Chromium Isotope Systematics in Modern and Ancient Microbialites
Abstract
:1. Introduction
2. Materials and Methods
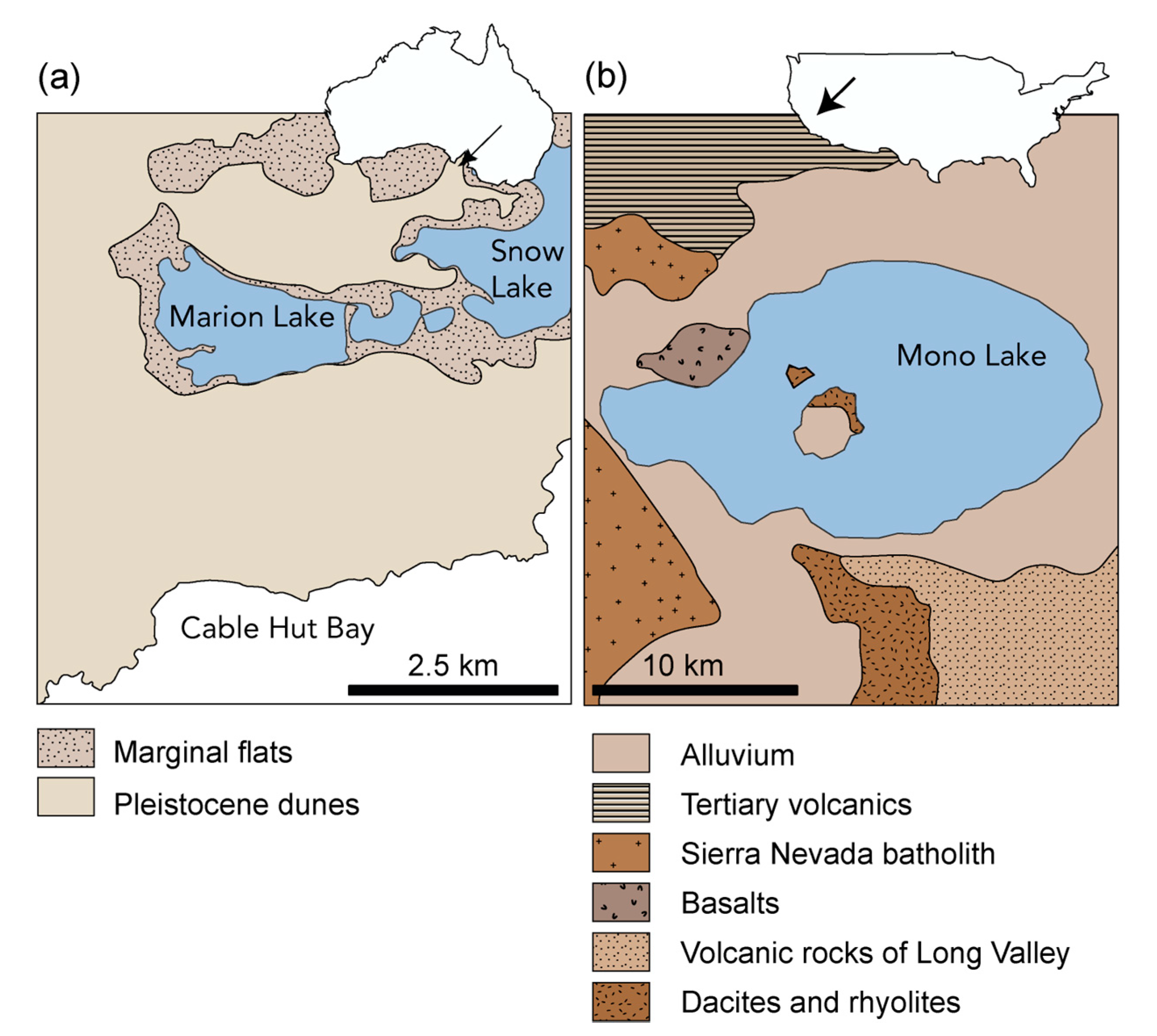
2.1. Sample Description
2.2. Sample Preparation
2.3. Chromium Isotope Analysis
2.4. Stable O and C Isotope Analysis
2.5. Elemental Concentrations and REY
3. Results
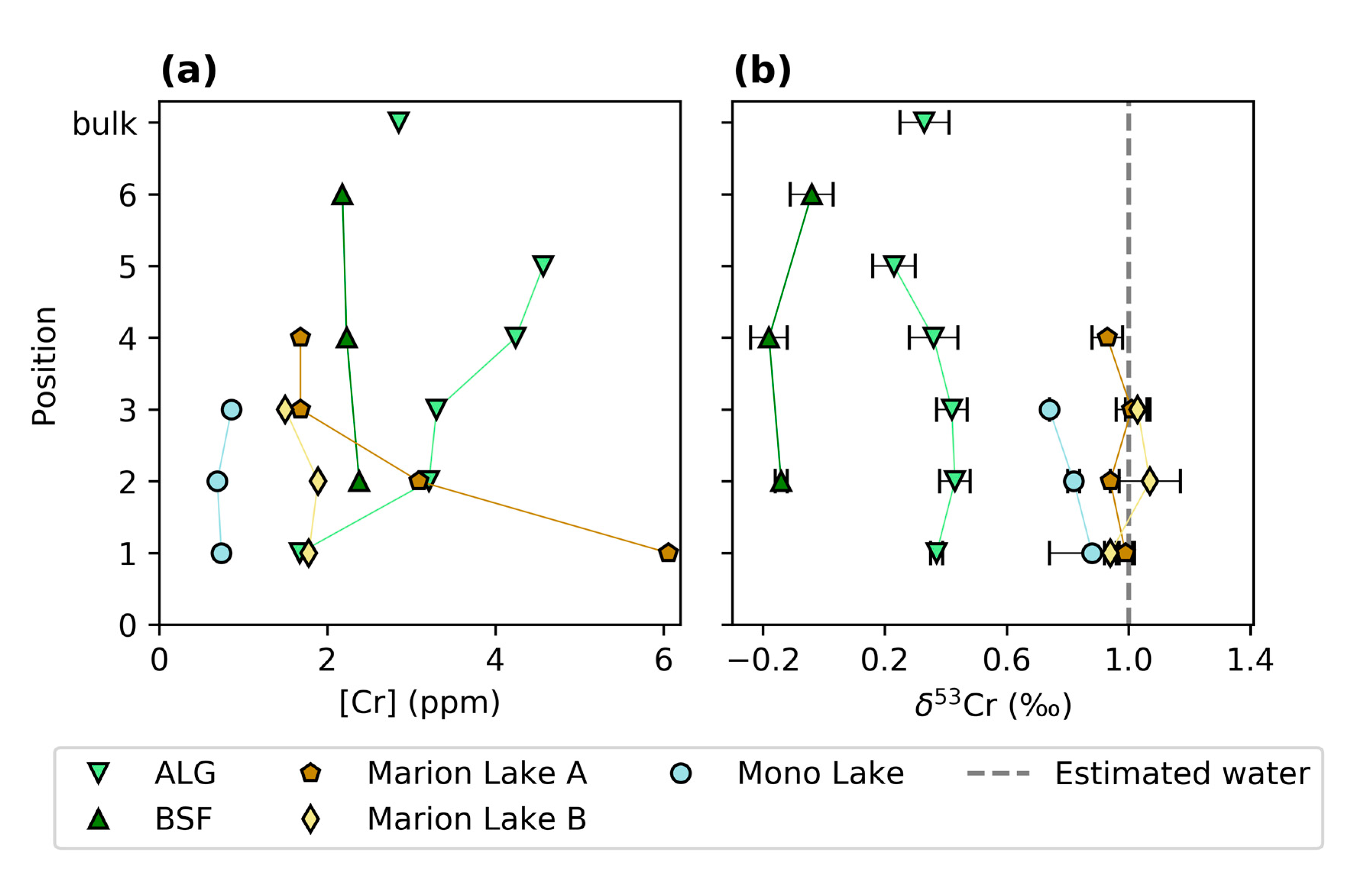
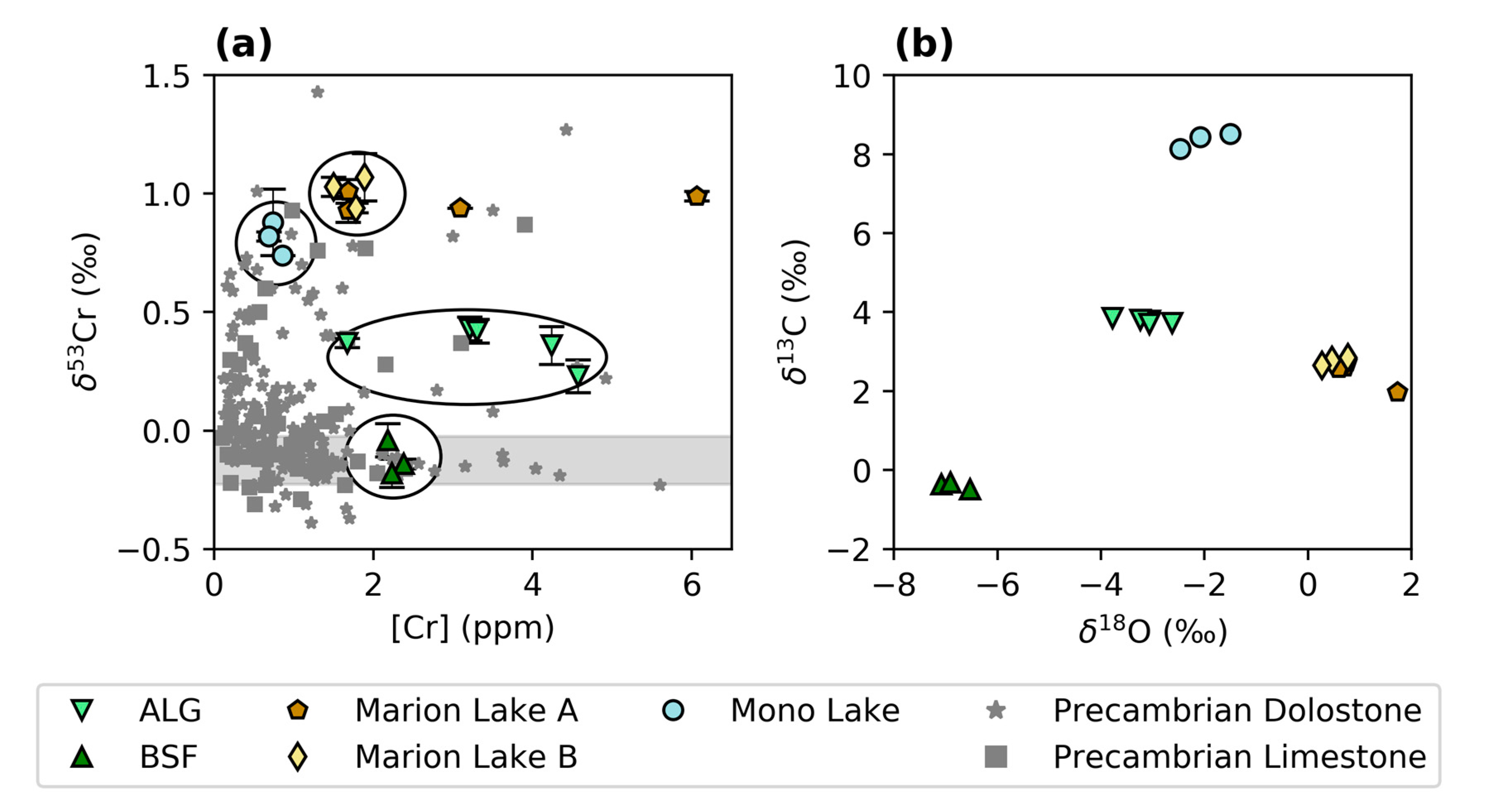
3.1. Chromium Concentrations and Isotope Compositions
3.2. Stable O and C Isotopes
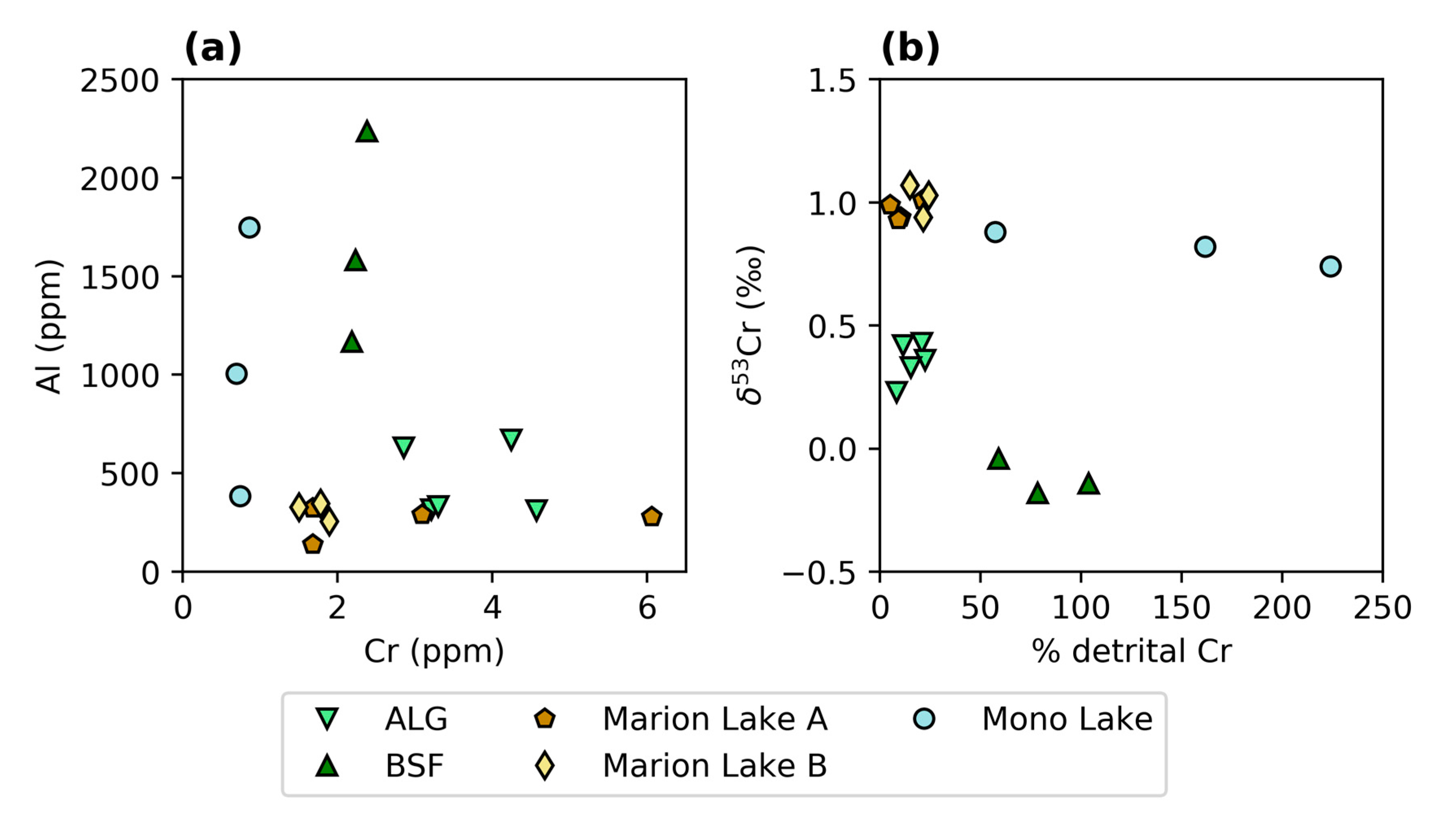
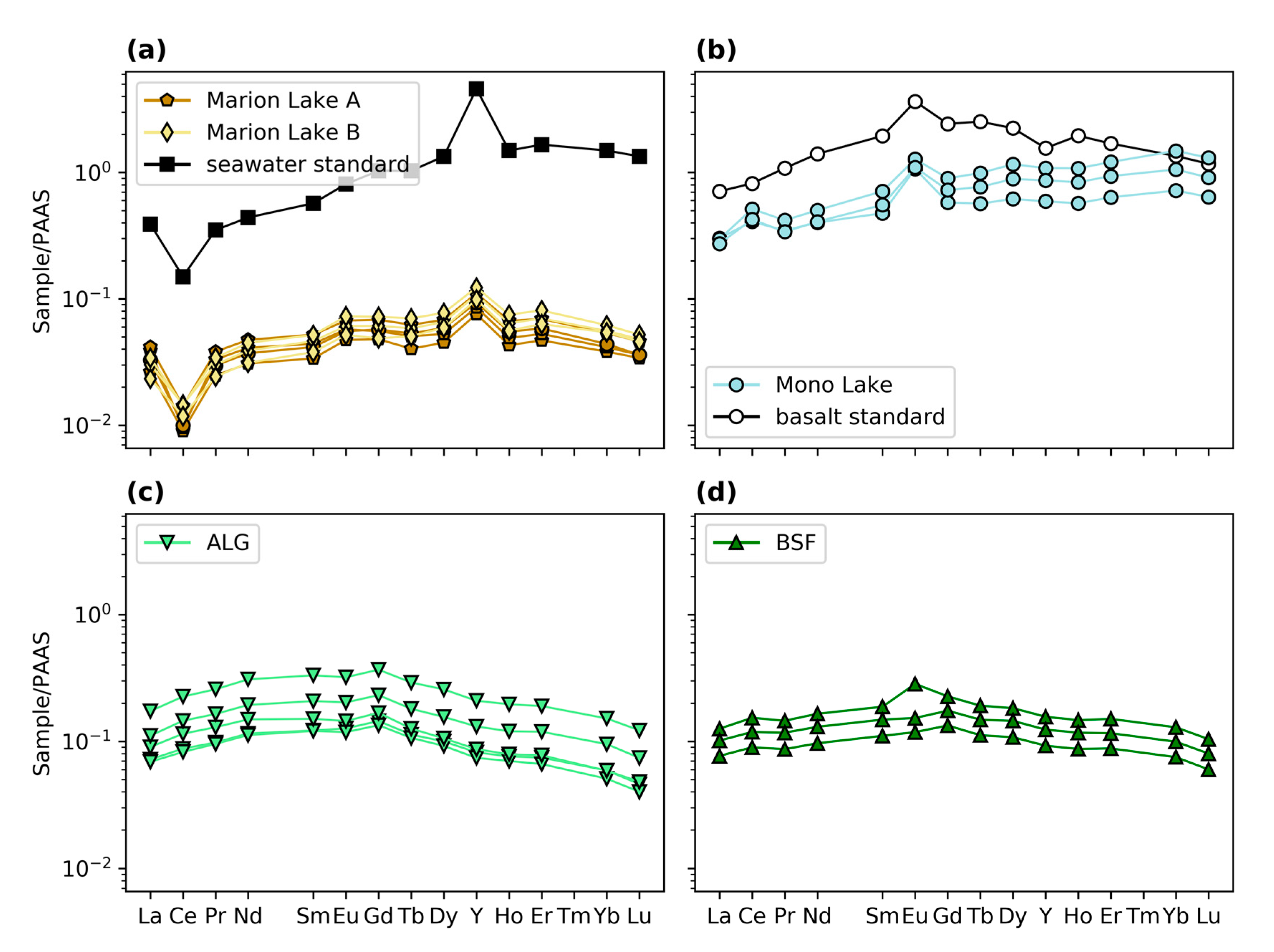
3.3. Elemental Concentrations and REY
4. Discussion
4.1. Modern Microbialites
4.1.1. Geochemistry of Modern Microbialites
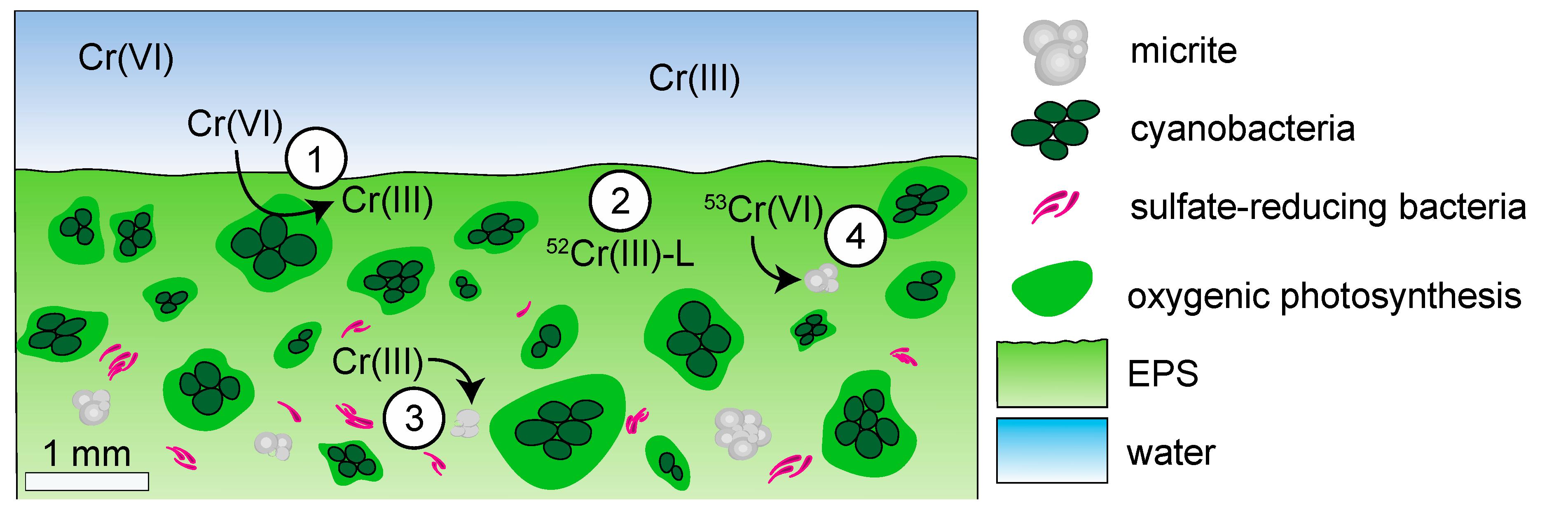
4.1.2. Biological Controls on δ53Cr Values in Modern Microbialites
4.1.3. Environmental Controls on δ53Cr Values in Modern Microbialites
4.1.4. Comparison of Cr in Modern Microbialites and Seawater
4.2. Ancient Microbialites
4.2.1. Diagenetic Alteration of Ancient Microbialites
4.2.2. Comparison of Modern and Ancient Microbialites
4.2.3. Biological and Environmental Controls on δ53Cr Values in Ancient Microbialites
4.2.4. Comparison of Abiotic and Microbial Carbonates from the Precambrian
5. Implications and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilleaudeau, G.J.; Voegelin, A.R.; Thibault, N.; Moreau, J.; Ullmann, C.V.; Klaebe, R.M.; Korte, C.; Frei, R. Stable isotope records across the Cretaceous–Paleogene transition, Stevns Klint, Denmark: New insights from the chromium isotope system. Geochim. Cosmochim. Acta 2018, 235, 305–332. [Google Scholar] [CrossRef]
- Crowe, S.A.; Døssing, L.N.; Beukes, N.J.; Bau, M.; Kruger, S.J.; Frei, R.; Canfield, D.E. Atmospheric oxygenation three billion years ago. Nature 2013, 501, 535–538. [Google Scholar] [CrossRef]
- Ellis, A.S.; Johnson, T.M.; Bullen, T.D. Chromium isotopes and the fate of hexavalent chromium in the environment. Science 2002, 295, 2060–2062. [Google Scholar] [CrossRef] [Green Version]
- Schauble, E.; Rossman, G.R.; Taylor, H.P. Theoretical estimates of equilibrium chromium-isotope fractionations. Chem. Geol. 2004, 205, 99–114. [Google Scholar] [CrossRef]
- Zink, S.; Schoenberg, R.; Staubwasser, M. Isotopic fractionation and reaction kinetics between Cr(III) and Cr(VI) in aqueous media. Geochim. Cosmochim. Acta 2010, 74, 5729–5745. [Google Scholar] [CrossRef]
- Basu, A.; Johnson, T.M.; Sanford, R.A. Cr isotope fractionation factors for Cr(VI) reduction by a metabolically diverse group of bacteria. Geochim. Cosmochim. Acta 2014, 142, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Sikora, E.R.; Johnson, T.M.; Bullen, T.D. Microbial mass-dependent fractionation of chromium isotopes. Geochim. Cosmochim. Acta 2008, 72, 3631–3641. [Google Scholar] [CrossRef]
