Coulsonite FeV2O4—A Rare Vanadium Spinel Group Mineral in Metamorphosed Massive Sulfide Ores of the Kola Region, Russia
Abstract
1. Introduction
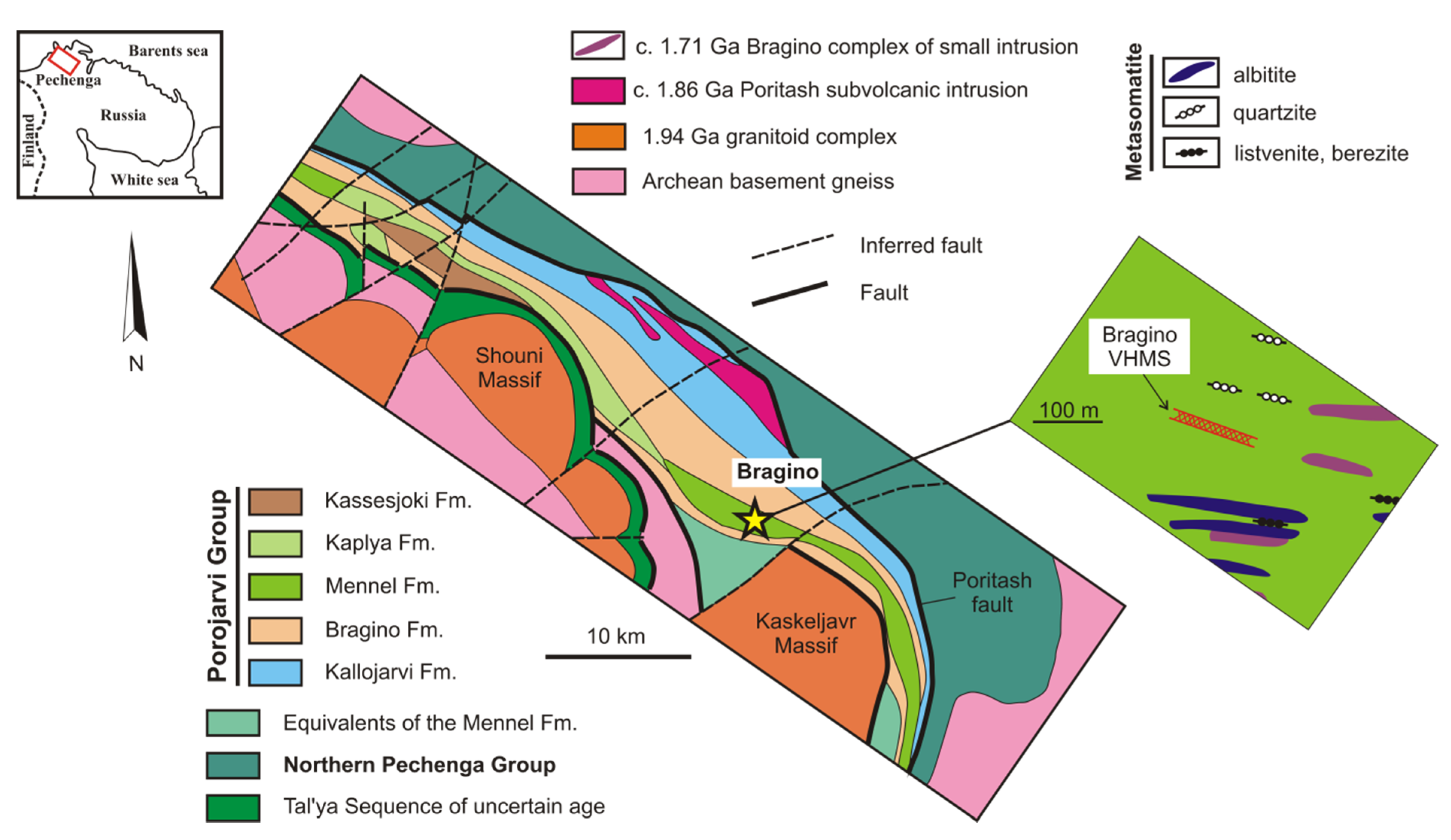
2. Geological Setting
3. Methods
4. Results
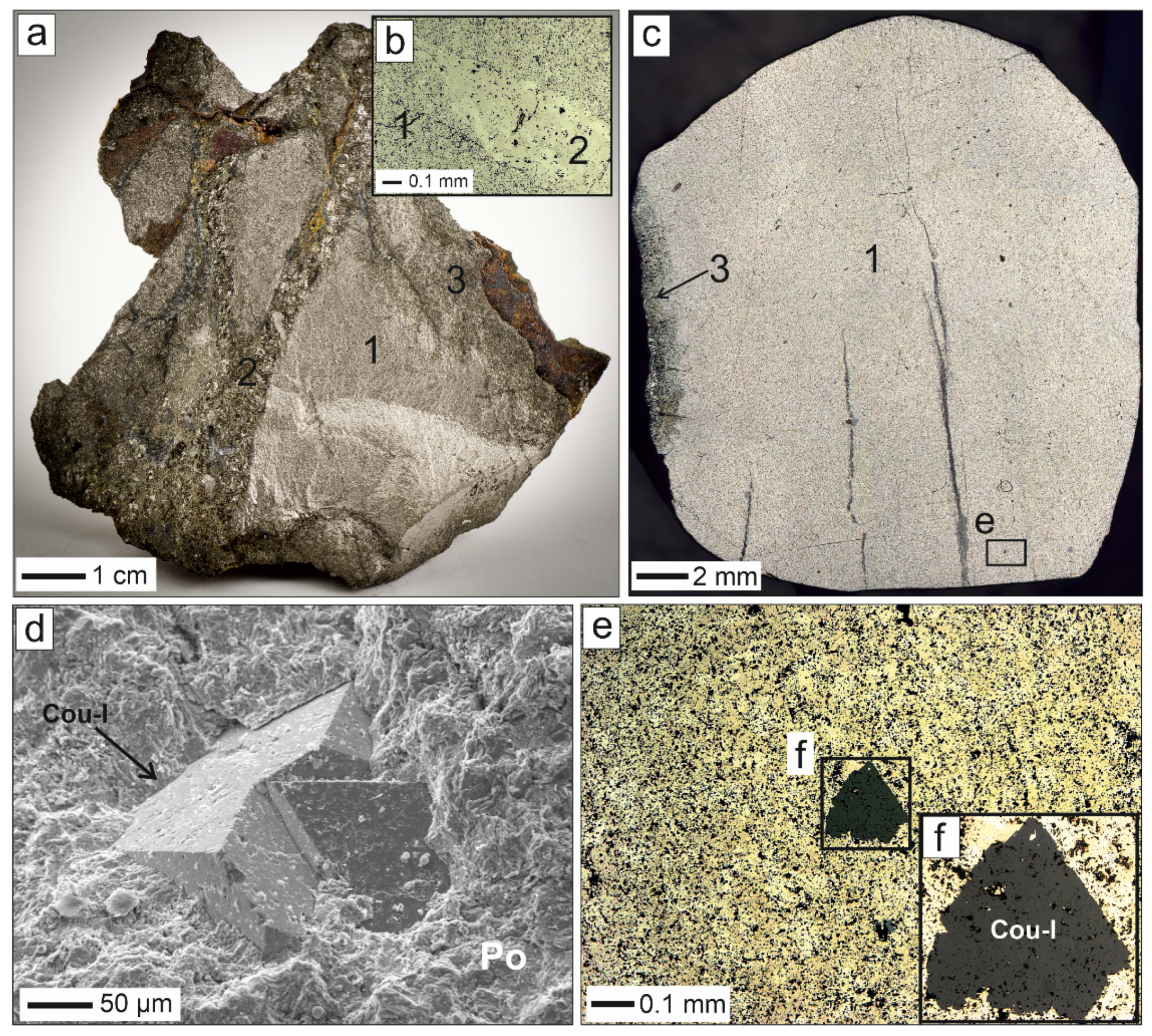
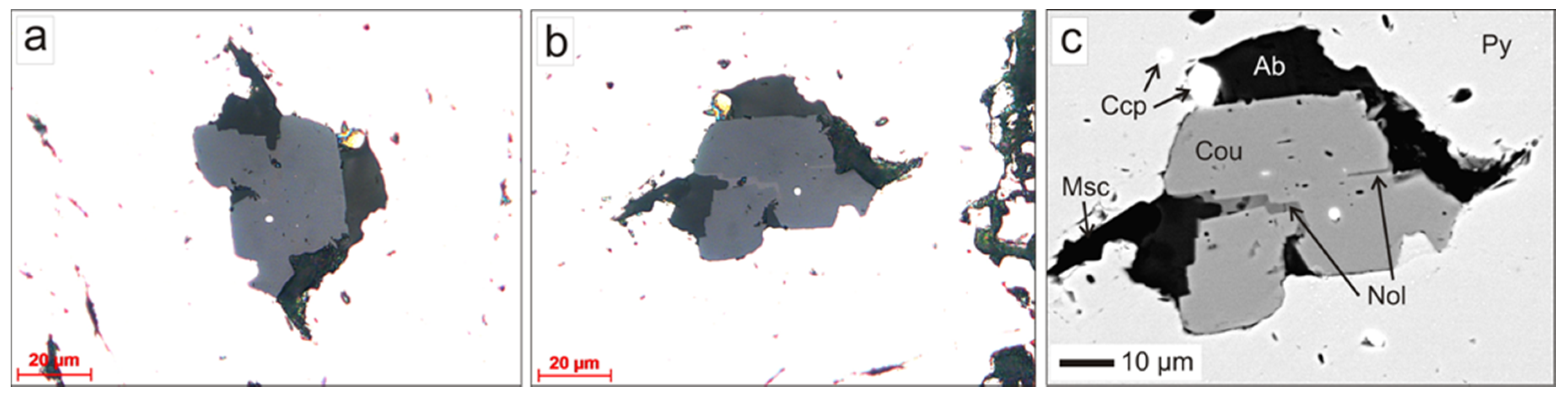
4.1. Coulsonite-I
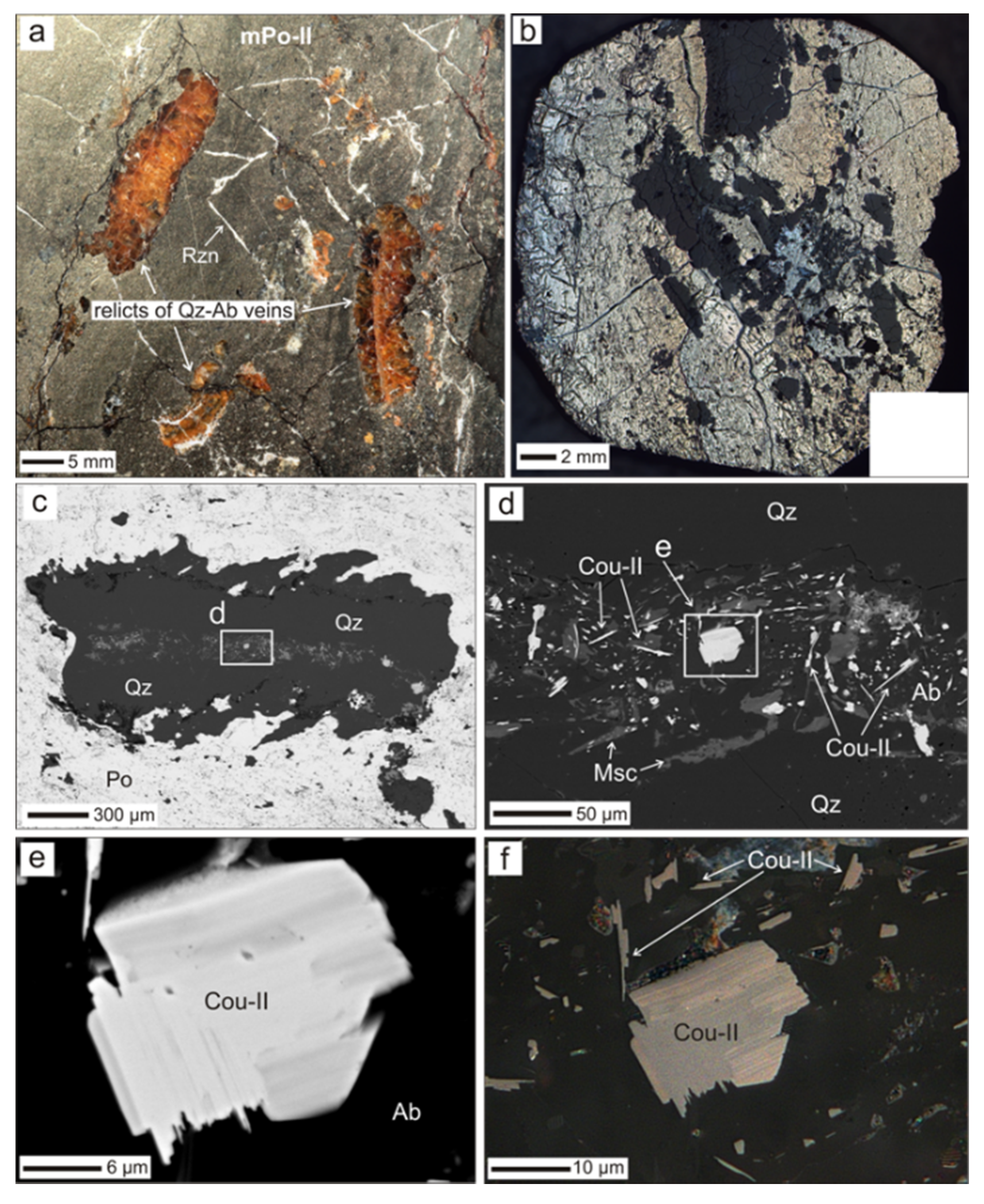
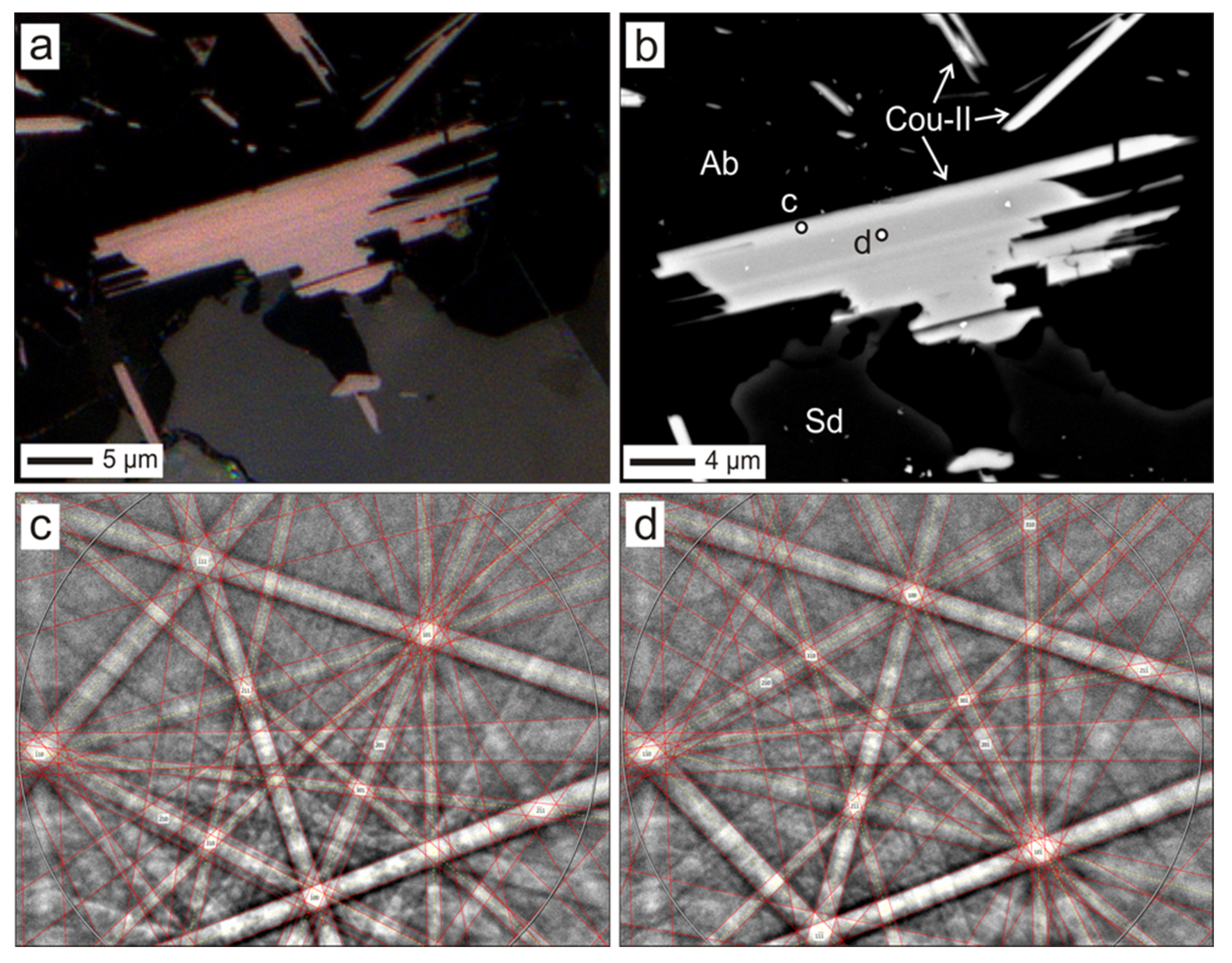
4.2. Coulsonite-II
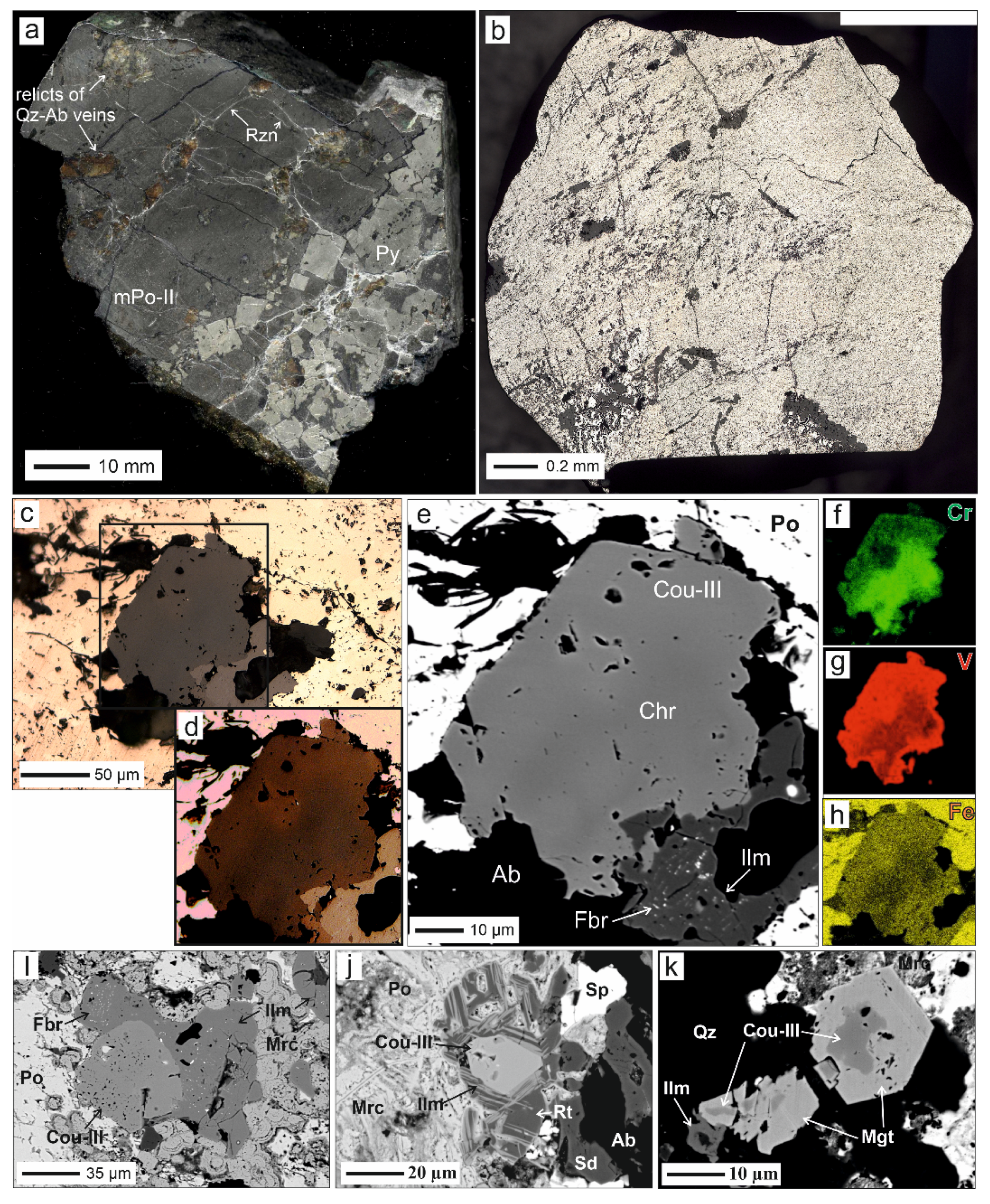
4.3. Coulsonite-III
5. Discussion
5.1. Genesis of Coulsonite of the Bragino Occurrence
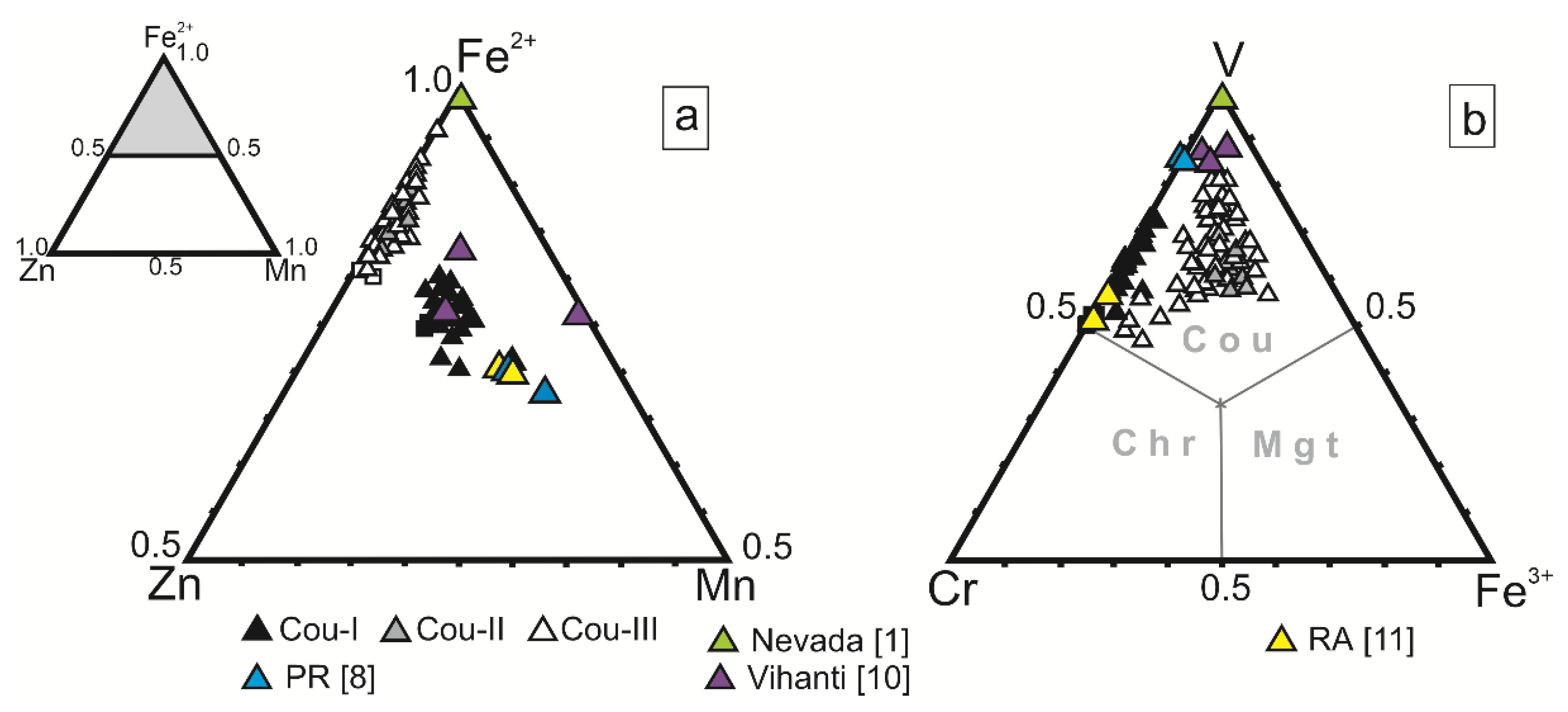
5.2. Chemical Composition of Coulsonite of the Bragino Occurrence
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Radtke, A.S. Coulsonite, FeV2O4, a spinel-type mineral from Lovelock, Nevada. Am. Mineral. 1962, 47, 1284–1291. [Google Scholar]
- Harris, C.; Hlongwane, W.; Gule, N.; Scheepers, R. Origin of tanzanite and associated gemstone mineralization at Merelani, Tanzania. S. Afr. J. Geol. 2014, 117, 15–30. [Google Scholar] [CrossRef]
- Olivier, B. The Geology and petrology of the Merelani tanzanite deposit, NE Tanzania. Ph.D. Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2006; 453p. [Google Scholar]
- Reznitsky, L.Z.; Skliarov, E.V.; Ushchapovskaia, Z.F. Natalyite Na(V,Cr)Si2O6-A new chromium-vanadium pyroxene from Slyudianka. Proc. Russ. Mineral. Soc. 1985, 114, 630–635. (In Russian) [Google Scholar]
- Reznitsky, L.Z.; Sklyarov, E.V.; Ushchapovskaya, Z.F. Magnesiocoulsonite MgV2O4-A new mineral species in the spinel group. Proc. Russ. Mineral. Soc. 1995, 124, 91–98. (In Russian) [Google Scholar]
- Reznitsky, L.Z.; Sklyarov, E.V.; Armbruster, T.; Ushchapovskaya, Z.F.; Galuskin, E.V.; Polekhovsky, Y.S.; Barash, I.G. The new mineral oxyvanite V3O5 and the oxyvanite-berdesinskiite V2TiO5 isomorphic join in metamorphic rocks of Sludyanka complex (South Baikal region). Proc. Russ. Mineral. Soc. 2009, 138, 70–81. (In Russian) [Google Scholar]
