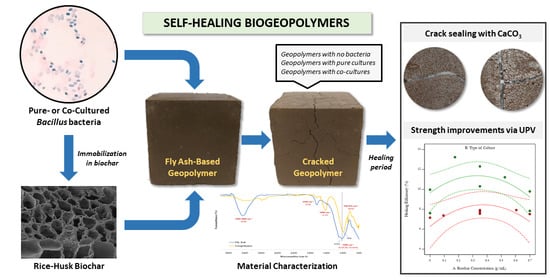
Self-Healing Biogeopolymers Using Biochar-Immobilized Spores of Pure- and Co-Cultures of Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Methods
2.3.1. Initial Characterization
2.3.2. Preparation of Spore Suspensions
2.3.3. Selection of a Suitable Healing Agent
2.3.4. Two-Factor Test on Immobilization and Co-Culturing
2.3.5. Characterization of the Geopolymers and the Mineral Precipitates
3. Results and Discussion
3.1. A Suitable Healing Agent for Geopolymers
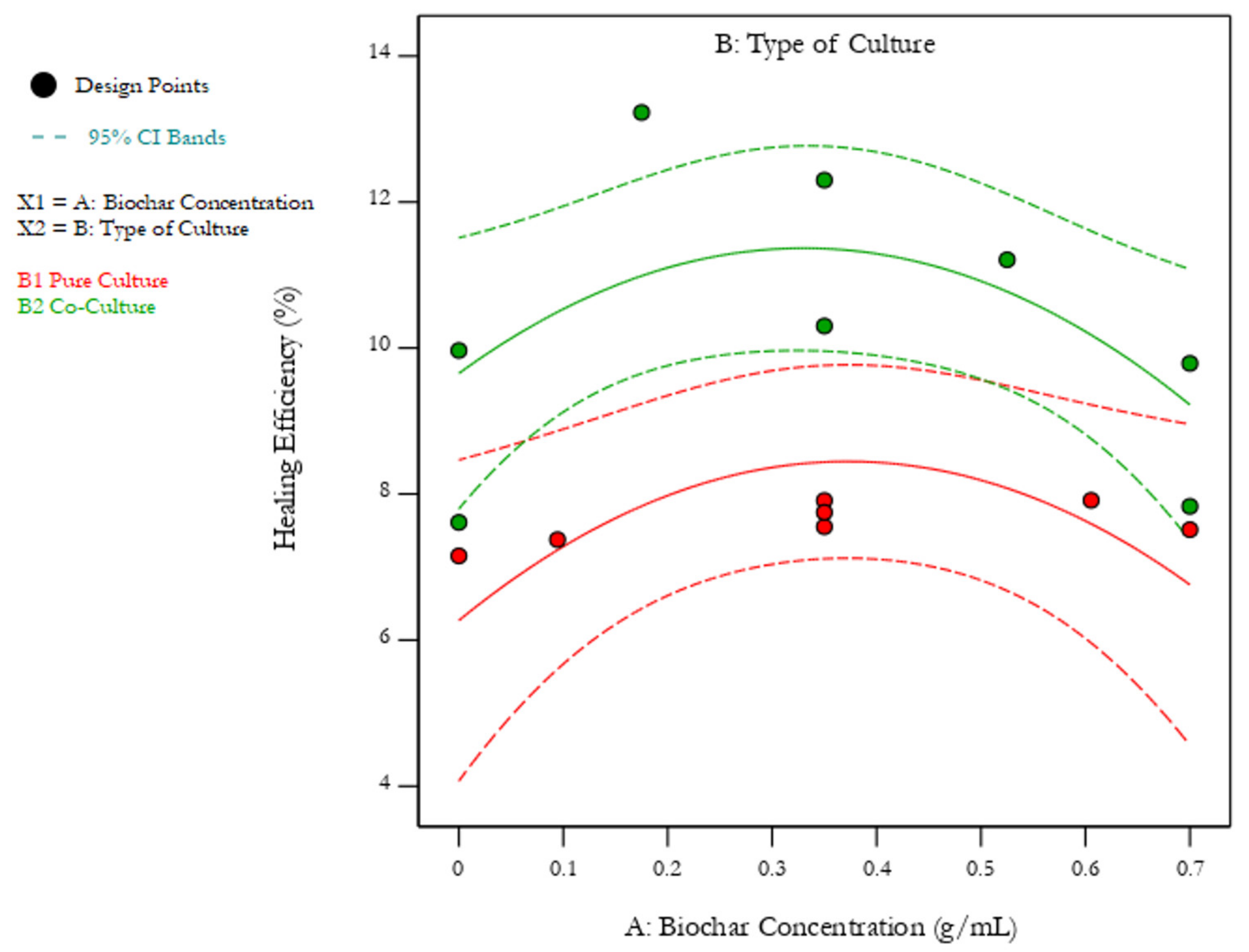
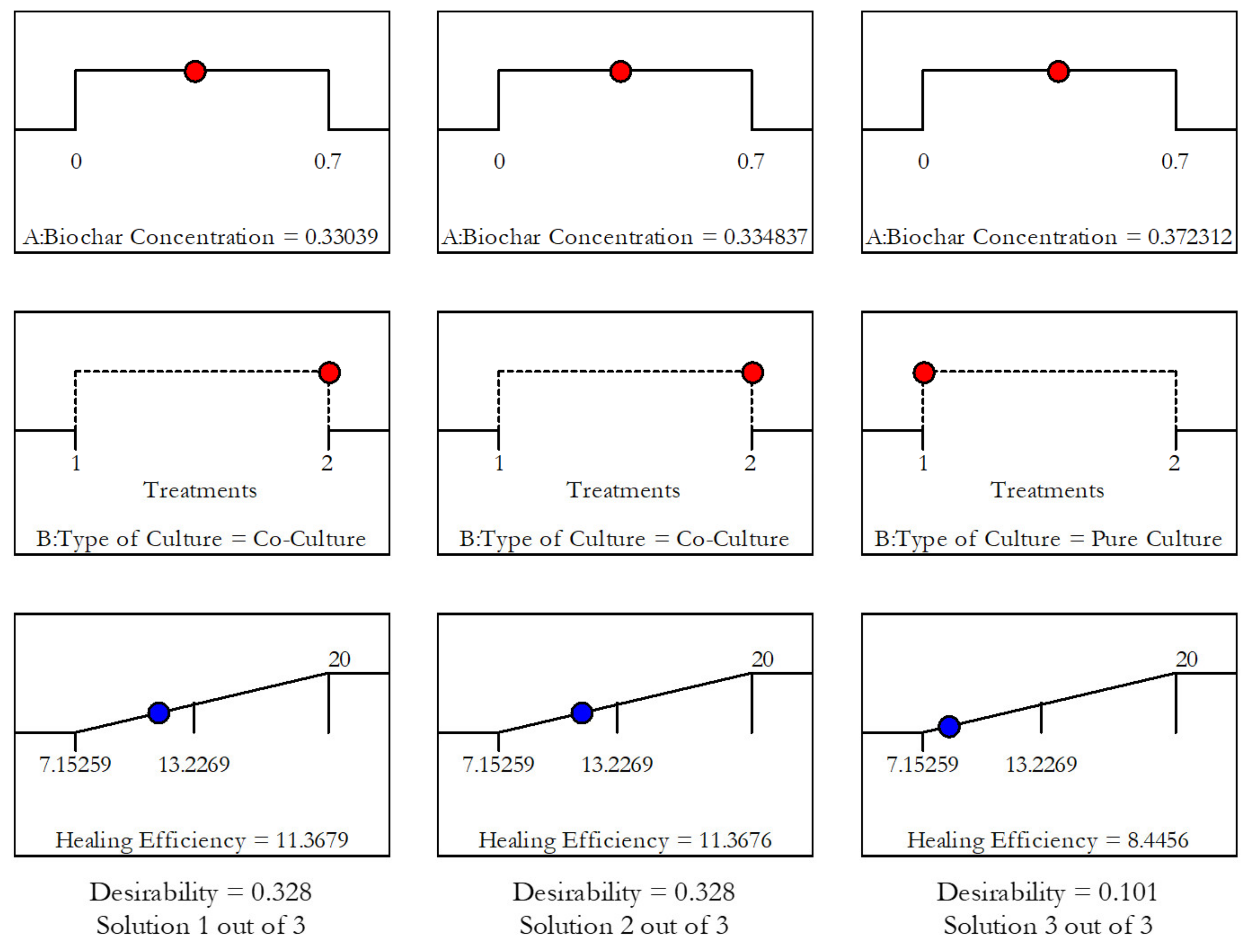
3.2. Effect of Biochar-Immobilization and Co-Culturing
3.2.1. Test Results for the Control Geopolymers
3.2.2. Test Results for the Bacteria-Containing Geopolymers
3.3. Characterization of the Geopolymers and the Mineral Precipitates
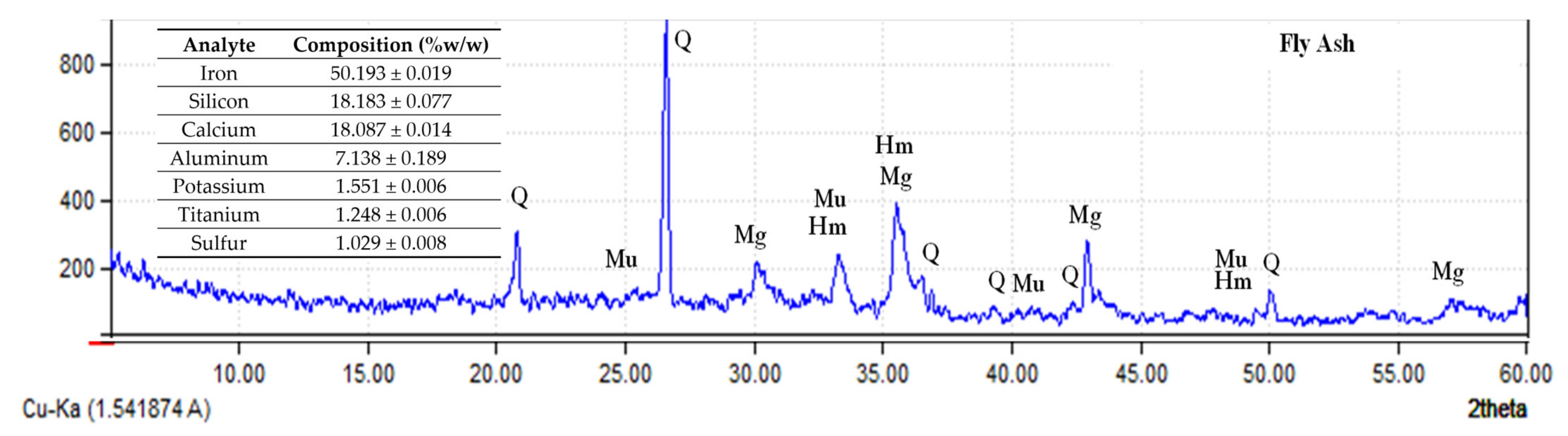
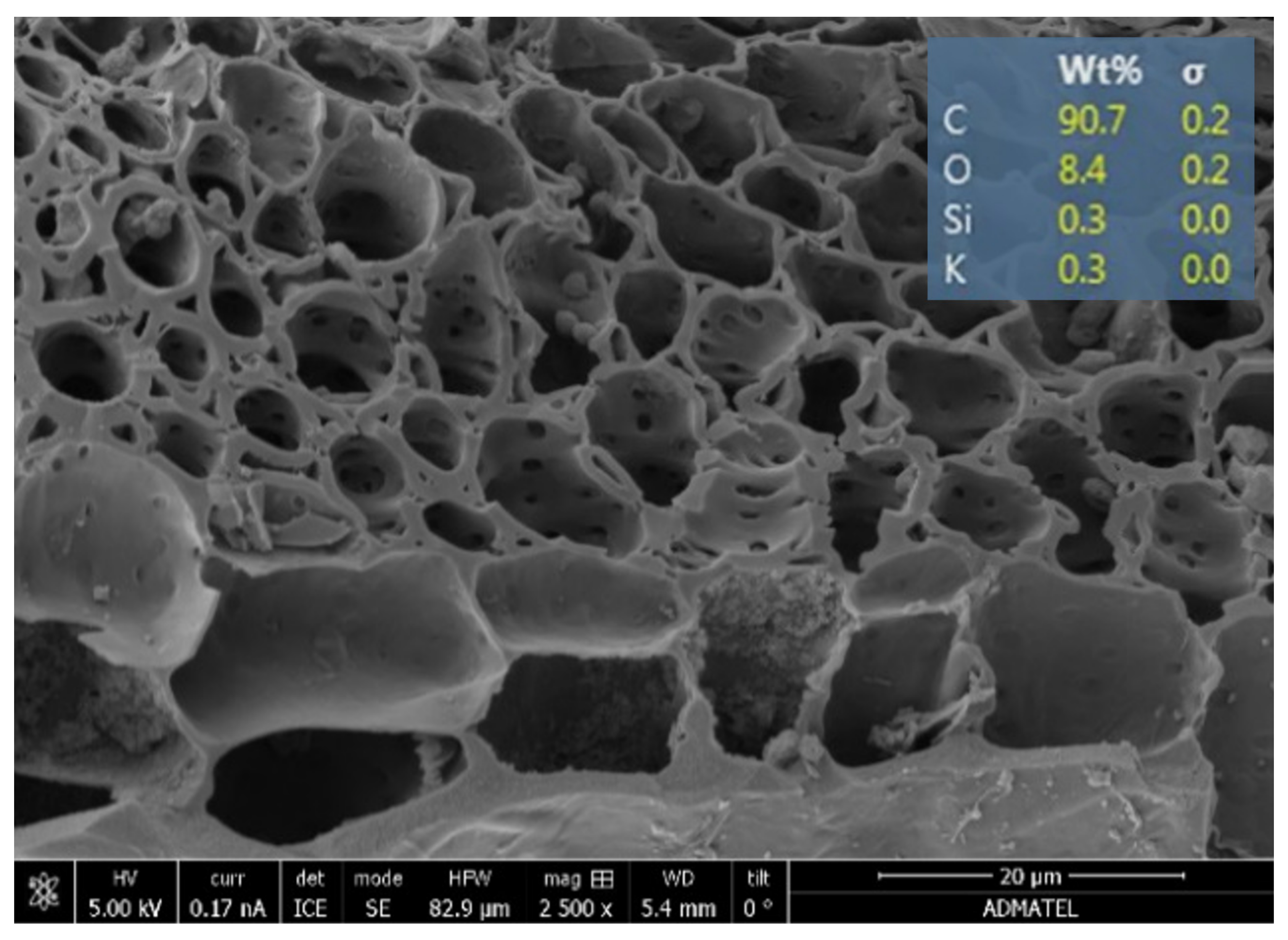
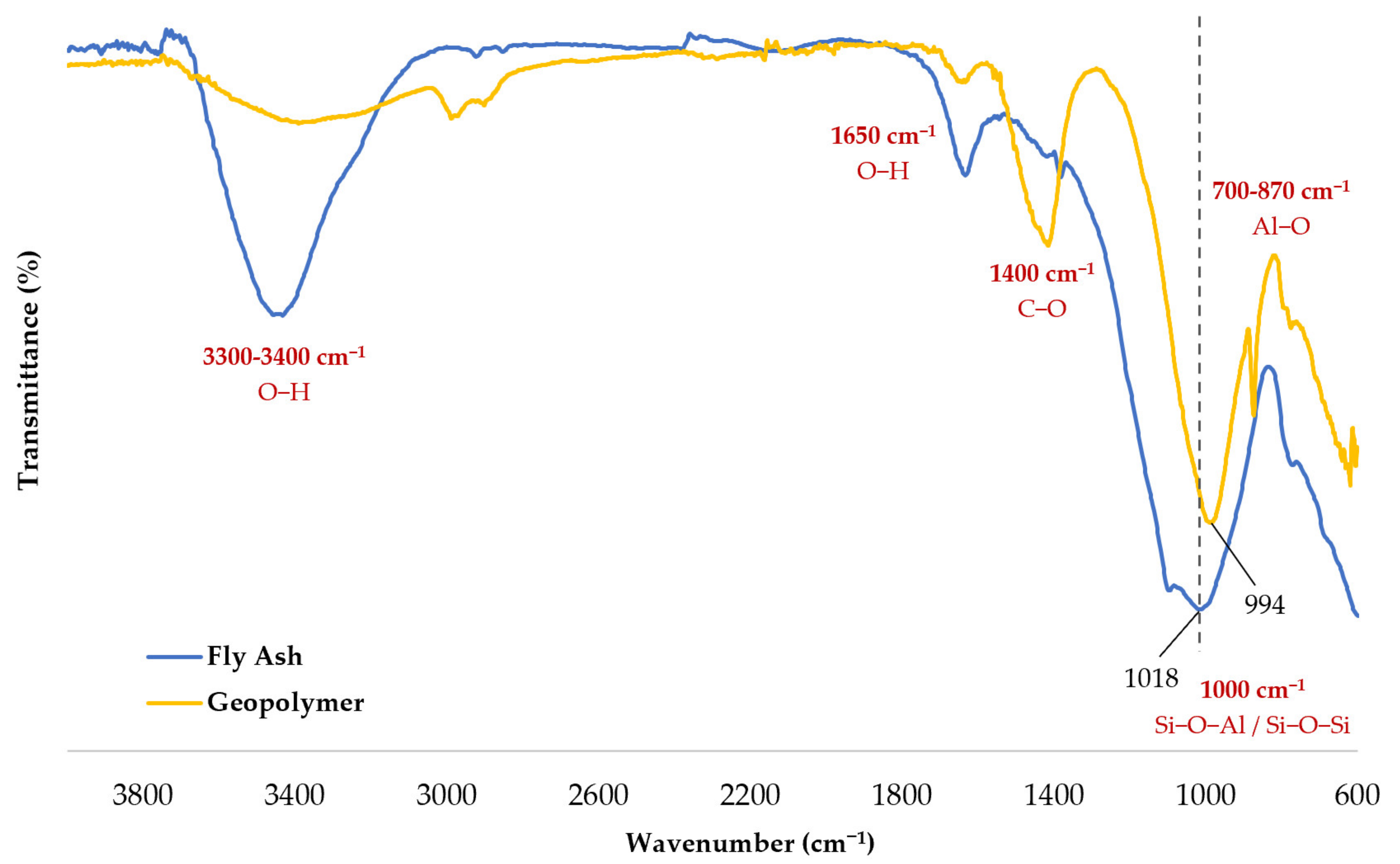
3.3.1. Confirmation of Samples as Geopolymers
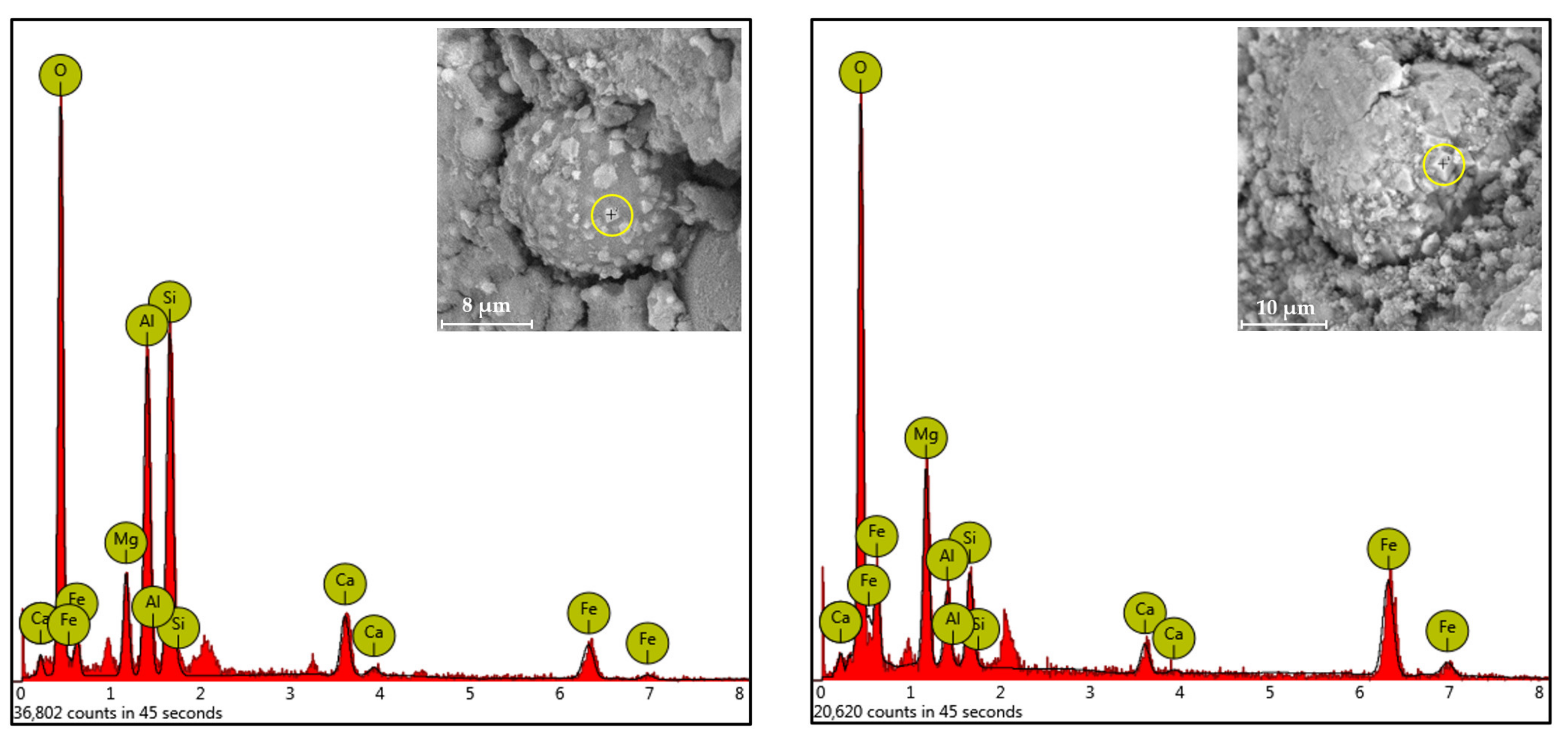
3.3.2. Analysis of the Precipitates from Bacterially Induced Mineralization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Experimental Design for the Two-Factor Test
| Run | Grams of Biochar per mL of Spore Suspension | Type of Culture |
|---|---|---|
| 1 | 0 | Pure Culture |
| 2 | 0.175 | Co-Culture |
| 3 | 0.7 | Pure Culture |
