Assessment of the Mode of Occurrence and Radiological Impact of Radionuclides in Nigerian Coal and Resultant Post-Combustion Coal Ash Using Scanning Electron Microscopy and Gamma-Ray Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
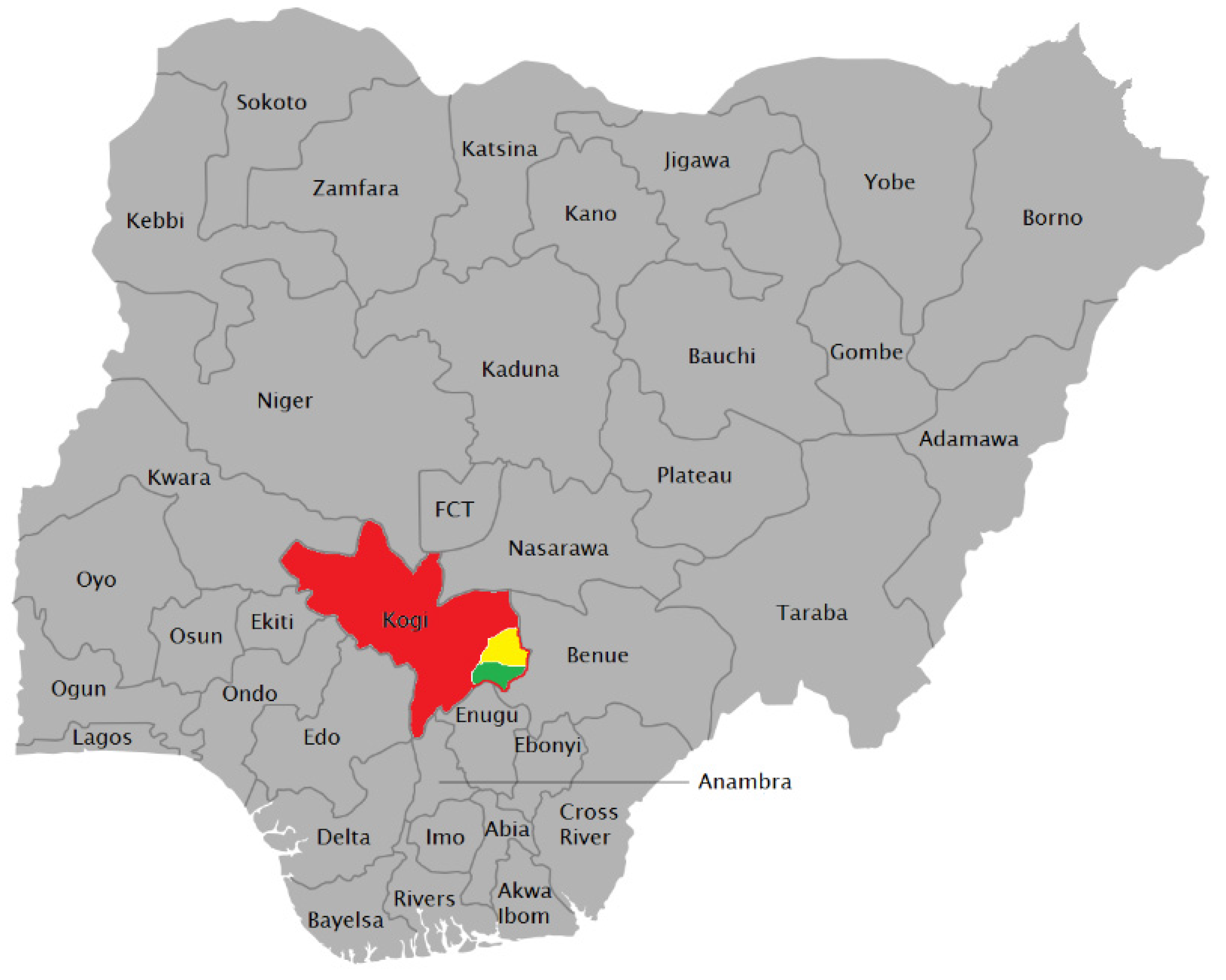
2.1. Samples and Samples Collection
2.2. Simulant Preparation
2.3. High Resolution Gamma-Ray Spectroscopy
Activity Concentration Determination
2.4. Radiological Hazard Indices
2.4.1. Radium Equivalent
2.4.2. Absorbed Dose
2.4.3. Annual Effective Dose Equivalent
2.5. Electron Microscopy Analysis
3. Results and Discussion
3.1. Gamma Spectroscopy
3.1.1. Activity Concentration
3.1.2. Radiological Hazard Indices
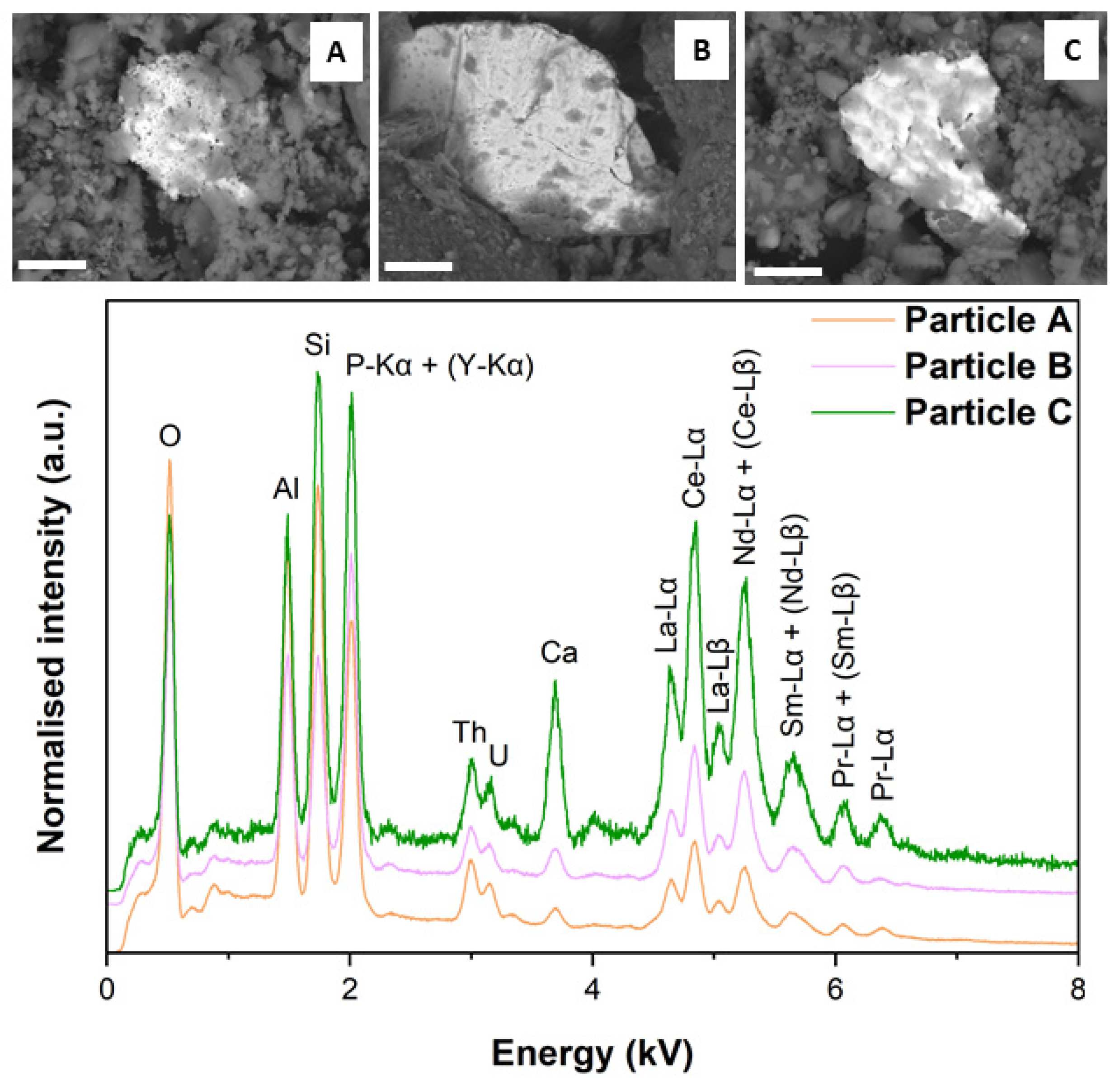
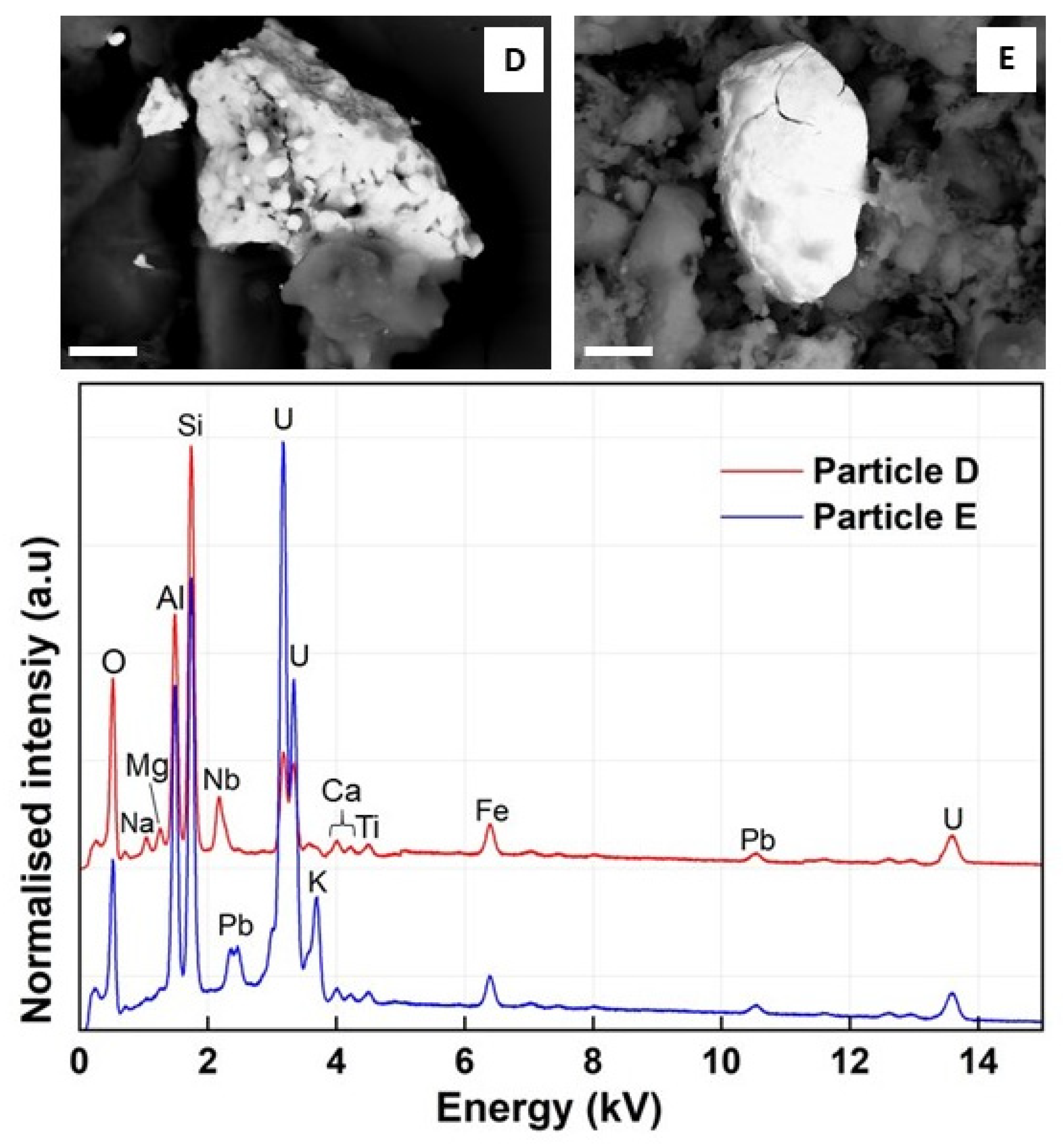
3.2. SEM–EDS
4. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nancy, E.L.; James, C.H.; Heileen, H.-K.; Ross, K.; Taggart, A.V. Naturally Occurring radioactive materials in coals and coal combustion residuals in the United States. Environ. Sci. Technol. 2015, 49, 11227–11233. [Google Scholar] [CrossRef]
- Ruhl, L.; Vengosh, A.; Dwyer, G.S.; Hsu-Kim, H.; Deonarine, A.; Bergin, M.; Kravchenko, J. Survey of the potential environmental and health impacts in the immediate aftermath of the coal ash spill in kingston, tennessee. Environ. Sci. Technol. 2009, 43, 6326–6333. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, P.; Yao, D.; Huang, W.; Tang, S.; Wang, K.; Liu, W.; Hu, Y.; Zhang, B.; Sha, J. Abundance, distribution, and modes of occurrence of uranium in Chinese coals. Minerals 2017, 7, 239. [Google Scholar] [CrossRef]
- Chen, J.; Chen, P.; Yao, D.; Huang, W.; Tang, S.; Wang, K.; Liu, W.; Hu, Y.; Li, Q.; Wang, R. Geochemistry of uranium in Chinese coals and the emission inventory of coal-fired power plants in China. Int. Geol. Rev. 2017, 621–637. [Google Scholar] [CrossRef]