- Cametti, G.; Armbruster, T.; Reznitsky, L.Z.; Sklyarov, E.V.; Della Ventura, G. Crystal structure and crystal-chemistry of vanadio-pargasite: A new amphibole from southern Lake Baikal, Siberia, Russia. Eur. J. Mineral. 2018, 30, 981–987. [Google Scholar] [CrossRef]
- Karpov, S.M.; Voloshin, A.V.; Savchenko, Y.E.; Selivanova, E.A.; Polekhovsky, Y.S. Coulsonite in the Pyrrhotite Canyon deposit (Kola peninsula): The first find in Russia. Dokl. Earth Sci. 2012, 446, 1064–1066. (In Russian) [Google Scholar] [CrossRef]
- Kompanchenko, A.A.; Voloshin, A.V.; Bazai, A.V.; Polekhovsky, Y.S. Evolution of Cr-V mineralization in volcanic massive sulfide ore of Bragino occurences (South Pechenga structure zone, the Kola region) on example of spinel group minerals. Proc. Russ. Miner. Soc. 2017, 5, 44–59. (In Russian) [Google Scholar]
- Sergeeva, N.E.; Eremin, N.I.; Dergachev, A.L. Vanadium mineralization in ore of the Vihanti massive sulfide base-metal deposit, Finland. Dokl. Earth Sci. 2011, 436, 210–212. [Google Scholar] [CrossRef]
- Höller, W.; Stumpfl, E.F. Cr-V oxides from the Rampura Agucha Pb-Zn-(Ag) deposit, Rajasthan, India. Can. Miner. 1995, 33, 745–752. [Google Scholar]
- Zakrzewski, M.A.; Burke, E.A.J.; Lustenhouwer, W.J. Vourelainenite, a new spinel, and associated minerals from the Sätra (Doverstorp) pyrite deposit, central Sweden. Can. Miner. 1982, 20, 281–290. [Google Scholar]
- Long, J.V.P.; Vourelainen, Y.; Kuovo, O. Karelianite, a new vanadium mineral. Am. Miner. 1963, 48, 33–41. [Google Scholar]
- Rouhunkoski, P. On the geology and geochemistry of the Vihanti zinc ore deposit, Finland. Bull. Comm. Geol. Finl. 1968, 236, 121. [Google Scholar]
- Rauhamäki, E.; Mäkelä, T.; Isomäki, O.P. Geology of the Vihanti mine. In Precambrian Ores of Finland. Guide to Excursions 078 A + C, Part 2 (Finland), Proceedings of the 26th International Geological Congress, Paris, France 7 July 1980; Häkli, T.A., Ed.; Geological Survey of Finland: Espoo, Finland, 1980; pp. 14–24. [Google Scholar]
