Phase Transitions in Natural Vanadinite at High Pressures
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. EPMA Data of the Vanadinite Sample
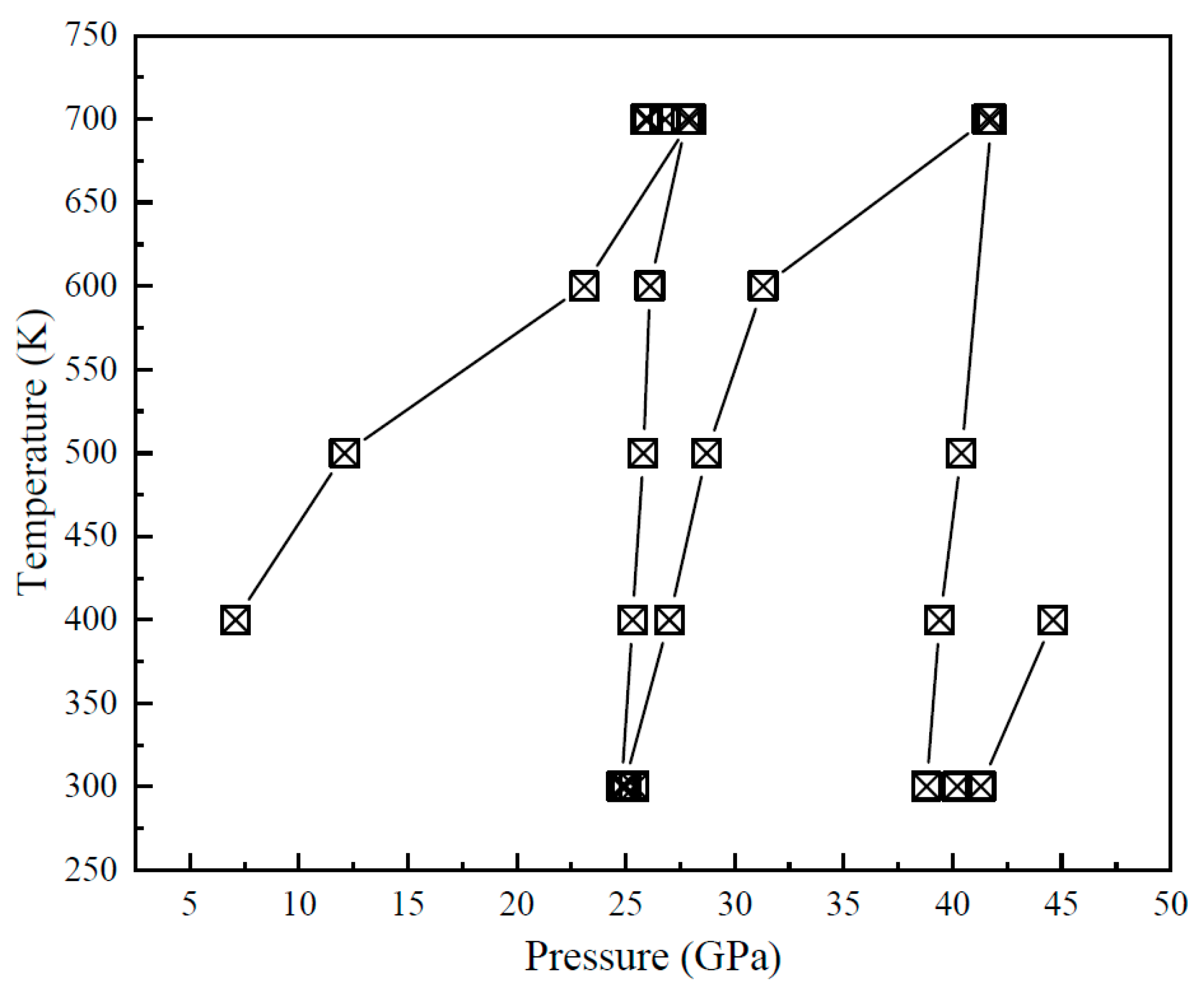
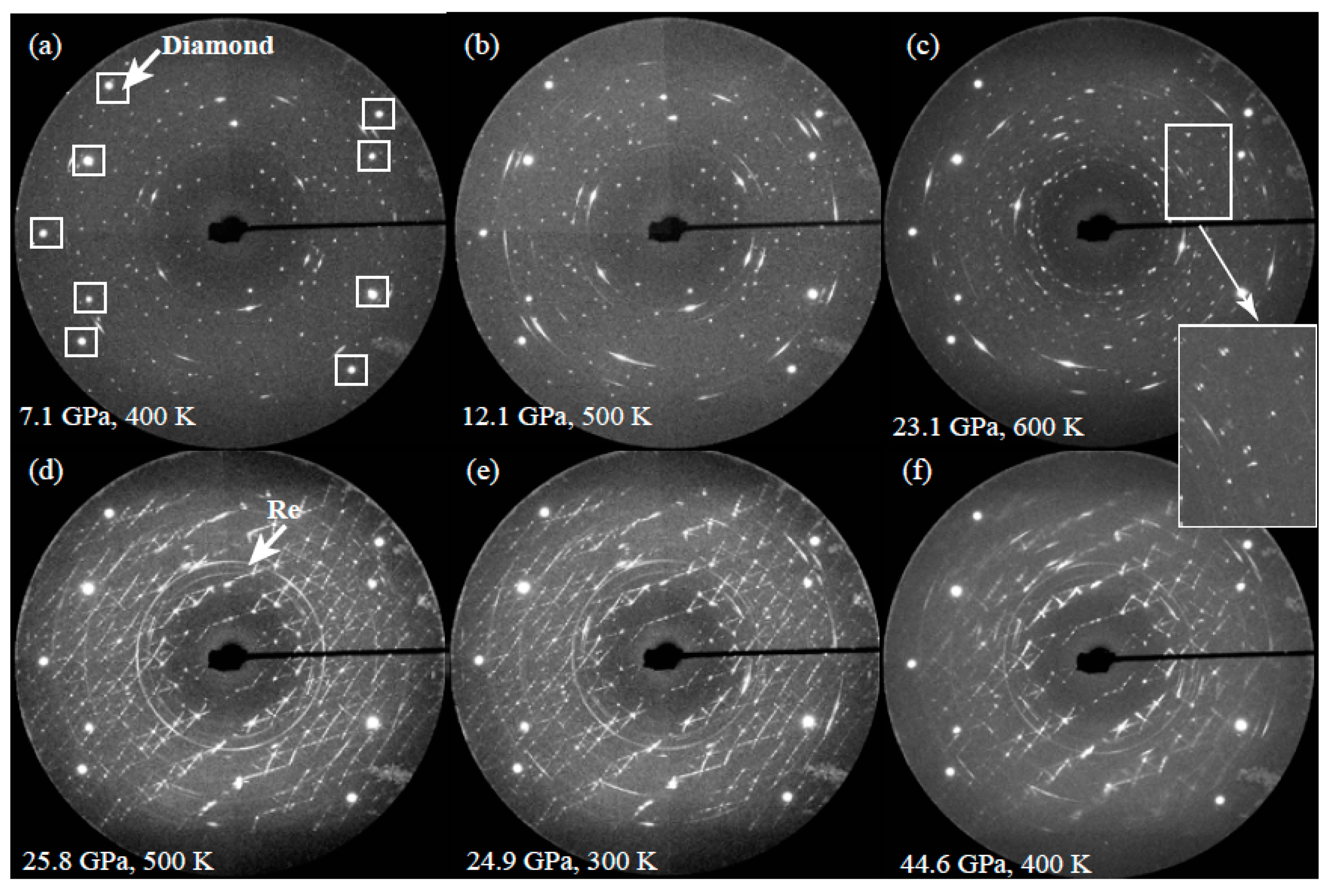
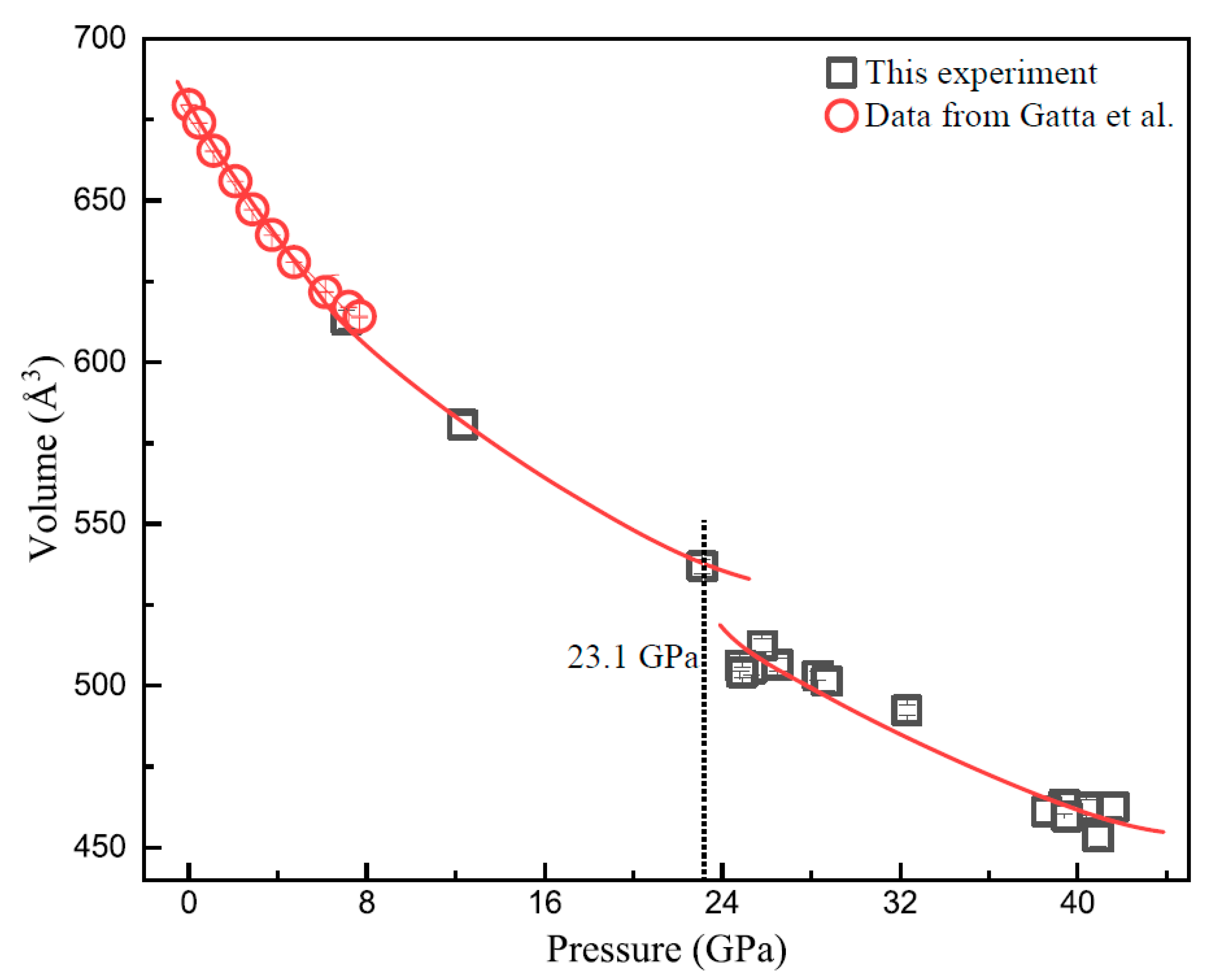
3.2. XRD Results of Vanadinite at HPHT Conditions
3.3. Raman Spectroscopy Studies of Vanadinite at Ambient Conditions and High Pressures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Wright, J.V.; Conca, J.L.; Peurrung, L.M. Evaluation of heavy metal remediation using mineral apatite. Water Air Soil Pollut. 1997, 98, 57–78. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Traina, S.J.; Logan, T.J.; Ryan, J.A. In situ lead immobilization by apatite. Environ. Sci. Technol. 1993, 27, 1803–1810. [Google Scholar] [CrossRef]
- Shi, Q.T.; Zhang, S.J.; Ge, J.; Wei, J.S.; Christodoulatos, C.; Korfiatis, G.P.; Meng, X.G. Lead immobilization by phosphate in the presence of iron oxides: Adsorption versus precipitation. Water Res. 2020, 179, 115853. [Google Scholar] [CrossRef]
- Reisfeld, R.; Gaft, M.; Boulon, G.; Panczer, G.; Jorgensen, C.K. Laser-induced luminescence of rare-earth elements in natural fluor-apatites. J. Lumin. 1996, 69, 343–353. [Google Scholar] [CrossRef]
- Kang, J.; Yang, Y.; Pan, S.; Yu, H.; Zhou, Z. Synthesis, crystal structure and optical properties of Ba5V3O12F. J. Mol. Struct. 2014, 1056, 79–83. [Google Scholar] [CrossRef]
- Raicevic, S.; Wright, J.V.; Veljkovic, V.; Conca, J.L. Theoretical stability assessment of uranyl phosphate and apatites: Selection of amendments for in situ remediation of uranium. Sci. Total Environ. 2006, 355, 13–24. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Montel, J.M. Phosphate and nuclear waste storage. Elements 2008, 4, 113–116. [Google Scholar] [CrossRef]
