Backtracking to Parent Maceral from Produced Bitumen with Raman Spectroscopy
Abstract
1. Introduction
- The liptinite group (Type I and II kerogen) includes primarily algal material or amorphous organic matter derived from algal or bacterial precursors,
- the vitrinite group (Type III kerogen) refers to organic matter that is derived from the woody tissue of post-Silurian vascular plants,
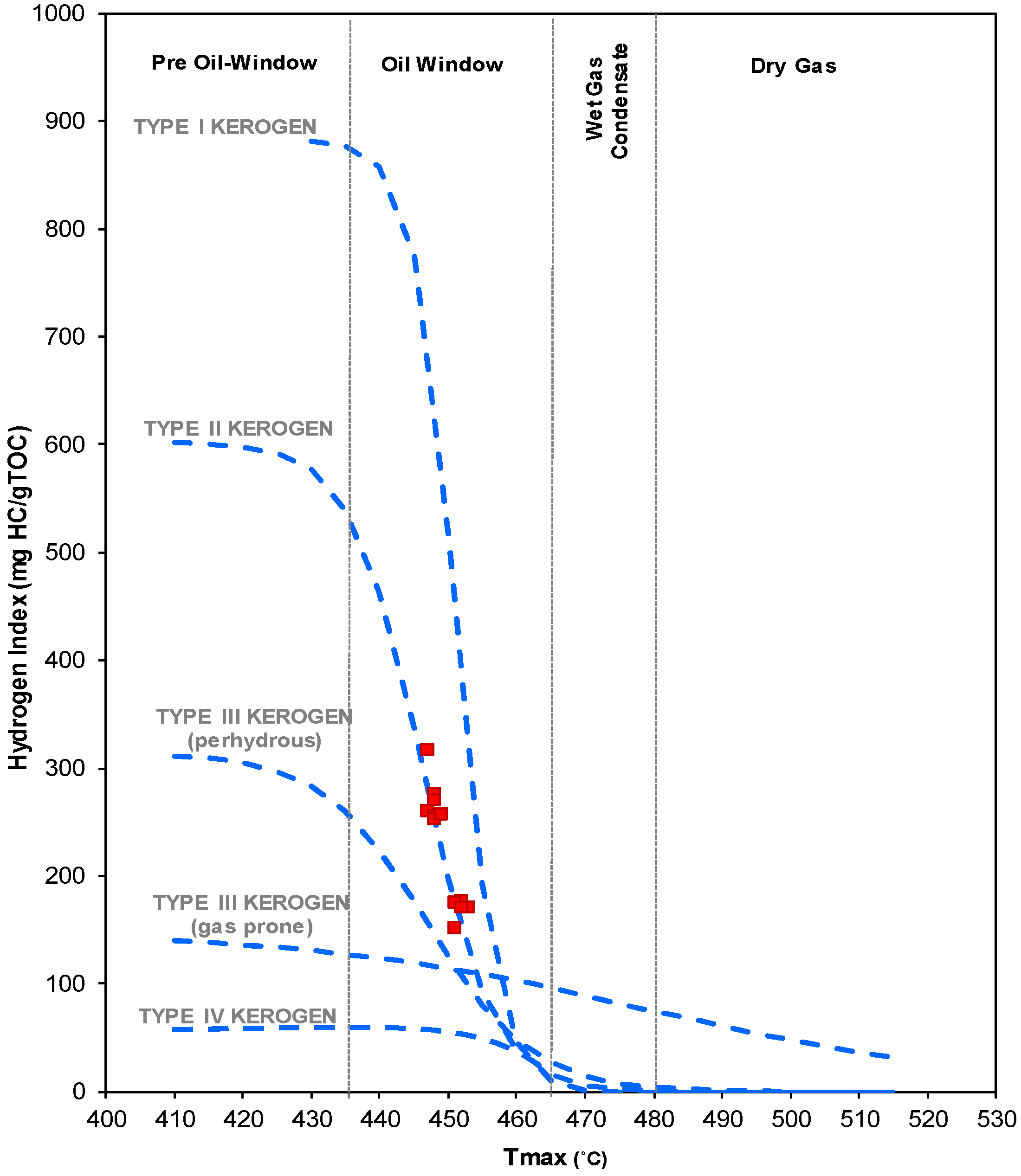
2. Samples and Measurements
3. Results and Discussion
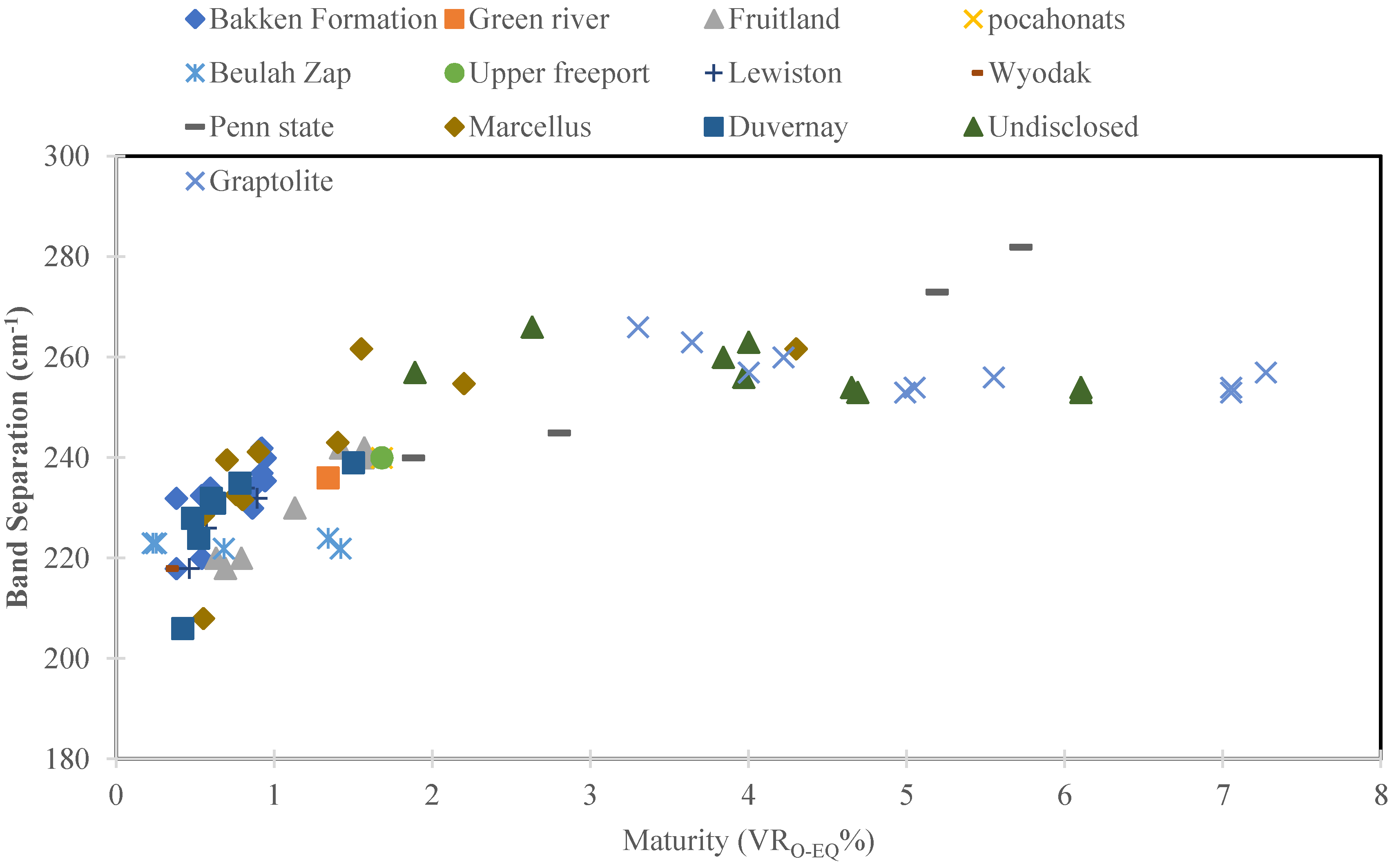
3.1. Raman Spectroscopy and Maturity
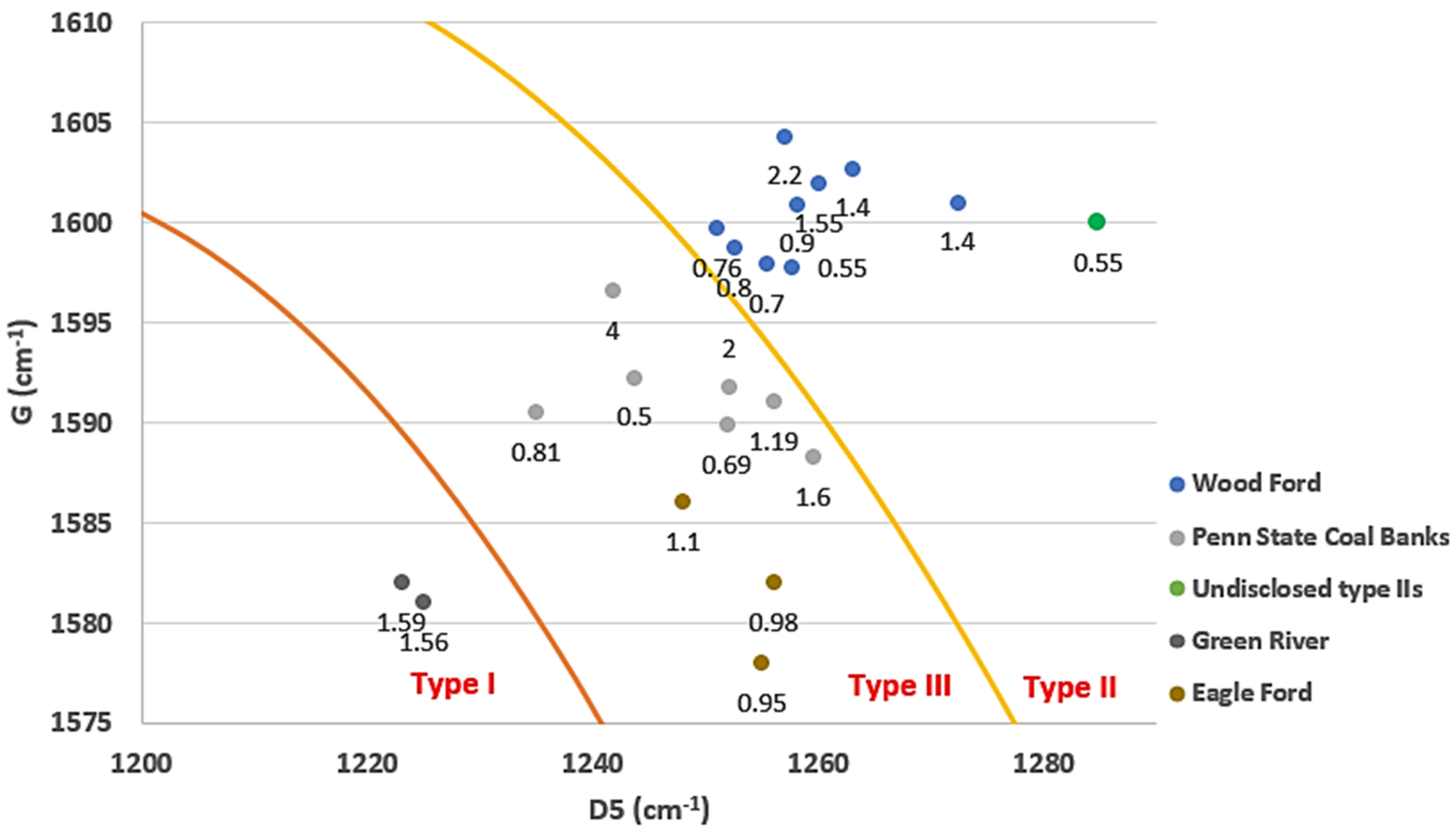
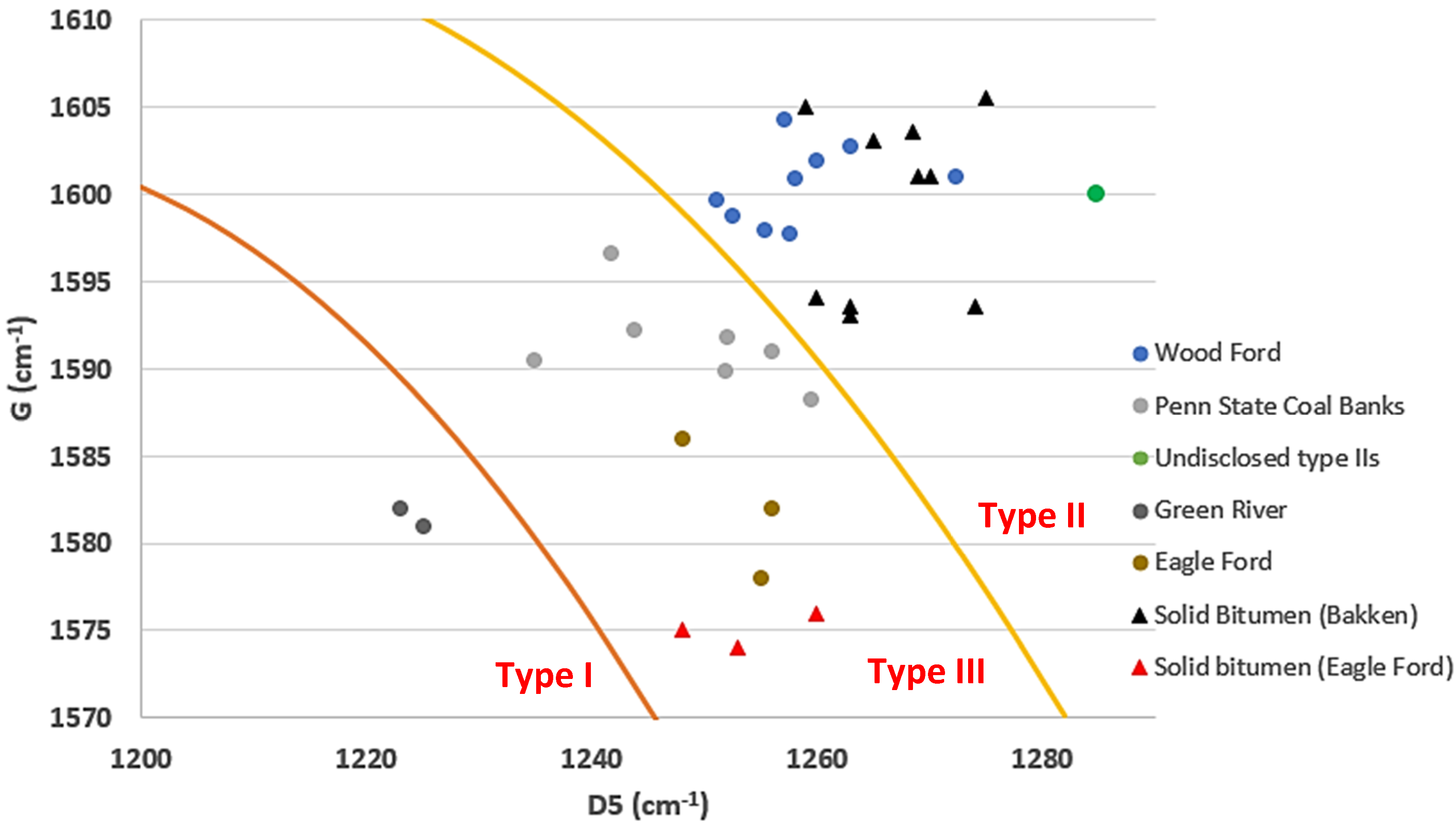
3.2. Raman Spectroscopy and Kerogen Typing
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hutton, A.; Bharati, S.; Robl, T. Chemical and petrographic classification of kerogen/macerals. Energy Fuels 1994, 8, 1478–1488. [Google Scholar] [CrossRef]
- Vandenbroucke, M.; Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Tissot, B.; Welte, D. Petroleum Formation and Occurrence: A New Approach to Oil and Gas Exploration; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Waples, D.W. Geochemistry in Petroleum Exploration; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Peters, K. Guidelines for evaluating petroleum source rock using programmed pyrolysis. AAPG Bull. 1986, 70, 318–329. [Google Scholar]
- Abarghani, A.; Ostadhassan, M.; Gentzis, T.; Carvajal-Ortiz, H.; Ocubalidet, S.; Bubach, B.; Mann, M.; Hou, X. Correlating Rock-EvalTM Tmax with bitumen reflectance from organic petrology in the Bakken Formation. Int. J. Coal Geol. 2019, 205, 87–104. [Google Scholar] [CrossRef]
- Spackman, W.; Moses, R.G. Nature and Occurrence of Ash Forming Minerals in Anthracite; No. SR-22; Department of Geology, University Park (USA), Pennsylvania State University: State College, PA, USA, 1960. [Google Scholar]
- Teichmuller, M.; Teichmuller, R. Stach’s Textbook of Coal Petrology; Gebruder Borntraeger: Stuttgart, Germany, 1982. [Google Scholar]
- Styan, W.B.T.; Bustin, R.M. Petrographyof some fraser river delta peat deposits: Coal maceral and microlithotype precursors in temperate-climate peats. Int. J. Coal Geol. 1983, 2, 321–370. [Google Scholar] [CrossRef]
- Teichmüller, M. The genesis of coal from the viewpoint of coal petrology. Int. J. Coal Geol. 1989, 12, 1–87. [Google Scholar] [CrossRef]
- International, Committee for Coal and Organic Petrology. The new vitrinite classification (ICCP System 1994). Fuel 1998, 77, 349–358. [Google Scholar] [CrossRef]
- International, Committee for Coal and Organic Petrology. The new inertinite classification (ICCP System 1994). Fuel 2001, 80, 459–471. [Google Scholar] [CrossRef]
- Taylor, G.H.; Teichmüller, M.; Davis, A.C.F.K.; Diessel, C.F.K.; Ralf, L.; Paul, R. Organic Petrology; Borntraeger: Berlin-Stuttgar, Germany, 1998. [Google Scholar]
