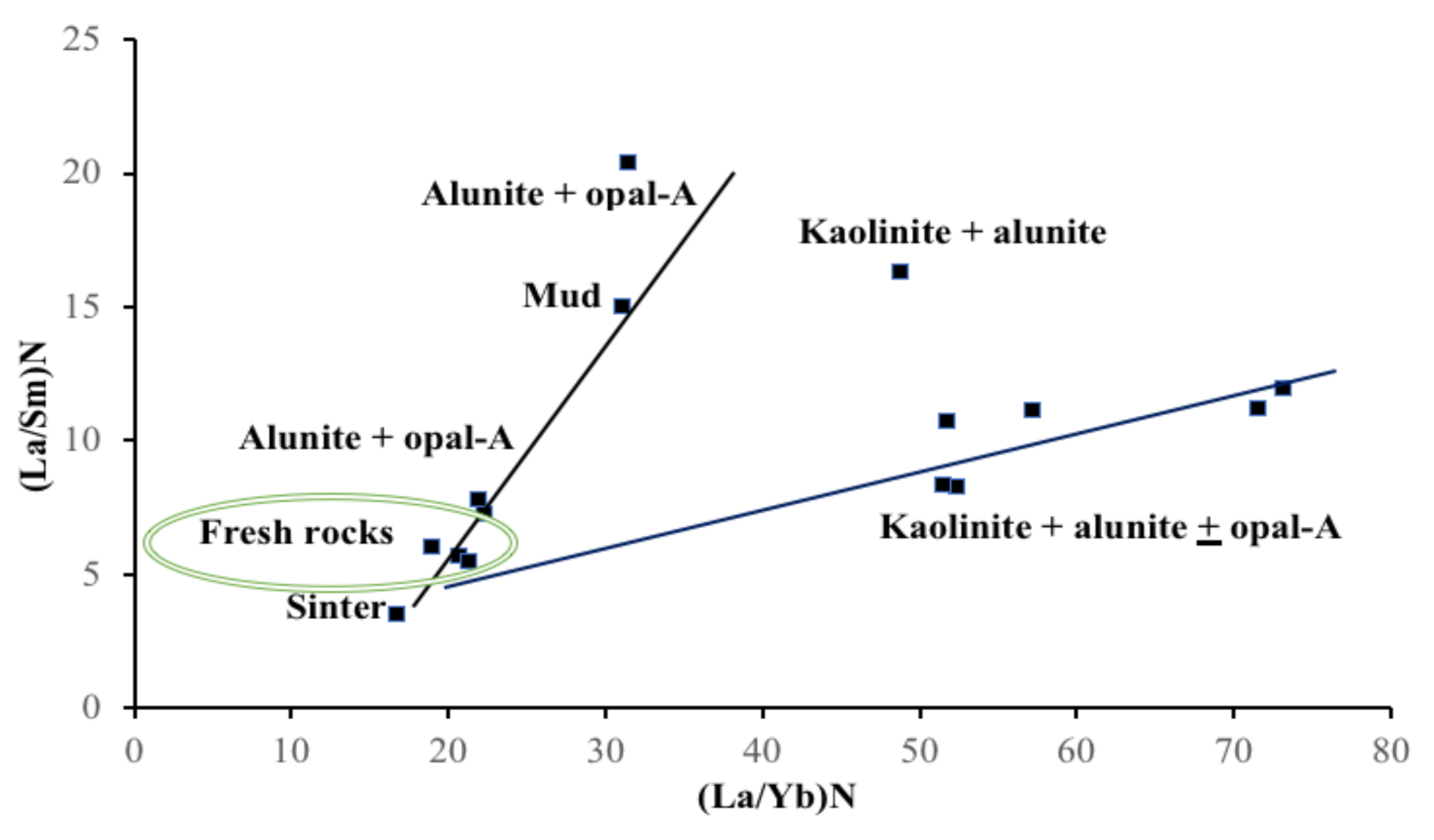
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giggenbach, W.F. Magma degassing and mineral deposition in hydrothermal systems along convergent plate boundaries. Econ. Geol. 1992, 97, 1927–1944. [Google Scholar]
- Christenson, B.W.; Wood, C.P. Evolution of a vent-hosted hydrothermal system beneath Ruapehu crater lake, New Zeeland. Bull. Volcanol. 1993, 55, 547–565. [Google Scholar] [CrossRef]
- Delmelle, P.; Bernard, A. Geochemistry, mineralogy, and chemical modelling of the acid crater lake of Kawah Ijen volcano, Indonesia. Geochim. Cosmochim. Acta 1994, 58, 2445–2460. [Google Scholar] [CrossRef]
- Africano, F.; Bernard, A. Acid alteration in the fumarolic environment of Usu volcano, Hokkaido, Japan. J. Volcanol. Geotherm. Res. 2000, 97, 475–495. [Google Scholar] [CrossRef]
- Berger, B.R.; Henley, R.W.; Lowers, H.A.; Pribil, M.J. The Lepanto Cu–Au deposit, Philippines: A fossil hyperacidic volcanic lake complex. J. Volcanol. Geotherm. Res. 2014, 271, 70–82. [Google Scholar] [CrossRef]
- Henley, R.W. Hyperacidic Volcanic Lakes, Metal Sinks and Magmatic Gas Expansion in Arc Volcanoes. In Volcanic Lakes, Advances in Volcanology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 155–184. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Isotopic shifts in waters from geothermal and volcanic systems along convergent plate boundaries and their origin. Earth Planet. Sci. Lett. 1992, 113, 495–510. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Aoki, M.; Shinohara, H. Flux of volatiles and ore-forming metals from the magmatic-hydrothermal system of Satsuma Iwojima volcano. Geology 1994, 22, 585–588. [Google Scholar] [CrossRef]
- Symonds, R.B.; Rose, W.I.; Bluth, G.J.S.; Gerlach, T.M. Volcanic-gas studies: Methods, results and applications. Rev. Mineral. 1994, 30, 1–66. [Google Scholar]
- Shinohara, H. A missing link between volcanic degassing and experimental studies on chloride partitioning. Chem. Geol. 2009, 263, 51–59. [Google Scholar] [CrossRef]
- Delmelle, P.; Henley Richard, W.R.; Opfergelt, S.; Detienne, M. Summit Acid Crater Lakes and Flank Instability in Composite Volcanoes. In Volcanic Lakes; Springer: Berlin/Heidelberg, Germany, 2015; pp. 289–305. [Google Scholar] [CrossRef]
- Muffler, L.J.P. Geothermal reservoir assessment. In Geothermal Systems: Principles and Case Histories; Rybach, L., Muffler, L.J.P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1981; p. 181. [Google Scholar]
- Henley, R.W.; McNabb, A. Magmatic vapor plumes and ground water interaction in porphyry copper emplacement. Econ. Geol. 1978, 73, 1–20. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Arribas, A.; Reynolds, T.J. Evolution of an intrusion-centered hydrothermal system; Far Southeast-Lepanto porphyry and epithermal Cu-Au deposits, Philippines. Econ. Geol. 1998, 93, 373–404. [Google Scholar] [CrossRef]