| 4 | 0.35 | Pure Culture |
| 5 | 0.35 | Co-Culture |
| 6 | 0.6055 | Pure Culture |
| 7 | 0.35 | Pure Culture |
| 8 | 0 | Co-Culture |
| 9 | 0.35 | Pure Culture |
| 10 | 0.0945 | Pure Culture |
| 11 | 0.525 | Co-Culture |
| 12 | 0.7 | Co-Culture |
| 13 | 0 | Co-Culture |
| 14 | 0.35 | Co-Culture |
| 15 | 0.7 | Co-Culture |
| Control Group | Grams of Biochar per mL of Distilled Water |
|---|---|
| Con-A | 0 |
| Con-B | 0.175 |
| Con-C | 0.525 |
| Con-D | 0.70 |
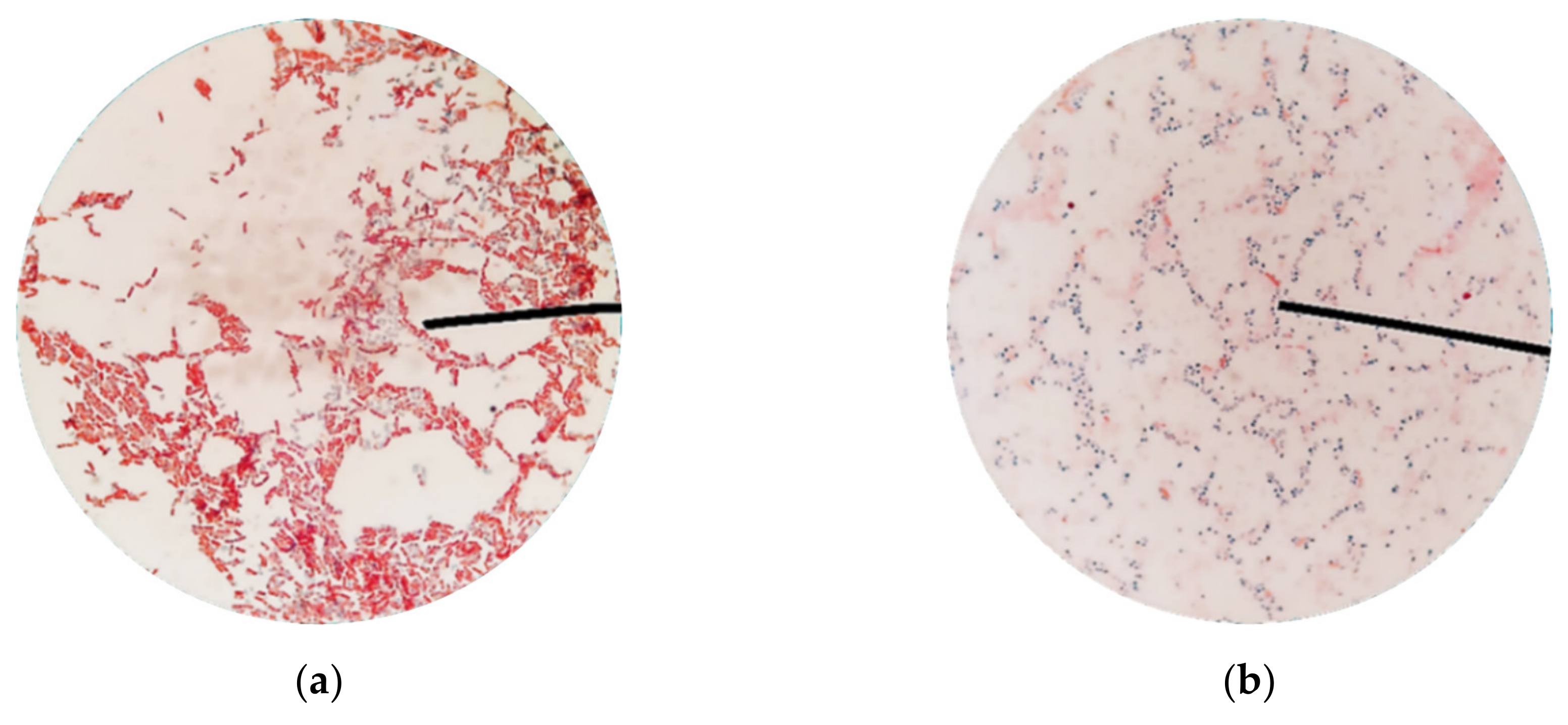
Appendix B. Schaeffer-Fulton Stains
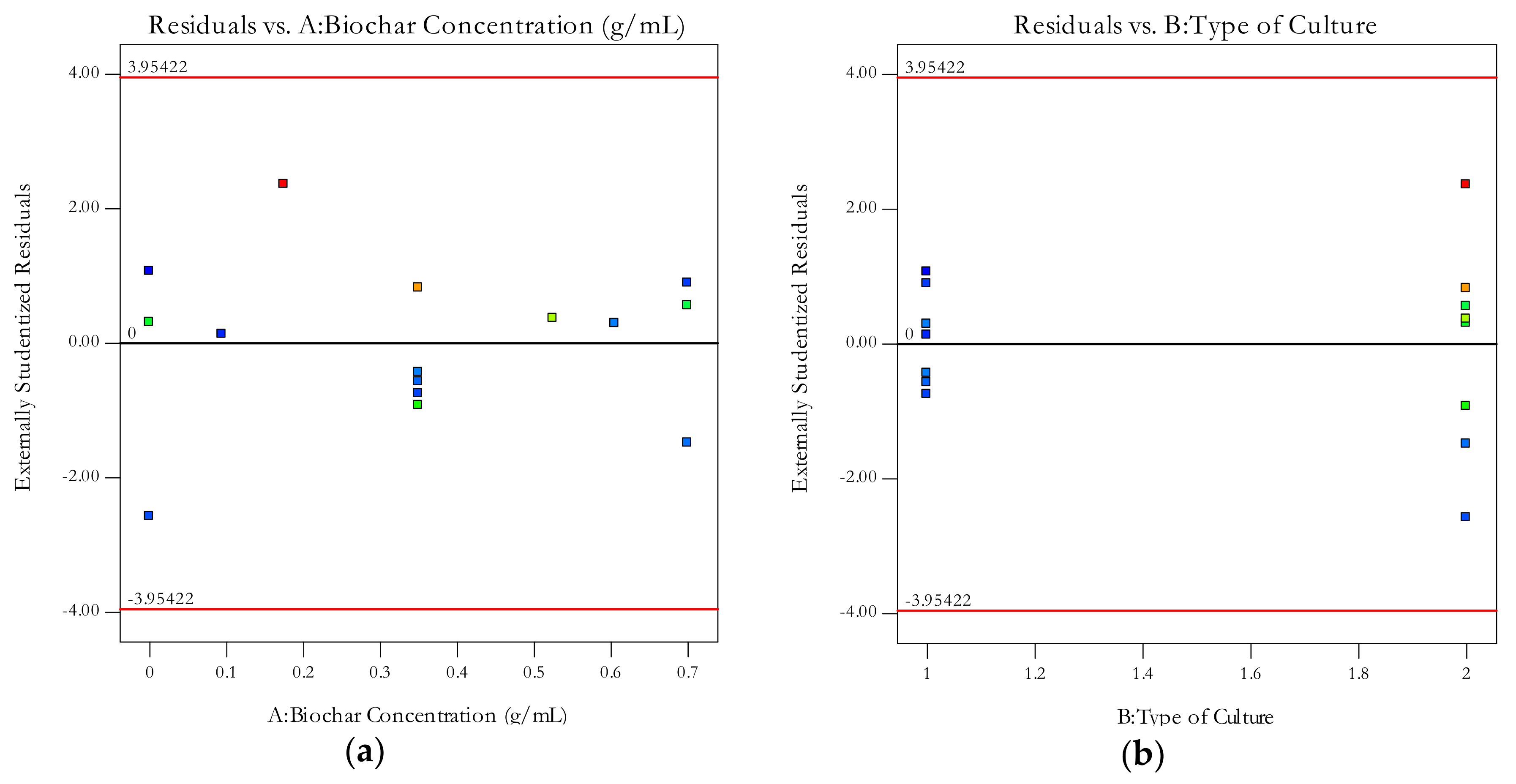
Appendix C. Analysis of Variance for the Two-Factor Test
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
| Model | 37.73 | 4 | 9.43 | 5.63 | 0.0122 |
| A—Biochar Concentration | 0.0017 | 1 | 0.0017 | 0.0010 | 0.9751 |
| B—Type of Culture | 31.28 | 1 | 31.28 | 18.68 | 0.0015 |
| AB | 0.3863 | 1 | 0.3863 | 0.2307 | 0.6413 |
| A2 | 10.44 | 1 | 10.44 | 6.23 | 0.0316 |
| Residual | 16.74 | 10 | 1.67 | ||
| Lack of Fit | 9.99 | 5 | 2.00 | 1.48 | 0.3391 |
| Pure Error | 6.75 | 5 | 1.35 | ||
| Cor Total | 54.47 | 14 |
References
- Burciaga-Díaz, O.; Magallanes-Rivera, R.X.; García, J.E. Alkali-activated slag-metakaolin pastes: Strength, structural, and microstructural characterization. J. Sustain. Cem. Mater. 2013, 2, 111–127. [Google Scholar] [CrossRef]
