Post-Magmatic Fluids Dominate the Mineralization of Dolomite Carbonatitic Dykes Next to the Giant Bayan Obo REE Deposit, Northern China
Abstract
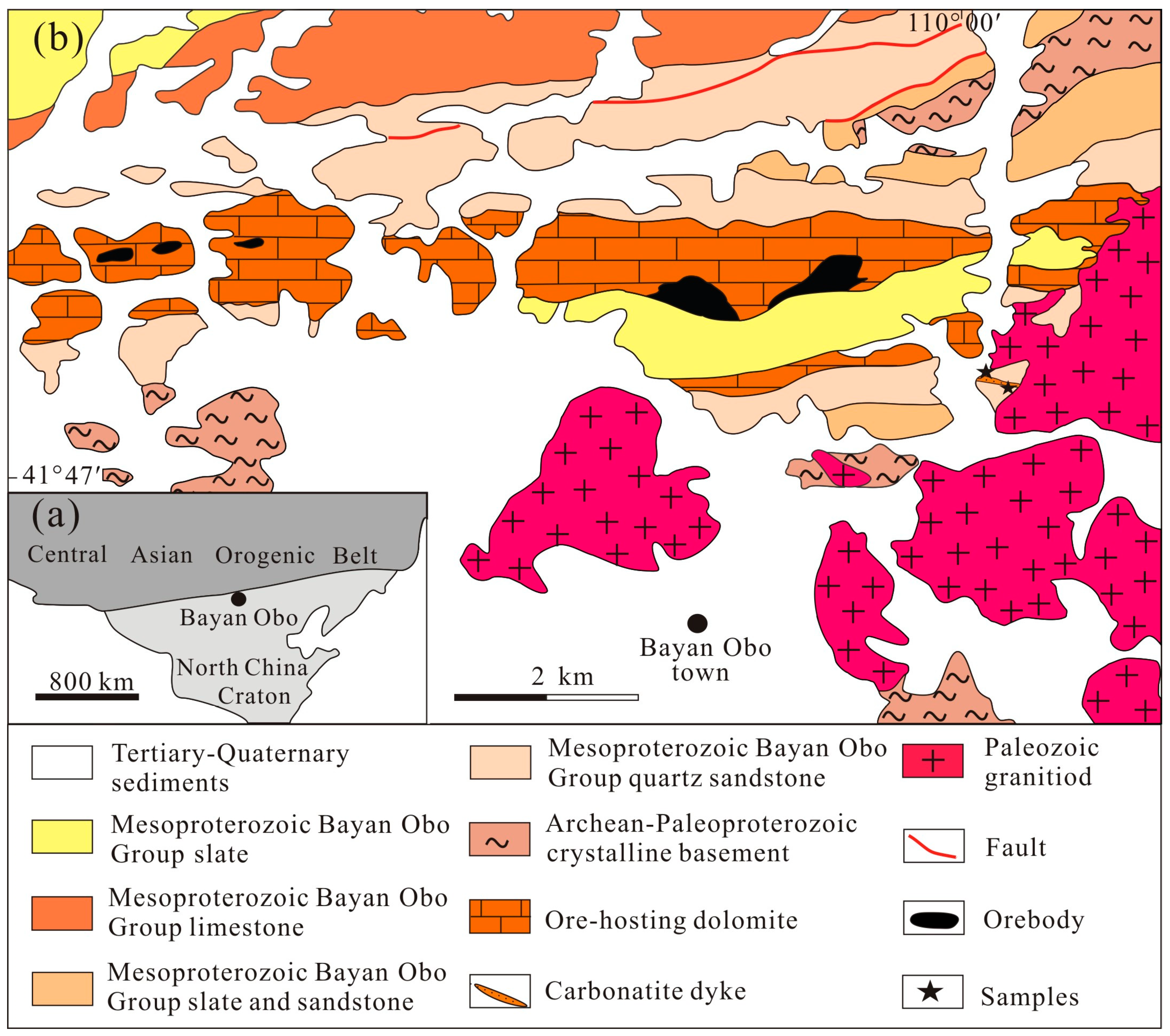
:1. Introduction
2. Analytical Methods
3. Results
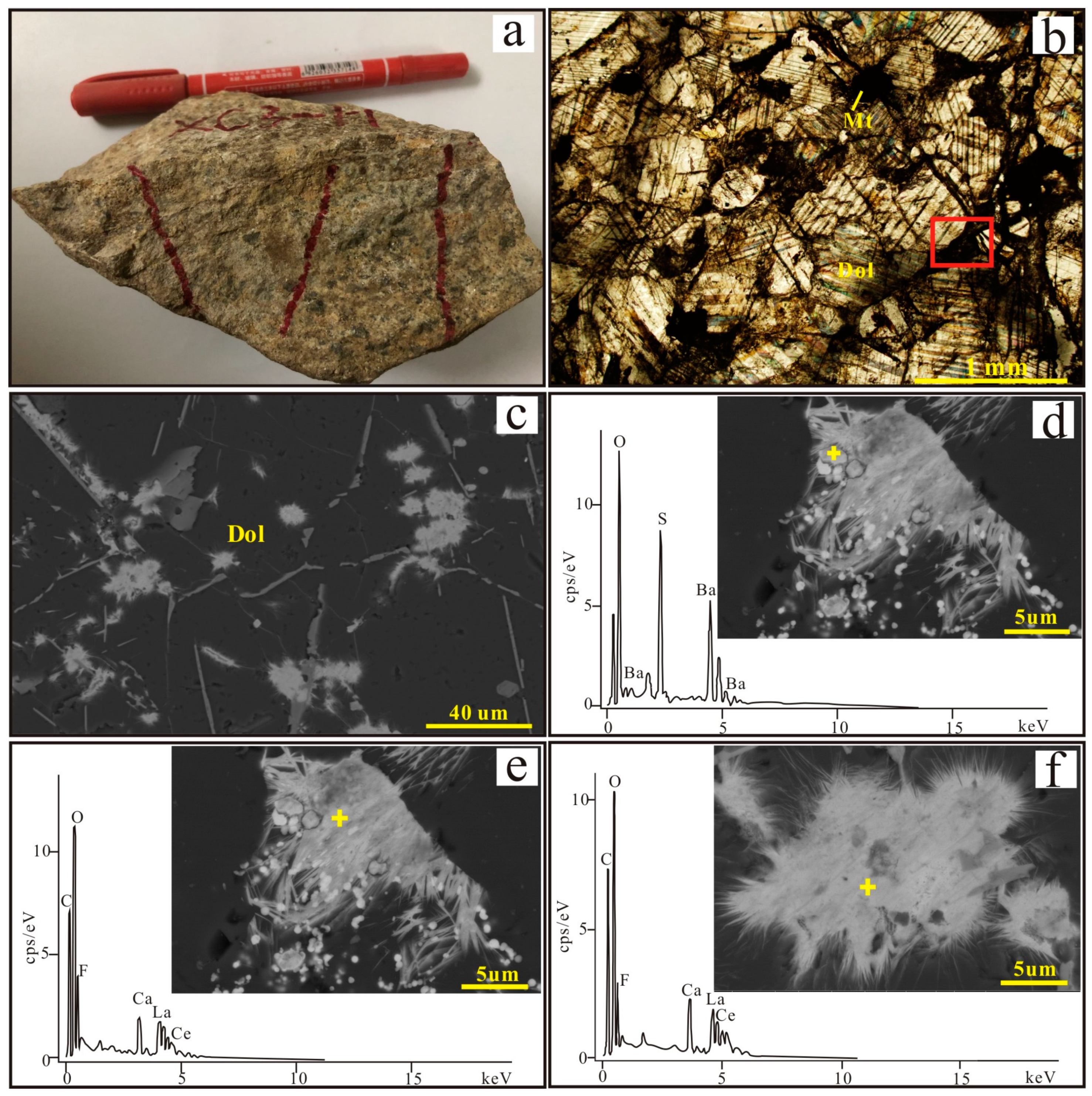
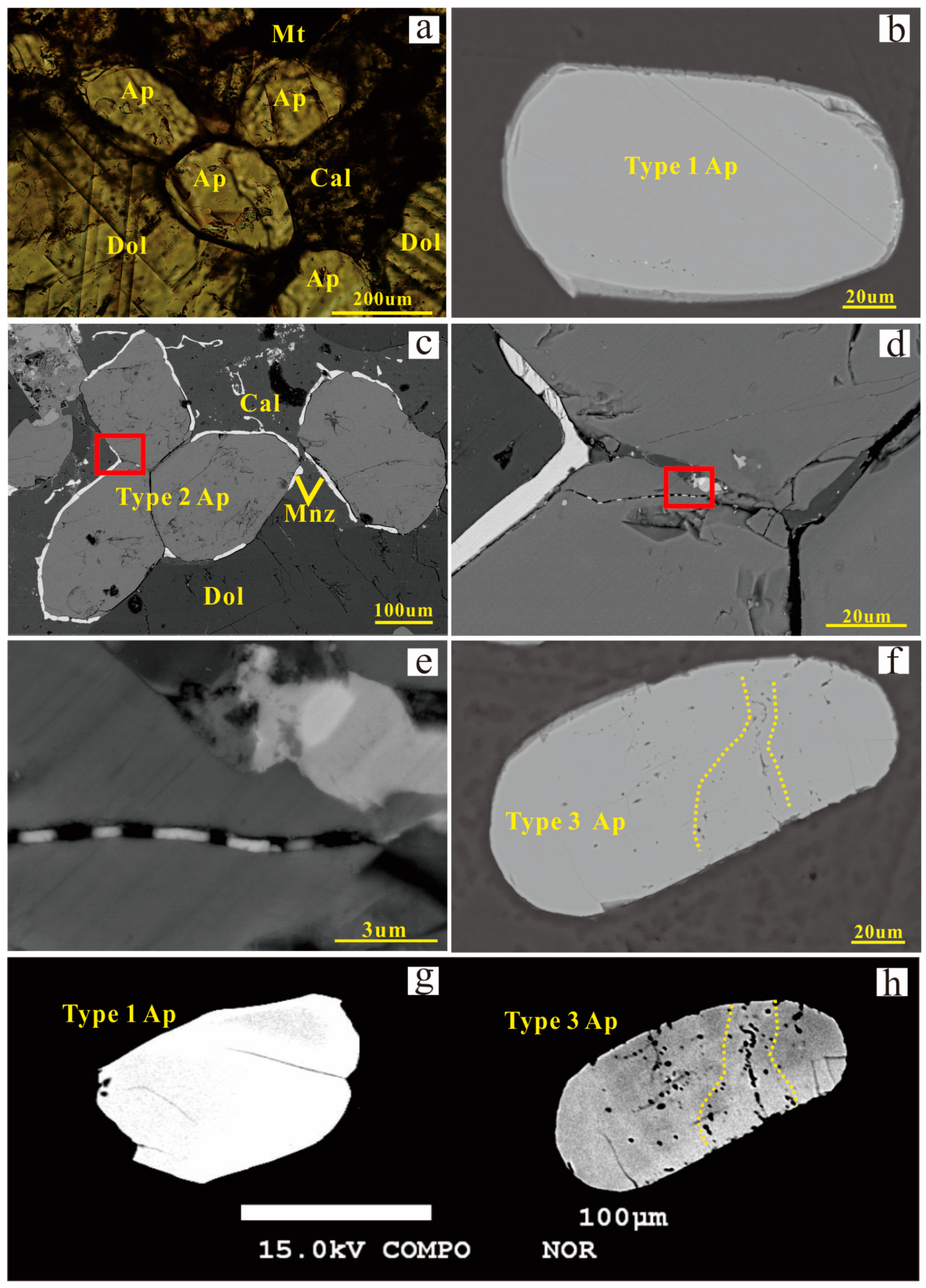
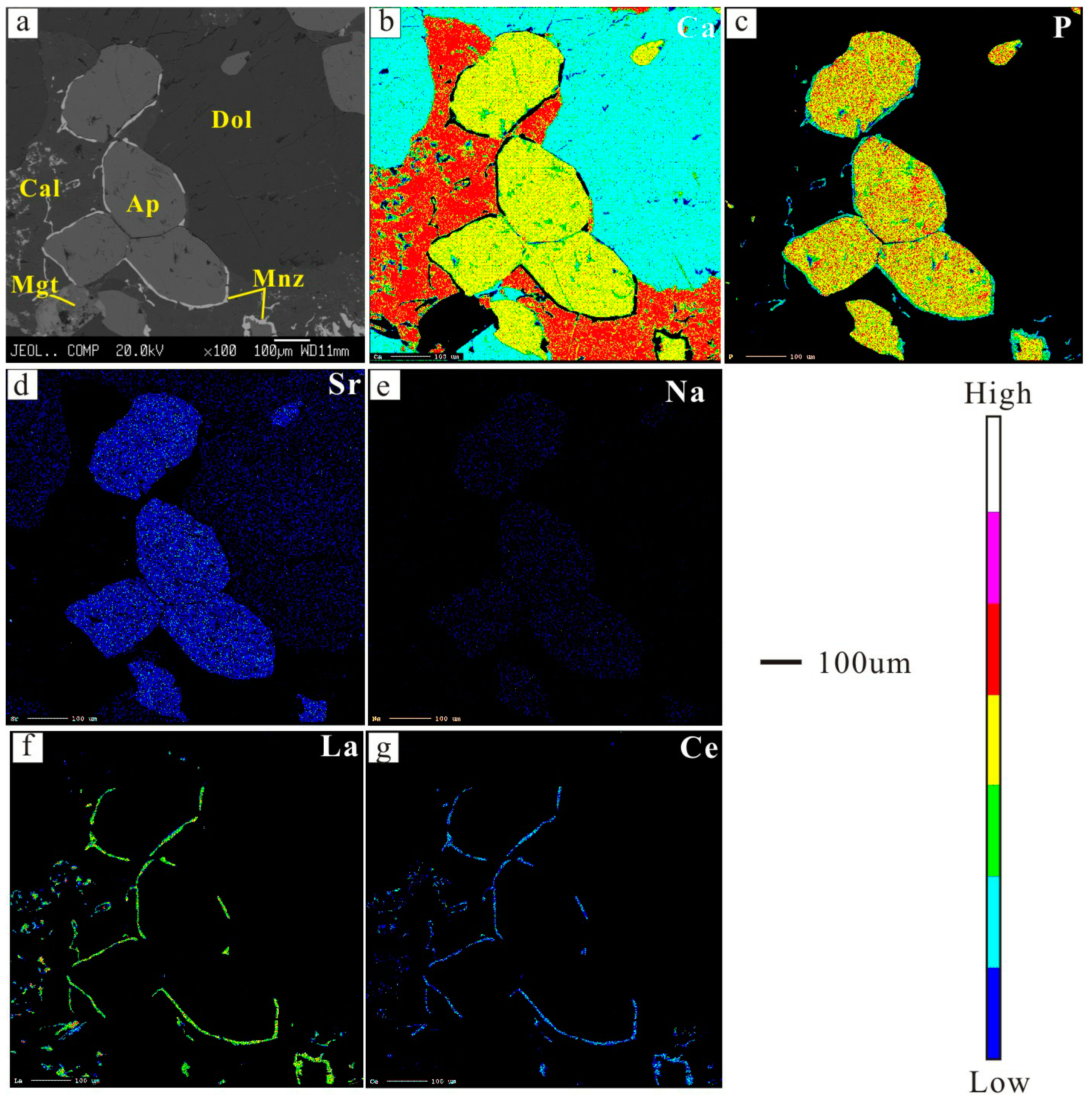
3.1. Petrography
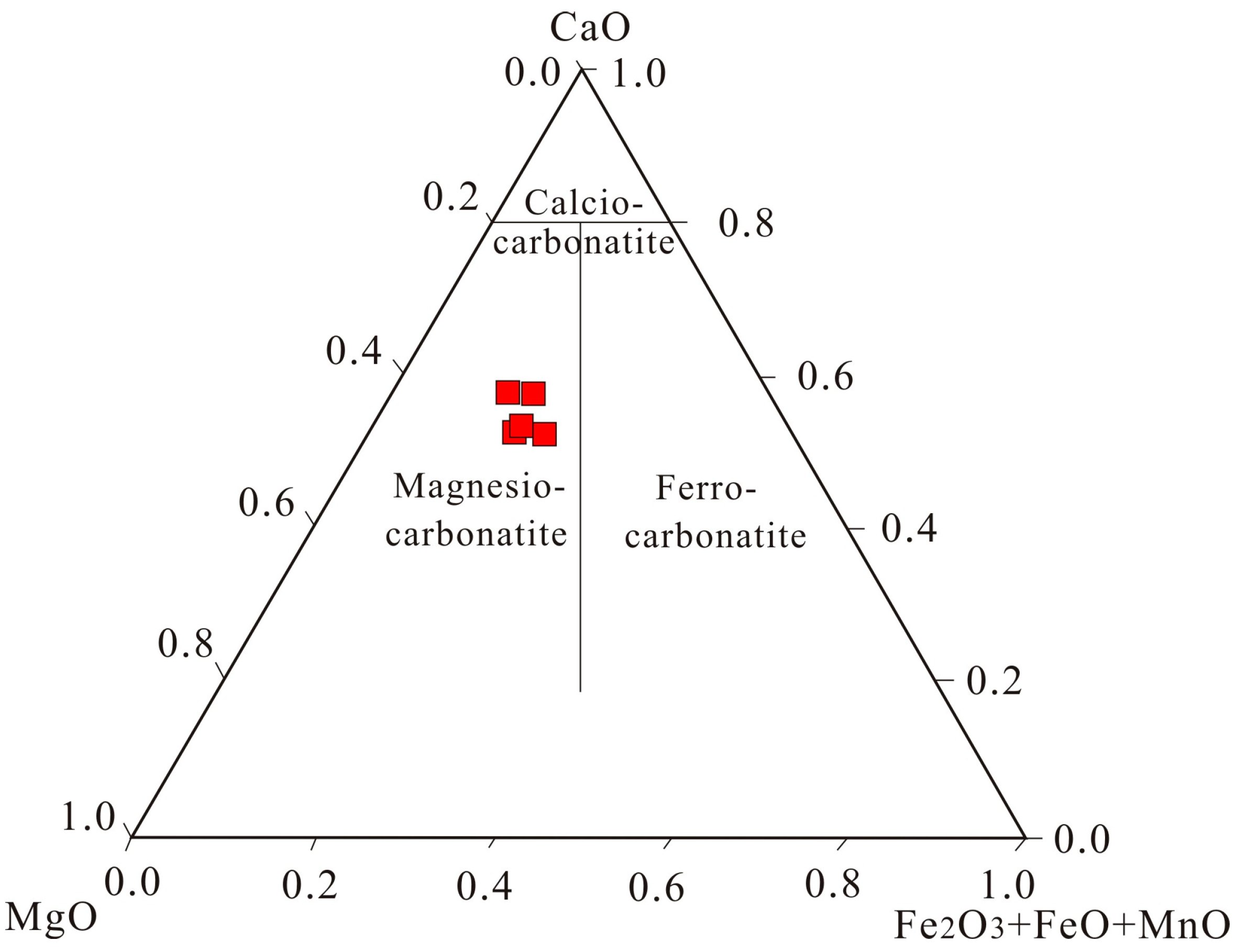
3.2. Bulk Rock Geochemistry
3.3. Mineral Chemistry of Dolomite and Apatite
4. Discussion
4.1. Formation of the Three Types of Apatite
4.2. The REE Mineralization of the Dol-Dyke
4.3. Implication for the Formation of the Bayan Obo Deposit
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drew, L.J.; Meng, Q.R.; Sun, W.J. The Bayan Obo iron rare-earth niobium deposits, Inner-Mongolia, China. Lithos 1990, 26, 43–65. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Keller, J.; Tao, K.; Wall, F.; William, C.T.; Zhang, P.S. Carbonatite dykes at Bayan Obo, Inner Mongolia, China. Miner. Petrol. 1992, 46, 195–228. [Google Scholar] [CrossRef]
- Zhu, X.K.; Sun, J.; Pan, C.X. Sm-Nd isotopic constraints on rare-earth mineralization in the Bayan Obo ore deposit, Inner Mongolia, China. Ore Geol. Rev. 2015, 64, 543–553. [Google Scholar] [CrossRef]
- Zhang, S.H.; Zhao, Y.; Liu, Y. A precise zircon Th-Pb age of carbonatite sills from the world’s largest Bayan Obo deposit: Implications for timing and genesis of REE-Nb mineralization. Precambrian Res. 2017, 291, 202–219. [Google Scholar] [CrossRef]
- Yang, X.Y.; Lai, X.D.; Pirajno, F.; Liu, Y.L.; Ling, M.X.; Sun, W.D. Genesis of the Bayan Obo Fe-REE-Nb formation in Inner Mongolia, North China Craton: A perspective review. Precambrian Res. 2017, 288, 39–71. [Google Scholar] [CrossRef]
- Chao, E.C.T.; Back, J.M.; Minkin, J.A.; Tasumoto, M.; Wang, J.W.; Conrad, J.E.; Mckee, E.H. The Sedimentary Carbonate-Hosted Giant Bayan Obo REE-Fe-Nb Ore Deposit of Inner Mongolia, China: Cornerstone Examples for Giant Polymetallic Ore Deposits of Hydrothermal Origin; Bulletin 2143; United States Government Publishing Office: Washington, DC, USA, 1997; p. 65.
- Lai, X.D.; Yang, X.Y.; Sun, W.D. Geochemical constraints on genesis of dolomite marble in the Bayan Obo REE–Nb–Fe deposit, Inner Mongolia: Implications for REE mineralization. J. Asian Earth Sci. 2012, 57, 90–102. [Google Scholar] [CrossRef]
- Lai, X.D.; Yang, X.D. Geochemical characteristics of the Bayan Obo giant REE Nb–Fe deposit and constraints on its genesis. J. S. Am. Earth Sci. 2013, 41, 99–112. [Google Scholar] [CrossRef]
- Lai, X.D.; Yang, X.Y.; Santosh, M.; Liu, Y.L.; Ling, M.X. New data of the Bayan Obo Fe-REE-Nb deposit, Inner Mongolia: Implications for ore genesis. Precambrian Res. 2015, 263, 108–122. [Google Scholar] [CrossRef]
