Unique Authigenic Mineral Assemblages and Planktonic Foraminifera Reveal Dynamic Cold Seepage in the Southern South China Sea
Abstract
1. Introduction
2. Materials and Methods
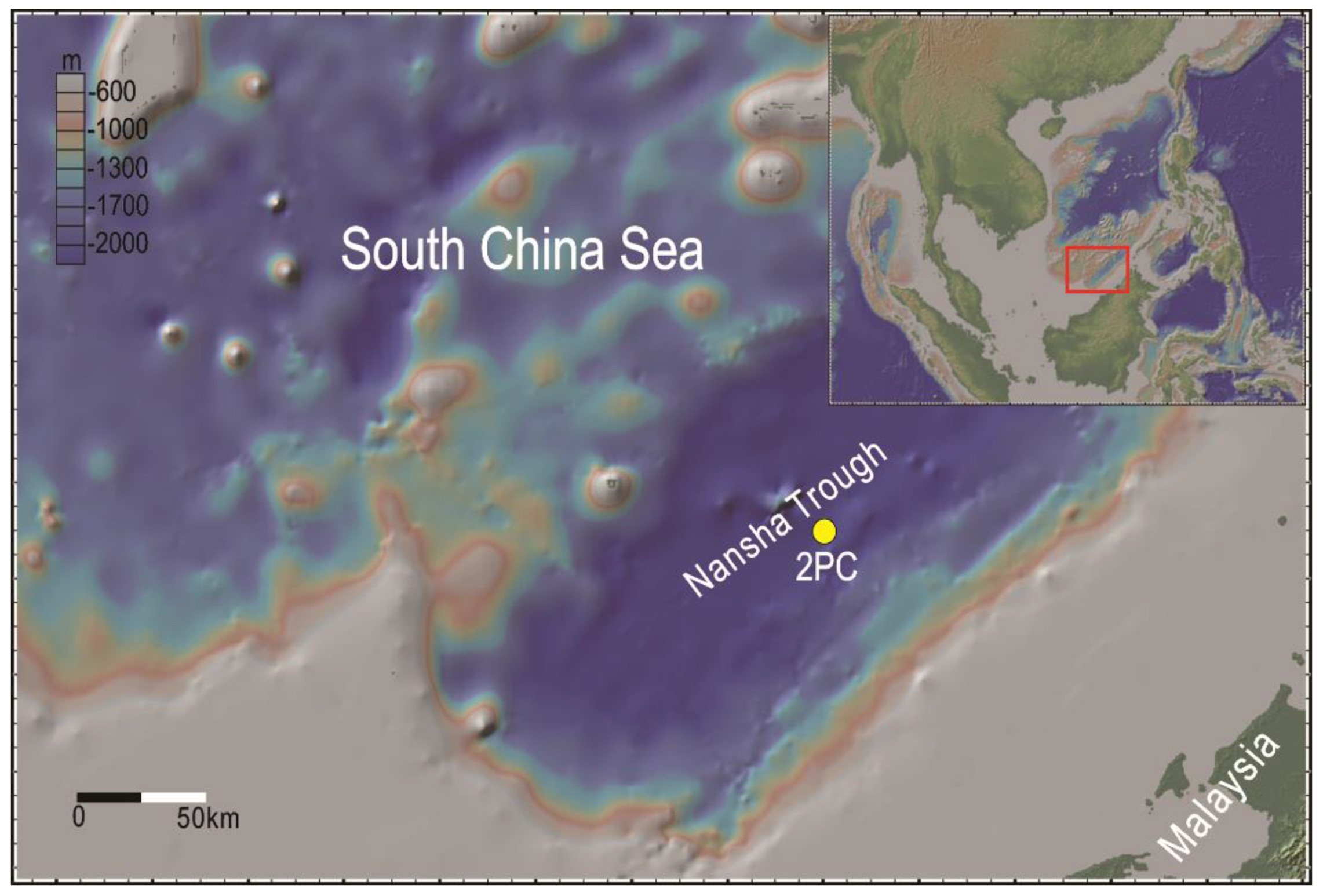
2.1. Study Area
2.2. Sampling and Analyses
3. Results
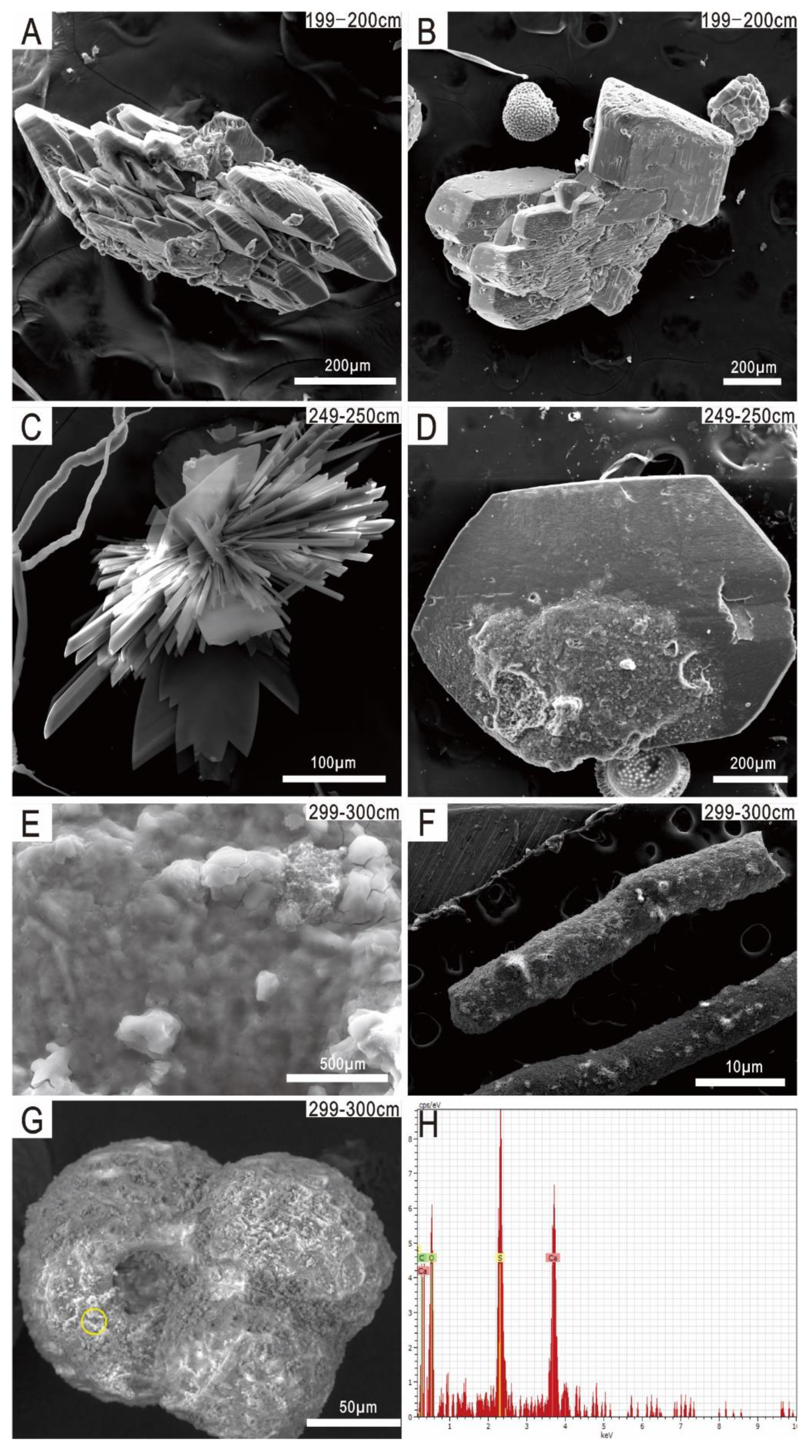
3.1. Morphological Characteristics of Authigenic Pyrite and Gypsum Aggregates
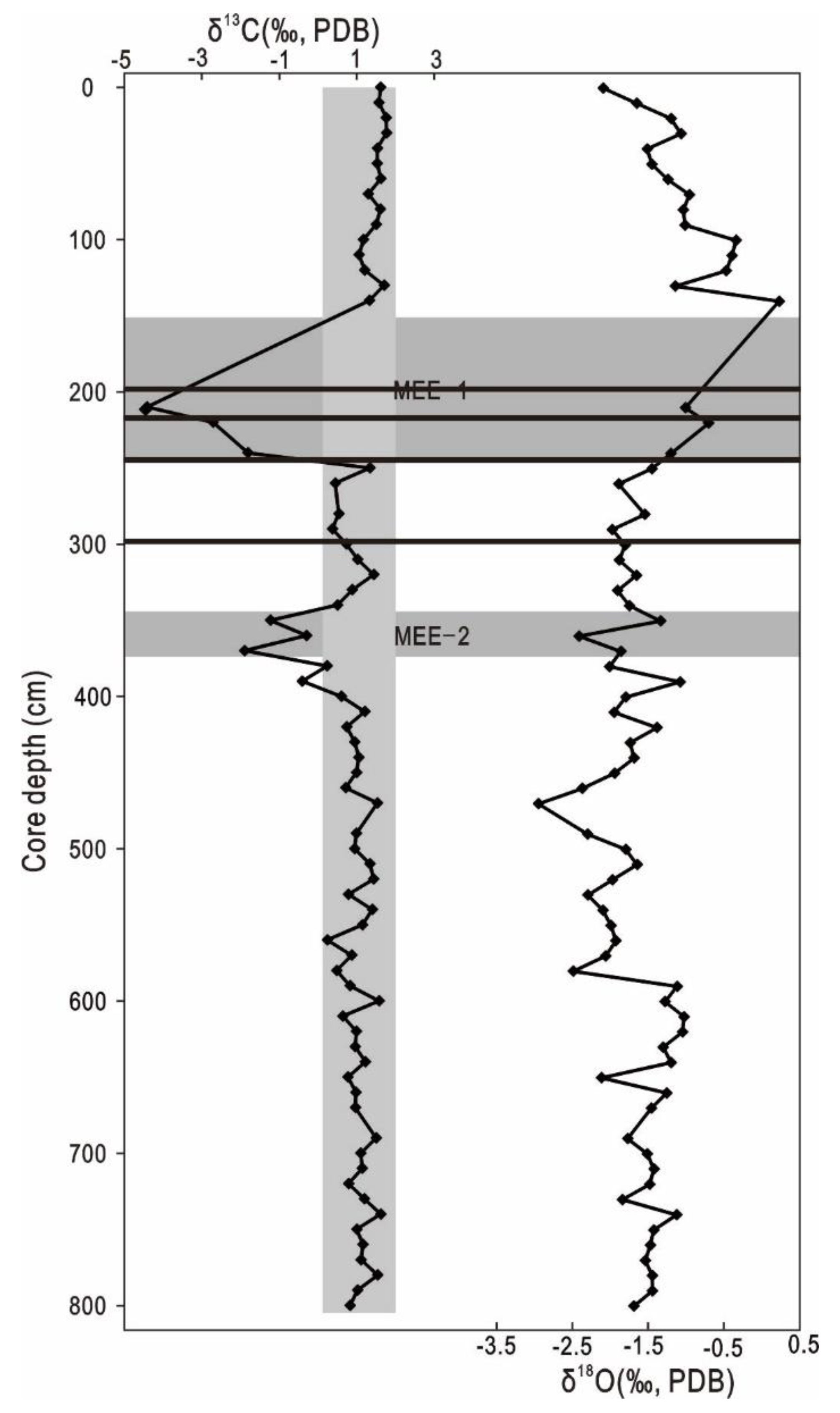
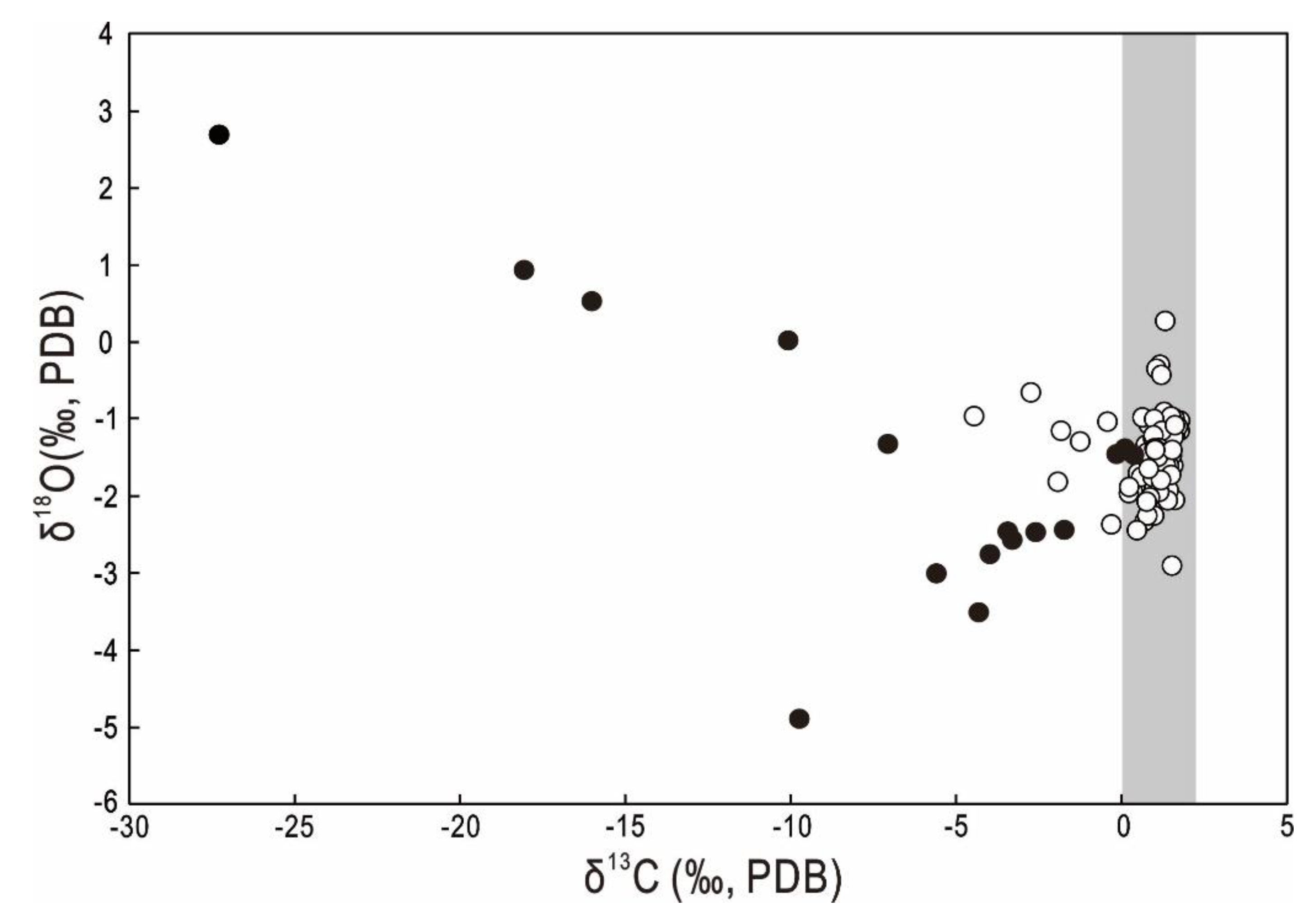
3.2. Stable Isotopic Compositions of Planktonic Foraminifera
4. Discussion
4.1. Paleo-Methane Release in the Nansha Trough
4.2. Authigenic Gypsum and Pyrite as Indicators of the Paleo-SMTZ
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suess, E.; Torres, M.E.; Bohrmann, G.; Collier, R.W.; Greinert, J.; Linke, P.; Rehder, G.; Tréhu, A.; Wallmann, K.; Winckler, G.; et al. Gas hydrate destabilization: Enhanced dewatering, benthic material turnover and large methane plumes at the Cascadia convergent margin. Earth Planet. Sci. Lett. 1999, 170, 1–15. [Google Scholar] [CrossRef]
- Judd, A.G. The global importance and context of methane escape from the seabed. Geo-Mar. Lett. 2003, 23, 147–154. [Google Scholar] [CrossRef]
- Judd, A.G.; Hovland, M.; Dimitrov, L.I.; Garcia Gil, S.; Jukes, V. The geological methane budget at continental margins and its influence on climate change. Geofluids 2002, 2, 109–126. [Google Scholar] [CrossRef]
- Torres, M.E.; McManus, J.; Hammond, D.E.; de Angelis, M.A.; Heeschen, K.U.; Colbert, S.L.; Tryon, M.D.; Brown, K.M.; Suess, E. Fluid and chemical fluxes in and out of sediments hosting methane hydrate deposits on Hydrate Ridge, OR, I: Hydrological provinces. Earth Planet. Sci. Lett. 2002, 201, 525–540. [Google Scholar] [CrossRef]
- Campbell, K.A. Hydrocarbon seep and hydrothermal vent paleoenvironments and paleontology: Past developments and future research directions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 232, 362–407. [Google Scholar] [CrossRef]
- Judd, A.G.; Hovland, M. Seabed Fluid Flow: The Impact of Geology, Biology and the Marine Environment; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Boetius, A.; Wenzhöfer, F. Seafloor oxygen consumption fuelled by methane from cold seeps. Nat. Geosci. 2013, 6, 725–734. [Google Scholar] [CrossRef]
- Hinrichs, K.-U.; Hayes, J.M.; Sylva, S.P.; Brewer, P.G.; DeLong, E.F. Methane-consuming archaebacteria in marine sediments. Nature 1999, 398, 802–805. [Google Scholar] [CrossRef]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gieseke, A.; Amann, G.; Jørgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. [Google Scholar] [CrossRef]
- Orphan, V.J.; House, C.H.; Hinrichs, K.-U.; McKeegan, K.D.; DeLong, E.F. Methane-consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science 2001, 293, 484–487. [Google Scholar] [CrossRef]
- Reeburgh, W.S. Oceanic methane biogeochemistry. Chem. Rev. 2007, 107, 486–513. [Google Scholar] [CrossRef]