- Heald, P.; Foley, N.K.; Hayba, D.O. Comparative anatomy of volcanic hosted epithermal deposits: Acid sulfate and adularia-sericite types. Econ. Geol. 1987, 82, 1–26. [Google Scholar] [CrossRef]
- Hemley, J.J.; Jones, J. Chemical aspects of hydrothermal alteration with emphasis on hydrogen metasomatism. Econ. Geol. 1964, 59, 538–569. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Herdianita, N.R.; Rodgers, K.A.; Browne, P.R.L. Routine procedures for characterizing modern and ancient silica sinter deposits. Geothermics 2000, 29, 367–375. [Google Scholar] [CrossRef]
- Herdianita, N.R.; Browne, P.R.L.; Rodgers, K.A.; Campbell, A. Mineralogical and textural changes accompanying ageing of silica sinter. Mineral. Depos. 2000, 35, 48–62. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Taran, Y.A. Modeling the formation of advanced argillic lithocaps: Volcanic vapor condensation above porphyry intrusions. Econ. Geol. 2013, 108, 1523–1540. [Google Scholar] [CrossRef]
- Giggenbach, W.F.; Gonfiantini, R.; Jangi, B.L.; Truesdell, A.H. Isotopic and chemical composition of Parbati Valley geothermal discharges, northwest Himalaya, India. Geothermics 1983, 12, 199–222. [Google Scholar] [CrossRef]
- Inskeep, W.; Nordstrom, D.K.; Mogk, D.; Rodman, A.; Macur, E.E.; Fouke, B.; Durães, N.; Guzman, M. Secondary Minerals associated with thermal soils and geothermal features of Yellowstone National Park. In Clays of Yellostone Park; Schroeder, P., Ed.; Clay Minerals Society: Boulder, CO, USA, 2010; pp. 29–47. [Google Scholar]
- Piochi, M.; Kilburn, C.R.J.; Di Vito, M.A.; Mormone, A.; Tramelli, A.; Troise, C.; De Natale, G. The volcanic and geothermally active Campi Flegrei caldera: An integrated multidisciplinary image of its buried structure. Int. J. Earth Sci. 2014, 10, 401–421. [Google Scholar] [CrossRef]
- Piochi, M.; Mormone, A.; Balassone, G.; Strauss, H.; Troise, C.; De Natale, G. Native sulfur, sulfates and sulfides from the active Campi Flegrei volcano (southern Italy): Genetic environments and degassing dynamics revealed by mineralogy and isotope geochemistry. J. Volcanol. Geotherm. Res. 2015, 304, 180–193. [Google Scholar] [CrossRef]
- Piochi, M.; Mormone, A.; Balassone, G. Hydrothermal alteration environments in the recent dynamics of the Ischia volcanic island (Southern Italy): Clues from repeated field, mineralogical and geochemical surveys across the 2017 earthquake of Casamicciola. J. Volcanol. Geotherm. Res. 2019, 376, 104–124. [Google Scholar] [CrossRef]
- Shakeri, A.; Ghoreyshinia, S.; Mehrabi, B.; Delavari, M. Rare earth elements geochemistry in springs from Taftan geothermal area SE Iran. J. Volcanol. Geotherm. Res. 2015, 304, 49–61. [Google Scholar] [CrossRef]
- White, D.E.; Hutchinson, R.A.; Keith, T.E.C. The Geology and Remarkable Thermal Activity of Norris Geyser Basin, Yellowstone National Park, Wyoming; U.S. Geological Survey (USGS): Reston, VA, USA, 1988; p. 1456.