- ISO-7404/5.2009. Methods for the Petrographic Analysis of Coals. Part 5: Method of Determining Microscopically the Reflectance of Vitrinite. 2009. Available online: https://www.iso.org/standard/42832.html (accessed on 27 July 2020).
- American Society for Testing and Materials (ASTM). Standard test method for microscopical determination of the reflectance of vitrinite dispersed in sedimentary rocks. Pet. Prod. Lubr. Fossil Fuels gaSeous Fuels Coal Coke 2014, 823–830. [Google Scholar] [CrossRef]
- Pickel, W.; Kus, J.; Flores, D.; Kalaitzidis, S.; Christanis, K.; Cardott, B.J.; Misz-Kennan, M.; Crosdale, P.; Wagner, N.; ICCP. Classification of liptinite-ICCP System 1994. Int. J. Coal Geol. 2017, 169, 40–61. [Google Scholar] [CrossRef]
- Lis, G.P.; Mastalerz, M.; Schimmelmann, A.; Lewan, M.D.; Artur Stankiewicz, B. FTIR absorption indices for thermal maturity in comparison with vitrinite reflectance R0 in type-II kerogens from Devonian black shales. Org. Geochem. 2005, 36, 1533–1552. [Google Scholar] [CrossRef]
- Abarghani, A.; Gentzis, T.; Shokouhimehr, M.; Liu, B.; Ostadhassan, M. Chemical heterogeneity of organic matter at nanoscale by AFM-based IR spectroscopy. Fuel 2020, 261, 116454. [Google Scholar] [CrossRef]
- Lafargue, E.; Marquis, F.; Pillot, D. Rock-Eval 6 applications in hydrocarbon exploration, production, and soil contamination studies. Revue de l’institut Français du Pétrole 1998, 53, 421–437. [Google Scholar] [CrossRef]
- Behar, F.; Beaumont, V.; De Penteado, H.L.B. Rock-Eval 6 technology: Performances and developments. Oil Gas Sci. Technol. 2001, 56, 111–134. [Google Scholar] [CrossRef]
- Kultaransingh, H.; Hathon, L.; Dixon, M.; Myers, M. Organic matter characterization in shales: A systematic empirical protocol. Micros. Microanal. 2017, 23, 2130–2131. [Google Scholar]
- Liu, Z.; Hathon, L.A.; Myers, M.T. Characterization of thermal maturity and organic material type With Raman Spectroscopy in shale reservoirs. In Proceedings of the SPWLA 58th Annual Logging Symposium, Oklahoma City, OK, USA, 17–21 June 2017; Society of Petrophysicists and Well-Log Analysts: Houston, TX, USA, 2017. [Google Scholar]
- John, R.; Nicholas, P. Cheremisinoff. Gasification Technologies: A Primer for Engineers and Scientists; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Chaudhuri, S.N. Coal Macerals. Encycl. Miner. Energy Policy 2016, 1–5. [Google Scholar]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide. Volume 2: Biomarkers and Isotopes in the Environment and Human History; Cambridge University Press: Cambridge, UK, 2005; Volume 75, p. 76. [Google Scholar]
- Hackley, P.C.; Cardott, B.J. Application of organic petrography in North American shale petroleum systems: A review. Int. J. Coal Geol. 2016, 163, 8–51. [Google Scholar] [CrossRef]
- Cardott, B.J.; Kidwai, M.A. Graptolite reflectance as a potential thermal-maturation indicator. Okla. Geol. Surv. Circ. 1991, 92, 203–209. [Google Scholar]
