Gradual Replacement of Ca2+ with Mg2+ Ions in Brushite for the Production of Ca1−xMgxHPO4·nH2O Materials
Abstract
1. Introduction
2. Experimental Methodology
2.1. Materials
2.2. Synthesis of Ca1−xMgxHPO4·nH2O
2.3. Characterization Techniques
3. Results and Discussion
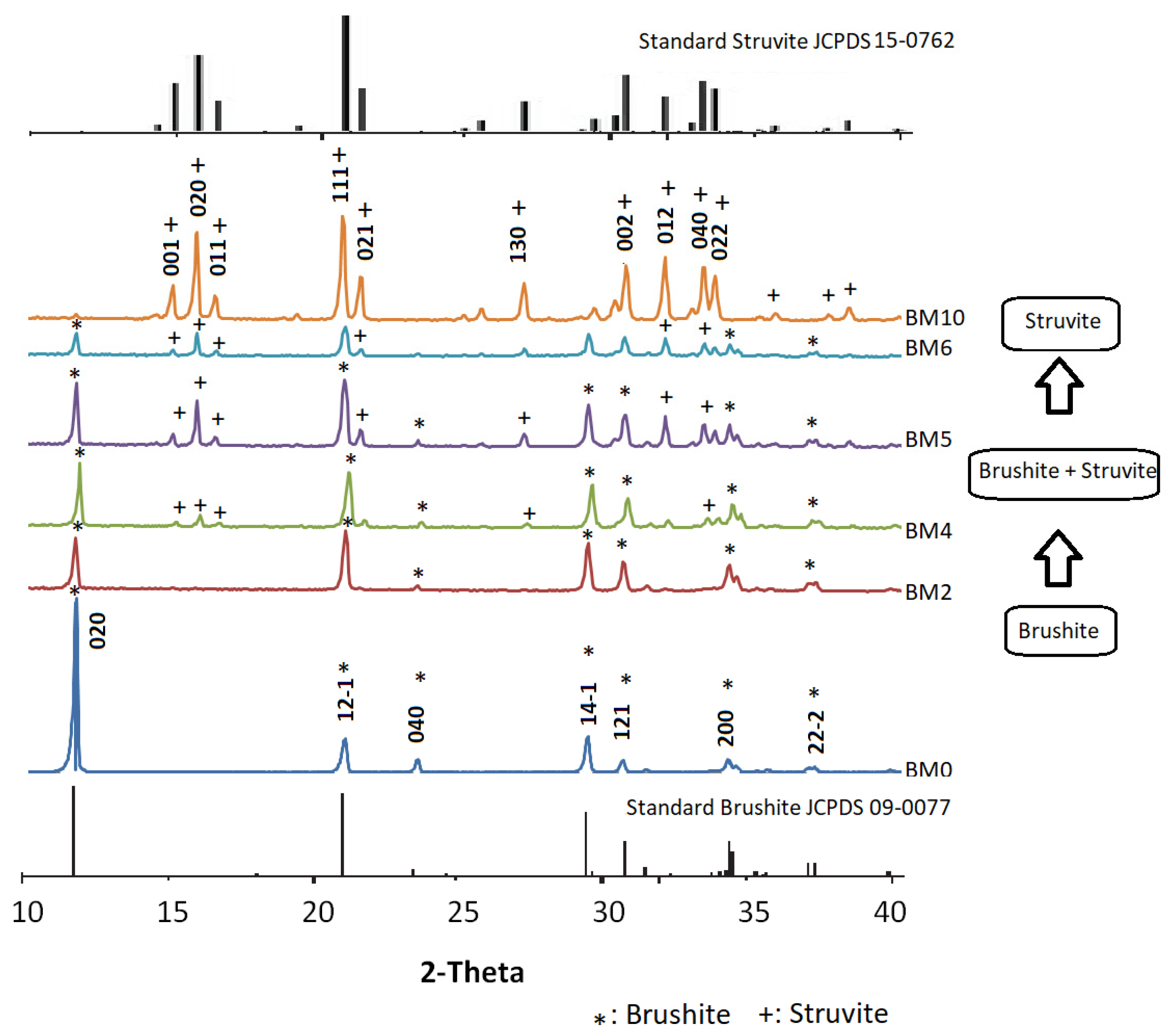
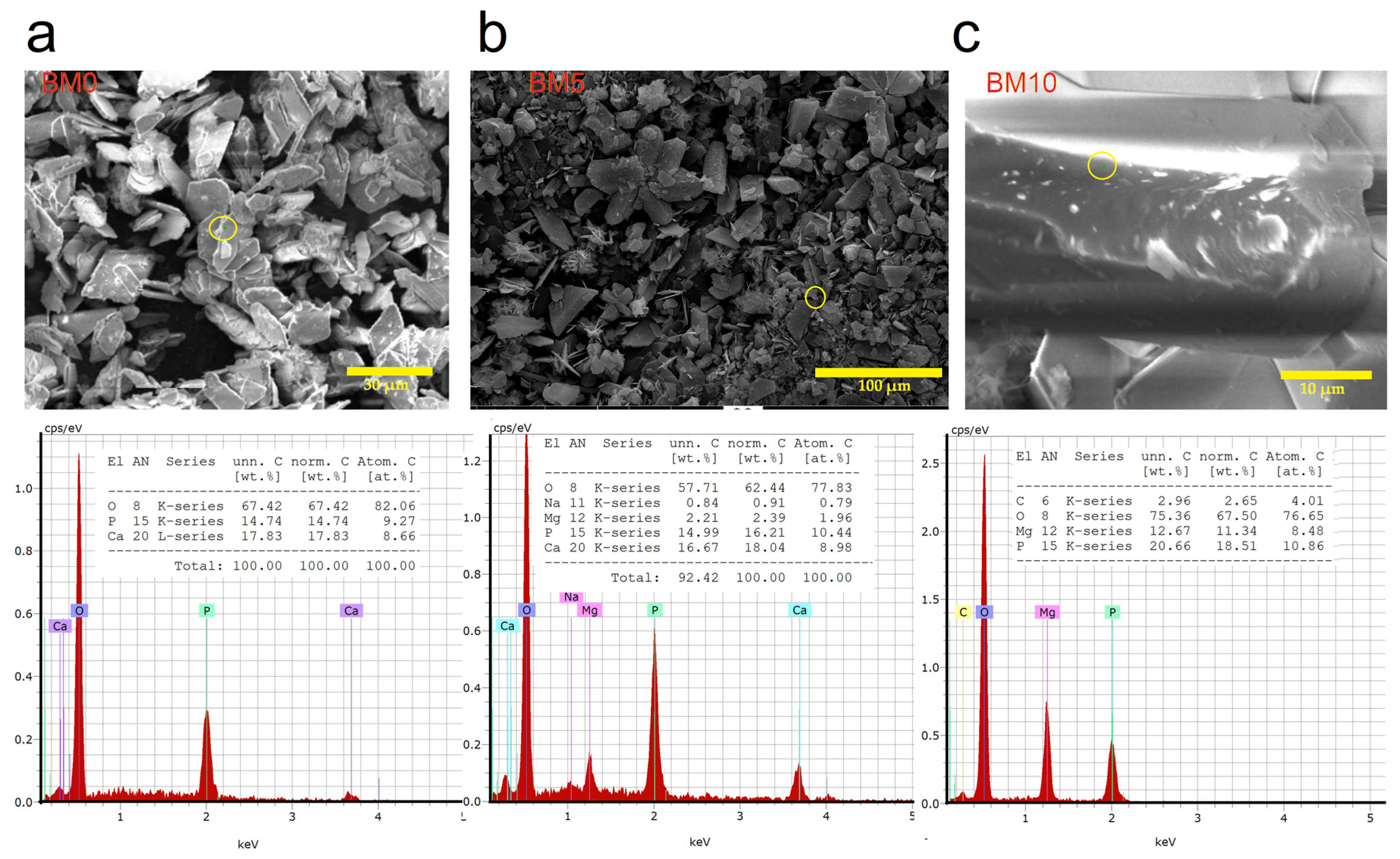
3.1. Mineralogical and Microstructural Analysis
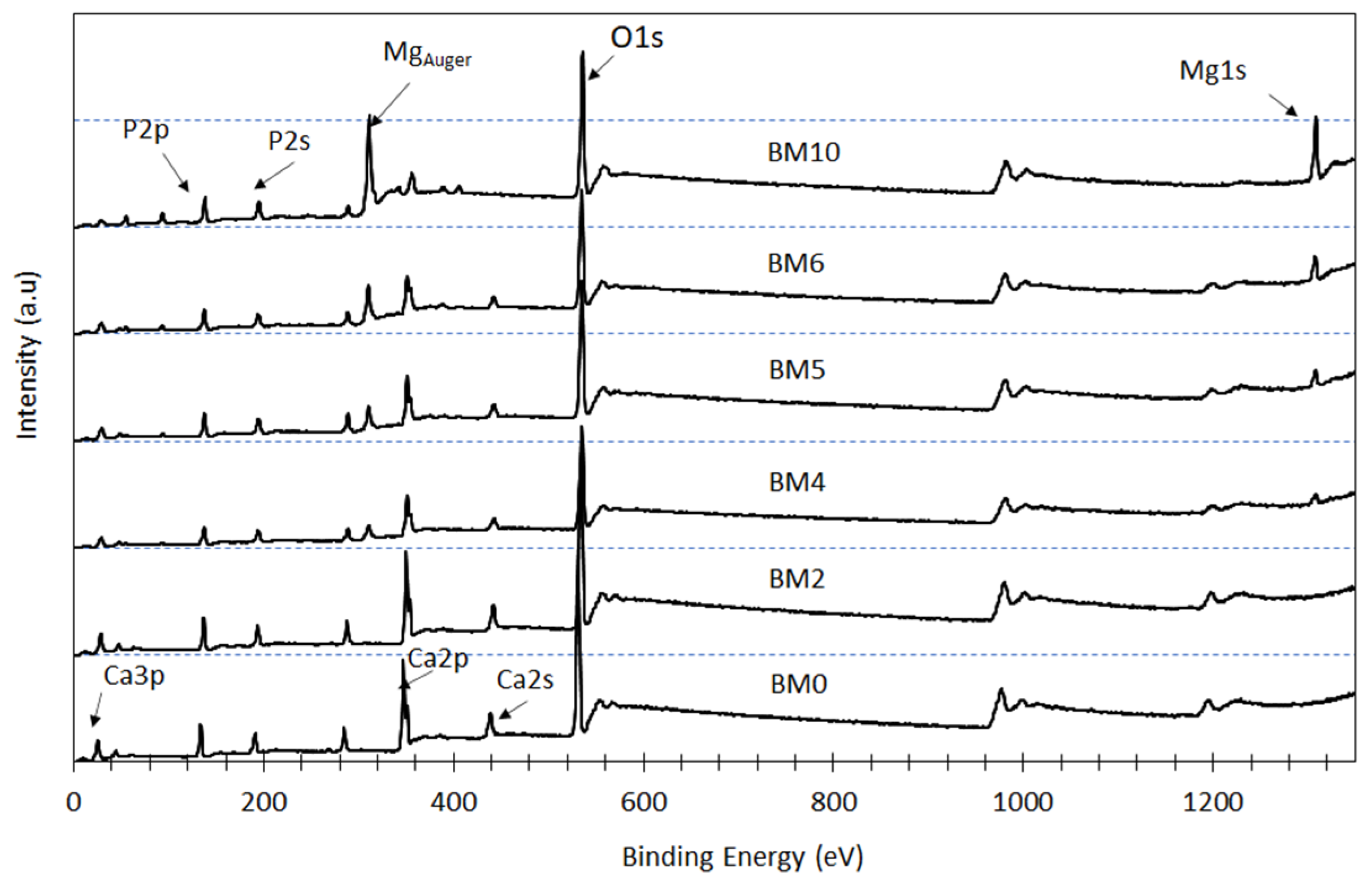
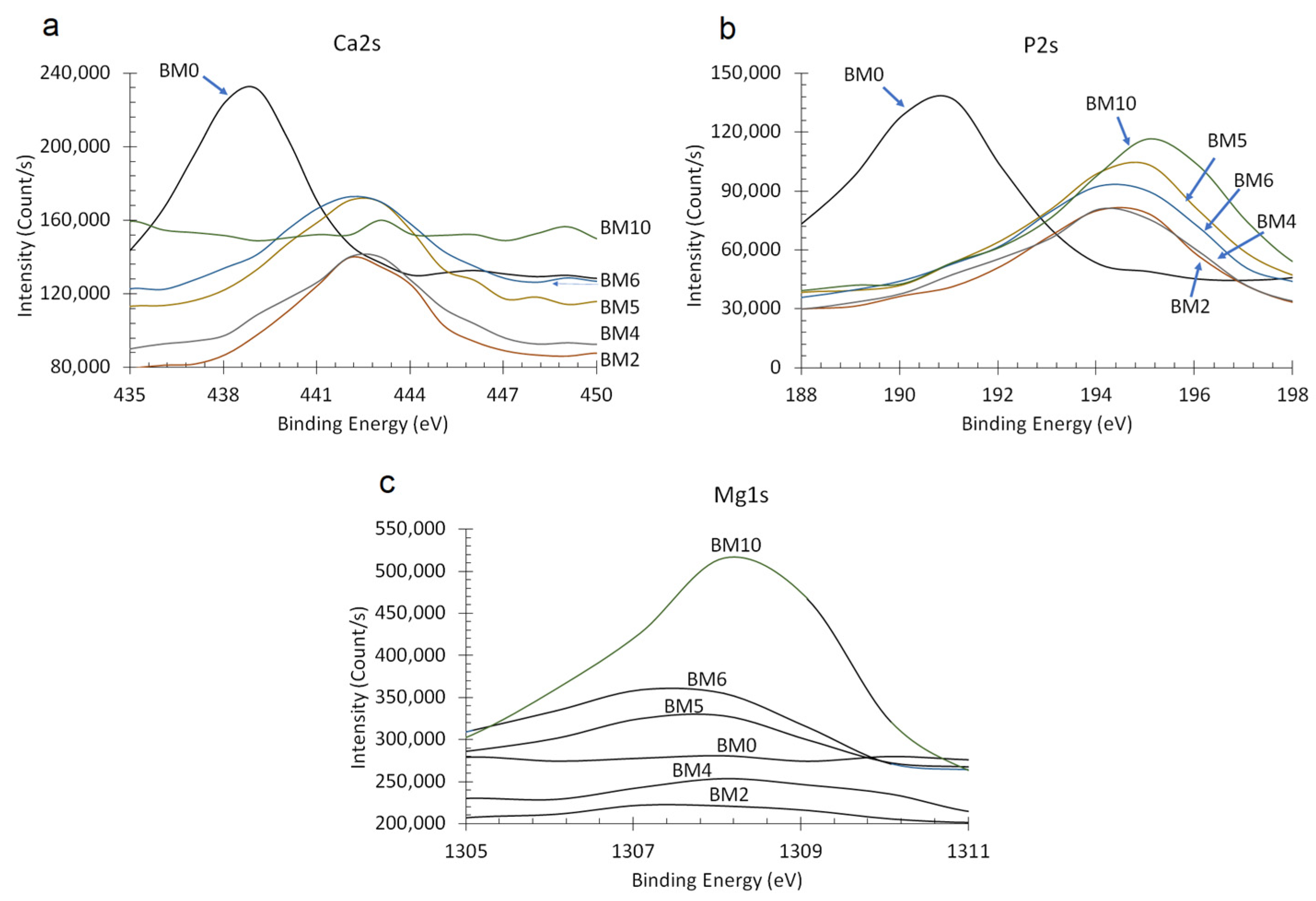
3.2. Elemental and Chemical Composition of Ca1−xMgx·HPO4·nH2O Compounds
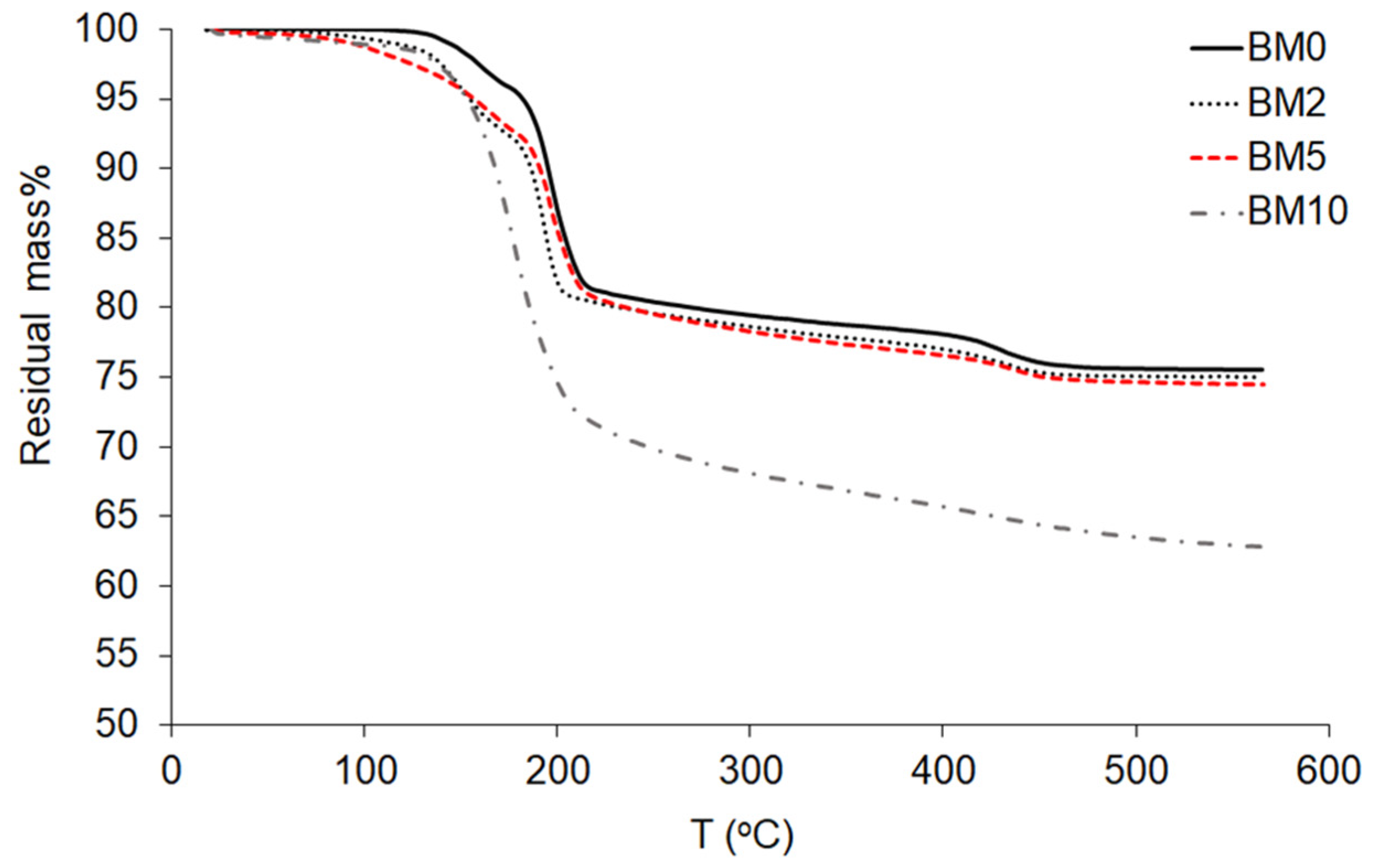
3.3. Thermo-Gravimetric Analysis (TGA)
3.4. Phase Evolution during the Precipitation of Ca1−xMgxHPO4·nH2O Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshaaer, M.; Kailani, M.H.; Ababneh, N.; Abu Mallouh, S.A.; Sweileh, B.; Awidi, A. Fabrication of porous bioceramics for bone tissue applications using luffa cylindrical fibres (LCF) as template. Process. Appl. Ceram. 2017, 11, 13–20. [Google Scholar] [CrossRef]
- Radwan, N.H.; Nasr, M.; Ishak, R.A.; Abdeltawa, N.F.; Awad, G.A. Chitosan-calcium phosphate composite scaffolds for control of postoperative osteomyelitis: Fabrication, characterization, and in vitro–in vivo evaluation. Carbohyd. Polym. 2020, 244, 116482. [Google Scholar] [CrossRef] [PubMed]
- Alshaaer, M.; Kailani, M.H.; Jafar, H.; Ababneh, N.; Awidi, A. Physicochemical and Microstructural Characterization of Injectable Load-Bearing Calcium Phosphate Scaffold. Adv. Mater. Sci. Eng. 2013, 149261. [Google Scholar] [CrossRef]
- Hurle, K.; Oliveira, J.; Reis, R.; Pina, S.; Goetz-Neunhoeffer, F. Ion-doped Brushite Cements for Bone Regeneration. Acta Biomater. 2021. [Google Scholar] [CrossRef]
- Khalifehzadeh, R.; Arami, H. Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Colloid Interface Sci. 2020, 279, 102157. [Google Scholar] [CrossRef]
- Shyong, Y.; Chang, K.; Lin, F. Calcium phosphate particles stimulate exosome secretion from phagocytes for the enhancement of drug delivery. Colloid Surf. B 2018, 1711, 391–397. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, R.; Li, D.; Qi, C.; Han, L.; Chen, M.; Fu, F.; Yuan, J.; Li, G. Effects of calcium magnesium phosphate fertilizer, biochar and spent mushroom substrate on compost maturity and gaseous emissions during pig manure composting. J. Environ. Manag. 2020, 267, 110649. [Google Scholar] [CrossRef]
- Alshaaer, M.; Cuypers, H.; Mosselmans, G.; Rahier, H.; Wastiels, J. Evaluation of a low temperature hardening Inorganic Phosphate Cement for high-temperature applications. Cement Concrete Res. 2011, 41, 38–45. [Google Scholar] [CrossRef]