- Johnstone, E.V.; Bailey, D.J.; Lawson, S.; Stennett, M.C.; Corkhill, C.L.; Kim, M.; Heo, J.; Matsumura, D.; Hyatt, N.C. Synthesis and characterization of iodovanadinite using PdI2, an iodine source for the immobilisation of radioiodine. RSC Adv. 2020, 10, 25116–25124. [Google Scholar] [CrossRef]
- Stoppa, F.; Liu, Y. Chemical composition and petrogenetic implications of apatites from some ultra-alkaline Italians rocks. Eur. J. Miner. 1995, 7, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Peng, G.; Luhr, J.F.; McGee, J.J. Factors controlling sulfur concentrations in volcanic apatite. Am. Mineral. 1997, 82, 1210–1224. [Google Scholar] [CrossRef]
- Zhai, S.; Liu, X.; Shieh, S.R.; Zhang, L.; Ito, E. Equation of state of γ-tricalcium phosphate, γ-Ca3(PO4)2, to lower mantle pressures. Am. Mineral. 2009, 94, 1388–1391. [Google Scholar] [CrossRef]
- Zhai, S.; Wu, X.; Xue, W. Pressure-dependent raman spectra of β-Ca3(PO4)2 whitlockite. Phys. Chem. Miner. 2015, 42, 303–308. [Google Scholar] [CrossRef]
- Gatta, G.D.; Lee, Y.; Kao, C.C. Elastic behavior of vanadinite, Pb10(VO4)6Cl2, a microporous non-zeolitic mineral. Phys. Chem. Miner. 2009, 36, 311–317. [Google Scholar] [CrossRef]
- Redfern, S.A.T.; Smith, S.E.; Maddrell, E.R. High-temperature breakdown of the synthetic iondine analogue of vanadinite, Pb5(VO4)3I: An apatite-related compound for iodine radioisotope immobilization? Miner. Mag. 2012, 76, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.W.; Wei, S.Y.; Liu, J.; Li, Y.C.; Xie, H.S. X-ray diffraction study of calcium-lead fluorapatite solid solution at high pressure: The composition dependence of the bulk modulus and its pressure derivative. High Temp. High Press. 2013, 42, 69–80. [Google Scholar]
- Fan, D.W.; Ma, M.N.; Wei, S.Y.; Chen, Z.Q.; Xie, H.S. In-situ synchrotron powder X-ray diffraction study of vanadinite at room temperature and high pressure. High Temp. High Press. 2013, 42, 441–449. [Google Scholar]
