Cross-Reactive and Lineage-Specific Single Domain Antibodies against Influenza B Hemagglutinin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Influenza Antigens and Immunisation of the Alpacas
2.2. Construction and Selection of the Phage-Displayed Libraries
2.3. Antibody Expression and Screening
2.4. Analysis Using Surface Plasmon Resonance
2.5. Next-Generation-Sequence-Assisted Single Domain Antibody Discovery
2.6. Lentiviral Pseudotype Assays
2.7. Construction and Screening of a Randomly Mutagenised HA Library Using Yeast Display
3. Results
3.1. Isolation and Characterisation of Cross-Reactive and Lineage-Specific Single Domain Antibodies against the Influenza B Hemagglutinin
3.2. Grouping sdAbs on the Basis of HA1 Head Domain or Stem Domain-Specific Binding
3.3. In Vitro Neutralisation Activity of Single Domain Antibodies
3.4. Identification of the B Victoria Lineage-Specific Epitope
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, H.; Thompson, W.W.; Viboud, C.G.; Ringholz, C.M.; Cheng, P.Y.; Steiner, C.; Abedi, G.R.; Anderson, L.J.; Brammer, L.; Shay, D.K. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin. Infect. Dis. 2012, 54, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.W.; Schmier, J.K.; Kuehn, C.M.; Ryan, K.J.; Oxford, J. The burden of influenza B: A structured literature review. Am. J. Public Health 2013, 103, e43–e51. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, T.; Ikonen, N.; Ziegler, T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999–2012. Clin. Infect. Dis. 2014, 59, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Cameroni, E.; Guarino, B.; Kallewaard, N.L.; Zhu, Q.; Lanzavecchia, A. Tackling influenza with broadly neutralizing antibodies. Curr. Opin. Virol. 2017, 24, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Skehel, J.J.; Wiley, D.C. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu. Rev. Biochem. 2000, 69, 531–569. