Microscopic Diffusion Characteristics of Linear Alkylbenzene Sulfonates on the Surface of Anthracite: The Influence of Different Attachment Sites of Benzene Ring in the Backbone
Abstract
:1. Introduction
2. Materials and Methods
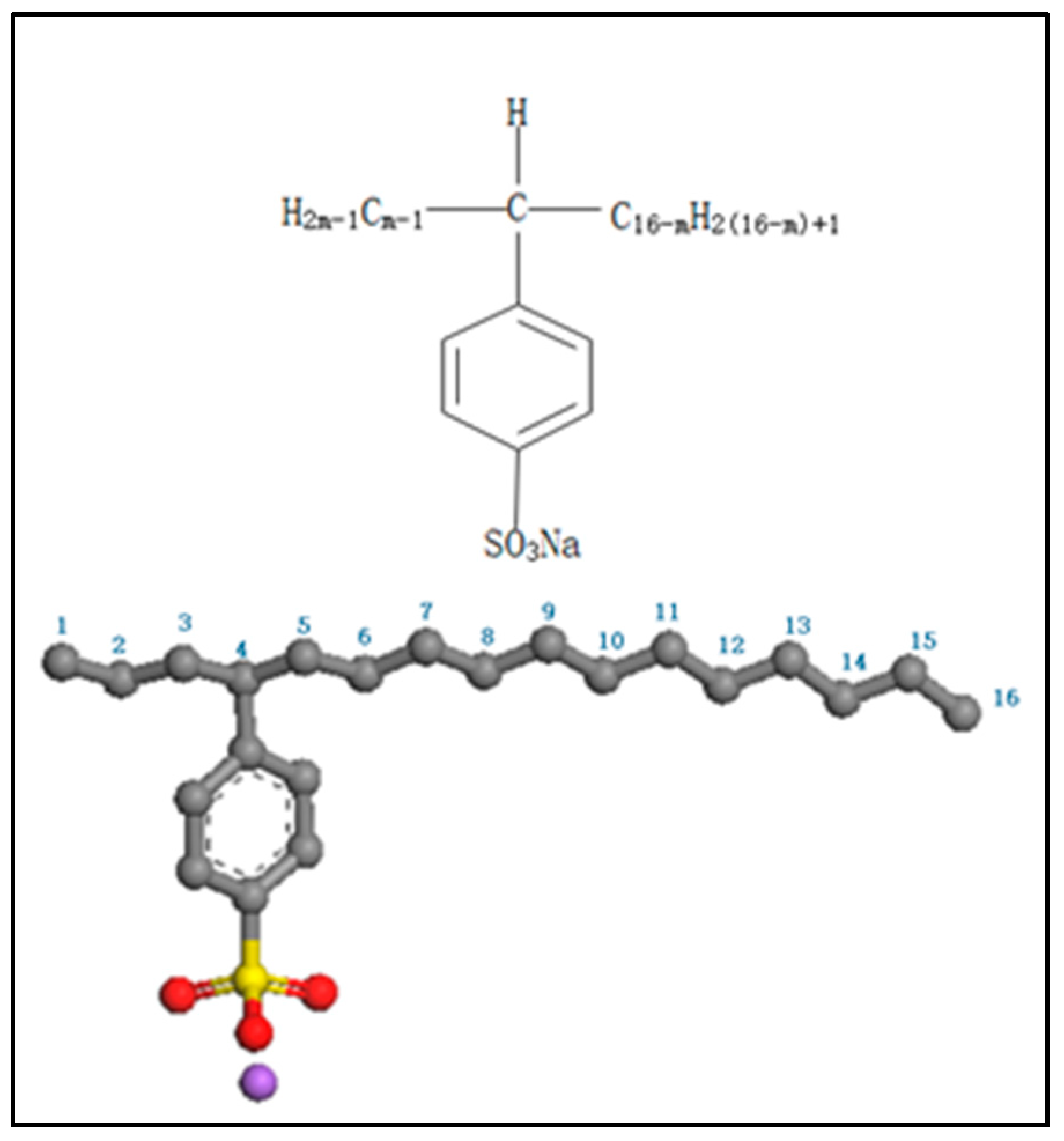
2.1. Materials
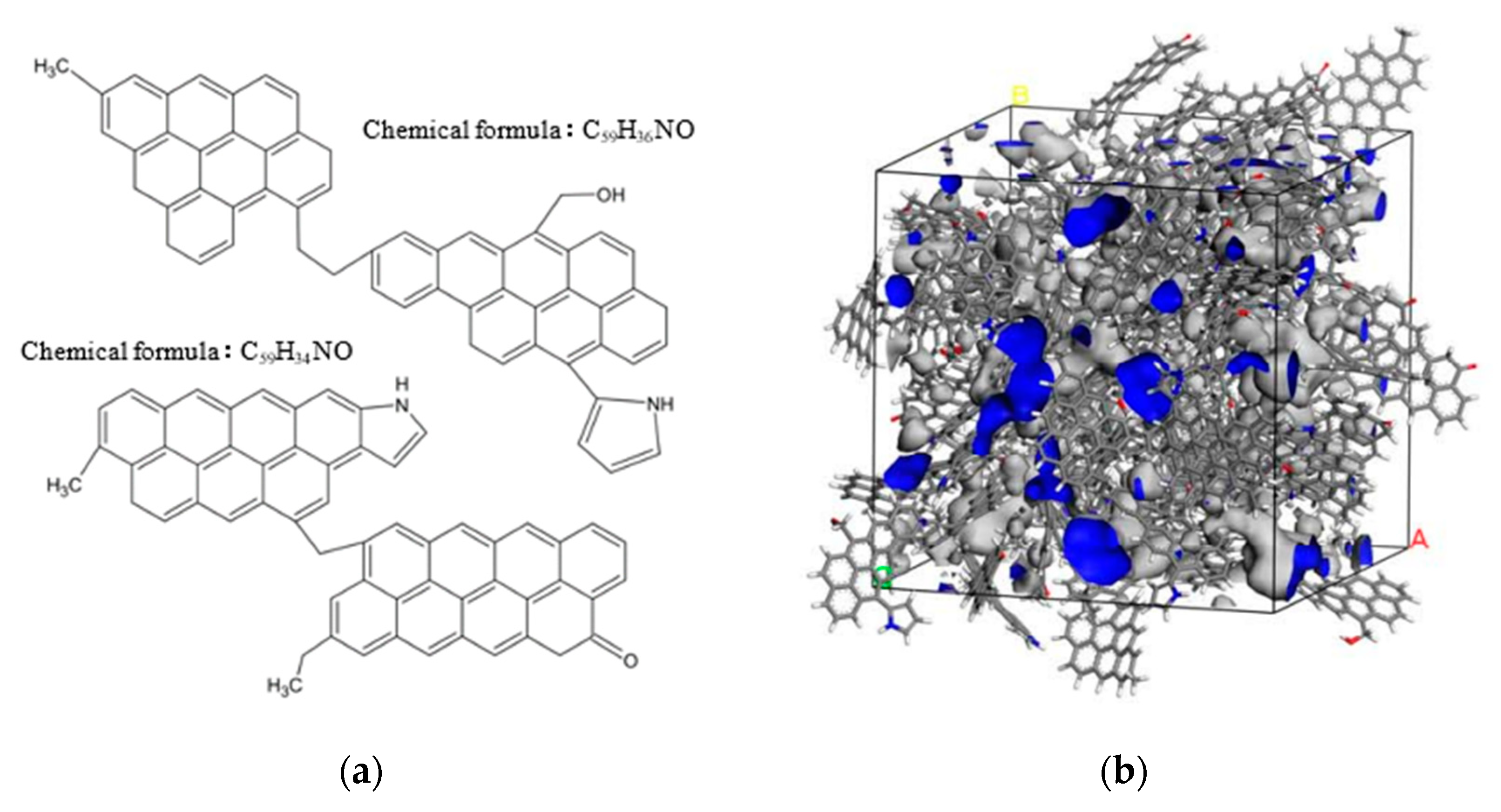
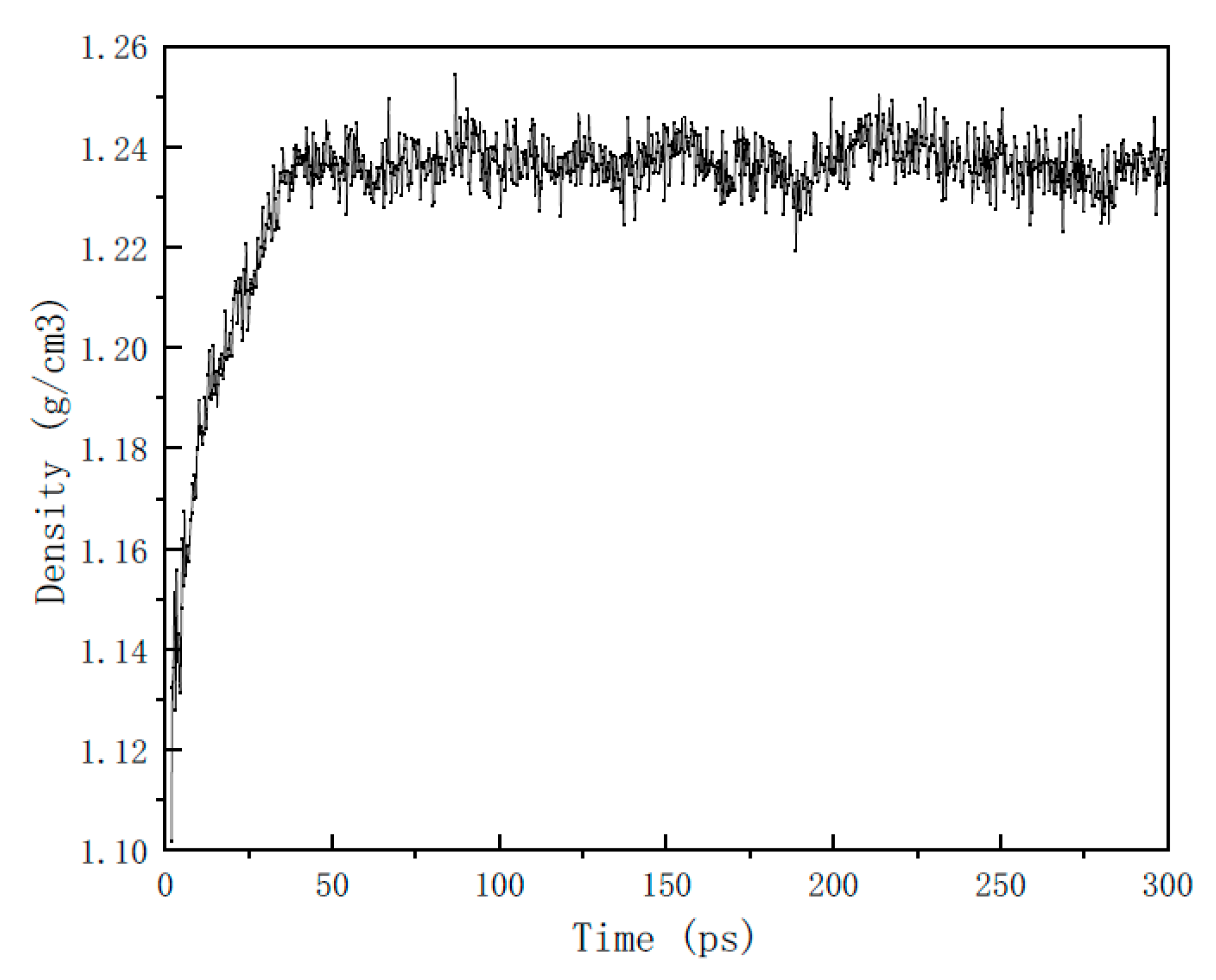
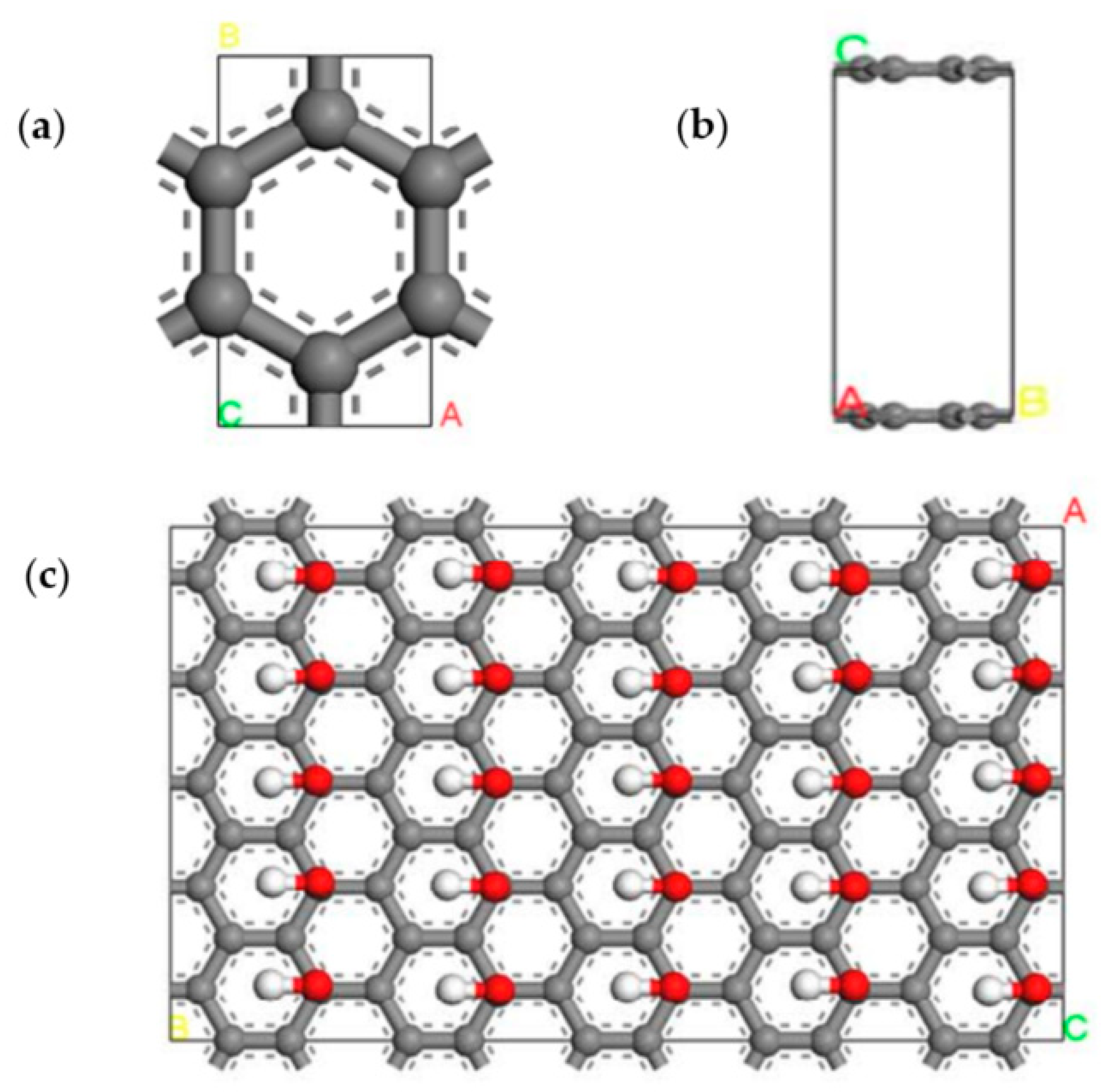
2.2. Computational Details
3. Results and Discussion
