Mg-Phengite in Carbonate Rock Syngenetically Formed from Hydrothermal Fluid: Micro-Textural Evidence and Mineral Chemistry
Abstract
1. Introduction
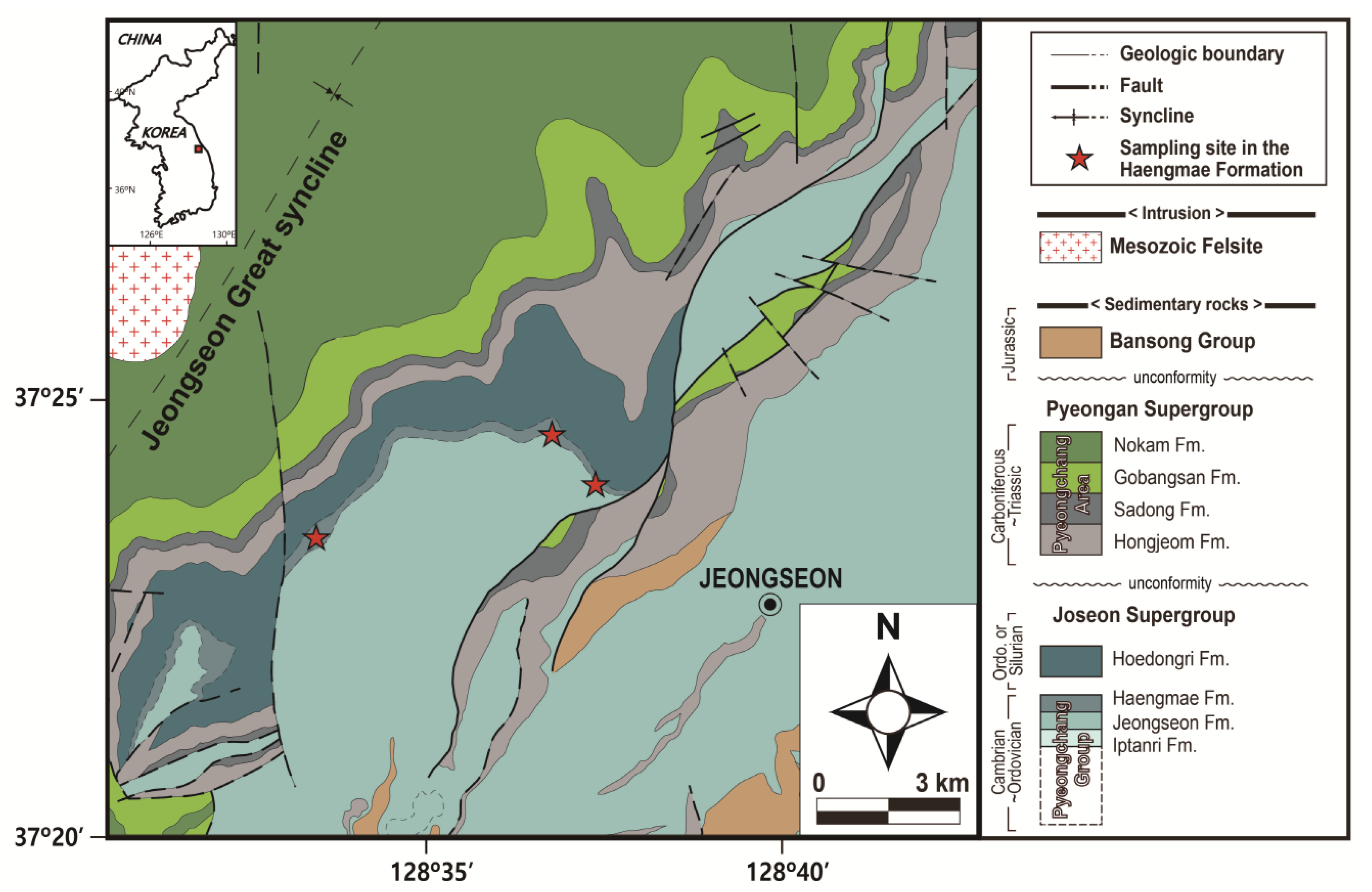
2. General Geology of Mg-Phengite Occurrence Area
3. Analytical Methods
4. Results
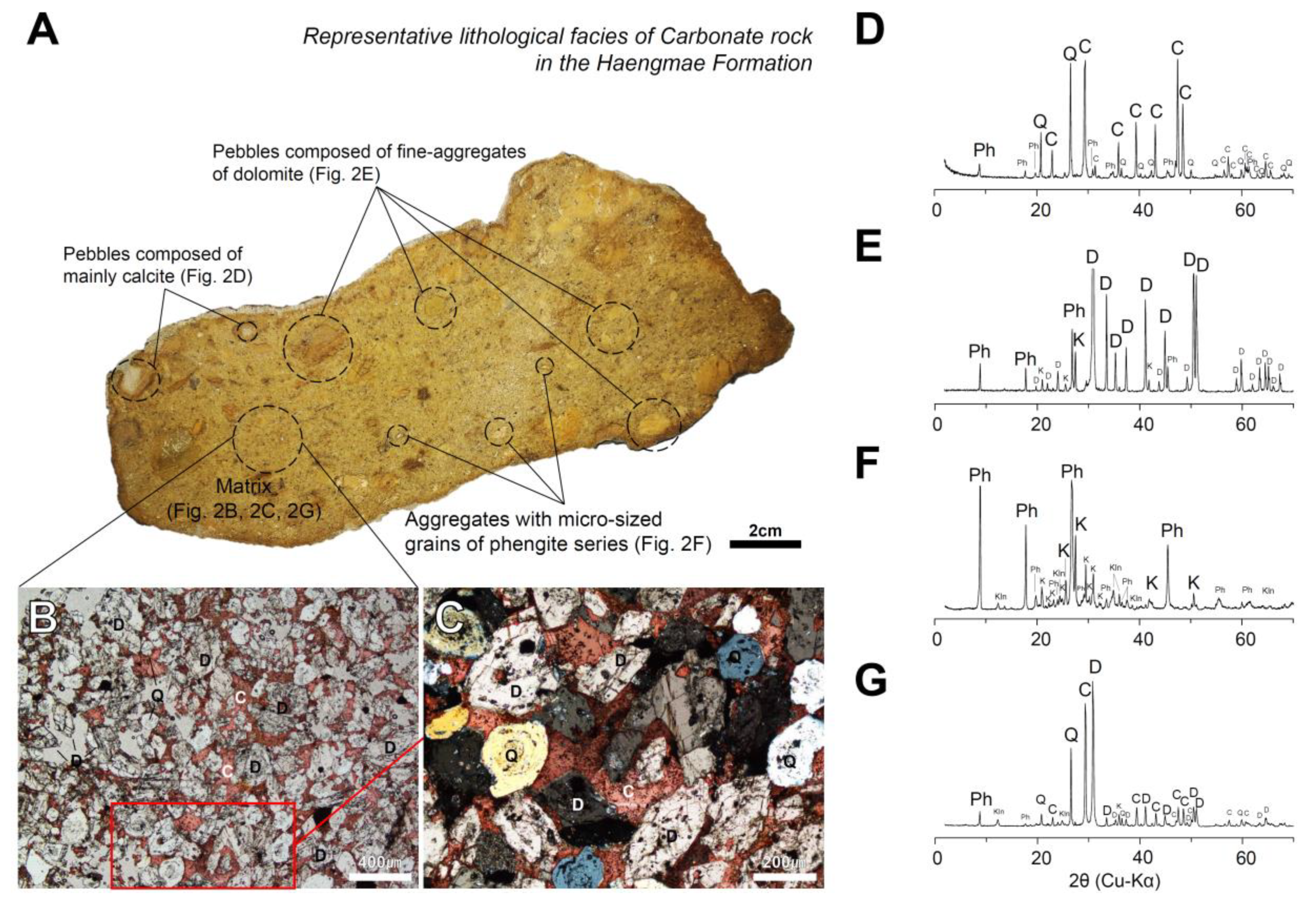
4.1. Characteristics of Haengmae Formation Containing Mg-Phengite
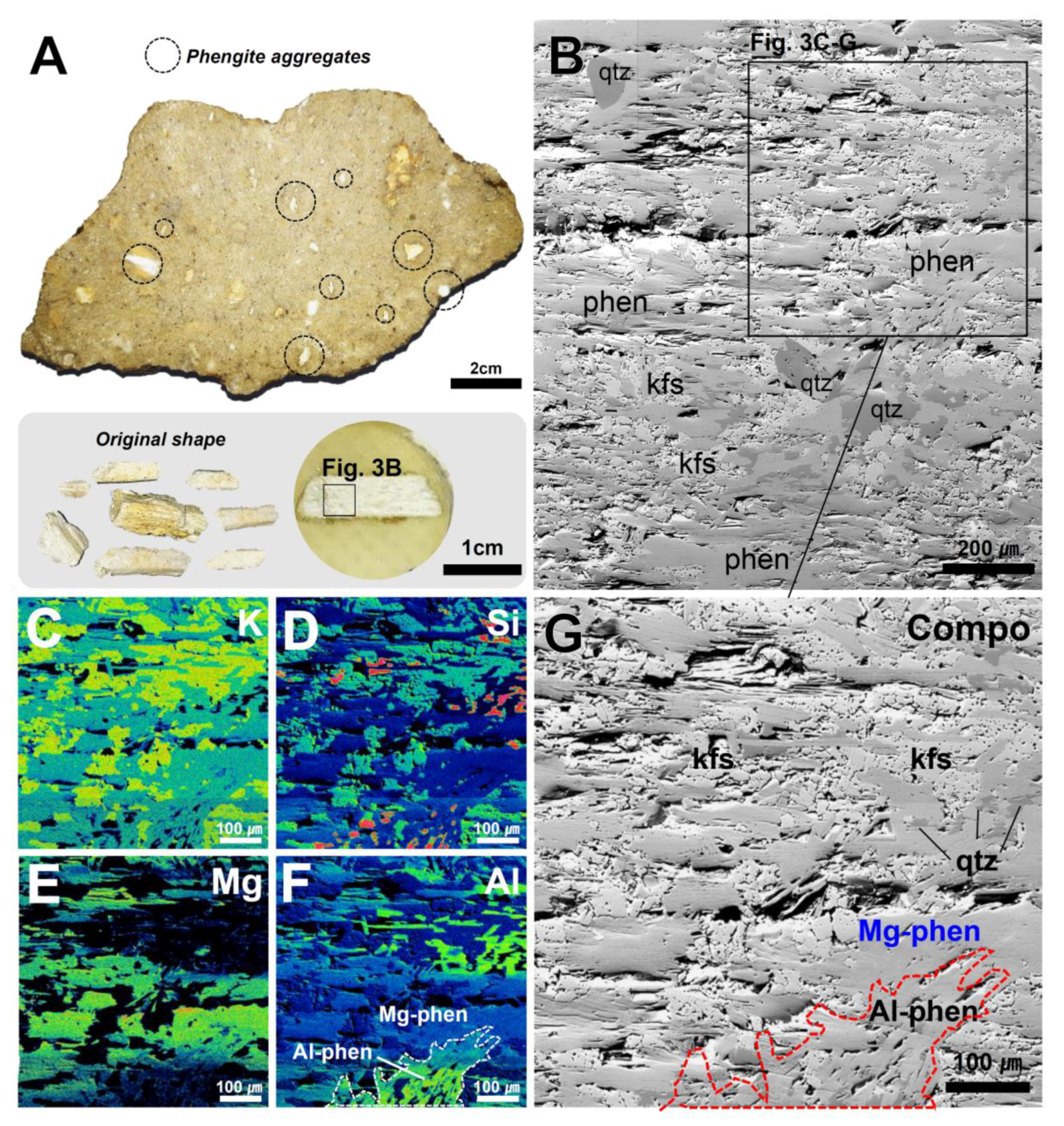
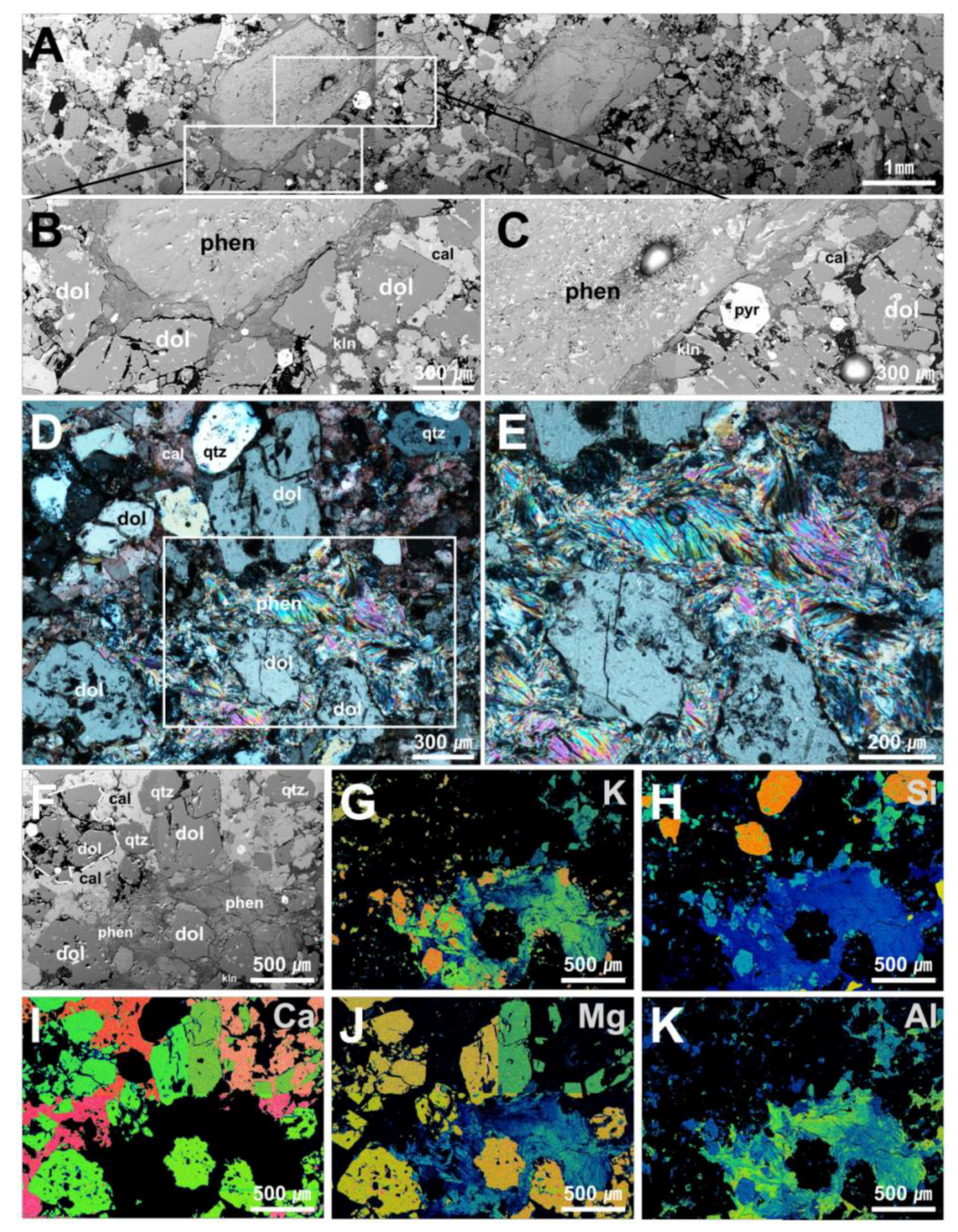
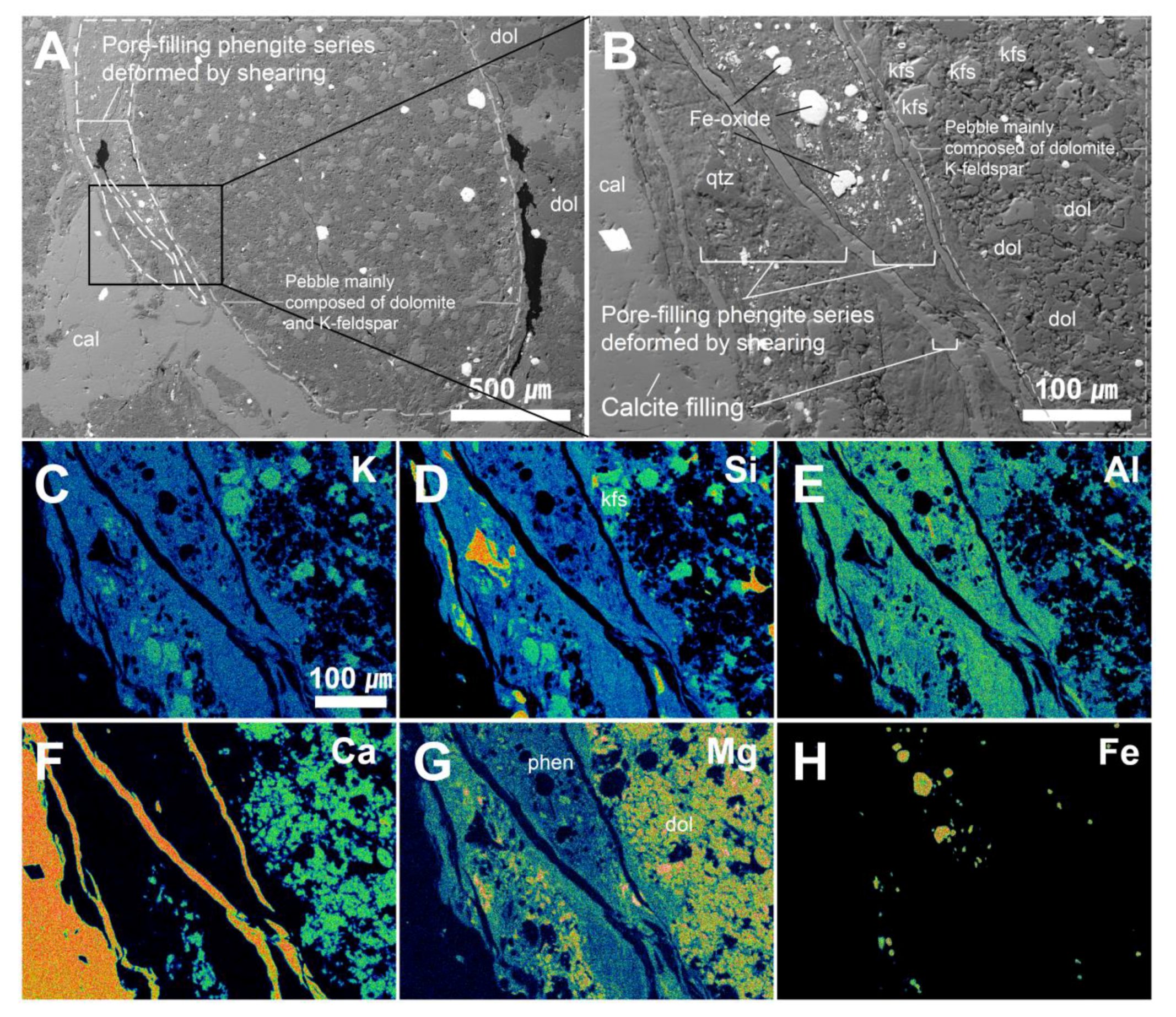
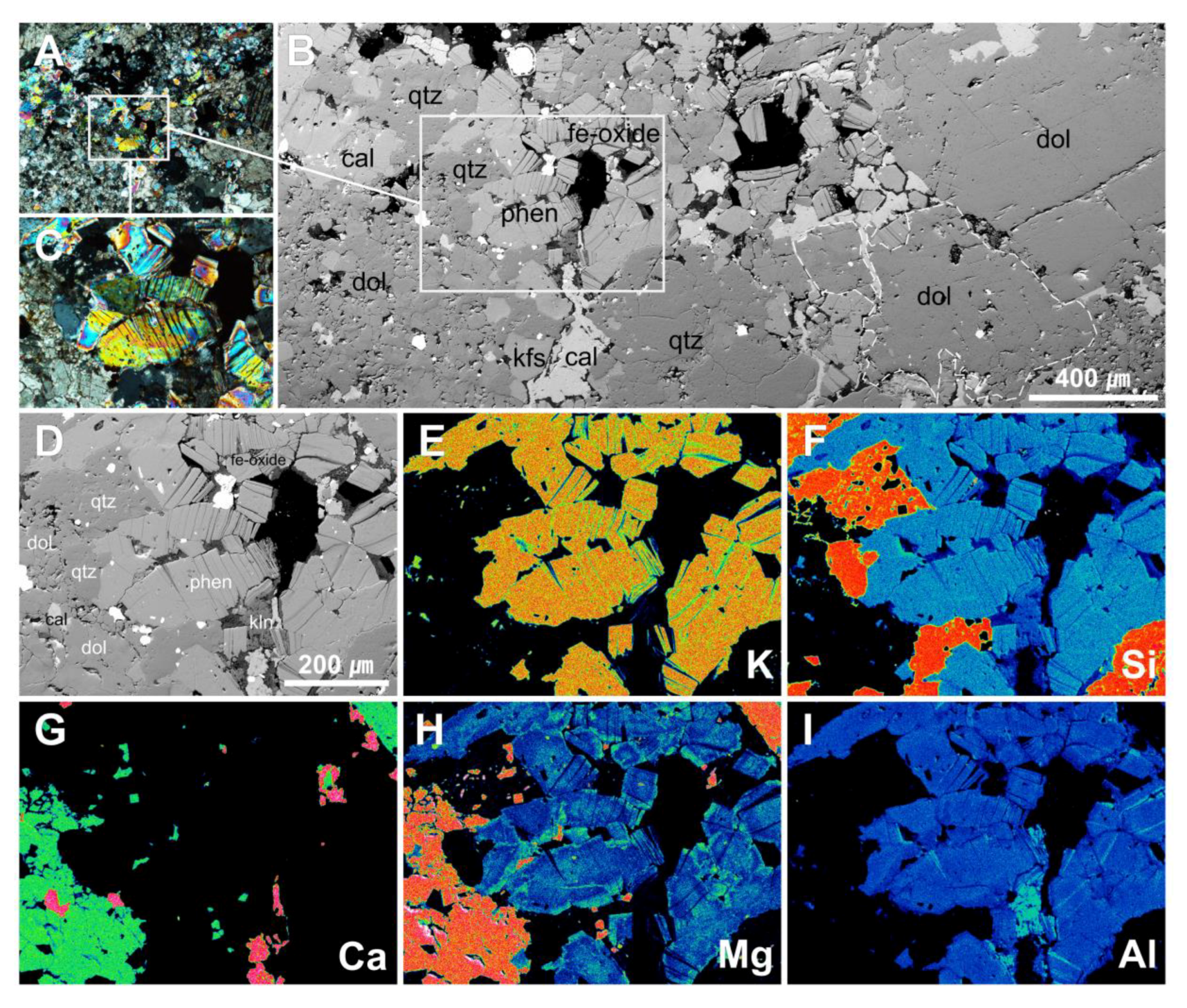
4.2. Micro-textural Analysis of Mg-Phengite
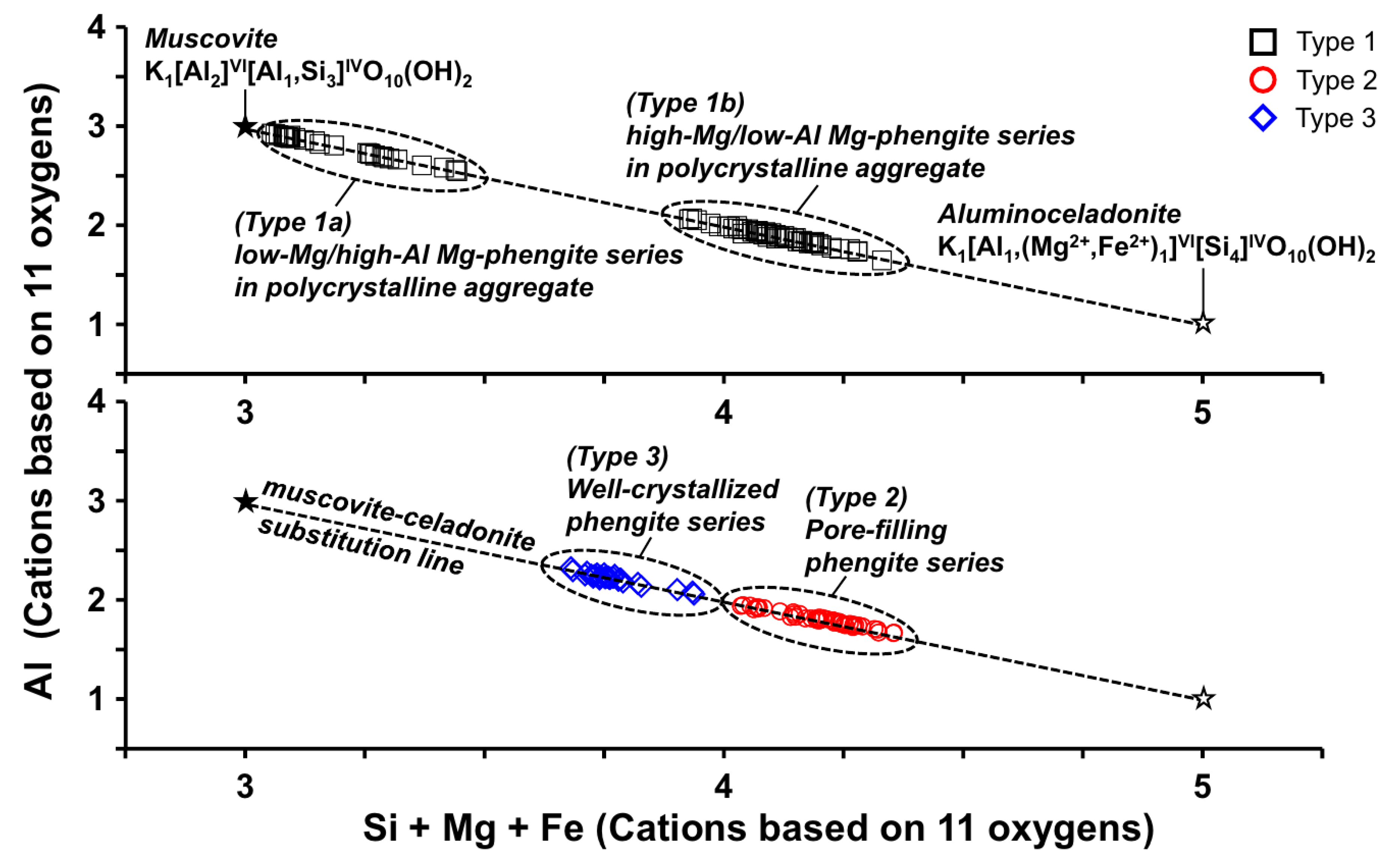
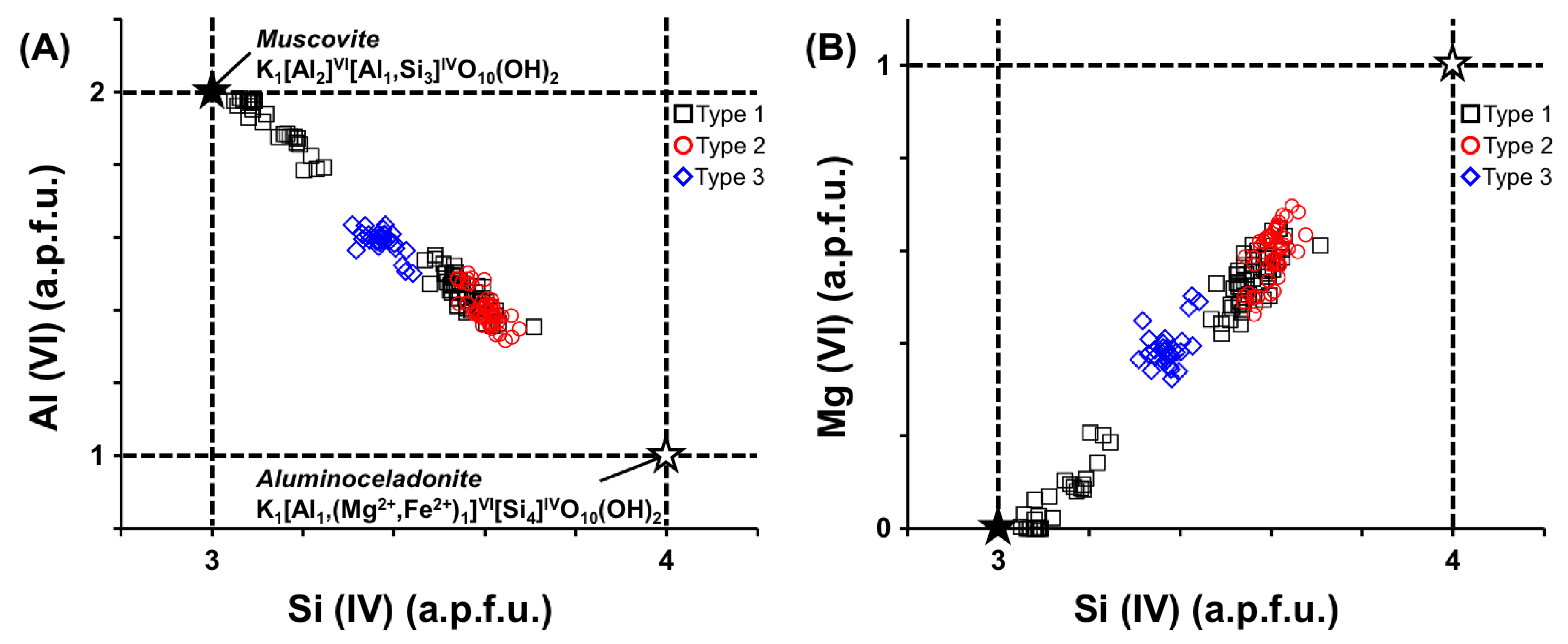
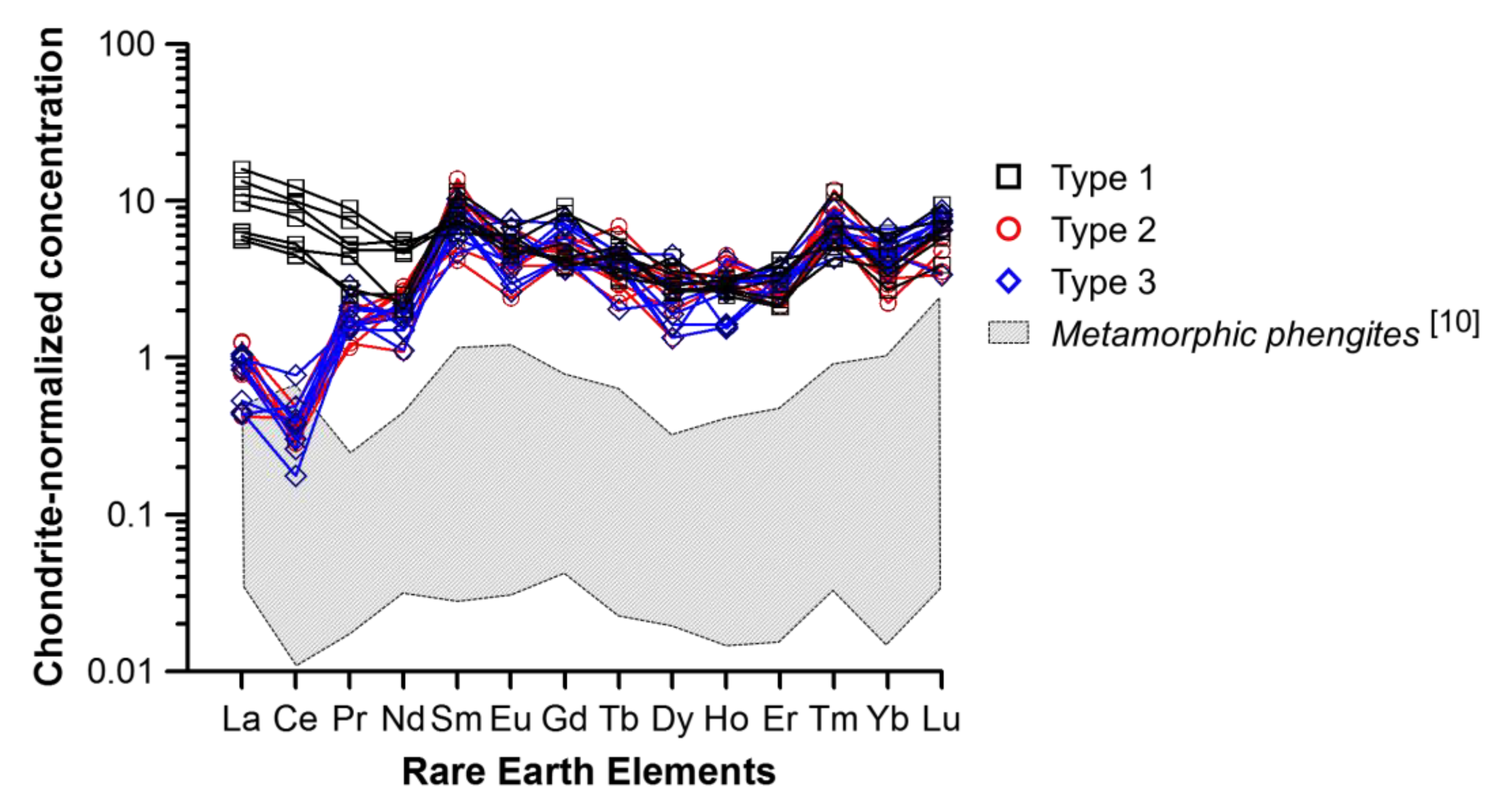
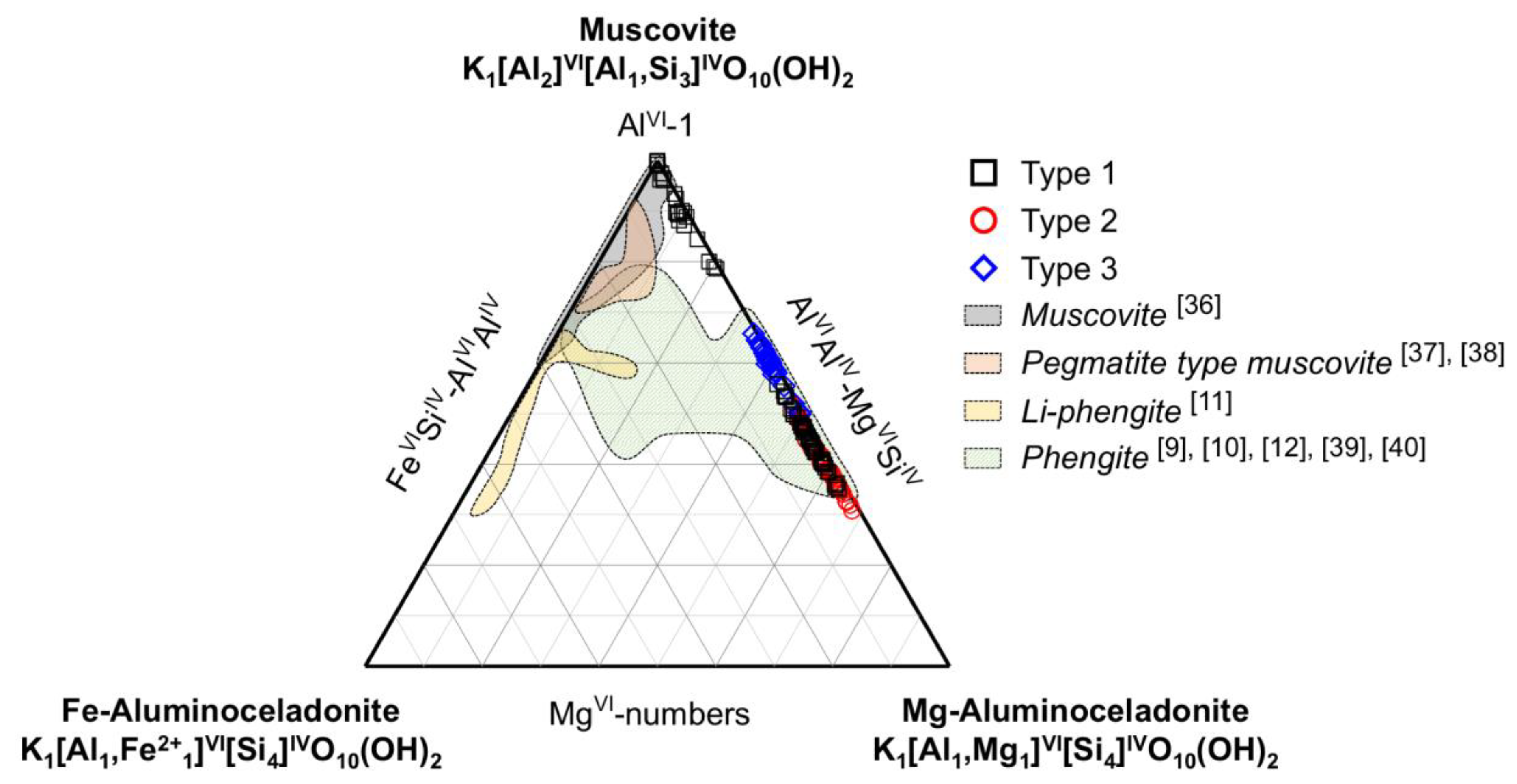
4.3. Mineral Chemistry of Phengite
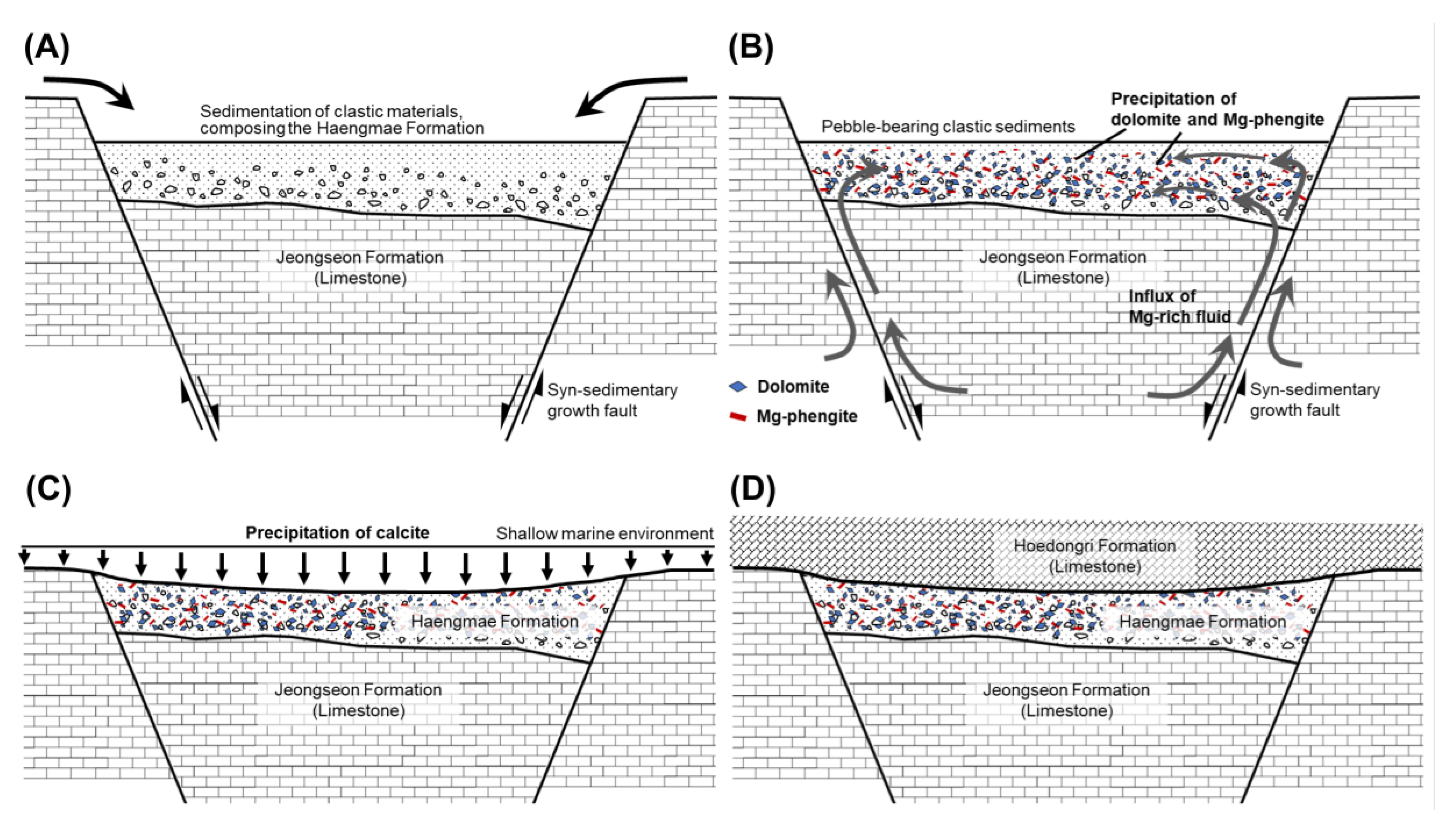
5. Discussion
6. Summary
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schaller, W.T. An interpretation of the composition of high-silica sericites. Mineral. Mag. 1950, 29, 406–415. [Google Scholar] [CrossRef][Green Version]
- Foster, M.D. Correlation of dioctahedral potassium micas on the basis of their charge relations. U. S. Geol. Surv. Bull. 1956, 1036-D, 57–67. [Google Scholar]
- Ernst, W.G. Significance of phengitic micas from low-grade schists. Am. Mineral. 1963, 48, 1357–1373. [Google Scholar]
- Rieder, M.; Cavazzini, G.; D’yakonov, Y.S.; Frank-Kamenetskii, V.A.; Gottardi, G.; Guggenheim, S.; Koval, P.W.; Mueller, G.; Neiva, A.M.; Radoslovich, E.W.; et al. Nomenclature of the Micas. Clays Clay Miner. 1998, 46, 586–595. [Google Scholar] [CrossRef]