- Moore, R. Volcanic geology and eruption frequency, S. Miguel, Azores. Bull. Volcanol. 1990, 52, 602–614. [Google Scholar] [CrossRef]
- Ferreira, T.; Oskarsson, N. Chemistry and isotopic composition of fumarole discharge of Furnas caldera. J. Volcanol. Geotherm. Res. 1999, 92, 169–179. [Google Scholar] [CrossRef]
- Cruz, J.V.; Coutinho, R.M.; Carvalho, M.R.; Oskarsson, N.; Gislason, S.R. Chemistry of waters from Furnas volcano, São Miguel, Azores: Fluxes of volcanic carbon dioxide and leached material. J. Volcanol. Geotherm. Res. 1999, 92, 151–167. [Google Scholar] [CrossRef]
- Searle, R. Tectonic pattern of the Azores spreading centre and triple junction. Earth Planet Sci. Lett. 1980, 51, 415–434. [Google Scholar] [CrossRef]
- Luis, J.F.; Miranda, J.M.; Galdeano, A.; Patriat, P.; Rossignol, J.C.; Mendes Victor, L.A. The Azores triple junction evolution since 10 Ma from an aerogmagnetic survey of mid-Atlantic Ridge. Earth Planet. Sci. Lett. 1994, 125, 439–459. [Google Scholar] [CrossRef]
- Marques, F.O.; Catalão, J.C.; DeMets, C.; Costa, A.C.G.; Hildenbrand, A. GPS and tectonic evidence for a diffuse plate boundary at the Azores Triple Junction. Earth Planet. Sci. Lett. 2013, 381, 177–187. [Google Scholar] [CrossRef]
- Moore, B.R. Geology of three late Quaternary stratovolcanoes on São Miguel, Azores. U.S. Geol. Surv. Bull. 1991, 1900, 1–46. [Google Scholar]
- Walker, G.P.L.; Croasdale, R. Two plinian-type eruptions in the Azores. J. Geol. Soc. Lond. 1971, 127, 17–55. [Google Scholar] [CrossRef]
- Calvert, A.T.C.; Moore, R.B.; McGeehin, J.P.; Rodrigues da Silva, A.M. Volcanic history and 40Ar/39Ar and 14C geochronology of Terceira Island, Azores, Portugal. J. Volcanol. Geotherm. Res. 2006, 156, 103–115. [Google Scholar] [CrossRef]
- Jeffery, A.J.; Gertisser, R.; O’Driscoll, B.; Pacheco, J.M.; Whitley, S.; Pimentel, S.A. Temporal evolution of a post-caldera, mildly peralkaline magmatic system: Furnas volcano, São Miguel, Azores. Contrib. Mineral. Petrol. 2016, 171, 42–59. [Google Scholar] [CrossRef]
- Cole, P.D.; Queiroz, G.; Wallenstein, N.; Gaspar, J.L.; Duncan, A.M.; Guest, J.E. An historic subplinian to phreatomagmatic eruption: The 1630 eruption of Furnas volcano, São Miguel, Azores. J. Volcanol. Geotherm. Res. 1999, 69, 117–135. [Google Scholar] [CrossRef]
- Guest, J.E.; Gaspar, J.L.; Cole, P.D.; Queiroz, G.; Duncan, M.; Wallenstein, N.; Ferreira, T.; Pacheco, J.M. Volcanic geology of Furnas volcano, São Miguel, Azores. J. Volcanol. Geotherm. Res. 1999, 92, 1–29. [Google Scholar] [CrossRef]
- Booth, B.; Walker, G.P.L.; Croasdale, R. A quantitative study of five thousand years of volcanism on São Miguel, Azores. Philos. Trans. R. Soc. Lond. 1978, 228, 271–319. [Google Scholar]
- Guest, J.E.; Pacheco, J.M.; Cole, P.D.; Duncan, A.M.; Wallenstein, N.; Queiroz, G.; Gaspar, J.L.; Ferreira, T. Volcanic geology of São Miguel Island (Azores Archipelago): Introduction. Geol. Soc. London Mem. 2015, 44, 125–134. [Google Scholar] [CrossRef]
- Cruz, J.V.; França, Z. Hydrogeochemistry of thermal and mineral water springs of the Azores archipelago (Portugal). J. Volcanol. Geotherm. Res. 2006, 151, 382–398. [Google Scholar] [CrossRef]
- Zbyszewski, G.; Almeida, F.M.; Ferreira, O.V.; Assunção, C.T. Carta Geológica de Portugal na escala 1:50,000. Notícia explicativa da folha B, S. Miguel (Açores). Serv. Geol. Port. Lisboa 1958, 37. [Google Scholar]
- Woitischek, J.; Dietzel, M.; Inguaggiato, C.; Böttcher, M.E.; Leis, L.; Cruz, V.J.; Gehre, M. Characterisation and origin of hydrothermal waters at São Miguel (Azores) inferred by chemical and isotopic composition. J. Volcanol. Geotherm. Res. 2017, 346, 104–117. [Google Scholar] [CrossRef]
- Jones, J.B.; Segnit, E.R. The nature of opal I. Nomenclature and constituent phases. J. Geol. Soc. Aust. 1971, 18, 57–68. [Google Scholar] [CrossRef]
- Flörke, O.W.; Graetsch, H.; Martin, B.; Roller, K.; Wirth, R. Nomenclature of micro- and non-crystalline silica minerals, based on structure and microstructure. Neues Jahrbh. Mineral. Abh. 1991, 163, 19–42. [Google Scholar]
- Graetsch, H. Structural characteristics of opaline and microcrystalline silica minerals. In Silica: Physical Behaviour, Geochemistry and Materials Applications; Heaney, P.J., Prewitt, C.T., Gibbs, G.V., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 1994; pp. 209–232. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The geochemical evolution of the continental crust. Rev. Geophisique 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Hinckley, D.N. Variability in “crystallinity” values among the kaolin deposits of the coastal plain of Georgia and South Carolina. Clays Clay Miner. 1963, 11, 229–235. [Google Scholar] [CrossRef]
- Bigham, J.M.; Schwertmann, U.; Traina, S.J.; Winland, R.L.; Wolf, M. Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim. Cosmochim. Acta 1996, 60, 185–195. [Google Scholar] [CrossRef]
- Marschall, H.R.; Dohmen, R.; Ludwig, T. Diffusion-induced fractionation of niobium and tantalum during continental crust formation. Earth Planet. Sci. Lett. 2013, 375, 361–371. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Wang, R.-C.; Xu, X.-S.; Zhao, K.-D. Mobility of high field strength elements (HFSE) in magmatic-, metamorphic-, and submarine-hydrothermal systems. Phys. Chem. Earth 2005, 30, 1020–1029. [Google Scholar] [CrossRef]