- Chi, M.-C.; Chang, J.-J.; Huang, R. Strength and drying shrinkage of alkali-activated slag paste and mortar. Adv. Civ. Eng. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Tigue, A.A.S.; Malenab, R.A.; Dungca, J.R.; Yu, D.E.C.; Promentilla, M.A.B. Chemical stability and leaching behavior of one-part geopolymer from soil and coal fly ash mixtures. Minerals 2018, 8, 411. [Google Scholar] [CrossRef]
- Malenab, R.A.; Ngo, J.P.S.; Promentilla, M.A.B. Chemical treatment of waste abaca for natural fiber-reinforced geopolymer composite. Materials 2017, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357. [Google Scholar] [CrossRef]
- Luo, J.; Chen, X.; Crump, J.; Zhou, H.; Davies, D.G.; Zhou, G.; Zhang, N.; Jin, C. Interactions of fungi with concrete: Significant importance for bio-based self-healing concrete. Constr. Build. Mater. 2018, 164, 275–285. [Google Scholar] [CrossRef]
- Skinner, H.; Ehrlich, H. Biomineralization. In Treatise on Geochemistry; Elsevier BV: Berlin, Germany, 2014; pp. 105–162. [Google Scholar]
- Benzerara, K. Biomineralization. In Encyclopedia of Astrobiology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2011; pp. 197–198. [Google Scholar]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed]
- Frankel, R. Biologically induced mineralization by bacteria. Rev. Miner. Geochem. 2003, 54, 95–114. [Google Scholar] [CrossRef]