- Zhai, S.; Shieh, S.R.; Xue, W.; Xie, T. Raman spectra of stronadelphite Sr5(PO4)3F at high pressures. Phys. Chem. Miner. 2015, 42, 579–585. [Google Scholar] [CrossRef]
- Zhai, S.; Li, Z.; Shieh, S.R.; Wang, C.; Xue, W. Pressure-dependent Raman spectra of Ba5(PO4)3Cl alforsite. Phys. Chem. Miner. 2018, 45, 353–359. [Google Scholar] [CrossRef]
- Kong, X.; Yuan, Z.; Yu, Y.; Gao, T.; Ma, S. Elastic, vibrational, and thermodynamic properties of Sr10(PO4)6F2 and Ca10(PO4)6F2 from first principles. Chin. Phys. B 2017, 26, 086301. [Google Scholar] [CrossRef]
- White, J.S. Vanadinite from Toussit, Morocco, and comments on endlichite. Miner. Rec. 1984, 15, 347–350. [Google Scholar]
- Laufek, F.; Skala, R.; Haloda, J.; Cisarova, I. Crystal structure of vanadinte: Refinement of anisotropic displacement parameters. J. Czech Geol. Soc. 2006, 51, 271–275. [Google Scholar]
- Beevers, C.A.; McIntyre, D.B. The atomic structure of fluor-apatite and its relation to that of tooth and bond materials. Mineral. Mag. J. Mineral. Soc. 1946, 27, 254–257. [Google Scholar] [CrossRef]
- Dai, Y.; Huges, J.M. Crystal-structure refinements of vanadinite and pyromorphite. Can. Miner. 1989, 27, 189–192. [Google Scholar]
- Okudera, H. Relationships among channel typology and atomic displacements in the structures of Pb5(BO4)3Cl with B = P (pyromorphite), V (vanadinite), and as (mimetite). Am. Mineral. 2013, 98, 1573–1579. [Google Scholar] [CrossRef]
- Comodi, P.; Liu, Y.; Zanazzi, P.F.; Montagnoli, M. Structural and vibrational behaviour of fluorapatite with pressure. Part I: In situ single-crystal X-ray diffraction investigation. Phys. Chem. Miner. 2001, 28, 219–224. [Google Scholar] [CrossRef]
- Comodi, P.; Liu, Y.; Frezzotti, M.L. Structural and vibrational behaviour of fluorapatite with pressure. Part II: In situ micro-raman spectroscopic investigation. Phys. Chem. Miner. 2001, 28, 225–231. [Google Scholar] [CrossRef]