- Münker, C.; Pfänder, J.A.; Weyer, S.; Buchl, A.; Kleine, T.; Mezger, K. Evolution of planetary cores and the Earth–Moon system from Nb/Ta systematics. Science 2003, 301, 84–87. [Google Scholar] [CrossRef]
- Wood, S.A. The geochemistry of rare earth elements and yttrium in geothermal waters. Soc. Econ. Geol. Spec. Pub. 2003, 10, 133–158. [Google Scholar]
- Peiffer, L.; Taran, Y.; Lounejeva, E.; Solís-Pichardo, E.; Rouwet, D.; Bernard-Romero, R.A. Tracing thermal aquifers of El Chichónn volcano-hydrothermal system (Mexico) with 87Sr/87Sr, Ca/Sr and REE. J. Volcanol. Geotherm. Res. 2011, 205, 55–66. [Google Scholar] [CrossRef]
- Wood, S.A. Rare element systematics of acidic geothermal waters from the Taupo Volcanic Zone, New Zealand. J. Geochem. Explor. 2006, 89, 424–427. [Google Scholar] [CrossRef]
- Fulignati, A.; Gioncada, A.; Sbrana, A. Rare-earth element (REE) behavior in the alteration facies of the active magmatic hydrothermal system of Vulcano (Aeolian Islands, Italy). J. Volcanol. Geotherm. Res. 1999, 88, 325–342. [Google Scholar] [CrossRef]
- Kulaksız, S.; Bau, M. Contrasting behaviour of anthropogenic gadolinium and natural rare earth elements in estuaries and the gadolinium input into the North Sea. Earth Planet. Sci. Lett. 2007, 260, 361–371. [Google Scholar] [CrossRef]
- Bau, M. Controls on the Fractionation of Isovalent Trace Elements in Magmatic and Aqueous Systems: Evidence from Y/Ho, Zr/Hf and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Bobos, I.; Gomes, C. Greisen and post-greisen alteration in the São Vicente de Pereira area. Can. Mineral. 1998, 36, 1615–1624. [Google Scholar]
- Bobos, I.; Duplay, J.; Rocha, J.; Gomes, C. Kaolinite to halloysite-7Å transformation in the kaolin deposit of São Vicente de Pereira, Portugal. Clays Clay Miner. 2001, 49, 597–605. [Google Scholar] [CrossRef]
- Gomes, C.S.F.; Massa, M.E. Allophane and sperullitic halloysite, weathering products of trachitic pumice fall-outs in the Caldeira Velha /S.Miguel-Azores). Miner. Petrog. Acta 1992, XXXVA, 283–288. [Google Scholar]
- Hay, R.L.; Iijima, A. Nature and origin of palagonite tuffs of the Honolulu group on Oahu, Hawaii. Mem. Geol. Soc. Am. 1968, 116, 331–376. [Google Scholar]
- Tomita, K.; Yamane, H.; Kawano, M. Synthesis of Smectite from volcanic glass at low temperature. Clays Clay Miner. 1993, 41, 655–661. [Google Scholar] [CrossRef]
- Berger, G.; Meunier, A.; Beaufort, D. Clay mineral formation on Mars: Chemical constraints and possiblecontribution of basalt out-gassing. Planet. Space Sci. 2014, 95, 25–32. [Google Scholar] [CrossRef]
- Berger, G.; Toplis, M.J.; Treguier, E.; d’Uston, C.; Pinet, P. Evidence in favor of small amounts of ephemeral and transient water during alteration at Meridiani Planum, Mars. Am. Mineral. 2009, 94, 1279–1282. [Google Scholar] [CrossRef]
- Mormone, A.; Ghiara, M.R.; Balassone, G.; Poichi, M.; Lonis, R.; Rossi, M. High-silica zeolites in pyroclastic flows from Central Sardinia (Italy): Clues on genetic processes and reserves from a mineralogical study. Miner. Petrol. 2018, 112, 767–788. [Google Scholar] [CrossRef]
- Ikehata, I.; Maruoka, T. Sulfur isotopic characteristics of volcanic products from the September 2014 Mount Ontake eruption, Japan. Earth Planets Space 2016, 68, 116. [Google Scholar] [CrossRef]
- Williams, S.N.; Sturchio, N.C.; Calvache, V.M.L.; Mendez, R.F.; Londonõ, C.A.; Garcia, N.P. Sulfur dioxide from Nevado del Ruiz volcano, Columbia: Total flux and isotopic constraints on its origin. J. Volcanol. Geotherm. Res. 1990, 42, 53–68. [Google Scholar] [CrossRef]
- Raymahashay, B.C. A geochemical study of rock alteration by hot springs in the Paint Pot Hill area, Yellowstone Park. Geochim. Cosmochim. Acta 1968, 32, 499–522. [Google Scholar] [CrossRef]
- Barker, W.W.; Welch, S.A.; Chu, S.; Banfield, J.F. Experimental observations of the effects of bacteria on aluminosilicate weathering. Am. Mineral. 1998, 83, 1551–1563. [Google Scholar] [CrossRef]
- Monterroso, C.; Alvarez, E.; Macías, F. Speciation and solubility control of Al and Fe in mine soil solutions. Sci. Total Environ. 1994, 158, 31–43. [Google Scholar] [CrossRef]
- Durães, N.; Bobos, I.; Ferreira da Silva, E. Speciation and precipitation of heavy metals in the acid mine waters from the Iberian Pyrite Belt (Portuguese sector). Environ. Sci. Pollut. Res. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, D.K. Aqueous pyrite oxidation and the consequent formation of secondary iron minerals. Acid Sulfate Weather 1982, 10, 37–56. [Google Scholar]
- Finlow-Bates, T.; Stumpfl, E.F. The behaviour of so-called immobile elements in hydrothermally altered rocks associated with volcanogenic submarine-exhalative ore deposits. Miner. Deposita. 1981, 16, 319–328. [Google Scholar] [CrossRef]
- Rubin, J.N.; Henry, C.D.; Price, J.G. The mobilization of zirconium and other “immobile” elements during hydrothermal alteration. Chem. Geol. 1993, 110, 29–47. [Google Scholar] [CrossRef]
- Aja, S.U.; Wood, S.A.; Williams-Jones, A.E. The aqueous geochemistry of Zr and the solubility of some Zr-bearing minerals. Appl. Geochem. 1995, 10, 603–620. [Google Scholar] [CrossRef]
- Brown, P.; Curti, E.; Grambow, B. Chemical Thermodynamics of Zirconium; Elsevier: Amsterdam, The Netherlands, 2005; Available online: http://www.oecdnea.org/dbtdb/pubs/vol8-zirconium.pdf (accessed on 23 March 2021).