- Tang, Y.; Elzinga, E.J.; Jae Lee, Y.; Reeder, R.J. Coprecipitation of chromate with calcite: Batch experiments and X-ray absorption spectroscopy. Geochim. Cosmochim. Acta 2007, 71, 1480–1493. [Google Scholar] [CrossRef]
- Rodler, A.S.; Sánchez-Pastor, N.; Fernández-Díaz, L.; Frei, R. Fractionation behavior of chromium isotopes during coprecipitation with calcium carbonate: Implications for their use as paleoclimatic proxy. Geochim. Cosmochim. Acta 2015, 164, 221–235. [Google Scholar] [CrossRef]
- Füger, A.; Bruggmann, S.; Frei, R.; Leis, A.; Dietzel, M.; Mavromatis, V. The role of pH on Cr(VI) partitioning and isotopic fractionation during its incorporation in calcite. Geochim. Cosmochim. Acta 2019, 265, 520–532. [Google Scholar] [CrossRef]
- Saad, E.M.; Wang, X.; Planavsky, N.J.; Reinhard, C.T.; Tang, Y. Redox-independent chromium isotope fractionation induced by ligand-promoted dissolution. Nat. Commun. 2017, 18, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraemer, D.; Frei, R.; Viehmann, S.; Bau, M. Applied Geochemistry Mobilization and isotope fractionation of chromium during water-rock interaction in presence of siderophores. Appl. Geochem. 2019, 102, 44–54. [Google Scholar] [CrossRef]
- Bonnand, P.; James, R.H.; Parkinson, I.J.; Connelly, D.P.; Fairchild, I.J. The chromium isotopic composition of seawater and marine carbonates. Earth Planet. Sci. Lett. 2013, 382, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Pereira, N.S.; Voegelin, A.R.; Paulukat, C.; Sial, A.N.; Ferreira, V.P.; Frei, R. Chromium-isotope signatures in scleractinian corals from the Rocas Atoll, Tropical South Atlantic. Geobiology 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Holmden, C.; Jacobson, A.D.; Sageman, B.B.; Hurtgen, M.T. Response of the Cr isotope proxy to Cretaceous Ocean Anoxic Event 2 in a pelagic carbonate succession from the Western Interior Seaway. Geochim. Cosmochim. Acta 2016, 186, 277–295. [Google Scholar] [CrossRef]
- Wang, X.; Planavsky, N.J.; Hull, P.M.; Tripati, A.E.; Zou, H.J.; Elder, L.; Henehan, M. Chromium isotopic composition of core-top planktonic foraminifera. Geobiology 2016, 1–14. [Google Scholar] [CrossRef]
- Farkaš, J.; Frýda, J.; Paulukat, C.; Hathorne, E.C.; Matoušková, Š.; Rohovec, J.; Frýdová, B.; Francová, M.; Frei, R. Chromium isotope fractionation between modern seawater and biogenic carbonates from the Great Barrier Reef, Australia: Implications for the paleo-seawater δ53Cr reconstruction. Earth Planet. Sci. Lett. 2018, 498, 140–151. [Google Scholar] [CrossRef]
- Frei, R.; Paulukat, C.; Bruggmann, S.; Klaebe, R.M. A systematic look at chromium isotopes in modern shells—Implications for paleo-environmental reconstructions. Biogeosciences 2018, 15, 4905–4922. [Google Scholar] [CrossRef] [Green Version]
