Effects of Hydraulic Gradient and Clay Type on Permeability of Clay Mineral Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample of Clay Mineral Materials
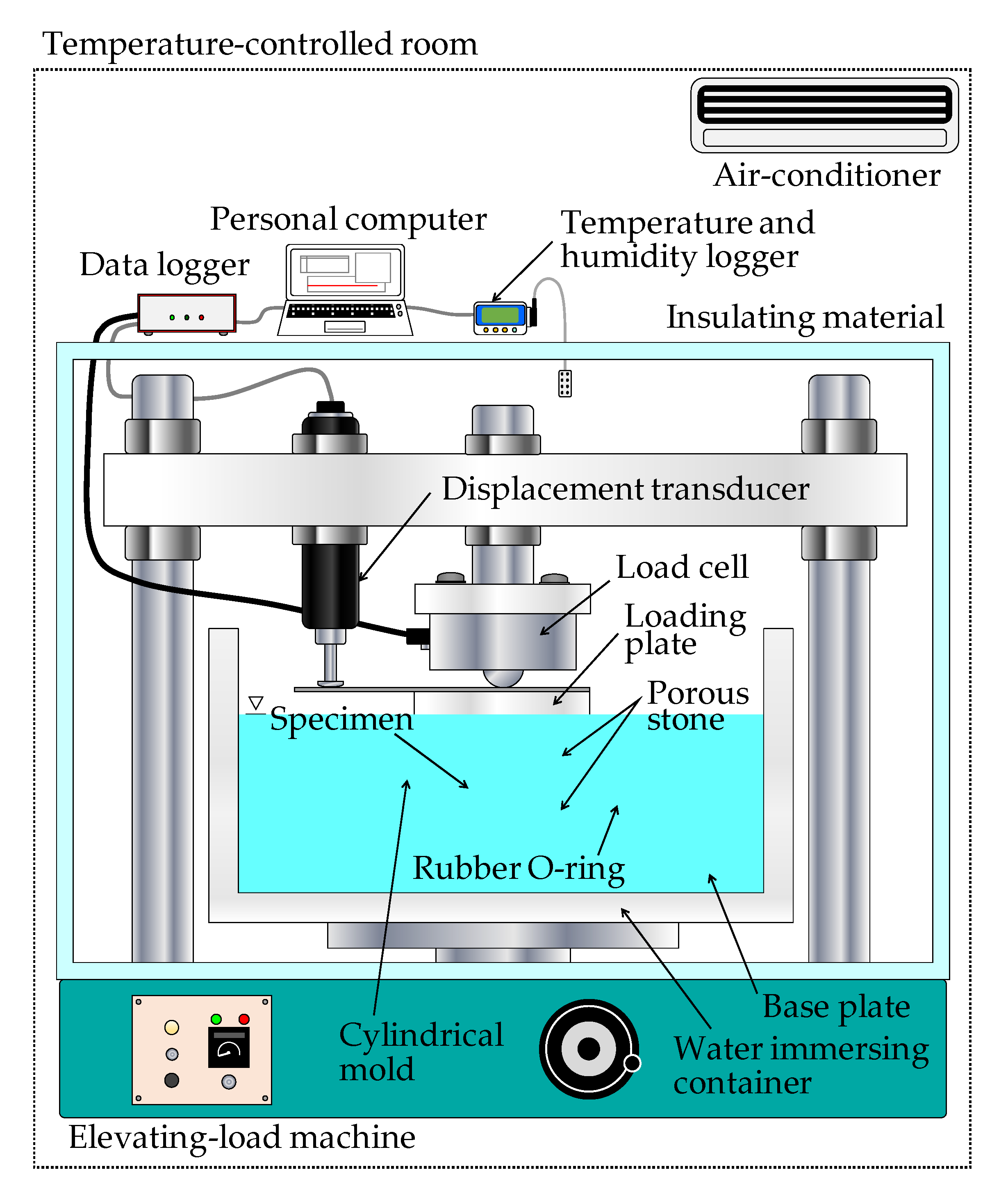
2.2. Experimental Procedure
2.2.1. One-Dimensional Swelling-Pressure Test
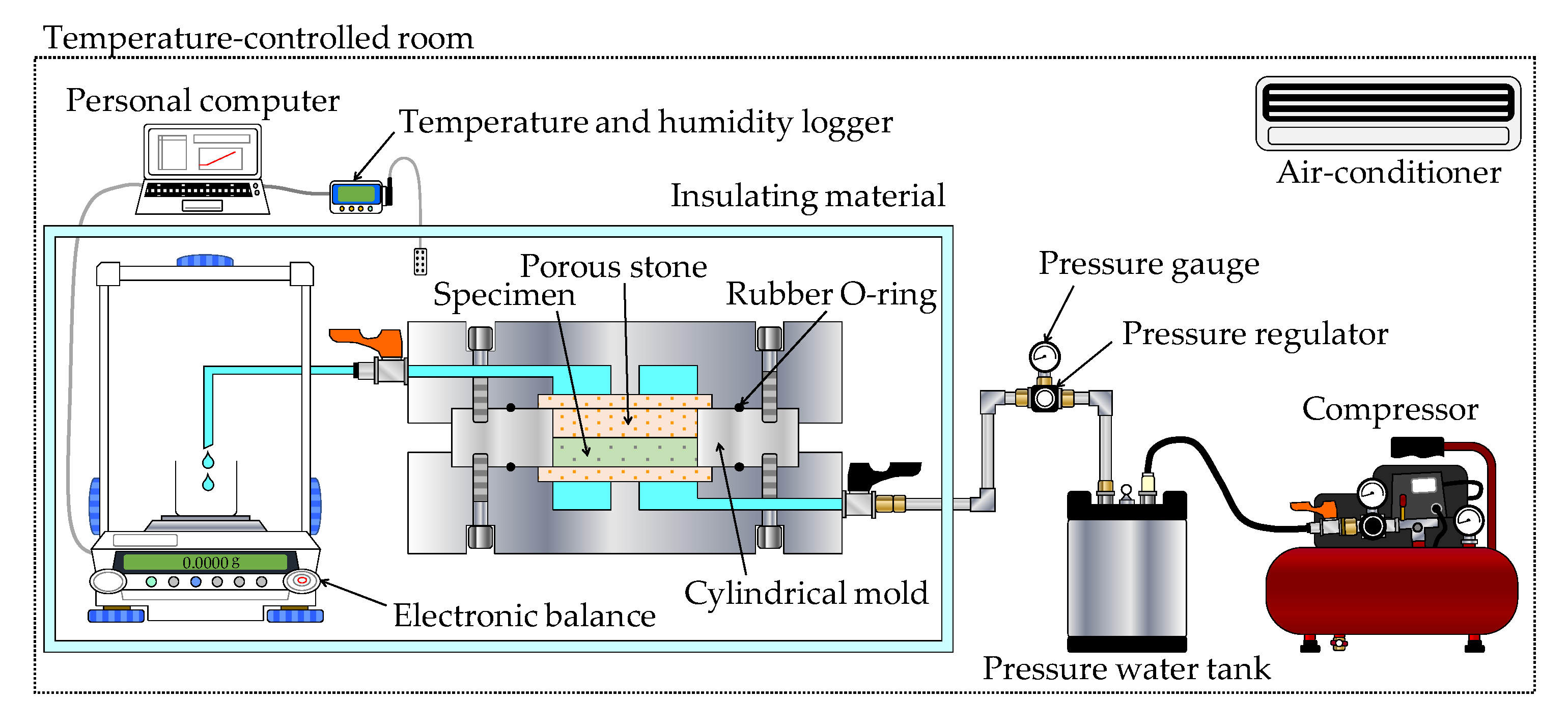
2.2.2. Constant Pressure Permeability Test
3. Results and Discussion
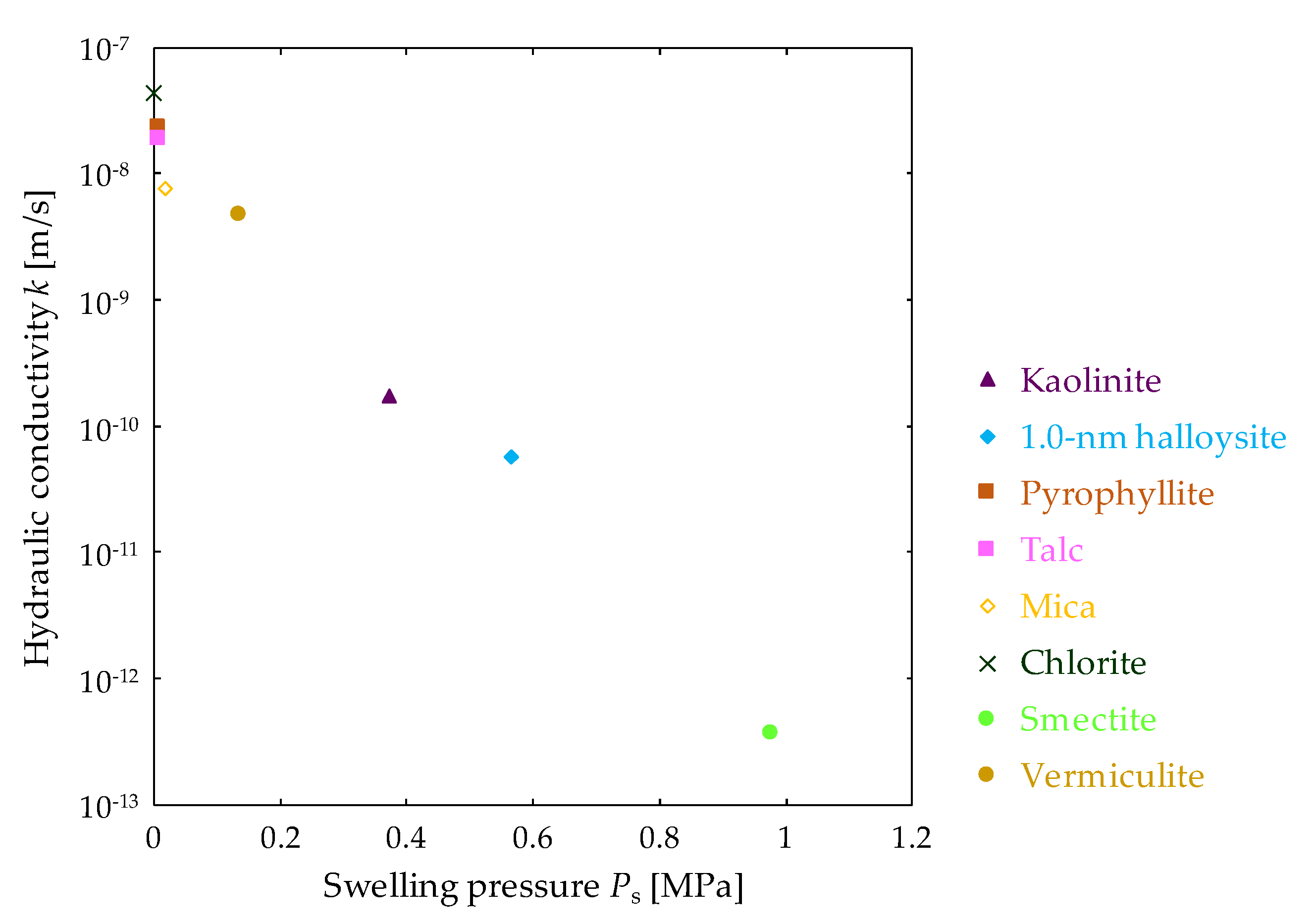
3.1. Swelling Pressure and Hydraulic Conductivity of Clay Mineral Materials
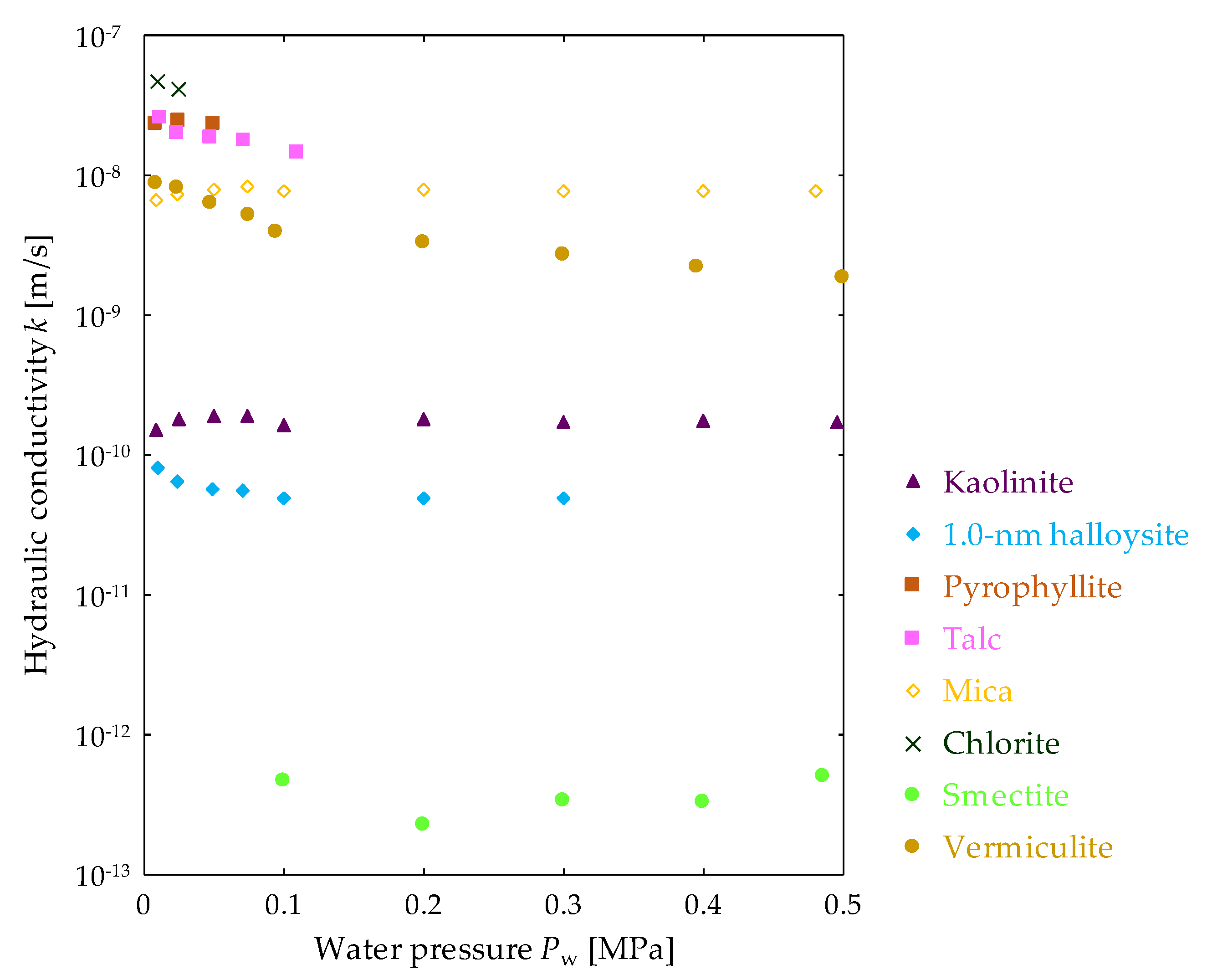
3.2. Effect of Water Pressure on Hydraulic Conductivity of Clay Mineral Materials
3.3. Estimation of Hydraulic Conductivity Using Kozeny–Carman Equation
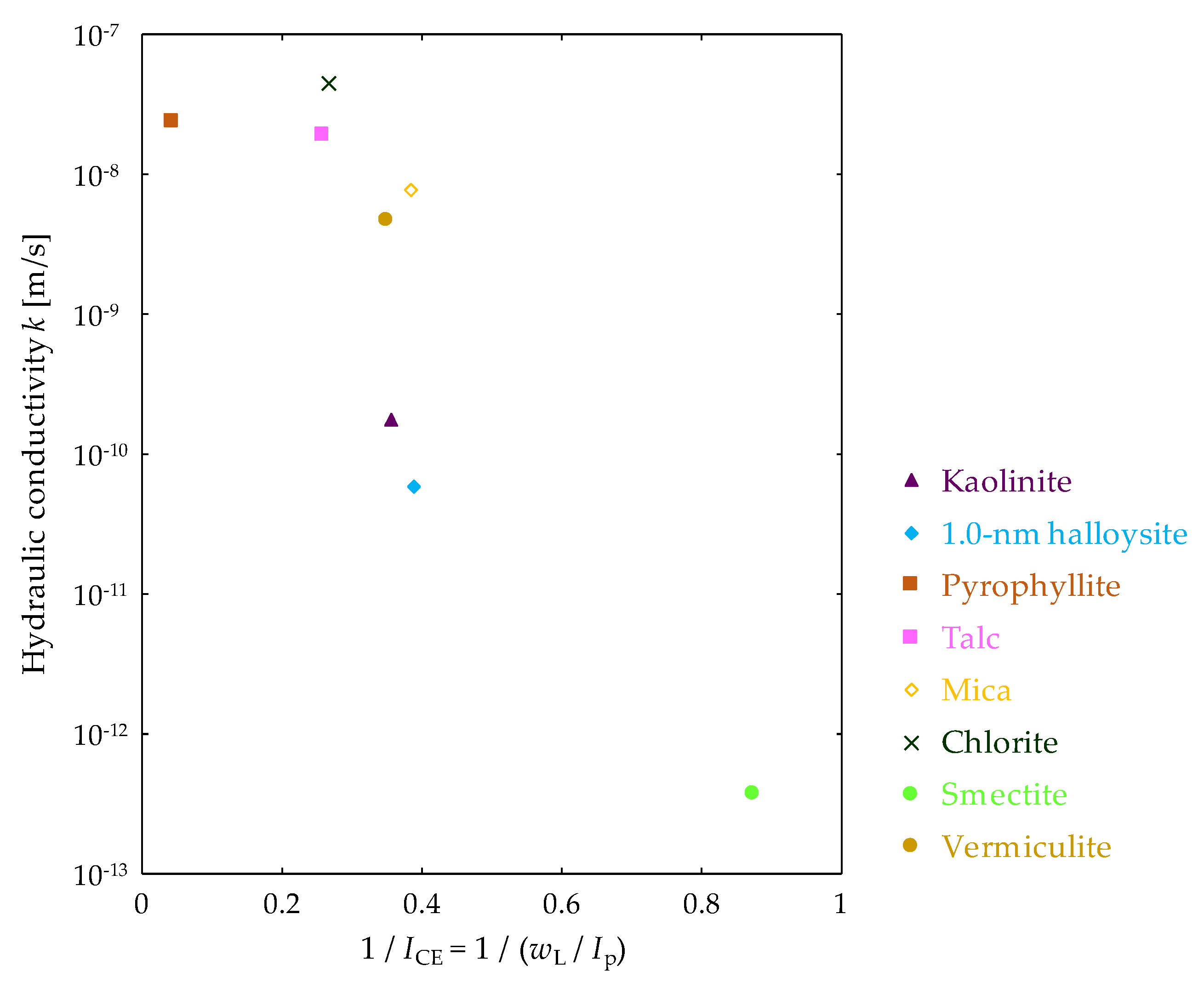
3.4. Relationship between Hydrogen Energy and Hydraulic Conductivity of Clay Mineral Materials
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Velde, B. Introduction to Clay Minerals: Chemistry, Origins, Uses and Environmental Significance; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1992; pp. 1–198. [Google Scholar]
- The Clay Science Society of Japan. Handbook of Clays and Clay Minerals, 3rd ed.; Gihodo Shuppan: Tokyo, Japan, 2009; pp. 1–990. (In Japanese) [Google Scholar]
- Bergaya, F.; Lagaly, G. (Eds.) Developments in Clay Science—Volume 5A, Handbook of Clay Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–874. [Google Scholar]