- Rieder, M.; Cavazzini, G.; D’yakonov, Y.D.; Frank-Kamenetskii, V.A.; Gottardi, G.; Guggenheim, S.; Müller, G.; Neiva, A.M.R.; Radoslovich, E.W.; Robert, J.L.; et al. Nomenclature of the micas. Can. Mineral. 1998, 36, 905–912. [Google Scholar] [CrossRef]
- Meunier, A.; Velde, B. Phengitization, sericitization and potassium–beidellite in a hydrothermal-altered granite. Clay Miner. 1982, 17, 285–299. [Google Scholar] [CrossRef]
- Eberl, D.D.; Srodon, J.; Lee, M.; Nadeau, P.H.; Northrop, H.R. Sericite from the Silverton caldera, Colorado: Correlation among structure, composition, origin, and particle thickness. Am. Mineral. 1987, 72, 914–934. [Google Scholar]
- Hitzman, M.W.; Orekes, N.; Einaudi, M.T. Geological characteristics and tectonic settings of Proterozoic iron oxide (Cu–U–Au-REE) deposits. Precambrian Res. 1992, 58, 241–287. [Google Scholar] [CrossRef]
- Tappert, M.C.; Rivard, B.; Giles, D.; Tappert, R.; Mauger, A. The mineral chemistry,near-infrared, and mid-infrared reflectance spectroscopy of phengite from theOlympicDam IOCG deposit, South Australia. Ore Geol. Rev. 2013, 53, 26–38. [Google Scholar] [CrossRef]
- El Korh, A.; Schmidt, S.T.; Ulianov, A.; Potel, S. Trace element partitioning in HP-LT metamorphic assemblages during subduction-related metamorphism, Ile de Groix,France: A detailed LA-ICP-MS study. J. Petrol. 2009, 50, 1107–1148. [Google Scholar] [CrossRef]
- Petrík, I.; Čík, Š.; Miglierini, M.; Vaculovič, T.; Dianiška, I.; Ozdín, D. Alpine oxidation of lithium micas in Permian S-type granites (Gemeric unit, Western Carpathians, Slovakia). Mineral. Mag. 2014, 78, 507–533. [Google Scholar] [CrossRef]