- Bruggmann, S.; Klaebe, R.M.; Paulukat, C.; Frei, R. Heterogeneity and incorporation of chromium isotopes in recent marine molluscs (Mytilus). Geobiology 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Burne, R.V.; Moore, L.S. Microbialites: Organosedimentary deposits of benthic microbial communities. Soc. Sediment. Geol. 1987, 2, 241–254. [Google Scholar] [CrossRef]
- Reid, R.P.; Visscher, P.T.; Decho, A.W.; Stolz, J.F.; Bebout, B.M.; Dupraz, C.; Macintyre, I.G.; Paerl, H.W.; Pinckney, J.L.; Prufert-Bebout, L.; et al. The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature 2000, 406, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Grotzinger, J.P.; Knoll, A.H. Stromatolites in Precambrian carbonates: Evolutionary mileposts or environmental dipsticks? Annu. Rev. Earth Planet. Sci. 1999, 27, 313–358. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, C.; Warthmann, R.; McKenzie, J.A.; Visscher, P.T.; Bittermann, A.G.; van Lith, Y. Lithifying microbial mats in Lagoa Vermelha, Brazil: Modern Precambrian relics? Sediment. Geol. 2006, 185, 175–183. [Google Scholar] [CrossRef]
- Perri, E.; Tucker, M.E.; Spadafora, A.; Universita, T. Carbonate organo-mineral micro- and ultrastructures in sub-fossil stromatolites: Marion lake, South Australia. Geobiology 2012, 10, 105–117. [Google Scholar] [CrossRef]
- Ueno, Y.; Isozaki, Y.; Yurimoto, H.; Maruyama, S. Carbon isotopic signatures of individual Archean microfossils (?) from Western Australia. Int. Geol. Rev. 2001, 43, 196–212. [Google Scholar] [CrossRef]
- Ueno, Y.; Maruyama, S.; Isozaki, Y. Early Archean (ca. 3.5 Ga) microfossils and 13C-depleted carbonaceous matter in the North Pole area, Western Australia: Field occurrence and geochemistry. In Geochemistry and the Origin of Life; Nakasima, S., Maruyama, S., Brack, A., Windley, B.F., Eds.; Universal Academic Press: New York, NY, USA, 2001; pp. 203–236. [Google Scholar]
- van Kranendonk, M.J.; Webb, G.E.; Kamber, B.S. Geological and trace element evidence for a marine sedimentary environment of deposition and biogenicity of 3.45 Ga stromatolitic carbonates in the Pilbara Craton, and support for a reducing Archaean ocean. Geobiology 2003, 1, 91–108. [Google Scholar] [CrossRef]
- Allwood, A.C.; Kamber, B.S.; Walter, M.R.; Burch, I.W.; Kanik, I. Trace elements record depositional history of an Early Archean stromatolitic carbonate platform. Chem. Geol. 2010, 270, 148–163. [Google Scholar] [CrossRef]
- Nutman, A.P.; Bennett, V.C.; Friend, C.R.L.; Van Kranendonk, M.J.; Chivas, A.R. Rapid emergence of life shown by discovery of 3700-million-year-old microbial structures. Nature 2016, 537, 535–538. [Google Scholar] [CrossRef] [Green Version]
- Riding, R. Microbial carbonates: The geological record of calcified bacterial-algal mats and biofilms. Sedimentology 2000, 47, 179–214. [Google Scholar] [CrossRef]
