Phase Evolution from Volborthite, Cu3(V2O7)(OH)2·2H2O, upon Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Chemical Composition
2.2.2. Thermal Analysis
2.2.3. In Situ High-Temperature Powder X-ray Diffraction
2.2.4. Ex Situ Powder X-ray Diffraction
2.2.5. Calculations of Crystal-Structure Complexity
3. Results
3.1. Chemical Composition
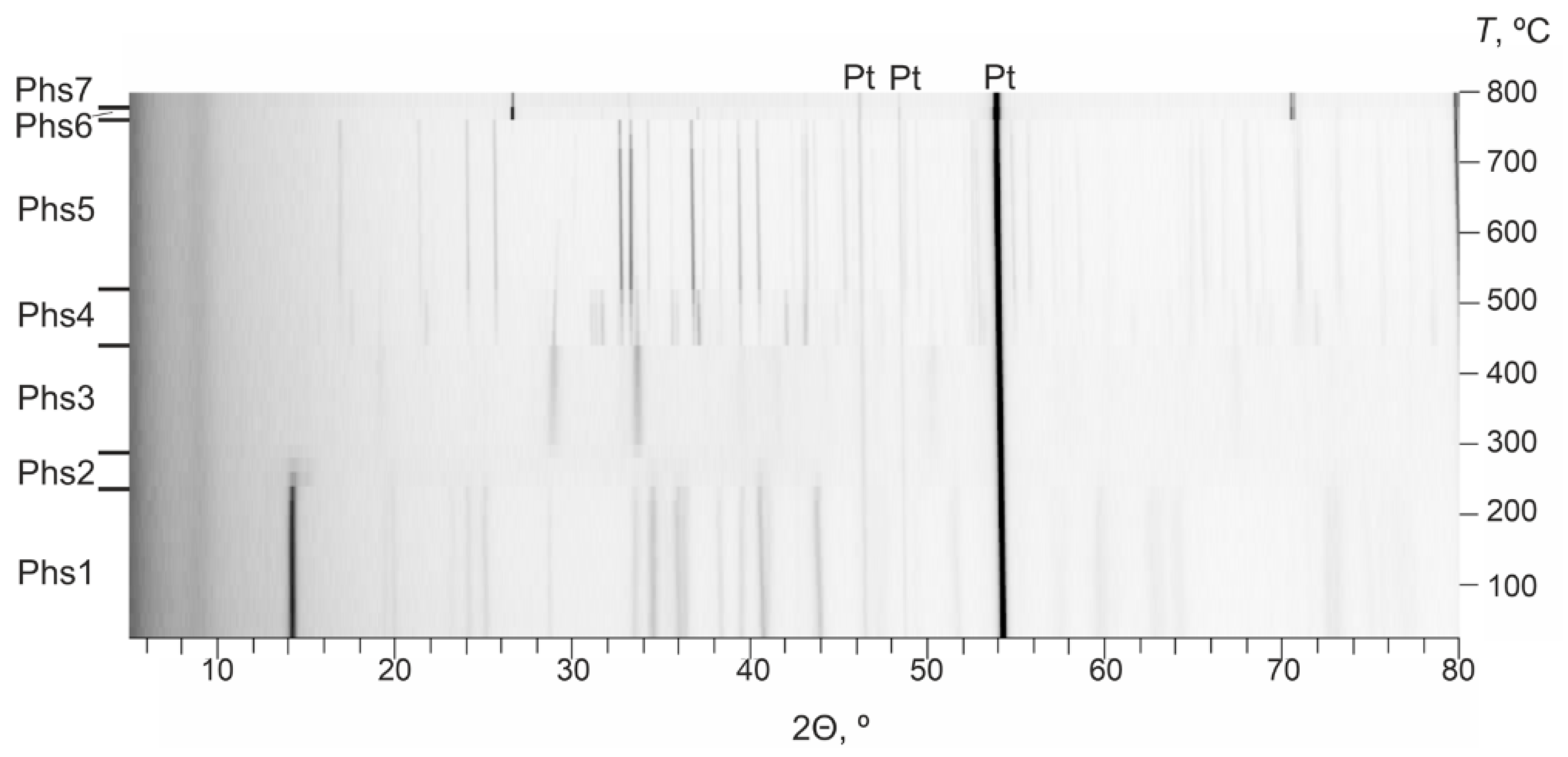
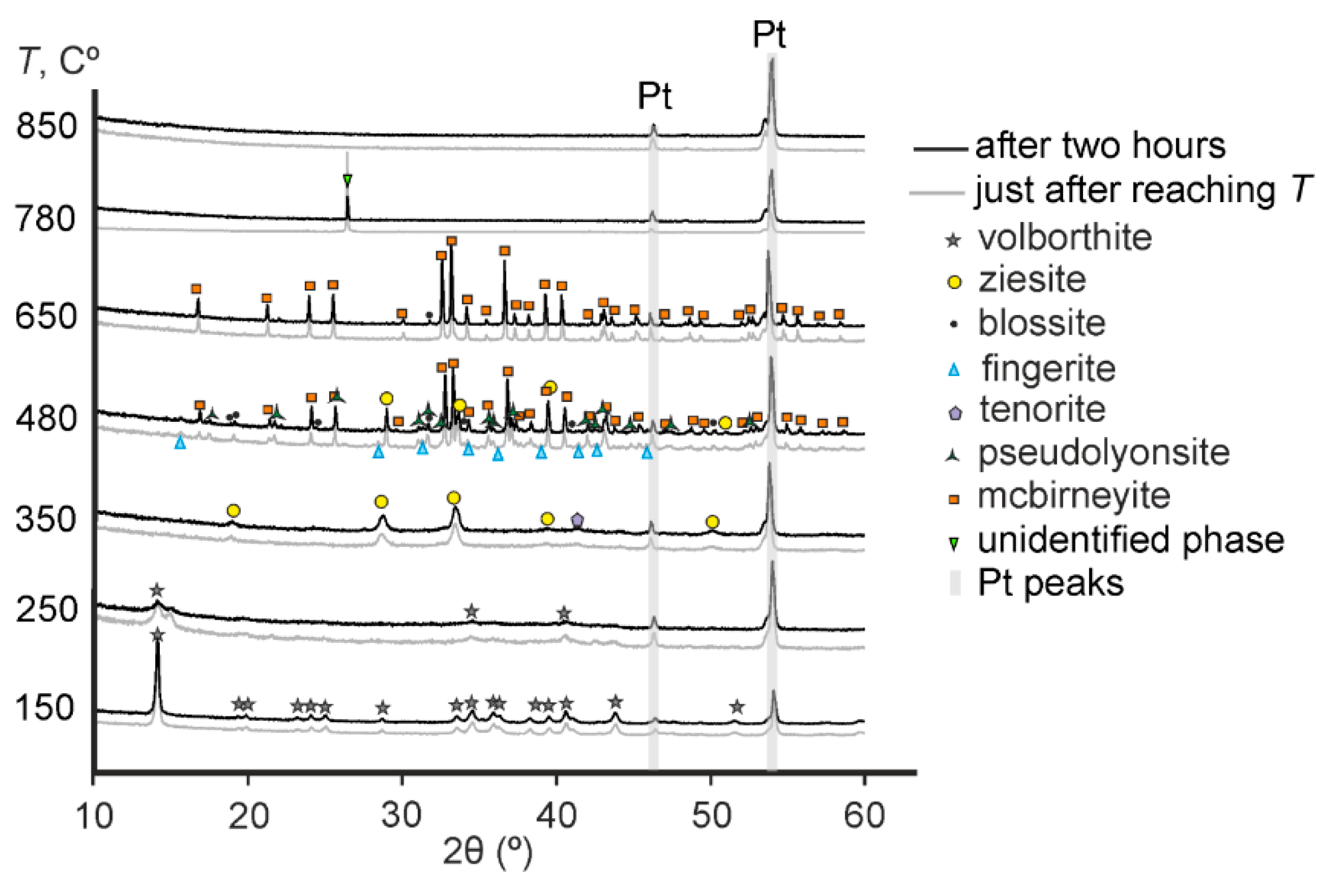
3.2. In Situ High-Temperature Powder X-ray Diffraction
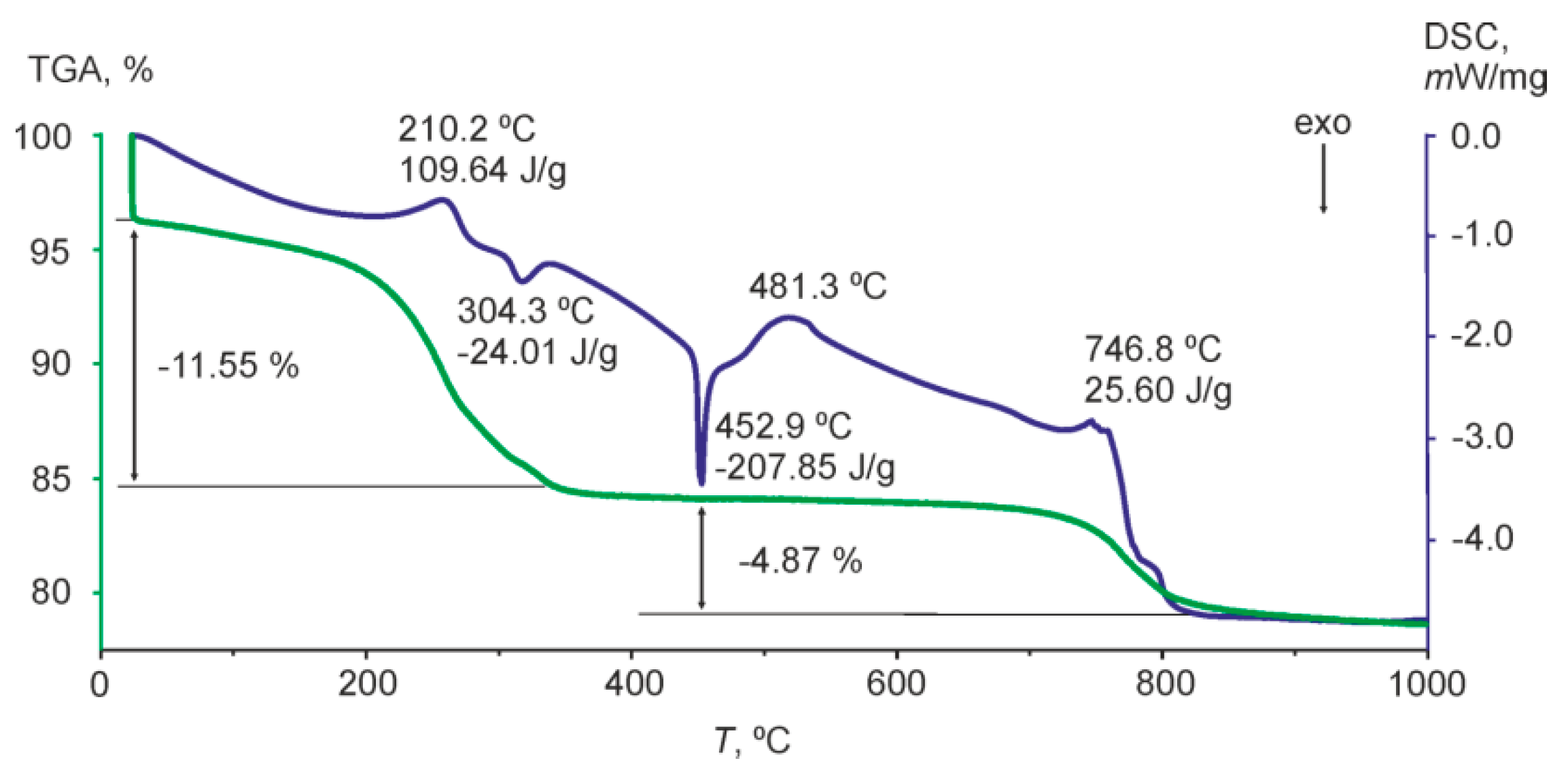
3.3. Thermal Analysis
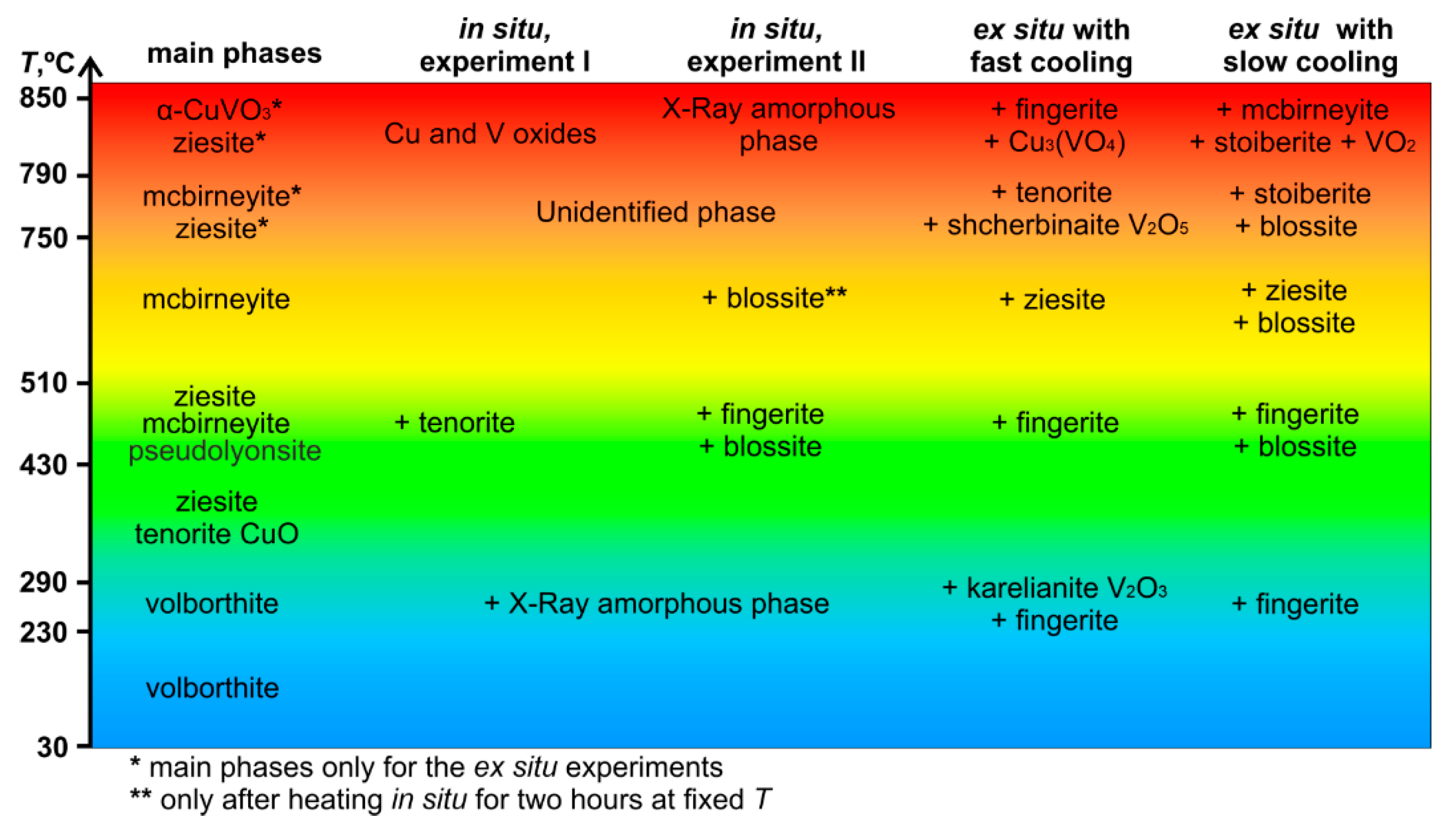
3.4. Phases Obtained by Ex Situ Annealing
3.5. Thermal Expansion Coefficients of Volborthite and Mcbirneyite at Elevated Temperatures
3.6. Crystal Structure Complexity
4. Discussion
4.1. General Trend of High-Temperature Transformation
4.2. The Influence of Heating Strategy to the Phase Composition
4.3. Crystal Structure Complexity of Copper Vanadates
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Volborth, A.; Hess, H. Ueber (das Volborthit), ein neues Vanadhaltiges Mineral. Bull. Académie Impériale Sci. St.-Pétersbourg 1838, 4, 21–23. [Google Scholar]
- Ginga, V.A.; Siidra, O.I.; Ugolkov, V.L.; Bubnova, R.S. Refinement of the Crystal Structure and Features of the Thermal Behavior of Volborthite Cu3V2O7(OH)2⋅2H2O from the Tyuya-Muyun Deposit, Kyrgyzstan. Zapiski Rossiyskogo Mineralogicheskogo Obshchestva 2021, 150, 115–133. (In Russian) [Google Scholar] [CrossRef]
- Hålenius, U.; Hatert, F.; Pasero, M.; Mills, S.J. New minerals and nomenclature modifications approved in 2016. Mineral. Mag. 2016, 80, 1135–1144. [Google Scholar] [CrossRef]
- Nenadkevich, K.A. Turanite and alaite, two new vanadium minerals. Bull. Académie Impériale Sci. St.-Pétersbourg 1909, 3, 185–187. [Google Scholar]