- Gandhi, S.M.; Paliwal, H.V.; Bhatnagar, S.N. Geology and ore reserve estimates of Rampura Agucha lead zinc deposit, Bhilwara District. J. Geol. Soc. India 1984, 25, 689–705. [Google Scholar]
- Deb, M.; Thorpe, R.L.; Cumming, G.L.; Wagner, P.A. Age, source and stratigraphic implications of Pb isotope data for conformable, sediment-hosted, base metal deposits in the Proterozoic Aravalli-Delhi orogenic belt, northwestern India. Precambrian Res. 1989, 43, 1–22. [Google Scholar] [CrossRef]
- Deb, M. Lithogeochemistry of rocks around Rampura Agucha massive zinc sulfide ore-body, NWIndia–implications for the evolution of a Proterozoic “Aulakogen”. In Metallogeny Related to Tectonics of the Proterozoic Mobile Belts; Sarkar, S.C., Ed.; Balkhema: Rotterdam, The Netherlands, 1992; pp. 1–35. [Google Scholar]
- Höller, W.; Gandhi, S.M. Origin of tourmaline and oxide minerals from the metamorphosed Rampura Agucha Zn-Pb-(Ag) deposit, Rajasthan, India. Miner. Petrol. 1997, 60, 99–110. [Google Scholar] [CrossRef]
- Allen, R.L.; Lunström, I.; Ripa, M.; Simeonov, A.; Christofferson, H. Facies analysis of a 1.9 Ga, contintental margin, back-arc, felsic caldera province with diverse Zn-Pb-Ag-(Cu-Au) sulfide and Feoxide deposits, Bergslagen region, Sweden. Econ. Geol. 1996, 91, 979–1008. [Google Scholar] [CrossRef]
- Peltola, E. Origin of Precambrian Copper Sulfides of the Outokumpu Disctrict, Finland. Econ. Geol. 1978, 73, 461–477. [Google Scholar] [CrossRef]
- Kompanchenko, A.A.; Voloshin, A.V.; Balagansky, V.V. Vanadium Mineralization in the Kola Region, Fennoscandian Shield. Minerals 2018, 8, 474. [Google Scholar] [CrossRef]