- Wei, S.; Ma, M.; Fan, D.; Yang, J.; Zhou, W.; Li, B.; Chen, Z.; Xie, H. Compressibility of mimetite and pyromorphite at high pressure. High Press. Res. 2013, 33, 27–34. [Google Scholar] [CrossRef]
- Chen, X.; Xu, X.; Qin, F.; Liu, Y.X. In-situ high-pressure single crystal X-ray diffraction of natural pyromorphite and mimetite. Nucl. Tech. 2019, 49, 020101. [Google Scholar]
- Chernorukov, N.G.; Knyazev, A.V.; Bulanov, E.N. Phase transitions and thermal expansion of apatite-structured compounds. Inorg. Mater. 2011, 47, 172–177. [Google Scholar] [CrossRef]
- Xue, W.; Zhai, K.; Lin, C.C.; Zhai, S. Raman spectroscopic study of stronadelphite Sr5(PO4)3F at various temperatures. Vib. Spectrosc. 2018, 98, 123–127. [Google Scholar] [CrossRef]
- Williams, Q.; Knittle, E. Infrared and Raman spectra of Ca5(PO4)3F2-fluorapatite at high pressures: Compression-induced changes in phosphate site and Davydov splittings. J. Phys. Chem. Solids 1996, 57, 417–422. [Google Scholar] [CrossRef]
- Zhang, D.; Dera, P.K.; Eng, P.J.; Stubbs, J.E.; Zhang, J.S.; Prakapenka, V.B.; Rivers, M.L. High pressure single crystal diffraction at PX^2. J. Vis. Exp. 2017, 119, e54660. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.J.; Zhu, F.; Zhang, J.S.; Zhang, D.Z.; Tkachev, S.; Prakapenka Vitali, B.; Chen, B. An externally-heated diamond anvil cell for synthesis and single-crystal elasticity determination of Ice-VII at high pressure-temperature conditions. J. Vis. Exp. 2020, 160, e61389. [Google Scholar] [CrossRef]
- Rivers, M.; Prakapenka, V.B.; Kubo, A.; Pullins, C.; Holl, C.M.; Jacobsen, S.D. The COMPRES/GSECARS gas-loading system for diamond anvil cells at the Advanced Photon Source. High Press. Res. 2008, 28, 273–292. [Google Scholar] [CrossRef]