- Webb, G.E.; Kamber, B.S. Rare earth elements in Holocene reefal microbialites: A new shallow seawater proxy. Geochim. Cosmochim. Acta 2000, 64, 1–9. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Hawkins, D.L.; Cortés, A. Do Archean chemical sediments record ancient seawater rare earth element patterns? Geochim. Cosmochim. Acta 2006, 70, 871–890. [Google Scholar] [CrossRef]
- Elderfield, H.; Upstill-Goddard, R.; Sholkovitz, E.R. The rare earth elements in rivers, estuaries, and coastal seas and their significance to the composition of ocean waters. Geochim. Cosmochim. Acta 1990, 54, 971–991. [Google Scholar] [CrossRef]
- Planavsky, N.; Bekker, A.; Rouxel, O.J.; Kamber, B.; Hofmann, A.; Knudsen, A.; Lyons, T.W. Rare Earth Element and yttrium compositions of Archean and Paleoproterozoic Fe formations revisited: New perspectives on the significance and mechanisms of deposition. Geochim. Cosmochim. Acta 2010, 74, 6387–6405. [Google Scholar] [CrossRef]
- Bau, M.; Möller, P. Rare earth element systematics of the chemically precipitated component in early precambrian iron formations and the evolution of the terrestrial atmosphere-hydrosphere-lithosphere system. Geochim. Cosmochim. Acta 1993, 57, 2239–2249. [Google Scholar] [CrossRef]
- Nothdurft, L.D.; Webb, G.E.; Kamber, B.S. Rare earth element geochemistry of Late Devonian reefal carbonates, Canning Basin, Western Australia: Confirmation of a seawater REE proxy in ancient limestones. Geochim. Cosmochim. Acta 2004, 68, 263–283. [Google Scholar] [CrossRef]
- Whiticar, M.J.; Suess, E. The cold carbonate connection between Mono Lake, California and the Bransfield Strait, Antarctica. Aquat. Geochem. 1998, 4, 429–454. [Google Scholar] [CrossRef] [Green Version]
- Klaebe, R.M.; Smith, M.P.; Fairchild, I.J.; Fleming, E.J.; Kennedy, M.J. Facies-dependent δ13C variation and diagenetic overprinting at the onset of the Sturtian glaciation in North-East Greenland. Precambrian Res. 2018, 319, 96–113. [Google Scholar] [CrossRef]
- Klaebe, R.M.; Kennedy, M.J.; Jarrett, A.J.M.; Brocks, J.J. Local paleoenvironmental controls on the carbon-isotope record defining the Bitter Springs Anomaly. Geobiology 2016, 15, 65–80. [Google Scholar] [CrossRef]
- von der Borch, C.C.; Bolton, B.; Warren, J.K. Environmental setting and microstructure of subfossil lithified stromatolites associated with evaporites. Sedimentology 1977, 24, 693–708. [Google Scholar] [CrossRef]
- Warren, J.K. The Hydrological Significance of Holocene Tepees, Stromatolites, and Boxwork Limestones in Coastal Salinas in South Australia. J. Sediment. Res. 1982, 52, 1171–1201. [Google Scholar]
- Pilkington, G. Resistivity Survey near Stenhouse Bay, Yorke Peninsula; South Australian Department of Mines and Energy: Adelaide, SA, Australia, 1977; Unpublished work.