- Pierre, C. Origin of the authigenic gypsum and pyrite from active methane seeps of the southwest African Margin. Chem. Geol. 2017, 449, 158–164. [Google Scholar] [CrossRef]
- Peckmann, J.; Reimer, A.; Luth, U.; Luth, C.; Hansen, B.T.; Heinicke, C.; Hoefs, J.; Reitner, J. Methane-derived carbonates and authigenic pyrite from the northwestern Black Sea. Mar. Geol. 2001, 177, 129–150. [Google Scholar] [CrossRef]
- Lim, Y.C.; Lin, S.; Yang, T.F.; Chen, Y.; Liu, C. Variations of methane induced pyrite formation in the accretionary wedge sediments offshore southwestern Taiwan. Mar. Petrol. Geol. 2011, 28, 1829–1837. [Google Scholar] [CrossRef]
- Pierre, C.; Blanc-Valleron, M.M.; Demange, J.; Boudouma, O.; Foucher, J.P.; Pape, T.; Himmler, T.; Fekete, N.; Spiess, V. Authigenic carbonates from active methane seeps offshore southwest Africa. Geo-Mar. Lett. 2012, 32, 501–513. [Google Scholar] [CrossRef]
- Zhang, M.; Konishi, H.; Xu, H.F.; Sun, X.M.; Lu, H.F.; Wu, D.D.; Wu, N.Y. Morphology and formation mechanism of pyrite induced by the anaerobic oxidation of methane from the continental slope of the NE South China Sea. J. Asian Earth Sci. 2014, 92, 293–301. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, X.; Lu, Y.; Xu, L.; Gong, J.; Lu, H.; Teichert, B.M.A.; Peckmann, J. Stable isotope patterns of coexisting pyrite and gypsum indicating variable methane flow at a seep site of the Shenhu area, South China Sea. J. Asian Earth Sci. 2016, 123, 213–223. [Google Scholar] [CrossRef]
- Tryon, M.D.; Brown, K.M. Fluid and chemical cycling at Bush Hill: Implications for gas- and hydrate-rich environments. Geochem. Geophys. Geosyst. 2004, 5, 1–7. [Google Scholar] [CrossRef]
- Solomon, E.A.; Kastner, M.; Jannasch, H.; Robertson, G.; Weinstein, Y. Dynamic fluid flow and chemical fluxes associated with a seafloor gas hydrate deposit on the northern Gulf of Mexico slope. Earth Planet. Sci. Lett. 2008, 270, 95–105. [Google Scholar] [CrossRef]
- Teichert, B.M.A.; Eisenhauer, A.; Bohrmann, G.; Haase-Schramm, A.; Bock, B.; Linke, P. U/Th systematics and ages of authigenic carbonates from Hydrate Ridge, Cascadia Margin: Recorders of fluid flow variations. Geochim. Cosmochim. Acta 2003, 67, 3845–3857. [Google Scholar] [CrossRef]
- Bhatnagar, G.; Chapman, W.G.; Dickens, G.R.; Dugan, B.; Hirasaki, G.J. Sulfate methane transition as a proxy for average methane hydrate saturation in marine sediments. Geophys. Res. Lett. 2008, 35, 1–15. [Google Scholar] [CrossRef]
- Feng, D.; Chen, D.; Peckmann, J. Rare earth elements in seep carbonates as tracers of variable redox conditions at ancient hydrocarbon seeps. Terra Nova 2009, 21, 49–56. [Google Scholar] [CrossRef]
- Feng, D.; Birgel, D.; Peckmann, J.; Roberts, H.H.; Joye, S.B.; Sassen, R.; Liu, X.-L.; Hinrichs, K.-U.; Chen, D. Time integrated variation of sources of fluids and seepage dynamics archived in authigenic carbonates from Gulf of Mexico Gas Hydrate Seafloor Observatory. Chem. Geol. 2014, 385, 129–139. [Google Scholar] [CrossRef]