- Dewaele, A.; Datchi, F.; Loubeyre, P.; Mezouar, M. High pressure—High temperature equations of state of neon and diamond. Phys. Rev. B 2008, 77, 094106. [Google Scholar] [CrossRef]
- Fei, Y.; Ricolleau, A.; Frank, M.; Mibe, K.; Shen, G.; Prakapenka, V. Toward an internally consistent pressure scale. Proc. Natl. Acad. Sci. USA 2007, 104, 9182–9186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prescher, C.; Prakapenka Vitali, B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Mao, H.K.; Bell, P.M. High-pressure physics: The 1-Megabar mark on the ruby R1 static pressure scale. Science 1976, 191, 851–852. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.L.; Crane, M.; Williams, P.A.; Kloprogge, J.T. Isomorphic substitution in vanadinite [Pb5(VO4)3Cl]-a Raman spectroscopic study. J.Raman. Spectrosc. 2003, 34, 214–220. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Liu, X.; Hu, X.; Li, S.; Wang, H. Solid solution between lead fluorapatite and lead fluorvanadate apatite: Mixing behavior, RAMAN feature and thermal expansivity. Phys. Chem. Miner. 2011, 38, 741–752. [Google Scholar] [CrossRef]
- Dove, M.T. Theory of displacive phase transitions in minerals. Am. Mineral. 1997, 82, 213–244. [Google Scholar] [CrossRef]
- Seto, Y.; Hamane, D.; Nagai, T.; Sata, N. Development of a software suite on X-ray diffraction experiments. Rev. High Press. Sci. Technol. 2010, 20, 269–276. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Downs, R.T.; Hall-Wallace, M. The American mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Zhai, S.M.; Xue, W.H.; Yamazaki, D.; Ma, F. Trace element composition in tuite decomposed from natural apatite in high-pressure and high-temperature experiments. Sci. China Earth Sci. 2014, 57, 2922–2927. [Google Scholar] [CrossRef]
- Jayaraman, A.; Wang, S.Y.; Sharma, S.K.; Cheong, S.W. A spectacular pressure-induced electronic transition in KTb(MoO4)2. Curr. Sci. 1996, 70, 232–234. [Google Scholar]