- Zhitova, E.S.; Anikin, L.P.; Sergeeva, A.V.; Ismagilova, R.M.; Rashidov, V.A.; Chubarov, V.M.; Kupchinenko, A.N. Volborthite Occurrence at the Alaid Volcano (Atlasov Island, Kuril Islands, Russia). Zapiski Rossiyskogo Mineralogicheskogo Obshchestva 2020, 149, 78–95. (In Russian) [Google Scholar] [CrossRef]
- Balassone, G.; Petti, C.; Mondillo, N.; Panikorovskii, T.L.; de Gennaro, R.; Cappelletti, P.; Altomare, A.; Corriero, N.; Cangiano, M.; D’Orazio, L. Copper minerals at Vesuvius Volcano (Southern Italy): A mineralogical review. Minerals 2019, 9, 730. [Google Scholar] [CrossRef] [Green Version]
- Pekov, I.V.; Zubkova, N.V.; Yapaskurt, V.O.; Polekhovsky, Y.S.; Britvin, S.N.; Turchkova, A.G.; Sidorov, E.G.; Pushcharovsky, D.Y. Kainotropite, Cu4Fe3+O2(V2O7)(VO4), a new mineral with a complex vanadate anion from fumarolic exhalations of the Tolbachik Volcano, Kamchatka, Russia. Canad. Miner. 2020, 58, 155–165. [Google Scholar] [CrossRef]
- Robinson, P.D.; Hughes, J.M.; Malinconico, M.L. Blossite, α-Cu2+2V5+2O7, a new fumarolic sublimate from Izalco volcano, El Salvador. Am. Mineral. 1987, 72, 397–400. [Google Scholar]
- Hughes, J.M.; Birnie, R.W. Ziesite, ß-Cu2V2O7, a new copper vanadate and fumarole temperature indicator. Am. Mineral. 1980, 65, 1146–1149. [Google Scholar]
- Finger, L.W. Fingerite, Cu11O2(VO4)6, a new vanadium sublimate from Izalco volcano, El Salvador: Crystal structure. Am. Mineral. 1985, 70, 197–199. [Google Scholar]
- Hughes, J.M.; Christian, B.S.; Finger, L.W.; Malinconico, L.L. Mcbirneyite, Cu3(VO4)2, a new sublimate mineral from the fumaroles of Izalco volcano, El Salvador. J. Volcanol. Geotherm. Res. 1987, 33, 183–190. [Google Scholar] [CrossRef]
