Effect of Ordinary Portland Cement and Water Glass on the Properties of Alkali Activated Fly Ash Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Techniques
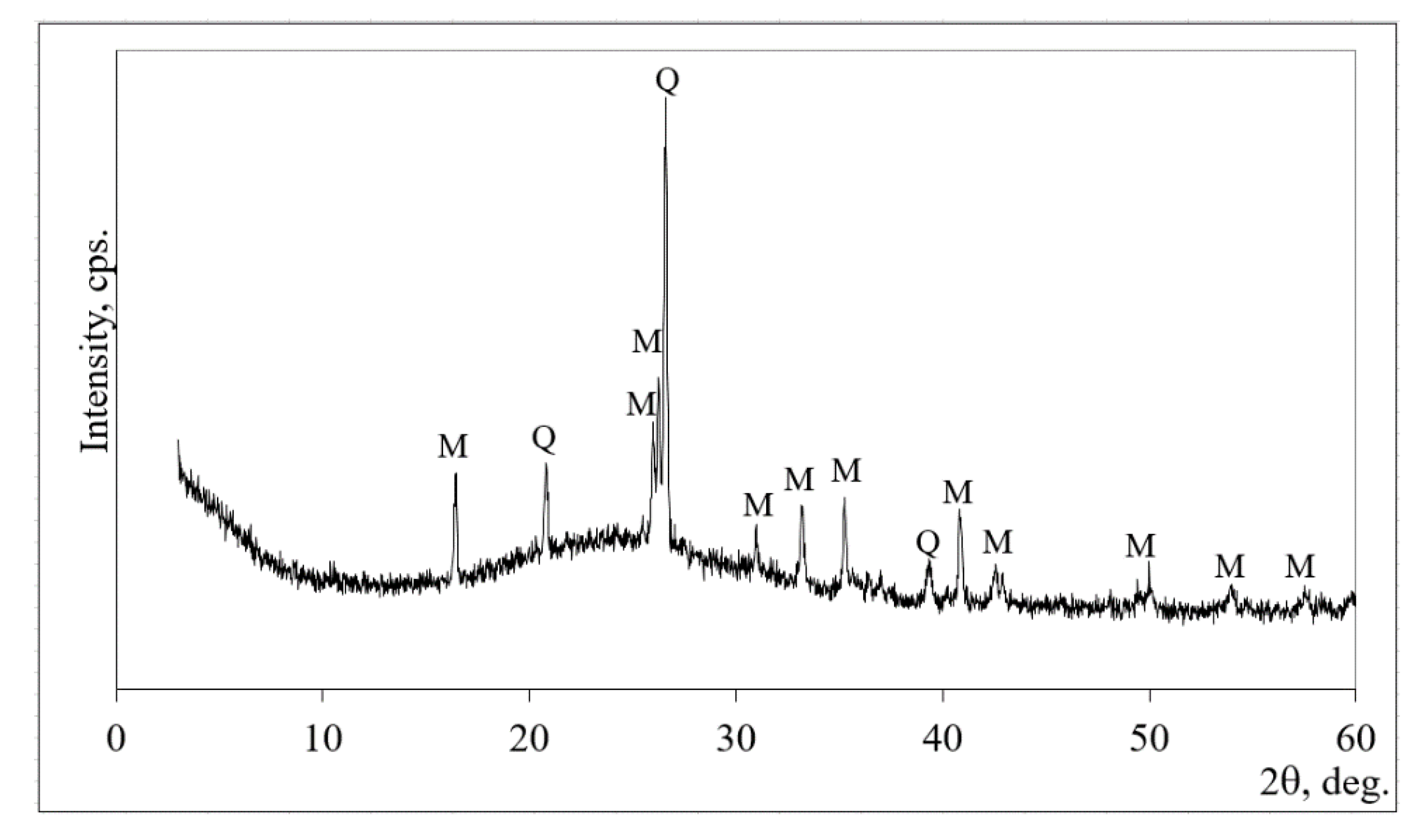
2.2. Materials and Sample Preparation