- Karangelos, D.; Petropoulos, N.; Anagnostakis, M.; Hinis, E.; Simopoulos, S. Radiological characteristics and investigation of the radioactive equilibrium in the ashes produced in lignite-fired power plants. J. Environ. Radioact. 2004, 77, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulou, M.; Papastefanou, C. Behavior of natural radionuclides in lignites and fly ashes. J. Environ. Radioact. 1992, 16, 261–271. [Google Scholar] [CrossRef]
- Arbuzov, S.I.; Maslov, S.G.; Volostnov, A.V.; Il’enok, S.S.; Arkhipov, V.S. Modes of Occurrence of Uranium and Thorium in Coals and Peats of Northern Asia. Solid Fuel Chem. 2012, 46, 52–66. [Google Scholar] [CrossRef]
- Salbu, B.; Lind, O.C.; Skipperud, L. Radionuclide speciation and its relevance in environmental impact assessments. J. Environ. Rad. 2004, 74, 233–242. [Google Scholar] [CrossRef]
- Kogi Power Station. 2012. Available online: www.sourcewatch.org/index.php/Kogi_power_station (accessed on 5 June 2018).
- Energy Mix Report. ETA-ZUMA Launches Coal Briquette Production Plant-Kogi State. 2018. Available online: www.energymixreport.com/eta-zuma-launches-coal-briquette-production-plant-kogi/ (accessed on 7 June 2018).
- Franklin Alli Cement Manufacturers Cut Power Cost with Investments in Coal. 2017. Available online: www.vanguardngr.com/2017/11/cement-manufacturers-cut-power-cost-coal-investments/ (accessed on 8 June 2018).
- Ministry of Solid Minerals Development, Federal Republic of Nigeria. Feasibility Study: Nigerian Coal Resource Development. Available online: https://electricityinnigeria.com/ (accessed on 18 March 2019).