- Ling, M.X.; Liu, Y.L.; Williams, I.S.; Teng, F.Z.; Yang, X.Y.; Ding, X.; Wei, G.J.; Xie, L.H.; Deng, W.F.; Sun, W.D. Formation of the world’s largest REE deposit through protracted fluxing of carbonatite by subduction-derived fluids. Sci. Rep. 2013, 3, 1776–1783. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.P.; Campbell, L.S.; Kynicky, J. A review of the genesis of the world class Bayan Obo Fe-REE-Nb deposits, Inner Mongolia, China: Multistage processes and outstanding questions. Ore Geol. Rev. 2015, 64, 459–476. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ling, M.X.; Williams, I.S.; Yang, X.Y.; Wang, C.Y.; Sun, W.D. The formation of the giant Bayan Obo REE-Nb-Fe deposit, North China, Mesoproterozoic carbonatite and overprinted Paleozoic dolomitization. Ore Geol. Rev. 2018, 92, 73–83. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Spiro, B.; Yang, X.M. Oxygen carbon and strontium isotope study of the carbonatitic dolomite host of the Bayan Obo Fe-Nb-REE deposit, Inner Mongolia, N. China. Mineral. Mag. 1997, 61, 531–541. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Yang, X.M.; Taylor, R.N.; Spiro, B.; Milton, J.A.; Zhang, P.S. New evidence for the magmatic origin of the Bayan Obo ore-bearing dolomite marble, Inner Mongolia, China, from a calcite-dolomite carbonatite dyke. Miner. Petrol. 2007, 90, 223–248. [Google Scholar] [CrossRef]
- Yuan, Z.X.; Bai, G.; Zhang, Z.Q. Trachytic rock and associated fenitization in the Bayan Obo ore deposit, Inner Mongolia, China: Evidence for magmatic–hydrothermal mineralization related to a carbonatitic complex. Acta Geol. Sin. Engl. Ed. 2000, 74, 148–153. [Google Scholar]
- Hao, Z.G.; Wang, X.B.; Li, Z.; Xiao, G.; Zhang, T.R. The carbonatitic type REE-Nb-Fe ore deposit-A rare case of Middle Proterozoic Paleo-volcanogenic mineralization in Bayan Obo, Inner Mongolia. Acta Geol. Sin. Engl. Ed. 2002, 76, 525–540. [Google Scholar]
- Yang, X.M.; Le Bas, M.J. Chemical compositions of carbonate minerals from Bayan Obo, Inner Mongolia, China: Implications for petrogenesis. Lithos 2004, 72, 97–116. [Google Scholar] [CrossRef]
- Wang, K.Y.; Zhang, J.E.; Yu, L.J.; Fang, A.M.; Dong, C.; Hu, F.Y. Fenitized wall rock geochemistry of the first carbonatite dyke at Bayan Obo, Inner Mongolia, China. Acta Geol. Sin. Engl. Ed. 2018, 92, 180–193. [Google Scholar] [CrossRef]
- Wang, K.Y.; Fang, A.M.; Zhang, J.E.; Yu, L.J.; Dong, C.; Zan, J.F.; Hao, M.Z.; Hu, F.Y. Genetic relationship between fenitized ores and hosting dolomite carbonatite of the Bayan Obo REE deposit, Inner Mongolia, China. J. Asian Earth Sci. 2019, 174, 189–204. [Google Scholar] [CrossRef]
- Yang, K.F.; Fan, H.R.; Santosh, M.; Hu, F.F.; Wang, K.Y. Mesoproterozoic mafic and carbonatitic dykes from the northern margin of the North China Craton: Implications for the final breakup of Columbia supercontinent. Tectonophysics 2011, 498, 1–10. [Google Scholar] [CrossRef]
- Yang, K.F.; Fan, H.R.; Santosh, M.; Wang, K.Y. Mesoproterozoic carbonatitic magmatism in the Bayan Obo deposit, Inner Mongolia, North China: Constraints for the mechanism of super accumulation of rare earth elements. Ore Geol. Rev. 2011, 40, 122–131. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, X.; Chen, Y.; Fang, N. Iron isotopic constraints on the genesis of Bayan Obo ore deposit, Inner Mongolia, China. Precambrian Res. 2013, 235, 88–106. [Google Scholar] [CrossRef]