- Wang, J.; Snoeck, D.; Van Vlierberghe, S.; Verstraete, W.; De Belie, N. Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr. Build. Mater. 2014, 68, 110–119. [Google Scholar] [CrossRef]
- Vijay, K.; Murmu, M.; Deo, S.V. Bacteria based self healing concrete—A review. Constr. Build. Mater. 2017, 152, 1008–1014. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Pang, S.D. Healing cement mortar by immobilization of bacteria in biochar: An integrated approach of self-healing and carbon sequestration. Cem. Concr. Compos. 2018, 86, 238–254. [Google Scholar] [CrossRef]
- Van Mullem, T.; Gruyaert, E.; Caspeele, R.; De Belie, N. First large scale application with self-healing concrete in belgium: Analysis of the laboratory control tests. Materials 2020, 13, 997. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, U.U.; Lahoti, M.; Chen, Z.; Qiu, J.; Cao, B.; Yang, E.-H. Viability of bacterial spores and crack healing in bacteria-containing geopolymer. Constr. Build. Mater. 2018, 169, 716–723. [Google Scholar] [CrossRef]
- Wulandari, K.D.; Ekaputri, J.J.; Triwulan, N.; Fujiyama, C.; Setiamarga, D. Effects of microbial agents to the properties of fly ash-based paste. In Proceedings of the MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 195, p. 01012. [Google Scholar]