- Mohammed, U.; Garba, U.M.; Priscilla, E.; Azare, K.I.; Usman, S.; Onoduku, Y.; Mohammad, S. Characterisation and ash chemistry of selected Nigerian coals for solid fuel combustion. Petoleum Coal. 2016, 58, 646–654. [Google Scholar]
- Afu, D.J.; Ifeola, E.O.; Adesida, P.A. Omelewu coal characterization for powering power plant at Dangote cement factory, Obajana, Kogi state, Nigeria. Am. J. Eng. Res. 2018, 7, 143–152. [Google Scholar]
- Wikipedia Map of Nigerian States with Names. 2019. Available online: https://en.wikipedia.org/wiki/File:Map_of_Nigerian_States_with_names.png (accessed on 4 July 2019).
- Stanislav, V.V.; Christina, G.V. Methods for Characterization of Composition of Fly Ashes from Coal-Fired Power Stations: A Critical Overview. Energy Fuels 2005, 19, 1084–1098. [Google Scholar] [CrossRef]
- Eckert & Ziegler. Reference & Calibration Sources Product Information. Available online: www.ezag.com/ (accessed on 4 January 2019).
- Ga-Ma and Associates Inc. Snap-on Lid Marinelli Beakers for Solid and Liquid Samples; Ga-Ma and Associates Inc.: Ocala, FL, USA.
- Lauer, N.; Vengosh, A.; Dai, S. Naturally Occurring Radioactive Materials in Uranium-Rich Coals and Associated Coal Combustion Residues from China. Environ. Sci. Technol. 2017, 51, 13487–13493. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.G. Practical Gamma-Ray Spectrometry; John Wiley and Sons: Sussex, UK, 2008. [Google Scholar]
- Isinkaye, O.M.; Nnamdi, N.; Jibiri, A.; Olomide, A. Radiological health assessment of natural radioactivity in the vicinity of Obajana cement factory, North Central Nigeria. J. Med. Phys. 2015, 52–59. [Google Scholar] [CrossRef] [PubMed]
- National Nuclear Data Center, Brookhaven National Laboratory, USA. Available online: http://www.nndc.bnl.gov/ (accessed on 19 January 2019).