- Michard, A. Rare earth element systematics in hydrothermal fluids. Geochim. Cosmochim. Acta 1989, 53, 745–750. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Rare earth elements and hydrothermal ore formation processes. Ore Geol. Rev. 1992, 7, 25–41. [Google Scholar] [CrossRef]
- Alderton, D.H.M.; Pearce, J.A.; Potts, P.J. Rare earth element mobility during granite alteration: Evidence from southeast England. Earth Planet. Sci. Lett. 1980, 49, 149–165. [Google Scholar] [CrossRef]
- Inguaggiato, C.; Censi, P.; Zuddas, P.; Londoño, J.M.; Chacón, Z.; Alzate, D.; Brusca, L.; D’Alessandro, W. Zirconium–hafnium and rare earth element signatures discriminating the effect of atmospheric fallout from hydrothermal input in volcanic lake water. Chem. Geol. 2016, 433, 1–11. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Europium redox equilibria in aqueous solution. Earth Planet. Sci. Lett. 1984, 67, 70–78. [Google Scholar] [CrossRef]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid–rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Lewis, A.J.; Palmer, M.A.; Sturchio, N.C.; Kemp, A.J. The rare earth element geochemistry of acid-sulfate and acid-sulfate-chloride geothermal systems from Yellowstone National Park, Wyoming, USA. Geochim. Cosmochim. Acta 1997, 61, 695–706. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Goodfellow, W.D.; Boyle, D.R. Hydrogeochemical, isotopic and rare earth element geochemistry of acid–sulphate and acid–sulphate–chloride geothermal systems from Yellowstone. Geochim. Cosmochim. Acta 2000, 61, 695–723. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, C. The effect of humic acid on the adsorption of REE on kaolin. Colloids Surf. A Physicochem. Eng. Asp. 2006, 290, 112–117. [Google Scholar] [CrossRef]
- Coppin, F.; Berger, G.; Bauer, A.; Castet, S.; Loubet, M. Sorption of lanthanides on smectite and kaolinite. Chem. Geol. 2002, 182, 57–68. [Google Scholar] [CrossRef]
- Ellis, A.J.; Mahon, W.A. Geochemistry of Geothermal Systems; Academic Press: New York, NY, USA, 1977; p. 392. [Google Scholar] [CrossRef]
- Merino, E.; Harvey, C.; Murray, H.H. Aqueous-Chemical Control of the Tetrahedral-Aluminum Content of Quartz, Halloysite, and other Low-Temperature Silicates. Clays Clay Miner. 1989, 37, 135–142. [Google Scholar] [CrossRef]
- Inoue, A.; Aoki, M.; Ito, H. Mineralogy of Ohyunuma explosion crater lake, Kokkaido, Japan. Clay Sci. 2000, 11, 169–187. [Google Scholar]
- Sposito, G. Chemical Equilibria and Kinetics in Soils; Oxford University Press: Oxford, UK, 1994; p. 260. [Google Scholar]
- Rimstidt, J.D.; Cole, D.R. Geothermal mineralization I: The mechanism of formation of the Beowawe, Nevada, siliceous sinter deposit. Am. J. Sci. 1983, 283, 861–875. [Google Scholar] [CrossRef]
- Fournier, R.O. The behaviour of silica in hydrothermal solutions. In Geology and Geochemistry of Epithermal Systems; Society of Economic Geologists: Littleton, CO, USA, 1985; pp. 45–60. [Google Scholar]





| Samples | Location | Mineral Assemblages |
|---|---|---|
| F0 | Caldeiras-Furnas | Kaolinite, alunite, opal-A |
| F1 | Caldeiras-Furnas | Alunite, halloysite, opal-A |
| F2 | Caldeiras-Furnas | Kaolinite, alunite ± opal-A |
| F3 | Caldeiras-Furnas | Alunite, opal-A |
| F4 | Caldeiras-Furnas | Opal-A, alunite |
| F5 | Caldeiras-Furnas | Alunite, opal-A |
| F6 | Caldeiras-Furnas | Opal-A, alunite |
| F7 | Caldeiras-Furnas | Kaolinite, alunite |
| F8 | Caldeiras-Furnas | Alunite, opal-A |
| F20 | Caldeiras-Furnas | Alunite, (Feldspar) |