- Fan, H.R.; Yang, K.F.; Hu, F.F.; Liu, S.; Wang, K.Y. The giant Bayan Obo Ree-Nb-Fe deposit, China: Ccontroversy and ore genesis. Geosci. Front. 2016, 7, 335–344. [Google Scholar] [CrossRef]
- Xie, Y.L.; Qu, Y.W.; Yang, Z.F.; Liang, P.; Zhong, R.C.; Wang, Q.W.; Xie, J.M.; Li, B.C. Giant Bayan Obo Fe-Nb-REE deposit: Progresses, Controversaries and new understandings. Miner. Depos. 2019, 38, 983–1003, (In Chinese with English abstract). [Google Scholar]
- Liu, S.; Fan, H.R.; Groves, D.I.; Yang, K.F.; Yang, Z.F.; Wang, Q.W. Multiphase carbonatite-related magmatic and metasomatic processes in the genesis of the ore-hosting dolomite in the giant Bayan Obo REE-Nb-Fe deposit. Lithos 2020, 354–355, 105359. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.Y.; Lu, J.; Jiang, S.Y.; Simonetti, A.; Xu, C.; Zhang, W. The formation of the ore-hosting dolomite marble from the giant Bayan Obo REE-Nb-Fe deposit, Inner Mongolia: Insights from micron-scale geochemical data. Miner. Depos. 2020, 55, 131–146. [Google Scholar] [CrossRef]
- Song, W.L.; Xu, C.; Smith, M.P.; Chakhmouradian, A.R.; Brenna, M.; Kynicky, J.; Chen, W.; Yang, Y.H.; Deng, M.; Tang, H.Y. Genesis of the world’s largest rare earth element deposit, Bayan Obo, China: Protracted mineralization evolution over 1 b.y. Geology 2018, 46, 323–326. [Google Scholar] [CrossRef]
- Yang, K.F.; Fan, H.R.; Pirajno, F.; Li, X.C. The Bayan Obo (China) giant REE accumulation conundrum elucidated by intense magmatic differentiation of carbonatite. Geology 2019, 47, 1198–1202. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.Y.; Fan, H.R.; Xie, Y.H. Geochemistry of REE and other trace elements of the carbonatite dykes at Bayan Obo, implication for its formation. Acta Petrol. Sin. 2002, 18, 340–348, (In Chinese with English abstract). [Google Scholar]
- Fan, H.R.; Hu, F.F.; Yang, K.F.; Pirajno, F.; Liu, X.; Wang, K.Y. Integrated U-Pb and Sm-Nd geochronology for a REE-rich carbonatite dyke at the giant Bayan Obo REE deposit, Northern China. Ore Geol. Rev. 2014, 63, 510–519. [Google Scholar] [CrossRef]
- Yang, X.M.; Yang, X.Y.; Zheng, Y.F.; Le Bas, M.J. A rare earth element-rich carbonatite dyke at Bayan Obo, Inner Mongolia, North China. Miner. Petrol. 2003, 78, 93–110. [Google Scholar] [CrossRef]
- Xiao, W.J.; Windley, B.F.; Hao, J.; Zhai, M.G. Accretion leading to collision and the Permian Solonker suture, Inner Mongolia, China: Termination of the central Asian orogenic belt. Tectonics 2003, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.R.; Yang, K.F.; Hu, F.F.; Wang, K.Y.; Zhai, M.G. Zircon geochronology of basement rocks from the Bayan Obo area, Inner Mongolia, and tectonic implications. Acta Petrol. Sin. 2010, 26, 1342–1350. (In Chinese) [Google Scholar]
- Bai, G.; Yuan, Z.X.; Wu, C.Y.; Zhang, Z.Q.; Zheng, L. Demonstration on the Geological Features and Genesis of the Bayan Obo Ore Deposit; Geological Publishing House: Beijing, China, 1996. (In Chinese) [Google Scholar]