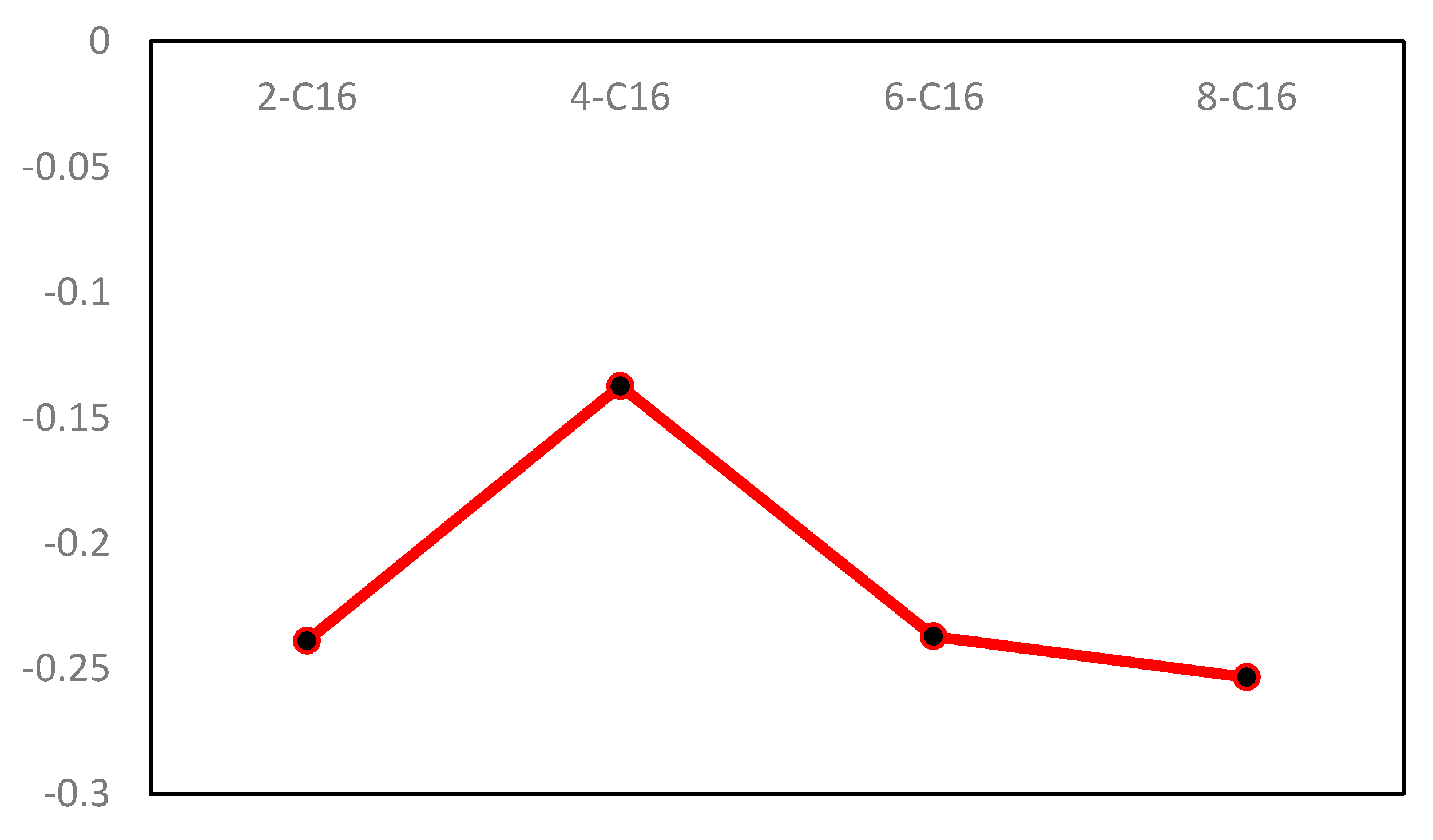
3.1. Surfactant/Anthracite Adsorption System
3.1.1. Interaction Energy
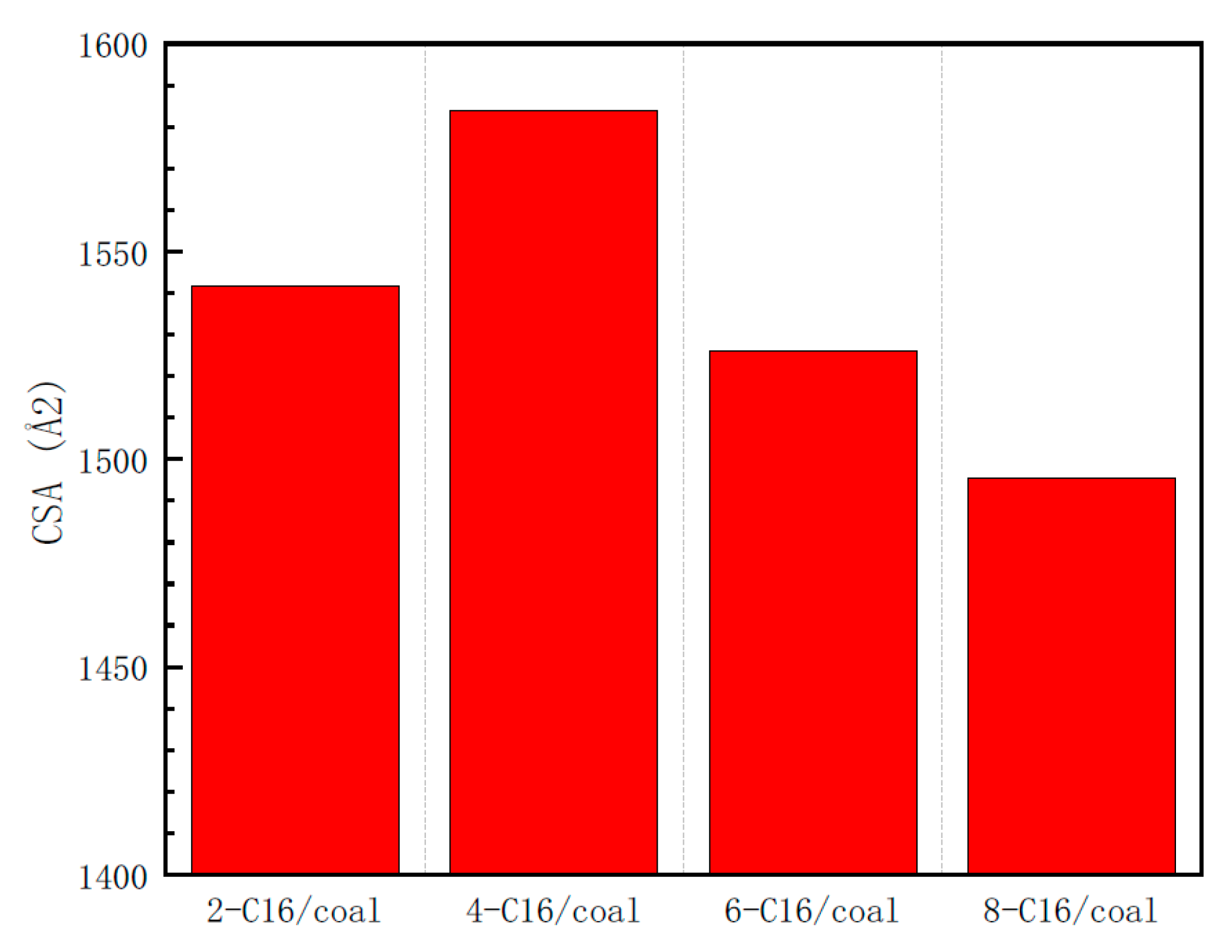
3.1.2. Contact Surface Area
3.2. Graphite Layer System
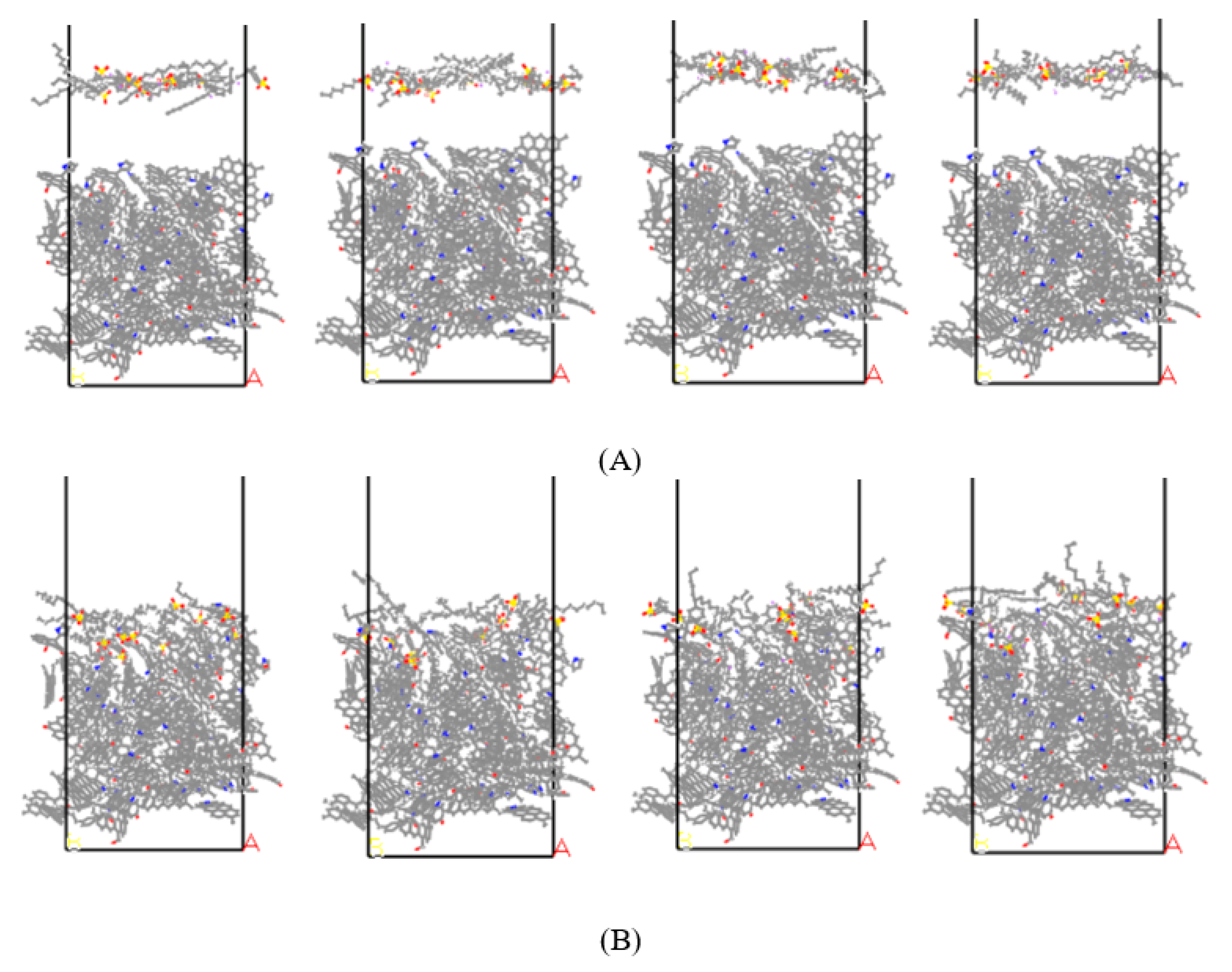
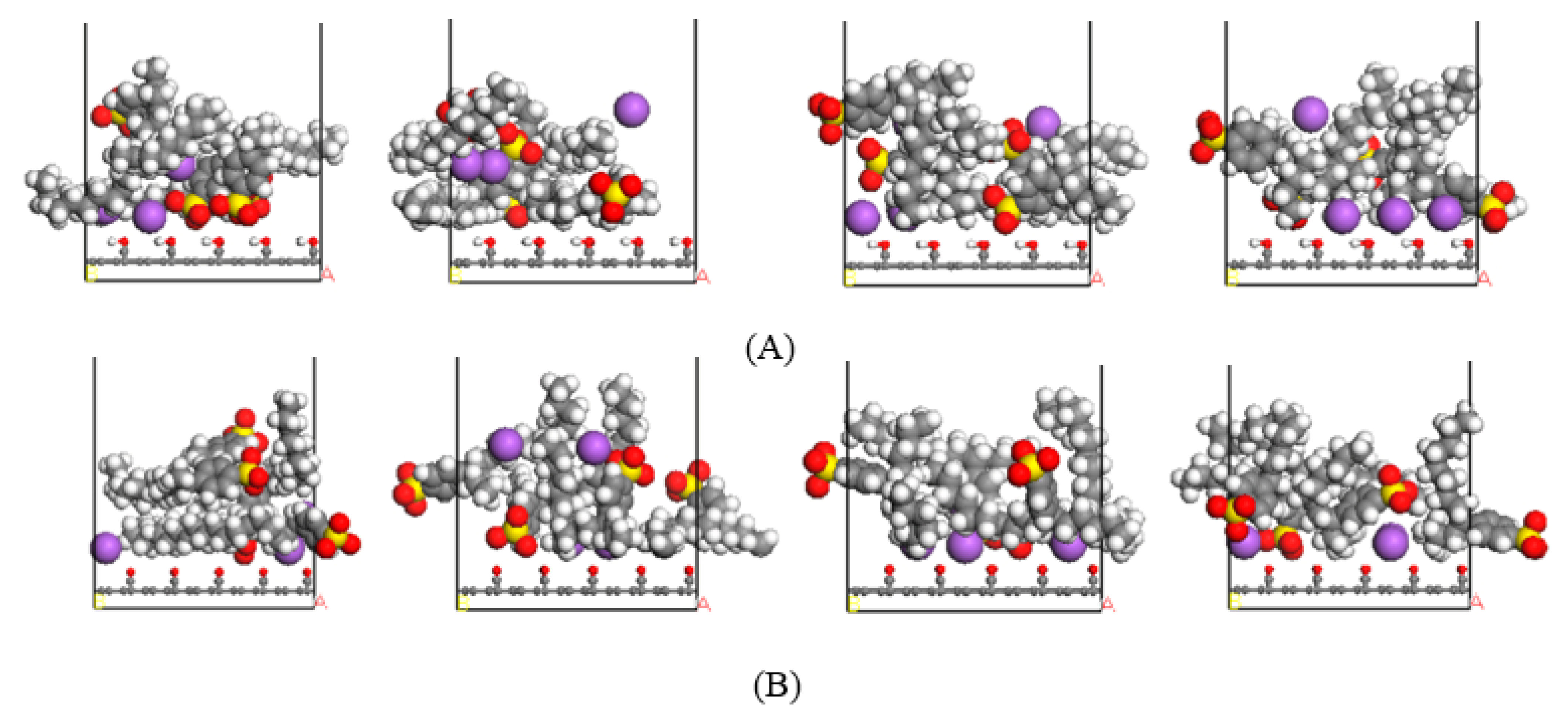
3.2.1. Adsorption Configuration
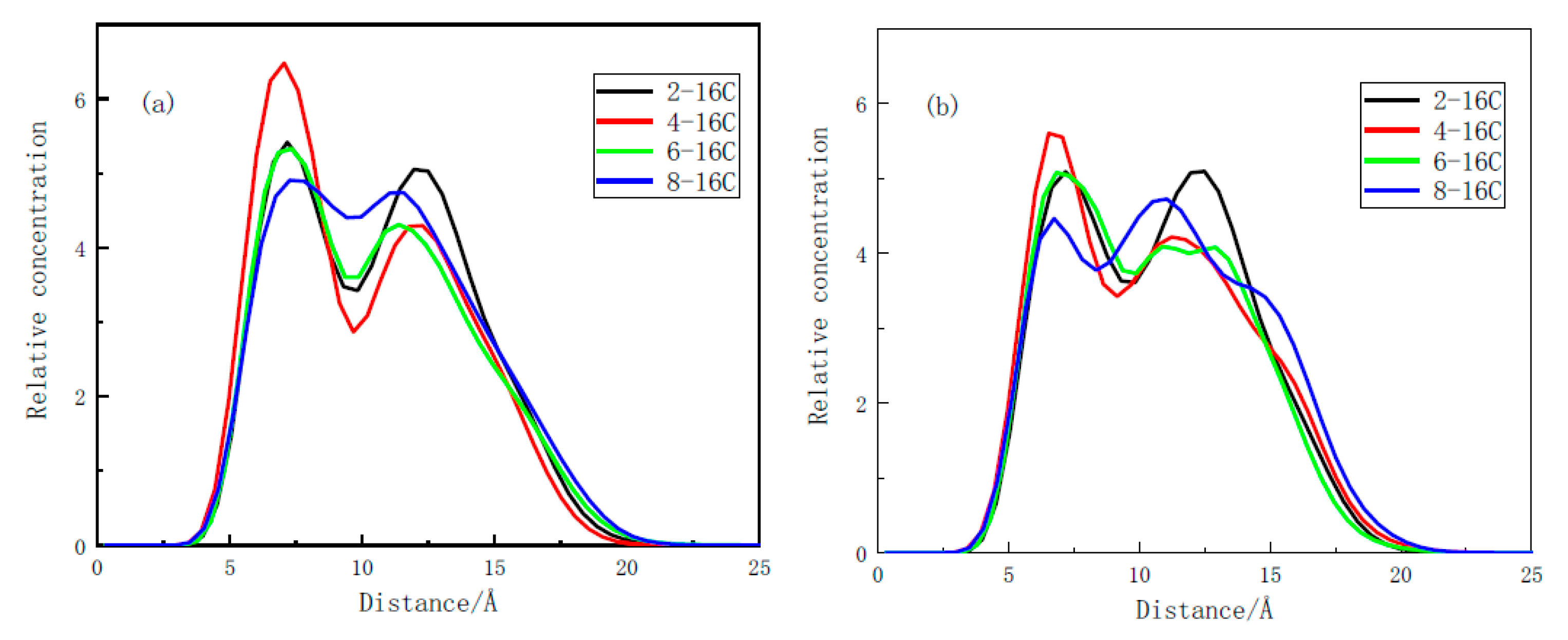
3.2.2. Relative Concentration Distribution
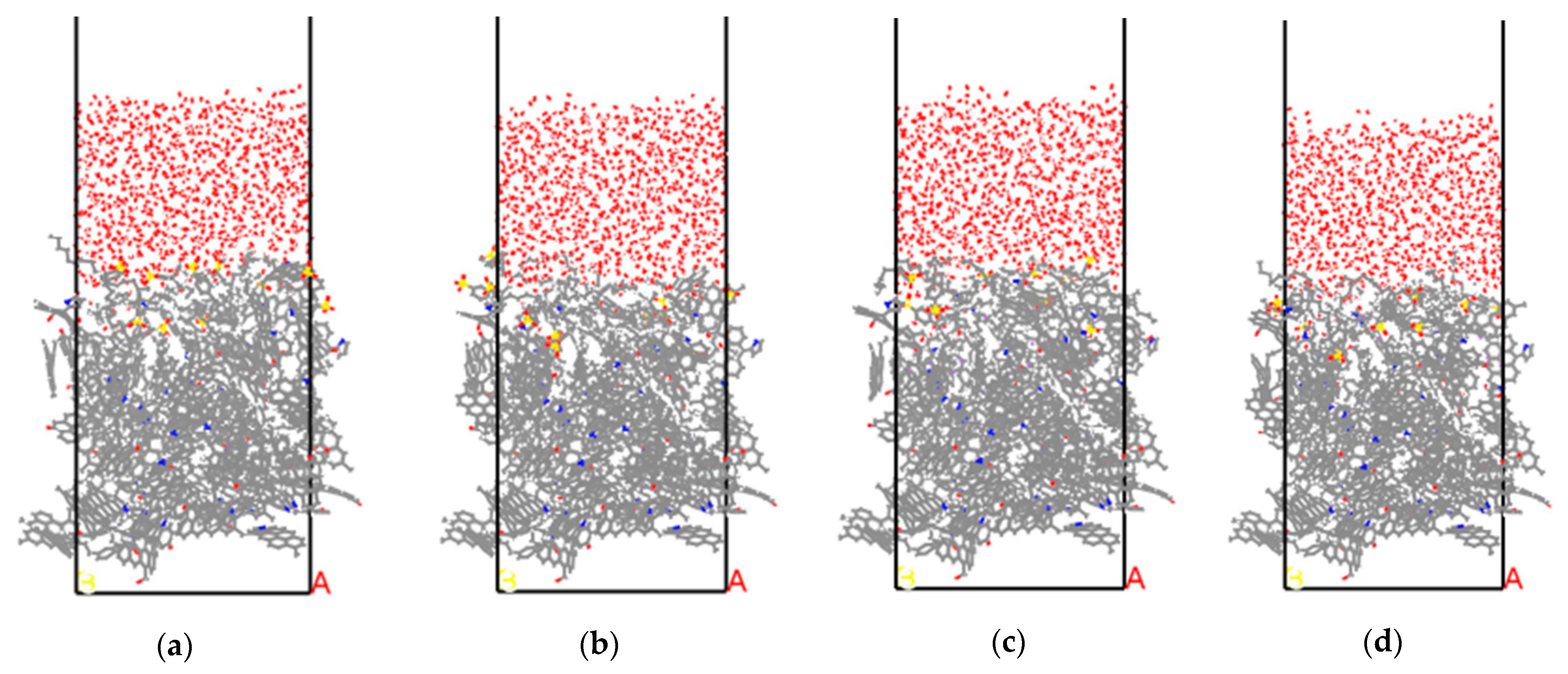
3.3. Water/Surfactant/Anthracite System