- Suárez-Ruiz, I.; Flores, D.; Filho, J.G.M.; Hackley, P.C. Review and update of the applications of organic petrology: Part 1, geological applications. Int. J. Coal Geol. 2012, 99, 54–112. [Google Scholar] [CrossRef]
- Jacob, H. Classification, structure, genesis and practical importance of natural solid oil bitumen (“migrabitumen”). Int. J. Coal Geol. 1989, 11, 65–79. [Google Scholar] [CrossRef]
- Bertrand, R.; Malo, M. Source rock analysis, thermal maturation and hydrocarbon generation in Siluro-Devonian rocks of the Gaspe Belt basin, Canada. Bull. Can. Pet. Geol. 2001, 49, 238–261. [Google Scholar] [CrossRef]
- Mählmann, R.F.; Frey, M. Standardisation, calibration and correlation of the Kübler-index and the vitrinite/bituminite reflectance: An inter-laboratory and field related study. Swiss J. Geosci. 2012, 105, 153–170. [Google Scholar]
- Daniel, R.; Littke, R.; Bruns, B.; Mahlstedt, N. Organic Geochemistry and petrography of Lower Cretaceous Wealden black shales of the Lower Saxony Basin: The transition from lacustrine oil shales to gas shales. Org. Geochem. 2013, 63, 18–36. [Google Scholar]
- Danielle, K.; Sanei, H.; Embry, A.; Ardakani, O.H.; Clarkson, C.R. Depositional environment and hydrocarbon potential of the Middle Triassic strata of the Sverdrup Basin, Canada. Int. J. Coal Geol. 2015, 147, 71–84. [Google Scholar]
- Mastalerz, M.; Drobniak, A.; Stankiewicz, A.B. Origin, properties, and implications of solid bitumen in source-rock reservoirs: A review. Int. J. Coal Geol. 2018, 195, 14–36. [Google Scholar] [CrossRef]
- Tissot, B.P.; Dietrich, H.W. From kerogen to petroleum. In Petroleum Formation and Occurrence; Springer: Berlin/Heidelberg, Germany, 1984; pp. 160–198. [Google Scholar]
- Tyson, R.V. Palynological kerogen classification. In Sedimentary Organic Matter; Springer: Dordrecht, The Netherlands, 1995; pp. 341–365. [Google Scholar]
- Vincent, A.J. Palynofacies Analysis of Middle Jurassic Sediments from the Inner Hebrides. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 1995. [Google Scholar]
- Mendonça Filho, J.G. Aplicação de estudos de palinofácies e fácies orgânica em rochas do Paleozóico da Bacia do Paraná, Sul do Brasil. Universidade Federal do Rio Grande do Sul. Tese de Doutorado 1999, 2, 338. [Google Scholar]
- Lewan, M.D. Laboratory simulation of petroleum formation. In Organic Geochemistry; Springer: Boston, MA, USA, 1993; pp. 419–442. [Google Scholar]
- Thomas, G.; Carvajal-Ortiz, H.; Tahoun, S.; Li, C.; Ostadhassan, M.; Xie, H.; Filho, J.G.M. A Multi-Component Approach to Study the Source-Rock Potential of the Bakken Shale in North Dakota, USA, Using Organic Petrology, Rock-Eval Pyrolysis, Palynofacies, LmPy-GCMSMS Geochemistry, and NMR Spectroscopy. 2017. Available online: http://archives.datapages.com/data/tsop/tsopv34-2017/gentzis.htm (accessed on 27 July 2020).