- Warren, J.K. The hydrological setting, occurrence and significance of gypsum in late Quaternary salt lakes in South Australia. Sedimentology 1982, 29, 603–637. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Lyons, W.B. The rare earth element geochemistry of Mono Lake water and the importance of carbonate complexing. Limnol. Oceanogr. 1994, 39, 1141–1154. [Google Scholar] [CrossRef]
- Oremland, R.S.; Stolz, J.F.; Hollibaugh, J.T. The microbial arsenic cycle in Mono Lake, California. Fems Microb. Ecol. 2004, 48, 15–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stam, M.C.; Mason, P.R.D.; Pallud, C.; Van Cappellen, P. Sulfate reducing activity and sulfur isotope fractionation by natural microbial communities in sediments of a hypersaline soda lake (Mono Lake, California). Chem. Geol. 2010, 278, 23–30. [Google Scholar] [CrossRef]
- Herbst, D.B. Potential salinity limitations on nitrogen fixation in sediments from Mono Lake, California. Int. J. Salt Lake Res. 1998, 7, 261–274. [Google Scholar] [CrossRef]
- Domagalski, J.L.; Eugster, H.P.; Jones, B.F. Trace metal geochemistry of Walker, Mono, and Great Salt Lakes. In Fluid-Mineral Interactions: A Tribute to H.P. Eugster; Spencer, R.J., Chou, I.M., Eds.; The Geochemical Society: Pergamon, Turkey, 1990; pp. 315–353. [Google Scholar]
- Scholl, D.W.; Taft, W.H. Algae, contributors to the formation of calcareous tufa, Mono Lake, California. J. Sediment. Petrol. 1964, 34, 309–319. [Google Scholar]
- Pedley, M.; Rogerson, M.; Middleton, R. Freshwater calcite precipitates from in vitro mesocosm flume experiments: A case for biomediation of tufas. Sedimentology 2009, 56, 511–527. [Google Scholar] [CrossRef]
- Brasier, A.; Wacey, D.; Rogerson, M.; Guagliardo, P.; Saunders, M.; Kellner, S.; Mercedes-Martin, R.; Prior, T.; Taylor, C.; Matthews, A.; et al. A microbial role in the construction of Mono Lake carbonate chimneys? Geobiology 2018, 16, 540–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frei, R.; Gaucher, C.; Døssing, L.N.; Sial, A.N. Chromium isotopes in carbonates - A tracer for climate change and for reconstructing the redox state of ancient seawater. Earth Planet. Sci. Lett. 2011, 312, 114–125. [Google Scholar] [CrossRef]
- Schoenberg, R.; Zink, S.; Staubwasser, M.; Blanckenburg, F. Von The stable Cr isotope inventory of solid Earth reservoirs determined by double spike MC-ICP-MS. Chem. Geol. 2008, 249, 294–306. [Google Scholar] [CrossRef]
- Rodler, A.S.; Hohl, S.V.; Guo, Q.; Frei, R. Chromium isotope stratigraphy of Ediacaran cap dolostones, Doushantuo. Chem. Geol. 2016, 436, 24–34. [Google Scholar] [CrossRef]
- Rodler, A.S.; Frei, R.; Gaucher, C.; Korte, C.; Rosing, S.A.; Germs, G.J.B. Multiproxy isotope constraints on ocean compositional changes across the late Neoproterozoic Ghaub glaciation, Otavi Group, Namibia. Precambrian Res. 2017, 298, 306–324. [Google Scholar] [CrossRef]
- D’Arcy, J.; Gilleaudeau, G.; Peralta, S.; Frei, R. Redox fluctuations in the Early Ordovician oceans: An insight from chromium stable isotopes. Chem. Geol. 2017, 448, 1–12. [Google Scholar] [CrossRef]
- Imai, A.; Gloyna, E.F. Effects of pH and oxidation state of chromium on the behavior of chromium in the activated sludge process. Water Res. 1990, 24, 1143–1150. [Google Scholar] [CrossRef]
- Bonnand, P.; Parkinson, I.J.; James, R.H.; Karjalainen, A.; Fehr, M.A. Accurate and precise determination of stable Cr isotope compositions in carbonates by double spike MC-ICP-MS. J. Anal. At. Spectrom. 2011, 26, 528. [Google Scholar] [CrossRef]
- Planavsky, N.J.; Reinhard, C.T.; Wang, X.; Thomson, D.; Mcgoldrick, P.; Rainbird, R.H.; Johnson, T.; Fischer, W.W.; Lyons, T.W. Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 2014, 346, 635–638. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Hoboken, NJ, USA, 1985. [Google Scholar]
- Nance, W.B.; Taylor, S.R. Rare earth element patterns and crustal sedimentary rocks. Geochim. Cosmochim. Acta 1976, 40, 1539–1551. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Bolhar, R.; Kamber, B.S.; Moorbath, S.; Fedo, C.M.; Whitehouse, M.J. Characterisation of early Archaean chemical sediments by trace element signatures. Earth Planet. Sci. Lett. 2004, 222, 43–60. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Comparing yttrium and rare earths in hydrothermal fluids from the Mid–Atlantic Ridge: Implications for Y and REE behaviour during near–vent mixing and for the Y/Ho ratio of Proterozoic seawater. Chem. Geol. 1999, 155, 77–90353. [Google Scholar] [CrossRef]
- Viehmann, S.; Hohl, S.V.; Kraemer, D.; Bau, M.; Walde, D.H.G.; Galer, S.J.G.; Jiang, S.; Meister, P. Metal cycling in Mesoproterozoic microbial habitats: Insights from trace elements and stable Cd isotopes in stromatolites. Gondwana Res. 2019, 67, 101–114. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Kamber, B.S. The behaviour of the rare earth elements during estuarine mixing—Revisited. Mar. Chem. 2006, 100, 147–161. [Google Scholar] [CrossRef]
- Gilleaudeau, G.J.; Frei, R.; Kaufman, A.J.; Kah, L.C.; Azmy, K.; Bartley, J.K.; Chernyavskiy, P.; Knoll, A.H. Oxygenation of the mid-Proterozoic atmosphere: Clues from chromium isotopes in carbonates. Geochem. Perspect. Lett. 2016, 2, 178–187. [Google Scholar] [CrossRef]