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Voss, J.; Gamblin, S.J.; Codoni, G.; Macagno, A.; Jarrossay, D.; Vachieri, S.G.; Pinna, D.; Minola, A.; Vanzetta, F.; et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 2011, 333, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Bhabha, G.; Elsliger, M.A.; Friesen, R.H.; Jongeneelen, M.; Throsby, M.; Goudsmit, J.; Wilson, I.A. Antibody recognition of a highly conserved influenza virus epitope. Science 2009, 324, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Hwang, W.C.; Perez, S.; Wei, G.; Aird, D.; Chen, L.M.; Santelli, E.; Stec, B.; Cadwell, G.; Ali, M.; et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Kashyap, A.K.; Steel, J.; Rubrum, A.; Bhabha, G.; Khayat, R.; Lee, J.H.; Dillon, M.A.; O’Neil, R.E.; Faynboym, A.M.; et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 2012, 489, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, C.; Laursen, N.S.; Kwaks, T.; Zuijdgeest, D.; Khayat, R.; Ekiert, D.C.; Lee, J.H.; Metlagel, Z.; Bujny, M.V.; Jongeneelen, M.; et al. Highly conserved protective epitopes on influenza B viruses. Science 2012, 337, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Yasugi, M.; Kubota-Koketsu, R.; Yamashita, A.; Kawashita, N.; Du, A.; Sasaki, T.; Nishimura, M.; Misaki, R.; Kuhara, M.; Boonsathorn, N.; et al. Human monoclonal antibodies broadly neutralizing against influenza B virus. PLoS Pathog. 2013, 9, e1003150. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.; Swem, L.R.; Park, S.; Nakamura, G.; Chiang, N.; Estevez, A.; Fong, R.; Kamen, L.; Kho, E.; Reichelt, M.; et al. A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action. Nat. Commun. 2017, 8, 14234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xu, L.; Dey, B.; Hessell, A.J.; Van, R.D.; Xiang, S.H.; Yang, X.; Zhang, M.Y.; Zwick, M.B.; Arthos, J.; et al. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature 2007, 445, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Hufton, S.E.; Risley, P.; Ball, C.R.; Major, D.; Engelhardt, O.G.; Poole, S. The breadth of cross sub-type neutralisation activity of a single domain antibody to influenza hemagglutinin can be increased by antibody valency. PLoS ONE 2014, 9, e103294. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, S.; Ying, T. Single-Domain Antibodies as Therapeutics against Human Viral Diseases. Front. Immunol. 2017, 8, 1802. [Google Scholar] [CrossRef] [PubMed]
- Gaiotto, T.; Hufton, S.E. Cross-Neutralising Nanobodies Bind to a Conserved Pocket in the Hemagglutinin Stem Region Identified Using Yeast Display and Deep Mutational Scanning. PLoS ONE 2016, 11, e0164296. [Google Scholar] [CrossRef] [PubMed]
- Rota, P.A.; Wallis, T.R.; Harmon, M.W.; Rota, J.S.; Kendal, A.P.; Nerome, K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990, 175, 59–68. [Google Scholar] [CrossRef]
- Weir, J.P.; Gruber, M.F. An overview of the regulation of influenza vaccines in the United States. Influenza Other Respir. Viruses 2016, 10, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Minor, P.D. Assaying the Potency of Influenza Vaccines. Vaccines 2015, 3, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sapienza, G.; Rossotti, M.A.; Tabares-da, R.S. Single-Domain Antibodies as Versatile Affinity Reagents for Analytical and Diagnostic Applications. Front. Immunol. 2017, 8, 977. [Google Scholar] [CrossRef] [PubMed]
- De Genst, E.; Silence, K.; Decanniere, K.; Conrath, K.; Loris, R.; Kinne, J.; Muyldermans, S.; Wyns, L. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc. Natl. Acad. Sci. USA 2006, 103, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, R.; Katsamba, P.S.; Nordin, H.; Pol, E.; Myszka, D.G. Analyzing a kinetic titration series using affinity biosensors. Anal. Biochem. 2006, 349, 136–147. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Glanville, J.; Ferrara, F.; Naranjo, L.; Gleasner, C.D.; Shen, X.; Bradbury, A.R.; Kiss, C. The antibody mining toolbox: An open source tool for the rapid analysis of antibody repertoires. MAbs 2014, 6, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Carnell, G.W.; Ferrara, F.; Grehan, K.; Thompson, C.P.; Temperton, N.J. Pseudotype-based neutralization assays for influenza: A systematic analysis. Front. Immunol. 