- Moukannaa, S.; Nazari, A.; Bagheri, A.; Loutou, M.; Sanjayan, J.G.; Hakkou, R. Alkaline fused phosphate mine tailings for geopolymer mortar synthesis: Thermal stability, mechanical and microstructural properties. J. Non Cryst. Solids 2019, 511, 76–85. [Google Scholar] [CrossRef]
- Kinnunen, P.; Ismailov, A.; Solismaa, S.; Sreenivasan, H.; Räisänen, M.-L.; Levänen, E.; Illikainen, M. Recycling mine tailings in chemically bonded ceramics—A review. J. Clean. Prod. 2018, 174, 634–649. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Rueda, C.; Cabarcos, E.L. Strontium Ions Substitution in Brushite Crystals: The Role of Strontium Chloride. J. Funct. Biomater. 2011, 2, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, Z.; Sun, A.; Huang, J.; Wu, W.; Chen, M.; Hao, X.; Huang, Z.; Lin, X.; Weng, S. Rapid construction of polyetheretherketone (PEEK) biological implants incorporated with brushite (CaHPO4·2H2O) and antibiotics for anti-infection and enhanced osseointegration. Mater. Sci. Eng. C Mater. 2020, 111. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.Y.; Roh, Y.; Lee, J.; Kim, J.; Lee, Y.; Bang, J.; Lee, Y.J. Optimizing Calcium Phosphates by the Control of pH and Temperature via Wet Precipitation. J. Nanosci. Nanotechnol. 2015, 15, 10008–10016. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Engqvist, H.; Persson, C. A ready-to-use acidic, brushite-forming calcium phosphate cement. Acta Biomater. 2018, 81, 304–314. [Google Scholar] [CrossRef]
- Mert, I.; Mandel, S.; Tas, A.C. Do cell culture solutions transform brushite (CaHP04.2H20) to octacalium phosphate (Ca8(HP04)2(P04)4 5H20). In Advances in Bioceramics and Porous Ceramics IV; Narayan, R., Colombo, P., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; pp. 79–94. [Google Scholar] [CrossRef]
- Patil, S.B.; Jena, A.; Bhargava, P. Influence of Ethanol Amount during Washing on Deagglomeration of Co-Precipitated Calcined Nanocrystalline 3YSZ Powders. Int. J. Appl. Ceram. Technol. 2013, 10, E247–E257. [Google Scholar] [CrossRef]
- Piva, R.H.; Piva, D.H.; Pierri, J.; Montedo, O.R.K.; Morelli, M.R. Azeotropic distillation, ethanol washing, and freeze drying on coprecipitated gels for production of high surface area 3Y–TZP and 8YSZ powders: A comparative study. Ceram. Int. 2015, 41, 14148–14156. [Google Scholar] [CrossRef]