- United Nation Scientific Committee on Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation; Report to the General Assembly with Scientific Annexes; UNSCEAR: New York, NY, USA, 2000. [Google Scholar]
- El-Taher, A. Gamma spectroscopic analysis and associated radiation hazards of building materials used in Egypt. Radiat. Prot. Dosim. 2009, 2, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Beretka, J.; Mathew, P.J. Natural radioactivity of Australian building materials, industrial wastes and by-products. Health Phys. 1985, 48, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jia, X.; Wang, F. Natural radioactivity of coal and its by-products in the Baoji coal-fired power plant, China. Curr. Sci. 2006, 91, 1508. [Google Scholar]
- Mandal, A.; Sengupta, D. Radioelemental study of Kolaghat thermal power plant, West Bengal, India: Possible environmental hazards. Environ. Geol. 2003, 44, 180–186. [Google Scholar] [CrossRef]
- Kisić, D.M.; Miletić, S.R.; Radonjić, V.D.; Radanović, S.B.; Filipovic, J.Z.; Gržetić, I.A. Natural radioactivity of coal and fly ash at the Nikola Tesla B TPP. Hem. Ind. 2013, 67, 729–738. [Google Scholar] [CrossRef]
- Cevik, U.; Damla, N.; Nezir, S. Radiological characterization of Cayirhan coal-fired power plant in Turkey. Fuel 2007, 2509–2513. [Google Scholar] [CrossRef]
- United Nation Scientific Committee on Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation; Report to the General Assembly with Scientific Annexes; UNSCEAR: New York, NY, USA, 1988. [Google Scholar]
- Labrincha, J.; Puertas, F.; Schroeyers, W.; Kovler, K. Naturally occurring radioactive materials in construction. Integr. Radiat. Prot. Reuse 2017, 183–252. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation; Report to the General Assembly Scientific Annexes A and B; UNSCEAR: New York, NY, USA, 2008; Volume I. [Google Scholar]
- Wang, Y.; Bagnoud, A.; Suvorona, E.; McGivney, E. Geochemical control on uranium (IV) mobility in a mining-impacted wetland. Environ. Sci. Technol. 2014, 48, 10062–10072. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, P.; Kyser, T.K. Effects of cationic substitutions and alteration in uraninite, and implications for the dating of uranium deposits. Can. Mineral. 2005, 43, 1005–1017. [Google Scholar] [CrossRef]
- Burns, P.C.; Finch, R. Uranium-Mineralogy, Geochemistry and the Environment; Reviews in Mineralogy 38; Mineralogical Society of America: Washington, DC, USA, 1999. [Google Scholar]
- Sani, R.K.; Peyton, B.M.; Dohnalkova, A.; Amonette, J.E. Re-oxidation of reduced uranium with iron (III) (hydr)oxides under sulfate-reducing conditions. Environ. Sci. Technol. 2005, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.U.; Ilton, E.S.; Veeramani, H.; Sharp, J.O.; Bernier-Latmani, R.; Schofield, E.J.; Bargar, J.R.; Glammar, D.E. Comparative dissolution kinetics of biogenic and chemogenic uraninite under oxidizing conditions in the presence of carbonate. Geochim. ET Cosmochim. Acta 2009, 73, 6065–6083. [Google Scholar] [CrossRef]
- Leay, L.; Potts, A.; Donoclift, T. Geopolymers from fly ash and their gamma irradiation. Mater. Lett. 2018, 227, 240–242. [Google Scholar] [CrossRef]
- Zoorob, S.E.; Cabrera, J.G. Design and construction of a road pavement using fly ash in hot rolled asphalt. Stud. Environ. Sci. 1997, 71, 149–165. [Google Scholar] [CrossRef]
- U.S. Department of Energy, National Energy Technology Laboratory. Characterization of Rare Earth Element Minerals in Coal Utilization By-products. 2017. Available online: https://edx.netl.doe.gov/dataset/characterization-of-rare-earth-element-minerals-in-coal-utilization-byproducts (accessed on 8 May 2019).