- Zelenski, M.E.; Zubkova, N.V.; Pekov, I.V.; Boldyreva, M.M.; Pushcharovsky, D.Y.; Nekrasov, A.N. Pseudolyonsite, Cu3(VO4)2, a new mineral species from the Tolbachik volcano, Kamchatka Peninsula, Russia. Eur. J. Mineral. 2011, 23, 475–481. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Yapaskurt, V.O.; Polekhovsky, Y.S.; Vigasina, M.F.; Britvin, S.N.; Turchkova, A.G.; Sidorov, E.G.; Pushcharovsky, D.Y. A new mineral borisenkoite, Cu3[(V,As)O4]2, and the isomorphous series borisenkoite–lammerite-β in fumarolic exhalations of the Tolbachik volcano, Kamchatka, Russia. Phys. Chem. Miner. 2020, 47, 17. [Google Scholar] [CrossRef]
- Birnie, R.W.; Hughes, J.M. Stoiberite, Cu5V2O10, a new copper vanadate from Izalco volcano, El Salvador, Central America. Am. Mineral. 1979, 64, 941–944. [Google Scholar]
- Palacio, L.A.; Silva, J.M.; Ribeiro, F.R.; Ribeiro, M.F. Catalytic oxidation of volatile organic compounds with a new precursor type copper vanadate. Catal. Today 2008, 133, 502–508. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Li, H.; Hu, H.; Han, J.; Shi, L.; Zhang, D. Highly dispersed V2O5/TiO2 modified with transition metals (Cu, Fe, Mn, Co) as efficient catalysts for the selective reduction of NO with NH3. Chin. J. Catal. 2015, 36, 1886–1899. [Google Scholar] [CrossRef]
- Yoshida, H.; Okamoto, Y.; Tayama, T.; Sakakibara, T.; Tokunaga, M.; Matsuo, A.; Narumi, Y.; Kindo, K.; Yoshida, M.; Takigawa, M.; et al. Magnetization “steps” on a kagome lattice in volborthite. J. Phys. Soc. Jpn. 2009, 78, 043704. [Google Scholar] [CrossRef] [Green Version]
- Hiroi, Z.; Ishikawa, H.; Yoshida, H.; Yamaura, J.I.; Okamoto, Y. Orbital transitions and frustrated magnetism in the kagome-type copper mineral volborthite. Inorg. Chem. 2019, 58, 11949–11960. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Masjedi-Arani, M.; Ghanbari, D.; Bagheri, S.; Salavati-Niasari, M. Novel chemical synthesis and characterization of copper pyrovanadate nanoparticles and its influence on the flame retardancy of polymeric nanocomposites. Sci. Rep. 2016, 6, 1–9. [Google Scholar]