- Birgel, D.; Feng, D.; Roberts, H.H.; Peckmann, J. Changing redox conditions at cold seeps as revealed by authigenic carbonates from Alaminos Canyon, northern Gulf of Mexico. Chem. Geol. 2011, 285, 82–96. [Google Scholar] [CrossRef]
- Himmler, T.; Bach, W.; Bohrmann, G.; Peckmann, J. Rare earth elements in authigenic methane-seep carbonates as tracers for fluid composition during early diagenesis. Chem. Geol. 2010, 277, 126–136. [Google Scholar] [CrossRef]
- Himmler, T.; Haley, B.A.; Torres, M.E.; Klinkhammer, G.P.; Bohrmann, G.; Peckmann, J. Rare earth element geochemistry in cold-seep pore waters of Hydrate Ridge, northeast Pacific Ocean. Geo-Mar. Lett. 2013, 33, 369–379. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, D.; Peckmann, J.; Roberts, H.H.; Chen, D. New insights into cerium anomalies and mechanisms of trace metal enrichment in authigenic carbonate from hydrocarbon seeps. Chem. Geol. 2014, 381, 55–66. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, D.; Chen, L.; Zheng, G.; Peckmann, J.; Chen, D. Using iron speciation in authigenic carbonates from hydrocarbon seeps to trace variable redox conditions. Mar. Petrol. Geol. 2015, 67, 111–119. [Google Scholar] [CrossRef]
- Crémière, A.; Pierre, C.; Blanc-Valleron, M.M.; Zitter, T.; Cagatay, N.M.; Henry, P. Methane-derived authigenic carbonates along the North Anatolian fault system in the Sea of Marmara (Turkey). Deep Sea Res. Part I: Oceanogr. Res. Pap. 2012, 66, 114–130. [Google Scholar] [CrossRef]
- Peckmann, J.; Goedert, J.L.; Heinrichs, T.; Hoefs, J.; Reitner, J. The Late Eocene ‘Whiskey Creek’ methane-seep deposit (western Washington State)-Part II: Petrology, stable isotopes, and biogeochemistry. Facies 2003, 48, 241–254. [Google Scholar] [CrossRef]
- Sassen, R.; Roberts, H.H.; Carney, R.; Milkov, A.V.; DeFreitas, D.A.; Lanoil, B.; Zhang, C.L. Free hydrocarbon gas, gas hydrate, and authigenic minerals in chemosynthetic communities of the northern Gulf of Mexico continental slope: Relation to microbial processes. Chem. Geol. 2004, 205, 195–217. [Google Scholar] [CrossRef]
- Wang, J.; Suess, E.; Rickert, D. Authigenic gypsum found in gas hydrate associated sediments from Hydrate Ridge, the eastern North Pacific. Sci. China Ser. D Earth Sci. 2004, 47, 280–288. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, W.; Chen, M.H.; Lu, J.; Gu, S.C. Formation of authigenic gypsum and pyrite assemblage and its siganificance to gas ventings in Nansha Trough, South China Sea. Mar. Geol. Quarter. Geol. 2007, 2, 91–100. [Google Scholar]
- Larrasoaña, J.C.; Roberts, A.P.; Musgrave, R.J.; Gràcia, E.; Piñero, E.; Vega, M.; Martínez-Ruiz, F. Diagenetic formation of greigite and pyrrhotite in gas hydrate marine sedimentary systems. Earth Planet. Sci. Lett. 2007, 261, 350–366. [Google Scholar] [CrossRef]
- Bayon, G.; Birot, D.; Ruffine, L.; Caprais, J.-C.; Ponzevera, E.; Bollinger, C.; Donval, J.-P.; Charlou, J.-L.; Voisset, M.; Grimaud, S. Evidence for intense REE scavenging at cold seeps from the Niger Delta margin. Earth Planet. Sci. Lett. 2011, 312, 443–452. [Google Scholar] [CrossRef]