2015, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Temperton, N.J.; Hoschler, K.; Major, D.; Nicolson, C.; Manvell, R.; Hien, V.M.; Ha, D.Q.; de Jong, M.; Zambon, M.; Takeuchi, Y.; et al. A sensitive retroviral pseudotype assay for influenza H5N1-neutralizing antibodies. Influenza Other Respir. Viruses 2007, 1, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Carnell, G.; Kinsley, R.; Bottcher-Friebertshauser, E.; Pohlmann, S.; Scott, S.; Fereidouni, S.; Corti, D.; Kellam, P.; Gilbert, S.; et al. Development and use of lentiviral Vectors Pseudotyped with Influenza B Haemagglutinins: Application to vaccine immunogenicity, mAb potency and sero-surveillance studies. bioRxiv 2018. [Google Scholar] [CrossRef]
- Ravn, U.; Didelot, G.; Venet, S.; Ng, K.T.; Gueneau, F.; Rousseau, F.; Calloud, S.; Kosco-Vilbois, M.; Fischer, N. Deep sequencing of phage display libraries to support antibody discovery. Methods 2013, 60, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.M.; Ruuls, R.C.; Nijman, I.J.; Niewold, T.A.; Frenken, L.G.; de Geus, B. Llama heavy-chain V regions consist of at least four distinct subfamilies revealing novel sequence features. Mol. Immunol. 2000, 37, 579–590. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, F.; Lu, M.; Tian, X.; Ma, J. Crystal structure of unliganded influenza B virus hemagglutinin. J. Virol. 2008, 82, 3011–3020. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Kondrashkina, E.; Wang, Q. Structural basis for the divergent evolution of influenza B virus hemagglutinin. Virology 2013, 446, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Stijlemans, B.; Conrath, K.; Cortez-Retamozo, V.; Van, X.H.; Wyns, L.; Senter, P.; Revets, H.; De Baetselier, P.; Muyldermans, S.; Magez, S. Efficient targeting of conserved cryptic epitopes of infectious agents by