ADME Considerations and Bioanalytical Strategies for Pharmacokinetic Assessments of Antibody-Drug Conjugates
Abstract
1. Introduction to Antibody-Drug Conjugates (ADC)
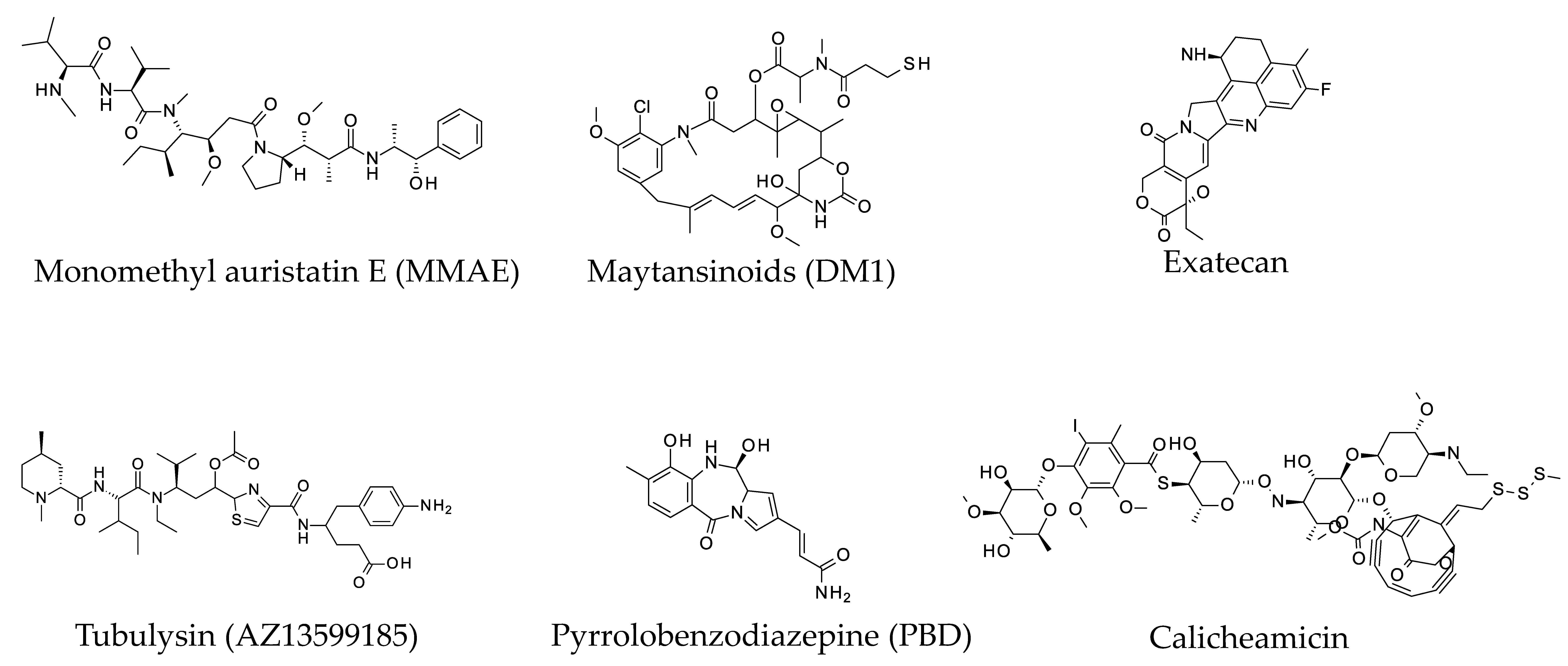
1.1. Cytotoxic Warheads
1.2. Linkers and Conjugation Sites for ADCs
2. ADME Considerations for Pre-Clinical and Clinical Development of ADC
3. Bioanalytical Platforms for ADCs
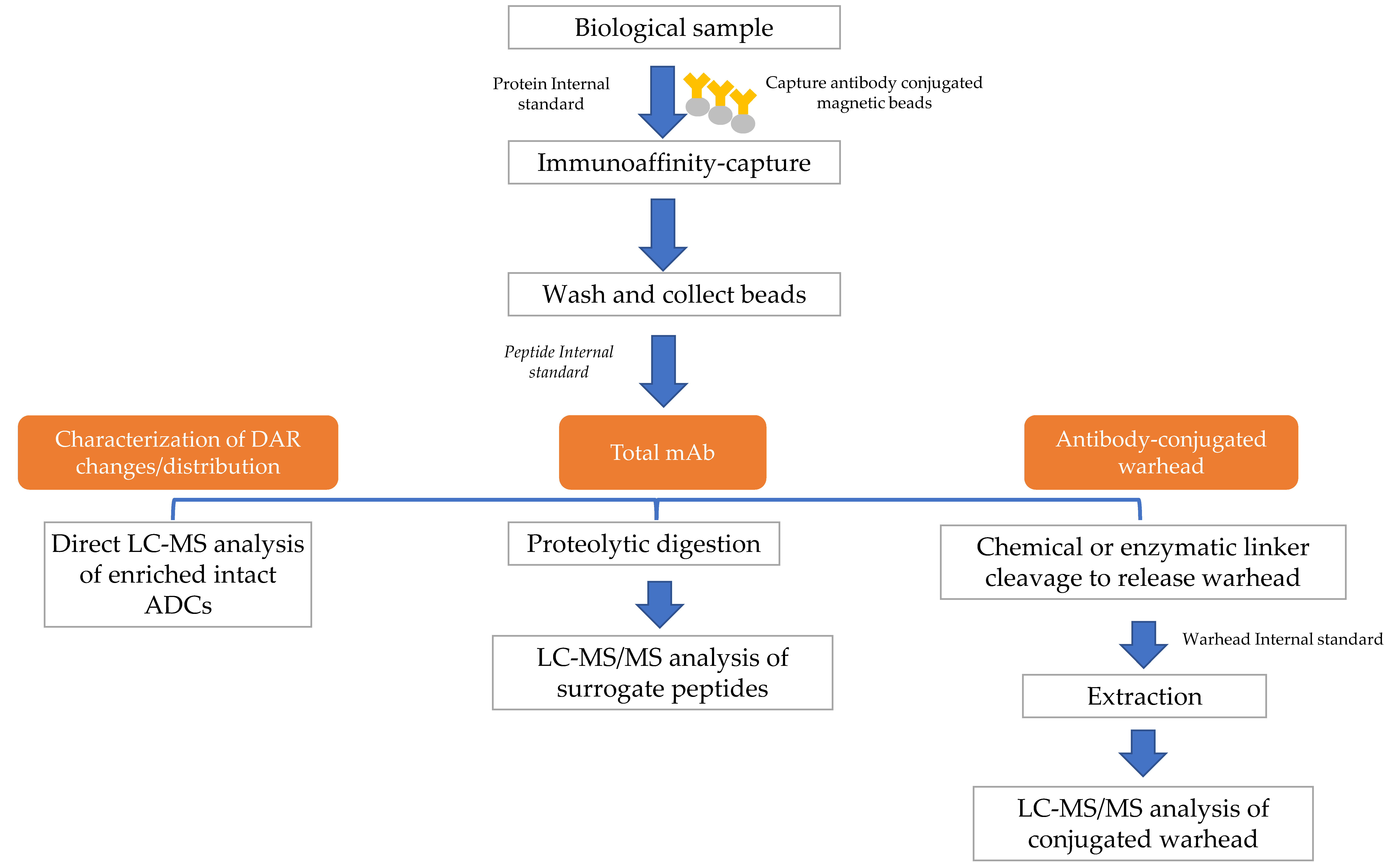
4. Hybrid LBA-LC-MS for ADC Analysis
4.1. Hybrid LBA-LC-MS of Surrogate Peptides of ADCs
4.2. Hybrid LBA-LC-MS of Intact ADCs
4.3. Hybrid LBA-LC-MS of Conjugated Drugs
5. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Deslandes, A. Comparative clinical pharmacokinetics of antibody-drug conjugates in first-in-human Phase 1 studies. mAbs 2014, 6, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Pettit, G.R.; Hamel, E. Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem. Pharmacol. 1990, 39, 1941–1949. [Google Scholar] [CrossRef]
- Senter, P.D.; Sievers, E.L. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nature Biotechnol. 2012, 30, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Chari, R.V.; Martell, B.A.; Gross, J.L.; Cook, S.B.; Shah, S.A.; Blattler, W.A.; McKenzie, S.J.; Goldmacher, V.S. Immunoconjugates containing novel maytansinoids: Promising anticancer drugs. Cancer Res. 1992, 52, 127–131. [Google Scholar] [PubMed]
- Lambert, J.M.; Chari, R.V. Ado-trastuzumab Emtansine (T-DM1): An antibody-drug conjugate (ADC) for HER2-positive breast cancer. J. Med. Chem. 2014, 57, 6949–6964. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.W.; Sasse, F.; Lunsdorf, H.; Elnakady, Y.A.; Reichenbach, H. Mechanism of action of tubulysin, an antimitotic peptide from myxobacteria. Chembiochem 2006, 7, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Oganesyan, V.; Peng, L.; Bee, J.S.; Li, J.; Perry, S.R.; Comer, F.; Xu, L.; Cook, K.; Senthil, K.; Clarke, L.; et al. Structural insights into the mechanism of action of a biparatopic anti-HER2 antibody. J. Boil. Chem. 2018, 293, 8439–8448. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Perry, S.R.; Muniz-Medina, V.; Wang, X.; Wetzel, L.K.; Rebelatto, M.C.; Hinrichs, M.J.; Bezabeh, B.Z.; Fleming, R.L.; Dimasi, N.; et al. A Biparatopic HER2-Targeting Antibody-Drug Conjugate Induces Tumor Regression in Primary Models Refractory to or Ineligible for HER2-Targeted Therapy. Cancer Cell 2016, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- A Phase 1/2 Study of MEDI4276 in Adults Subjects with Select HER2-Expressing Advanced Solid Tumors. Available online: https://ClinicalTrials.gov/show/NCT02576548 (accessed on 23 May 2018).