- Fan, Z.; Yang, X.; Li, G.; Zhao, Y.; Shen, J. Preparation and properties of copper vanadate materials. J. Adv. Phys. Chem. 2015, 4, 52–65. [Google Scholar] [CrossRef]
- Newhouse, P.F.; Boyd, D.A.; Shinde, A.; Guevarra, D.; Zhou, L.; Soedarmadji, E.; Li, G.; Neaton, J.B.; Gregoire, J.M. Solar fuel photoanodes prepared by inkjet printing of copper vanadates. J. Mater. Chem. A 2016, 4, 7483–7494. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Yan, Q.; Shinde, A.; Guevarra, D.; Newhouse, P.F.; Becerra-Stasiewicz, N.; Chatman, S.M.; Haber, J.A.; Neaton, J.B.; Gregoire, J.M. High throughput discovery of solar fuels photoanodes in the CuO–V2O5 system. Adv. Energy Mater. 2015, 5, 1500968. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.K.; Sotelo, P.; Sarker, H.P.; Galante, M.T.; Kormányos, A.; Longo, C.; Macaluso, R.T.; Huda, M.N.; Janáky, C.; Rajeshwar, K. Rapid one-pot synthesis and photoelectrochemical properties of copper vanadates. ACS Appl. Energy Mater. 2019, 2, 2837–2847. [Google Scholar] [CrossRef]
- Bruker-AXS. TopasV4.2: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker-AXS: Karlsruhe, Germany, 2009. [Google Scholar]
- Lafontaine, M.A.; Le Bail, A.; Férey, G. Copper-containing minerals—I. Cu3V2O7(OH)2·2H2O: The synthetic homolog of volborthite; Crystal structure determination from X-ray and neutron data; Structural correlations. J. Solid State Chem. 1990, 85, 220–227. [Google Scholar] [CrossRef]
- Coing-Boyat, J. Structure de la variete ordinaire, triclinique, de l’orthovanadate de cuivre(II), Cu3(VO4)2. Acta Cryst. 1982, B38, 1546–1548. [Google Scholar] [CrossRef]