- Kompanchenko, A.A.; Voloshin, A.V.; Bazai, A.V. Mineral composition of paleoproterozoic metamorphosed massive sulfide ore of the Kola region on example of Bragino occurrence in South Pechenga. Proc. Russ. Miner. Soc. 2019, 5, 74–88. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Melezhik, V.A.; Sturt, B.A. General geology and evolutionary history of the early Proterozoic Polmak-Pasvik-Pechenga-Imandra/Varzuga-Ust’Ponoy Greenstone Belt in the north-eastern Baltic Shield. Earth Sci. Rev. 1994, 36, 205–241. [Google Scholar] [CrossRef]
- Glebovitsky, V.A. (Ed.) Early Precambrian of the Baltic Shield; Nauka: Saint-Petersburg, Russia, 2005; p. 711. ISBN 5-02-024950-5. (In Russian) [Google Scholar]
- Melezhik, V.A.; Prave, A.R.; Hanski, E.J.; Fallick, A.E.; Lepland, A.; Kump, L.R.; Srauss, H. (Eds.) The Palaeoproterozoic of Fennoscandia as Context for the Fennoscandian Arctic Russia—Drilling Early Earth Project. In Reading the Archive of Earth’s Oxygenation; Springer: Heidelberg, Germany, 2013; p. 490. [Google Scholar]
- Mints, M.V.; Dokukina, K.A.; Konilov, A.N.; Philippova, I.B.; Zlobin, V.L.; Babayants, P.S.; Belousova, E.A.; Blokh, V.I.; Bogina, M.M.; Bush, D.A.; et al. East European Craton: Early Precambrian History and 3D Models of Deep Crustal Structure; Geological Society of America Special Paper 510; Geological Society of America: Boulder, CO, USA, 2015; p. 433. [Google Scholar]
- Skuf’in, P.K.; Theart, H.F.J. Geochemical and tectono-magmatic evolution of the volcano-sedimentary rocks of Pechenga and other greenstone fragments within the Kola Greenstone Belt, Russia. Precambrian Res. 2005, 141, 1–48. [Google Scholar] [CrossRef]
- Skuf’in, P.K.; Elizarov, D.V.; Zhavkov, V.A. Geological and geochemical pecularities of volcanics of the the South Pechenga structural zone. Proc. Murm. State Technol. Univ. 2009, 12, 416–435. (In Russian) [Google Scholar]
- Pouret, O.; Dia, A. Vanadium. In Encyclopedia of Geochemistry; White, W.M., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Balashov, Y.A. Paleoproterozoic geochronology of the Pechenga-Varzuga structure, Kola Peninsula. Petrology 1996, 4, 3–25. (In Russian) [Google Scholar]