- Gambino, F.; Borghi, A.; d’Atri, A.; Martire, L.; Cavallo, M.; Appolonia, L.; Croveri, P. Minero-petrographic characterization of Chianocco Marble employed for Palazzo Madama Façade in Turin (Northwest Italy). Sustainability 2019, 11, 4229. [Google Scholar] [CrossRef]
- Crowley, M.S.; Roy, R. Crystalline solubility in the muscovite and phlogopite groups. Am. Mineral. 1964, 49, 348–362. [Google Scholar]
- Cibin, G.; Cinque, G.; Marcelli, A.; Mottana, A.; Sassi, R. The octahedral sheet of metamoprhic 2M1-phengites: A combined EMPA and AXANES study. Am. Mineral. 2008, 93, 414–425. [Google Scholar] [CrossRef]
- Frey, M.; Hunziker, J.C.; Jager, E.; Stern, W.B. Regional distribution of white K-mica polymorphs and their phengite content in the central Alps. Contrib. Mineral. Petrol. 1983, 83, 185–197. [Google Scholar] [CrossRef]
- Gouzu, C.; Itaya, T.; Takeshita, H. Interlayer cation vacancies of phengites in calcshists from the Piemonte zone, wester Alps, Italy. J. Mineral. Petrol. Sci. 2005, 100, 142–149. [Google Scholar] [CrossRef][Green Version]
- Massonne, H.J.; Schreyer, W. Phengite geobarometry based on the limiting assemblage with K-feldspar, phlogopite, and quartz. Contrib. Mineral. Petrol. 1987, 96, 212–224. [Google Scholar] [CrossRef]
- Shau, Y.-H.; Feather, M.E.; Essene, E.J.; Peacor, D.R. Genesis and solvus relations of submicroscopically intergrown paragonite and phengite in a blueschist from nothern California. Contrib. Mineral. Petrol. 1991, 106, 367–378. [Google Scholar] [CrossRef][Green Version]
- Velde, B. Phengite micas: Synthesis, stability, and natural occurence. Am. J. Sci. 1965, 263, 886–913. [Google Scholar] [CrossRef]
- Velde, B. Si+4 content of natural phengites. Contrib. Mineral. Petrol. 1967, 14, 250–258. [Google Scholar] [CrossRef]