- Belousov, R.I.; Filatov, S.K. Algorithm for calculating the thermal expansion tensor and constructing the thermal expansion diagram for crystals. Glass Phys. Chem. 2007, 33, 271–275. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Firsova, V.A.; Filatov, S.K. Software for Determining the Thermal Expansion Tensor and the Graphic Representation of Its Characteristic Surface (ThetaToTensor-TTT). Glass Phys. Chem. 2013, 39, 347–350. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Topological complexity of crystal structures: Quantitative approach. Acta Crystallogr. 2012, A68, 393–398. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals and mineral parageneses: Information and its evolution in the mineral world. In Highlights in Mineralogical Crystallography; Danisi, R., Armbruster, T., Eds.; Walter de Gruyter GmbH: Berlin, Germany, 2015; pp. 31–73. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity and configurational entropy of crystalline solids. Acta Crystallogr. 2016, B72, 274–276. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Hughes, J.M.; Brown, M.A. The crystal structure of ziesite, beta-Cu2V2O7, a thortveitite-type structure with a non-linear X-O-X inter-tetrahedral bond. Neues Jahrbuch fur Mineralogie Monatshefte 1989, 1, 41–47. [Google Scholar]
- Shannon, R.D.; Calvo, C. Crystal Structure of a New Form of Cu3V2O8. Can. J. Chem. 1972, 50, 3944–3949. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D.; Calvo, C. Crystal structure of Cu5V2O10. Acta Cryst. 1973, B29, 1338–1345. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Yang, H.; Wang, D.; Chen, A.; Dai, W.-L.; Zhao, X.; Yang, J.; Wang, X. Activation of Kagome lattice-structured Cu3V2O7(OH)2·2H2O volborthite via hydrothermal crystallization for boosting visible light-driven water oxidation. Phys. Chem. Chem. Phys. 2018, 20, 24561–24569. [Google Scholar] [CrossRef]
- Filatov, S.K. General concept of increasing crystal symmetry with an increase in temperature. Crystallogr. Rep. 2011, 56, 953–961. [Google Scholar] [CrossRef]