| Coal Samples | 40K | 232Th | 228Ra | 226Ra | Total Radium |
|---|---|---|---|---|---|
| OMC1 | 81.40 ± 4.98 | 32.23 ± 1.27 | 27.00 ± 1.67 | 32.35 ± 1.17 | 59.35 |
| OMC2 | 80.24 ± 4.84 | 31.94 ± 1.25 | 27.33 ± 1.67 | 30.22 ± 1.09 | 57.55 |
| OMC3 | 53.40 ± 3.64 | 28.77 ± 1.14 | 24.92 ± 1.53 | 28.56 ± 1.03 | 53.48 |
| OMC4 | 105.95 ± 5.67 | 46.88 ± 1.74 | 37.93 ± 2.21 | 47.06 ± 1.64 | 84.99 |
| OMC5 | 90.09 ± 5.15 | 44.71 ± 1.68 | 36.89 ±2.17 | 45.03 ± 1.57 | 81.92 |
| OMC6 | 88.75 ± 5.08 | 43.72 ± 1.64 | 36.33 ± 2.13 | 46.74 ± 1.63 | 83.07 |
| OMC7 | 90.10 ± 5.00 | 46.37 ± 1.72 | 38.56 ± 2.23 | 45.74 ± 1.59 | 84.30 |
| OMC8 | 96.52 ± 5.50 | 48.25 ± 1.81 | 41.54 ± 2.42 | 50.99 ± 1.78 | 92.53 |
| OMC9 | 84.10 ± 4.93 | 36.55 ± 1.40 | 31.43 ± 1.88 | 40.46 ± 1.42 | 71.89 |
| OMC10 | 50.35 ± 3.31 | 66.56 ± 2.40 | 55.98 ± 3.14 | 59.22 ± 2.03 | 115.20 |
| OMC11 | 45.91 ± 3.39 | 33.06 ± 1.29 | 27.55 ± 1.69 | 30.95 ± 1.11 | 58.50 |
| OMC12 | 83.02 ± 4.87 | 41.25 ± 1.56 | 35.28 ± 2.08 | 43.97 ± 1.54 | 79.25 |
| OMC13 | 80.19 ± 4.85 | 41.85 ± 1.59 | 35.14 ± 2.09 | 45.34 ± 1.59 | 80.48 |
| OMC14 | 78.12 ± 4.76 | 42.07 ± 1.60 | 35.47 ± 2.10 | 45.44 ± 1.60 | 80.91 |
| OMC15 | 81.68 ± 4.91 | 42.20 ± 1.60 | 35.60 ± 2.11 | 45.57 ± 1.60 | 81.17 |
| Min. Max. | 45.91 ± 3.39 105.95 ± 5.67 | 28.77 ± 1.14 66.56 ± 2.40 | 27.00 ± 1.67 55.98 ± 3.14 | 28.56 ± 1.03 59.22 ± 2.03 | 53.48 115.20 |
| Mean | 79.32 ± 16.91 | 41.76 ± 9.17 | 35.13 ± 7.32 | 42.51 ± 8.54 | 77.64 |
| UNSCEAR 2000 soil average [23] | 400 | 30 | 30 | 35 | 65 |
| % of UNSCEAR 2000 | 20 | 139 | 117 | 121 | 119 |
| Coal Samples | 40K | 232Th | 228Ra | 226Ra | Total Radium |
|---|---|---|---|---|---|
| OKC1 | 82.09 ± 6.31 | 18.43 ± 0.92 | 16.83 ± 1.30 | 13.84 ± 0.63 | 30.67 |
| OKC2 | 46.60 ± 3.80 | 12.57 ± 0.63 | 13.26 ± 0.98 | 19.43 ± 0.76 | 32.69 |
| OKC3 | 77.11 ± 6.50 | 16.59 ± 0.90 | 16.52 ± 1.34 | 12.08 ± 0.59 | 28.60 |
| OKC4 | 165.99 ± 9.74 | 22.11 ± 1.04 | 20.89 ± 1.50 | 20.12 ± 0.83 | 41.01 |
| OKC5 | 174.21 ± 10.27 | 21.27 ± 1.03 | 19.43 ± 1.45 | 16.21 ± 0.70 | 35.64 |
| OKC6 | 116.26 ± 7.69 | 21.10 ± 1.00 | 18.87 ± 1.40 | 23.52 ± 0.95 | 42.39 |
| OKC7 | 224.58 ± 12.48 | 27.14 ± 1.26 | 27.68 ± 1.92 | 16.86 ± 0.74 | 44.54 |
| OKC8 | 139.32 ± 8.53 | 25.38 ± 1.14 | 21.80 ± 1.53 | 27.52 ± 1.07 | 49.32 |
| OKC9 | 145.05 ± 9.02 | 16.97 ± 0.87 | 18.42 ± 1.37 | 12.67 ± 0.59 | 31.09 |
| OKC10 | 119.32 ± 7.97 | 17.99 ± 0.91 | 18.05 ± 1.36 | 15.57 ± 0.69 | 33.62 |
| OKC11 | 127.04 ± 7.43 | 16.27 ± 0.77 | 14.07 ± 1.04 | 18.23 ± 0.73 | 32.30 |
| OKC12 | 192.01 ± 11.66 | 23.04 ± 1.13 | 19.66 ± 1.52 | 16.20 ± 0.74 | 35.86 |
| OKC13 | 74.63 ± 5.75 | 19.36 ± 0.94 | 18.55 ± 1.37 | 13.95 ± 0.62 | 32.50 |
| OKC14 | 87.53 ± 6.86 | 4.82 ± 0.40 | 5.03 ± 0.61 | 5.02 ± 0.32 | 10.05 |
| OKC15 | 72.61 ± 5.65 | 13.50 ± 0.72 | 13.47 ± 1.07 | 11.63 ± 0.54 | 25.10 |
| Min. Max. | 46.60 ± 3.80 224.58 ± 12.48 | 4.82 ± 0.40 27.14 ± 1.26 | 5.03 ± 0.61 27.68 ± 1.92 | 5.02 ± 0.32 27.52 ± 1.07 | 10.05 49.32 |
| Mean | 122.96 ± 50.80 | 18.43 ± 5.54 | 17.50 ± 4.99 | 16.19 ± 5.33 | 33.69 |
| UNSCEAR 2000 soil average [23] | 400 | 30 | 30 | 35 | 65 |
| % of UNSCEAR 2000 | 31 | 61 | 58 | 46 | 52 |
| Coal Ash Samples | 40K | 232Th | 228Ra | 226Ra | Total Radium |
|---|---|---|---|---|---|
| OKA1 | 1330.79 ± 68.14 | 241.29 ± 7.56 | 218.87 ± 11.86 | 187.93 ± 7.08 | 406.80 |
| OKA2 | 1146.74 ± 60.37 | 160.13 ± 6.88 | 146.84 ± 9.74 | 190.42 ± 7.09 | 337.26 |
| OKA3 | 991.76 ± 54.23 | 169.20 ± 7.14 | 140.09 ± 9.31 | 180.38 ± 6.79 | 320.26 |