| F21 | Caldeiras-Furnas | Sinter (opal-A, alunite) |
| F22 | Caldeiras-Furnas | Sinter (opal-A, alunite) |
| Mud | Lagoa das Furnas | Smectite, kaolinite, alunite, (Feldspar) |
| Trachyte pumice | Caldeiras-Furnas | Volcanic glassy, feldspar |
| Trachyte | Caldeiras-Furnas | Feldspar, clinopyroxene, Fe-Ti oxides, amphibole |
| Oxides | Feldspar n = 5 | Feldspar n = 5 | Feldspar n = 5 | Feldspar n = 5 | Feldspar n = 5 | Feldspar n = 5 | Feldspar n = 5 | Alunite n = 5 | Alunite n = 5 | Alunite n = 5 | Alunite n = 5 | Alunite n = 5 | Kaolinite n = 5 | Kaolinite n = 5 | Kaolinite n =5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 67.63 | 65.78 | 65.58 | 65.97 | 65.95 | 66.02 | 65.28 | 0.2 | 0.35 | 0.56 | 0.10 | 0.16 | 45.24 | 44.15 | 45.2 |
| TiO2 | 0.28 | 0.12 | 0.14 | 0.19 | 0.15 | 0.14 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 |
| Al2O3 | 20.69 | 21.35 | 21.07 | 20.59 | 20.09 | 20.66 | 20.71 | 48.35 | 52.35 | 49.28 | 49.59 | 50.14 | 37.98 | 38.29 | 38.37 |
| FeO | 0.53 | 0.31 | 0.34 | 0.35 | 0.37 | 0.32 | 0.22 | 0.01 | 0.02 | 0.06 | 0.05 | 0.03 | 0.19 | 0.06 | 0.11 |
| MnO | 0.00 | 0.00 | 0.02 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MgO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CaO | 0.40 | 1.17 | 1.04 | 0.59 | 0.28 | 0.75 | 0.99 | 0.03 | 0.01 | 0.03 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 |
| Na2O | 4.54 | 6.28 | 6.15 | 5.91 | 5.75 | 6.30 | 5.55 | 0.77 | 0.21 | 0.56 | 0.23 | 0.34 | 0.00 | 0.00 | 0.00 |
| K2O | 6.39 | 4.30 | 5.48 | 6.09 | 7.71 | 6.21 | 7.42 | 2.1 | 2 | 2.54 | 2.36 | 2.44 | 0.00 | 0.00 | 0.00 |
| SO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 34.89 | 35.32 | 35.32 | 36.51 | 35.82 | 0.00 | 0.00 | 0.00 |
| Total | 100.47 | 99.32 | 99.81 | 99.68 | 100.32 | 100.41 | 100.29 | 86.35 | 90.26 | 88.34 | 88.86 | 88.94 | 83,41 | 83.47 | 83.68 |
| Si | 2.979 | 2.926 | 2.923 | 2.946 | 2.952 | 2.955 | 2.922 | 0.00 | 0.01 | 0.02 | 0.00 | 0.0 | 1.99 | 1.97 | 1.98 |
| Ti | 0.009 | 0.004 | 0.005 | 0.006 | 0.005 | 0.005 | 0.004 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al | 1.074 | 1.119 | 1.107 | 1.084 | 1.059 | 1.090 | 1.092 | 1.76 | 1.82 | 1.76 | 1.75 | 1.75 | 1.97 | 2.01 | 2.01 |
| Fe | 0.020 | 0.012 | 0.013 | 0.013 | 0.014 | 0.012 | 0.008 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mn | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mg | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ca | 0.019 | 0.056 | 0.050 | 0.028 | 0.013 | 0.036 | 0.048 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Na | 0.388 | 0.542 | 0.532 | 0.512 | 0.499 | 0.547 | 0.482 | 0.05 | 0.12 | 0.03 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 |
| K | 0.359 | 0.244 | 0.312 | 0.347 | 0.440 | 0.355 | 0.424 | 0.08 | 0.08 | 0.1 | 0.09 | 0.09 | 0.00 | 0.00 | 0.00 |
| S | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.95 | 0.88 | 0.91 | 0.93 | 0.92 | 0.00 | 0.00 | 0.00 |
| Total | 4.848 | 4.903 | 4.941 | 4.935 | 4.983 | 4.999 | 4.979 | 2.83 | 2.91 | 2.73 | 2.78 | 2.78 | 3.96 | 3.98 | 3.99 |
| An | 2.5 | 6.6 | 5.6 | 3.2 | 1.4 | 3.9 | 5.0 | ||||||||
| Ab | 50.6 | 64.4 | 59.5 | 57.7 | 52.4 | 58.3 | 50.6 | ||||||||
| Or | 46.9 | 29.0 | 34.9 | 39.1 | 46.2 | 37.8 | 44.4 | ||||||||
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Samples | Cu | Pb | Zn | As | Ba | Ni | Cr | Co | Cs | Ga | Hf | Nb | Rb | Sn | Sr | Ta | Th | Tl | U | V | W | Zr | Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fo | 9 | 11 | 34 | 5 | 247.5 | 11 | 6 | 0.4 | 0.6 | 47.8 | 30 | 259.21 | 44.14 | 8 | 176.9 | 13.1 | 25.6 | 0.2 | 4.2 | 43 | 4.1 | 1336.3 | 26.1 |