- Müller, J.; Speziale, S.; Efthimiopoulos, I.; Jahn, S.; Koch-Müller, M. Raman spectroscopy of siderite at high pressure: Evidence for a sharp spin transition. Am. Mineral. 2016, 101, 2638–2644. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Li, X.; Song, H.; Xu, J.; Zhang, D.; Wu, X. Pressure-induced phase transition in Mn(Ta,Nb)2O6: An experimental investigation and first-principle study. Inorg. Chem. 2020, 59, 18122–18130. [Google Scholar] [CrossRef]
- Bartholomai, G.; Klee, W.E. The vibrational spectra of pyromorphite, vanadinite and mimetite. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1978, 34, 831–843. [Google Scholar] [CrossRef]
- Łodziński, M.; Sitarz, M. Chemical and spectroscopic characterization of some phosphate accessory minerals from pegmatites of the Sowie Góry Mts, SW Poland. J. Mol. Struct. 2009, 924, 442–447. [Google Scholar] [CrossRef]
- Solecka, U.; Bajda, T.; Topolska, J.; Zelek-Pogudz, S.; Manecki, M. Raman and Fourier transform infrared spectroscopic study of pyromorphirte-vanadinite solid solutions. Spectro. Acta A 2018, 190, 96–103. [Google Scholar] [CrossRef]
- Toumi, M.; Smiri-Dogguy, L.; Bulou, A. Crystal structure and polarized Raman spectra of Ca6Sm2Na2(PO4)6F2. J. Solid State Chem. 2000, 149, 308–313. [Google Scholar] [CrossRef]
- Bajda, T.; Mozgawab, W.; Maneckia, M.; Flis, J. Vibrational spectroscopic study of mimetite—Pyromorphite solid solutions. Polyhedron 2011, 30, 2479–2485. [Google Scholar] [CrossRef]
- Giera, A.; Manecki, M.; Bajda, T.; Rakovan, J.; Kwaśniak-Kominek, M.; Marchlewski, T. Arsenate substitution in lead hydroxyl apatites: A raman spectroscopic study. Spectrochim. Acta A 2016, 152, 370–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzechnik, A.; McMillan, P.F. High pressure behavior of Sr3(VO4)2 and Ba3(VO4)2. J. Solid State Chem. 1997, 132, 156–162. [Google Scholar] [CrossRef]