- Kocherla, M. Authigenic gypsum in gas hydrate associated sediments from the east coast of India (Bay of Bengal). Acta Geol. Sin. 2013, 87, 749–760. [Google Scholar] [CrossRef]
- Pirlet, H.; Wehrmann, L.M.; Brunner, B.; Frank, N.; Dewanckele, J.; Van Rooij, D.; Foubert, A.; Swennen, R.; Naudts, L.; Boone, M.; et al. Diagenetic formation of gypsum and dolomite in a cold-water coral mound in the Porcupine Seabight, off Ireland. Sedimentology 2010, 57, 786–805. [Google Scholar] [CrossRef]
- Gooday, A.J. Benthic foraminifera (Protista) as tools in deep-water palaeoceanography: Environmental influences on faunal characteristics. Adv. Mar. Biol. 2003, 46, 1–90. [Google Scholar]
- Jorissen, F.J.; Fontanier, C.; Thomas, E. Paleoceanographical proxies based on deep-sea benthic foraminiferal assemblage characteristics. In Proxies in Late Cenozoic Paleoceanography; Elsevier: Amsterdam, The Netherlands, 2007; Volume 1, 862p. [Google Scholar]
- Wefer, G.; Heinze, P.M.; Berger, W.H. Clues to ancient methane release. Nature 1994, 369, 282. [Google Scholar] [CrossRef]
- Panieri, G.; Camerlenghi, A.; Conti, S.; Pini, G.A.; Cacho, I. Methane seepages recorded in benthic foraminifera from Miocene seep carbonates, Northern Apennines (Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 284, 271–282. [Google Scholar] [CrossRef]
- Fontanier, C.; Koho, K.A.; Goni-Urriza, M.S.; Deflandre, B.; Galaup, S.; Ivanovsky, A.; Gayet, N.; Dennielou, B.; Gremare, A.; Bichon, S.; et al. Benthic foraminifera from the deep-water Niger Delta (Gulf of Guinea): Assessing present-day and past activity of hydrate pockmarks. Deep-Sea Res. Part I 2014, 94, 87–106. [Google Scholar] [CrossRef]
- Panieri, G.; James, R.H.; Camerlenghi, A.; Westbrook, G.K.; Consolaro, C.; Cacho, I.; Cesari, V.; Cervera, C.S. Record of methane emissions from the West Svalbard continental margin during the last 23.500 yrs revealed by δ13C of benthic foraminifera. Global Planet. Chang. 2014, 122, 151–160. [Google Scholar] [CrossRef]
- Hill, T.M.; Kennett, J.P.; Valentine, D.L. Isotopic evidence for the incorporation of methane-derived carbon into foraminifera from modern methane seeps, Hydrate Ridge, Northeast Pacific. Geochim. Cosmochim. Acta 2004, 68, 4619–4627. [Google Scholar] [CrossRef]
- Hinrichs, K.U.; Hmelo, L.R.; Sylva, S.P. Molecular fossil record of elevated methane levels in late Pleistocene coastal waters. Science 2003, 299, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Keigwin, L.D. Late Pleistocene-Holocene paleoceanography and ventilation of the Gulf of California. J. Oceanogr. 2002, 58, 421–432. [Google Scholar] [CrossRef]
- Kennett, J.P.; Cannariato, K.G.; Hendy, I.L.; Behl, R.J. Carbon isotopic evidence for methane hydrate instability during Quaternary interstadials. Science 2000, 88, 128–133. [Google Scholar] [CrossRef]