- Thomas, G.; Carvajal-Ortiz, H.; Ocubalidet, S.G.; Wawak, B. Organic Petrology Characteristics of Selected Shale Oil and Shale Gas Reservoirs in the USA: Examples from “The Magnificent Nine”. In Geology: Current and Future Developments: The Role of Organic Petrology in the Exploration of Conventional and Unconventional Hydrocarbon Systems; Bentham Science Publishers: Sharjah, UAE, 2017; Volume 1, pp. 131–168. [Google Scholar]
- Houseknecht, D.W.; Bensley, D.F.; Hathon, L.A.; Kastens, P.H. Rotational reflectance properties of Arkoma Basin dispersed vitrinite: Insights for understanding reflectance populations in high thermal maturity regions. Org. Geochem. 1993, 20, 187–196. [Google Scholar] [CrossRef]
- Guedes, A.; Valentim, B.; Prieto, A.C.; Rodrigues, S.; Noronha, F. Micro-Raman spectroscopy of collotelinite, fusinite and macrinite. Int. J. Coal Geol. 2010, 83, 415–422. [Google Scholar] [CrossRef]
- Mastalerz, M.; Marc Bustin, R. Application of reflectance micro-Fourier transform infrared spectrometry in studying coal macerals: Comparison with other Fourier transform infrared techniques. Fuel 1995, 74, 536–542. [Google Scholar] [CrossRef]
- Mastalerz, M.; Marc Bustin, R. Electron microprobe and micro-FTIR analyses applied to maceral chemistry. Int. J. Coal Geol. 1993, 24, 333–345. [Google Scholar] [CrossRef]
- Ward, C.R.; Gurba, L.W. Chemical composition of macerals in bituminous coals of the Gunnedah Basin, Australia, using electron microprobe analysis techniques. Int. J. Coal Geol. 1999, 39, 279–300. [Google Scholar] [CrossRef]
- Marqués, M.; Suárez-Ruiz, I.; Flores, D.; Guedes, A.; Rodrigues, S. Correlation between optical, chemical and micro-structural parameters of high-rank coals and graphite. Int. J. Coal Geol. 2009, 77, 377–382. [Google Scholar] [CrossRef]
- Wilkins, R.W.T.; Diessel, C.K.F.; Buckingham, C.P. Comparison of two petrographic methods for determining the degree of anomalous vitrinite reflectance. Int. J. Coal Geol. 2002, 52, 45–62. [Google Scholar] [CrossRef]
- Guedes, A.; Valentim, B.; Prieto, A.C.; Noronha, F. Raman spectroscopy of coal macerals and fluidized bed char morphotypes. Fuel 2012, 97, 443–449. [Google Scholar] [CrossRef]
- Mumm, A.S.; İnan, S. Microscale organic maturity determination of graptolites using Raman spectroscopy. Int. J. Coal Geol. 2016, 162, 96–107. [Google Scholar] [CrossRef]
- Guedes, A.; Noronha, F.; Carmelo Prieto, A. Characterisation of dispersed organic matter from lower Palaeozoic metasedimentary rocks by organic petrography, X-ray diffraction and micro-Raman spectroscopy analyses. Int. J. Coal Geol. 2005, 62, 237–249. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Chopin, C.; Rouzaud, J.N. Raman spectra of carbonaceous material in metasediments: A new geothermometer. J. Metamorph. Geol. 2002, 20, 859–871. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Petitet, J.-P.; Froigneux, E.; Moreau, M.; Rouzaud, J.-N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
- Hinrichs, R.; Brown, M.T.; Vasconcellos, M.A.Z.; Abrashev, M.V.; Kalkreuth, W. Simple procedure for an estimation of the coal rank using micro-Raman spectroscopy. Int. J. Coal Geol. 2014, 136, 52–58. [Google Scholar] [CrossRef]
- Schito, A.; Romano, C.; Corrado, S.; Grigo, D.; Poe, B. Diagenetic thermal evolution of organic matter by Raman spectroscopy. Org. Geochem. 2017, 106, 57–67. [Google Scholar] [CrossRef]
- Khatibi, S.; Ostadhassan, M.; Tuschel, D.; Gentzis, T.; Carvajal-Ortiz, H. Evaluating molecular evolution of kerogen by Raman Spectroscopy: Correlation with optical microscopy and rock-eval pyrolysis. Energies 2018, 11, 1406. [Google Scholar] [CrossRef]