- Akhmedov, A.M.; Voronyaeva, L.V.; Pavlov, V.A.; Krupenik, V.A.; Kuznetsov, V.A.; Sveshnikova, K.Y. The gold content of the South Pechenga structural zone (the Kola Peninsula): Types of manifestations and prospects for identifying industrial gold contents. Reg. Geol. Metallog. 2004, 20, 143–165. (In Russian) [Google Scholar]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Miner. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. (Eds.) Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 2001. [Google Scholar]
- Spiridonov, E.M. Titanium coulsonite from Kalgoorlie deposit. Dokl. AS USSR 1979, 245, 447–449. (In Russian) [Google Scholar]
- Gatehouse, B.M.; Grey, I.E.; Nickel, E.H. The crystal chemistry of nolanite, (V,Fe,Ti,Al)10O14(OH)2, from Kalgoorlie, Western Australia. Am. Miner. 1983, 68, 833–839. [Google Scholar]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist Crystal Structure Database. Am. Miner. 2003, 88, 247–250. [Google Scholar]
- Maslennikov, V.V.; Ayupova, N.R.; Maslennikova, S.P.; Tret’yakov, G.A.; Melekestzeva, I.Y. Toxic Elements in Massive Sulfide Systems; Institute of Mineralogy, the Ural Branch of Russian Academy of Sciences: Miass, Russia, 2014; p. 340. ISBN 978-5-7691-2411-2. (In Russian) [Google Scholar]
- Butler, I.B.; Nesbitt, R.W. Trace element distribution in the chalcopyrite wall of a black smoker chimney: Insight from laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Earth Planet. Sci. Lett. 1999, 167, 335–345. [Google Scholar] [CrossRef]
- Zaykov, V.V.; Maslennikov, V.V.; Zaykova, E.V.; Herrington, R. Ore-Formation and Ore-Facies Analyses of Massive Sulphide Deposits of the Ural Paleoocean; Institute of Mineralogy, the Ural Branch of Russian Academy of Sciences: Miass, Russia, 2001; p. 315. (In Russian) [Google Scholar]