3. Results and Discussion
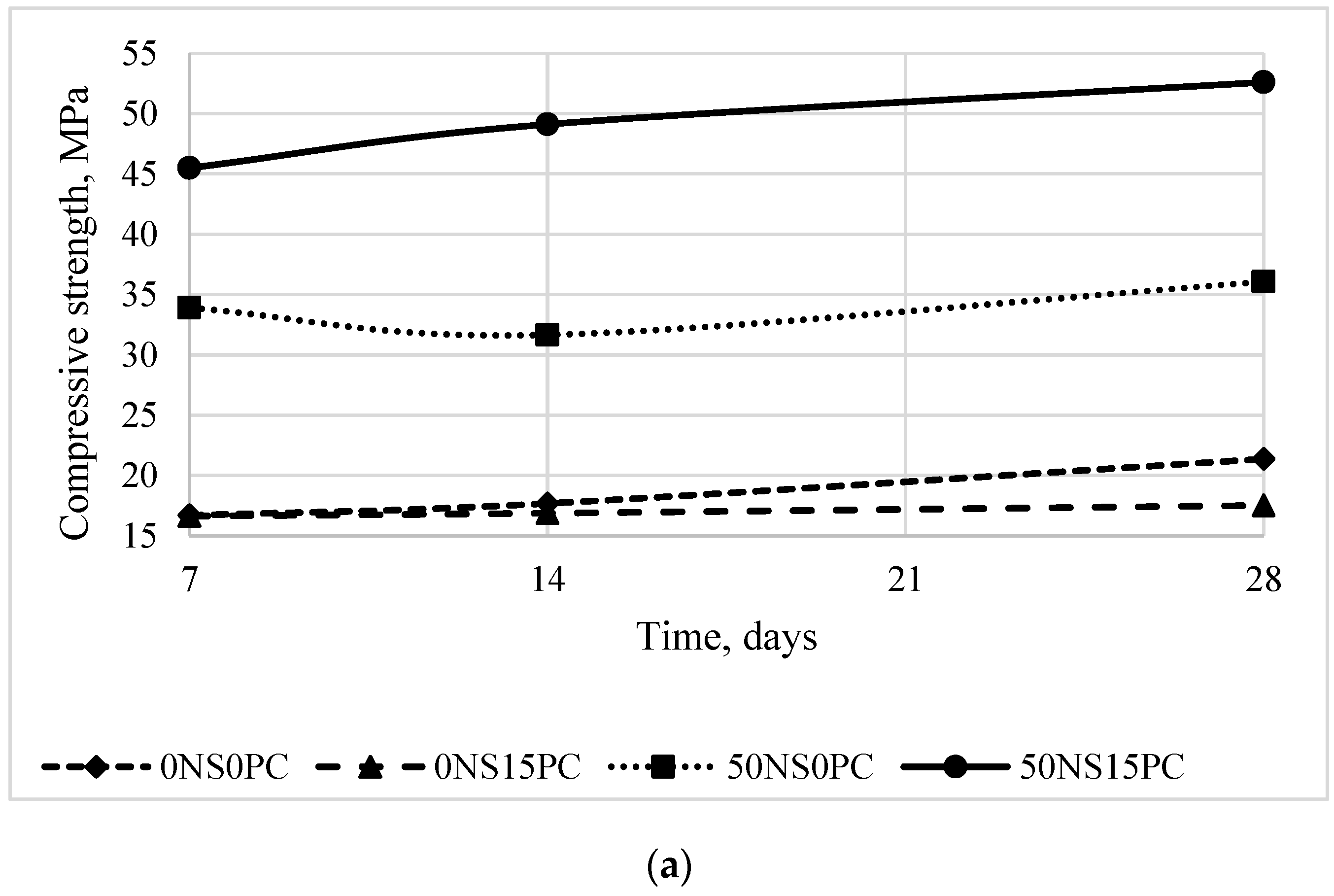
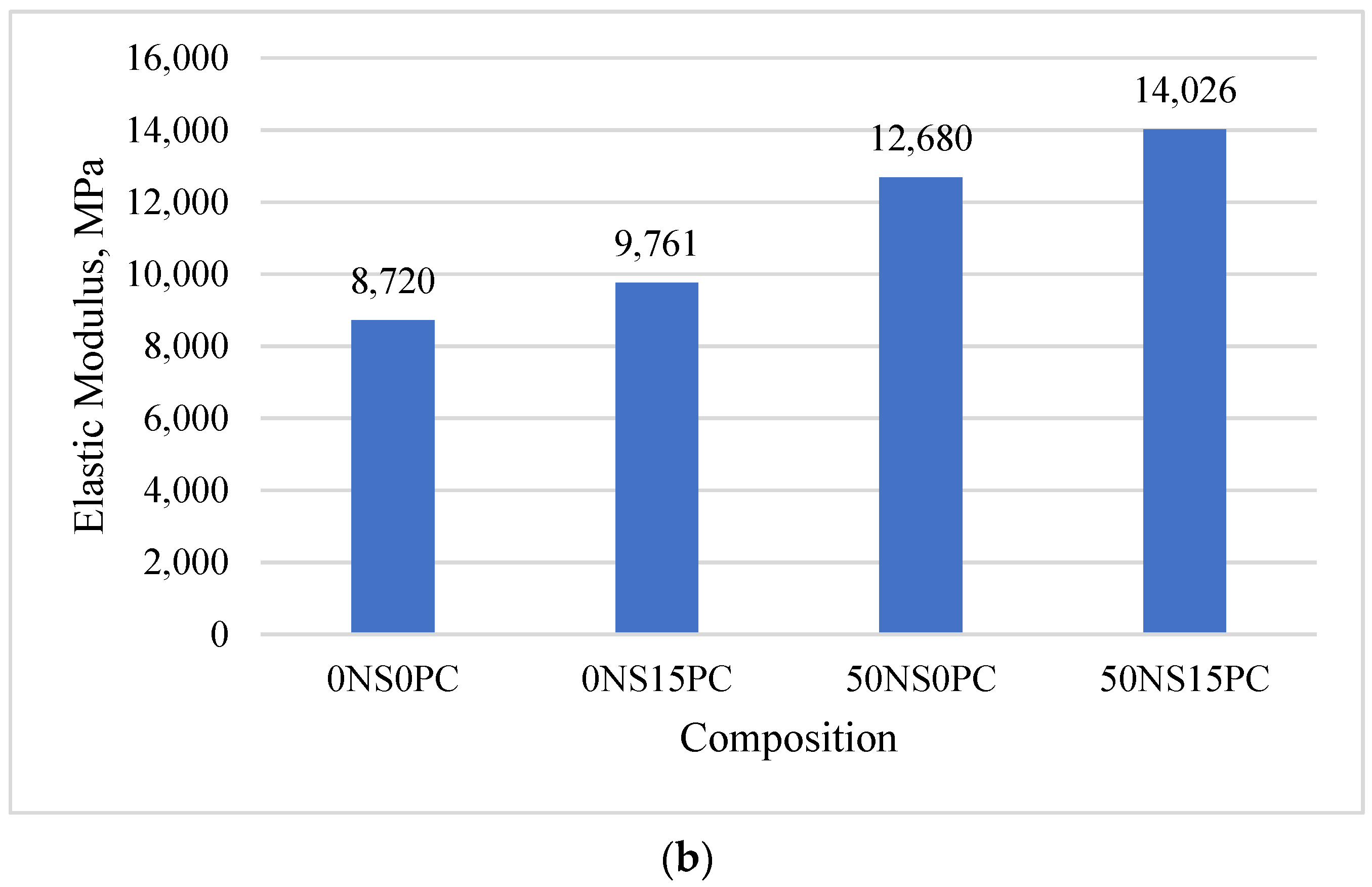