| F1 | 11 | 8 | 17 | 5 | 119.9 | 15 | 5 | 0.6 | 0.8 | 39.8 | 47.5 | 361.69 | 23.87 | 12 | 68.2 | 20.2 | 24.1 | 0.5 | 7.6 | 25 | 8.7 | 1958.6 | 33.4 |
| F2 | 3 | 4 | 4 | 5 | 294.4 | 9 | 4 | 0.3 | 0.6 | 50.2 | 35.2 | 305.38 | 25.87 | 10 | 207 | 16.3 | 25.3 | 0.4 | 5.3 | 30 | 5.6 | 1537 | 27.1 |
| F3 | 5 | 8 | 6 | 5 | 211.1 | 13 | 8 | 0.6 | 0.6 | 43.2 | 25.8 | 226.8 | 24.52 | 10 | 140.7 | 11.9 | 12.2 | 0.6 | 4 | 23 | 3.6 | 1101.1 | 14.3 |
| F4 | 4 | 6 | 4 | 5 | 211.8 | 9 | 5 | 0.6 | 0.6 | 39.6 | 32.5 | 287.02 | 25.55 | 8 | 113.3 | 15.1 | 11 | 0.4 | 4.6 | 24 | 4 | 1359.7 | 10 |
| F5 | 2 | 7 | 5 | 5 | 104 | 16 | 11 | 0.5 | 0.7 | 31.8 | 28.2 | 220.74 | 17.96 | 5 | 64.3 | 11.6 | 13.3 | 0.2 | 2.6 | 17 | 2.6 | 1254.8 | 9.4 |
| F6 | 2 | 4 | 3 | 5 | 182.1 | 12 | 3 | 0.4 | 0.4 | 35 | 26.6 | 246.8 | 22.72 | 8 | 117.4 | 12.2 | 13.2 | 0.4 | 2.9 | 13 | 4.1 | 1179.7 | 13.3 |
| F7 | 9 | 12 | 7 | 5 | 282 | 8 | 12 | 0.5 | 0.2 | 89.6 | 54.2 | 477.45 | 9.71 | 10 | 224.5 | 20.1 | 29.3 | 0.2 | 4.3 | 16 | 7.6 | 2414.4 | 35.6 |
| F8 | 4 | 5 | 2 | 5 | 169.1 | 10 | 7 | 3.5 | 0.8 | 28.5 | 22.4 | 202.58 | 19.41 | 7 | 85.9 | 10.1 | 19.9 | 0.3 | 7.3 | 21 | 3.3 | 996.1 | 31.9 |
| F20 | 5 | 3 | 23 | 5 | 24.3 | 5 | 4 | 0.3 | 1.9 | 37.9 | 29.3 | 270.5 | 218.64 | 8 | 11.1 | 13.8 | 23.6 | 0.1 | 7.3 | 10 | 4 | 1285.2 | 70 |
| F21 | 3 | 4 | 2 | 5 | 310.2 | 11 | 8 | 0.4 | 0.8 | 10.2 | 28.4 | 214.7 | 29.36 | 5 | 50.3 | 9.9 | 9.4 | 0.2 | 2.7 | 23 | 4.3 | 1235.3 | 12.9 |
| F22 | 1 | 3 | 74 | 5 | 279.5 | 9 | 5 | 1.5 | 0.4 | 28.7 | 9.8 | 78.77 | 117.48 | 3 | 85.7 | 3.8 | 7.7 | 0.1 | 2.3 | 20 | 0.5 | 424.6 | 21.5 |
| Mud | 3 | 3 | 8 | 5 | 360.6 | 14 | 6 | 0.4 | 0.4 | 84.2 | 70 | 623.8 | 17.72 | 11 | 184.4 | 27.1 | 32.8 | 0.2 | 5.6 | 34 | 8.5 | 3192.5 | 53.9 |
| Trachyte pumice | 4 | 11 | 66 | 5 | 169.1 | 4 | 3 | 1.8 | 2.1 | 112.8 | 34 | 326.75 | 221.32 | 9 | 126.52 | 21.3 | 34 | 0.6 | 7.8 | 38 | 5.1 | 1690.26 | 74 |
| Trachyte | 6 | 9 | 82 | 5 | 286.3 | 8 | 6 | 1 | 0.9 | 94.7 | 51 | 391.56 | 263.98 | 6 | 148.31 | 27.5 | 41 | 0.9 | 6.3 | 59 | 7.4 | 2189 | 112 |
| Samples | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F0 | 245.2 | 473.2 | 44.87 | 132.2 | 14.5 | 0.99 | 8.01 | 1.03 | 6.11 | 1.06 | 3.16 | 0.47 | 3.2 | 0.5 | 26.1 |
| F1 | 132.3 | 262.8 | 27.71 | 85.3 | 11.6 | 1.02 | 7.19 | 1.14 | 7.06 | 1.39 | 3.98 | 0.59 | 3.99 | 0.58 | 33.4 |
| F2 | 235.4 | 409.6 | 39.66 | 131.1 | 18 | 1.24 | 9.37 | 1.21 | 6.68 | 1.14 | 3.33 | 0.44 | 3.03 | 0.47 | 27.1 |
| F3 | 114.6 | 178.2 | 16.58 | 57.8 | 8.7 | 0.55 | 5.28 | 0.63 | 3.6 | 0.56 | 1.62 | 0.23 | 1.5 | 0.23 | 14.3 |
| F4 | 88.1 | 123.4 | 10.63 | 33.2 | 5 | 0.38 | 2.7 | 0.4 | 2.19 | 0.39 | 1.18 | 0.16 | 1.04 | 0.16 | 10 |
| F5 | 122 | 192.1 | 16.33 | 48.4 | 6.9 | 0.46 | 3.13 | 0.44 | 2.72 | 0.38 | 1.1 | 0.16 | 1.15 | 0.15 | 9.4 |
| F6 | 139.9 | 201.2 | 17.31 | 53.6 | 7.4 | 0.56 | 4.11 | 0.52 | 3.19 | 0.54 | 1.46 | 0.2 | 1.29 | 0.21 | 13.3 |
| F7 | 279.2 | 357.7 | 27.15 | 76.7 | 10.8 | 0.85 | 6.4 | 1.07 | 6.75 | 1.37 | 4.17 | 0.61 | 3.86 | 0.64 | 35.6 |
| F8 | 148.5 | 242.2 | 18.15 | 37.5 | 4.6 | 0.58 | 4.71 | 0.79 | 5.55 | 1.12 | 3.44 | 0.5 | 3.18 | 0.49 | 31.9 |
| F20 | 184.2 | 345.4 | 35.03 | 123.8 | 20.5 | 0.92 | 14.61 | 2.24 | 14.16 | 2.61 | 7.26 | 1.07 | 5.98 | 0.94 | 70 |
| F21 | 45.7 | 79.9 | 7.37 | 23.1 | 3.7 | 0.4 | 2.32 | 0.36 | 2.51 | 0.47 | 1.4 | 0.21 | 1.4 | 0.21 | 12.9 |
| F22 | 48.4 | 148.3 | 11.92 | 47.6 | 8.8 | 1.9 | 6.16 | 0.84 | 4.96 | 0.86 | 2.32 | 0.32 | 1.95 | 0.33 | 21.5 |