- Roberts, D.E.; Hudson, G.R.T. The Olympic Dam copper–uranium–gold deposit, Roxby Downs, South Australia. Econ. Geol. 1983, 78, 799–822. [Google Scholar] [CrossRef]
- Jang, Y.; Cheong, H.J. Structural geometry of the Pyeongchang-Jeongseon Area of the Northwestern Taebaeksan Zone, Okcheon Belt. Econ. Environ. Geol. 2019, 52, 541–554, (In Korean with English abstract). [Google Scholar]
- Jang, Y. Structural Style of the Phanerozoic Polyphase Orogenic Belt in the Western Taebaeksan Zone, Okcheon Belt, Korea: Insights from Multidisciplinary Analyses. Ph.D. Thesis, Yonseic University, Seoul, Korea, 2018. [Google Scholar]
- Cheong, C.H.; Lee, H.Y.; Koh, I.S.; Lee, J.D. A study on stratigraphy and sedimentological environments of the lower Paleozoic sequences in South Korea (Chiefly in Jeongseon Area). J. Natl. Acad. Sci. Repub. Korea (Nat. Sci.) 1979, 18, 123–159. (In Korean) [Google Scholar]
- Lee, H.Y. Discovery of Silurian conodont fauna from South Korea. J. Geol. Soc. Korea 1980, 16, 114–123. [Google Scholar]
- Son, C.M.; Cheong, J.G. Geology of the Northwestern part of Pyeongchang district, Gangweon-do, Korea. J. Geol. Soc. Korea 1971, 7, 103–116, (In Korean with English abstract). [Google Scholar]
- Kim, J.M. A Study on Stratigraphy and Paleontology of the Lower Paleozoic Sequence in the Kogilri-Daehwari Area, Pyeongchang-Gun, Kangweon-Do (Chiefly by Means of Conodont Study). Master’s Thesis, Yonsei University, Seoul, Korea, 1982. (In Korean with English abstract). [Google Scholar]
- Seo, K.S. A Study on Stratigraphy and Paleontology of the Lower Paleozoic Sequences in the Daewhari and Anmiri Area, Pyeongchang-Gun, Kangwon-Do (Chiefly by Means of Conodont Study). Master’s Thesis, Yonsei University, Seoul, Korea, 1983. (In Korean with English abstract). [Google Scholar]