- Goldsmith, J.R. A “simplexity principle” and its relation to “ease” of crystallization. J. Geol. 1953, 61, 439–451. [Google Scholar] [CrossRef]


| Mineral | Symmetry | Chemical Formula | Geological Setting | References |
|---|---|---|---|---|
| OH/H2O-Containing Copper Vanadates | ||||
| Volborthite | Monoclinic | Cu3(V2O7)(OH)2·2H2O | Hypergene, paleofumarolic(?) or hypergene after fumarolic minerals | [1,2,5,6,7] |
| Molinelloite | Triclinic | Cu(H2O)(OH)V4+O(V5+O4) | Hypergene | [3] |
| Turanite | Triclinic | Cu5(VO4)2(OH)4 | [4] | |
| Anhydrous Copper Vanadates | ||||
| Blossite | Orthorhombic | Cu2(V2O7) | Fumarolic | [8] |
| Ziesite | Monoclinic | [9] | ||
| Fingerite | Triclinic | Cu11(VO4)6O2 | [10] | |
| Mcbirneyite | Triclinic | Cu3(VO4)2 | [11] | |
| Pseudolyonsite | Monoclinic * | [12] | ||
| Borisenkoite | Monoclinic * | Cu3[(V,As)O4]2 | [13] | |
| Stoiberite | Monoclinic | Cu5(VO4)2O2 | [14] | |
| Phase | Volborthite | Ziesite | Blossite | Pseudolyonsite | Mcbirneyite | Fingerite | Stoiberite |
|---|---|---|---|---|---|---|---|
| Chemical formula | Cu3(V2O7)(OH)2∙ 2H2O | Cu2(V2O7) | Cu3V2O8 | Cu11O2(VO4)6 | Cu5O2(VO4)2 | ||
| Symmetry | Monoclinic | Monoclinic | Orthorhombic | Monoclinic | Triclinic | Triclinic | Monoclinic |
| Space group | C2/m | C2/c | Fdd2 | P21/c | P-1 | P-1 | P21/c |
| a (Å) | 10.607 | 7.689 | 20.676 | 6.249 | 5.196 | 8.158 | 8.393 |
| b (Å) | 5.864 | 8.029 | 8.392 | 7.994 | 5.355 | 8.269 | 6.065 |
| c (Å) | 7.214 | 10.106 | 6.446 | 6.378 | 6.505 | 8.044 | 6.446 |
| α (°) | 90 | 90 | 90 | 90 | 69.22 | 107.1 | 90 |
| β (°) | 94.88 | 110.252 | 90 | 111.49 | 88.69 | 91.4 | 108.09 |
| γ (°) | 90 | 90 | 90 | 90 | 68.08 | 106.4 | 90 |
| V (Å3) | 447.08 | 585.35 | 1118.46 | 296.44 | 155.73 | 494.12 | 781.77 |