- Vikentiev, I.V. Precious metal and telluride mineralogy of large volcanic-hosted massive sulfide deposits in the Ural. Miner. Petrol. 2006, 87, 305–326. [Google Scholar] [CrossRef]
- Vikent’ev, I.V.; Yudovskaya, M.A.; Moloshag, V.P. Speciation of noble metals and conditions of their concentration in massive sulfide ores of the Urals. Geol. Ore Depos. 2006, 48, 77–107. [Google Scholar] [CrossRef]
- Moloshag, V.P.; Grabezhev, A.I.; Vikent’ev, I.V.; Gulyaeva, T.Y. Ore formation facies of massive sulphide ore deposits and copper-gold-porphyry deposits of Urals. Litosfera 2004, 2, 30–51. (In Russian) [Google Scholar]
- Spry, P.G.; Scott, S.D. Zincian spinel and staurolite in the Appalachians and Scandinavian caledonides. Can. Miner. 1986, 24, 147–163. [Google Scholar]
| Characteristics/Varieties of Coulsonite | Ore Type | Morphology | Mineral Paragenesis | Special Features of Chemical Composition | Physical Properties | Alleged Genesis |
|---|---|---|---|---|---|---|
| Coulsonite [8] | Banded pyrrhotite ore | Corroded crystals and growth up to 100 µm | Rim of mukhinite or/and goldmanite, V-bearing epidote and grossular | High Mn content, low Fe and Cr content | Microhardness close to reference, coefficient of reflection accepted by author of this work as a standart | Metamorphic |
| coulsonite-III | Massive pyrrhotite ore of type II and massive pyrite ore | Various, crystals and growth up to 100–150 µm | 1. W-V-bearing rutile, ilmenite 2. V-bearing ilmenite, ferberite 3. Rim of magnetite 4. Nolanite | Minimal Mn content, high variation of Cr, Fe and V content | Increased microhardness 577 kg/mm2, coefficient of reflection lower than that to coulsonite | Metamorphic |
| coulsonite-II | Relics of quartz–albite veins in the massive pyrrhotite ore of type II and massive pyrite | Lamellar (needle-like in slice) crystals and growth up to 10 × 30 µm | V-bearing muscovite, roscoelite, crictonite group minerals, rutile, byrudite, tivanite, thortveitite, siderite | Increased Fe content, and Ti in some crystals | High-titanium crystals have the highest coefficient of reflection | Hydrothermal |