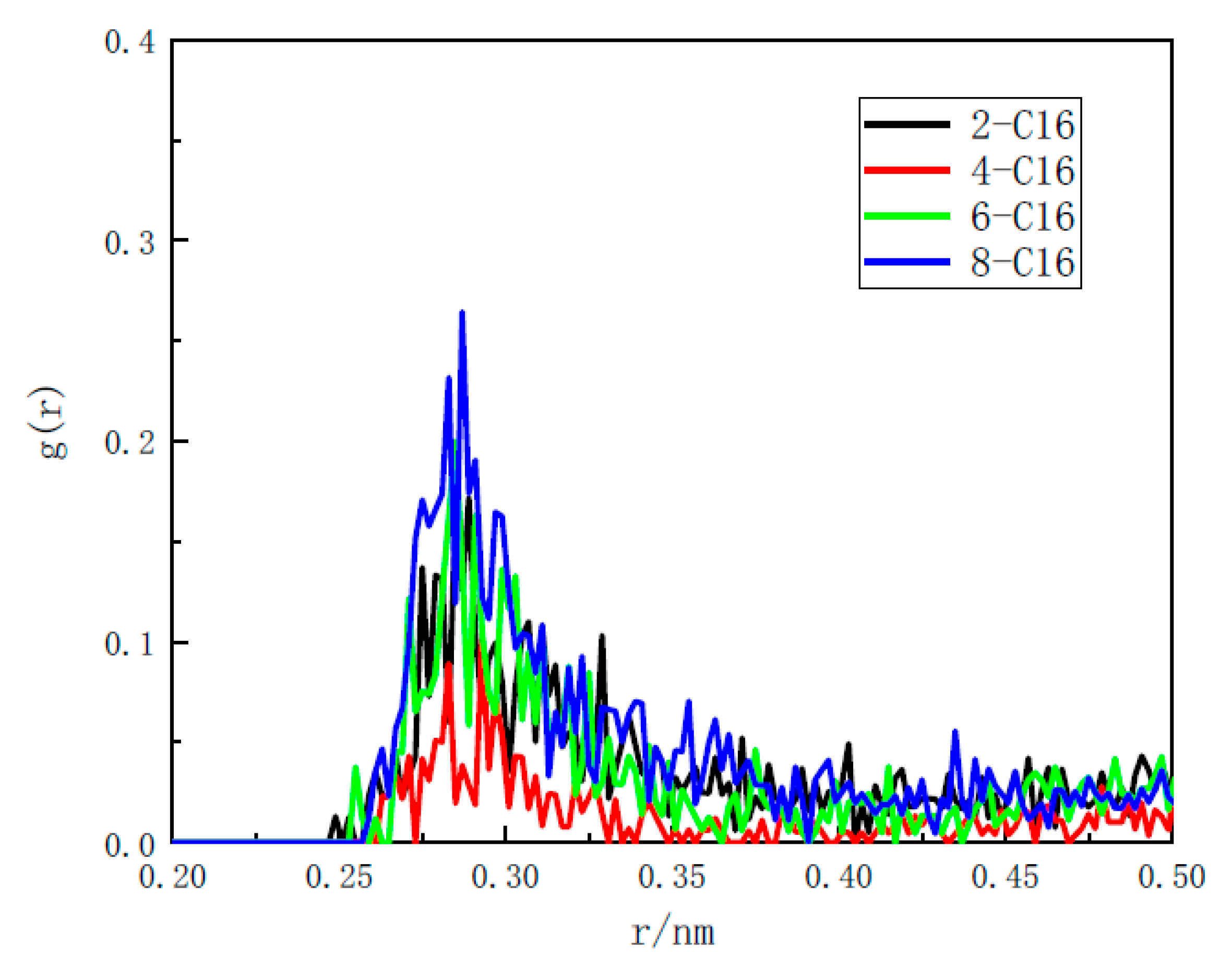
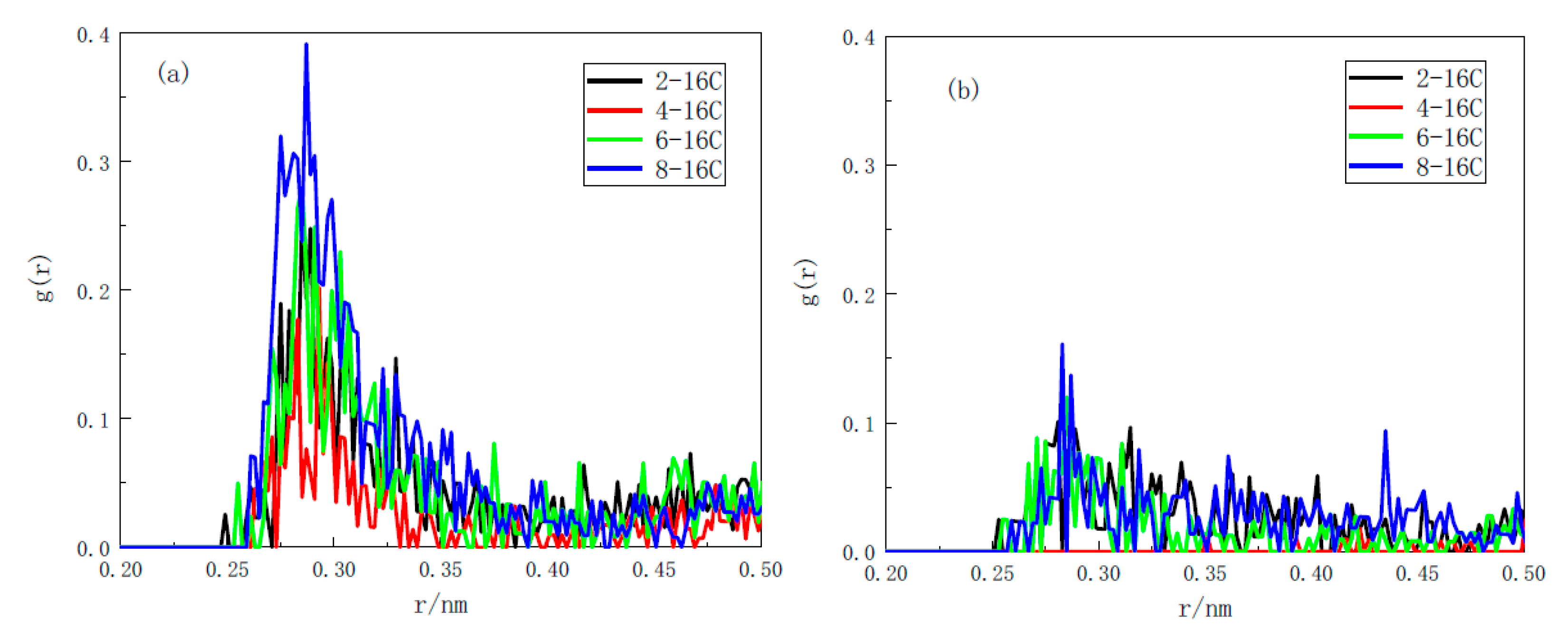
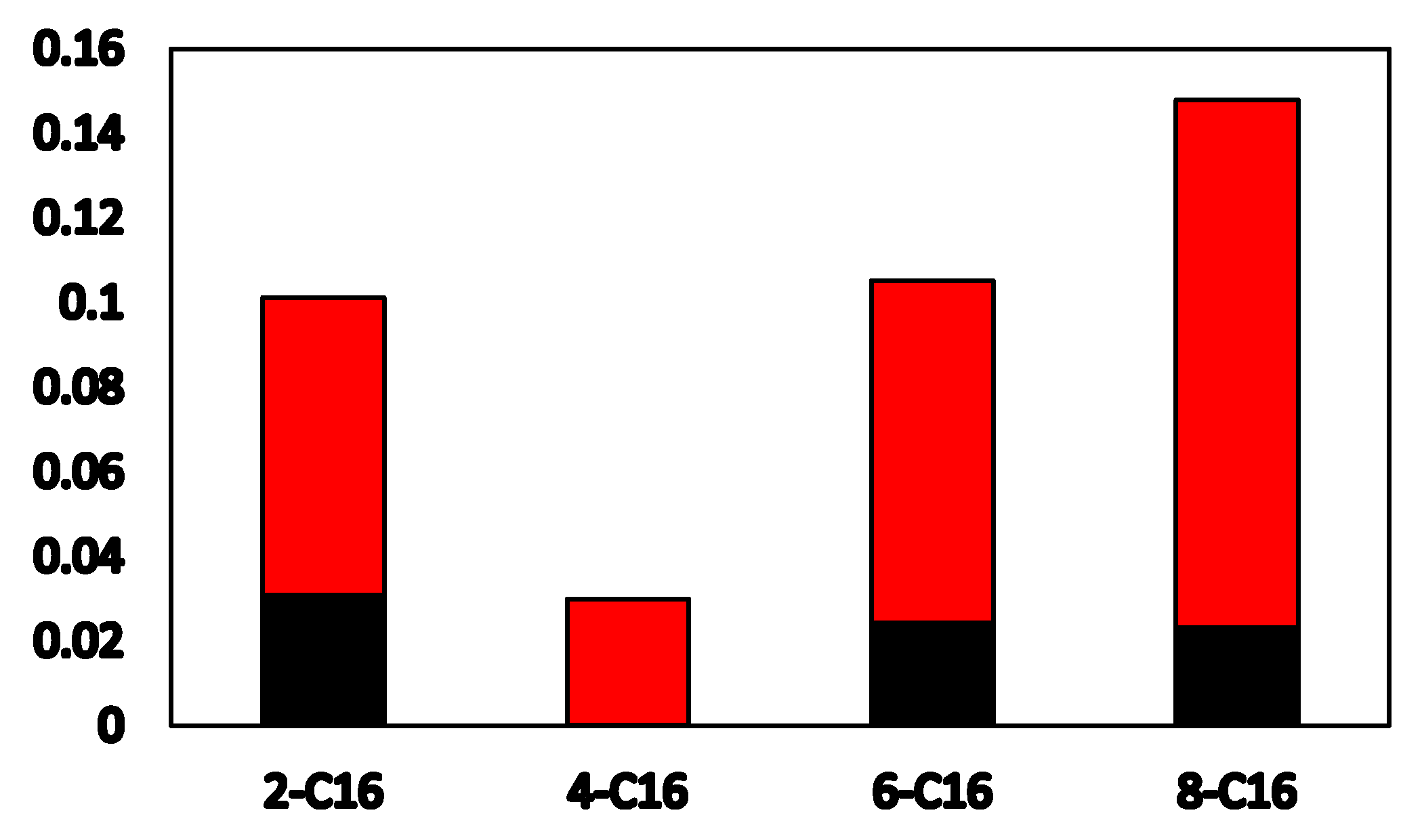
3.3.1. Radial Distribution Function
3.3.2. Surfactant Molecular Orientation
4. Conclusions
- (1)
- The benzene ring has different attachment sites in the backbone and different adsorption strengths. By analyzing the interaction energy of the surfactant–anthracite system and the CSA, it is found that the total interaction energy of the 4-C16/anthracite system is the lowest, while the contact surface area between 4-C16 and anthracite is the largest. It shows that 4-C16 has the highest adsorption strength on the surface of anthracite. The difference in adsorption strength is due to the different structure of the surfactant.
- (2)
- Through the analysis of the adsorption configuration and relative concentration distribution of the graphite layer system, it is found that the reason for the highest adsorption strength of 4-C16 on the surface of anthracite is that it has the highest adsorption strength near the oxygen-containing functional groups on the surface of anthracite.
- (3)
- Through the RDF and coordination number analysis of the water/surfactant/anthracite system, it is found that 4-C16 has the highest adsorption strength in the solution and the strongest ability to “isolate” water molecules. The reason is that (a) 4-C16 can densely cover the ketone groups on the surface of anthracite, completely “isolating” water from contact with the ketone groups; (b) 4-C16 has the best effect of “isolating” water near the hydroxyl group. Through surfactant orientation analysis, it is found that the angle between the long-chain branch of 4-C16 and the Z axis is the smallest, and the degree of order in the Z axis is the highest. This is the reason why the layered structure formed by 4-C16 is denser.