- Kosyl, K.M.; Paszkowicz, W.; Ermakova, O.; Wlodarczyk, D.; Suchocki, A.; Minikayev, R.; Domagala, J.Z.; Shekhovtsov, A.N.; Kosmyna, M.; Popescu, C.; et al. Equation of state and amorphization of Ca9R(VO4)7 (R = La, Nd, Gd): A combined high-pressure X-ray diffraction and Raman spectroscopy study. Inorg. Chem. 2018, 57, 13115–13127. [Google Scholar] [CrossRef]
- Gao, J.; Wu, W.; Jia, L.; Wang, C.P.; Liu, Y.; Xu, C.; Chen, F.; Fei, C.; Su, W. Raman and infrared spectra to monitor the phase transition of natural kyanite under static compression. J. Raman Spectrosc. 2020, 51, 2102–2111. [Google Scholar] [CrossRef]

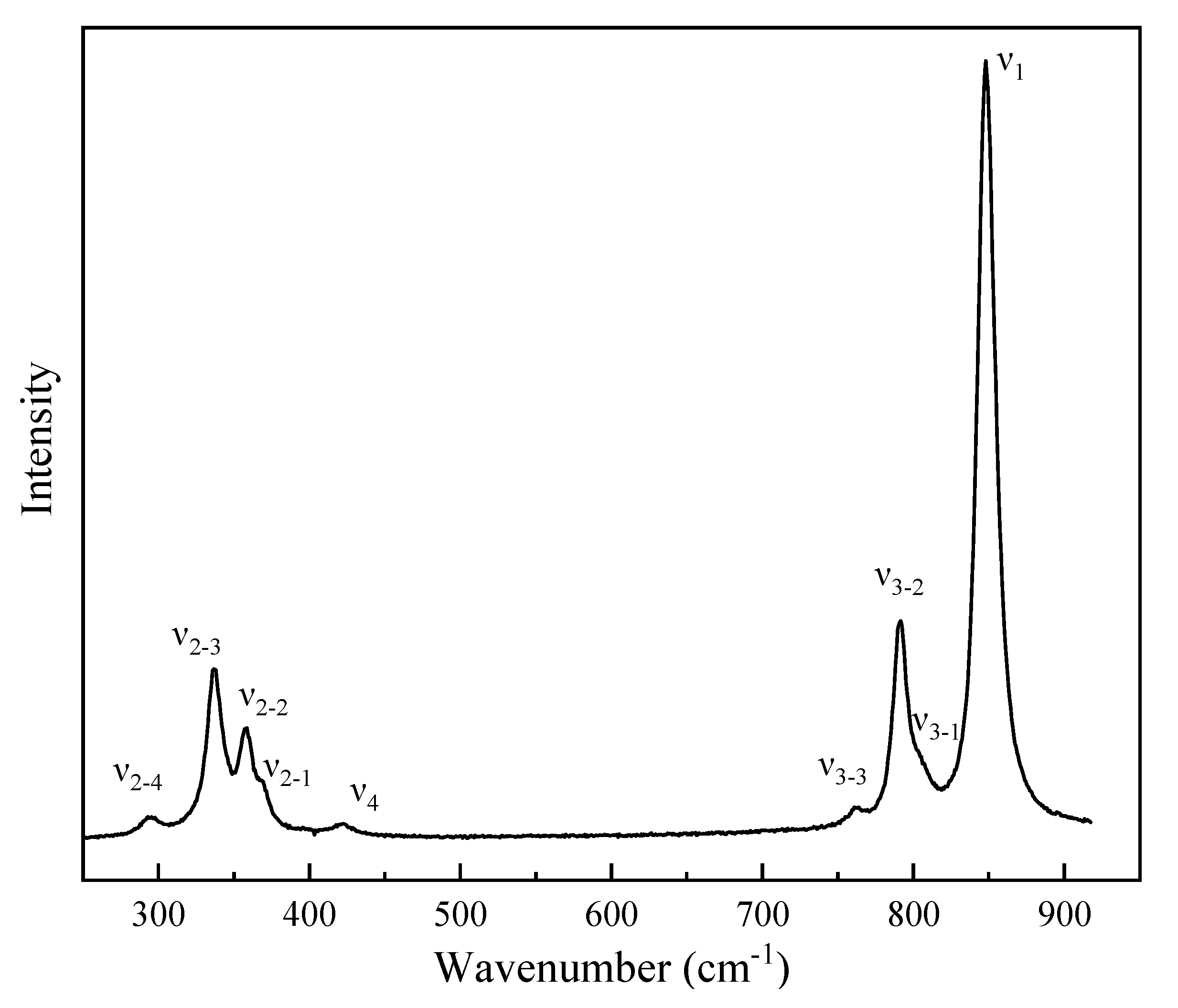

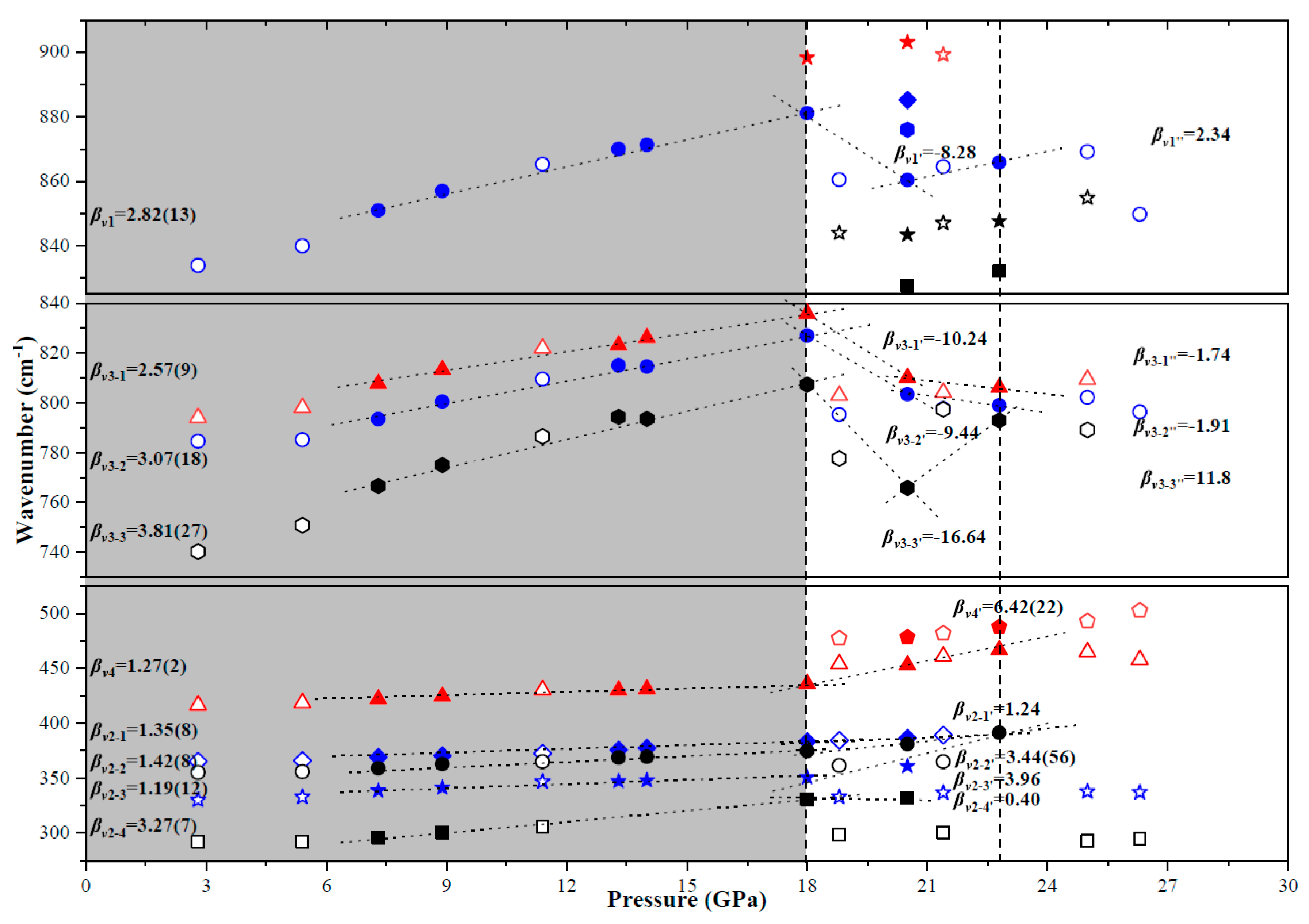
| P/GPa | ν1 | ν3-1 | ν3-2 | ν3-3 | ν4 | ν2-1 | ν2-2 | ν2-3 | ν2-4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compression | 10−4 | 848.0 | 802.0 | 791.0 | 760.0 | 420.0 | 367.0 | 357.0 | 336.1 | 295.2 | ||||||
| 7.3 | 850.9 | 807.7 | 793.5 | 766.6 | 422.1 | 368.9 | 358.9 | 338.2 | 296.0 | |||||||
| 8.9 | 857.0 | 813.3 | 800.5 | 775.0 | 424.4 | 370.3 | 362.7 | 341.1 | 300.4 | |||||||
| 13.3 | 870.0 | 823.1 | 815.1 | 794.3 | 430.1 | 375.5 | 368.6 | 347.1 | * | |||||||
| 14.0 | 871.3 | 826.1 | 814.5 | 793.6 | 430.9 | 377.5 | 369.3 | 347.8 | * | |||||||
| 18.0 | 898.3 | 881.1 | 835.8 | 827.0 | 807.4 | 435.7 | 383.3 | 374.6 | 350.7 | 330.7 | ||||||
| 20.5 | 903.1 | 885.2 | 876.0 | 860.4 | 843.4 | 827.6 | 810.2 | 803.4 | 765.8 | 478.5 | 452.8 | 386.4 | 380.9 | 360.6 | 331.7 | |
| 22.8 | 865.8 | 847.6 | 832.1 | 806.2 | 799.0 | 792.9 | 487.9 | 466.8 | 391.2 | |||||||
| 26.3 | Almost amorphous phase | |||||||||||||||
| Decompression | 26.3 | 849.7 | 796.4 | 502.8 | 457.9 | 337.1 | 294.4 | |||||||||
| 25.0 | 869.1 | 854.8 | 844.0 | 809.4 | 802.2 | 789.2 | 492.9 | 464.8 | 337.8 | 292.6 | ||||||