- Mirtchi, A.A.; Lemaitre, J.; Terao, N. Calcium phosphate cements: Study of the β-tricalcium phosphate—monocalcium phosphate system. Biomaterials 1989, 10, 475–480. [Google Scholar] [CrossRef]
- Pina, S.; Olhero, S.; Gheduzzi, S.; Miles, A.; Ferreira, J. Influence of setting liquid composition and liquid-to-powder ratio on properties of a Mg-substituted calcium phosphate cement. Acta Biomater. 2009, 5, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Lagier, R.; Baud, C.A. Magnesium Whitlockite, a Calcium Phosphate Crystal of Special Interest in Pathology. Pathol. Res. Pract. 2003, 199, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Ferreira, J.M.F. Ionic Substitutions in Biphasic Hydroxyapatite and β-Tricalcium Phosphate Mixtures: Structural Analysis by Rietveld Refinement. J. Am. Ceram. Soc. 2008, 91, 1–12. [Google Scholar] [CrossRef]
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Rebelo, A.H.S.; Valério, P.; Ferreira, J.M.F. Rietveld structure and in vitro analysis on the influence of magnesium in biphasic (hydroxyapatite and β-tricalcium phosphate) mixtures. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 404–411. [Google Scholar] [CrossRef]
- Kannan, S.; Lemos, A.F.; Rocha, J.H.G.; Ferreira, J.M.F. Characterization and Mechanical Performance of the Mg-Stabilized β-Ca3(PO4)2 Prepared from Mg-Substituted Ca-Deficient Apatite. J. Am. Ceram. Soc. 2006, 89, 2757–2761. [Google Scholar] [CrossRef]
- Kannan, S.; Ferreira, J.M.F. Synthesis and Thermal Stability of Hydroxyapatite−β-Tricalcium Phosphate Composites with Cosubstituted Sodium, Magnesium, and Fluorine. Chem. Mater. 2006, 18, 198–203. [Google Scholar] [CrossRef]
- Torres, P.M.C.; Abrantes, J.C.C.; Kaushal, A.; Pina, S.; Döbelin, N.; Bohner, M.; Ferreira, J.M.F. Influence of Mg-doping, calcium pyrophosphate impurities and cooling rate on the allotropic α ↔ β—tricalcium phosphate phasetransformations. J. Eur. Ceram. Soc. 2016, 36, 817–827. [Google Scholar] [CrossRef]
- Sayahi, M.; Santos, J.; El-Feki, H.; Charvillat, C.; Bosc, F.; Karacan, I.; Milthorpe, B.; Drouet, C. Brushite (Ca,M)HPO4·2H2O doping with bioactive ions (M = Mg2+, Sr2+, Zn2+, Cu2+, and Ag+): A new path to functional biomaterials? Mater. Today Chem. 2020, 16, 100230. [Google Scholar] [CrossRef]
- Wu, F.; Wei, J.; Guo, H.; Chen, F.; Hong, H.; Liu, C. Self-setting bioactive calcium-magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008, 4, 1873–1884. [Google Scholar] [CrossRef]