- Griffith, W.P. Raman spectroscopy of minerals. Nature 1969, 224, 264. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P. Raman spectroscopy of minerals and mineral pigments in archaeometry. J. Raman Spectrosc. 2016, 47, 499–530. [Google Scholar] [CrossRef]
- Tuschel, D. Raman spectroscopy of oil shale. Spectroscopy 2013, 28, 20–27. [Google Scholar]
- Wilkins, R.W.T.; Boudou, R.; Sherwood, N.; Xiao, X. Thermal maturity evaluation from inertinites by Raman spectroscopy: The ‘RaMM’technique. Int. J. Coal Geol. 2014, 128, 143–152. [Google Scholar] [CrossRef]
- Waples, D.W. Organic Geochemistry for Exploration Geologists; Burgess Pub. Co.: Minneapolis, MN, USA, 1981. [Google Scholar]
- Ferrari, A.C.; Robertson, J.F. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Fang, H.L. Maturity trends in Raman spectra from kerogen and coal. Energy Fuels 2001, 15, 653–658. [Google Scholar] [CrossRef]
- Sauerer, B.; Craddock, P.R.; AlJohani, M.D.; Alsamadony, K.L.; Abdallah, W. Fast and accurate shale maturity determination by Raman spectroscopy measurement with minimal sample preparation. Int. J. Coal Geol. 2017, 173, 150–157. [Google Scholar] [CrossRef]
- Henry, D.G.; Jarvis, I.; Gillmore, G.; Stephenson, M. A rapid method for determining organic matter maturity using laser Raman spectroscopy: Application to Carboniferous organic-rich mudstones and coals. Int. J. Coal Geol. 2019, 203, 87–98. [Google Scholar] [CrossRef]
- Khatibi, S.; Ostadhassan, M.; Aghajanpour, A. Raman spectroscopy: An analytical tool for evaluating organic matter. J. Oil Gas Petrochem. Sci. 2018, 1, 28–33. [Google Scholar] [CrossRef]
- Khatibi, S.; Ostadhassan, M.; Tuschel, D.; Gentzis, T.; Bubach, B.; Carvajal-Ortiz, H. Raman spectroscopy to study thermal maturity and elastic modulus of kerogen. Int. J. Coal Geol. 2018, 185, 103–118. [Google Scholar] [CrossRef]
- Gorbanenko, O.O.; Ligouis, B. Changes in optical properties of liptinite macerals from early mature to post mature stage in Posidonia Shale (Lower Toarcian, NW Germany). Int. J. Coal Geol. 2014, 133, 47–59. [Google Scholar] [CrossRef]
- Hackley, P.C.; Araujo, C.V.; Borrego, A.G.; Bouzinos, A.; Cardott, B.J.; Cook, A.J.; Eble, C. Standardization of reflectance measurements in dispersed organic matter: Results of an exercise to improve interlaboratory agreement. Mar. Pet. Geol. 2015, 59, 22–34. [Google Scholar] [CrossRef]
- Carvajal-Ortiz, H.; Gentzis, T. Critical considerations when assessing hydrocarbon plays using Rock-Eval pyrolysis and organic petrology data: Data quality revisited. Int. J. Coal Geol. 2015, 152, 113–122. [Google Scholar] [CrossRef]
- Tuinstra, F.; Lo Koenig, J. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Yui, T.-F.; Huang, E.; Xu, J. Raman spectrum of carbonaceous material: A possible metamorphic grade indicator for low-grade metamorphic rocks. J. Metamorph. Geol. 1996, 14, 115–124. [Google Scholar] [CrossRef]
- Marshall, C.P.; Edwards, H.G.M.; Jehlicka, J. Understanding the application of Raman spectroscopy to the detection of traces of life. Astrobiology 2010, 10, 229–243. [Google Scholar] [CrossRef]
- Ferralis, N.; Matys, E.D.; Knoll, A.H.; Hallmann, C.; Summons, R.E. Rapid, direct and non-destructive assessment of fossil organic matter via microRaman spectroscopy. Carbon 2016, 180, 440–449. [Google Scholar] [CrossRef]