single domain antibodies. African trypanosomes as paradigm. J. Biol. Chem. 2004, 279, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Vil, M.D.; Jimenez, X.; Iacolina, M.; Zhang, H.; Zhu, Z. Single variable domain-IgG fusion. A novel recombinant approach to Fc domain-containing bispecific antibodies. J. Biol. Chem. 2006, 281, 10706–10714. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, K.; Dreier, T.; Silence, K.; de Haard, H.; Lauwereys, M.; Casteels, P.; Beirnaert, E.; Jonckheere, H.; Van de Wiele, C.; Staelens, L.; et al. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum. 2006, 54, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Balazs, A.B.; Bloom, J.D.; Hong, C.M.; Rao, D.S.; Baltimore, D. Broad protection against influenza infection by vectored immunoprophylaxis in mice. Nat. Biotechnol. 2013, 31, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Deal, C.E.; Balazs, A.B. Engineering humoral immunity as prophylaxis or therapy. Curr. Opin. Immunol. 2015, 35, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, L.I.; De Filette, M.; Hultberg, A.; Verrips, T.; Temperton, N.; Weiss, R.A.; Vandevelde, W.; Schepens, B.; Vanlandschoot, P.; Saelens, X. Nanobodies with in vitro neutralizing activity protect mice against H5N1 influenza virus infection. J. Infect. Dis. 2011, 203, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Soto, J.; Vasudevan, A.; Schmeisser, F.; Alvarado-Facundo, E.; Wang, W.; Weiss, C.D.; Weir, J.P. Determination of influenza B identity and potency in quadrivalent inactivated influenza vaccines using lineage-specific monoclonal antibodies. PLoS ONE 2017, 12, e0175733. [Google Scholar] [CrossRef] [PubMed]
- Chao, G.; Cochran, J.R.; Wittrup, K.D. Fine epitope mapping of anti-epidermal growth factor receptor antibodies through random mutagenesis and yeast surface display. J. Mol. Biol. 2004, 342, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Chen, T.F.; de Picciotto, S.; Yang, N.J.; Tzeng, A.; Santos, M.S.; Van Deventer, J.A.; Traxlmayr, M.W.; Wittrup, K.D. Protein Engineering and Selection Using Yeast Surface Display. Methods Mol. Biol. 2015, 1319, 3–36. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Higashi, N.; Nakagawa, N. Detection of antigenic variants of the influenza B virus by melting curve analysis with LCGreen. J. Virol. Methods 2008, 148, 296–299. [Google Scholar] [CrossRef] [PubMed]
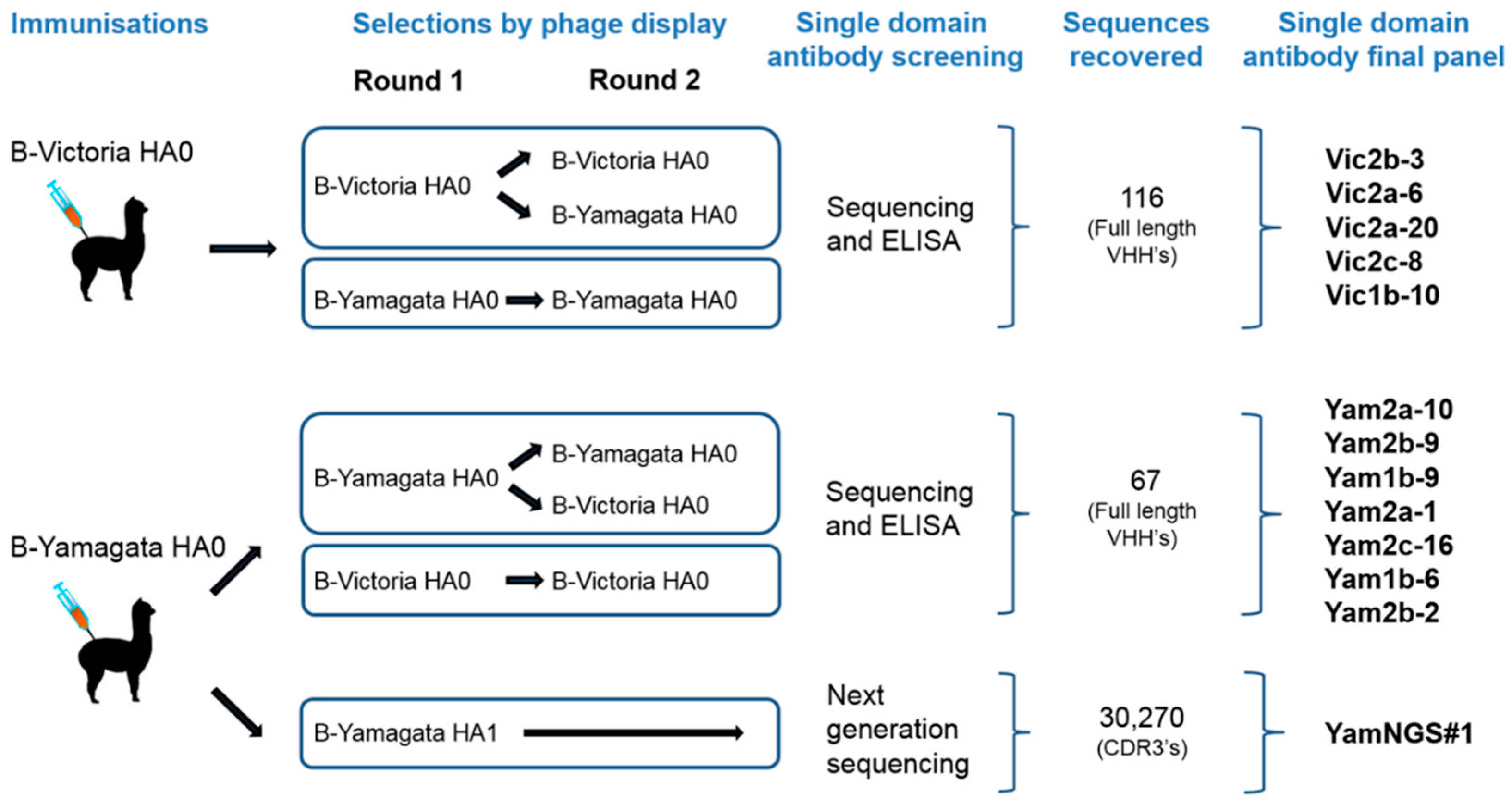
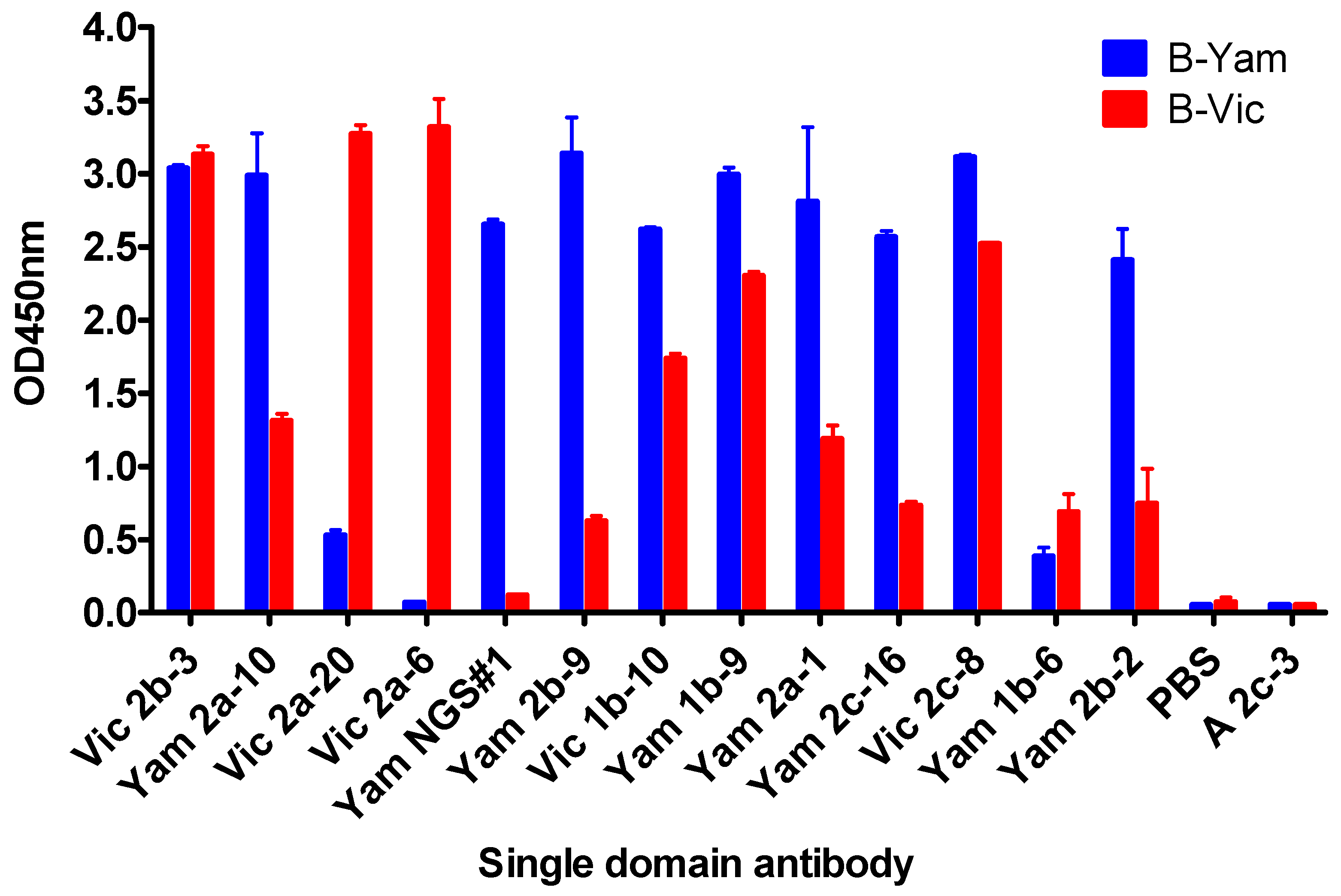
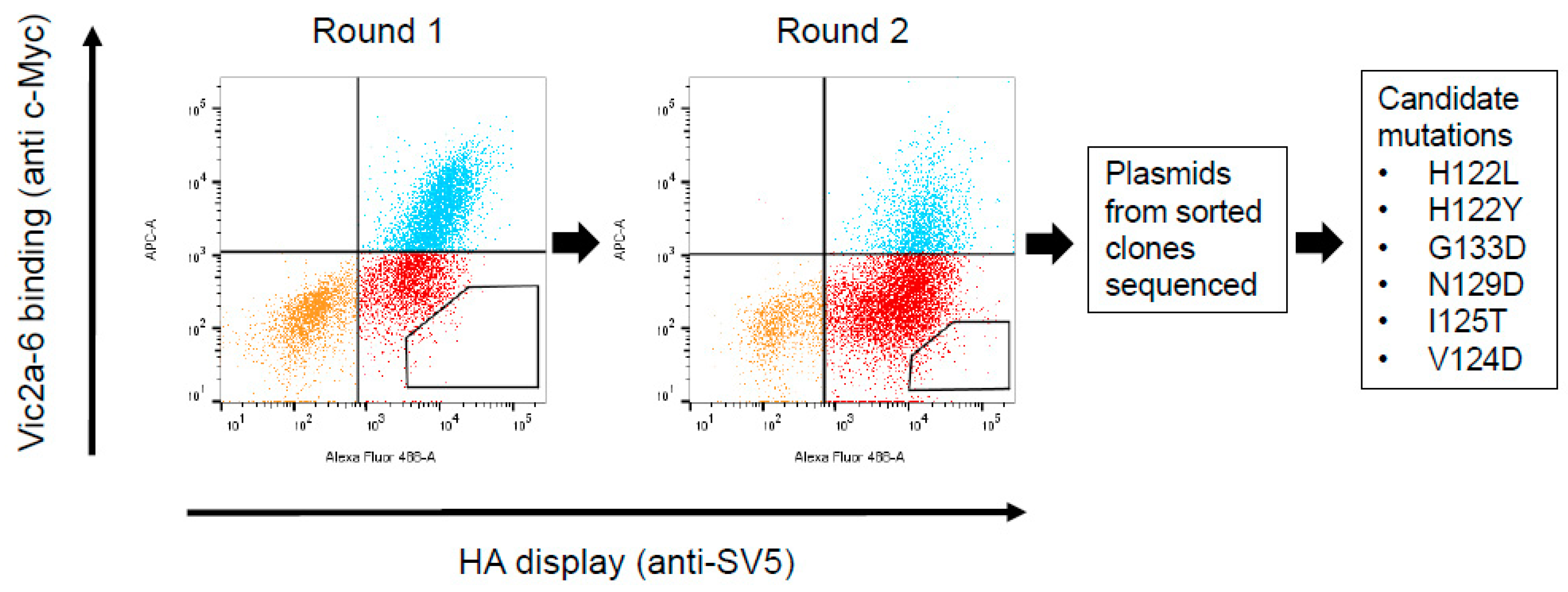
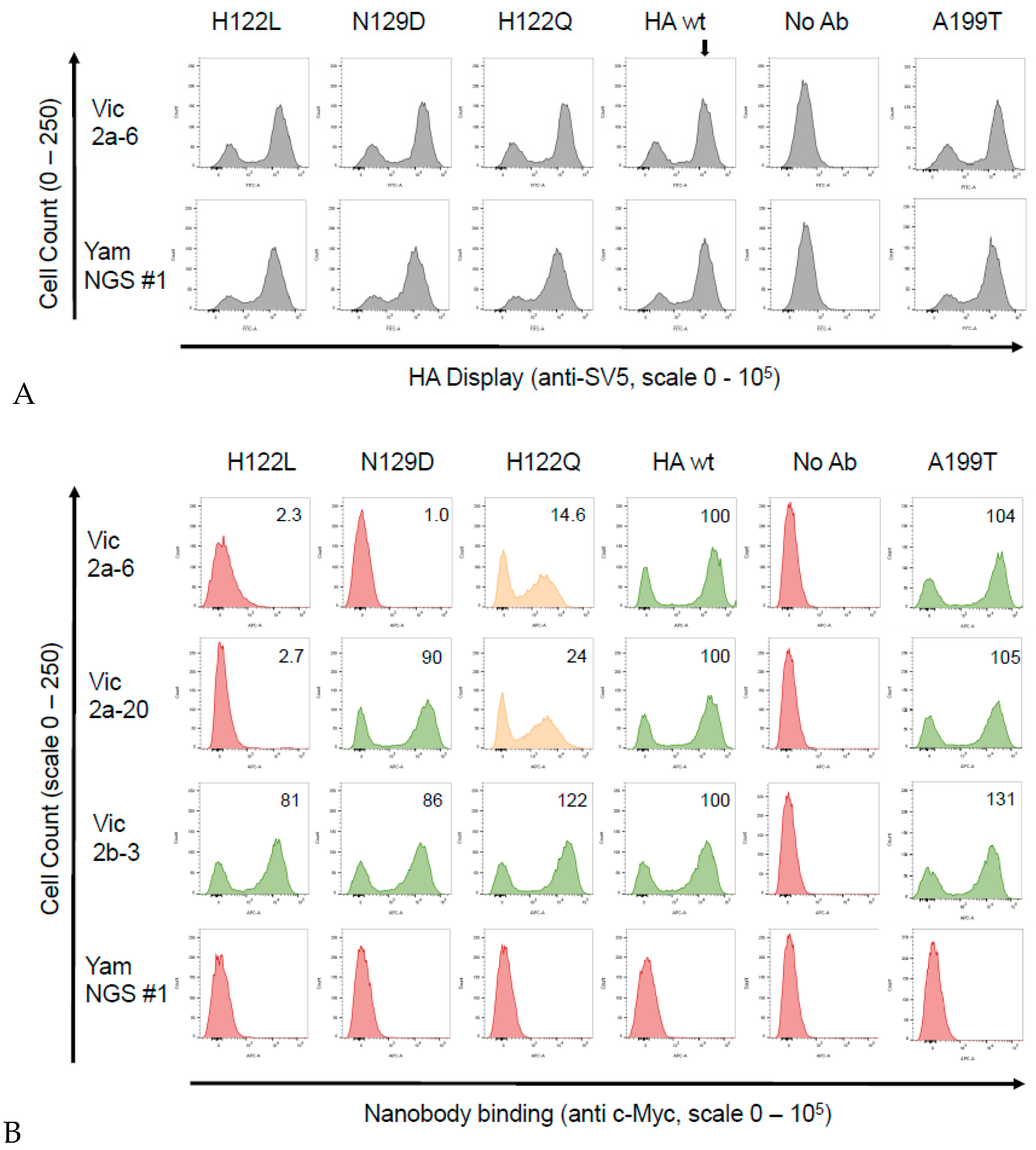
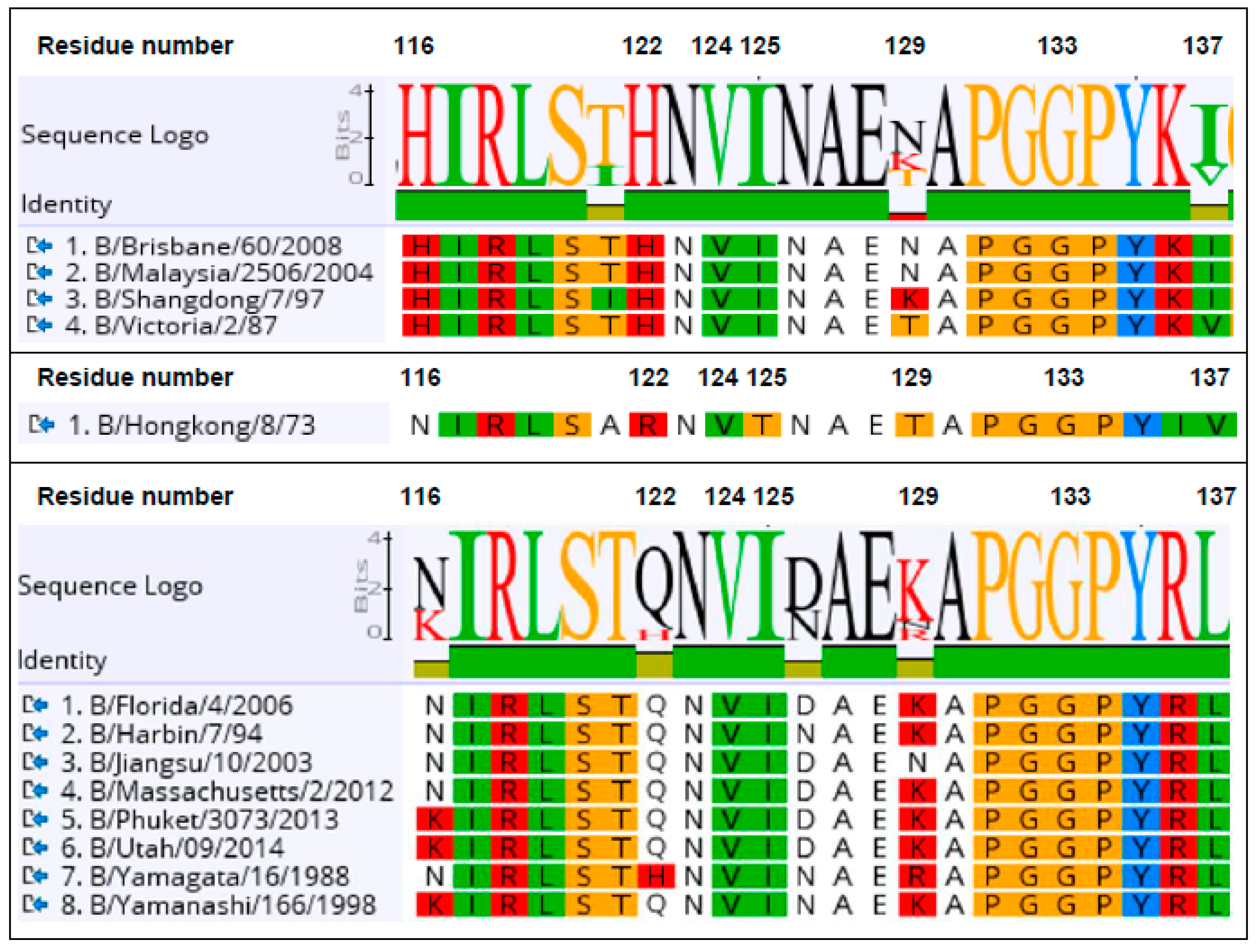
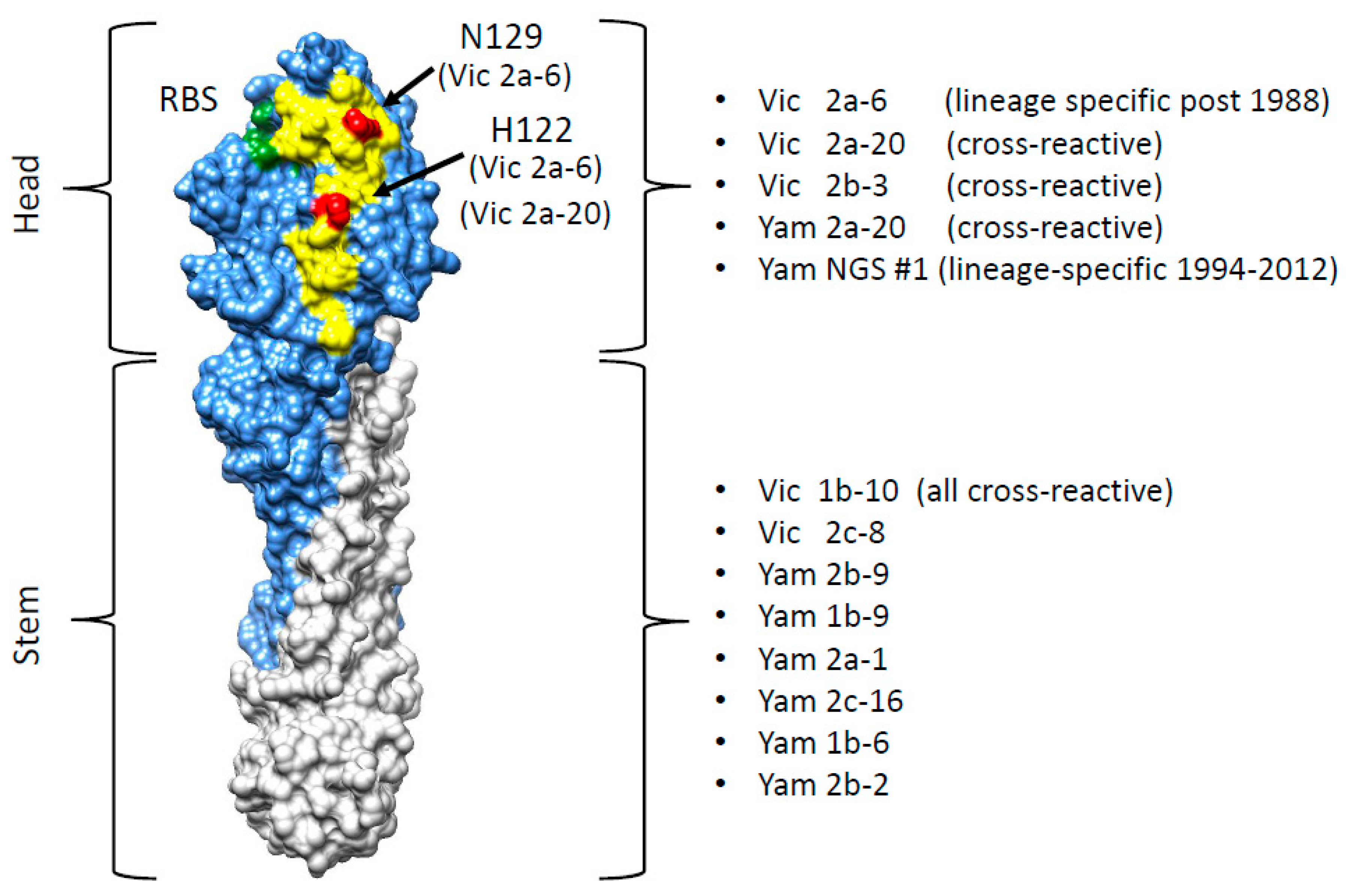
| Nanobody | CDR3 Sequence | B-Yamagata | B-Victoria |
|---|---|---|---|
| Vic2b-3 | AADAVVAGPDDEYDY | + | + |
| Yam2a-10 | NVGFNY | + | + |
| Vic2a-20 | ASKGTDYIDGIYYISYQFNS | + | + |
| Vic2a-6 | VASPFSTGRATLPYQYPY | − | + |
| YamNGS#1 | AAASLCSFSSNDYFY | + | − |
| Yam2b-9 | AAGSGGRYDY | + | + |
| Vic1b-10 | NAPTYSN | + | + |
| Yam1b-9 | ALGDFTGLTNLRQAFYDF | + | + |
| Yam2a-1 | NFPRSSS | + | + |
| Yam2c-16 | NTHDY | + | + |
| Vic2c-8 | ALGDFSGSLWAYEYDF | + | + |
| Yam1b-6 | AAAKGGGAYSMISAYTY | + | + |
| Yam2b-2 | RLDHWLVSGY | + | + |
| Antigen Standard | Year | Vic2b-3 | Yam2a-10 | Vic2a20 | Vic2a-6 | YamNGS#1 | Yam2b-9 | Vic1b-10 | Yam1b-9 | Yam2a-1 | Yam2c-16 | Vic2c-8 | Yam1b-6 | Yam2b-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B/Brisbane/60/2008 | 2008 | 0.34 | 0.16 | 2.30 | 2.42 | 0.08 | 0.45 | 0.79 | 0.30 | 0.82 | 0.45 | 0.29 | 0.50 | 0.07 |
| B/Malaysia/2506/2004 | 2004 | 0.07 | 0.08 | 3.54 | 1.93 | 0.09 | 0.68 | 1.39 | 0.40 | 1.43 | 0.72 | 0.40 | 0.59 | 0.07 |
| B/Shangdong/7/97 | 1997 | 0.39 | 0.14 | 2.58 | 0.32 | 0.06 | 0.69 | 0.96 | 0.30 | 0.92 | 0.51 | 0.28 | 0.31 | 0.08 |
| B/Victoria/2/87 | 1987 | 0.43 | 0.20 | 3.31 | 0.86 | 0.09 | 0.56 | 1.27 | 0.83 | 1.35 | 1.15 | 0.77 | 1.04 | 0.11 |
| B/HongKong/8/73 | 1973 | 0.46 | 0.39 | 0.06 | 0.07 | 0.13 | 0.34 | 1.48 | 0.66 | 1.41 | 0.72 | 0.56 | 0.29 | 0.09 |