- Ishida, R.; Tsutsumi, S. Physical properties of smectite bearing soft rocks (Part 2): Texture and swelling pressure. J. Clay Sci. Soc. Jpn. 1994, 34, 22–34. (In Japanese) [Google Scholar]
- Kohno, M.; Maeda, H. Relationship between cylinder (longitudinal) point load strength and uniaxial compression strength for smectite-bearing fine tuffs in a soft and semi-hard rock boundary area: Example of the Upper Miocene Ikutawara Formation from the Ikutahara-Minami landslide area. J. Jpn. Landslide Soc. 2010, 47, 17–25. (In Japanese) [Google Scholar]
- Kohno, M.; Maeda, H.; Kage, I.; Kotake, J.; Nii, M. Method of axial point load strength test for smectite-bearing rocks. JSCE J. Geotech. Geoenviron. Eng. (Doboku Gakkai Ronbunshuu C) 2010, 66, 859–868. (In Japanese) [Google Scholar] [CrossRef]
- Kohno, M.; Maeda, H. Relationship between point load strength index and uniaxial compressive strength of hydrothermally altered rocks. Int. J. Rock Mech. Min. Sci. 2012, 50, 147–157. [Google Scholar] [CrossRef]
- Kahraman, S.; Aloglu, A.S.; Aydin, B.; Saygin, E. The effect of clay content on the strength of clay-bearing rocks. In Proceedings of the 9th Asian Rock Mechanics Symposium, Bali, Indonesia, 18–20 October 2016. [Google Scholar]