- Chatterjee, A.; Chattopadhyay, B.; Mandal, S. Bacterium amended 100% fly ash geopolymer. In Proceedings of the International Conference on Sustainable Materials and Structures for Civil Infrastructures (SMSCI2019), Madhya Pradesh, India, 4–15 March 2019; AIP Publishing: New York, NY, USA, 2019; Volume 2158, p. 020013. [Google Scholar]
- Wulandari, K.D.; Ekaputri, J.J.; Triwulan, N.; Kurniawan, S.B.; Primaningtyas, W.E.; Abdullah, S.R.S.; Ismail, N.I.; Imron, M.F. Effect of microbes addition on the properties and surface morphology of fly ash-based geopolymer paste. J. Build. Eng. 2021, 33, 101596. [Google Scholar] [CrossRef]
- Son, H.M.; Kim, H.Y.; Park, S.; Lee, H. Ureolytic/Non-ureolytic bacteria co-cultured self-healing agent for cementitious materials crack repair. Materials 2018, 11, 782. [Google Scholar] [CrossRef]
- Arafa, S.A.; Ali, A.Z.M.; Awal, A.A.; Loon, L.Y. Optimum mix for fly ash geopolymer binder based on workability and compressive strength. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 140, p. 012157. [Google Scholar]
- Nain, N.; Surabhi, R.; Yathish, N.; Krishnamurthy, V.; Deepa, T.; Tharannum, S. Enhancement in strength parameters of concrete by application of Bacillus bacteria. Constr. Build. Mater. 2019, 202, 904–908. [Google Scholar] [CrossRef]