| B/Yamagata/16/88 | 1988 | 0.83 | 1.76 | 2.41 | 1.07 | 0.09 | 1.59 | 1.85 | 0.98 | 1.79 | 1.01 | 0.87 | 0.71 | 0.12 |
| B/Yamanashi/166/98 | 1994 | 0.74 | 1.08 | 0.11 | 0.07 | 0.07 | 1.70 | 2.16 | 1.34 | 2.41 | 1.35 | 1.24 | 1.70 | 0.14 |
| B/Harbin/7/94 | 1994 | 0.28 | 0.26 | 0.14 | 0.09 | 0.44 | 0.38 | 0.57 | 0.27 | 0.52 | 0.36 | 0.27 | 0.30 | 0.06 |
| B/Jiangsu/10/2003 | 2003 | 0.46 | 0.71 | 1.16 | 0.08 | 0.59 | 0.66 | 1.32 | 0.53 | 1.33 | 0.46 | 0.53 | 0.23 | 0.06 |
| B/Florida/4/2006 | 2006 | 0.42 | 0.40 | 0.16 | 0.07 | 0.83 | 0.62 | 0.64 | 0.20 | 0.68 | 0.31 | 0.18 | 0.32 | 0.07 |
| B/Mass’/02/2012 | 2012 | 0.53 | 0.82 | 0.79 | 0.05 | 1.00 | 3.03 | 1.82 | 0.94 | 1.96 | 0.85 | 0.84 | 1.57 | 0.10 |
| B/Phuket/3073/2013 | 2013 | 0.58 | 1.22 | 0.15 | 0.06 | 0.11 | 1.01 | 0.94 | 0.55 | 0.96 | 0.45 | 0.52 | 0.32 | 0.08 |
| B/Utah/9/2014 | 2014 | 0.90 | 1.36 | 0.14 | 0.07 | 0.10 | 0.31 | 1.13 | 0.52 | 1.20 | 0.43 | 0.50 | 0.21 | 0.05 |
| A/Brisbane/10/2007 | 2007 | 0.05 | 0.06 | 0.07 | 0.07 | 0.07 | 0.06 | 0.06 | 0.07 | 0.06 | 0.07 | 0.06 | 0.07 | 0.07 |
| Single Domain Antibody | B Yam HA1 1 B/Florida /4/2006 | B Yam HA0 2 B/Florida /4/2006 | B Vic HA1 1 B/Brisbane /60/2008 | B Vic HA0 2 B/Brisbane /60/2008 | Domain | Specificity |
|---|---|---|---|---|---|---|
| Vic2b-3 | 0.27 nM | 1.57 nM | 0.06 nM | 0.11 nM | Head | Cross reactive |
| Yam2a-10 | 3.49 nM | 6.70 nM | + 3 | 3.30 nM | Head | Cross reactive |
| Vic2a-20 | 173 nM | - | 0.79 nM | 0.48 nM | Head | Cross reactive |
| Vic2a-6 | - | - | 0.07 nM | 0.08 nM | Head | B-Vic lineage |
| YamNGS#1 | 11.8 nM | 2.39 nM | - | - | Head | B-Yam lineage |
| Yam2b-9 | - | 2.13 nM | - | 10.0 nM | Stem | Cross reactive |
| Vic1b-10 | - | 0.28 nM | - | 1.1 nM | Stem | Cross reactive |
| Yam1b-9 | - | 0.10 nM | - | 0.08 nM | Stem | Cross reactive |
| Yam2a-1 | - | 0.14 nM | - | 7.40 nM | Stem | Cross reactive |
| Yam2c-16 | - | 1.46 nM | - | 0.76 nM | Stem | Cross reactive |
| Vic2c-8 | - | 0.13 nM | - | 0.37 nM | Stem | Cross reactive |