3.1. Mechanical Properties
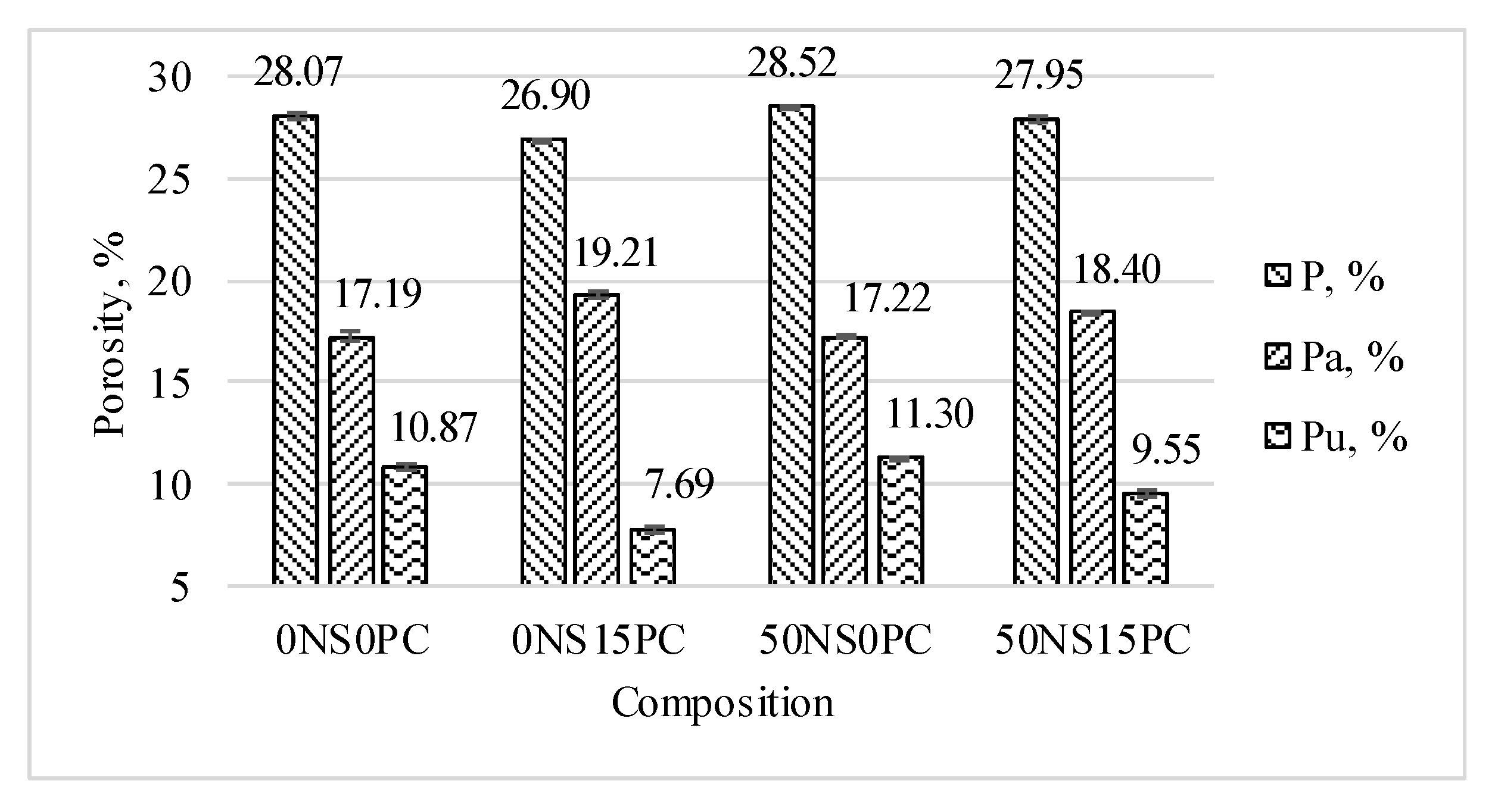
3.2. Porosity
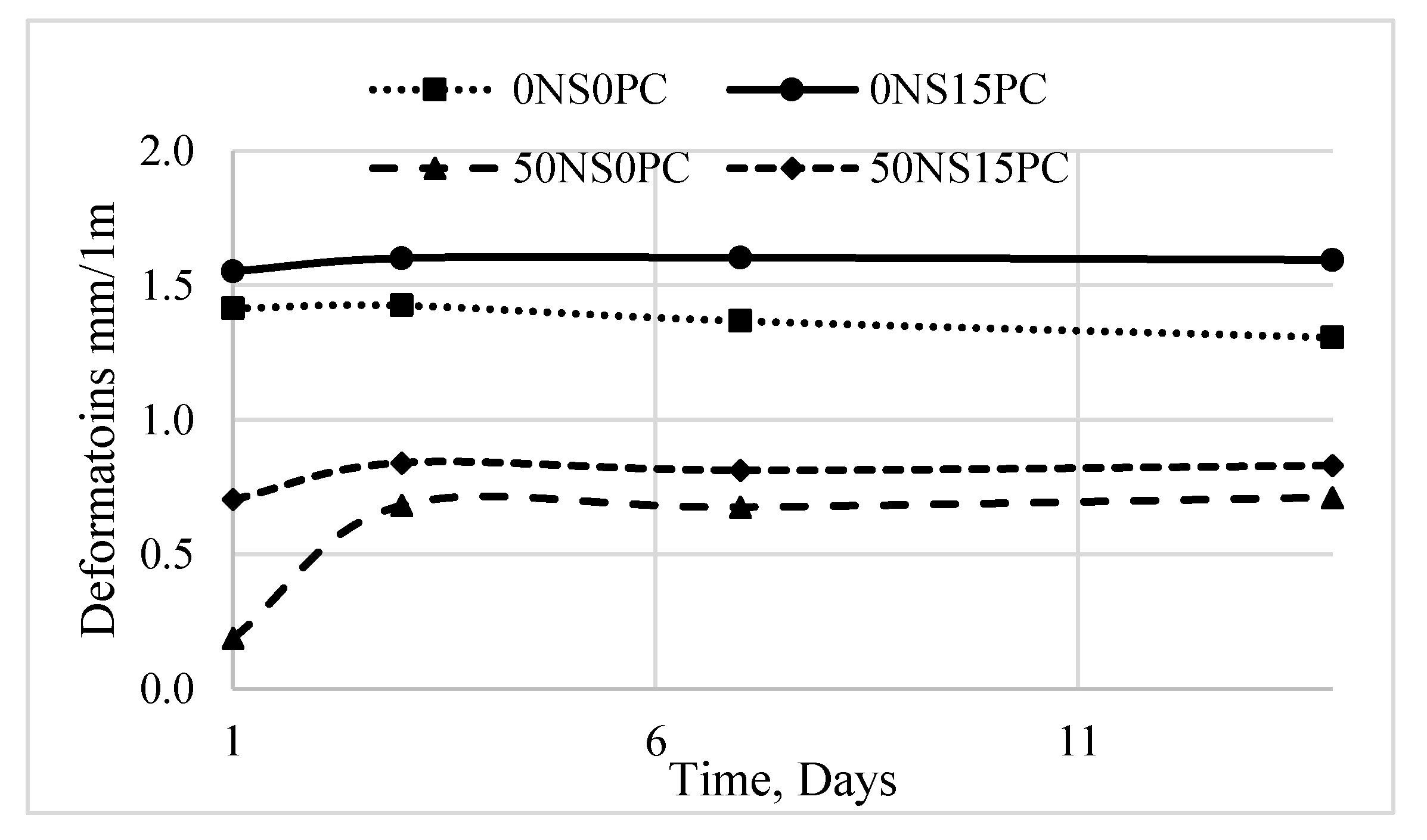