- Qiu, Y.Z.; Wen, H.J.; Qin, C.J. Precise determination of the age of Hercynian granitoids and discovery of Yanshanian granitoids. Acta Miner. Sin. 2011, 31, 631–632, (In Chinese with English abstract). [Google Scholar]
- Ling, M.X.; Zhang, H.; Li, H.; Liu, Y.L.; Liu, J.; Li, L.Q.; Li, Y.C.; Yang, X.Y.; Sun, W.D. The Permian-Triassic granitoids in Bayan Obo, North China Craton: A geochemical and geochronological study. Lithos 2014, 190–191, 430–439. [Google Scholar] [CrossRef]
- Hu, Z.C.; Gao, S.; Liu, Y.S.; Hu, S.H.; Chen, H.H.; Yuan, H.L. Signal enhancement in laser ablation ICP-MS by addition of nitrogen in the central channel gas. J. Anal. At. Spectrom. 2008, 23, 1093–1101. [Google Scholar] [CrossRef]
- Woolley, A.R.; Kempe, D.R.C. Carbonatites: Nomenclature, average chemical compositions, and element distribution. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 1–14. [Google Scholar]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalt: Implications for mantle compositions and processes. In Magmatism in the Ocean Basins; Saunders, A.D., Norry, M.J., Eds.; Geological Society of London Special Publication: London, UK, 1989; Volume 42, pp. 313–314. [Google Scholar]
- Zirner, A.L.K.; Marks, M.A.W.; Wenzel, T.; Jacob, D.E.; Markl, G. Rare earth elements in apatite as a monitor of magmatic and metasomatic processes: The Ilímaussaq complex, South Greenland. Lithos 2015, 228–229, 12–22. [Google Scholar] [CrossRef]
- Li, X.C.; Zhou, M.F. Multiple stages of hydrothermal REE remobilization recorded in fluorapatite in the Paleoproterozoic Yinachang Fe–Cu–(REE) deposit, Southwest China. Geochim. Cosmochim. Acta 2015, 166, 53–73. [Google Scholar] [CrossRef]
- Harlov, D.E. Apatite: A fingerprint for metasomatic processes. Elements 2015, 11, 171–176. [Google Scholar] [CrossRef]
- Broomfendley, S.; Styles, M.T.; Appleton, J.D.; Gunn, G.; Wall, F. Evidence for dissolution-reprecipitation of apatite and preferential LREE mobility in carbonatite-derived late-stage hydrothermal processes. Am. Mineral. 2016, 101, 596–611. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Li, Y.K.; Wu, Z.J.; Bai, Y.; Wang, A.J. Two metasomatic events recorded in apatite from the ore-hosting dolomite marble and implications for genesis of the giant Bayan Obo REE deposit, Inner Mongolia, Northern China. J. Asian Earth Sci. 2019, 172, 56–65. [Google Scholar] [CrossRef]
- Roeder, P.L.; MacArthur, D.; Ma, X.-P.; Palmer, G.R.; Mariano, A.N. Cathodoluminescence and microprobe study of rare-earth elements in apatite. Am. Mineral. 1987, 72, 801–811. [Google Scholar]
- Xing, C.M.; Wang, C.Y. Cathodoluminescence images and trace element compositions of fluorapatite from the Hongge layered intrusion in SW China: A record of prolonged crystallization and overprinted fluid metasomatism. Am. Mineral. 2017, 102, 1390–1401. [Google Scholar] [CrossRef]
- Betkowski, W.B.; Harlov, D.E.; Rakovan, J.F. Hydrothermal mineral replacement reactions for an apatite-monazite assemblage in alkali-rich fluids at 300–600 °C and 100 Mpa. Am. Mineral. 2016, 101, 2620–2637. [Google Scholar] [CrossRef]