- Yi, M.S. A Study on Stratigraphy and Paleontology of the Lower Paleozoic Sequence in the Bangrimri and Jujinri Area, Pyeongchang-Gun, Kangweon-Do (Chiefly by Means of Conodont Study). Master’s Thesis, Yonsei University, Seoul, Korea, 1983. (In Korean with English abstract). [Google Scholar]
- Park, C.; Kim, N.; Song, Y. Micro-textural and mineralogical Characteristics of the Haengmae Formation. In Proceedings of the 2019 Joint International Conference of the Geological Science and Technology of Korea, Jeju, South Korea, 17–19 April 2019; p. 236. (In Korean). [Google Scholar]
- Choi, S.-J. A Study on Biostratigraphy and Micropaleontology of the Hoedongri and Haengmae Formation in Pyeonganri Area, Pyeongchang-Gun, Gangwon-Do. Master’s Thesis, Yonsei University, Seoul, Korea, 1980. (In Korean with English abstract). [Google Scholar]
- Pearce, N.; Perkins, W.; Westgate, J.; Gorton, M.; Jackson, S.; Neal, C.; Chenery, S. A compilation of new and published major and trace element data for NIST SRM 610 and NIST SRM 612 glass reference materials. Geostand. Newsl. 1997, 21, 115–144. [Google Scholar] [CrossRef]
- Bishop, B.P.; Bird, D.K. Variation in sericite compositions from fracture zones within the Coso Hot Springs geothermal system. Geochim. Cosmochim. Acta 1987, 1245–1256. [Google Scholar] [CrossRef]
- Evans, A.M. Ore geology and industrial minerals: An introduction (third edition). Blackwell Sci. 1993, 1993, 1–389. [Google Scholar]