- (4)
- The study of alkyl sulfonate isomers on the surface of anthracite can help in better understanding the influence of the surfactant structure on the adsorption strength. The research results have important theoretical and practical significance for the development of new surfactants and the enrichment and development of the basic theory of coal wettability.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Sun, C.; Kou, J.; Zhao, H.; Wei, D.; Xing, Y. Enhancing the leaching of chalcopyrite using Acidithiobacillus ferrooxidans under the induction of surfactant triton X-100. Minerals 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Xu, J.; Geng, P.; Li, J. Carrier flotation of low-rank coal with polystyrene. Minerals 2020, 10, 452. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Wang, C.; Zhou, H.; Zhang, L.; Zhao, S. Characterizing the impact of surfactant structure on interfacial tension: A molecular dynamics study. J. Mol. Model. 2017, 23, 112. [Google Scholar] [CrossRef]
- Chen, C.-L.; Liao, Y.-F.; Lu, F.; Zheng, Y.-S.; Peng, Y.-Y.; Ding, C.-W.; Tong, Q.-X. Facile synthesis, surface activity, wettability and ultrahigh foaming properties of novel nonionic Gemini fluorocarbon surfactants. J. Mol. Liq. 2020, 302, 112469. [Google Scholar] [CrossRef]
- Askari, A.; Vahabzadeh, F.; Mardanpour, M.M. Quantitative determination of linear alkylbenzene sulfonate (LAS) concentration and simultaneous power generation in a microbial fuel cell-based biosensor. J. Clean. Prod. 2021, 294, 126349. [Google Scholar] [CrossRef]
- Zhao, X.; Gong, L.; Liao, G.; Luan, H.; Chen, Q.; Liu, D.; Feng, Y. Micellar solubilization of petroleum fractions by heavy alkylbenzene sulfonate surfactant. J. Mol. Liq. 2021, 329, 115519. [Google Scholar] [CrossRef]
- Franco-Belussi, L.; Jones-Costa, M.; Salla, R.F.; de Souza, B.F.S.; Pinto-Vidal, F.A.; de Oliveira, C.R.; Silva-Zacarin, E.C.M.; Abdalla, F.C.; Duarte, I.C.S.; De Oliveira, C. Hepatotoxicity of the anionic surfactant linear alkylbenzene sulphonate (LAS) in bullfrog tadpoles. Chemosphere 2021, 266, 129014. [Google Scholar] [CrossRef]
- Chai, L.; Yang, L.; Zhang, Y.; Zhou, Y.; Wang, F.; Wu, Z. Antagonism or synergism? Responses of Hydrocharis dubia (Bl.) Backer to linear alkylbenzene sulfonate, naphthalene and their joint exposure. Ecotoxicol. Environ. Saf. 2020, 200, 110747. [Google Scholar] [CrossRef]
- He, X.; Guvench, O.; MacKerell, A.D., Jr.; Klein, M.L. Atomistic simulation study of linear alkylbenzene sulfonates at the water/air interface. J. Phys. Chem. B 2010, 114, 9787–9794. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.A.; Huggett, A.; Jones, C.; Ortego, G. Surface activity and performance properties of gemini salts of linear alkylbenzene sulfonate in aqueous solution. J. Surfactants Deterg. 2021, 24, 563–574. [Google Scholar] [CrossRef]
- Lee, T.; Park, U.; Puligundla, P.; Mok, C. Corona discharge plasma-based degradation of simulated residual linear alkylbenzene sulphonate and dodecyl benzene sulfonate surfactants. Int. J. Environ. Sci. Technol. 2019, 17, 1171–1178. [Google Scholar] [CrossRef]
- Kishimoto, N.; Hamamoto, S. Removal of linear alkylbenzene sulfonate (LAS) by a cetyltrimethylammonium bromide (CTAB)-aided coagulation-filtration process. Environ. Technol. 2020, 42, 1–9. [Google Scholar] [CrossRef]
- Wang, X.P.; Liu, X.C.; Huo, Y.Q.; Niu, J.P. Properties of binary mixture of cetyl biphenyl ether disulfonate and linear alkylbenzene sulfonate. Tenside Surfactants Deterg. 2020, 57, 259–264. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M.; Sepehrian, M.; Maghsoodlu, M. Facile hydrothermal synthesis of hierarchical sodium P zeolite as a nonphosphate detergent builder. J. Surfactants Deterg. 2021, 24, 85–97. [Google Scholar] [CrossRef]
- Gang, H.; Bian, P.; He, X.; He, X.; Bao, X.; Mu, B.; Li, Y.; Yang, S. Mixing of surfactin, an anionic biosurfactant, with alkylbenzene sulfonate, a chemically synthesized anionic surfactant, at the n-decane /water interface. J. Surfactants Deterg. 2021, 24, 445–457. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, G.; Li, S.; Wang, C.; Liu, R.; Jiang, W. Molecular dynamics simulation and experimental characterization of anionic surfactant: Influence on wettability of low-rank coal. Fuel 2020, 279, 118323. [Google Scholar] [CrossRef]
- Han, W.; Zhou, G.; Xing, M.; Yang, Y.; Zhang, X.; Miao, Y.; Wang, Y. Experimental investigation on physicochemical characteristics of coal treated with synthetic sodium salicylate-imidazole ionic liquids. J. Mol. Liq. 2021, 327, 114822. [Google Scholar] [CrossRef]
- Li, L.; He, M.; Li, Z.; Ma, C.; Yu, H.; You, X. Wettability effect of ethoxylated nonyl phenol with different ethylene oxide chain length on Shendong long-flame coal surface. Mater. Today Commun. 2021, 26, 101697. [Google Scholar] [CrossRef]
- Meng, J.; Yin, F.; Li, S.; Zhong, R.; Sheng, Z.; Nie, B. Effect of different concentrations of surfactant on the wettability of coal by molecular dynamics simulation. Int. J. Min. Sci. Technol. 2019, 29, 577–584. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, X.; Shah, S.; Wu, X. Experimental study of influence of natural surfactant soybean phospholipid on wettability of high-rank coal. Fuel 2019, 239, 1–12. [Google Scholar] [CrossRef]
- Yuan, M.; Nie, W.; Zhou, W.; Yan, J.; Bao, Q.; Guo, C.; Tong, P.; Zhang, H.; Guo, L. Determining the effect of the non-ionic surfactant AEO9 on lignite adsorption and wetting via molecular dynamics (MD) simulation and experiment comparisons. Fuel 2020, 278, 118339. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Fan, M.; Zhang, L. The effect of ethylene oxide groups in dodecyl ethoxyl ethers on low rank coal flotation: An experimental study and simulation. Powder Technol. 2019, 344, 684–692. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, C.; Zhang, Y.; Zhou, G.; Zhang, S.; Wang, D. Micromechanism of coal dust wettability and its effect on the selection and development of dust suppressants. Process. Saf. Environ. Prot. 2017, 111, 726–732. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Cheng, Y.; Xu, G. Decrease in hydrophilicity and moisture readsorption of lignite: Effects of surfactant structure. Fuel 2020, 273, 117812. [Google Scholar] [CrossRef]
- Ni, G.H.; Qian, S.; Meng, X.; Hui, W.; Xu, Y.H.; Cheng, W.M.; Gang, W. Effect of NaCl-SDS compound solution on the wettability and functional groups of coal. Fuel 2019, 257, 116077. [Google Scholar]
- Chang, Z.; Chen, X.; Peng, Y. The interaction between diesel and surfactant triton X-100 and their adsorption on coal surfaces with different degrees of oxidation. Powder Technol. 2019, 342, 840–847. [Google Scholar] [CrossRef]
- Dey, S. Enhancement in hydrophobicity of low rank coal by surfactants—A critical overview. Fuel Process. Technol. 2012, 94, 151–158. [Google Scholar] [CrossRef]
- Zhou, P.; Hou, J.; Yan, Y.; Wang, J. The effect of surfactant adsorption on surface wettability and flow resistance in slit nanopore: A molecular dynamics study. J. Colloid Interface Sci. 2018, 513, 379–388. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Xia, Y.; Liu, S. Wettability modification of wender lignite by adsorption of dodecyl poly ethoxylated surfactants with different degree of ethoxylation: A molecular dynamics simulation study. J. Mol. Graph. Model. 2017, 76, 106–117. [Google Scholar] [CrossRef]
- Zhang, H.; Xi, P.; Zhuo, Q.; Liu, W. Construction of molecular model and adsorption of collectors on bulianta coal. Molecules 2020, 25, 4030. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Yan, K. Adsorption of collectors on model surface of wiser bituminous coal: A molecular dynamics simulation study. Miner. Eng. 2015, 79, 31–39. [Google Scholar] [CrossRef]
- Chen, X.L.; Yan, G.C.; Yang, X.L.; Feng, Z.Z.; Wei, S. Molecular Dynamics Simulation of the Effect of SDS/SDBS on the Wettability of Anthracite. Coal Science and Technology. Available online: https://kns.cnki.net/kcms/detail/11.2402.TD.20210623.1502.004.html (accessed on 23 June 2021).