- Qi, T.; Weng, J.; Yu, F.; Zhang, W.; Li, G.; Qin, H.; Tan, Z.; Zeng, H. Insights into the Role of Magnesium Ions in Affecting Osteogenic Differentiation of Mesenchymal Stem Cells. Biol. Trace Elem. Res. 2021, 199, 559–567. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, J.; Tian, Y.; Huang, B.; Yuan, Y.; Liu, C. Magnesium modification up-regulates the bioactivity of bone morphogenetic protein-2 upon calcium phosphate cement via enhanced BMP receptor recognition and Smad signaling pathway. Colloids Surf. B Biointerfaces 2016, 145, 140–151. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Kolmas, J.; Przekora, A. Biological Response to Macroporous Chitosan-Agarose Bone Scaffolds Comprising Mg- and Zn-Doped Nano-Hydroxyapatite. Int. J. Mol. Sci. 2019, 20, 3835. [Google Scholar] [CrossRef]
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Pina, S.; Torres, P.M.C.; Ferreira, J.M.F. Synthesis and structural characterization of strontium—and magnesium-co-substituted b-tricalcium phosphate. Acta Biomater. 2010, 6, 571–576. [Google Scholar] [CrossRef]
- Smoak, M.M.; Mikos, A.G. Advances in biomaterials for skeletal muscle engineering and obstacles still to overcome. Mater. Today Bio 2020, 7, 100069. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Bassous, N.; Harb, S.; Palo-Nieto, C.; Ruiz-Esparza, G.U.; Marciano, F.R.; Webster, T.J.; Furtado, A.S.A.; Lobo, A.O. Advances in dual functional antimicrobial and osteoinductive biomaterials for orthopaedic applications. Nanomedicine 2020, 24, 102143. [Google Scholar] [CrossRef]
- Liu, T.; Xu, J.; Pan, X.; Ding, Z.; Xie, H.; Wang, X.; Xie, H. Advances of adipose-derived mesenchymal stem cells-based biomaterial scaffolds for oral and maxillofacial tissue engineering. Bioact. Mater. 2021, 6, 2467–2478. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.-Q.; Willhammar, T.; Sun, B.; Hedin, N.; Gale, J.D.; Gebauer, D. Introducing the crystalline phase of dicalcium phosphate monohydrate. Nat. Commun. 2020, 11, 1546. [Google Scholar] [CrossRef] [PubMed]
- Cheary, R.W.; Coelho, A.A. A Fundamental Parameters Approach of X-Ray Line-Profile Fitting. J. Appl. Cryst. 1992, 25, 109–121. [Google Scholar] [CrossRef]
- Nosrati, H.; Le, D.Q.S.; Emameh, R.Z.; Perez, M.C.; Bünger, C.E. Nucleation and growth of brushite crystals on the graphene sheets applicable in bone cement. Bol. Soc. Esp. Cerám. Vidr. 2020. [Google Scholar] [CrossRef]