- Yang, K.; Huntington, J.F.; Cudahy, T.J.; Mason, P.; Scott, K.M. Spectrally mapping the compositional variation of white micas in hydrothermal systems and the application in mineral exploration. In Proceedings of the 2001 Geoscience and Remote Sensing Symposium, International Geoscience and Remote Sensing Symposium, Sydney, Australia, 9–13 July 2001; pp. 3294–3296. [Google Scholar]
- Psyrillos, A.; Howe, J.H.; Manning, D.A.C.; Burley, S.D. Geological controls on kaolin particle shape and consequences for mineral processing. Clay Miner. 1999, 34, 193–208. [Google Scholar] [CrossRef]
- Deveaud, S.; Millot, R.; Villaros, A. The genesis of LCT-type granitic pegmatites, as illustrated by lithium isotopes in micas. Chem. Geol. 2015, 411, 97–111. [Google Scholar] [CrossRef]
- Hulsbosch, N.; Hertogen, J.; Dewaele, S.; André, L.; Muchez, P. Alkali metal and rare earth element evolution of rock-forming minerals from the Gatumba area pegmatites (Rwanda): Quantitative assessment of crystal-melt fractionation in the regional zonation of pegmatite groups. Geochim. Cosmochim. Acta 2014, 132, 349–374. [Google Scholar] [CrossRef]
- Di Vincenzo, G.; Tonarini, S.; Lombardo, B.; Castelli, D.; Ottolini, L. Comparison of 40 Ar/39 Ar and Rb–Sr data on phengites from the UHP Brossaco-Isasca unit (Dora Maira Massif, Italy): Implications for dating white mica. J. Petrol. 2006, 47, 1439–1465. [Google Scholar] [CrossRef]
- Aoki, K.; Windley, B.F.; Sato, K.; Sawaki, Y.; Kawai, T.; Shibuya, T.; Kumagai, H.; Suzuki, K.; Maruyama, S. Chemical composition and K-Ar age of phengite from Barrovian metapelites, Loch Leven, Scotland. J. Geol. Soc. Jpn. 2013, 6, 437–442. [Google Scholar] [CrossRef][Green Version]