- Flynn, M.J.; Zammarchi, F.; Tyrer, P.C.; Akarca, A.U.; Janghra, N.; Britten, C.E.; Havenith, C.E.; Levy, J.N.; Tiberghien, A.; Masterson, L.A.; et al. ADCT-301, a Pyrrolobenzodiazepine (PBD) Dimer-Containing Antibody-Drug Conjugate (ADC) Targeting CD25-Expressing Hematological Malignancies. Mol. Cancer Ther. 2016, 15, 2709–2721. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.; Lloyd, C.; Dimasi, N.; Toader, D.; Marwood, R.; Lewis, L.; Bannister, D.; Jovanovic, J.; Fleming, R.; D’Hooge, F.; et al. Preclinical Evaluation of MEDI0641, a Pyrrolobenzodiazepine-Conjugated Antibody-Drug Conjugate Targeting 5T4. Mol. Cancer Ther. 2017, 16, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Kemp, G.C.; Tiberghien, A.C.; Patel, N.V.; D’Hooge, F.; Nilapwar, S.M.; Adams, L.R.; Corbett, S.; Williams, D.G.; Hartley, J.A.; Howard, P.W. Synthesis and in vitro evaluation of SG3227, a pyrrolobenzodiazepine dimer antibody-drug conjugate payload based on sibiromycin. Bioorg. Med. Chem. Lett. 2017, 27, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Kung Sutherland, M.S.; Walter, R.B.; Jeffrey, S.C.; Burke, P.J.; Yu, C.; Kostner, H.; Stone, I.; Ryan, M.C.; Sussman, D.; Lyon, R.P.; et al. SGN-CD33A: A novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 2013, 122, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Zammarchi, F.; Williams, D.G.; Havenith, C.E.G.; Monks, N.R.; Tyrer, P.; D’Hooge, F.; Fleming, R.; Vashisht, K.; Dimasi, N.; et al. Antitumor Activity of MEDI3726 (ADCT-401), a Pyrrolobenzodiazepine Antibody-Drug Conjugate Targeting PSMA, in Preclinical Models of Prostate Cancer. Mol. Cancer Ther. 2018, 17, 2176–2186. [Google Scholar] [CrossRef] [PubMed]
- Damle, N.K.; Frost, P. Antibody-targeted chemotherapy with immunoconjugates of calicheamicin. Curr. Opin. Pharmacol. 2003, 3, 386–390. [Google Scholar] [CrossRef]
- De Vries, J.F.; Zwaan, C.M.; De Bie, M.; Voerman, J.S.; den Boer, M.L.; van Dongen, J.J.; van der Velden, V.H. The novel calicheamicin-conjugated CD22 antibody inotuzumab ozogamicin (CMC-544) effectively kills primary pediatric acute lymphoblastic leukemia cells. Leukemia 2012, 26, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Dijoseph, J.F.; Dougher, M.M.; Armellino, D.C.; Kalyandrug, L.; Kunz, A.; Boghaert, E.R.; Hamann, P.R.; Damle, N.K. CD20-specific antibody-targeted chemotherapy of non-Hodgkin’s B-cell lymphoma using calicheamicin-conjugated rituximab. Cancer Immunol. Immunother. 2007, 56, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Ricart, A.D. Antibody-drug conjugates of calicheamicin derivative: Gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin. Cancer Res. 2011, 17, 6417–6427. [Google Scholar] [CrossRef] [PubMed]