- Harlov, D.E.; Wirth, R.; Förster, H.J. An experimental study of dissolution–reprecipitation in fluorapatite: Fluid infiltration and the formation of monazite. Contrib. Mineral. Petrol. 2005, 150, 268–286. [Google Scholar] [CrossRef]
- Trofanenko, J. The nature and origin of the REE mineralization in the Wicheeda carbonatite, British Columbia, Canada. Econ. Geol. 2016, 111, 199–223. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Kynicky, J.; Chakhmouradian, A.N.; Campbell, I.H.; Allen, C.M. Trace-element modeling of the magmatic evolution of rare-earth-rich carbonatite from the Miaoya deposit, Central China. Lithos 2010, 118, 145–155. [Google Scholar] [CrossRef]
- Prokopyev, I.R.; Borisenko, A.S.; Borovikov, A.A.; Pavlova, G.G. Origin of REE-rich ferrocarbonatites in southern Siberia (Russia): Implications based on melt and fluid inclusions. Mineral. Petrol. 2016, 110, 845–859. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y. Mechanisms of element precipitation in carbonatite-related rare-earth element deposits: Evidence from fluid inclusions in the Maoniuping deposit, Sichuan Province, southwestern China. Ore Geol. Rev. 2019, 107, 218–238. [Google Scholar] [CrossRef]
- Campbell, L.S.; Compston, W.; Sircombe, K.N.; Wilkinson, C.C. Zircon from the East Orebody of the Bayan Obo Fe–Nb–REE deposit, China, and SHRIMP ages for carbonatite-related magmatism and REE mineralization events. Contrib. Mineral. Petrol. 2014, 168, 1040–1062. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Yang, X.; Wang, S.; Öztürk, H. Mineralogical and geochemical study of apatite and dolomite from the Bayan Obo giant Fe-REE-Nb deposit in Inner Mongolia: New evidences for genesis. Ore Geol. Rev. 2019, 109, 381–406. [Google Scholar] [CrossRef]

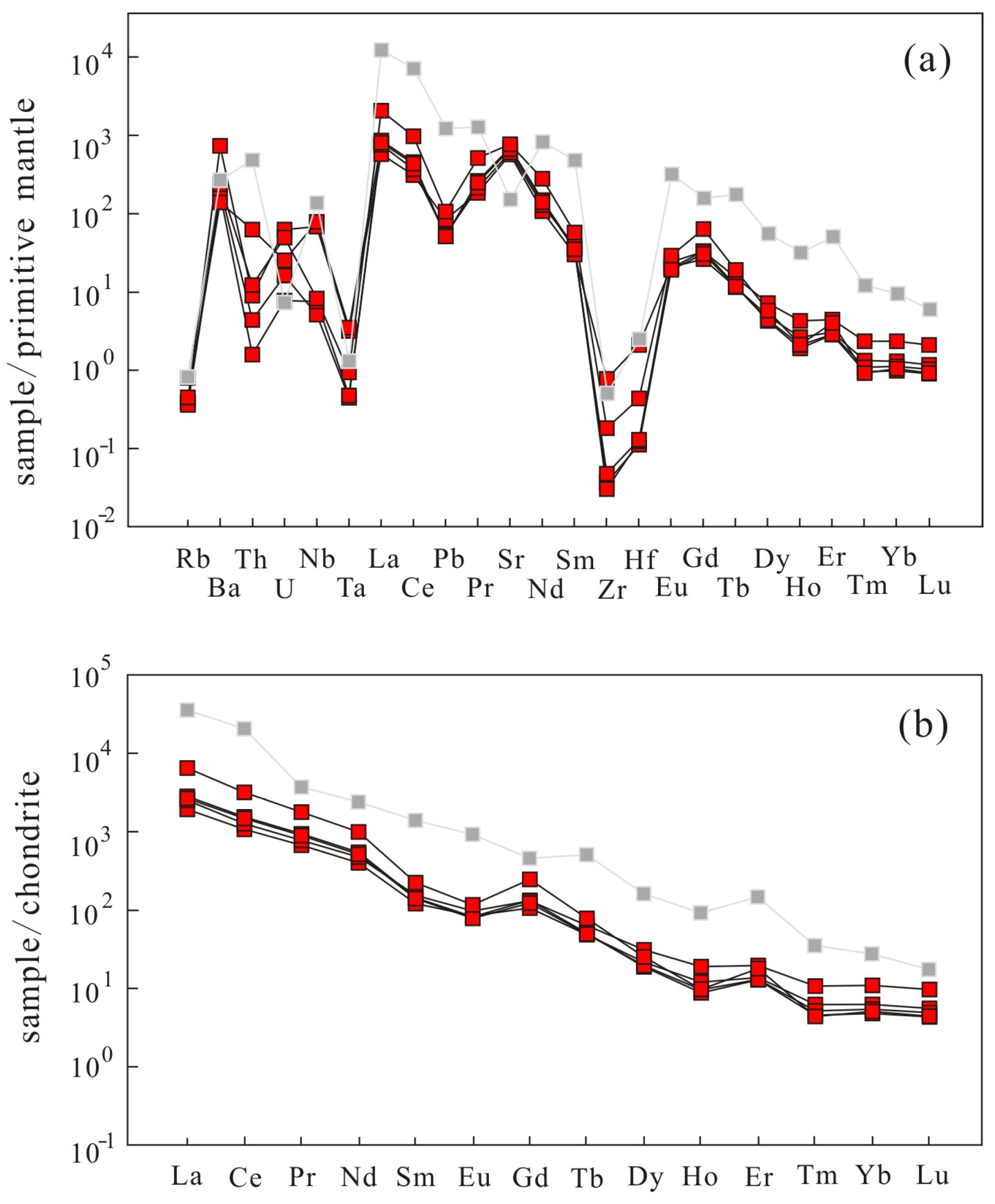
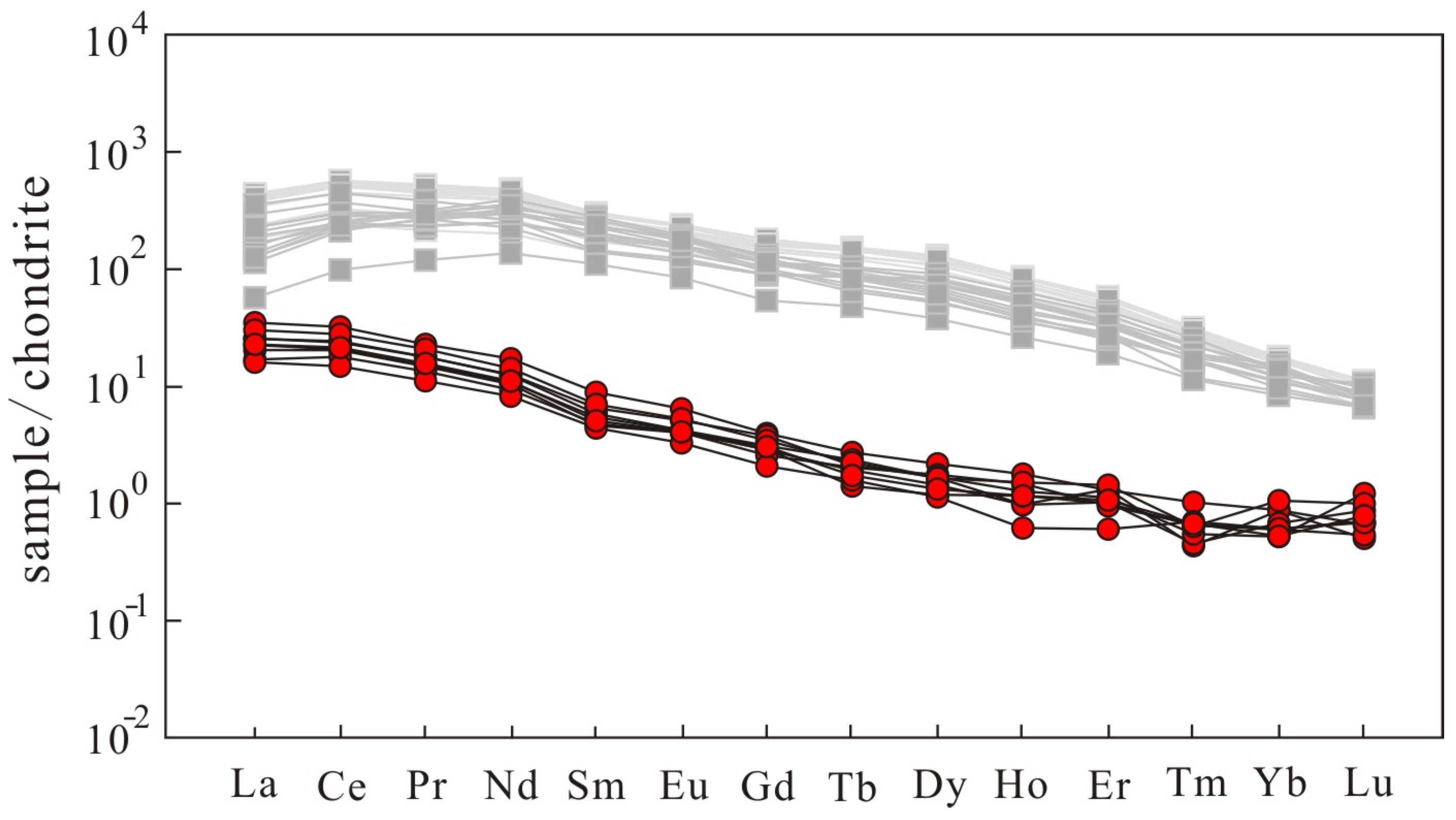
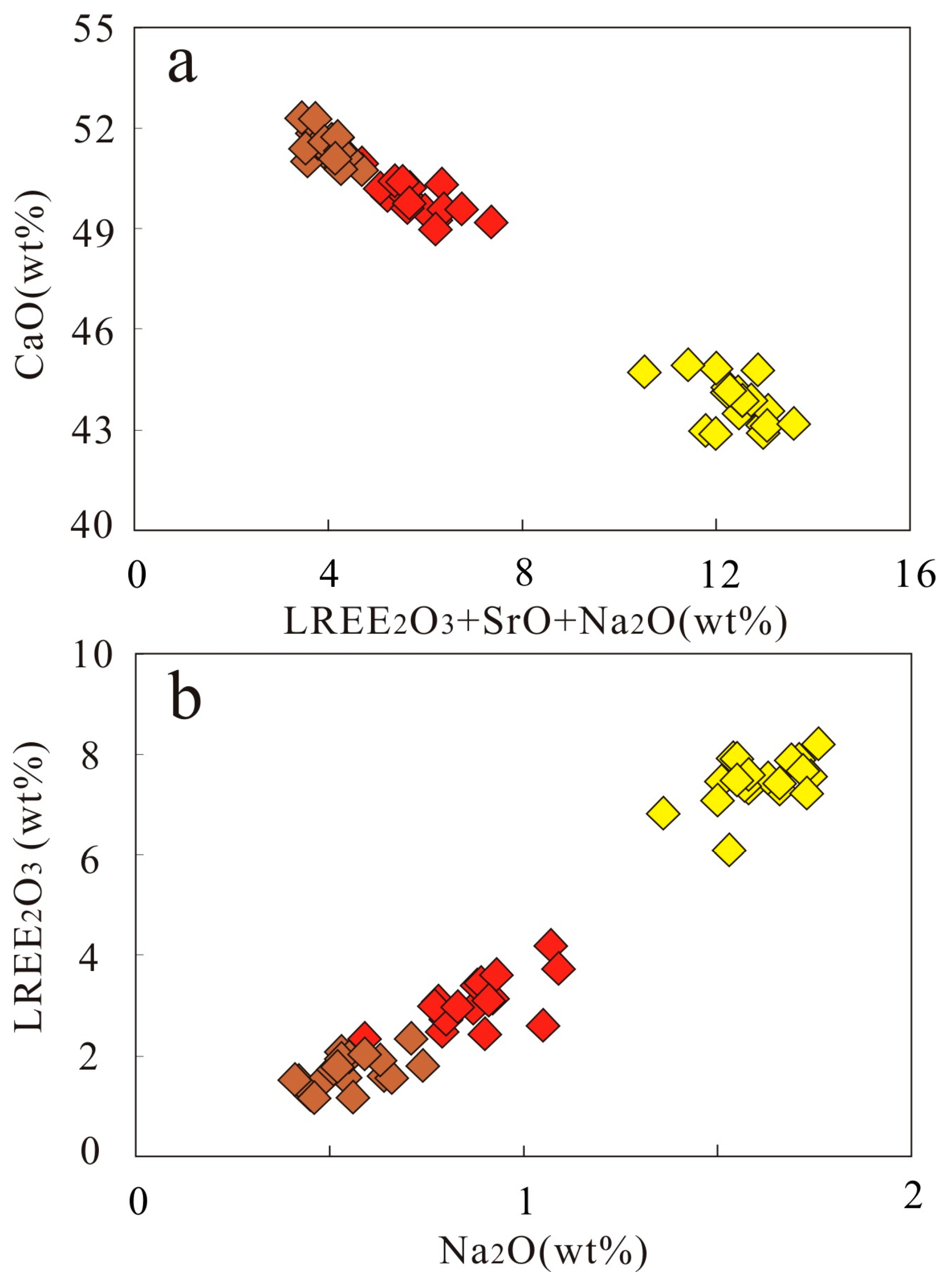
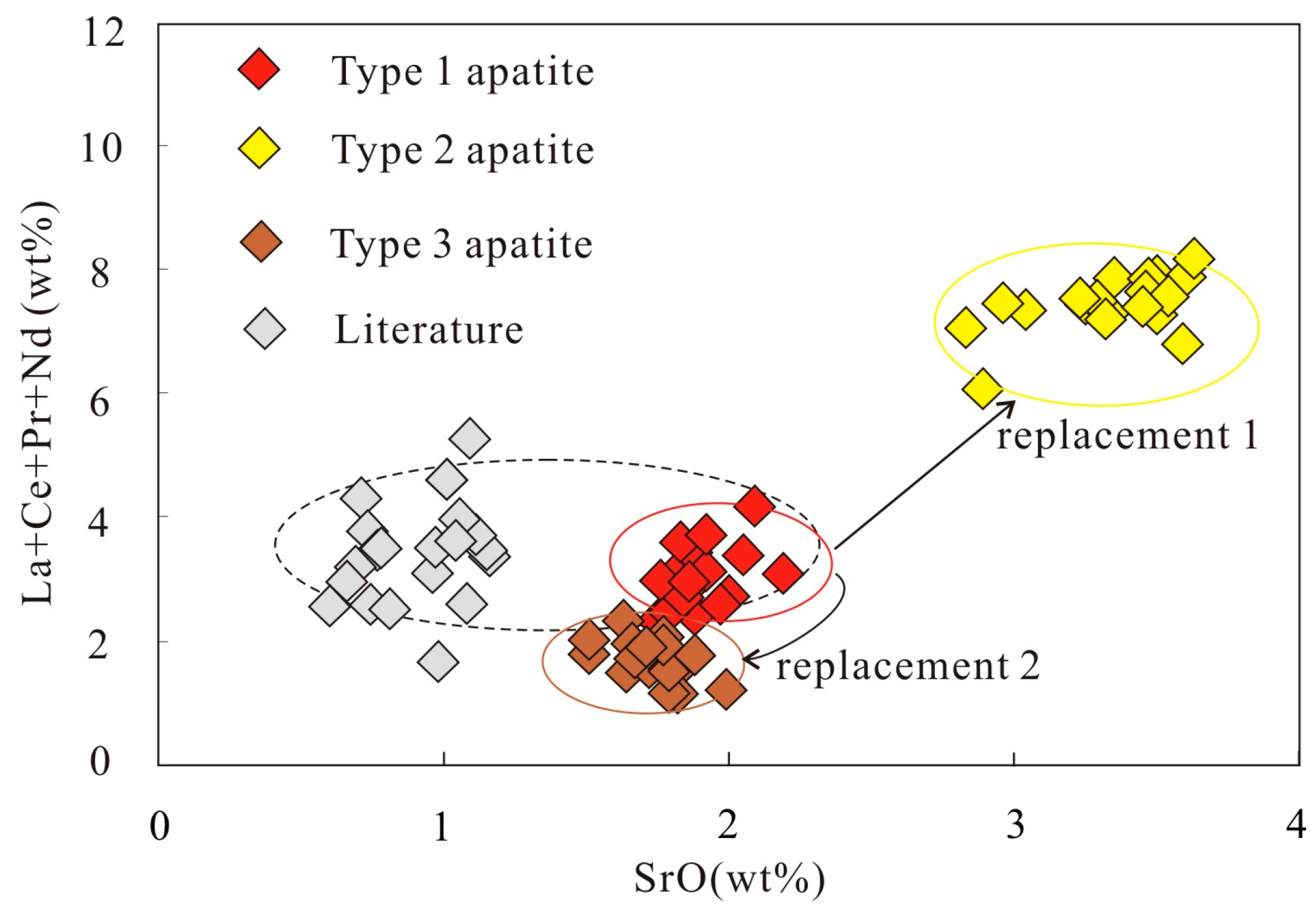
| Sample | XC3-1-1 | XC3-1-2 | XC3-1-3 | XC3-1-4 | XC3-1-5 |
|---|---|---|---|---|---|
| SiO2 | 0.89 | 0.49 | 0.61 | 0.34 | 2.81 |
| Al2O3 | 0.06 | 0.03 | 0.06 | 0.02 | 0.03 |
| Fe2O3 | 6.36 | 6.62 | 4.66 | 6.32 | 8.51 |
| FeO | 1.97 | 2.07 | 1.98 | 2.11 | 2.05 |
| MgO | 14.89 | 16.90 | 16.21 | 17.50 | 15.45 |
| CaO | 32.87 | 30.59 | 32.44 | 29.96 | 29.43 |
| NaO | bd | bd | bd | bd | bd |
| K2O | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| MnO | 0.80 | 0.90 | 0.70 | 0.91 | 0.61 |
| TiO2 | 0.02 | 0.01 | 0.02 | 0.03 | 0.05 |
| P2O5 | 0.30 | 0.09 | 0.37 | 0.08 | 0.33 |
| LOI | 41.55 | 42.44 | 43.02 | 43.00 | 40.98 |
| Sc | 23.4 | 24.1 | 28.0 | 23.3 | 32.4 |
| Pb | 24.6 | 12.3 | 12.9 | 12.3 | 19.7 |
| Rb | 0.81 | 0.47 | 0.38 | 0.41 | 0.41 |
| Ba | 2005 | 1198 | 1533 | 1803 | 5833 |
| Th | 1.45 | 6.84 | 0.21 | 0.55 | 1.08 |
| U | 1.35 | 0.72 | 0.23 | 0.47 | 1.69 |
| Nb | 8.4 | 71.2 | 7.7 | 5.3 | 62.3 |
| Ta | 0.06 | 0.21 | 0.03 | 0.03 | 0.19 |
| Zr | 3.51 | 0.98 | 0.64 | 0.77 | 13.90 |
| Hf | 0.22 | 0.07 | 0.07 | 0.06 | 0.99 |
| Sr | 18,400 | 16,000 | 14,700 | 16,800 | 13,800 |