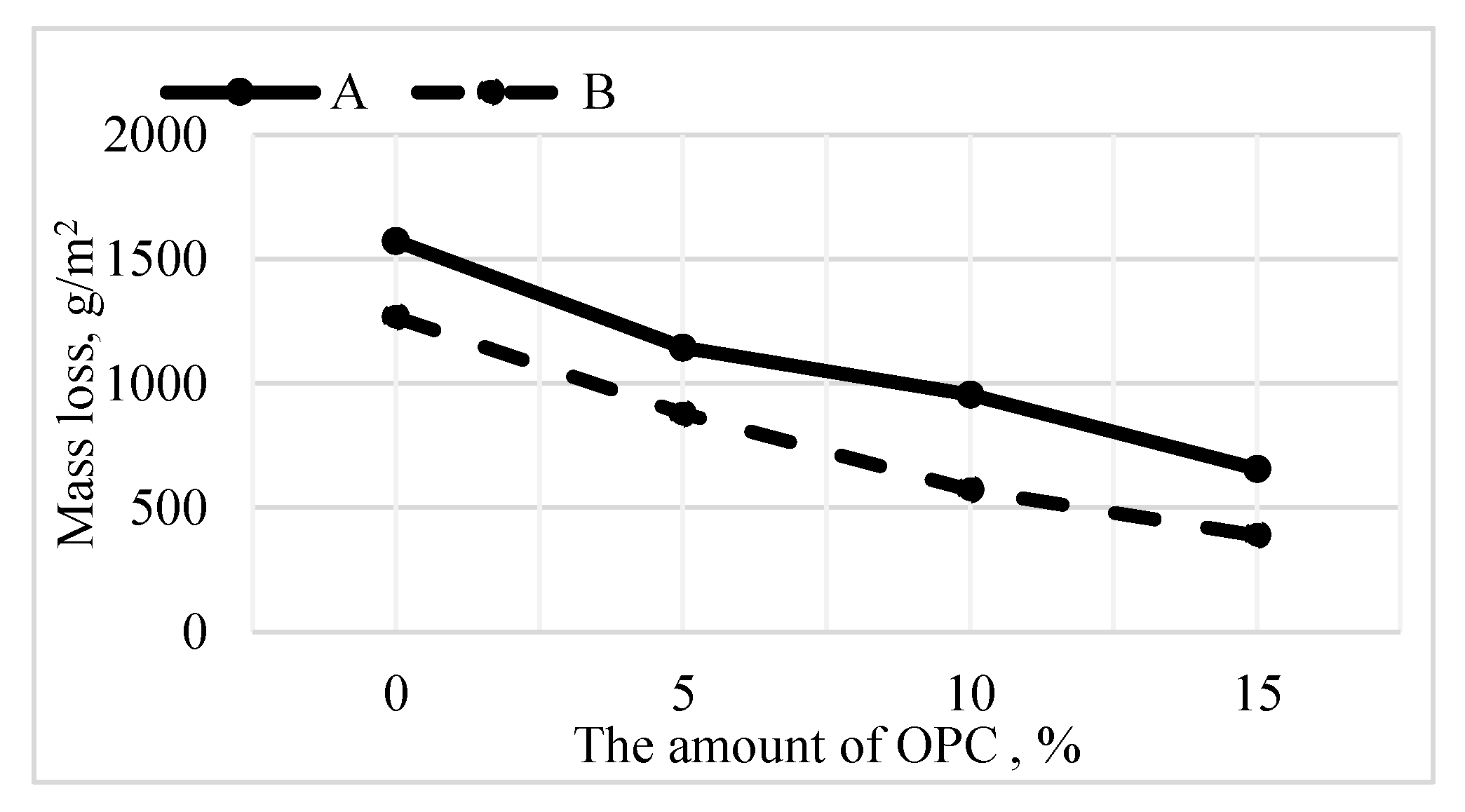
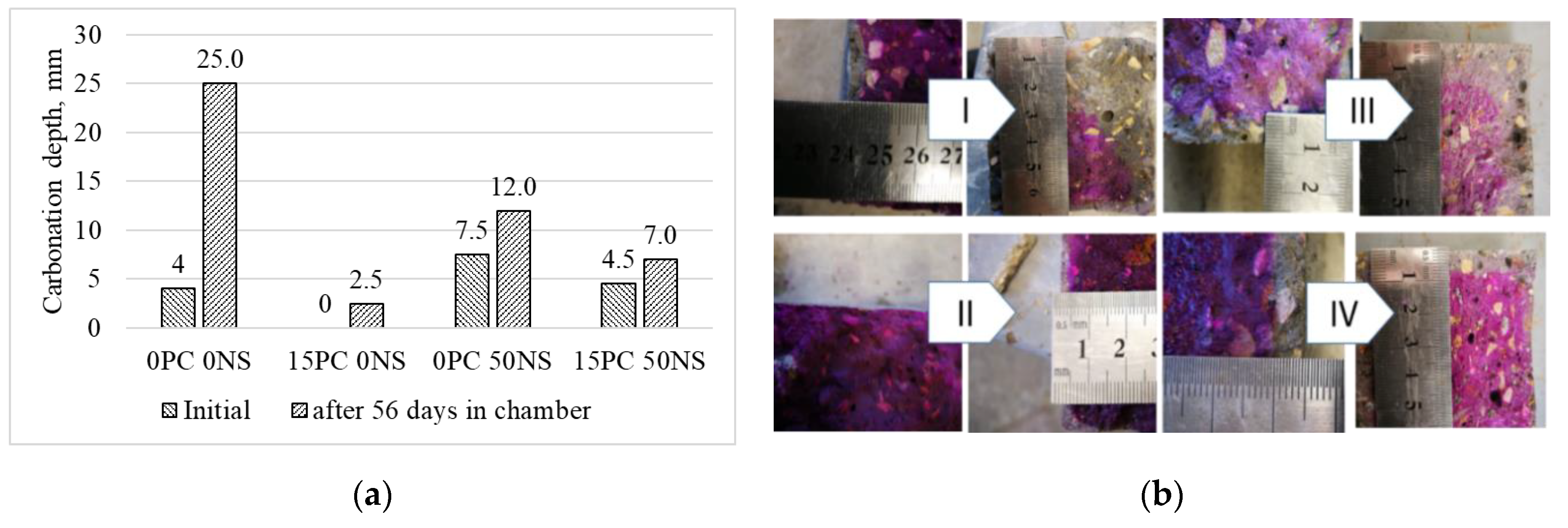
3.3. Durability of Alkali-Activated Materials
4. Conclusions
- The expansive deformations are less significant when there are conditions promoting ASR.