- Bacterial Concrete—A Sustainable Solution for Concrete Maintenance. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 227–232. [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Kalaw, M.E.; Culaba, A.B.; Hinode, H.; Kurniawan, W.; Gallardo, S.M.; Promentilla, M.A.B. Optimizing and characterizing geopolymers from Ternary Blend of Philippine Coal Fly Ash, coal bottom ash and rice hull ash. Materials 2016, 9, 580. [Google Scholar] [CrossRef]
- Khan, M.I.; Azizli, K.A.; Sufian, S.; Siyal, A.A.; Man, Z. Sodium silicate free geopolymer as coating material: Adhesion to steel. In Proceedings of the 1st International Electronic Conference on Materials, 26 May–10 June 2014; MDPI: Basel, Switzerland, 2014. [Google Scholar]
- Rosas-Casarez, C.; Arredondo-Rea, S.; Gómez-Soberón, J.; Alamaral-Sánchez, J.; Corral-Higuera, R.; Chinchillas-Chinchillas, M.; Acuña-Agüero, O. Experimental study of XRD, FTIR and TGA techniques in geopolymeric materials. Int. J. Adv. Comput. Sci. Its Appl. 2014, 4, 25–30. [Google Scholar]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Ruan, S.; Qiu, J.; Weng, Y.; Yang, Y.; Yang, E.-H.; Chu, J.; Unluer, C. The use of microbial induced carbonate precipitation in healing cracks within reactive magnesia cement-based blends. Cem. Concr. Res. 2019, 115, 176–188. [Google Scholar] [CrossRef]
- Suleiman, A.R.; Nelson, A.J.; Nehdi, M.L. Visualization and quantification of crack self-healing in cement-based materials incorporating different minerals. Cem. Concr. Compos. 2019, 103, 49–58. [Google Scholar] [CrossRef]
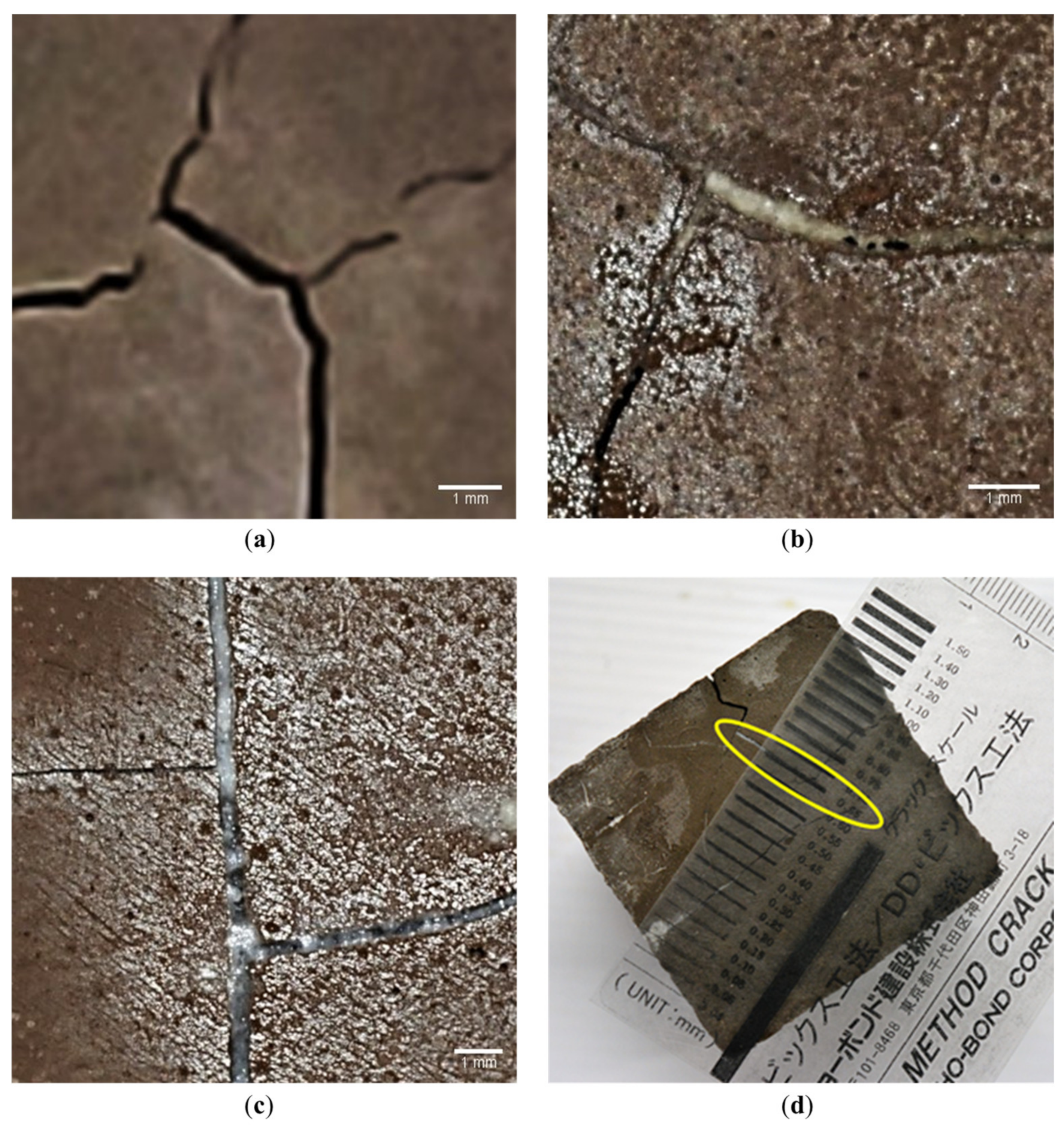