- Wei, W.; Frei, R.; Gilleaudeau, J.; Li, D.; Wei, G.; Chen, X. Oxygenation variations in the atmosphere and shallow seawaters of the Yangtze Platform during the Ediacaran Period: Clues from Cr-isotope and Ce-anomaly in carbonates. Precambrian Res. 2018, 313, 78–90. [Google Scholar] [CrossRef]
- Rodler, A.S.; Frei, R.; Gaucher, C.; Germs, G.J.B. Chromium isotope, REE and redox-sensitive trace element chemostratigraphy across the late Neoproterozoic Ghaub glaciation, Otavi Group, Namibia. Precambrian Res. 2016, 286, 234–249. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Telfeyan, K.; Chevis, D.A.; Rosenheim, B.E.; Leybourne, M.L. Rare earth elements in stromatolites—1. Evidence that modern terrestrial stromatolites fractionate rare earth elements during incorporation from ambient waters. In Evolution of Archean Crust and Early Life; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany, 2014; pp. 385–411. [Google Scholar]
- van de Flierdt, T.; Pahnke, K.; Basak, C.; Coles, B.; Colin, C.; Crocket, K.; Frank, M.; Frank, N.; Goldstein, S.L.; Goswami, V.; et al. GEOTRACES intercalibration of neodymium isotopes and rare earth element concentrations in seawater and suspended particles. Part 1: Reproducibility of results for the international intercomparison. Limnol. Oceanogr. Methods 2012, 234–251. [Google Scholar] [CrossRef] [Green Version]
- Frimmel, H.E. Trace element distribution in Neoproterozoic carbonates as palaeoenvironmental indicator. Chem. Geol. 2009, 258, 338–353. [Google Scholar] [CrossRef]
- Sforna, M.C.; Daye, M.; Philippot, P.; Somogyi, A.; van Zuilen, M.A.; Medjoubi, K.; Gérard, E.; Jamme, F.; Dupraz, C.; Braissant, O.; et al. Patterns of metal distribution in hypersaline microbialites during early diagenesis: Implications for the fossil record. Geobiology 2017, 15, 259–279. [Google Scholar] [CrossRef]
- Valdespino-Castillo, P.M.; Hu, P.; Merino-Ibarra, M.; López-Gómez, L.M.; Cerqueda-García, D.; González-De Zayas, R.; Pi-Puig, T.; Lestayo, J.A.; Holman, H.Y.; Falcón, L.I. Exploring biogeochemistry and microbial diversity of extant microbialites in Mexico and Cuba. Front. Microbiol. 2018, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Remmelzwaal, S.R.C.; Yu, A.; Parkinson, I.J.; Schmidt, D.N.; Titelboim, D.; Abramovich, S.; Roepert, A.; Kienhuis, M.; Polerecky, L.; Goring-harford, H.; et al. Post-depositional overprinting of chromium in foraminifera. Earth Planet. Sci. Lett. 2019, 515, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Souza-Egipsy, V.; Wierzchos, J.; Ascaso, C.; Nealson, K.H. Mg–silica precipitation in fossilization mechanisms of sand tufa endolithic microbial community, Mono Lake (California). Chem. Geol. 2005, 217, 77–87. [Google Scholar] [CrossRef]
- Connelly, D.P.; Statham, P.J.; Knap, A.H. Seasonal changes in speciation of dissolved chromium in the surface Sargasso Sea. Deep-Sea Res. I 2006, 53, 1975–1988. [Google Scholar] [CrossRef]
- Sander, S.; Koschinsky, A. Onboard-ship redox speciation of chromium in diffuse hydrothermal fluids from the North Fiji Basin. Mar. Chem. 2000, 71, 83–102. [Google Scholar] [CrossRef]
- Sander, S.; Koschinsky, A. Metal flux from hydrothermal vents increased by organic complexation. Nat. Publ. Group 2011, 4, 145–150. [Google Scholar] [CrossRef]
- Spadafora, A.; Perri, E.; Mckenzie, J.A.; Vasconcelos, C. Microbial biomineralization processes forming modern Ca:Mg carbonate stromatolites. Sedimentology 2010, 57, 27–40. [Google Scholar] [CrossRef]
- Pace, A.; Bourillot, R.; Bouton, A.; Vennin, E.; Galaup, S.; Bundeleva, I.; Patrier, P.; Dupraz, C.; Thomazo, C.; Sansjofre, P.; et al. Microbial and diagenetic steps leading to the mineralisation of Great Salt Lake microbialites. Nat. Publ. Group 2016, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, A.; Bourillot, R.; Bouton, A.; Vennin, E.; Braissant, O.; Dupraz, C.; Duteil, T.; Bundeleva, I.; Patrier, P.; Galaup, S.; et al. Formation of stromatolite lamina at the interface of oxygenic-anoxygenic photosynthesis. Geobiology 2018, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rickli, J.; Janssen, D.J.; Hassler, C.; Ellwood, M.J.; Jaccard, S.L. Chromium biogeochemistry and stable isotope distribution in the Southern Ocean. Geochim. Cosmochim. Acta 2019, 262, 188–206. [Google Scholar] [CrossRef]
- Talbot, M.R. A review of the palaeohydrological interpretation of carbon and oxygen isotopic ratios in primary lacustrine carbonates. Chem. Geol. 1990, 80, 261–279. [Google Scholar] [CrossRef]
- Li, H.; Ku, T.; Stott, L.D.; Anderson, R.F. Stable isotope studies on Mono Lake (California). 1. δl80 in lake sediments as proxy for climatic change during the last 150 years Mono Lake hydrology. Limnol. Oceanogr. 1997, 42, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Meyer, E.E.; Quicksall, A.N.; Landis, J.D.; Link, P.K.; Bostick, B.C. Trace and rare earth elemental investigation of a Sturtian cap carbonate, Pocatello, Idaho: Evidence for ocean redox conditions before and during carbonate deposition. Precambrian Res. 2012, 192–195, 89–106. [Google Scholar] [CrossRef]