| OKA4 | 900.92 ± 51.20 | 127.69 ± 5.75 | 114.43 ± 7.98 | 143.12 ± 5.55 | 257.55 |
| OKA5 | 1159.41 ± 62.15 | 152.40 ± 6.68 | 135.92 ± 9.23 | 156.34 ± 6.06 | 292.26 |
| OKA6 | 1165.76 ± 62.68 | 161.95 ± 7.03 | 141.50 ± 9.57 | 140.71 ± 5.53 | 282.21 |
| OKA7 | 698.89 ± 41.08 | 177.87 ± 7.32 | 151.83 ± 9.76 | 172.86 ± 6.47 | 324.69 |
| OKA8 | 882.02 ± 49.40 | 180.89 ± 7.51 | 147.79 ± 9.67 | 176.79 ± 6.63 | 324.58 |
| OKA9 | 1412.72 ± 74.87 | 163.33 ± 7.32 | 158.41 ± 10.77 | 176.85 ± 6.85 | 335.26 |
| OKA10 | 1360.02 ± 71.68 | 136.41 ± 6.29 | 133.83 ± 9.31 | 154.85 ± 6.08 | 288.68 |
| OKA11 | 1137.84 ± 62.58 | 133.34 ± 6.12 | 125.18 ± 8.79 | 157.93 ± 6.15 | 283.11 |
| OKA12 | 1119.74 ± 64.46 | 145.70 ± 6.77 | 138.15 ± 9.79 | 145.71 ± 5.87 | 283.86 |
| OKA13 | 485.95 ± 33.47 | 145.12 ± 6.34 | 137.55 ± 9.18 | 116.86 ± 4.67 | 254.41 |
| OKA14 | 622.25 ± 41.66 | 103.56 ± 5.09 | 104.09 ± 7.65 | 99.38 ± 4.24 | 203.47 |
| OKA15 | 519.97 ± 35.55 | 100.30 ± 4.78 | 81.99 ± 6.21 | 101.49 ± 4.23 | 183.48 |
| Min. Max. | 485.95 ± 33.47 1360.02 ± 71.68 | 100.30 ± 4.78 241.29 ± 7.56 | 81.99 ± 6.21 218.87 ± 11.86 | 99.38 ± 4.24 190.42 ± 7.09 | 183.48 406.80 |
| Mean | 995.65 ± 301.03 | 153.28 ± 34.15 | 138.41 ± 29.72 | 153.44 ± 29.36 | 291.86 |
| UNSCEAR 1988 coal ash average [30] | 265 | 70 | 130 | 240 | - |
| UNSCEAR 2000 soil average [23] | 400 | 30 | 30 | 35 | 65 |
| % of UNSCEAR 2000 | 249 | 511 | 461 | 438 | 449 |
| Coal Ash Samples | 40K | 232Th | 228Ra | 226Ra | Total Radium |
|---|---|---|---|---|---|
| OMA1 | 295.00 ± 21.68 | 130.49 ± 5.50 | 124.48 ± 8.02 | 222.84 ± 7.95 | 347.32 |
| OMA2 | 280.90 ± 23.34 | 127.58 ± 5.78 | 140.06 ± 9.27 | 225.17 ± 8.22 | 365.23 |
| OMA3 | 395.27 ± 25.74 | 122.17 ± 5.18 | 117.32 ± 7.59 | 201.43 ± 7.24 | 318.75 |
| OMA4 | 332.90 ± 25.31 | 123.73 ± 5.46 | 111.64 ± 7.64 | 215.51 ± 7.87 | 327.15 |
| OMA5 | 451.19 ± 31.07 | 120.53 ± 5.46 | 125.88 ± 8.47 | 216.81 ± 7.93 | 342.69 |
| OMA6 | 371.47 ± 24.79 | 112.07 ± 4.80 | 98.39 ± 6.58 | 212.78 ± 7.62 | 311.17 |
| OMA7 | 199.53 ± 19.75 | 129.73 ± 5.83 | 121.90 ± 8.41 | 230.72 ± 8.48 | 352.62 |
| OMA8 | 324.30 ± 25.68 | 137.84 ± 6.05 | 128.01 ± 8.65 | 253.57 ± 9.18 | 381.58 |
| OMA9 | 351.50 ± 25.87 | 155.93 ± 6.53 | 131.69 ± 8.67 | 261.51 ± 9.34 | 393.20 |
| OMA10 | 299.89 ± 24.48 | 157.59 ± 6.73 | 141.05 ± 9.36 | 283.75 ± 10.19 | 424.80 |
| OMA11 | 232.33 ± 18.93 | 129.90 ± 5.47 | 111.01 ± 7.33 | 272.47 ± 9.63 | 383.48 |
| OMA12 | 310.47 ± 22.31 | 140.72 ± 5.82 | 124.83 ± 8.03 | 257.19 ± 9.10 | 382.02 |
| OMA13 | 298.25 ± 20.97 | 135.88 ± 5.56 | 118.41 ± 7.56 | 257.92 ± 9.07 | 376.33 |
| OMA14 | 267.12 ± 19.06 | 129.90 ± 5.29 | 111.33 ± 7.10 | 250.21 ± 8.79 | 361.54 |
| OMA15 | 284.26 ± 19.83 | 124.72 ± 5.13 | 112.78 ± 7.18 | 251.42 ± 8.83 | 364.20 |
| Min. Max. | 199.53 ± 19.75 451.19 ± 31.07 | 112.07 ± 4.80 155.93 ± 6.53 | 98.39 ± 6.58 141.05 ± 9.36 | 201.43 ± 7.24 283.75 ± 10.19 | 311.17 424.80 |
| Mean | 312.96 ± 62.88 | 131.92 ± 12.35 | 121.25 ± 11.13 | 240.89 ± 24.53 | 362.14 |
| UNSCEAR 1988 fly ash average [30] | 265 | 70 | 130 | 240 | - |
| UNSCEAR 2000 soil average [23] | 400 | 30 | 30 | 35 | 65 |
| % of UNSCEAR 2000 | 78 | 440 | 404 | 688 | 557 |
| Coal Samples | Absorbed Dose D (nGyh−1) | Annual Effective Dose Equivalent AEDE (µSvy−1) | Radium Equivalent Raeq (Bqkg−1) |
|---|---|---|---|
| OMC1 | 37.81 ± 0.96 | 46.37 ± 1.18 | 84.71 ± 2.19 |
| OMC2 | 36.60 ± 0.93 | 44.89 ± 1.14 | 82.07 ± 2.13 |
| OMC3 | 32.80 ± 0.85 | 40.22 ± 1.04 | 73.81 ± 1.95 |
| OMC4 | 54.48 ± 1.32 | 66.81 ± 1.62 | 122.26 ± 3.01 |