| Mud | 276.1 | 376.5 | 29.69 | 82.1 | 11.6 | 0.94 | 7.49 | 1.35 | 9.3 | 1.87 | 5.92 | 0.96 | 5.98 | 0.93 | 53.9 |
| Trachyte | 146 | 232 | 28.3 | 101 | 17 | 1.79 | 12 | 1.8 | 11 | 2.3 | 6.4 | 0.85 | 4.6 | 0.62 | 74 |
| Trachyte pumice | 178.6 | 346.1 | 37.43 | 119.82 | 18.77 | 0.61 | 15.89 | 2.33 | 13.81 | 2.55 | 7.11 | 0.91 | 6.34 | 0.93 | 112 |
| Samples | SUMREE | LREE | HREE | MREE | (LREE/HRRE)N | (La/Yb)N | (La/Ce)N | Ce/Ce* | Eu/Eu* | (La/Sm)N | (Tb/Yb)N | (Gd/Gd)N | Y/Ho | Y/Dy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fo | 934.50 | 895.47 | 7.33 | 31.7 | 18.36 | 51.78 | 1.35 | 1.06 | 0.28 | 10.64 | 1.38 | 0.80 | 24.62 | 4.27 |
| F1 | 546.65 | 508.11 | 9.14 | 29.4 | 9.46 | 22.41 | 1.31 | 1.02 | 0.34 | 7.18 | 1.22 | 0.79 | 24.03 | 4.73 |
| F2 | 860.67 | 815.76 | 7.27 | 37.64 | 17.06 | 52.50 | 1.49 | 0.99 | 0.29 | 8.23 | 1.71 | 0.77 | 23.77 | 4.057 |
| F3 | 390.08 | 367.18 | 3.58 | 19.32 | 9.80 | 51.63 | 1.67 | 0.96 | 0.25 | 8.29 | 1.79 | 0.87 | 25.53 | 3.97 |
| F4 | 268.93 | 255.33 | 2.54 | 11.06 | 7.48 | 57.24 | 1.86 | 0.94 | 0.32 | 11.09 | 1.64 | 0.75 | 25.64 | 4.56 |
| F5 | 395.42 | 378.83 | 2.56 | 14.03 | 10.91 | 71.68 | 1.65 | 1.01 | 0.30 | 11.13 | 1.64 | 0.68 | 24.74 | 3.46 |
| F6 | 431.49 | 412.01 | 3.16 | 16.32 | 11.47 | 73.28 | 1.81 | 0.96 | 0.31 | 11.90 | 1.72 | 0.81 | 24.63 | 4.17 |
| F7 | 777.27 | 740.75 | 9.28 | 27.24 | 14.13 | 48.88 | 2.03 | 0.96 | 0.31 | 16.27 | 1.18 | 0.75 | 25.98 | 5.27 |
| F8 | 471.31 | 446.35 | 7.61 | 17.35 | 9.05 | 31.56 | 1.59 | 1.09 | 0.38 | 20.32 | 1.06 | 0.98 | 28.48 | 5.75 |
| F20 | 758.72 | 688.43 | 15.25 | 55.04 | 9.52 | 20.81 | 1.39 | 1.01 | 0.16 | 5.65 | 1.60 | 0.86 | 26.82 | 4.94 |
| F21 | 169.05 | 156.07 | 3.22 | 9.76 | 4.22 | 22.06 | 1.49 | 1.02 | 0.42 | 7.77 | 1.09 | 0.80 | 27.45 | 5.14 |
| F22 | 284.66 | 256.22 | 4.92 | 23.52 | 5.60 | 16.77 | 0.85 | 1.45 | 0.79 | 3.46 | 1.84 | 0.90 | 25 | 4.33 |
| Mud | 810.73 | 764.39 | 13.79 | 32.55 | 11.60 | 31.20 | 1.912 | 0.97 | 0.31 | 14.98 | 0.96 | 0.76 | 28.82 | 5.79 |
| Trachyte | 565.66 | 507.3 | 12.47 | 45.89 | 11.72 | 21.45 | 1.64 | 0.84 | 0.38 | 5.40 | 1.67 | 0.87 | 32.17 | 6.73 |
| Trachyte pumice | 751.2 | 681.95 | 15.29 | 53.96 | 12.39 | 19.04 | 1.34 | 0.99 | 0.11 | 5.99 | 1.57 | 0.96 | 43.92 | 8.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobos, I.; Gomes, C. Mineralogy and Geochemistry (HFSE and REE) of the Present-Day Acid-Sulfate Types Alteration from the Active Hydrothermal System of Furnas Volcano, São Miguel Island, The Azores Archipelago. Minerals 2021, 11, 335. https://doi.org/10.3390/min11040335
Bobos I, Gomes C. Mineralogy and Geochemistry (HFSE and REE) of the Present-Day Acid-Sulfate Types Alteration from the Active Hydrothermal System of Furnas Volcano, São Miguel Island, The Azores Archipelago. Minerals. 2021; 11(4):335. https://doi.org/10.3390/min11040335
Chicago/Turabian StyleBobos, Iuliu, and Celso Gomes. 2021. "Mineralogy and Geochemistry (HFSE and REE) of the Present-Day Acid-Sulfate Types Alteration from the Active Hydrothermal System of Furnas Volcano, São Miguel Island, The Azores Archipelago" Minerals 11, no. 4: 335. https://doi.org/10.3390/min11040335
APA StyleBobos, I., & Gomes, C. (2021). Mineralogy and Geochemistry (HFSE and REE) of the Present-Day Acid-Sulfate Types Alteration from the Active Hydrothermal System of Furnas Volcano, São Miguel Island, The Azores Archipelago. Minerals, 11(4), 335. https://doi.org/10.3390/min11040335