- Mackensen, A.; Wollenburg, J.; Licari, L. Low δ13C in tests of live epibenthic and endobenthic foraminifera at a site of active methane seepage. Paleoceanography 2006, 21. [Google Scholar] [CrossRef]
- Ohkushi, K.; Ahagon, N.; Uchida, M.; Shibata, Y. Foraminiferal isotope anomalies from northwestern Pacific marginal sediments. Geochem. Geophys. Geosyst. 2005, 6. [Google Scholar] [CrossRef]
- Uchida, M.; Ohkushi, K.; Kimoto, K.; Inagaki, F.; Ishimura, T.; Tsunogai, U.; TuZino, T.; Shibata, Y. Radiocarbon-based carbon source quantification of anomalous isotopic foraminifera in last glacial sediments in the western North Pacific. Geochem. Geophys. Geosyst. 2008, 9. [Google Scholar] [CrossRef]
- Katz, M.E.; Pak, D.K.; Dickens, G.R.; Miller, K.G. The source and fate of massive carbon input during the latest Paleocene thermal maximum. Science 1999, 286, 1531–1533. [Google Scholar] [CrossRef]
- Hesselbo, S.P.; Grocke, D.R.; Jenkyns, H.C.; Bjerrum, C.J.; Farrimond, P.; Morgans Bell, H.S.; Green, O.R. Massive dissociation of gas hydrate during a Jurassic oceanic anoxic event. Nature 2000, 406, 392–395. [Google Scholar] [CrossRef]
- Jahren, A.H.; Arens, N.C.; Sarmiento, G.; Guerrero, J.; Amundson, R. Terrestrial record of methane hydrate dissociation in the Early Cretaceous. Geology 2001, 29, 159–162. [Google Scholar] [CrossRef]
- Padden, M.; Weissert, H.; de Rafelis, M. Evidence for Late Jurassic release of methane from gas hydrate. Geology 2001, 29, 223–226. [Google Scholar] [CrossRef]
- Rathburn, A.E.; Pérez, M.E.; Martin, J.B.; Day, S.A.; Mahn, C.; Gieskes, J.; Ziebis, W.; Williams, D.; Bahls, A. Relationships between the distribution and stable isotopic composition of living benthic foraminifera and cold methane seep biogeochemistry in Monterey Bay, California. Geochem. Geophys. Geosyst. 2003, 4, 1106. [Google Scholar] [CrossRef]
- Panieri, G. Benthic foraminifera associated with a hydrocarbon seep in the Rockall Trough (NE Atlantic). Geobios 2005, 38, 247–255. [Google Scholar]
- Panieri, G.; Camerlenghi, A.; Cacho, I.; Cervera, C.S.; Canals, M.; Lafuerza, S.; Herrera, G. Tracing seafloor methane emissions with benthic foraminifera: Results from the Ana submarine landslide (Eivissa Channel, Western Mediterranean Sea). Mar. Geol. 2012, 291–294, 97–112. [Google Scholar] [CrossRef]
- Panieri, G.; Graves, C.A.; James, R.H. Paleo-methane emissions recorded in foraminifera near the landward limit of the gas hydrate stability zone offshore western Svalbard. Geochem. Geophys. Geosyst. 2016, 17, 521–537. [Google Scholar] [CrossRef]
- Bernhard, J.M.; Buck, K.R.; Barry, J.P. Monterey Bay cold-seep biota: Assemblages, abundance, and ultrastructure of living foraminifera. Deep Sea Res. Part I: Oceanogr. Res. Pap. 2001, 48, 2233–2249. [Google Scholar]
- Sibuet, M.; Olu, K. Biogeography, biodiversity and fluid dependence of deep-sea cold-seep communities at active and passive margins. Deep-Sea Res. Part II 1998, 45, 517–567. [Google Scholar] [CrossRef]