| coulsonite-I | Massive pyrrhotite ore type 1 | Octahedral crystals and growth up to 300–500 µm | W-V-bearing rutile, V-bearing senaite, V-bearing phlogopite | Increased Zn and Mn content, almost complete absence of Fe3+, uniformly high Cr content | High microhardness 743 kg/mm2, low chromite-like coefficient of reflection | Metamorphic |
| Samples | Coulsonite-I | Coulsonite-II | Coulsonite-III | Coulsonite * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Max Cr | Min Cr | Av (22) | Max Ti | Min Ti | Av (9) | Max Cr | Min Cr | Av (36) | ||
| SiO2 | n.d. | n.d. | 0.01 | n.d. | n.d. | n.d. | 0.05 | n.d. | 0.01 | n.d. |
| TiO2 | 0.29 | 0.44 | 0.22 | 18.77 | 0.53 | 8.77 | 0.54 | n.d. | 0.90 | n.d. |
| Al2O3 | 0.99 | n.d. | 1.02 | 0.62 | 1.64 | 1.15 | 2.46 | 0.49 | 0.89 | n.d. |
| Cr2O3 | 32.87 | 20.21 | 24.99 | 7.07 | 11.57 | 9.05 | 28.04 | 7.11 | 10.79 | 9.90 |
| V2O3 | 32.91 | 46.14 | 39.98 | 40.70 | 40.03 | 39.47 | 28.51 | 50.08 | 43.38 | 55.60 |
| FeO | 25.69 | 27.33 | 27.21 | 31.30 | 42.86 | 38.66 | 35.82 | 39.61 | 40.83 | 25.63 |
| ZnO | 4.48 | 3.32 | 3.79 | 0.72 | 3.11 | 2.12 | 4.12 | 2.04 | 2.32 | 2.74 |
| MnO | 2.34 | 2.55 | 2.31 | n.d. | 0.18 | 0.10 | 0.09 | n.d. | 0.02 | 5.08 |
| Total | 99.57 | 99.99 | 99.53 | 99.18 | 99.92 | 99.32 | 99.63 | 99.33 | 99.15 | 99.06 ** |
| apfu | based on three cations and four oxygens | |||||||||
| Si | - | - | - | - | - | - | 0.002 | - | - | - |
| Ti | 0.01 | 0.01 | - | 0.50 | 0.02 | - | 0.02 | - | - | - |
| Al | 0.04 | - | - | 0.03 | 0.07 | - | 0.11 | 0.02 | - | - |
| Cr | 0.97 | 0.59 | - | 0.20 | 0.35 | - | 0.84 | 0.22 | - | 0.29 |
| V | 0.98 | 1.38 | - | 1.15 | 1.24 | - | 0.86 | 1.54 | - | 1.67 |
| Fe3+ | - | 0.02 | - | 0.13 | 0.32 | - | 0.17 | 0.22 | - | - |