| Y | 13.7 | 14.5 | 30.8 | 13.6 | 18.3 |
| La | 1531 | 626 | 592 | 665 | 454 |
| Ce | 1936 | 900 | 771 | 941 | 654 |
| Pr | 164 | 82 | 72 | 87 | 62 |
| Nd | 451 | 234 | 216 | 248 | 182 |
| Sm | 33 | 21 | 23 | 21 | 18 |
| Eu | 6.5 | 4.4 | 5.5 | 4.6 | 4.7 |
| Gd | 49 | 24 | 26 | 26 | 21 |
| Tb | 2.8 | 1.8 | 2.3 | 1.8 | 1.7 |
| Dy | 6.2 | 4.8 | 7.6 | 4.6 | 5.3 |
| Ho | 0.53 | 0.52 | 1.03 | 0.48 | 0.65 |
| Er | 2.9 | 2.1 | 3.1 | 2.1 | 2.2 |
| Tm | 0.11 | 0.13 | 0.26 | 0.11 | 0.15 |
| Yb | 0.81 | 0.87 | 1.75 | 0.77 | 1.00 |
| Lu | 0.11 | 0.12 | 0.24 | 0.11 | 0.14 |
| Sample | Sc | Ti | V | Cr | Mn | Rb | Ba | Th | U | Zr | Hf | Pb | Sr | Y |
| BY022-1 | 3.7 | bd | 0.18 | 7.28 | 4766 | bd | 82.7 | 0.003 | 0.050 | bd | 0.014 | 1.75 | 5727 | 2.54 |
| BY022-2 | 4.8 | 0.77 | 0.34 | 5.69 | 4982 | 0.006 | 89.6 | 0.004 | 0.063 | 0.162 | 0.009 | 2.33 | 5927 | 3.79 |
| BY022-3 | 3.2 | 0.02 | 0.22 | 5.15 | 4967 | bd | 90.0 | 0.003 | 0.142 | bd | 0.008 | 2.98 | 5597 | 3.66 |
| BY022-5 | 2.0 | 0.10 | 0.15 | 4.78 | 4654 | 0.008 | 72.3 | 0.003 | 0.001 | bd | 0.004 | 0.79 | 5230 | 2.30 |
| BY022-6 | 42.5 | 6.73 | 11.03 | 6.21 | 4847 | 0.022 | 76.1 | 0.005 | 0.176 | 0.002 | 0.065 | 2.73 | 5120 | 3.27 |
| BY022-7 | 5.1 | 0.31 | 0.33 | 8.61 | 4912 | 0.005 | 97.9 | 0.011 | 1.380 | bd | bd | 1.29 | 5673 | 2.96 |
| BY022-8 | 3.5 | 0.19 | 0.29 | 6.26 | 4793 | bd | 76.8 | 0.005 | 0.149 | 0.164 | 0.004 | 3.8 | 5957 | 3.23 |
| BY022-9 | 4.7 | 0.12 | 0.32 | 7.07 | 4694 | 0.017 | 96 | 0.005 | 0.007 | bd | bd | 2.24 | 5418 | 3.32 |
| BY022-10 | 5.9 | 0.42 | 0.52 | 6.18 | 5151 | 0.021 | 85.8 | 0.003 | 0.035 | 0.202 | 0.007 | 2.13 | 6036 | 4.79 |
| Sample | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
| BY022-1 | 19.5 | 46.9 | 4.9 | 16.2 | 2.1 | 0.62 | 1.57 | 0.15 | 0.73 | 0.14 | 0.37 | 0.03 | 0.16 | 0.04 |
| BY022-2 | 27.1 | 63.7 | 6.9 | 21.7 | 3.1 | 0.84 | 1.81 | 0.20 | 0.94 | 0.19 | 0.52 | 0.03 | 0.37 | 0.05 |
| BY022-3 | 22.3 | 52.7 | 5.7 | 18.8 | 2.8 | 0.81 | 2.01 | 0.19 | 0.97 | 0.15 | 0.39 | 0.03 | 0.16 | 0.07 |
| BY022-5 | 13.0 | 30.6 | 3.3 | 11.5 | 1.8 | 0.48 | 1.01 | 0.13 | 0.61 | 0.07 | 0.19 | 0.03 | 0.19 | 0.03 |
| BY022-6 | 13.8 | 37.9 | 4.1 | 13.3 | 2.0 | 0.62 | 1.29 | 0.18 | 1.02 | 0.18 | 0.32 | 0.03 | 0.19 | 0.03 |
| BY022-7 | 17.1 | 44.2 | 4.6 | 15.1 | 1.9 | 0.61 | 1.48 | 0.12 | 0.64 | 0.14 | 0.34 | 0.03 | 0.19 | 0.03 |
| BY022-8 | 19.2 | 44.9 | 4.7 | 15.8 | 2.3 | 0.64 | 1.45 | 0.17 | 0.81 | 0.11 | 0.35 | 0.02 | 0.22 | 0.04 |
| BY022-9 | 22.2 | 54.2 | 5.8 | 18.5 | 2.5 | 0.65 | 1.6 | 0.21 | 0.95 | 0.11 | 0.47 | 0.02 | 0.29 | 0.02 |
| BY022-10 | 32.2 | 75.4 | 7.7 | 27.1 | 4.1 | 1.06 | 2.13 | 0.25 | 1.31 | 0.23 | 0.47 | 0.05 | 0.3 | 0.03 |
| Element | P2O5 | SiO2 | La2O3 | Ce2O3 | Pr2O3 | Nd2O3 | CaO | SrO | Na2O | F | Cl | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 1 apatite | ||||||||||||
| BY22-1 | 41.83 | bd | 1.93 | 3.72 | 0.38 | 1.06 | 44.94 | 2.83 | 1.50 | 1.80 | bd | 99.28 |
| BY22-2 | 42.32 | bd | 1.10 | 3.61 | 0.45 | 0.94 | 44.73 | 2.89 | 1.53 | 2.25 | bd | 98.88 |