- Zwaan, C.M.; Reinhardt, D.; Jurgens, H.; Huismans, D.R.; Hahlen, K.; Smith, O.P.; Biondi, A.; van Wering, E.R.; Feingold, J.; Kaspers, G.J. Gemtuzumab ozogamicin in pediatric CD33-positive acute lymphoblastic leukemia: First clinical experiences and relation with cellular sensitivity to single agent calicheamicin. Leukemia 2003, 17, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Park, G.; Park, J.B.; Kim, S.; Kim, H.; Chung, J. An anti-EGFR x cotinine bispecific antibody complexed with cotinine-conjugated duocarmycin inhibits growth of EGFR-positive cancer cells with KRAS mutations. Exp. Mol. Med. 2018, 50, 67. [Google Scholar] [CrossRef] [PubMed]
- Takegawa, N.; Nonagase, Y.; Yonesaka, K.; Sakai, K.; Maenishi, O.; Ogitani, Y.; Tamura, T.; Nishio, K.; Nakagawa, K.; Tsurutani, J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int. J. Cancer 2017, 141, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.A.; Flynn, M.J.; Bingham, J.P.; Corbett, S.; Reinert, H.; Tiberghien, A.; Masterson, L.A.; Antonow, D.; Adams, L.; Chowdhury, S.; et al. Pre-clinical pharmacology and mechanism of action of SG3199, the pyrrolobenzodiazepine (PBD) dimer warhead component of antibody-drug conjugate (ADC) payload tesirine. Sci. Rep. 2018, 8, 10479. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.A.; Hamaguchi, A.; Coffils, M.; Martin, C.R.; Suggitt, M.; Chen, Z.; Gregson, S.J.; Masterson, L.A.; Tiberghien, A.C.; Hartley, J.M.; et al. SG2285, a novel C2-aryl-substituted pyrrolobenzodiazepine dimer prodrug that cross-links DNA and exerts highly potent antitumor activity. Cancer Res. 2010, 70, 6849–6858. [Google Scholar] [CrossRef] [PubMed]
- Shor, B.; Gerber, H.P.; Sapra, P. Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies. Mol. Immunol. 2015, 67, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Abe, Y.; Iguchi, T.; Yamaguchi, J.; Terauchi, T.; Kitamura, M.; Goto, K.; Goto, M.; Oitate, M.; Yukinaga, H.; et al. Wide application of a novel topoisomerase I inhibitor-based drug conjugation technology. Bioorganic Med. Chem. Lett. 2016, 26, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Sapra, P.; Hooper, A.T.; O’Donnell, C.J.; Gerber, H.P. Investigational antibody drug conjugates for solid tumors. Expert Opin. Investing. Drugs 2011, 20, 1131–1149. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, X.; Jia, S.; Weeks, A.M.; Hornsby, M.; Lee, P.S.; Nichiporuk, R.V.; Iavarone, A.T.; Wells, J.A.; Toste, F.D.; et al. Redox-based reagents for chemoselective methionine bioconjugation. Science 2017, 355, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nelson, C.G.; Nair, R.R.; Hazlehurst, L.; Moroni, T.; Martinez-Acedo, P.; Nanna, A.R.; Hymel, D.; Burke, T.R., Jr.; Rader, C. Stable and Potent Selenomab-Drug Conjugates. Cell Chem. Biol. 2017, 24, 433–442.e6. [Google Scholar] [CrossRef] [PubMed]