| OMC5 | 51.57 ± 1.27 | 63.24 ± 1.55 | 115.90 ± 2.90 |
| OMC6 | 51.70 ± 1.26 | 63.41 ± 1.55 | 116.09 ± 2.88 |
| OMC7 | 52.90 ± 1.29 | 64.87 ± 1.58 | 118.99 ± 2.95 |
| OMC8 | 56.73 ± 1.39 | 69.57 ± 1.70 | 127.42 ± 3.17 |
| OMC9 | 44.28 ± 1.09 | 54.30 ± 1.34 | 99.20 ± 2.48 |
| OMC10 | 69.66 ± 1.73 | 85.43 ± 2.12 | 158.28 ± 4.00 |
| OMC11 | 36.18 ± 0.94 | 44.37 ± 1.16 | 81.76 ± 2.17 |
| OMC12 | 48.69 ± 1.20 | 59.71 ± 1.47 | 109.35 ± 2.74 |
| OMC13 | 49.57 ± 1.23 | 60.79 ± 1.50 | 111.36 ± 2.80 |
| OMC14 | 49.66 ± 1.23 | 60.90 ± 1.51 | 111.62 ± 2.82 |
| OMC15 | 49.95 ± 1.23 | 61.26 ± 1.51 | 112.21 ± 2.82 |
| Min. Max. | 32.80 ± 0.85 69.66 ± 1.73 | 40.22 ± 1.04 85.43 ± 2.12 | 73.81 ± 1.95 158.28 ± 4.00 |
| Mean | 48.17 ± 9.18 | 59.08 ± 11.66 | 108.33 ± 21.63 |
| World average [23,25] | 59 | 70 | ˂370 |
| % of world average | 81 | 84 | 29 |
| Coal Samples | Absorbed Dose (D)(nGyh−1) | Annual Effective Dose Equivalent (AEDE)(µSvy−1) | Radium Equivalent (Raeq)(Bqkg−1) |
|---|---|---|---|
| OKC1 | 20.95 ± 0.68 | 25.69 ± 0.83 | 46.52 ± 1.54 |
| OKC2 | 18.51 ± 0.54 | 22.70 ± 0.66 | 40.99 ± 1.21 |
| OKC3 | 18.82 ± 0.67 | 23.08 ± 0.82 | 41.74 ± 1.50 |
| OKC4 | 29.57 ± 0.84 | 36.27 ± 1.03 | 64.52 ± 1.86 |
| OKC5 | 27.60 ± 0.82 | 33.85 ± 1.01 | 60.04 ± 1.81 |
| OKC6 | 28.46 ± 0.81 | 33.90 ± 1.00 | 62.65 ± 1.82 |
| OKC7 | 33.55 ± 0.98 | 41.14 ± 1.21 | 72.96 ± 2.17 |
| OKC8 | 33.85 ± 0.92 | 41.52 ± 1.13 | 74.54 ± 2.06 |
| OKC9 | 22.15 ± 0.70 | 27.17 ± 0.86 | 48.11 ± 1.54 |
| OKC10 | 23.03 ± 0.72 | 28.25 ± 0.88 | 50.48 ± 1.60 |
| OKC11 | 23.55 ± 0.65 | 28.88 ± 0.80 | 51.28 ± 1.44 |
| OKC12 | 29.41 ± 0.91 | 36.07 ± 1.11 | 63.93 ± 1.99 |
| OKC13 | 21.25 ± 0.68 | 26.06 ± 0.83 | 47.38 ± 1.55 |
| OKC14 | 8.88 ± 0.40 | 10.89 ± 0.49 | 18.65 ± 0.84 |
| OKC15 | 16.55 ± 0.55 | 20.30 ± 0.68 | 36.53 ± 1.24 |
| Min. Max. | 8.88 ± 0.40 33.85 ± 0.92 | 10.89 ± 0.49 41.52 ± 1.13 | 18.65 ± 0.84 74.54 ± 2.06 |
| Mean | 23.74 ± 6.78 | 29.12 ± 8.31 | 52.02 ± 14.81 |
| World average [23,25] | 59 | 70 | ˂370 |
| % of world average | 40 | 42 | 14 |
| Coal Ash Samples | D (nGyh−1) | AEDE (µSvy−1) | Raeq (Bqkg−1) |
|---|---|---|---|
| OKA1 | 288.06 ± 6.29 | 353.27 ± 7.72 | 635.45 ± 13.95 |
| OKA2 | 232.51 ± 5.86 | 285.15 ± 7.19 | 507.70 ± 12.99 |
| OKA3 | 226.88 ± 5.79 | 278.26 ± 7.10 | 498.70 ± 12.95 |
| OKA4 | 180.81 ± 4.82 | 221.75 ± 5.91 | 395.09 ± 10.67 |
| OKA5 | 212.63 ± 5.55 | 260.76 ± 6.81 | 463.55 ± 12.28 |
| OKA6 | 211.44 ± 5.60 | 259.31 ± 6.87 | 462.06 ± 12.45 |
| OKA7 | 216.44 ± 5.61 | 265.44 ± 6.87 | 481.03 ± 12.71 |
| OKA8 | 227.71 ± 5.85 | 279.27 ± 7.17 | 503.38 ± 13.18 |
| OKA9 | 239.27 ± 6.27 | 293.44 ± 7.69 | 519.19 ± 13.77 |
| OKA10 | 210.65 ± 5.59 | 258.34 ± 6.86 | 454.64 ± 12.18 |
| OKA11 | 200.95 ± 5.34 | 246.44 ± 6.55 | 436.22 ± 11.73 |
| OKA12 | 202.01 ± 5.59 | 247.75 ± 6.86 | 440.28 ± 12.36 |
| OKA13 | 161.91 ±4.61 | 198.56 ± 5.66 | 361.80 ± 10.52 |
| OKA14 | 134.41 ± 4.04 | 164.84 ± 4.95 | 295.38 ± 9.01 |
| OKA15 | 129.15 ± 3.79 | 158.39 ± 4.65 | 284.96 ± 8.49 |
| Min. Max. | 129.15 ± 3.79 288.06 ± 6.29 | 158.39 ± 4.65 353.27 ± 7.72 | 284.96 ± 8.49 635.45 ± 13.95 |
| Mean | 204.99 ± 40.79 | 251.40 ± 50.02 | 449.30 ± 89.15 |
| World average [23,25] | 59 | 70 | ˂370 |
| % of world average | 347 | 359 | 121 |
| Coal Ash Samples | D (nGyh−1) | AEDE (µSvy−1) | Raeq (Bqkg−1) |
|---|---|---|---|
| OMA1 | 194.07 ± 5.03 | 238.01 ± 6.17 | 432.16 ± 11.31 |
| OMA2 | 192.80 ± 5.25 | 236.45 ± 6.44 | 429.24 ± 11.80 |
| OMA3 | 183.33 ± 4.70 | 224.84 ± 5.77 | 406.57 ± 10.54 |
| OMA4 | 188.18 ± 5.02 | 230.78 ± 6.16 | 418.08 ± 11.26 |
| OMA5 | 191.78 ± 5.10 | 235.20 ± 6.25 | 423.91 ± 11.38 |