| BY22-3 | 42.07 | bd | 2.24 | 3.72 | 0.49 | 1.04 | 44.84 | 2.96 | 1.55 | 2.01 | 0.05 | 100.21 |
| BY22-4 | 41.50 | bd | 2.40 | 4.28 | 0.59 | 0.94 | 43.19 | 3.63 | 1.76 | 2.18 | 0.02 | 99.57 |
| BY22-5 | 42.34 | bd | 1.78 | 4.13 | 0.53 | 0.79 | 44.18 | 3.32 | 1.73 | 2.24 | 0.04 | 100.14 |
| BY22-6 | 41.51 | bd | 2.26 | 3.75 | 0.51 | 0.91 | 43.88 | 3.45 | 1.66 | 2.55 | 0.04 | 99.45 |
| BY22-7 | 41.94 | bd | 1.76 | 4.22 | 0.47 | 1.15 | 43.89 | 3.54 | 1.58 | 2.32 | bd | 99.98 |
| BY22-8 | 41.14 | bd | 2.62 | 3.72 | 0.47 | 0.87 | 44.79 | 3.46 | 1.72 | 2.68 | 0.02 | 100.36 |
| BY22-9 | 42.37 | bd | 2.00 | 3.77 | 0.49 | 1.12 | 42.89 | 3.04 | 1.57 | 3.34 | bd | 99.19 |
| BY22-10 | 42.03 | bd | 2.22 | 4.03 | 0.59 | 1.05 | 43.13 | 3.47 | 1.69 | 2.37 | 0.03 | 99.61 |
| BY22-11 | 41.90 | bd | 2.55 | 4.11 | 0.57 | 0.69 | 43.58 | 3.60 | 1.55 | 2.17 | bd | 99.81 |
| BY22-12 | 41.68 | bd | 2.15 | 4.24 | 0.37 | 0.81 | 43.87 | 3.23 | 1.74 | 3.00 | 0.04 | 99.99 |
| BY22-13 | 41.88 | bd | 1.84 | 4.17 | 0.50 | 1.02 | 44.13 | 3.29 | 1.63 | 2.68 | 0.02 | 100.03 |
| BY22-14 | 41.68 | bd | 2.20 | 4.44 | 0.53 | 0.76 | 42.92 | 3.50 | 1.54 | 3.22 | bd | 99.44 |
| BY22-15 | 42.39 | bd | 1.60 | 3.74 | 0.40 | 1.09 | 42.98 | 3.59 | 1.36 | 2.88 | bd | 98.89 |
| BY22-16 | 42.33 | bd | 2.11 | 3.79 | 0.46 | 0.97 | 44.28 | 3.33 | 1.58 | 2.12 | 0.03 | 100.11 |
| BY22-17 | 41.49 | bd | 2.09 | 3.74 | 0.69 | 0.79 | 43.50 | 3.50 | 1.66 | 2.68 | bd | 99.01 |
| BY22-18 | 42.20 | bd | 1.70 | 4.45 | 0.50 | 1.25 | 43.15 | 3.35 | 1.71 | 2.40 | 0.03 | 99.73 |
| BY22-19 | 42.26 | bd | 2.09 | 4.10 | 0.44 | 0.84 | 44.13 | 3.25 | 1.51 | 3.02 | bd | 100.37 |
| Type 2 apatite | ||||||||||||
| XC3-4 | 41.3 | bd | 0.63 | 1.74 | 0.26 | 0.48 | 48.99 | 2.19 | 0.91 | 3.17 | 0.02 | 98.40 |
| XC3-5 | 41.86 | bd | 1.19 | 1.94 | 0.17 | 0.44 | 49.59 | 1.92 | 1.09 | 3.55 | 0.02 | 100.33 |
| XC3-6 | 42.07 | bd | 1.24 | 1.11 | 0.23 | 0.40 | 49.77 | 1.86 | 0.83 | 2.51 | 0.02 | 99.03 |
| XC3-7 | 41.20 | bd | 0.92 | 1.20 | 0.18 | 0.31 | 49.63 | 1.97 | 1.05 | 3.64 | 0.01 | 98.65 |
| XC3-8 | 41.60 | bd | 0.96 | 1.44 | 0.25 | 0.35 | 50.41 | 1.76 | 0.77 | 3.09 | 0.02 | 99.38 |
| XC3-9 | 41.56 | bd | 1.03 | 1.96 | 0.16 | 0.47 | 49.59 | 1.83 | 0.93 | 3.01 | 0.01 | 99.35 |
| XC3-10 | 42.04 | bd | 0.70 | 1.58 | 0.14 | 0.31 | 50.43 | 1.84 | 0.8 | 2.35 | 0.02 | 99.22 |
| XC3-11 | 42.20 | bd | 0.79 | 1.71 | 0.23 | 0.42 | 49.55 | 1.92 | 0.92 | 3.00 | bd | 99.58 |
| XC3-12 | 41.71 | bd | 1.28 | 2.00 | 0.30 | 0.62 | 49.20 | 2.09 | 1.07 | 2.88 | bd | 100.01 |
| XC3-16 | 42.05 | bd | 1.09 | 1.73 | 0.29 | 0.35 | 49.27 | 1.87 | 0.89 | 2.42 | 0.01 | 98.99 |
| XC3-17 | 41.59 | bd | 0.98 | 1.56 | 0.19 | 0.36 | 50.23 | 1.81 | 0.78 | 2.30 | 0.01 | 98.88 |
| XC3-19 | 41.79 | bd | 0.74 | 1.15 | 0.13 | 0.47 | 50.21 | 1.79 | 0.79 | 3.01 | 0.01 | 98.83 |
| XC3-21 | 42.05 | bd | 1.02 | 1.69 | 0.30 | 0.40 | 50.33 | 2.05 | 0.88 | 2.76 | bd | 100.32 |
| XC3-22 | 41.75 | bd | 0.51 | 1.73 | 0.16 | 0.35 | 50.04 | 2.00 | 0.80 | 2.98 | 0.01 | 99.12 |
| XC3-33 | 42.14 | bd | 0.72 | 1.38 | 0.08 | 0.26 | 49.99 | 1.88 | 0.90 | 2.96 | bd | 99.13 |