- Carbonation depth is reduced.
- The amount of material detached due to repetitive freeze and thaw is reduced.
Author Contributions
Funding
Conflicts of Interest
References
- Król, M.; Ro, P.; Chlebda, D.; Mozgawa, W. Molecular and Biomolecular Spectroscopy In fl uence of alkali metal cations/type of activator on the structure of alkali-activated fly ash—ATR-FTIR studies. Spectrochim. Acta Part A 2018, 198, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.R.; Lee, N.K.; Park, S.M.; Abbas, N.; Lee, H.K. Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential. J. Hazard Mater. 2018, 353, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Rozek, P.; Krol, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Askarian, M.; Tao, Z.; Adam, G.; Samali, B. Mechanical properties of ambient cured one-part hybrid OPC-geopolymer concrete. Constr. Build. Mater. 2018, 186, 330–337. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N–A–S–H and C–A–S–H gels, Study in the ternary. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Maltseva, O.; Palomo, Á.; Fernández-Jiménez, A.N.A. Cimenturi hubride alcaline partea I: Fundamente. Hybrid alkaline cements. Rev. Romana Mater. 2012, 42, 330–335. [Google Scholar]
- Palomo, A.; Krivenko, P.; Kavalerova, E.; Maltseva, O. A review on alkaline activation: New analytical perspectives. Mater. Construcción 2018, 64, 22. [Google Scholar] [CrossRef] [Green Version]
- Nath, P.; Sarker, P.K. Use of OPC to improve setting and early strength properties of low calcium fly ash geopolymer concrete cured at room temperature. Cem. Concr. Compos. 2015, 55, 205–214. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Flexural strength and elastic modulus of ambient-cured blended low-calcium fly ash geopolymer concrete. Constr. Build. Mater. 2017, 130, 22–31. [Google Scholar] [CrossRef]
- Nuaklong, P.; Sata, V.; Wongsa, A.; Srinavin, K.; Chindaprasirt, P. Recycled aggregate high calcium fly ash geopolymer concrete with inclusion of OPC and nano-SiO2. Constr. Build. Mater. 2018, 174, 244–252. [Google Scholar] [CrossRef]
- Pouhet, R.; Cyr, M. Alkali—silica reaction in metakaolin-based geopolymer mortar. Mater. Struct. 2015, 48, 571–583. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Allouche, E. Effect of Alkali Silica Reaction (ASR) in Geopolymer Concrete. In Proceedings of the World of Coal Ash (WOCA) Conference, Denver, CO, USA, 9–12 May 2011. [Google Scholar]
- Shi, Z.; Shi, C.; Wan, S.; Ou, Z. Effect of alkali dosage on alkali-silica reaction in sodium hydroxide activated slag mortars. Constr. Build. Mater. 2017, 143, 16–23. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y. Resistance of alkali-activated slag concrete to alkali—Aggregate reaction. Cem. Concr. Res. 2001, 31, 331–334. [Google Scholar] [CrossRef]
- Tänzer, R.; Jin, Y.; Stephan, D. Effect of the inherent alkalis of alkali activated slag on the risk of alkali silica reaction. Cem. Concr. Res. 2017, 98, 82–90. [Google Scholar] [CrossRef]
- Riahi, S.; Nazari, A.; Zaarei, D.; Khalaj, G.; Bohlooli, H.; Kaykha, M.M. Compressive strength of ash-based geopolymers at early ages designed by Taguchi method. Mater. Des. 2012, 37, 443–449. [Google Scholar] [CrossRef]