| OMA6 | 181.48 ± 4.67 | 222.57 ± 5.73 | 401.64 ± 10.43 |
| OMA7 | 193.27 ± 5.33 | 237.03 ± 6.54 | 431.60 ± 12.00 |
| OMA8 | 213.93 ± 5.70 | 262.36 ± 6.99 | 475.65 ± 12.77 |
| OMA9 | 229.66 ± 5.94 | 281.65 ± 7.29 | 511.56 ± 13.36 |
| OMA10 | 238.78 ± 6.30 | 292.84 ± 7.73 | 532.20 ± 14.15 |
| OMA11 | 214.03 ± 5.60 | 262.49 ± 6.86 | 476.12 ± 12.49 |
| OMA12 | 216.76 ± 5.56 | 265.84 ± 6.82 | 482.33 ± 12.45 |
| OMA13 | 213.67 ± 5.44 | 262.04 ± 6.67 | 475.19 ± 12.17 |
| OMA14 | 205.20 ± 5.23 | 251.65 ± 6.41 | 456.54 ± 11.69 |
| OMA15 | 203.34 ± 5.19 | 249.38 ± 6.36 | 451.66 ± 11.58 |
| Min. Max. | 181.48 ± 4.67 238.78 ± 6.30 | 222.57 ± 5.73 292.84 ± 7.73 | 401.64 ± 10.43 532.20 ± 14.15 |
| Mean | 204.02 ± 16.90 | 250.21 ± 20.73 | 453.63 ± 38.20 |
| World average [23,25] | 59 | 70 | ˂370 |
| % of world average | 346 | 357 | 123 |
| Element | Particle A | Particle B | Particle C | Particle D | Particle E |
|---|---|---|---|---|---|
| O | 17.77 ± 8.00 | 24.96 ± 8.06 | 13.58 ± 8.35 | 27.34 ± 9.30 | 20.11± 7.45 |
| Al | 8.79 ± 8.19 | 5.85 ± 8.05 | 9.29 ± 8.04 | 4.99 ± 7.67 | 5.12 ± 7.71 |
| Si | 9.99 ± 7.40 | 7.20 ± 6.91 | 10.80 ± 7.23 | 19.95 ± 6.69 | 33.78 ± 6.83 |
| P | 3.98 ± 7.41 | 12.74 ± 6.02 | 10.10 ± 6.74 | ND | ND |
| Nb | ND | ND | ND | 14.32 ± 4.45 | ND |
| Pb | ND | ND | ND | 2.45 ± 10.78 | 8.32 ± 8.81 |
| Fe | ND | ND | ND | 4.36 ± 2.88 | 1.39 ± 7.03 |
| Ti | ND | ND | ND | 0.18 ± 17.12 | 0.13 ± 14.41 |
| Th | 2.44 ± 12.63 | 2.61 ± 12.36 | 2.33 ± 11.20 | ND | ND |
| U | 1.34 ± 11.91 | 1.35 ± 11.95 | 0.82 ± 21.31 | 28.86 ± 1.44 | 34.93 ± 2.09 |
| K | ND | ND | ND | 0.12 ± 16.13 | 2.37 ± 6.88 |
| Ca | 1.17 ± 8.13 | 1.06 ± 10.87 | 1.00 ± 12.05 | 0.10 ± 15.21 | 0.15 ± 17.12 |
| Y | 2.60 ± 9.84 | 3.81 ± 8.62 | 1.94 ± 9.11 | ND | ND |
| La | 12.87 ± 4.99 | 11.36 ± 5.78 | 13.61 ± 5.13 | ND | ND |
| Ce | 24.52 ± 2.94 | 21.13 ± 3.42 | 24.45 ± 2.96 | ND | ND |
| Nd | 10.96 ± 6.44 | 9.01 ± 7.08 | 11.04 ± 6.05 | ND | ND |
| Sm | 4.76 ± 11.91 | 2.60 ± 11.28 | 2.60 ± 9.34 | ND | ND |
| Pm | 1.52 ± 15.13 | 1.86 ± 17.82 | 2.99 ± 15.46 | ND | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okeme, I.C.; Scott, T.B.; Martin, P.G.; Satou, Y.; Ojonimi, T.I.; Olaluwoye, M.O. Assessment of the Mode of Occurrence and Radiological Impact of Radionuclides in Nigerian Coal and Resultant Post-Combustion Coal Ash Using Scanning Electron Microscopy and Gamma-Ray Spectroscopy. Minerals 2020, 10, 241. https://doi.org/10.3390/min10030241
Okeme IC, Scott TB, Martin PG, Satou Y, Ojonimi TI, Olaluwoye MO. Assessment of the Mode of Occurrence and Radiological Impact of Radionuclides in Nigerian Coal and Resultant Post-Combustion Coal Ash Using Scanning Electron Microscopy and Gamma-Ray Spectroscopy. Minerals. 2020; 10(3):241. https://doi.org/10.3390/min10030241
Chicago/Turabian StyleOkeme, Ilemona C., Thomas B. Scott, Peter G. Martin, Yukihiko Satou, Theophilus I. Ojonimi, and Moromoke O. Olaluwoye. 2020. "Assessment of the Mode of Occurrence and Radiological Impact of Radionuclides in Nigerian Coal and Resultant Post-Combustion Coal Ash Using Scanning Electron Microscopy and Gamma-Ray Spectroscopy" Minerals 10, no. 3: 241. https://doi.org/10.3390/min10030241
APA StyleOkeme, I. C., Scott, T. B., Martin, P. G., Satou, Y., Ojonimi, T. I., & Olaluwoye, M. O. (2020). Assessment of the Mode of Occurrence and Radiological Impact of Radionuclides in Nigerian Coal and Resultant Post-Combustion Coal Ash Using Scanning Electron Microscopy and Gamma-Ray Spectroscopy. Minerals, 10(3), 241. https://doi.org/10.3390/min10030241