- Kohno, M.; Maeda, H. Estimate of uniaxial compressive strength of hydrothermally altered soft rocks based on strength index tests. Geomaterials 2018, 8, 147–157. [Google Scholar] [CrossRef][Green Version]
- Kohno, M.; Maeda, H. Correlations between point load strength index and physical properties of hydrothermally altered rocks. Int. J. Min. Eng. Miner. Process. 2018, 7, 1–13. [Google Scholar]
- Kohno, M.; Nishimura, T. Effect of clay mineral type on the physical and mechanical properties of rock materials. In Proceedings of the 2019 ISRM Specialized Conference, the 5th ISRM Young Scholars’ Symposium on Rock Mechanics and International Symposium on Rock Engineering for Innovative Future (YSRM2019 & REIF2019), Okinawa, Japan, 1–4 December 2019. [Google Scholar]
- Komine, H.; Ogata, N. Experimental study on swelling characteristics of compacted bentonite. Can. Geotech. J. 1994, 31, 478–490. [Google Scholar] [CrossRef]
- Mollins, L.H.; Stewart, D.I.; Cousens, T.W. Predicting the properties of bentonite–sand mixtures. Clay Miner. 1996, 31, 243–252. [Google Scholar] [CrossRef]
- Komine, H.; Ogata, N. Experimental study on swelling characteristics of sand–bentonite mixture for nuclear waste disposal. Soils Found. 1999, 39, 83–97. [Google Scholar] [CrossRef]
- Cho, W.J.; Lee, J.O.; Chun, K.S. The temperature effects on hydraulic conductivity of compacted bentonite. Appl. Clay Sci. 1999, 14, 47–58. [Google Scholar] [CrossRef]