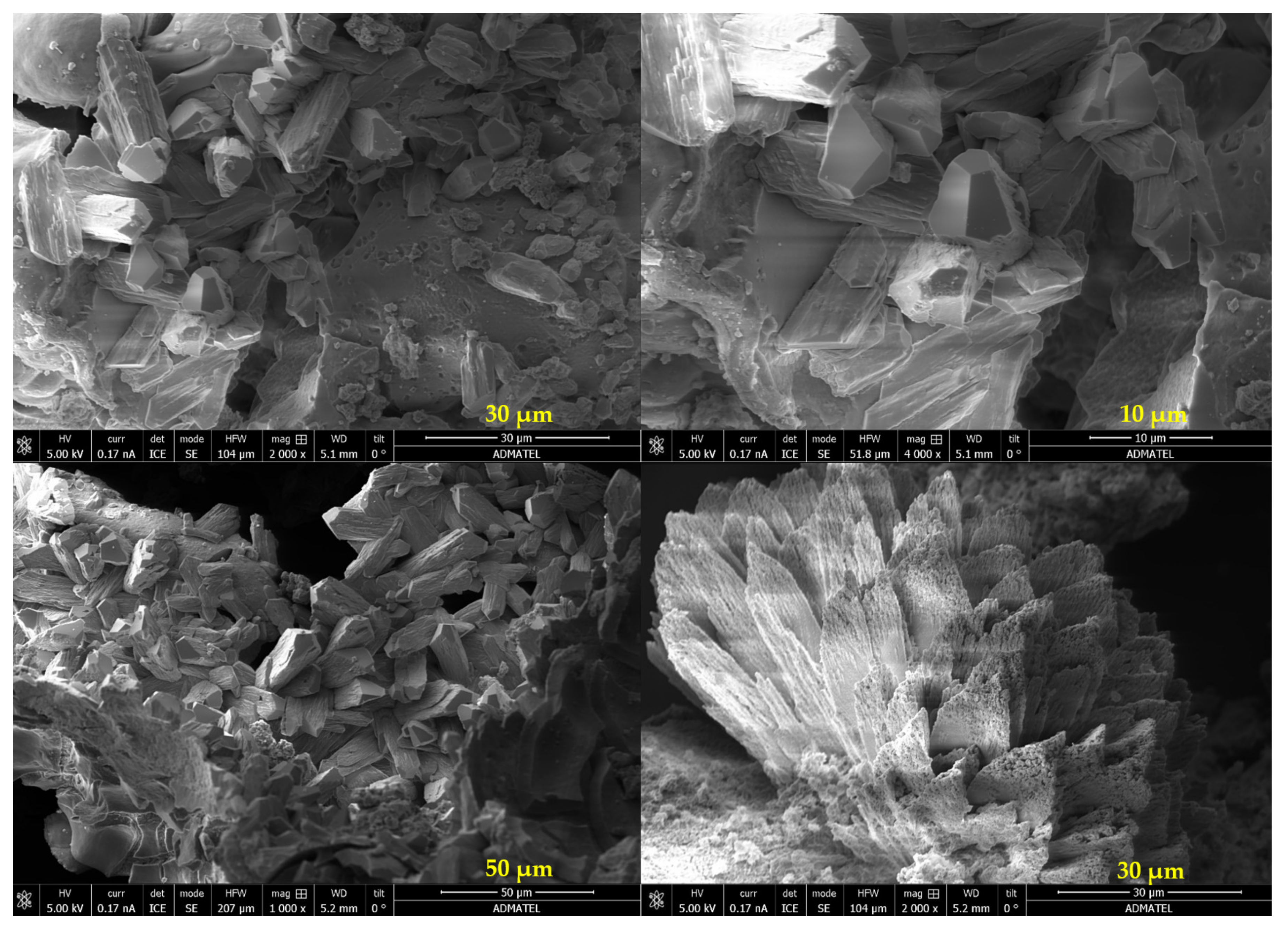
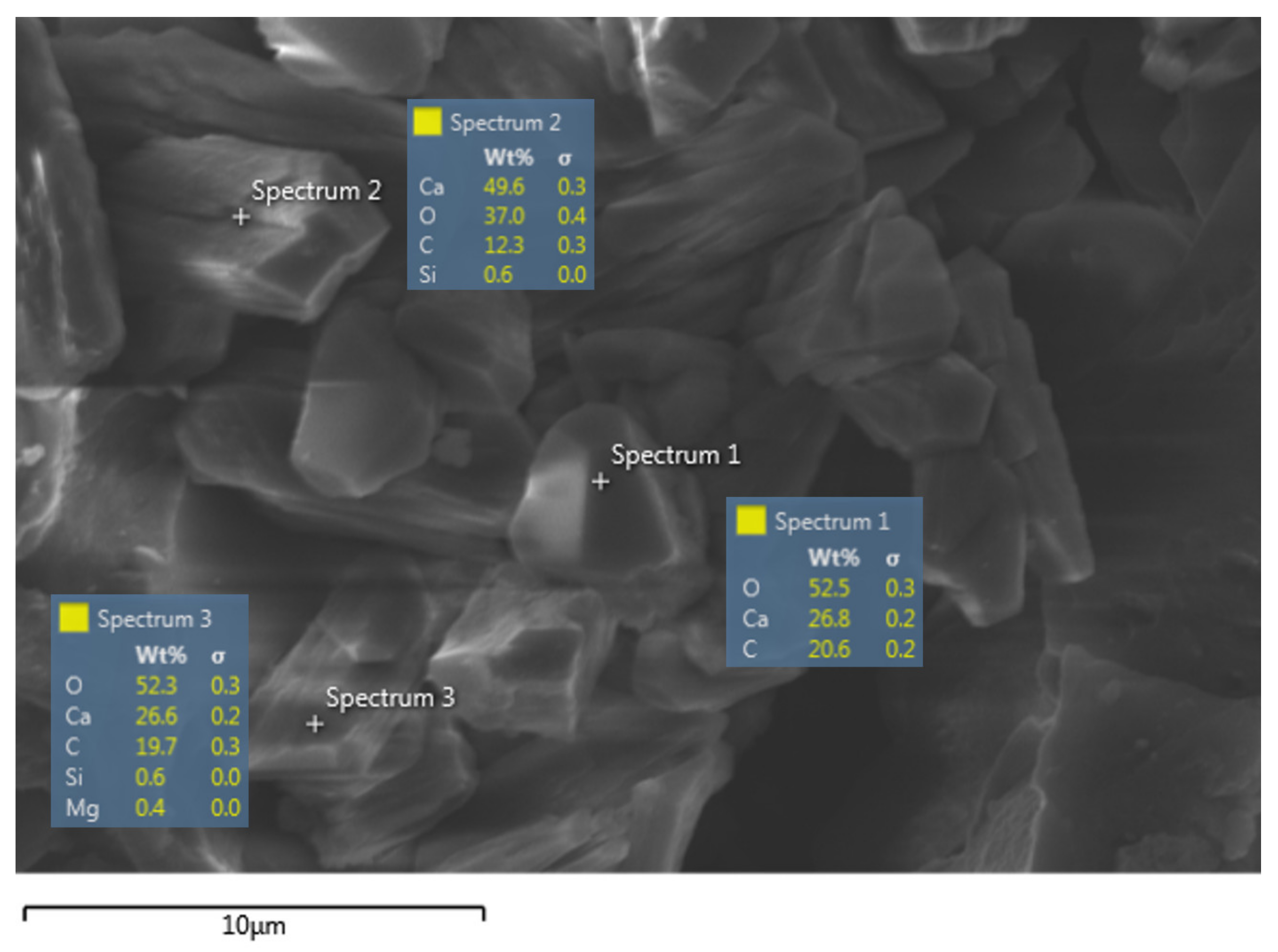

| Geopolymer Precursor | Healing Agent | Key Findings | Year of Publication | Reference |
|---|---|---|---|---|
| Metakaolin | Sporosarcinapasteurii | Sealing of 89 ± 3-µm crack widths with CaCO3 | 2018 | [15] |
| Fly Ash | Solution of S. pasteurii and yeast from a fungi | Geopolymer pores were filled with CaCO3, causing improvements in their mechanical properties | 2018 | [16] |
| Fly Ash | Genetically-modified B. subtilis | 70.9%, 40.0%, and 68.87% increase in compressive strength, ultrasonic pulse velocity, and acid resistance, respectively, after 28 days | 2019 | [17] |
| Fly Ash | Solution of S. pasteurii and Rhizopus oligosporus | 43.75% increase in compressive strength; higher amount of closed porosity for all specimens with microbes | 2021 | [18] |
| Control Group | Biochar Concentration (g/mL) | Mean Healing Efficiency (%) |
|---|---|---|
| Con-A | 0 | 2.12 |
| Con-B | 0.175 | 2.33 |
| Con-C | 0.525 | 2.70 |
| Con-D | 0.7 | 2.61 |
| Run | Biochar Concentration (g/mL) | Type of Culture | Mean Healing Efficiency (%) |
|---|---|---|---|
| 1 | 0 | Pure Culture | 7.15 |
| 10 | 0.0945 | Pure Culture | 7.38 |
| 4 | 0.35 | Pure Culture | 7.55 |
| 7 | 0.35 | Pure Culture | 7.75 |
| 9 | 0.35 | Pure Culture | 7.91 |
| 6 | 0.6055 | Pure Culture | 7.91 |