- Qian, J.X. Paleoceanography for the Late Quaternary in the South China Sea; China Science Press: Beijing, China, 1999; pp. 39–55. [Google Scholar]
- Jian, Z.; Wang, L. Late quaternary benthic foraminifera and deep-water paleoceanography in the South China Sea. Mar. Micropaleontol. 1997, 32, 127–154. [Google Scholar] [CrossRef]
- Jian, Z.; Zhao, Q.; Cheng, X.; Wang, J.; Wang, P.; Su, X. Pliocene-Pleistocene stable isotope and paleoceanographic changes in the northern South China Sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 193, 425–442. [Google Scholar] [CrossRef]
- Formolo, M.J.; Lyons, T.W.; Zhang, C.L.; Kelley, C.A.; Sassen, R.; Horita, J.; Cole, D.R. Quantifying carbon sources in the formation of authigenic carbonates at gas hydrate sites in the Gulf of Mexico. Chem. Geol. 2004, 205, 253–264. [Google Scholar] [CrossRef]
- Heinz, P.; Sommer, S.; Pfannkuche, O.; Hemleben, C. Living benthic foraminifera in sediments influenced by gas hydrates at the Cascadia convergent margin, NE Pacific. Mar. Ecol. Prog. Ser. 2005, 304, 77–89. [Google Scholar] [CrossRef]
- Rathburn, A.E.; Levin, L.A.; Tryon, M.; Gieskes, J.M.; Martin, J.M.; Perez, M.E.; Fodrie, F.J.; Neira, C.; Fryer, G.J.; Mendoza, G.; et al. Geological and biological heterogeneity of the Aleutian margin (1965–4822 m). Prog. Oceanogr. 2009, 80, 22–50. [Google Scholar] [CrossRef]
- Panieri, G. Benthic foraminifera response to methane release in an Adriatic Sea pockmark. Riv. Ital. Paleontol. E Stratigr. 2003, 109, 549–562. [Google Scholar]
- Rathburn, A.E.; Levin, L.A.; Held, Z.; Lohmann, K.C. Benthic foraminifera associated with cold methane seeps on the northern California margin: Ecology and stable isotopic composition. Mar. Micropaleontol. 2000, 38, 247–266. [Google Scholar] [CrossRef]
- Sen Gupta, B.K.; Platon, E.; Bernhard, J.M.; Aharon, P. Foraminiferal colonization of hydrocarbon-seep bacterial mats and underlying sediment, Gulf of Mexico slope. J. Foraminifer. Res. 1997, 27, 292–300. [Google Scholar] [CrossRef]
- Torres, M.E.; Mix, A.C.; Kinports, K.; Haley, B.A.; Klinkhammer, G.P.; Mcmanus, J.; De Angelis, M. Is methane venting at the seafloor recorded by δ13C of benthic foraminifera shells? Paleoceanography 2003, 18. [Google Scholar] [CrossRef]
- Li, N.; Yang., X.; Peng., J.; Zhou., Q.; Chen., D. Paleo-cold seep activity in the southern South China Sea: Evidence from the geochemical and geophysical records of sediments. J. Asian Earth Sci. 2018, 168, 106–111. [Google Scholar] [CrossRef]
- Liu, H.; Yan, P.; Sun, Y. Layer-block tectonics of the Nansha microplate. Geol. China 2002, 29, 374–381. [Google Scholar]
- Ingram, G.M.; Chisholm, T.J.; Grant, C.J.; Hedlund, C.A.; Stuart-Smith, P.; Teasdale, J. Deepwater North West Borneo: Hydrocarbon accumulation in an active fold and thrust belt. Mar. Petrol. Geol. 2004, 21, 879–887. [Google Scholar] [CrossRef]