| Yam1b-6 | - | 5.43 nM | - | 0.60 nM | Stem | Cross reactive |
| Yam2b-2 | - | 10.0 nM | - | 1.36 nM | Stem | Cross reactive |
| Single Domain Antibody | B/Yamagata /16/1988 (Yamagata) IC50 [nM] | B/Victoria /2/1987 (Victoria) IC50 [nM] | B/Florida /4/2006 (Yamagata) IC50 [nM] | B/Brisbane /60/2008 (Victoria) IC50 [nM] |
|---|---|---|---|---|
| Vic2b-3 | 104 | 81 | 246 | 240 |
| Yam2a-10 | - | - | - | - |
| Vic2a-20 | - | 1.1 | - | 112 |
| Vic2a-6 | 315 | 159 | - | 10 |
| YamNGS1#1 | - | - | NT | NT |
| Yam2b-9 | 30.5 | - | NT | NT |
| Vic1b-10 | 0.2 | 15.5 | NT | NT |
| Yam1b-9 | 31.7 | 81.8 | NT | NT |
| Yam2a-1 | 81.8 | 6 | NT | NT |
| Yam2c-16 | NT | NT | NT | NT |
| Vic2c-8 | 202.5 | 228 | NT | NT |
| Yam1b-6 | 26.6 | 12.6 | NT | NT |
| Yam2b-2 | - | 113.5 | NT | NT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramage, W.; Gaiotto, T.; Ball, C.; Risley, P.; Carnell, G.W.; Temperton, N.; Cheung, C.Y.; Engelhardt, O.G.; Hufton, S.E. Cross-Reactive and Lineage-Specific Single Domain Antibodies against Influenza B Hemagglutinin. Antibodies 2019, 8, 14. https://doi.org/10.3390/antib8010014
Ramage W, Gaiotto T, Ball C, Risley P, Carnell GW, Temperton N, Cheung CY, Engelhardt OG, Hufton SE. Cross-Reactive and Lineage-Specific Single Domain Antibodies against Influenza B Hemagglutinin. Antibodies. 2019; 8(1):14. https://doi.org/10.3390/antib8010014
Chicago/Turabian StyleRamage, Walter, Tiziano Gaiotto, Christina Ball, Paul Risley, George W. Carnell, Nigel Temperton, Chung Y. Cheung, Othmar G. Engelhardt, and Simon E. Hufton. 2019. "Cross-Reactive and Lineage-Specific Single Domain Antibodies against Influenza B Hemagglutinin" Antibodies 8, no. 1: 14. https://doi.org/10.3390/antib8010014
APA StyleRamage, W., Gaiotto, T., Ball, C., Risley, P., Carnell, G. W., Temperton, N., Cheung, C. Y., Engelhardt, O. G., & Hufton, S. E. (2019). Cross-Reactive and Lineage-Specific Single Domain Antibodies against Influenza B Hemagglutinin. Antibodies, 8(1), 14. https://doi.org/10.3390/antib8010014