- Baludikay, B.K.; François, C.; Sforna, M.C.; Beghin, J.; Cornet, Y.; Storme, J.-Y.; Fagel, N.; Fontaine, F.; Littke, R.; Baudet, D.; et al. Raman microspectroscopy, bitumen reflectance and illite crystallinity scale: Comparison of different geothermometry methods on fossiliferous Proterozoic sedimentary basins (DR Congo, Mauritania and Australia). Int. J. Coal Geol. 2018, 191, 80–94. [Google Scholar] [CrossRef]
- Ostadhassan, M.; Liu, K.; Li, C.; Khatibi, S. Fine Scale Characterization of Shale Reservoirs: Methods and Challenges; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Cesare, B.; Maineri, C. Fluid-present anatexis of metapelites at El Joyazo (SE Spain): Constraints from Raman spectroscopy of graphite. Contrib. Miner. Petrol. 1999, 135, 41–52. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Li, Z.; Fredericks, P.M.; Ward, C.R.; Rintoul, L. Chemical functionalities of high and low sulfur Australian coals: A case study using micro attenuated total reflectance–Fourier transform infrared (ATR-FTIR) spectrometry. Org. Geochem. 2010, 41, 554–558. [Google Scholar] [CrossRef]
- Aileen, M.-R.; Grotheer, H.; Bourdet, J.; Suvorova, A.; Grice, K.; McCuaig, T.C.; Greenwood, P.F. Evidence and origin of different types of sedimentary organic matter from a Paleoproterozoic orogenic Au deposit. Precambrian Res. 2017, 299, 319–338. [Google Scholar]
- Spötl, C.; Houseknecht, D.W.; Jaques, R.C. Kerogen maturation and incipient graphitization of hydrocarbon source rocks in the Arkoma Basin, Oklahoma and Arkansas: A combined petrographic and Raman spectrometric study. Org. Geochem. 1998, 28, 535–542. [Google Scholar] [CrossRef]
- Trudinger, P.A.; Walter, M.R.; Ralph, B.J. (Eds.) Biogeochemistry of Ancient and Modern Environments: Proceedings of the Fourth International Symposium on Environmental Biogeochemistry (ISEB) and, Conference on Biogeochemistry in Relation to the Mining Industry and Environmental Pollution (Leaching Conference), Held in Canberra, Australia, 26 August–4 September 1979; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Van Krevelen, D.W. Graphical-statistical method for the study of structure and reaction processes of coal. Fuel 1950, 29, 269–284. [Google Scholar]
- Scott, A.C.; Glasspool, I.J. Observations and experiments on the origin and formation of inertinite group macerals. Int. J. Coal Geol. 2007, 70, 53–66. [Google Scholar] [CrossRef]
- Hower, J.C.; O’Keefe, J.M.K.; Watt, M.A.; Pratt, T.J.; Eble, C.F.; Stucker, J.D.; Richardson, A.R.; Kostova, I.J. Notes on the origin of inertinite macerals in coals: Observations on the importance of fungi in the origin of macrinite. Int. J. Coal Geol. 2009, 80, 135–143. [Google Scholar] [CrossRef]
- Bustin, R.M.; Link, C.; Goodarzi, F. Optical properties and chemistry of graptolite periderm following laboratory simulated maturation. Org. Geochem. 1989, 14, 355–364. [Google Scholar] [CrossRef]
- Borrego, A.G.; Hagemann, H.W.; Prado, J.G.; Guillén, M.D.; Blanco, C.G. Comparative petrographic and geochemical study of the Puertollano oil shale kerogens. Org. Geochem. 1996, 24, 309–321. [Google Scholar] [CrossRef]
- Crelling, J.C.; Dutcher, R.R. Principles and Applications of Coal Petrology: Short Course Notes; No. 8; Society of Economic Paleontologists & Mineralogists: New York, NY, USA, 1980. [Google Scholar]
- Cardott, B.J. Thermal maturity of Woodford Shale gas and oil plays, Oklahoma, USA. Int. J. Coal Geol. 2012, 103, 109–119. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Y.; Mastalerz, M. Comparative optical properties of vitrinite and other macerals from Upper Devonian–Lower Mississippian New Albany Shale: Implications for thermal maturity. Int. J. Coal Geol. 2016, 168, 222–236. [Google Scholar] [CrossRef]