| Polycrystalline Aggregate Type | Pore-Filling Type | Well-Crystallized Type | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 1a * (n = 30) | Type 1b * (n = 50) | Type 2 (n = 50) | Type 3 (n = 30) | |||||||||
| Min. | Max. | Average | Min. | Max. | Average | Min. | Max. | Average | Min. | Max. | Average | |
| SiO2 | 44.50 | 47.99 | 45.75 | 51.06 | 55.86 | 52.91 | 52.10 | 55.56 | 53.70 | 48.66 | 51.35 | 49.91 |
| TiO2 | 0.00 | 0.03 | 0.01 | 0.00 | 0.42 | 0.03 | 0.00 | 0.27 | 0.03 | 0.13 | 0.50 | 0.34 |
| Al2O3 | 30.46 | 36.87 | 34.70 | 21.05 | 25.95 | 23.85 | 21.00 | 24.53 | 22.82 | 26.01 | 29.12 | 27.83 |
| FeO | 0.00 | 0.37 | 0.12 | 0.20 | 0.35 | 0.28 | 0.19 | 0.38 | 0.23 | 0.08 | 0.24 | 0.12 |
| MnO | 0.00 | 0.04 | 0.01 | 0.00 | 0.03 | 0.00 | 0.00 | 0.03 | 0.01 | 0.00 | 0.02 | 0.00 |
| MgO | 0.00 | 1.98 | 0.56 | 4.19 | 6.46 | 5.37 | 4.58 | 7.01 | 5.90 | 3.18 | 5.05 | 3.86 |
| CaO | 0.00 | 0.09 | 0.01 | 0.00 | 0.20 | 0.03 | 0.00 | 0.11 | 0.05 | 0.07 | 0.63 | 0.13 |
| Na2O | 0.02 | 0.11 | 0.07 | 0.00 | 0.10 | 0.05 | 0.02 | 0.17 | 0.06 | 0.11 | 0.34 | 0.17 |
| K2O | 10.86 | 11.58 | 11.22 | 11.13 | 11.67 | 11.33 | 10.60 | 11.63 | 11.28 | 10.83 | 11.32 | 11.08 |
| sum | 87.98 | 94.55 | 92.44 | 93.01 | 95.25 | 93.86 | 92.59 | 95.45 | 94.07 | 92.45 | 94.82 | 93.44 |
| Si (IV) | 3.05 | 3.25 | 3.13 | 3.47 | 3.71 | 3.56 | 3.54 | 3.68 | 3.60 | 3.31 | 3.44 | 3.37 |
| Al (IV) | 0.75 | 0.95 | 0.87 | 0.29 | 0.53 | 0.44 | 0.32 | 0.46 | 0.40 | 0.56 | 0.69 | 0.63 |
| Sum (IV) | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Al (VI) | 1.78 | 1.98 | 1.92 | 1.35 | 1.55 | 1.45 | 1.32 | 1.50 | 1.40 | 1.50 | 1.63 | 1.59 |
| Ti (VI) | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.03 | 0.02 |