| Fe2+ | 0.84 | 0.86 | - | 0.98 | 0.92 | - | 0.89 | 0.96 | - | 0.81 |
| Zn | 0.10 | 0.07 | - | 0.02 | 0.08 | - | 0.11 | 0.04 | - | 0.08 |
| Mn | 0.06 | 0.07 | - | - | 0.01 | - | 0.003 | - | - | 0.16 |
| Λ, nm | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 400 | 16.19 | 17.71 | 15.78 | 14.93 | 16.64 |
| 420 | 16.12 | 17.24 | 15.79 | 15.01 | 16.44 |
| 440 | 16.09 | 16.85 | 15.81 | 15.08 | 16.28 |
| 460 | 16.06 | 16.6 | 15.83 | 15.14 | 16.21 |
| 480 | 16.04 | 16.39 | 15.85 | 15.2 | 16.18 |
| 500 | 16.01 | 16.24 | 15.88 | 15.25 | 16.18 |
| 520 | 15.98 | 16.14 | 15.93 | 15.3 | 16.23 |
| 540 | 15.95 | 16.07 | 15.98 | 15.34 | 16.3 |
| 560 | 15.93 | 15.98 | 16.01 | 15.37 | 16.38 |
| 580 | 15.91 | 15.92 | 16.06 | 15.4 | 16.46 |
| 600 | 15.88 | 15.86 | 16.12 | 15.42 | 16.54 |
| 620 | 15.89 | 15.8 | 16.21 | 15.45 | 16.67 |
| 640 | 15.9 | 15.74 | 16.33 | 15.48 | 16.83 |
| 660 | 15.94 | 15.74 | 16.45 | 15.49 | 17.03 |
| 680 | 16.03 | 15.8 | 16.56 | 15.51 | 17.26 |
| 700 | 16.13 | 15.99 | 16.66 | 15.53 | 17.5 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kompanchenko, A.A. Coulsonite FeV2O4—A Rare Vanadium Spinel Group Mineral in Metamorphosed Massive Sulfide Ores of the Kola Region, Russia. Minerals 2020, 10, 843. https://doi.org/10.3390/min10100843
Kompanchenko AA. Coulsonite FeV2O4—A Rare Vanadium Spinel Group Mineral in Metamorphosed Massive Sulfide Ores of the Kola Region, Russia. Minerals. 2020; 10(10):843. https://doi.org/10.3390/min10100843
Chicago/Turabian StyleKompanchenko, Alena A. 2020. "Coulsonite FeV2O4—A Rare Vanadium Spinel Group Mineral in Metamorphosed Massive Sulfide Ores of the Kola Region, Russia" Minerals 10, no. 10: 843. https://doi.org/10.3390/min10100843
APA StyleKompanchenko, A. A. (2020). Coulsonite FeV2O4—A Rare Vanadium Spinel Group Mineral in Metamorphosed Massive Sulfide Ores of the Kola Region, Russia. Minerals, 10(10), 843. https://doi.org/10.3390/min10100843