- Li, H.; Yao, Q.-Z.; Wang, Y.-Y.; Li, Y.-L.; Zhou, G.-T. Biomimetic synthesis of struvite with biogenic morphology and implication for pathological biomineralization. Sci. Rep. 2015, 5, 7718. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Martinelli, L.; Tasso, F.; Sprocati, A.R.; Pinzari, F.; Liu, Z.; Downs, R.T.; Sun, H.J. A new biogenic, struvite-related phosphate, the ammonium-analog of hazenite, (NH4)NaMg2(PO4)2·14H2O. Am. Mineral. 2014, 99, 1761–1766. [Google Scholar] [CrossRef]
- Tansel, T.; Lunn, G.; Monje, O. Struvite formation and decomposition characteristics for ammonia and phosphorus recovery: A review of magnesium-ammonia-phosphate interactions. Chemosphere 2018, 194, 504–514. [Google Scholar] [CrossRef]
- Schuiling, R.D.; Andrade, A. Recovery of struvite from calf manure. Environ. Technol. 1999, 20, 765–768. [Google Scholar] [CrossRef]
- Meira, R.C.S.; Paz, S.P.A.; Corrêa, J.A.M. XRD-Rietveld analysis as a tool for monitoring struvite analog precipitation from wastewater: P, Mg, N and K recovery for fertilizer production. J. Mater. Res. Technol. 2020, 9, 15202–15213. [Google Scholar] [CrossRef]
- Yan, H.; Shih, K. Effects of calcium and ferric ions on struvite precipitation: A new assessment based on quantitative X-ray diffraction analysis. Water Res. 2016, 95, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Alshaaer, M. Microstructural characteristics and long-term stability of wollastonite-based chemically bonded phosphate ceramics. Int. J. Appl. Ceram. Technol. 2021, 18, 319–331. [Google Scholar] [CrossRef]
- Kumar, M.; Xie, J.; Chittur, K.; Riley, C. Transformation of modified brushite to hydroxyapatite in aqueous solution: Effects of potassium substitution. Biomaterials 1999, 20, 1389–1399. [Google Scholar] [CrossRef]
- Tamimi, F.; Le Nihouannen, D.; Eimar, H.; Sheikh, Z.; Komarova, S.; Barralet, J. The effect of autoclaving on the physical and biological properties of dicalcium phosphate dihydrate bioceramics: Brushite vs. monetite. Acta Biomater. 2012, 8, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Thant Zin, M.M.; Kim, D.-J. Simultaneous recovery of phosphorus and nitrogen from sewage sludge ash and food wastewater as struvite by Mg-biochar. J. Hazard. Mater. 2021, 403, 123704. [Google Scholar] [CrossRef] [PubMed]
- Yuantao, L.; Bing, C.; Zhaohui, Q.; Dong, P.; Aminul Haque, M. Experimental research on properties and microstructures of magnesium-iron phosphate cement. Constr. Build. Mater. 2020, 257. [Google Scholar] [CrossRef]
- Qoku, E.; Scheibel, M.; Bier, T.; Gerz, A. Phase development of different magnesium phosphate cements at room temperature and elevated temperatures. Constr. Build. Mater. 2021, 272, 121654. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yoo, B.-H.; Lim, S.J.; Kim, T.-H.; Kim, S.-K.; Kim, J.Y. Development and validation of an equilibrium model for struvite formation with calcium co-precipitation. J. Cryst. Growth 2013, 372, 129–137. [Google Scholar] [CrossRef]