- Bruins, J.J.; Westphal, A.H.; Albada, B.; Wagner, K.; Bartels, L.; Spits, H.; van Berkel, W.J.H.; van Delft, F.L. Inducible, Site-Specific Protein Labeling by Tyrosine Oxidation-Strain-Promoted (4 + 2) Cycloaddition. Bioconjug. Chem. 2017, 28, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Axup, J.Y.; Bajjuri, K.M.; Ritland, M.; Hutchins, B.M.; Kim, C.H.; Kazane, S.A.; Halder, R.; Forsyth, J.S.; Santidrian, A.F.; Stafin, K.; et al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc. Natl. Acad. Sci. USA 2012, 109, 16101–16106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Stefano, J.E.; Manning, C.; Kyazike, J.; Chen, B.; Gianolio, D.A.; Park, A.; Busch, M.; Bird, J.; Zheng, X.; et al. Site-specific antibody-drug conjugation through glycoengineering. Bioconjug. Chem. 2014, 25, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, E.S.; Heibeck, T.H.; Gill, A.; Li, X.; Murray, C.J.; Madlansacay, M.R.; Tran, C.; Uter, N.T.; Yin, G.; Rivers, P.J.; et al. Production of site-specific antibody-drug conjugates using optimized non-natural amino acids in a cell-free expression system. Bioconjug. Chem. 2014, 25, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ramakrishnan, B.; Li, J.; Wang, Y.; Feng, Y.; Prabakaran, P.; Colantonio, S.; Dyba, M.A.; Qasba, P.K.; Dimitrov, D.S. Site-specific antibody-drug conjugation through an engineered glycotransferase and a chemically reactive sugar. mAbs 2014, 6, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Gebleux, R.; Waldmeier, L.; Hell, T.; Escher, M.; Wolter, F.I.; Grawunder, U.; Beerli, R.R. Highly Potent, Anthracycline-based Antibody-Drug Conjugates Generated by Enzymatic, Site-specific Conjugation. Mol. Cancer Ther. 2017, 16, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, J.; Jin, Y.; Vance, J.; Read, J.; Wang, X.; Wan, Y.; Zhou, H.; Ou, W.; Klock, H.E.; Peters, E.C.; et al. Optimization of an Enzymatic Antibody-Drug Conjugation Approach Based on Coenzyme A Analogs. Bioconjug. Chem. 2017, 28, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Behrens, C.R.; Liu, B. Methods for site-specific drug conjugation to antibodies. mAbs 2014, 6, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Nanna, A.R.; Li, X.; Walseng, E.; Pedzisa, L.; Goydel, R.S.; Hymel, D.; Burke, T.R., Jr.; Roush, W.R.; Rader, C. Harnessing a catalytic lysine residue for the one-step preparation of homogeneous antibody-drug conjugates. Nat. Commun. 2017, 8, 1112. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, J.D.; Pillow, T.H.; Chen, J.; Fan, F.; He, C.; Wang, Y.; Yan, G.; Yao, H.; Xu, Z.; Martin, S.; et al. Development of Efficient Chemistry to Generate Site-Specific Disulfide-Linked Protein- and Peptide-Payload Conjugates: Application to THIOMAB Antibody-Drug Conjugates. Bioconjug. Chem. 2017, 28, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Dimasi, N.; Fleming, R.; Zhong, H.; Bezabeh, B.; Kinneer, K.; Christie, R.J.; Fazenbaker, C.; Wu, H.; Gao, C. Efficient Preparation of Site-Specific Antibody-Drug Conjugates Using Cysteine Insertion. Mol. Pharm. 2017, 14, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Junutula, J.R.; Raab, H.; Clark, S.; Bhakta, S.; Leipold, D.D.; Weir, S.; Chen, Y.; Simpson, M.; Tsai, S.P.; Dennis, M.S.; et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nature Biotechnol. 2008, 26, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Boylan, N.J.; Zhou, W.; Proos, R.J.; Tolbert, T.J.; Wolfe, J.L.; Laurence, J.S. Conjugation site heterogeneity causes variable electrostatic properties in Fc conjugates. Bioconjug. Chem. 2013, 24, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.Q.; Xu, K.; Liu, L.; Raab, H.; Bhakta, S.; Kenrick, M.; Parsons-Reponte, K.L.; Tien, J.; Yu, S.F.; Mai, E.; et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nature Biotechnol. 2012, 30, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Strop, P.; Liu, S.H.; Dorywalska, M.; Delaria, K.; Dushin, R.G.; Tran, T.T.; Ho, W.H.; Farias, S.; Casas, M.G.; Abdiche, Y.; et al. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem. Biol. 2013, 20, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Dorywalska, M.; Strop, P.; Melton-Witt, J.A.; Hasa-Moreno, A.; Farias, S.E.; Galindo Casas, M.; Delaria, K.; Lui, V.; Poulsen, K.; Sutton, J.; et al. Site-Dependent Degradation of a Non-Cleavable Auristatin-Based Linker-Payload in Rodent Plasma and Its Effect on ADC Efficacy. PLoS ONE 2015, 10, e0132282. [Google Scholar] [CrossRef] [PubMed]
- Dorywalska, M.; Strop, P.; Melton-Witt, J.A.; Hasa-Moreno, A.; Farias, S.E.; Galindo Casas, M.; Delaria, K.; Lui, V.; Poulsen, K.; Loo, C.; et al. Effect of attachment site on stability of cleavable antibody drug conjugates. Bioconjug. Chem. 2015, 26, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Lyon, R.P.; Bovee, T.D.; Doronina, S.O.; Burke, P.J.; Hunter, J.H.; Neff-LaFord, H.D.; Jonas, M.; Anderson, M.E.; Setter, J.R.; Senter, P.D. Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nature Biotechnol. 