- Nasemann, P.; Janssen, D.J.; Rickli, J.; Grasse, P.; Frank, M.; Jaccard, S.L. Chromium reduction and associated stable isotope fractionation restricted to anoxic shelf waters in the Peruvian Oxygen Minimum Zone. Geochim. Cosmochim. Acta 2020, 285, 207–224. [Google Scholar] [CrossRef]
- Farkaš, J.; Chrastny, V.; Novak, M.; Cadkova, E.; Pasava, J.; Chakrabarti, R.; Jacobsen, S.B.; Ackermann, L.; Bullen, T.D. Chromium isotope variations (δ53/52 Cr) in mantle-derived sources and their weathering products: Implications for environmental studies and the evolution of δ53/52 Cr in the Earth’s mantle over geologic time. Geochim. Cosmochim. Acta 2013, 123, 74–92. [Google Scholar] [CrossRef]
- D’Arcy, J.D.; Babechuk, M.G.; Nørbye, L.; Gaucher, C.; Frei, R. Processes controlling the chromium isotopic composition of river water: Constraints from basaltic river catchments. Geochim. Cosmochim. Acta 2016, 186, 296–315. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Planavsky, N. Cr isotope systematics in the Connecticut River estuary. Chem. Geol. 2019, 506, 29–39. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Reinhard, C.T.; Planavsky, N.J. Chromium isotope systematics in the Connecticut River. Chem. Geol. 2017, 456, 98–111. [Google Scholar] [CrossRef]
- Cantine, M.D.; Knoll, A.H.; Bergmann, K.D. Carbonates before skeletons: A database approach. Earth-Sci. Rev. 2020, 201. [Google Scholar] [CrossRef]
- Scheiderich, K.; Amini, M.; Holmden, C.; Francois, R. Global variability of chromium isotopes in seawater demonstrated by Pacific, Atlantic, and Arctic Ocean samples. Earth Planet. Sci. Lett. 2015, 423, 87–97. [Google Scholar] [CrossRef]
- Fike, D.A.; Grotzinger, J.P.; Pratt, L.M.; Summons, R.E. Oxidation of the Ediacaran Ocean. Nature 2006, 444, 744–747. [Google Scholar] [CrossRef]
- Frei, R.; Gaucher, C.; Poulton, S.W.; Canfield, D.E. Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes. Nature 2009, 461, 250–253. [Google Scholar] [CrossRef]
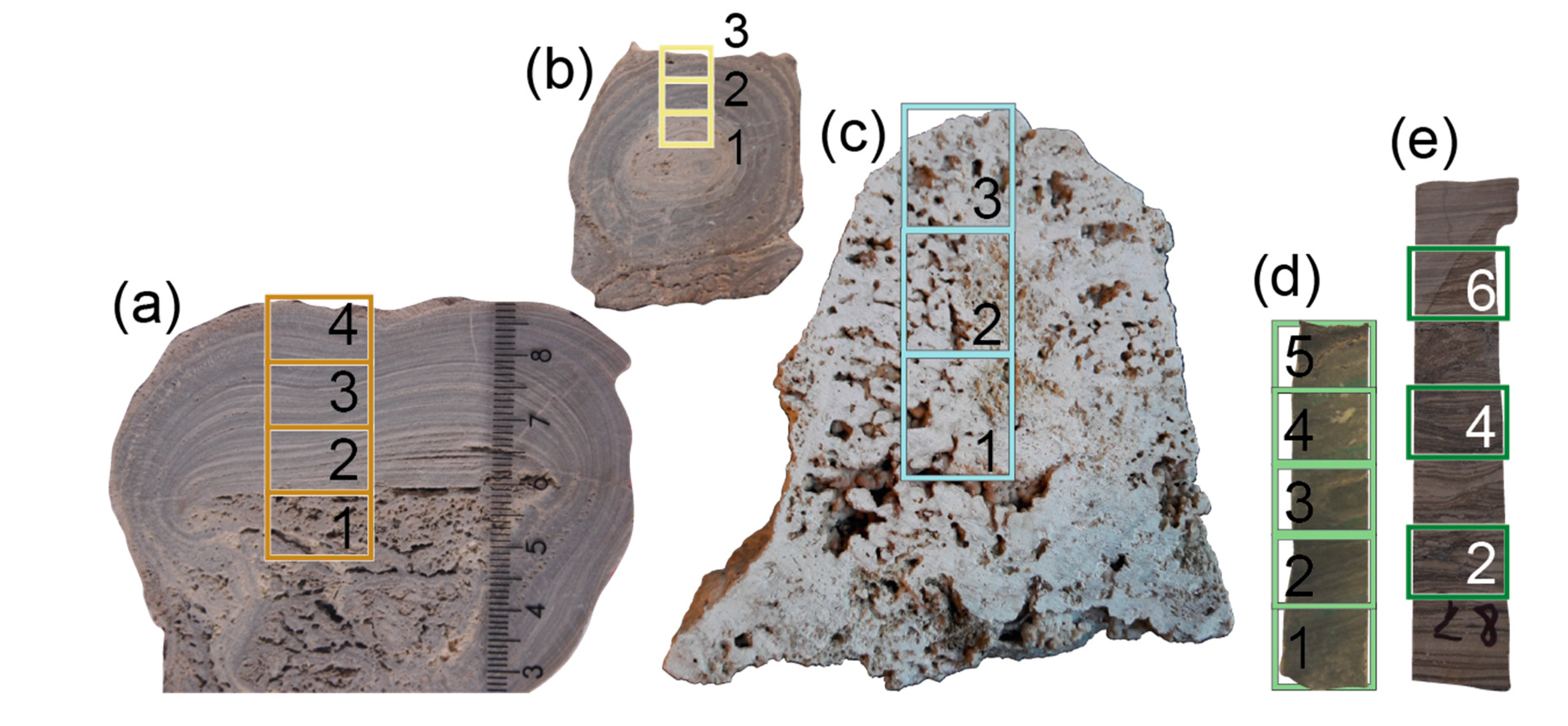
| Sample ID | Location | Sample Type | Position | Sample Characterization | Age |
|---|---|---|---|---|---|
| MRN-A-1 | Marion Lake, Australia | carbonate profile A | 1 | Fine-grained, thinly laminated microbialites formed in a hypersaline lake with marine connection | Recent |
| MRN-A-2 | carbonate profile A | 2 | |||
| MRN-A-3 | carbonate profile A | 3 | |||
| MRN-A-4 | carbonate profile A | 4 | |||
| MRN-B-1 | carbonate profile B | 1 | |||
| MRN-B-2 | carbonate profile B | 2 | |||
| MRN-B-3 | carbonate profile B | 3 | |||
| Mono-1 | Mono Lake, USA | carbonate profile | 1 | Tufa formed in an inland, alkaline freshwater lake | Recent |
| Mono-2 | carbonate profile | 2 | |||
| Mono-3 | carbonate profile | 3 | |||
| ALG-B | Andrée Land Group, Greenland | carbonate bulk | 7 | Dolomitized stromatolites formed on a carbonate ramp in a marine environment | ≈720 Ma |
| ALG-1 | carbonate profile | 1 | |||
| ALG-2 | carbonate profile | 2 | |||
| ALG-3 | carbonate profile | 3 | |||
| ALG-4 | carbonate profile | 4 | |||
| ALG-5 | carbonate profile | 5 | |||
| BSF-2 | Bitter Springs Formation, Australia | carbonate profile | 2 | Cyclic stromatolitic carbonates formed under restricted marine to alkaline lacustrine conditions | ≈800 Ma |
| BSF-4 | carbonate profile | 4 | |||
| BSF-6 | carbonate profile | 6 |
| Sample ID | Cr | δ53Cr | 2SD | δ13C | SD | δ18O | SD |
|---|---|---|---|---|---|---|---|
| ppm (^nM) | ‰ | ‰ | ‰ | ||||
| MRN-A-1 | 6.06 | 0.99 | 0.02 | 2.61 | 0.05 | 0.71 | 0.06 |
| MRN-A-2 | 3.09 | 0.94 | 0.00 | 1.97 | 0.10 | 1.73 | 0.17 |
| MRN-A-3 | 1.68 | 1.01 | 0.05 | 2.74 | 0.08 | 0.75 | 0.10 |
| MRN-A-4 | 1.68 | 0.93 | 0.05 | 2.58 | 0.07 | 0.59 | 0.14 |
| MRN-B-1 | 1.78 | 0.94 | 0.02 | 2.78 | 0.08 | 0.46 | 0.07 |
| MRN-B-2 | 1.89 | 1.07 | 0.10 | 2.85 | 0.08 | 0.77 | 0.17 |
| MRN-B-3 | 1.50 | 1.03 | 0.04 | 2.66 | 0.09 | 0.27 | 0.12 |
| Mono-1 | 0.74 | 0.85 | 0.14 | 8.14 | 0.08 | −2.47 | 0.14 |
| Mono-2 | 0.69 | 0.79 | 0.02 | 8.43 | 0.05 | −2.08 | 0.11 |
| Mono-3 | 0.86 | 0.71 | 0.00 | 8.52 | 0.11 | −1.50 | 0.11 |
| ALG-B | 2.85 | 0.33 | 0.08 | 4.27 | 0.09 | −3.54 | 0.18 |
| ALG-1 | 1.67 | 0.37 | 0.02 | 3.73 | 0.13 | −2.63 | 0.15 |
| ALG-2 | 3.21 | 0.43 | 0.05 | 3.79 | 0.13 | −3.06 | 0.20 |
| ALG-3 | 3.30 | 0.42 | 0.05 | 3.81 | 0.14 | −3.24 | 0.17 |
| ALG-4 | 4.24 | 0.36 | 0.08 | 3.70 | 0.14 | −3.07 | 0.19 |
| ALG-5 | 4.57 | 0.23 | 0.07 | 3.80 | 0.11 | −3.78 | 0.17 |
| BSF-1 | 2.38 | −0.14 | 0.02 | −0.48 | 0.15 | −6.53 | 0.21 |
| BSF-2 | 2.23 | −0.18 | 0.06 | −0.35 | 0.03 | −7.09 | 0.08 |
| BSF-3 | 2.18 | −0.04 | 0.07 | −0.31 | 0.06 | −6.91 | 0.12 |
| Sample ID | Cr | Al | Cr/Al × 1000 | Crdet | Crauth | Crdet | ƒ | δ53Cr | δ53Crauth |
|---|---|---|---|---|---|---|---|---|---|
| ppm | ppm | ppm | ppm | % | ‰ | ‰ | |||
| MRN-A-1 | 6.06 | 280 | 22 | 0.31 | 5.75 | 5.08 | 0.05 | 0.99 | 0.99 |
| MRN-A-2 | 3.09 | 290 | 11 | 0.32 | 2.77 | 10.3 | 0.10 | 0.94 | 0.95 |
| MRN-A-3 | 1.68 | 324 | 5 | 0.36 | 1.32 | 21.3 | 0.21 | 1.01 | 1.09 |
| MRN-A-4 | 1.68 | 139 | 12 | 0.15 | 1.52 | 9.14 | 0.09 | 0.93 | 0.94 |
| MRN-B-1 | 1.78 | 347 | 5 | 0.38 | 1.40 | 21.5 | 0.21 | 0.94 | 1.02 |
| MRN-B-2 | 1.89 | 256 | 7 | 0.28 | 1.61 | 14.9 | 0.15 | 1.07 | 1.11 |
| MRN-B-3 | 1.50 | 329 | 5 | 0.36 | 1.13 | 24.2 | 0.24 | 1.03 | 1.15 |