| Z | 2 | 4 | 8 | 2 | 1 | 1 | 4 |
| Dcalc (g/cm3) | 3.525 | 3.87 | 4.051 | 4.711 | 4.484 | 4.774 | 4.96 |
| IG (bits/atom) | 3.187 | 2.550 | 2.550 | 2.777 | 2.777 | 4.450 | 4.087 |
| IG,total (bits/cell) | 70.107 | 56.107 | 56.107 | 72.211 | 36.106 | 191.329 | 277.947 |
| Reference | [25] | [35] | [8] | [36] | [26] | [10] | [37] |
| Temperature Range (Temperature of Annealing), °C | In Situ Phase (HT XRD (I)) | In Situ Phase (HT XRD (II)) | Phase Notation | Ex Situ Phase (Annealing in Oven with Fast Cooling) | Ex Situ Phase (Annealing in Oven with Slow Cooling) |
|---|---|---|---|---|---|
| 30–230 (150) | Volborthite | Volborthite | S1 | Volborthite | Volborthite |
| 230–290 (250) | Volborthite, X-ray amorphous phase | Volborthite, X-ray amorphous phase | S2 | Volborthite, fingerite, karelianite | Volborthite, fingerite |
| 290–430 (350) | Ziesite and tenorite | Ziesite and tenorite | S3 | Ziesite and tenorite | Ziesite and tenorite |
| 430–510 (480) | Ziesite, tenorite, pseudolyonsite and mcbirneyite | Mcbirneyite, ziesite, pseudolyonsite, blossite and fingerite | S4 | Mcbirneyite, ziesite, pseudolyonsite and fingerite | Mcbirneyite, ziesite, pseudolyonsite, blossite and fingerite |
| 510–750 (650) | Mcbirneyite | Mcbirneyite, blossite | S5 | Mcbirneyite and ziesite | Mcbirneyite, blossite and ziesite |
| 750–790 (780) | Unidentified phase | Unidentified phase | S6 | Mcbirneyite, shcherbinaite, ziesite and tenorite | Mcbirneyite, stoiberite, ziesite and blossite |
| 790–800 (850) | Copper and vanadium oxides | X-ray amorphous phase | S7 | α-CuVO3, ziesite, fingerite, Cu3VO4 | Mcbirneyite, stoiberite, ziesite, α-CuVO3, VO2 |
| Phase | α1 | α2 | α3 | <α1a | <α2b | <α3c | αa | αb | αc | αα | αβ | αγ | αV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volborthite | −16.5(1.3) | 30.7(2.5) | 20.7(1.7) | 35.3 | 0 | 39.9 | −4.1(6) | 30.7(7) | 5.4(1.5) | - | −21.6(4) | - | 34.9(2.5) |