- Kannan, S.; Ventura, J.M.G.; Lemos, A.F.; Barba, A.; Ferreira, J.M.F. Effect of sodium addition on the preparation of hydroxyapatites and biphasic ceramics. Ceram. Int. 2008, 34, 7–13. [Google Scholar] [CrossRef]
- El-dek, S.I.; Mansour, S.F.; Ahmed, M.A.; Ahmed, M.K. Microstructural features of flower like Fe brushite. Prog. Nat. Sci. 2017, 27, 520–526. [Google Scholar] [CrossRef]
- El Hazzat, M.; El Hamidi, A.; Halim, M.; Arsalane, S. Complex evolution of phase during the thermal investigation of Brushite-type calcium phosphate CaHPO4·2H2O. Materialia 2021. [Google Scholar] [CrossRef]
- Perwitasari, D.S.; Muryanto, S.; Jamari, J.; Bayuseno, A.P. Kinetics and morphology analysis of struvite precipitated from aqueous solution under the influence of heavy metals: Cu2+, Pb2+, Zn2+. J. Environ. Chem. Eng. 2018, 6, 37–43. [Google Scholar] [CrossRef]
- Karbakhshravari, M.; Abeysiriwardana-Arachchige, I.S.A.; Henkanatte-Gedera, S.M.; Cheng, F.; Papelis, C.; Brewer, C.E.; Nirmalakhandan, N. Recovery of struvite from hydrothermally processed algal biomass cultivated in urban wastewaters. Resour. Conserv. Recy. 2020, 163, 105089. [Google Scholar] [CrossRef]
- Bayuseno, A.P.; Perwitasari, D.A.; Muryanto, S.; Tauviqirrahman, M.; Jamari, J. Kinetics and morphological characteristics of struvite (MgNH4PO4·6H2O) under the influence of maleic acid. Heliyon 2020, 6, 03533. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lia, J.; Li, B.; Zhang, D.; Zhao, N.; Tang, S. Comparison of different K-struvite crystallization processes for simultaneous potassium and phosphate recovery from source-separated urine. Sci. Total Environ. 2019, 651, 787–795. [Google Scholar] [CrossRef]
- Alshaaer, M.; Cuypers, H.; Rahier, H.; Wastiels, J. Production of monetite-based Inorganic Phosphate Cement (M-IPC) using hydrothermal post curing (HTPC). Cem. Concr. Res. 2011, 41, 30–37. [Google Scholar] [CrossRef]
- Gashti, M.P.; Stir, M.; Jurg, H. Growth of strontium hydrogen phosphate/gelatin composites: A biomimetic approach. New J. Chem. 2016, 40, 5495–5500. [Google Scholar] [CrossRef]
- Tortet, L.; Gavarri, J.R.; Nihoul, G. Study of Protonic Mobility in CaHPO4 2H2O (Brushite) and CaHPO4 (Monetite) by Infrared Spectroscopy and Neutron Scattering. J. Solid State Chem. 1997, 132, 6–16. [Google Scholar] [CrossRef]