| XC3-34 | 41.63 | bd | 0.63 | 1.79 | 0.23 | 0.30 | 49.89 | 1.87 | 0.87 | 2.72 | 0.01 | 98.80 |
| XC3-36 | 41.95 | bd | 0.84 | 0.98 | 0.22 | 0.31 | 50.96 | 1.75 | 0.59 | 2.62 | 0.01 | 99.22 |
| Type 3 apatite | ||||||||||||
| XC3-1 | 42.51 | bd | 0.71 | 0.88 | 0.09 | 0.36 | 51.10 | 1.51 | 0.59 | 3.12 | 0.01 | 99.61 |
| XC3-2 | 42.20 | bd | 0.52 | 0.86 | 0.11 | 0.30 | 51.75 | 1.88 | 0.52 | 3.08 | 0.01 | 99.97 |
| XC3-3 | 42.18 | bd | 0.45 | 0.89 | 0.21 | 0.37 | 50.79 | 1.71 | 0.63 | 2.61 | 0.02 | 98.79 |
| XC3-13 | 42.28 | bd | 0.70 | 0.82 | 0.11 | 0.32 | 51.34 | 1.77 | 0.53 | 3.04 | bd | 99.71 |
| XC3-14 | 42.29 | bd | 0.38 | 0.91 | 0.18 | 0.27 | 51.60 | 1.67 | 0.51 | 3.53 | 0.01 | 99.92 |
| XC3-15 | 43.15 | bd | 0.72 | 0.80 | 0.15 | 0.31 | 51.73 | 1.66 | 0.55 | 2.30 | 0.02 | 100.48 |
| XC3-18 | 42.89 | bd | 0.58 | 0.52 | 0.08 | 0.35 | 52.29 | 1.79 | 0.41 | 2.62 | 0.03 | 100.53 |
| XC3-20 | 42.62 | bd | 0.86 | 0.73 | 0.19 | 0.31 | 51.18 | 1.77 | 0.53 | 2.43 | 0.01 | 99.65 |
| XC3-24 | 42.17 | bd | 0.21 | 0.62 | 0.09 | 0.26 | 51.4 | 1.79 | 0.56 | 2.32 | 0.01 | 98.51 |
| XC3-25 | 42.44 | bd | 0.23 | 0.57 | 0.09 | 0.28 | 52.31 | 1.82 | 0.46 | 2.94 | 0.01 | 99.92 |
| XC3-26 | 42.04 | bd | 0.23 | 1.00 | 0.13 | 0.21 | 51.28 | 1.79 | 0.66 | 3.43 | 0.01 | 99.38 |
| XC3-27 | 42.07 | bd | 0.63 | 0.65 | 0.08 | 0.22 | 51.54 | 1.72 | 0.49 | 2.66 | 0.01 | 98.95 |
| XC3-28 | 41.99 | bd | 0.70 | 1.13 | 0.15 | 0.37 | 50.76 | 1.63 | 0.71 | 2.89 | 0.03 | 99.15 |
| XC3-29 | 42.22 | bd | 0.35 | 0.88 | 0.06 | 0.22 | 51.02 | 1.64 | 0.42 | 3.26 | 0.01 | 98.71 |
| XC3-30 | 42.45 | bd | 0.33 | 0.88 | 0.16 | 0.44 | 51.66 | 1.51 | 0.74 | 3.38 | 0.01 | 100.20 |
| XC3-31 | 41.50 | bd | 0.28 | 1.02 | 0.10 | 0.21 | 51.65 | 1.83 | 0.64 | 2.63 | 0.02 | 98.78 |
| XC3-32 | 41.96 | bd | 0.23 | 0.95 | 0.10 | 0.30 | 51.30 | 1.78 | 0.54 | 2.67 | bd | 98.78 |
| XC3-35 | 42.08 | bd | 0.33 | 0.58 | 0.06 | 0.26 | 51.86 | 1.99 | 0.45 | 3.16 | 0.01 | 99.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Li, Y.; Chuan, M.; Li, R.; Ke, C.; Wu, Z. Post-Magmatic Fluids Dominate the Mineralization of Dolomite Carbonatitic Dykes Next to the Giant Bayan Obo REE Deposit, Northern China. Minerals 2020, 10, 1117. https://doi.org/10.3390/min10121117
Hu L, Li Y, Chuan M, Li R, Ke C, Wu Z. Post-Magmatic Fluids Dominate the Mineralization of Dolomite Carbonatitic Dykes Next to the Giant Bayan Obo REE Deposit, Northern China. Minerals. 2020; 10(12):1117. https://doi.org/10.3390/min10121117
Chicago/Turabian StyleHu, Le, Yike Li, Maoshan Chuan, Ruiping Li, Changhui Ke, and Zhongjian Wu. 2020. "Post-Magmatic Fluids Dominate the Mineralization of Dolomite Carbonatitic Dykes Next to the Giant Bayan Obo REE Deposit, Northern China" Minerals 10, no. 12: 1117. https://doi.org/10.3390/min10121117
APA StyleHu, L., Li, Y., Chuan, M., Li, R., Ke, C., & Wu, Z. (2020). Post-Magmatic Fluids Dominate the Mineralization of Dolomite Carbonatitic Dykes Next to the Giant Bayan Obo REE Deposit, Northern China. Minerals, 10(12), 1117. https://doi.org/10.3390/min10121117