- Sivapullaiah, P.V.; Sridharan, A.; Stalin, V.K. Hydraulic conductivity of bentonite–sand mixtures. Can. Geotech. J. 2000, 37, 406–413. [Google Scholar] [CrossRef]
- Komine, H.; Ohashi, R.; Yasuhara, K.; Murakami, S. Thermal influences on swelling-pressure and swelling-deformation of bentonites and its factors. JSCE J. Geotech. Geoenviron. Eng. (Doboku Gakkai Ronbunshuu C) 2007, 63, 731–741. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Shirazi, S.M.; Kazama, H.; Salman, F.A.; Othman, F.; Akib, S. Permeability and swelling characteristics of bentonite. Int. J. Phys. Sci. 2010, 5, 1647–1659. [Google Scholar]
- Suzuki, K.; Asano, H.; Yahagi, R.; Kobayashi, I.; Sellin, P.; Svemar, C.; Holmqvist, M. Experimental investigations of piping phenomena in bentonite-based buffer materials for an HLW repository. Clay Miner. 2013, 48, 363–382. [Google Scholar] [CrossRef]
- Okubo, S.; Takahashi, K.; Nakashima, H. The relationship between the expandability of clay mineral and the permeability of Takinoue pyroclastic layer “T1 member” in Yufutsu Oil and Gas Field, Hokkaido, Japan. J. Jpn. Assoc. Pet. Technol. 2011, 76, 209–218. [Google Scholar] [CrossRef][Green Version]
- Pusch, R. Is Montmorillonite-Rich Clay of MX-80 Type the Ideal Buffer for Isolation of HLW? SKB Technical Report SKB-TR-99-33; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 1999; pp. 1–67.