- Dosen, A.; Giese, R.F. Thermal decomposition of brushite, CaHPO4·2H2O to monetite CaHPO4 and the formation of an amorphous phase. Am. Mineral. 2011, 96, 368–373. [Google Scholar] [CrossRef]
- Kannan, S.; Lemos, I.A.F.; Rocha, J.H.G.; Ferreira, J.M.F. Synthesis and characterization of magnesium substituted biphasic mixtures of controlled hydroxyapatite/b-tricalcium phosphate ratios. J. Solid State Chem. 2005, 178, 3190–3196. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J. Thermal stability of the ‘cave’ mineral brushite CaHPO4·2H2O—Mechanism of formation and decomposition. Thermochim. Acta 2011, 521, 14–17. [Google Scholar] [CrossRef]
- Hövelmann, J.; Stawski, T.M.; Besselink, R.; Freeman, H.M.; Dietmann, K.M.; Mayanna, S.; Pauw, B.R.; Benning, L.G. A template-free and low temperature method for the synthesis of mesoporous magnesium phosphate with uniform pore structure and high surface area. Nanoscale 2019, 11, 6939–6951. [Google Scholar] [CrossRef] [PubMed]
- Prywer, J.; Sieroń, L.; Czylkowska, A. Struvite Grown in Gel, Its Crystal Structure at 90 K and Thermoanalytical Study. Crystals 2019, 9, 89. [Google Scholar] [CrossRef]
- Wang, C.; Hao, X. Small-Scale Formation of Struvite by Electrochemical Deposition and Its Characterization. In Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; pp. 1–4. [Google Scholar] [CrossRef]
- Alshaaer, M.; Abdel-Fattah, E.; Saadeddin, I.; Al Battah, F.; Issa, K.I.; Saffarini, G. The effect of natural fibres template on the chemical and structural properties of Biphasic Calcium Phosphate scaffold. Mater. Res. Express 2020, 7, 065405. [Google Scholar] [CrossRef]
- Alshaaer, M.; Afify, A.S.; Moustapha, M.E.; Hamad, N.; Hammouda, G.A.; Rocha, F. Effect of full-scale substitution of strontium for calcium on the microstructure of brushite: (CaxSr1-x)HPO4·nH2O System. Clay Miner. 2021. [Google Scholar] [CrossRef]
- Ding, H.; Pan, H.; Xu, X.; Tang, R. Toward a Detailed Understanding of Magnesium Ions on Hydroxyapatite Crystallization Inhibition. Cryst. Growth Des. 2014, 14, 763–769. [Google Scholar] [CrossRef]
- Nielsen, M.R.; Sand, K.K.; Rodriguez-Blanco, J.D.; Bovet, N.; Generosi, J.; Dalby, K.N.; Stipp, S.L.S. Inhibition of Calcite Growth: Combined Effects of Mg2+ and SO42–. Cryst. Growth Des. 2016, 16, 6199–6207. [Google Scholar] [CrossRef]
- Muhammed, M.; Mujeeb, R.; Surya, R.D.; Rekha, P. Investigation on growth and morphology of in vitro generated struvite crystals. Biocatal. Agric. Biotechnol. 2019, 17, 566–570. [Google Scholar] [CrossRef]