- Lloyd, N.A.; Rangan, B.A. Geopolymer Concrete with Fly Ash. In Proceedings of the Second International Conference Sustainable Construction Materials and Technologies, Ancoma, Italy, 28–30 June 2010; pp. 1493–1504. [Google Scholar]
- Samantasinghar, S.; Singh, S.P. Hardened Properties of Fly Ash–Slag Blended Geopolymer Paste and Mortar. Int. J. Concr. Struct. Mater. 2019, 13, 47. [Google Scholar] [CrossRef]
- Bocullo, V.; Vaičiukynienė, D.; Borg, R.P.; Briguglio, C. Alkaline Activation of Hybrid Cements Binders Based on Industrial by-Products. J. Sustain. Archit. Civ. Eng. 2017, 19, 65–73. [Google Scholar]
- Grinys, A.; Bocullo, V.; Gumuliauskas, A. Research of Alkali Silica Reaction in Concrete With Active Mineral Additives. J. Sustain. Archit. Civ. Eng. 2014, 6, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Puertas, F.; Palacios, M.; Vázquez, T. Carbonation process of alkali-activated slag mortars. J. Mater. Sci. 2006, 41, 3071–3082. [Google Scholar] [CrossRef]
- Alaa, M. A synopsis of carbonation of alkali-activated materials. Greem Mater. 2019, 7, 1–19. [Google Scholar]
| Oxide | CaO | SiO2 | Al2O3 | Fe2O3 | K2O | MgO | SO3 | Na2O | TiO2 | ZrO2 | ZnO | MnO | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 3.68 | 49.47 | 27.45 | 7.38 | 4.54 | 1.70 | 0.92 | 0.95 | 1.66 | 0.147 | 0.05 | 0.063 | 1.99 |
| OPC | 61.32 | 19.51 | 5.25 | 3.36 | 1.01 | 3.84 | 4.30 | 0.94 | 0.13 | 0.016 | - | 0.042 | 0.282 |
| Ingredients | NaOH Activation | NaOH + Na2OnSiO2 ∙ mH2O Activation | ||
|---|---|---|---|---|
| 0NS0PC | 0NS15PC | 50NS0PC | 50NS15PC | |
| Sand 0/4 | 583 * | 583 * | 583 * | 583 * |
| Sand 0/4 | 1312 ** | 1312 ** | 1312 ** | 1312 ** |
| Sand 0/4 | 1180 *** | 1180 *** | 1180 *** | 1180 *** |
| Gravel 4/16 | 729 * | 729 * | 729 * | 729 * |
| Gravel 4/16 | 0 ** | 0 ** | 0 ** | 0 ** |
| Gravel 4/16 | 0 *** | 0 *** | 0 *** | 0 *** |
| Water | 238 | 238 | 119 | 119 |
| FA | 719 | 611 | 719 | 611 |
| NaOH | 166 | 166 | 83 | 83 |
| Water glass | 0 | 0 | 202 | 202 |
| OPC | 0 | 108 | 0 | 108 |
| Glass cullet (4/2) | 131 *** | 131 *** | 131 *** | 131 *** |
| Superplasticizer | 0 | 0 | 0 | 13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocullo, V.; Vaičiukynienė, D.; Gečys, R.; Daukšys, M. Effect of Ordinary Portland Cement and Water Glass on the Properties of Alkali Activated Fly Ash Concrete. Minerals 2020, 10, 40. https://doi.org/10.3390/min10010040
Bocullo V, Vaičiukynienė D, Gečys R, Daukšys M. Effect of Ordinary Portland Cement and Water Glass on the Properties of Alkali Activated Fly Ash Concrete. Minerals. 2020; 10(1):40. https://doi.org/10.3390/min10010040
Chicago/Turabian StyleBocullo, Vytautas, Danutė Vaičiukynienė, Ramūnas Gečys, and Mindaugas Daukšys. 2020. "Effect of Ordinary Portland Cement and Water Glass on the Properties of Alkali Activated Fly Ash Concrete" Minerals 10, no. 1: 40. https://doi.org/10.3390/min10010040
APA StyleBocullo, V., Vaičiukynienė, D., Gečys, R., & Daukšys, M. (2020). Effect of Ordinary Portland Cement and Water Glass on the Properties of Alkali Activated Fly Ash Concrete. Minerals, 10(1), 40. https://doi.org/10.3390/min10010040