- Sellin, P.; Leupin, O.X. The use of clay as an engineered barrier in radioactive-waste management a review. Clays Clay Miner. 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Kato, M.; Nara, Y.; Okazaki, Y.; Kohno, M.; Sato, T.; Sato, T.; Takahashi, M. Application of the transient pulse method to measure clay permeability. Mater. Trans. 2018, 59, 1427–1432. [Google Scholar] [CrossRef]
- Kohno, M.; Nara, Y.; Kato, M.; Nishimura, T. Effects of clay-mineral type and content on the hydraulic conductivity of bentonite-sand mixtures made of Kunigel bentonite from Japan. Clay Miner. 2018, 53, 721–732. [Google Scholar] [CrossRef]
- Kitagawa, R.; Nishido, H. Orientation analysis and formation ages of fractures filled with clay minerals (clay veins) in Hiroshima and Shimane Prefectures, southwest Japan. J. Jpn. Soc. Eng. Geol. 1994, 35, 60–68. [Google Scholar] [CrossRef]
- Ikari, M.J.; Saffer, D.M.; Marone, C. Frictional and hydrologic properties of clay-rich fault gouge. J. Geophys. Res. 2009, 114, B05409. [Google Scholar] [CrossRef]
- Nishimoto, S.; Yoshida, H. Hydrothermal alteration of deep fractured granite: Effects of dissolution and precipitation. Lithos 2010, 115, 153–162. [Google Scholar] [CrossRef]
- Maeda, H.; Fukushima, Y.; Naya, H.; Yamamoto, Y.; Kohno, M. A palygorskite vein found in conglomerate of the Upper Eocene-Lower Origocene Rikubetsu Formatiion in northeastern Hokkaido, Japan. Clay Sci. 2012, 16, 15–18. [Google Scholar]
- Maeda, H.; Kohno, M. An interstratified illite/smectite mineral vein found in chaotic rock from the Nikoro Group in the accretionary Tokoro Belt, northeastern Hokkaido, Japan. Clay Sci. 2015, 19, 5–9. [Google Scholar]
- Ishibashi, M.; Yoshida, H.; Sasao, E.; Yuguchi, T. Long term behavior of hydrogeological structures associated with faulting: An example from the deep crystalline rock in the Mizunami URL, Central Japan. Eng. Geol. 2016, 208, 114–127. [Google Scholar] [CrossRef]
- Niwa, M.; Shimada, K.; Tamura, H.; Shibata, K.; Sueoka, S.; Yasue, K.; Ishimaru, T.; Umeda, K. Thermal constraints on clay growth in fault gouge and their relationship with fault-zone evolution and hydrothermal alteration: Case study of gouges in the Kojaku granite, central Japan. Clays Clay Miner. 2016, 64, 86–107. [Google Scholar] [CrossRef]
- Ueki, T.; Tanabe, H.; Niwa, M.; Ishimaru, T.; Shimada, K. Observed and Analyzed Data for Clay Veins in Granite; Japan Atomic Energy Agency Report JAEA-Data/Code 2016-010; Japan Atomic Energy Agency: Ibaraki, Japan, 2016; pp. 1–292. (In Japanese) [Google Scholar]
- Maciel, I.B.; Dettori, A.; Balsamo, F.; Bezerra, F.H.R.; Vieira, M.M.; Nogueira, F.C.C.; Salvioli-Mariani, E.; Sousa, J.A.B. Structural control on clay mineral authigenesis in faulted arkosic sandstone of the Rio do Peixe basin, Brazil. Minerals 2018, 8, 408. [Google Scholar] [CrossRef]
- Nara, Y.; Kato, M.; Niri, R.; Kohno, M.; Sato, T.; Fukuda, D.; Sato, T.; Takahashi, M. Permeability of granite including macro-fracture naturally filled with fine-grained minerals. Pure Appl. Geophys. 2018, 175, 917–927. [Google Scholar] [CrossRef]