- Mählmann, R.F.; Le Bayon, R. Vitrinite and vitrinite like solid bitumen reflectance in thermal maturity studies: Correlations from diagenesis to incipient metamorphism in different geodynamic settings. Int. J. Coal Geol. 2016, 157, 52–73. [Google Scholar] [CrossRef]
- Hackley, P.C.; Lewan, M. Understanding and distinguishing reflectance measurements of solid bitumen and vitrinite using hydrous pyrolysis: Implications to petroleum assessment. AAPG Bull. 2018, 102, 1119–1140. [Google Scholar] [CrossRef]
- Liu, D.; Xiao, X.; Tian, H.; Min, Y.; Zhou, Q.; Cheng, P.; Shen, J. Sample maturation calculated using Raman spectroscopic parameters for solid organics: Methodology and geological applications. Chin. Sci. Bull. 2013, 58, 1285–1298. [Google Scholar] [CrossRef]
- Hampartsoumian, E.; Nimmo, W.; Rosenberg, P.; Thomsen, E.; Williams, A. Evaluation of the chemical properties of coals and their maceral group constituents in relation to combustion reactivity using multi-variate analyses. Fuel 1998, 77, 735–748. [Google Scholar] [CrossRef]
- Dembicki, H. Practical Petroleum Geochemistry for Exploration and Production; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Altun, N.E.; Hicyilmaz, C.; Hwang, J.-Y.; Bagci, A.S.; Kok, M.V. Oil shales in the world and Turkey; reserves, current situation and future prospects: A review. Oil Shale 2006, 23, 211–228. [Google Scholar]
- Kelemen, S.R.; Afeworki, M.; Gorbaty, M.L.; Sansone, M.; Kwiatek, P.J.; Walters, C.C.; Freund, H.; Siskin, M.; Bence, A.E.; Curry, D.J.; et al. Direct characterization of kerogen by X-ray and solid-state 13 C nuclear magnetic resonance methods. Energy Fuels 2007, 21, 1548–1561. [Google Scholar] [CrossRef]
- Orr, W.L. Kerogen/asphaltene/sulfur relationships in sulfur-rich Monterey oils. Org. Geochem. 1986, 10, 499–516. [Google Scholar] [CrossRef]
- Gentzis, T.; Goodarzi, F. A review of the use of bitumen reflectance in hydrocarbon exploration with examples from Melville Island, Arctic Canada. In Applications of Thermal Maturity Studies to Energy Exploration; Rocky Mountain Section (SEPM): Denver, CO, USA, 1990; pp. 23–36. [Google Scholar]
- Khorasani, G.K.; Michelsen, J.K. The thermal evolution of solid bitumens, bitumen reflectance, and kinetic modeling of reflectance: Application in petroleum and ore prospecting. Energy Sources 1993, 15, 181–204. [Google Scholar] [CrossRef]
- Schoenherr, J.; Littke, R.; Urai, J.L.; Kukla, P.A.; Rawahi, Z. Polyphase thermal evolution in the Infra-Cambrian Ara Group (South Oman Salt Basin) as deduced by maturity of solid reservoir bitumen. Org. Geochem. 2007, 38, 1293–1318. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Walters, C.C.; Kwiatek, P.J.; Freund, H.; Afeworki, M.; Sansone, M.; Lamberti, W.A.; Pottorf, R.J.; Machel, H.G.; Peters, K.E.; et al. Characterization of solid bitumens originating from thermal chemical alteration and thermochemical sulfate reduction. Geochim. Cosmochim. Acta 2010, 74, 5305–5332. [Google Scholar] [CrossRef]

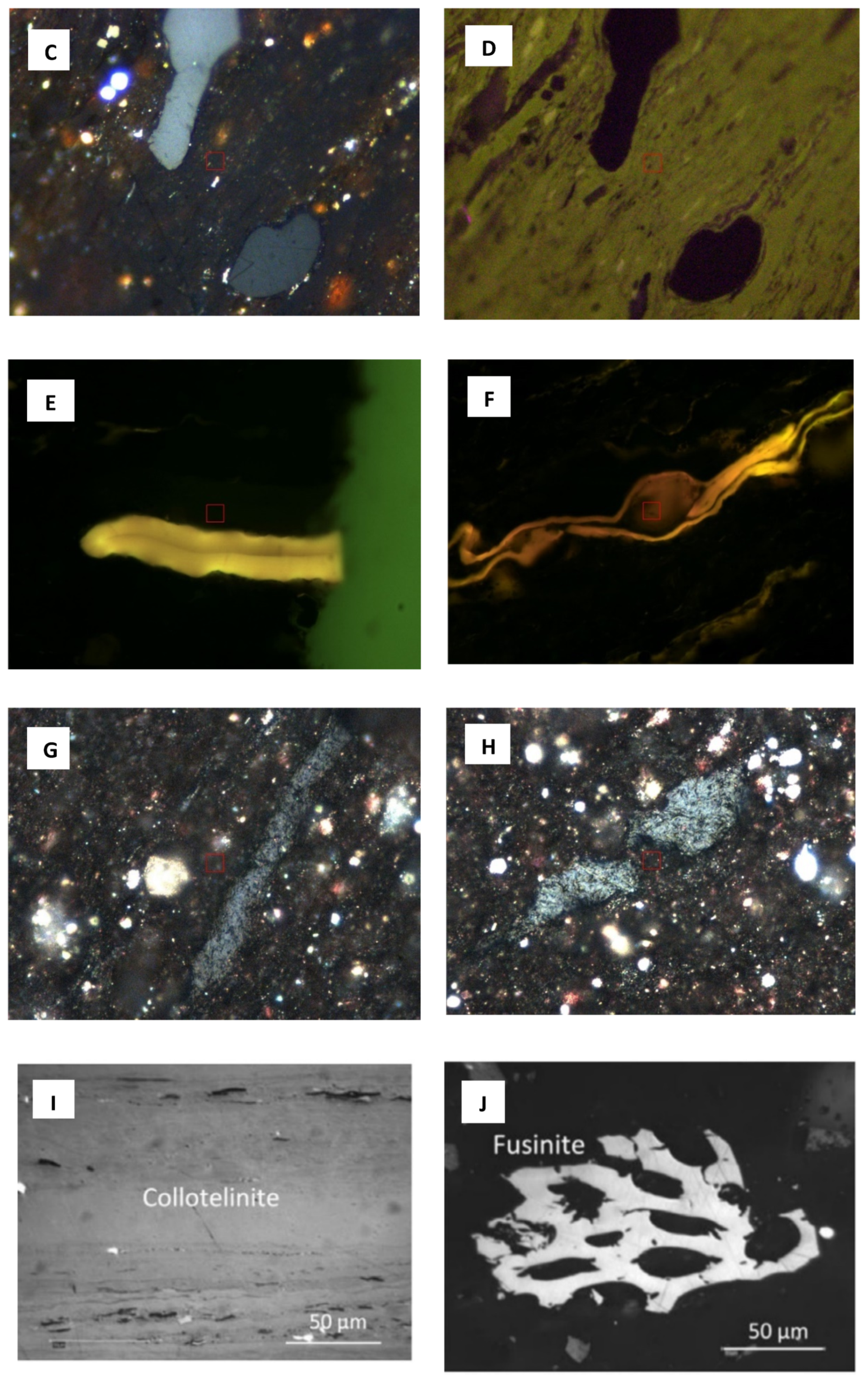
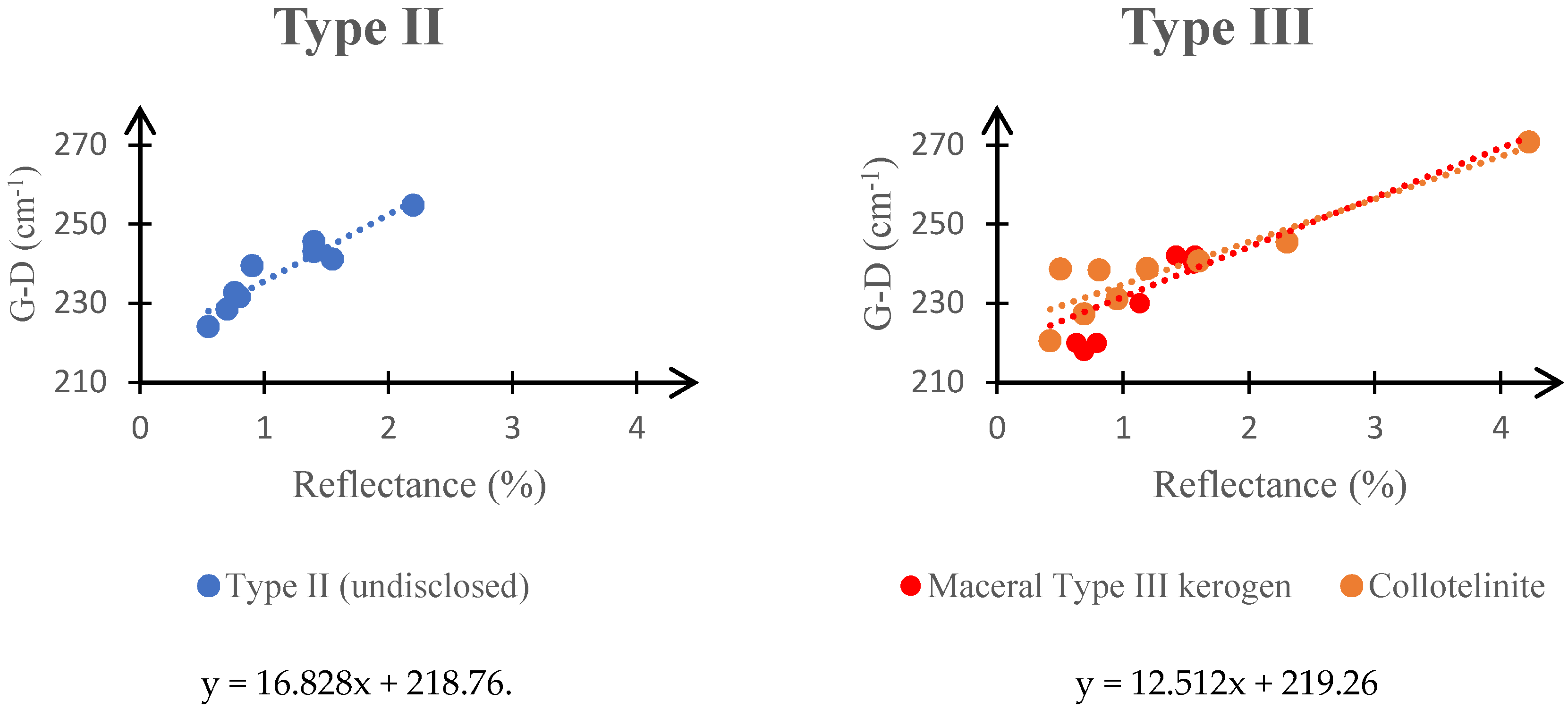
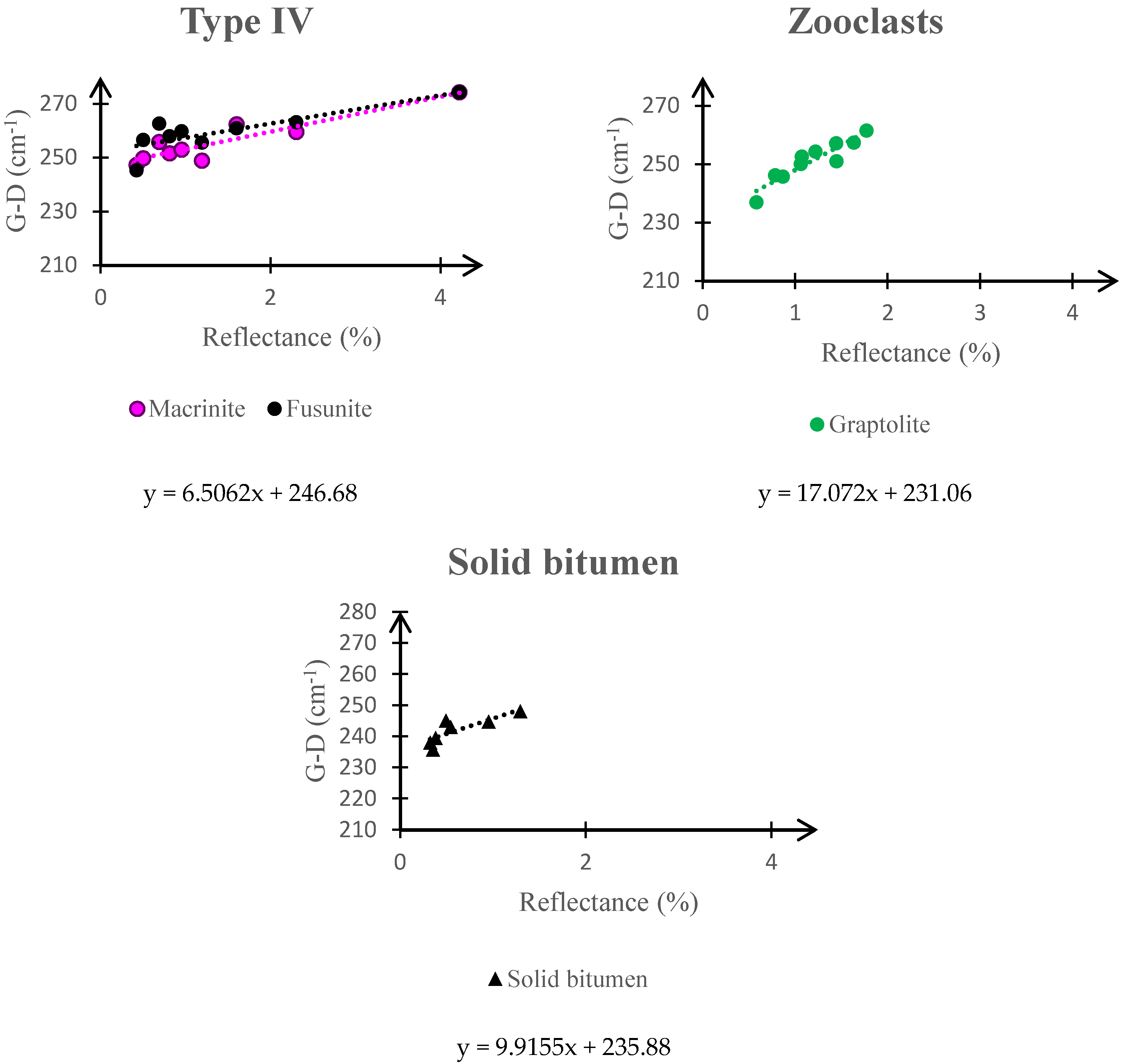
| Maceral Group | Kerogen Type | Grayness | Some of the Macerals in the Group |
|---|---|---|---|
| Liptinite | I and II | Dark gray | Alginite, Sporinite, Cutinite, Suberinite, Resinite, Liptodetrinite |
| Vitrinite | III | Medium to light gray | Collotelinite, Vitrodetrinite, Telinite |
| Inertinite | IV | White and can be very bright | Fusinite, Macrinite, Micrinite, Funginite |
| Zooclast | - | Medium to light gray | Graptolite, Scolecodont |