| Product ID | NaH2PO4·2H2O | Ca(NO3)2·4H2O | Mg(NO3)2·6H2O | Mg/Ca Molar Ratio |
|---|---|---|---|---|
| BM0 | 1 | 1 | 0 | 0 |
| BM2 | 1 | 0.8 | 0.2 | 0.25 |
| BM4 | 1 | 0.6 | 0.4 | 0.67 |
| BM5 | 1 | 0.5 | 0.5 | 1.0 |
| BM6 | 1 | 0.4 | 0.6 | 1.5 |
| BM10 | 1 | 0 | 1 | - |
| Product ID | Brushite wt% | a (Å) | b (Å) | c (Å) | (β°) | V (Å3) |
|---|---|---|---|---|---|---|
| BM0 | 100.0 | 5.8145 | 15.1693 | 6.2399 | 116.392 | 492.83 |
| BM2 | 100.0 | 5.8165 | 15.1904 | 6.2465 | 116.382 | 492.78 |
| BM4 | 100.0 | 5.8191 | 15.1908 | 6.2518 | 116.403 | 492.51 |
| BM5 | 97.1 | 5.8160 | 15.1764 | 6.2482 | 116.429 | 492.01 |
| BM6 | 20.9 | 5.8153 | 15.1677 | 6.2470 | 116.442 | |
| BM10 | 0.0 | - | - | - | - |
| Product ID | Struvite wt% | a (Å) | b (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|---|
| BM0 | 0.0 | - | - | - | - |
| BM2 | 0.0 | - | - | - | - |
| BM4 | 0.0 | - | - | - | - |
| BM5 | 2.9 | 6.9560 | 6.1378 | 11.2041 | - |
| BM6 | 79.1 | 6.9589 | 6.1364 | 11.2034 | 478.12 |
| BM10 | 100.0 | 6.9501 | 6.1359 | 11.2014 | 477.54 |
| Mg/Ca Ratio | Crystal Structure | Crystal Size (µm) | Compounds Formed |
|---|---|---|---|
| ≤0.25 | Plate-like | ~10 | Brushite |
| 0.25 < x ≤ 1.5 | Plate-like orthorhombic | ~2 | Brushite + Struvite |
| >1.5 | Orthorhombic | ~20 | Struvite |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaaer, M.; Issa, K.; Alanazi, A.; Mallouh, S.A.; Afify, A.S.; Moustapha, M.E.; Komnitsas, K. Gradual Replacement of Ca2+ with Mg2+ Ions in Brushite for the Production of Ca1−xMgxHPO4·nH2O Materials. Minerals 2021, 11, 284. https://doi.org/10.3390/min11030284
Alshaaer M, Issa K, Alanazi A, Mallouh SA, Afify AS, Moustapha ME, Komnitsas K. Gradual Replacement of Ca2+ with Mg2+ Ions in Brushite for the Production of Ca1−xMgxHPO4·nH2O Materials. Minerals. 2021; 11(3):284. https://doi.org/10.3390/min11030284
Chicago/Turabian StyleAlshaaer, Mazen, Khalil Issa, Abdulaziz Alanazi, Saida Abu Mallouh, Ahmed S. Afify, Moustapha E. Moustapha, and Kostas Komnitsas. 2021. "Gradual Replacement of Ca2+ with Mg2+ Ions in Brushite for the Production of Ca1−xMgxHPO4·nH2O Materials" Minerals 11, no. 3: 284. https://doi.org/10.3390/min11030284
APA StyleAlshaaer, M., Issa, K., Alanazi, A., Mallouh, S. A., Afify, A. S., Moustapha, M. E., & Komnitsas, K. (2021). Gradual Replacement of Ca2+ with Mg2+ Ions in Brushite for the Production of Ca1−xMgxHPO4·nH2O Materials. Minerals, 11(3), 284. https://doi.org/10.3390/min11030284