- Warr, L.N.; Cox, S. Clay mineral transformations and weakening mechanisms along the Alpine Fault, New Zealand. Geol. Soc. Lond. Spec. Publ. 2001, 186, 85–101. [Google Scholar] [CrossRef]
- Haines, S.H.; van der Pluijm, B.A.; Ikari, M.J.; Saffer, D.M.; Marone, C. Clay fabric intensity in natural and artificial fault gouges: Implications for brittle fault zone processes and sedimentary basin clay fabric evolution. J. Geophys. Res. 2009, 114, B05406. [Google Scholar] [CrossRef]
- Haines, S.H.; van der Pluijm, B.A. Patterns of mineral transformations in clay gouge, with examples from low-angle normal fault rocks in the western USA. J. Struct. Geol. 2012, 43, 2–32. [Google Scholar] [CrossRef]
- Haines, S.H.; Kaproth, B.; Marone, C.; Saffer, D.; van der Pluijm, B. Shear zones in clay-rich fault gouge: A laboratory study of fabric development and evolution. J. Struct. Geol. 2013, 51, 206–225. [Google Scholar] [CrossRef]
- Laurich, B.; Urai, J.L.; Desbois, G.; Vollmer, C.; Nussbaum, C. Microstructural evolution of an incipient fault zone in Opalinus Clay: Insights from an optical and electron microscopic study of ion-beam polished samples from the Main Fault in the Mt-Terri Underground Research Laboratory. Solid Earth 2014, 67, 107–128. [Google Scholar] [CrossRef]
- Wang, G.; Mitchell, T.M.; Meredith, P.G.; Nara, Y.; Wu, Z. Influence of gouge thickness on permeability of macro-fractured basalt. J. Geophys. Res. 2016, 121, 8472–8487. [Google Scholar] [CrossRef]
- Laurich, B.; Urai, J.L.; Vollmer, C.; Nussbaum, C. Deformation mechanisms and evolution of the microstructure of gouge in the Main Fault in Opalinus Clay in the Mont Terri rock laboratory (CH). J. Struct. Geol. 2018, 9, 1–24. [Google Scholar] [CrossRef]
- Pérez-Flores, P.; Wang, G.; Mitchell, T.M.; Meredith, P.G.; Nara, Y.; Sarkar, V.; Cembrano, J. The effect of offset on fracture permeability of rocks from the Southern Andes Volcanic Zone, Chile. J. Struct. Geol. 2017, 104, 142–158. [Google Scholar] [CrossRef]
- Pusch, R. Bentonite Clay: Environmental Properties and Applications; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–334. [Google Scholar]
- Hasibo, S. Influence of mobile particles in spft clay on permeability. In Proceedings of the International Symposium of soil structure, Gothenburg, Sweden, 1–2 August 1973; pp. 132–135. [Google Scholar]
- Kozeny, J. Ueber kapillare Leitung des Wassers im Boden. Sitzungsber. Akad. Wiss. Wien 1927, 136, 271–306. [Google Scholar]
- Carman, P.C. Fluid flow through a granular bed. Trans. Inst. Chem. Eng. 1937, 15, 150–156. [Google Scholar] [CrossRef]
- Shirozu, H. Clay Mineralogy: The Basis of Clay Science, New ed.; Asakura Publishing: Tokyo, Japan, 2012; pp. 1–185. (In Japanese) [Google Scholar]
- The Japanese Geotechnical Society. Test Method for liquid limit and plastic limit of soils (JGS 0141-2009). In Japanese Geotechnical Society Standards: Laboratory Testing Standards of Geomaterials; Maruzen Publishing: Tokyo, Japan, 2015; Volume 1. [Google Scholar]
- Malfoy, C.; Besq, A.; Pantet, A.; Monnet, P. The physico-chemical and rheological characterizations of two industrial bentonites, 2001 A Clay Odyssey. In Proceedings of the 12th International Clay Conference, Bahia Blanca, Argentina, 22–28 July 2001; pp. 285–292. [Google Scholar]