| Fe (VI) | 0.00 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.00 | 0.01 | 0.01 |
| Mn (VI) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mg (VI) | 0.00 | 0.21 | 0.06 | 0.42 | 0.65 | 0.54 | 0.46 | 0.70 | 0.59 | 0.32 | 0.50 | 0.39 |
| Sum (VI) | 1.98 | 2.00 | 1.98 | 1.98 | 2.02 | 2.00 | 1.98 | 2.03 | 2.01 | 1.98 | 2.04 | 2.00 |
| Ca | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.05 | 0.01 |
| Na | 0.00 | 0.02 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.02 | 0.01 | 0.01 | 0.04 | 0.02 |
| K | 0.96 | 1.00 | 0.98 | 0.96 | 0.99 | 0.97 | 0.90 | 1.00 | 0.96 | 0.93 | 0.98 | 0.96 |
| Sum | 0.98 | 1.01 | 0.99 | 0.98 | 1.01 | 0.98 | 0.91 | 1.01 | 0.98 | 0.97 | 1.02 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Kim, N.; Choi, S.-J.; Song, Y. Mg-Phengite in Carbonate Rock Syngenetically Formed from Hydrothermal Fluid: Micro-Textural Evidence and Mineral Chemistry. Minerals 2020, 10, 668. https://doi.org/10.3390/min10080668
Park C, Kim N, Choi S-J, Song Y. Mg-Phengite in Carbonate Rock Syngenetically Formed from Hydrothermal Fluid: Micro-Textural Evidence and Mineral Chemistry. Minerals. 2020; 10(8):668. https://doi.org/10.3390/min10080668
Chicago/Turabian StylePark, Chaewon, Namsoo Kim, Sung-Ja Choi, and Yungoo Song. 2020. "Mg-Phengite in Carbonate Rock Syngenetically Formed from Hydrothermal Fluid: Micro-Textural Evidence and Mineral Chemistry" Minerals 10, no. 8: 668. https://doi.org/10.3390/min10080668
APA StylePark, C., Kim, N., Choi, S.-J., & Song, Y. (2020). Mg-Phengite in Carbonate Rock Syngenetically Formed from Hydrothermal Fluid: Micro-Textural Evidence and Mineral Chemistry. Minerals, 10(8), 668. https://doi.org/10.3390/min10080668