- Wei, S.; Yan, G.C.; Zhang, Z.Q.; Liu, M.S.; Zhang, Y.F. Molecular structure analysis of jincheng anthracite coal. J. China Coal Soc. 2018, 43, 555–562. [Google Scholar]
- Zheng, M.; Li, X.; Liu, J.; Guo, L. Initial chemical reaction simulation of coal pyrolysis via. ReaxFF molecular dynamics. Energy Fuels 2013, 27, 2942–2951. [Google Scholar] [CrossRef]
- Yan, G.; Ren, G.; Bai, L.; Feng, J.; Zhang, Z. Molecular model construction and evaluation of jincheng anthracite. ACS Omega 2020, 5, 10663–10670. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, D.M.; Cai, Y.D.; Zhao, B.; Qiu, Y.K.; Zhou, Y.F. Scale-span pore structure heterogeneity of high volatile bituminous coal and anthracite by FIB-SEM and X-ray mu-CT. J. Nat. Gas. Sci. Eng. 2020, 81, 103443. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Qu, G.; Cheng, J.; Xue, C.; Gao, X.; Sun, T.; Ding, W. Molecular dynamics simulation of the aggregation behavior of N-Dodecyl-N,N-Dimethyl-3-Ammonio-1-Propanesulfonate/sodium dodecyl benzene sulfonate surfactant mixed system at oil/water interface. Colloids Surf. A Physicochem. Eng. Asp. 2017, 531, 73–80. [Google Scholar] [CrossRef]
- Anvari, M.H.; Liu, Q.; Xu, Z.; Choi, P. Molecular dynamics study of hydrophilic sphalerite (110) surface as modified by normal and branched butylthiols. Langmuir 2018, 34, 3363–3373. [Google Scholar] [CrossRef]
- Rai, B.; Sathish, P.; Tanwar, J.; Pradip; Moon, K.; Fuerstenau, D. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals. J. Colloid Interface Sci. 2011, 362, 510–516. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhou, H.T.; Ge, J.J.; Xu, G.Y. Aggregation behavior of double-chained anionic surfactant 1-C-m-C-9-SO3Na at air/liquid interface: Molecular dynamics simulation. Acta Phys. Chim. Sin. 2017, 33, 1214–1222. [Google Scholar] [CrossRef]
- Kubelka, J.; Bai, S.; Piri, M. Effects of surfactant charge and molecular structure on wettability alteration of calcite: Insights from molecular dynamics simulations. J. Phys. Chem. B 2021, 125, 1293–1305. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhang, R.; Xing, Y.; Gui, X. Enhancement of the surface hydrophobicity of low-rank coal by adsorbing DTAB: An experimental and molecular dynamics simulation study. Fuel 2019, 239, 145–152. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, S.; Yan, H.; Zhao, X. Mechanism of oil detachment from a silica surface in aqueous surfactant solutions: Molecular dynamics simulations. J. Phys. Chem. B 2012, 116, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, X.; Song, D.; Nie, B. Effect of microstructure on electrical property of coal surface. Appl. Surf. Sci. 2019, 483, 713–720. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Wang, L.; Cao, X.-L.; Song, X.-W.; Zhang, L.; Zhao, S.; Yu, J.-Y. Influence of alkyl chain length and position on microenvironmental properties of alkylbenzenesulfonates micelles. J. Dispers. Sci. Technol. 2009, 30, 65–67. [Google Scholar] [CrossRef]
- Cui, X.-H.; Wang, L.; Cao, X.-L.; Zhao, F.-L.; Luo, L.; Zhang, L.; Zhao, S.; Yu, J.-Y. Effect of additional alkyl substituents on the adsorption properties of sodium branched-alkylbenzenesulfonates. J. Dispers. Sci. Technol. 2008, 29, 1153–1157. [Google Scholar] [CrossRef]
- He, X.; Shinoda, W.; DeVane, R.; Anderson, K.L.; Klein, M.L. Paramaterization of a coarse-grained model for linear alkylbenzene sulfonate surfactants and molecular dynamics studies of their self-assembly in aqueous solution. Chem. Phys. Lett. 2010, 487, 71–76. [Google Scholar] [CrossRef]
- Wang, W.; Liang, L.; Peng, Y.; Holuszko, M. Surface chemical heterogeneity of low rank coal characterized by micro-FTIR and its correlation with hydrophobicity. Minerals 2021, 11, 239. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Fan, M.; Zhang, L. Decrease of hydrophilicity of lignite using CTAB: Effects of adsorption differences of surfactant onto mineral composition and functional groups. Fuel 2017, 197, 474–481. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Hao, M.; Li, B.; Liu, S. Microscopic spreading characteristics of non-polar oil droplet on low rank coal surface: Effects of surfactant pretreatment and oxygen-containing groups. J. Mol. Liq. 2021, 325, 115232. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Li, B.; Xie, Z.; Guo, J.; Liu, S. Experimental and molecular dynamics simulation study on the enhancement of low rank coal flotation by mixed collector. Fuel 2020, 266, 117046. [Google Scholar] [CrossRef]
- Zhang, R.; Xing, Y.; Xia, Y.; Luo, J.; Tan, J.; Rong, G.; Gui, X. New insight into surface wetting of coal with varying coalification degree: An experimental and molecular dynamics simulation study. Appl. Surf. Sci. 2020, 511, 145610. [Google Scholar] [CrossRef]
- Yan, H.; Guo, X.-L.; Yuan, S.-L.; Liu, C.-B. Molecular dynamics study of the effect of calcium ions on the monolayer of SDC and SDSn surfactants at the vapor/liquid interface. Langmuir 2011, 27, 5762–5771. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Yang, C.-L.; Wang, M.-S.; Chen, B.-D.; Ma, X.G. Crystallization of alkane melts induced by carbon nanotubes and graphene nanosheets: A molecular dynamics simulation study. Phys. Chem. Chem. Phys. 2011, 13, 15476–15482. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Chen, H.; Wang, P.; Xie, Z.; Yao, Y.; Yan, Y.; Zhang, J. Effect of surfactant headgroups on the oil/water interface: An interfacial tension measurement and simulation study. J. Mol. Struct. 2013, 1052, 50–56. [Google Scholar] [CrossRef]
- Parra, J.G.; Iza, P.; Dominguez, H.; Schott, E.; Zarate, X. Effect of triton X-100 surfactant on the interfacial activity of ionic surfactants SDS, CTAB and SDBS at the air/water interface: A study using molecular dynamic simulations. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125284. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.-L.; Liu, G.-S. Molecular dynamics simulation of primary ammonium ions with different alkyl chains on the muscovite (001) surface. Int. J. Miner. Process. 2015, 145, 48–56. [Google Scholar] [CrossRef]
- Takeda, K.; Andoh, Y.; Shinoda, W.; Okazaki, S. Molecular behavior of linear alkylbenzene sulfonate in hydrated crystal, tilted gel, and liquid crystal phases studied by molecular dynamics simulation. Langmuir 2019, 35, 10877–10884. [Google Scholar] [CrossRef]
- Stanishneva-Konovalova, T.; Sokolova, O. Molecular dynamics simulations of negatively charged DPPC/DPPI lipid bilayers at two levels of resolution. Comput. Theor. Chem. 2015, 1058, 61–66. [Google Scholar] [CrossRef]
- Vermeer, L.; De Groot, B.L.; Reat, V.; Milon, A.; Czaplicki, J. Acyl chain order parameter profiles in phospholipid bilayers: Computation from molecular dynamics simulations and comparison with 2H NMR experiments. Eur. Biophys. J. 2007, 36, 919–931. [Google Scholar] [CrossRef] [Green Version]

| Proximate Analysis | Ultimate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | FCd | C | H | O | N | S |
| 0.66 | 23.05 | 12.86 | 67.05 | 91.51 | 3.89 | 2.10 | 1.71 | 0.79 |
| Model | EV/(kcal·mol−1) | EL/(kcal·mol−1) | E/(kcal·mol−1) |
|---|---|---|---|
| 2-C16/Anthracite | −277.92 | −2.23 | −280.15 |
| 4-C16/Anthracite | −293.77 | −6.54 | −300.31 |
| 6-C16/Anthracite | −273.07 | −3.01 | −276.08 |
| 8-C16/Anthracite | −257.94 | −3.29 | −261.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yan, G.; Yang, X.; Xu, G.; Wei, S. Microscopic Diffusion Characteristics of Linear Alkylbenzene Sulfonates on the Surface of Anthracite: The Influence of Different Attachment Sites of Benzene Ring in the Backbone. Minerals 2021, 11, 1045. https://doi.org/10.3390/min11101045
Chen X, Yan G, Yang X, Xu G, Wei S. Microscopic Diffusion Characteristics of Linear Alkylbenzene Sulfonates on the Surface of Anthracite: The Influence of Different Attachment Sites of Benzene Ring in the Backbone. Minerals. 2021; 11(10):1045. https://doi.org/10.3390/min11101045
Chicago/Turabian StyleChen, Xuanlai, Guochao Yan, Xianglin Yang, Guang Xu, and Shuai Wei. 2021. "Microscopic Diffusion Characteristics of Linear Alkylbenzene Sulfonates on the Surface of Anthracite: The Influence of Different Attachment Sites of Benzene Ring in the Backbone" Minerals 11, no. 10: 1045. https://doi.org/10.3390/min11101045
APA StyleChen, X., Yan, G., Yang, X., Xu, G., & Wei, S. (2021). Microscopic Diffusion Characteristics of Linear Alkylbenzene Sulfonates on the Surface of Anthracite: The Influence of Different Attachment Sites of Benzene Ring in the Backbone. Minerals, 11(10), 1045. https://doi.org/10.3390/min11101045