- Komine, H.; Oyamada, T.; Ozaki, T.; Iso, S. Discussion on water migration and swelling of compacted powder bentonites. J JSCE Ser. C (Geosph. Eng.) 2018, 74, 63–75. (In Japanese) [Google Scholar] [CrossRef]
- Jacinto, A.C.; Villar, M.V.; Ledesma, A. No AccessInfluence of water density on the water-retention curve of expansive clays. Geotechnique 2012, 62, 657–667. [Google Scholar] [CrossRef]
- Martin, R.T. Adsorbed water on clay: A review. Clays Clay Miner. 1960, 9, 28–70. [Google Scholar] [CrossRef]
- Komine, H. Inductive consideration on water density between montmorillonite mineral layers on the basis of previous one-dimensional self-sealing experiments. In Proceedings of the 48th Japan National Conference on Geotechnical Engineering, Toyama, Japan, 23–25 July 2013; pp. 459–460. (In Japanese). [Google Scholar]
- Terzaghi, C.H. New facts about surface-friction. Phys. Rev. 1920, 16, 54–61. [Google Scholar] [CrossRef]
- Achari, G.; Joshi, R.C.; Bentley, L.R.; Chatterji, S. Prediction of the hydraulic conductivity of clays using the electric double layer theory. Can. Geotech. J. 1999, 36, 783–792. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 1–592. [Google Scholar]
- Tanai, K.; Kikuchi, H.; Nakamura, K.; Tanaka, Y.; Hironaga, M. Survey on Current Status of Laboratory Test Method and Experimental Consideration for Establishing Standardized Procedure of Material Containing Bentonite—Report of Collaboration Research Between JAEA and CRIEPI- (Joint Research); Japan Atomic Energy Agency Report JAEA-Research 2010-025; Japan Atomic Energy Agency: Ibaraki, Japan, 2010; pp. 1–186. (In Japanese)
- Nakamura, K.; Tanaka, Y.; Hironaga, M. Survey on Current Status of Laboratory Test Method and Experimental Consideration for Material Containing Bentonite; CRIEPI Research Report N10026; CRIEPI: Tokyo, Japan, 2011; pp. 1–37. (In Japanese) [Google Scholar]
- Pusch, R. The Buffer and Backfill Handbook—Part 1: Definitions, Basic Relationships, and Laboratory Methods; SKB Technical Report SKB-TR-02-20; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2002; pp. 1–263. [Google Scholar]
- Dixon, J.B.; Weed, S.B. (Eds.) Minerals in Soil Environments, 2nd ed.; Soil Science Society of America: Madison, WI, USA, 1989; pp. 635–674. [Google Scholar]
- Ren, X.; Zhao, Y.; Deng, Q.; Kang, J.; Li, D.; Wang, D. A relation of hydraulic conductivity-void ratio for soils based on Kozeny-Carman equation. Eng. Geol. 2016, 213, 89–97. [Google Scholar] [CrossRef]
- Kobayashi, I.; Owada, H.; Ishii, T.; Iizuka, A. Evaluation of specific surface area of bentonite-engineered barriers for Kozeny-Carman law. Soils Found. 2017, 57, 683–697. [Google Scholar] [CrossRef]
- Mizuno, K.; Endo, K.; Hongo, T.; Nishigaki, M.; Kamon, M. Evaluation of impermeability for clayey mixture soil using consistency limits. In Proceedings of the 39th Japan National Conference on Geotechnical Engineering, Niigata, Japan, 7–9 July 2004; pp. 303–304. (In Japanese). [Google Scholar]

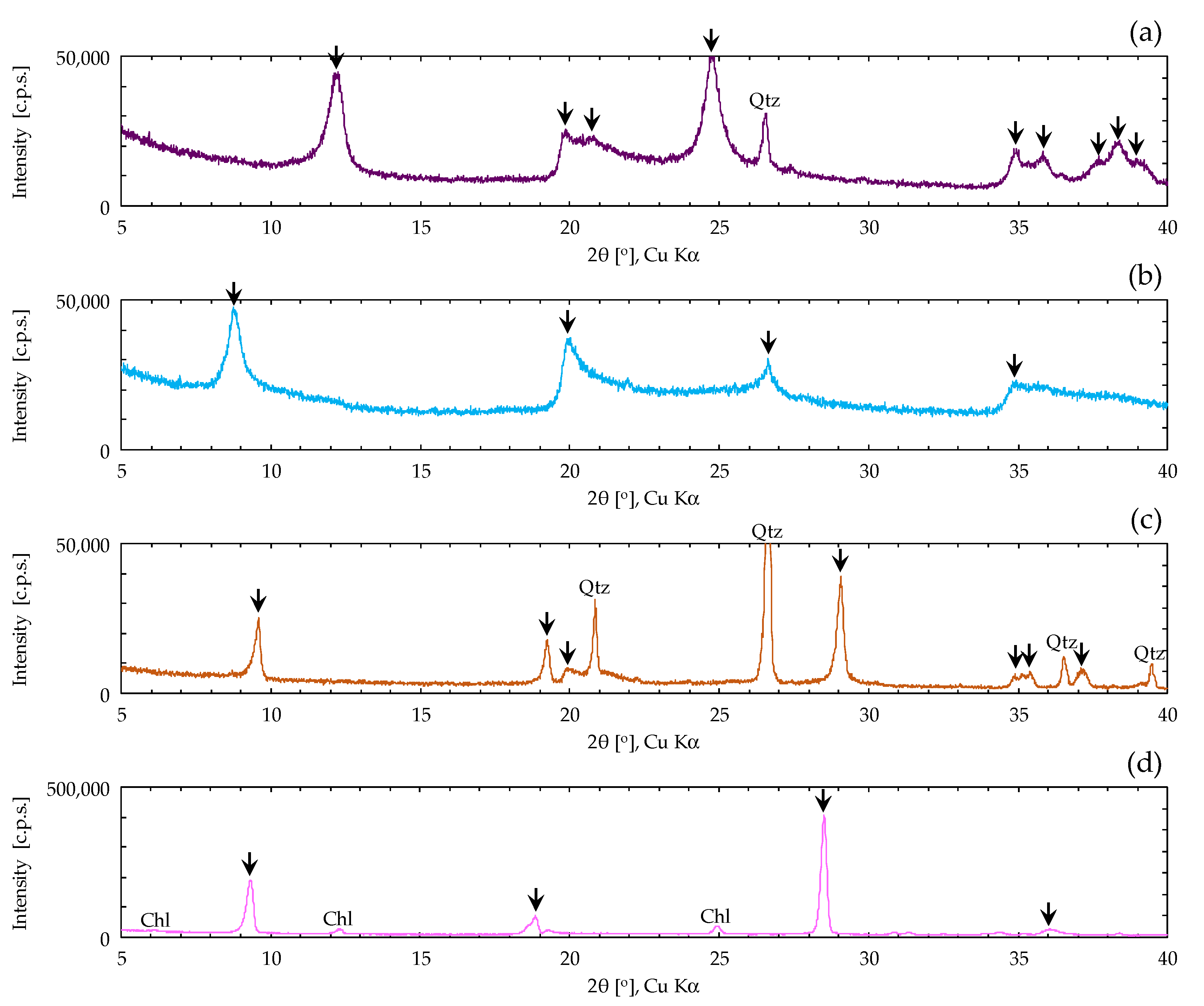
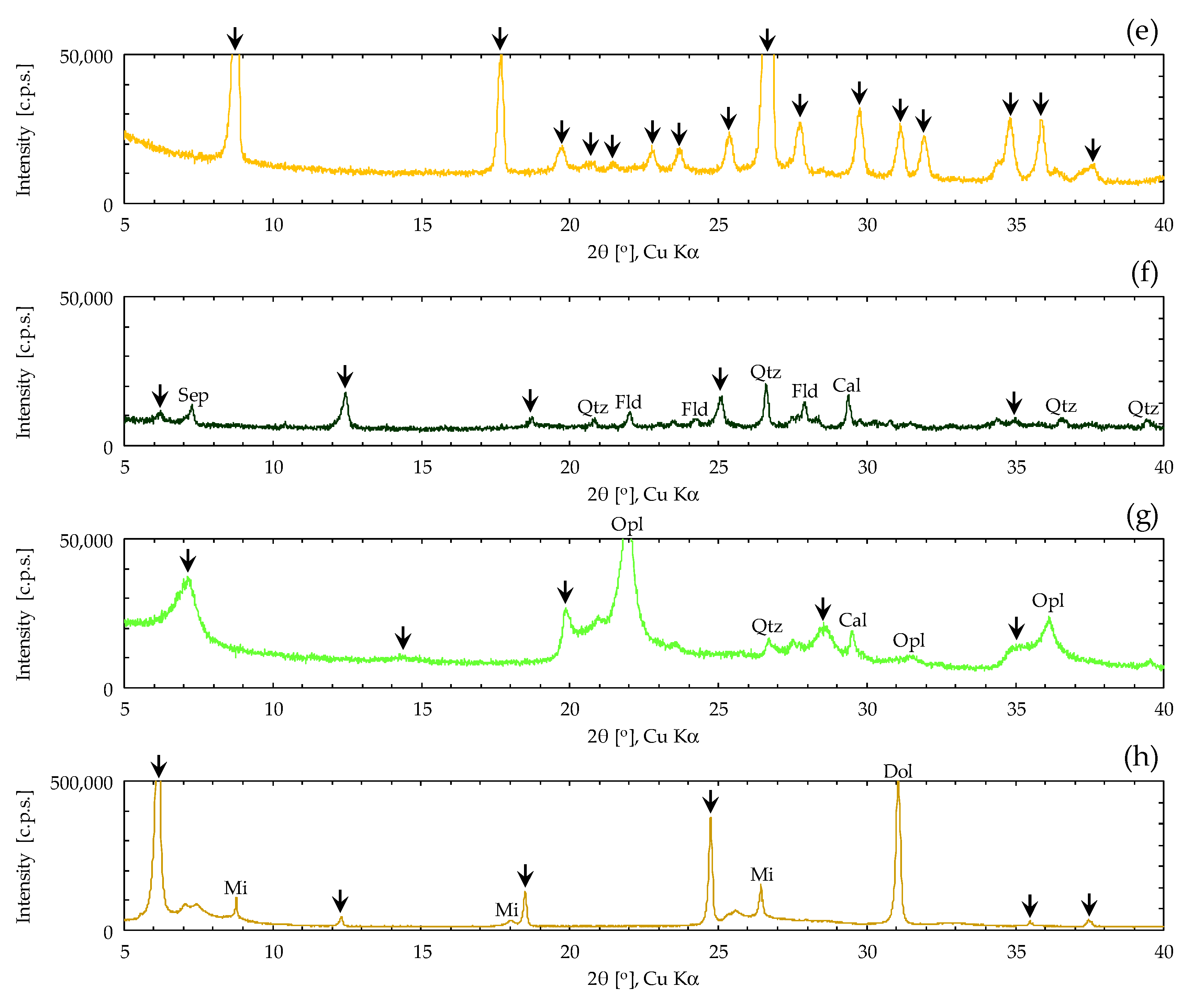
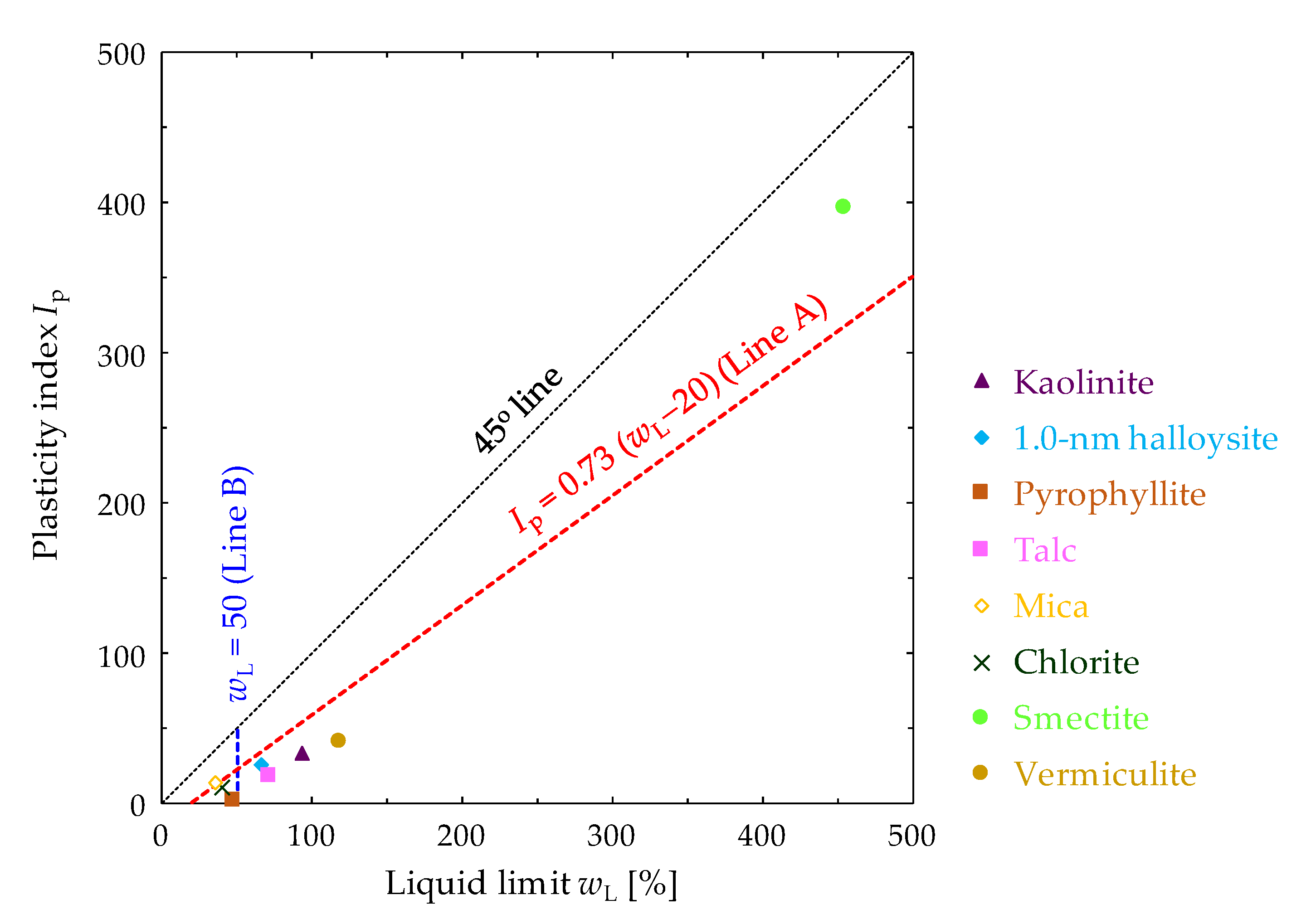