| Mono-1 | 0.74 | 383 | 2 | 0.42 | 0.31 | 57.3 | 0.57 | 0.85 | 2.61 |
| Mono-2 | 0.69 | 1006 | 1 | 1.11 | −0.42 | 162 | 1.61 | 0.79 | 7.12 |
| Mono-3 | 0.86 | 1748 | 0 | 1.92 | −1.06 | 224 | 2.24 | 0.71 | 3.44 |
| ALG-1 | 1.67 | 317 | 5 | 0.35 | 1.32 | 20.8 | 0.21 | 0.37 | 0.40 |
| ALG-2 | 3.21 | 332 | 10 | 0.37 | 2.85 | 11.4 | 0.11 | 0.43 | 0.44 |
| ALG-3 | 3.30 | 672 | 5 | 0.74 | 2.56 | 22.4 | 0.22 | 0.42 | 0.47 |
| ALG-4 | 4.24 | 313 | 14 | 0.34 | 3.89 | 8.13 | 0.08 | 0.36 | 0.36 |
| ALG-5 | 4.57 | 632 | 7 | 0.70 | 3.88 | 15.2 | 0.15 | 0.23 | 0.24 |
| BSF-1 | 2.38 | 2238 | 1 | 2.46 | −0.09 | 104 | 1.04 | −0.14 | −14.29 |
| BSF-2 | 2.23 | 1582 | 1 | 1.74 | 0.49 | 78.2 | 0.78 | −0.18 | −0.87 |
| BSF-3 | 2.18 | 1168 | 2 | 1.28 | 0.90 | 58.8 | 0.59 | −0.04 | 0.13 |
| Sample ID | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | NdSN/YbSN | Y/Ho | YSN/ HoSN | ErSN/ YbSN | YbSN/ PrSN | CeSN/ Ce*SN | EuSN/ Eu*SN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BHVO-2 1 | 0.72 | 0.83 | 1.09 | 1.41 | 1.96 | 3.67 | 2.44 | 2.55 | 2.27 | 1.56 | 1.96 | 1.69 | 1.36 | 1.37 | 1.18 | 1.03 | 22.32 | 0.80 | 1.24 | 1.25 | 1.07 | 1.71 |
| JDo-1 2 | 0.21 | 0.03 | 0.12 | 0.14 | 0.14 | 0.16 | 0.22 | 0.18 | 0.19 | 0.39 | 0.18 | 0.18 | 0.12 | 0.12 | 0.09 | 1.19 | 59.62 | 2.13 | 1.49 | 1.03 | 0.30 | 1.06 |
| MRN-A-1 | 0.03 | 0.01 | 0.02 | 0.03 | 0.03 | 0.05 | 0.05 | 0.04 | 0.05 | 0.08 | 0.04 | 0.05 | 0.03 | 0.04 | 0.03 | 0.81 | 49.30 | 1.76 | 1.23 | 1.55 | 0.48 | 1.32 |
| MRN-A-2 | 0.04 | 0.01 | 0.03 | 0.04 | 0.04 | 0.06 | 0.06 | 0.05 | 0.05 | 0.09 | 0.05 | 0.05 | 0.04 | 0.04 | 0.04 | 0.99 | 48.94 | 1.75 | 1.27 | 1.25 | 0.37 | 1.24 |
| MRN-A-3 | 0.04 | 0.01 | 0.04 | 0.05 | 0.05 | 0.07 | 0.07 | 0.06 | 0.07 | 0.11 | 0.07 | 0.07 | 0.05 | 0.05 | 0.05 | 0.88 | 46.16 | 1.65 | 1.29 | 1.40 | 0.49 | 1.21 |
| MRN-A-4 | 0.03 | 0.01 | 0.03 | 0.04 | 0.04 | 0.06 | 0.06 | 0.05 | 0.06 | 0.09 | 0.06 | 0.06 | 0.04 | 0.04 | 0.04 | 0.84 | 47.47 | 1.70 | 1.33 | 1.48 | 0.45 | 1.22 |
| MRN-B-1 | 0.03 | 0.01 | 0.03 | 0.04 | 0.05 | 0.06 | 0.06 | 0.06 | 0.07 | 0.11 | 0.06 | 0.07 | 0.05 | 0.06 | 0.05 | 0.70 | 47.13 | 1.68 | 1.26 | 1.84 | 0.68 | 1.21 |
| MRN-B-2 | 0.03 | 0.01 | 0.03 | 0.04 | 0.05 | 0.07 | 0.07 | 0.07 | 0.08 | 0.12 | 0.08 | 0.08 | 0.06 | 0.06 | 0.05 | 0.72 | 45.89 | 1.64 | 1.31 | 1.81 | 0.61 | 1.26 |
| MRN-B-3 | 0.02 | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | 0.05 | 0.05 | 0.06 | 0.10 | 0.06 | 0.06 | 0.05 | 0.05 | 0.05 | 0.57 | 49.23 | 1.76 | 1.16 | 2.25 | 0.69 | 1.23 |
| Mono-1 | 0.30 | 0.41 | 0.35 | 0.40 | 0.48 | 1.07 | 0.58 | 0.57 | 0.62 | 0.59 | 0.57 | 0.64 | 0.54 | 0.72 | 0.64 | 0.56 | 29.02 | 1.04 | 0.88 | 2.07 | 1.40 | 2.11 |
| Mono-2 | 0.30 | 0.51 | 0.42 | 0.50 | 0.71 | 1.28 | 0.90 | 0.99 | 1.16 | 1.08 | 1.08 | 1.21 | 1.09 | 1.48 | 1.30 | 0.34 | 28.11 | 1.00 | 0.82 | 3.54 | 1.54 | 1.59 |
| Mono-3 | 0.27 | 0.43 | 0.34 | 0.41 | 0.55 | 1.10 | 0.72 | 0.77 | 0.89 | 0.87 | 0.84 | 0.94 | 0.81 | 1.06 | 0.91 | 0.39 | 28.95 | 1.03 | 0.89 | 3.11 | 1.57 | 1.76 |
| ALG-1 | 0.13 | 0.15 | 0.15 | 0.16 | 0.19 | 0.28 | 0.23 | 0.19 | 0.18 | 0.16 | 0.15 | 0.15 | 0.11 | 0.13 | 0.10 | 1.28 | 29.76 | 1.06 | 1.17 | 0.89 | 1.22 | 1.50 |
| ALG-2 | 0.10 | 0.12 | 0.12 | 0.13 | 0.15 | 0.15 | 0.17 | 0.15 | 0.15 | 0.12 | 0.12 | 0.12 | 0.09 | 0.10 | 0.08 | 1.31 | 29.57 | 1.06 | 1.17 | 0.85 | 1.14 | 1.03 |
| ALG-3 | 0.08 | 0.09 | 0.09 | 0.10 | 0.11 | 0.12 | 0.13 | 0.11 | 0.11 | 0.09 | 0.09 | 0.09 | 0.07 | 0.08 | 0.06 | 1.29 | 29.75 | 1.06 | 1.17 | 0.87 | 1.17 | 1.07 |
| ALG-4 | 0.07 | 0.09 | 0.10 | 0.12 | 0.12 | 0.13 | 0.14 | 0.11 | 0.10 | 0.08 | 0.08 | 0.07 | 0.05 | 0.06 | 0.05 | 1.96 | 30.18 | 1.08 | 1.26 | 0.60 | 1.09 | 1.06 |
| ALG-5 | 0.07 | 0.08 | 0.10 | 0.11 | 0.12 | 0.12 | 0.13 | 0.11 | 0.09 | 0.07 | 0.07 | 0.07 | 0.05 | 0.05 | 0.04 | 2.21 | 29.59 | 1.06 | 1.31 | 0.53 | 1.05 | 1.02 |
| BSF-1 | 0.17 | 0.23 | 0.26 | 0.31 | 0.33 | 0.32 | 0.37 | 0.29 | 0.26 | 0.21 | 0.20 | 0.19 | 0.13 | 0.15 | 0.12 | 2.03 | 29.84 | 1.07 | 1.25 | 0.59 | 1.09 | 1.01 |
| BSF-2 | 0.09 | 0.11 | 0.13 | 0.15 | 0.15 | 0.14 | 0.17 | 0.13 | 0.11 | 0.09 | 0.08 | 0.08 | 0.05 | 0.06 | 0.05 | 2.54 | 30.73 | 1.10 | 1.33 | 0.45 | 1.05 | 1.02 |
| BSF-3 | 0.11 | 0.15 | 0.16 | 0.19 | 0.21 | 0.20 | 0.23 | 0.18 | 0.16 | 0.13 | 0.12 | 0.12 | 0.08 | 0.10 | 0.07 | 2.04 | 30.57 | 1.09 | 1.25 | 0.58 | 1.08 | 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruggmann, S.; Rodler, A.S.; Klaebe, R.M.; Goderis, S.; Frei, R. Chromium Isotope Systematics in Modern and Ancient Microbialites. Minerals 2020, 10, 928. https://doi.org/10.3390/min10100928
Bruggmann S, Rodler AS, Klaebe RM, Goderis S, Frei R. Chromium Isotope Systematics in Modern and Ancient Microbialites. Minerals. 2020; 10(10):928. https://doi.org/10.3390/min10100928
Chicago/Turabian StyleBruggmann, Sylvie, Alexandra S. Rodler, Robert M. Klaebe, Steven Goderis, and Robert Frei. 2020. "Chromium Isotope Systematics in Modern and Ancient Microbialites" Minerals 10, no. 10: 928. https://doi.org/10.3390/min10100928
APA StyleBruggmann, S., Rodler, A. S., Klaebe, R. M., Goderis, S., & Frei, R. (2020). Chromium Isotope Systematics in Modern and Ancient Microbialites. Minerals, 10(10), 928. https://doi.org/10.3390/min10100928