| Volborthite * | −21.9(1) | 28.8(1) | 22.6(1) | 39.1 | 0 | 43.9 | −4.2(5) | 28.7(5) | 1.2(8) | - | −26.7(1) | - | 29.5(1) |
| Mcbirneyite | 12.4(4) | 15.8(6) | −1.01(4) | 36.7 | 35.7 | 19.7 | 11.6(3) | 11.0(4) | 0.7(4) | 8.8(2) | 6.0(3) | 1.2(1) | 27.2(1.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismagilova, R.M.; Zhitova, E.S.; Krivovichev, S.V.; Sergeeva, A.V.; Nuzhdaev, A.A.; Anikin, L.P.; Krzhizhanovskaya, M.G.; Nazarova, M.A.; Kupchinenko, A.N.; Zolotarev, A.A.; et al. Phase Evolution from Volborthite, Cu3(V2O7)(OH)2·2H2O, upon Heat Treatment. Minerals 2021, 11, 1312. https://doi.org/10.3390/min11121312
Ismagilova RM, Zhitova ES, Krivovichev SV, Sergeeva AV, Nuzhdaev AA, Anikin LP, Krzhizhanovskaya MG, Nazarova MA, Kupchinenko AN, Zolotarev AA, et al. Phase Evolution from Volborthite, Cu3(V2O7)(OH)2·2H2O, upon Heat Treatment. Minerals. 2021; 11(12):1312. https://doi.org/10.3390/min11121312
Chicago/Turabian StyleIsmagilova, Rezeda M., Elena S. Zhitova, Sergey V. Krivovichev, Anastasia V. Sergeeva, Anton A. Nuzhdaev, Leonid P. Anikin, Mariya G. Krzhizhanovskaya, Maria A. Nazarova, Anastasia N. Kupchinenko, Andrey A. Zolotarev, and et al. 2021. "Phase Evolution from Volborthite, Cu3(V2O7)(OH)2·2H2O, upon Heat Treatment" Minerals 11, no. 12: 1312. https://doi.org/10.3390/min11121312
APA StyleIsmagilova, R. M., Zhitova, E. S., Krivovichev, S. V., Sergeeva, A. V., Nuzhdaev, A. A., Anikin, L. P., Krzhizhanovskaya, M. G., Nazarova, M. A., Kupchinenko, A. N., Zolotarev, A. A., Kutyrev, A. V., Bukhanova, D. S., Kuznetsov, R. A., & Khanin, D. A. (2021). Phase Evolution from Volborthite, Cu3(V2O7)(OH)2·2H2O, upon Heat Treatment. Minerals, 11(12), 1312. https://doi.org/10.3390/min11121312