| 3 | 0.7 | Pure Culture | 7.51 |
| 8 | 0 | Co-Culture | 9.97 |
| 13 | 0 | Co-Culture | 7.61 |
| 2 | 0.175 | Co-Culture | 13.23 |
| 5 | 0.35 | Co-Culture | 12.30 |
| 14 | 0.35 | Co-Culture | 10.30 |
| 11 | 0.525 | Co-Culture | 11.21 |
| 12 | 0.7 | Co-Culture | 9.79 |
| 15 | 0.7 | Co-Culture | 7.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doctolero, J.Z.S.; Beltran, A.B.; Uba, M.O.; Tigue, A.A.S.; Promentilla, M.A.B. Self-Healing Biogeopolymers Using Biochar-Immobilized Spores of Pure- and Co-Cultures of Bacteria. Minerals 2020, 10, 1114. https://doi.org/10.3390/min10121114
Doctolero JZS, Beltran AB, Uba MO, Tigue AAS, Promentilla MAB. Self-Healing Biogeopolymers Using Biochar-Immobilized Spores of Pure- and Co-Cultures of Bacteria. Minerals. 2020; 10(12):1114. https://doi.org/10.3390/min10121114
Chicago/Turabian StyleDoctolero, Jadin Zam S., Arnel B. Beltran, Marigold O. Uba, April Anne S. Tigue, and Michael Angelo B. Promentilla. 2020. "Self-Healing Biogeopolymers Using Biochar-Immobilized Spores of Pure- and Co-Cultures of Bacteria" Minerals 10, no. 12: 1114. https://doi.org/10.3390/min10121114
APA StyleDoctolero, J. Z. S., Beltran, A. B., Uba, M. O., Tigue, A. A. S., & Promentilla, M. A. B. (2020). Self-Healing Biogeopolymers Using Biochar-Immobilized Spores of Pure- and Co-Cultures of Bacteria. Minerals, 10(12), 1114. https://doi.org/10.3390/min10121114