- Berner, U.; Faber, E. Hydrocarbon gases in surface sediments of the South China Sea. In Marine Geology and Geophysics of the South China Sea; Jin, X.L., Ed.; China Ocean Press: Beijing, China, 1990; pp. 199–211. [Google Scholar]
- Cheng, X.; Huang, B.; Jian, Z.; Zhao, Q.; Tian, J.; Li, J. Foraminiferal isotopic evidence for monsoonal activity in the South China Sea: A present-LGM comparison. Mar. Micropaleontol. 2005, 54, 125–139. [Google Scholar] [CrossRef]
- Wan, S.; Feng, D.; Chen, F.; Zhuang, C.; Chen, D. Foraminifera from gas hydrate-bearing sediments of the northeastern South China Sea: Proxy evaluation and application for methane release activity. J. Asian Earth Sci. 2018, 168, 125–136. [Google Scholar] [CrossRef]
- Martinson, D.G.; Pisias, N.G.; Hays, J.D.; Imbrie, J.; Moore, T.C.; Shachleton, N.J. Age Dating and the Orbital Theory of the Ice Ages: Development of a High-Resolution 0 to 300,000 Year Chronostratigraghy. Quat. Res. 1987, 27, 1–29. [Google Scholar] [CrossRef]
- Li, N.; Di, P.; Feng, D.; Chen, D. Persistent deep-water oxygen depletion caused by methane seepage during the last glacial maximum: Evidence from the South China Sea. Ore Geol. Rev. 2020, submitted. [Google Scholar]
- Hoareau, G.; Monnin, C.; Odonne, F. The stability of gypsum in marine sediments using the entire ODP/IODP porewater composition database. Mar. Geol. 2011, 279, 87–97. [Google Scholar] [CrossRef]
- Cita, M.B. Exhumation of Messinian evaporites in the deep-sea and creation of deep anoxic brine-filled collapsed basins. Sediment. Geol. 2006, 188–189, 357–378. [Google Scholar] [CrossRef]
- Joye, S.B.; MacDonald, I.R.; Montoya, J.P.; Peccini, M. Geophysical and geochemical signatures of Gulf of Mexico seafloor brines. Biogeosciences 2005, 2, 295–309. [Google Scholar] [CrossRef]
- Balci, N.; Shanks III, W.C.; Mayer, B.; Mandernack, K.W. Oxygen and sulfur isotope systematics of sulfate produced by bacterial and abiotic oxidation of pyrite. Geochim. Cosmochim. Acta 2007, 71, 3796–3811. [Google Scholar] [CrossRef]
- Schippers, A.; Jørgensen, B.B. Oxidation of pyrite and iron sulfide by manganese dioxide in marine sediments. Geochim. Cosmochim. Acta 2001, 65, 915–922. [Google Scholar] [CrossRef]
- Canfield, D.E.; Thamdrup, B. The production of 34S-depleted sulfide during bacterial disproportionation of elemental sulfur. Science 1994, 266, 1973–1975. [Google Scholar] [CrossRef]
- Thamdrup, B.; Finster, K.; Hansen, J.W.; Bak, F. Bacterial disproportionation of elemental sulfur coupled to chemical reduction of iron or manganese. Appl. Environ. Microbiol. 1993, 59, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Pirlet, H.; Wehrmann, L.M.; Foubert, A.; Brunner, B.; Blamart, D.; De Mol, L.; Van Rooij, D.; Dewanckele, J.A.N.; Cnudde, V.; Swennen, R.; et al. Unique authigenic mineral assemblages reveal different diagenetic histories in two neighbouring cold-water coral mounds on Pen Duick Escarpment, Gulf of Cadiz. Sedimentology 2012, 59, 578–604. [Google Scholar] [CrossRef]