| Clay Mineral Samples | ρs [g/cm3] | Sm [m2/g] | wL [%] | wp [%] | Ip |
|---|---|---|---|---|---|
| Kaolinite | 2.577 | 40.42 | 92.69 | 59.77 | 32.92 |
| 1.0 nm halloysite | 2.736 | 86.55 | 66.28 | 40.50 | 25.79 |
| Pyrophyllite | 2.718 | 8.1 | 47.60 | 45.54 | 2.06 |
| Talc | 2.786 | 4.24 | 71.25 | 52.82 | 18.42 |
| Mica | 2.904 | 4.85 | 35.98 | 22.17 | 13.81 |
| Chlorite | 2.841 | 5.2 | 39.59 | 29.05 | 10.54 |
| Smectite | 2.648 | 594.27 [49] | 455.02 | 58.09 | 396.93 |
| Vermiculite | 2.663 | 7.73 | 118.35 | 77.04 | 41.32 |
| Clay Mineral Samples | Ps [MPa] | k [m/s] |
|---|---|---|
| Kaolinite | 0.37 | 1.8 × 10−10 |
| 1.0 nm halloysite | 0.56 | 5.8 × 10−11 |
| Pyrophyllite | 0.01 | 2.4 × 10−8 |
| Talc | 0.01 | 1.9 × 10−8 |
| Mica | 0.02 | 7.7 × 10−9 |
| Chlorite | <0.002 | 4.5 × 10−8 |
| Smectite | 0.98 | 3.7 × 10−13 |
| Vermiculite | 0.13 | 4.7 × 10−9 |
| Clay-Mineral Samples | Water Pressure Pw Settings [MPa] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.025 | 0.05 | 0.075 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| Kaolinite | 1.5 × 10−10 | 1.8 × 10−10 | 1.9 × 10−10 | 1.9 × 10−10 | 1.7 × 10−10 | 1.8 × 10−10 | 1.7 × 10−10 | 1.8 × 10−10 | 1.7 × 10−10 |
| (0.009) | (0.025) | (0.05) | (0.074) | (0.1) | (0.2) | (0.3) | (0.4) | (0.495) | |
| 1.0-nm halloysite | 8.1 × 10−11 | 6.5 × 10−11 | 5.8 × 10−11 | 5.6 × 10−11 | 4.9 × 10−11 | 4.9 × 10−11 | 5.0 × 10−11 | W.P. | |
| (0.01) | (0.024) | (0.049) | (0.071) | (0.1) | (0.2) | (0.3) | (0.4) | ||
| Pyrophyllite | 2.3 × 10−8 | 2.5 × 10−8 | 2.0 × 10−8 | W.P. | |||||
| (0.009) | (0.025) | (0.05) | (0.075) | ||||||
| Talc | 2.6 × 10−8 | 2.0 × 10−8 | 1.9 × 10−8 | 1.8 × 10−8 | 1.4 × 10−8 | W.P. | |||
| (0.012) | (0.024) | (0.048) | (0.072) | (0.11) | (0.2) | ||||
| Mica | 6.7 × 10−9 | 7.3 × 10−9 | 8.0 × 10−9 | 8.4 × 10−9 | 7.7 × 10−9 | 7.9 × 10−9 | 7.8 × 10−9 | 7.6 × 10−9 | 7.7 × 10−9 |
| (0.009) | (0.024) | (0.05) | (0.074) | (0.1) | (0.2) | (0.3) | (0.4) | (0.48) | |
| Chlorite | 4.7 × 10−8 | 4.2 × 10−8 | W.P. | ||||||
| (0.01) | (0.025) | (0.05) | |||||||
| Smectite | - | - | - | - | 4.6 × 10−13 | 2.3 × 10−13 | 3.3 × 10−13 | 3.3 × 10−13 | 5.0 × 10−13 |
| - | - | - | - | (0.1) | (0.2) | (0.3) | (0.4) | (0.485) | |
| Vermiculite | 8.7 × 10−9 | 8.2 × 10−9 | 6.4 × 10−9 | 5.2 × 10−9 | 4.0 × 10−9 | 3.3 × 10−9 | 2.7 × 10−9 | 2.2 × 10−9 | 1.9 × 10−9 |
| (0.009) | (0.024) | (0.048) | (0.075) | (0.095) | (0.2) | (0.3) | (0.395) | (0.5) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohno, M. Effects of Hydraulic Gradient and Clay Type on Permeability of Clay Mineral Materials. Minerals 2020, 10, 1064. https://doi.org/10.3390/min10121064
Kohno M. Effects of Hydraulic Gradient and Clay Type on Permeability of Clay Mineral Materials. Minerals. 2020; 10(12):1064. https://doi.org/10.3390/min10121064
Chicago/Turabian StyleKohno, Masanori. 2020. "Effects of Hydraulic Gradient and Clay Type on Permeability of Clay Mineral Materials" Minerals 10, no. 12: 1064. https://doi.org/10.3390/min10121064
APA StyleKohno, M. (2020). Effects of Hydraulic Gradient and Clay Type on Permeability of Clay Mineral Materials. Minerals, 10(12), 1064. https://doi.org/10.3390/min10121064