| Secondary products | - | Medium to light gray | Solid bitumen |
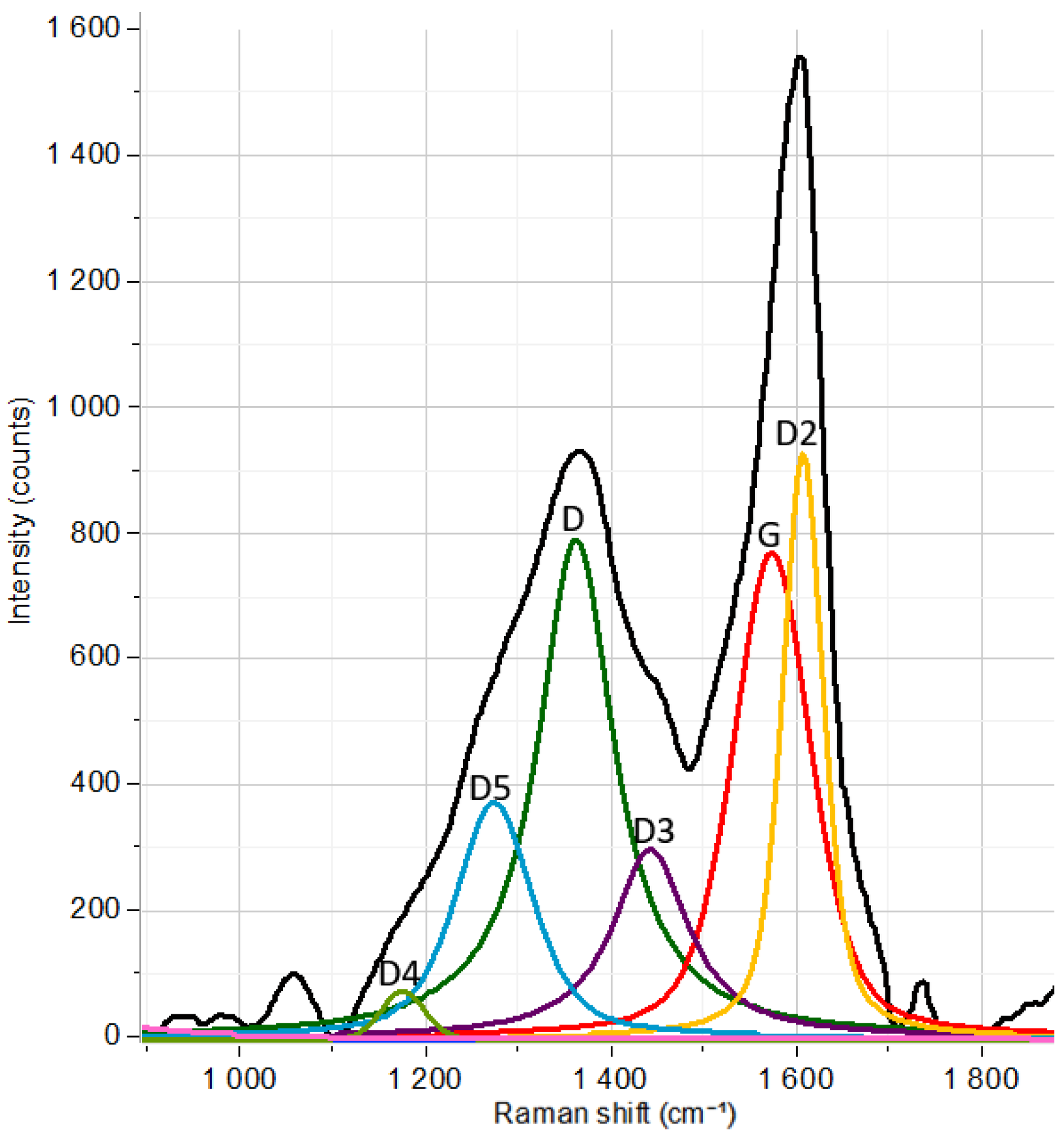
| Raman Band Shift | Description of the Band Assignment |
|---|---|
| G (around 1600 cm−1) |
|
| D (around 1350 cm−1) |
|
| D2 (around 1620 cm−1) |
|
| D3 (around 1500 cm−1) |
|
| D4 (around 1150 cm−1) |
|
| D5 (around 1265 cm−1) |
|
| VRO-Eq. | Type | D (cm−1) | G (cm−1) | D5 (cm−1) |
|---|---|---|---|---|
| 1.56 | I (Green river) | 1349 | 1581 | 1225 |
| 1.59 | I (Green river) | 1350 | 1582 | 1223 |
| 0.5 | IIS (undisclosed) | 1377 | 1600 | 1284.8 |
| 2.2 | II (Woodford) | 1349.5 | 1604.3 | 1257 |
| 0.76 | II (Woodford) | 1367 | 1599.7 | 1251 |
| 0.8 | II (Woodford) | 1367 | 1598.7 | 1252.5 |
| 0.55 | II (Woodford) | 1373.5 | 1597.7 | 1257.6 |
| 0.7 | II (Woodford) | 1369.3 | 1597.9 | 1255.4 |
| 0.9 | II (Woodford) | 1361.3 | 1600.9 | 1258.1 |
| 1.55 | II (Woodford) | 1360.7 | 1601.9 | 1260 |
| 4.3 | II (Woodford) | 1340.2 | 1601.9 | 1259 |
| 1.4 | II (Woodford) | 1359.6 | 1602.7 | 1263 |
| 1.4 | II (Woodford) | 1355.4 | 1601 | 1272.3 |
| 0.5 | III (Penn State Coal) | 1353.4 | 1592.2 | 1243.6 |
| 0.69 | III (Penn State Coal) | 1362.4 | 1589.8 | 1251.8 |
| 0.81 | III (Penn State Coal) | 1351.9 | 1590.4 | 1234.9 |
| 1.19 | III (Penn State Coal) | 1352.1 | 1591.1 | 1256.1 |
| 1.6 | III (Penn State Coal) | 1347.5 | 1588.2 | 1259.5 |
| 2 | III (Penn State Coal) | 1346.2 | 1591.7 | 1252.1 |
| 4 | III (Penn State Coal) | 1325.8 | 1596.5 | 1241.7 |
| 0.92 | Solid bitumen (Bakken) | 1354 | 1594 | 1260 |
| 0.94 | Solid bitumen (Bakken) | 1361 | 1603 | 1265 |
| 0.86 | Solid bitumen (Bakken) | 1358 | 1593.5 | 1263 |
| 0.54 | Solid bitumen (Bakken) | 1367 | 1601 | 1269 |
| 0.38 | Solid bitumen (Bakken) | 1361 | 1593.5 | 1274 |
| 0.59 | Solid bitumen (Bakken) | 1361 | 1593 | 1263 |
| 0.71 | Solid bitumen (Bakken) | 1365 | 1601 | 1270 |
| 1.08 | Solid bitumen (Bakken) | 1362 | 1605.5 | 1275 |
| 1.09 | Solid bitumen (Bakken) | 1360 | 1603.5 | 1268.5 |
| 0.91 | Solid bitumen (Bakken) | 1365 | 1605 | 1259 |
| 0.95 | III (Eagle ford) | 1354 | 1578 | 1255 |
| 0.98 | III (Eagle ford) | 1353 | 1582 | 1256 |
| 1.1 | III (Eagle ford) | 1350 | 1586 | 1248 |
| NA | Solid bitumen (Eagle Ford) | 1357 | 1575 | 1248 |
| NA | Solid bitumen (Eagle Ford) | 1362 | 1574 | 1253 |
| NA | Solid bitumen (Eagle Ford) | 1360 | 1576 | 1260 |
| Kerogen Type | %Carbon | %Hydrogen | %Oxygen | %Sulfur | %Nitrogen |
|---|---|---|---|---|---|
| Type I | 80 | 10.9 | 4.9 | 1.3 | 1.6 |
| Type II | 68.9 | 7.3 | 6.6 | 10.6 | 1.5 |
| Type III | 56.2 | 4.4 | 27.8 | 2.4 | 1.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatibi, S.; Abarghani, A.; Liu, K.; Guedes, A.; Valentim, B.; Ostadhassan, M. Backtracking to Parent Maceral from Produced Bitumen with Raman Spectroscopy. Minerals 2020, 10, 679. https://doi.org/10.3390/min10080679
Khatibi S, Abarghani A, Liu K, Guedes A, Valentim B, Ostadhassan M. Backtracking to Parent Maceral from Produced Bitumen with Raman Spectroscopy. Minerals. 2020; 10(8):679. https://doi.org/10.3390/min10080679
Chicago/Turabian StyleKhatibi, Seyedalireza, Arash Abarghani, Kouqi Liu, Alexandra Guedes, Bruno Valentim, and Mehdi Ostadhassan. 2020. "Backtracking to Parent Maceral from Produced Bitumen with Raman Spectroscopy" Minerals 10, no. 8: 679. https://doi.org/10.3390/min10080679
APA StyleKhatibi, S., Abarghani, A., Liu, K., Guedes, A., Valentim, B., & Ostadhassan, M. (2020). Backtracking to Parent Maceral from Produced Bitumen with Raman Spectroscopy. Minerals, 10(8), 679. https://doi.org/10.3390/min10080679