- Haffert, L.; Haeckel, M.; Liebetrau, V.; Berndt, C.; Hensen, C.; Nuzzo, M.; Reitz, A.; Scholz, F.; Schönfeld, J.; Perez-Garcia, C.; et al. Fluid evolution and authigenic mineral paragenesis related to salt diapirism the Mercator mud volcano in the Gulf of Cadiz. Geochim. Cosmochim. Acta 2013, 106, 261–286. [Google Scholar] [CrossRef]
- Harouaka, K.; Eisenhauer, A.; Fantle, M.S. Experimental investigation of Ca isotopic fractionation during abiotic gypsum precitpitation. Geochim. Cosmochim. Acta 2014, 129, 157–176. [Google Scholar] [CrossRef]
- Vogel, M.B.; Marais, D.J.; Parenteau, M.N.; Jahnke, L.; Turk, K.A.; Kubo, M.D.Y. Biological influences on modern sulfates: Textures and composition of gypsum deposits from Guerrero Negro, Baja California Sur, Mexico. Sediment. Geol. 2010, 223, 265–280. [Google Scholar] [CrossRef]
- Xie, L.; Wang, J.S.; Wu, N.Y.; Wu, D.D.; Wang, Z.; Zhu, X.W.; Hu, J.; Chen, H.R.; Lin, Q. Characteristics of authigenic pyrites in shallow core sediments in the Shenhu area of the northern South China Sea: Implications for a possible mud volcano environment. Sci. China Earth Sci. 2013, 56, 541–548. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. The size distribution of framboidal pyrite in modern sediments: An indicator of redox conditions. Geochim. Cosmochim. Acta 1996, 60, 3897–3912. [Google Scholar] [CrossRef]
- Rao, V.P.; Kessarkar, P.M.; Patil, S.K.; Ahmad, S.M. Rock magnetic and geochemical record in a sediment core from the eastern Arabian Sea: Diagenetic and environmental implications during the late Quaternary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 270, 46–52. [Google Scholar] [CrossRef]
- Butler, I.B.; Rickard, D. Framboidal pyrite formation via the oxidation of iron (II) monosulfide by hydrogen sulphide. Geochim. Cosmochim. Acta 2000, 64, 2665–2672. [Google Scholar] [CrossRef]
- Ohfuji, H.; Rickard, D. Experimental syntheses of framboids—A review. Earth Sci. Rev. 2005, 71, 147–170. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Di, P.; Li, N.; Chen, F.; Su, X.; Zhang, J. Unique Authigenic Mineral Assemblages and Planktonic Foraminifera Reveal Dynamic Cold Seepage in the Southern South China Sea. Minerals 2020, 10, 275. https://doi.org/10.3390/min10030275
Zhou Y, Di P, Li N, Chen F, Su X, Zhang J. Unique Authigenic Mineral Assemblages and Planktonic Foraminifera Reveal Dynamic Cold Seepage in the Southern South China Sea. Minerals. 2020; 10(3):275. https://doi.org/10.3390/min10030275
Chicago/Turabian StyleZhou, Yang, Pengfei Di, Niu Li, Fang Chen, Xin Su, and Jinpeng Zhang. 2020. "Unique Authigenic Mineral Assemblages and Planktonic Foraminifera Reveal Dynamic Cold Seepage in the Southern South China Sea" Minerals 10, no. 3: 275. https://doi.org/10.3390/min10030275
APA StyleZhou, Y., Di, P., Li, N., Chen, F., Su, X., & Zhang, J. (2020). Unique Authigenic Mineral Assemblages and Planktonic Foraminifera Reveal Dynamic Cold Seepage in the Southern South China Sea. Minerals, 10(3), 275. https://doi.org/10.3390/min10030275