| 21.4 | 899.2 | 864.5 | 847.0 | 833.1 | 804.1 | 797.6 | 797.4 | 481.9 | 460.8 | 389.2 | 364.9 | 336.7 | 300.5 | |||
| 18.8 | 860.5 | 843.9 | 829.5 | 802.9 | 795.3 | 777.6 | 477.3 | 453.8 | 384.2 | 361.5 | 332.8 | 298.5 | ||||
| 11.4 | 865.3 | 821.9 | 809.5 | 786.6 | 430.2 | 372.4 | 365.0 | 346.7 | 305.6 | |||||||
| 5.4 | 839.9 | 798.0 | 785.1 | 750.8 | 418.4 | 366.0 | 355.8 | 332.8 | 292.5 | |||||||
| 2.8 | 833.9 | 794.0 | 784.6 | 740.2 | 416.5 | 365.3 | 355.1 | 330.1 | 292.1 | |||||||
| νi a | (dvi/dp)T b | ɤi c | Assignment |
|---|---|---|---|
| 848 | 2.82(13) | 0.189 | ν1 symmetric stretching vibration |
| 802 | 2.57(9) | 0.183 | ν3 antisymmetric stretching vibration |
| 791 | 3.07(18) | 0.221 | |
| 760 | 3.81(27) | 0.286 | |
| 420 | 1.27(2) | 0.172 | ν4 OVO bending vibration |
| 367 | 1.35(8) | 0.149 | ν2 OVO s bending vibration |
| 357 | 1.42(8) | 0.210 | |
| 336 | 1.19(12) | 0.202 | |
| 295 | 3.27(7) | 0.631 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Dai, L.; Lai, X.; Zhu, F.; Zhang, D.; Hu, Y.; Tkachev, S.; Chen, B. Phase Transitions in Natural Vanadinite at High Pressures. Minerals 2021, 11, 1217. https://doi.org/10.3390/min11111217
Liu Y, Dai L, Lai X, Zhu F, Zhang D, Hu Y, Tkachev S, Chen B. Phase Transitions in Natural Vanadinite at High Pressures. Minerals. 2021; 11(11):1217. https://doi.org/10.3390/min11111217
Chicago/Turabian StyleLiu, Yingxin, Liyun Dai, Xiaojing Lai, Feng Zhu, Dongzhou Zhang, Yi Hu, Sergey Tkachev, and Bin Chen. 2021. "Phase Transitions in Natural Vanadinite at High Pressures" Minerals 11, no. 11: 1217. https://doi.org/10.3390/min11111217
APA StyleLiu, Y., Dai, L., Lai, X., Zhu, F., Zhang, D., Hu, Y., Tkachev, S., & Chen, B. (2021). Phase Transitions in Natural Vanadinite at High Pressures. Minerals, 11(11), 1217. https://doi.org/10.3390/min11111217