2015, 33, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Tibbitts, J. Pharmacokinetic considerations for antibody drug conjugates. Pharm. Res. 2012, 29, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Boswell, C.A.; Mundo, E.E.; Zhang, C.; Stainton, S.L.; Yu, S.F.; Lacap, J.A.; Mao, W.; Kozak, K.R.; Fourie, A.; Polakis, P.; et al. Differential effects of predosing on tumor and tissue uptake of an 111In-labeled anti-TENB2 antibody-drug conjugate. J. Nucl. Med. 2012, 53, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Alley, S.C.; Benjamin, D.R.; Jeffrey, S.C.; Okeley, N.M.; Meyer, D.L.; Sanderson, R.J.; Senter, P.D. Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjug. Chem. 2008, 19, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Martin, K.; Theurich, S.; Schreiner, J.; Savic, S.; Terszowski, G.; Lardinois, D.; Heinzelmann-Schwarz, V.A.; Schlaak, M.; Kvasnicka, H.M.; et al. Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol. Res. 2014, 2, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Muller, P.; Schreiner, J.; Prince, S.S.; Lardinois, D.; Heinzelmann-Schwarz, V.A.; Thommen, D.S.; Zippelius, A. The microtubule-depolymerizing agent ansamitocin P3 programs dendritic cells toward enhanced anti-tumor immunity. Cancer Immunol. Immunother. 2014, 63, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C. Immunobiology: The Immune System in Health and Disease, 6th ed.; Garland Science: New York, NY, USA, 2005; p. xxiii. 823p. [Google Scholar]
- Gerber, H.P.; Sapra, P.; Loganzo, F.; May, C. Combining antibody-drug conjugates and immune-mediated cancer therapy: What to expect? Biochem. Pharmacol. 2016, 102, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hock, M.B.; Thudium, K.E.; Carrasco-Triguero, M.; Schwabe, N.F. Immunogenicity of antibody drug conjugates: Bioanalytical methods and monitoring strategy for a novel therapeutic modality. AAPS J. 2015, 17, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.V.; Iyer, S. Preclinical Pharmacokinetic Considerations for the Development of Antibody Drug Conjugates. Pharm. Res. 2015, 32, 3470–3479. [Google Scholar] [CrossRef] [PubMed]
- Tibbitts, J.; Canter, D.; Graff, R.; Smith, A.; Khawli, L.A. Key factors influencing ADME properties of therapeutic proteins: A need for ADME characterization in drug discovery and development. mAbs 2016, 8, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Hamblett, K.J.; Senter, P.D.; Chace, D.F.; Sun, M.M.; Lenox, J.; Cerveny, C.G.; Kissler, K.M.; Bernhardt, S.X.; Kopcha, A.K.; Zabinski, R.F.; et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 2004, 10, 7063–7070. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, L.; Dere, R.; Mai, E.; Erickson, R.; Hendricks, A.; Lin, K.; Junutula, J.R.; Kaur, S. Characterization of the drug-to-antibody ratio distribution for antibody-drug conjugates in plasma/serum. Bioanalysis 2013, 5, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Alley, S.C.; Okeley, N.M.; Senter, P.D. Antibody-drug conjugates: Targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 2010, 14, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Polson, A.G.; Ho, W.Y.; Ramakrishnan, V. Investigational antibody-drug conjugates for hematological malignancies. Expert Opin. Investig. Drugs 2011, 20, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Shin, Y.G.; Shah, D.K. Application of Pharmacokinetic-Pharmacodynamic Modeling and Simulation for Antibody-Drug Conjugate Development. Pharm. Res. 2015, 32, 3508–3525. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Zhao, B. Absorption, distribution, metabolism, and excretion considerations for the development of antibody-drug conjugates. Drug Metab. Dispos. 2014, 42, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Birdsall, R.E.; McCarthy, S.M.; Janin-Bussat, M.C.; Perez, M.; Haeuw, J.F.; Chen, W.; Beck, A. A sensitive multidimensional method for the detection, characterization, and quantification of trace free drug species in antibody-drug conjugate samples using mass spectral detection. mAbs 2016, 8, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Dowell, J.A.; Korth-Bradley, J.; Liu, H.; King, S.P.; Berger, M.S. Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J. Clin. Pharm. 2001, 41, 1206–1214. [Google Scholar] [CrossRef]
- Kaur, S.; Xu, K.; Saad, O.M.; Dere, R.C.; Carrasco-Triguero, M. Bioanalytical assay strategies for the development of antibody-drug conjugate biotherapeutics. Bioanalysis 2013, 5, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Stephan, J.P.; Kozak, K.R.; Wong, W.L. Challenges in developing bioanalytical assays for characterization of antibody-drug conjugates. Bioanalysis 2011, 3, 677–700. [Google Scholar] [CrossRef] [PubMed]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Chunduri, L.A.A.; Kurdekar, A.; Haleyurgirisetty, M.K.; Bulagonda, E.P.; Kamisetti, V.; Hewlett, I.K. Femtogram Level Sensitivity achieved by Surface Engineered Silica Nanoparticles in the Early Detection of HIV Infection. Sci. Rep. 2017, 7, 7149. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.Y.; Kim, J.H.; Baek, D.S.; Kim, S.J.; Kang, S.; Yang, W.S.; Song, J.A.; Lee, M.S.; Kim, S.; Kim, Y.S. Production of recombinant human procollagen type I C-terminal propeptide and establishment of a sandwich ELISA for quantification. Sci. Rep. 2017, 7, 15946. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.R.; Verhaeghen, K.; Roels, S.; Stange, G.; Ling, Z.; Pipeleers, D.; Gorus, F.K.; Martens, G.A. An analytical comparison of three immunoassay platforms for subpicomolar detection of protein biomarker GAD65. PLoS ONE 2018, 13, e0193670. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.K.; Joyce, A.; Spengler, M.; Yang, T.Y.; Zhuang, Y.; Fjording, M.S.; Mikulskis, A. Emerging technologies to increase ligand binding assay sensitivity. AAPS J. 2015, 17, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Ochoa, L.; Hammond, L.A.; Patnaik, A.; Edwards, T.; Takimoto, C.; Smith, L.; de Bono, J.; Schwartz, G.; Mays, T.; et al. Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: A phase I, pharmacokinetic, and biologic correlative study. J. Clin. Oncol. 2003, 21, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Rotmensch, S.; Cole, L.A. False diagnosis and needless therapy of presumed malignant disease in women with false-positive human chorionic gonadotropin concentrations. Lancet 2000, 355, 712–715. [Google Scholar] [CrossRef]
- Hoofnagle, A.N.; Wener, M.H. The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J. Immunol. Methods 2009, 347, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.A.; Takeuchi, M.; Kazarosyan, M.; Wang, C.C.; Guttler, R.B.; Singer, P.A.; Fatemi, S.; LoPresti, J.S.; Nicoloff, J.T. Serum thyroglobulin autoantibodies: Prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 1998, 83, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Kozhich, A.; Passmore, D.; Gu, H.; Wong, R.; Zambito, F.; Rangan, V.S.; Myler, H.; Aubry, A.F.; Arnold, M.E.; et al. Quantitative bioanalysis of antibody-conjugated payload in monkey plasma using a hybrid immuno-capture LC-MS/MS approach: Assay development, validation, and a case study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1002, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ortiz, R.; Tran, L.; Hall, M.; Spahr, C.; Walker, K.; Laudemann, J.; Miller, S.; Salimi-Moosavi, H.; Lee, J.W. General LC-MS/MS method approach to quantify therapeutic monoclonal antibodies using a common whole antibody internal standard with application to preclinical studies. Anal. Chem. 2012, 84, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.Q.; Bumbaca, D.; Saad, O.; Yue, Q.; Pastuskovas, C.V.; Khojasteh, S.C.; Tibbitts, J.; Kaur, S.; Wang, B.; Chu, Y.W.; et al. Catabolic fate and pharmacokinetic characterization of trastuzumab emtansine (T-DM1): An emphasis on preclinical and clinical catabolism. Curr. Drug Metab. 2012, 13, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, L.; Saad, O.M.; Baudys, J.; Williams, L.; Leipold, D.; Shen, B.; Raab, H.; Junutula, J.R.; Kim, A.; et al. Characterization of intact antibody-drug conjugates from plasma/serum in vivo by affinity capture capillary liquid chromatography-mass spectrometry. Anal. Biochem. 2011, 412, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Debaene, F.; Boeuf, A.; Wagner-Rousset, E.; Colas, O.; Ayoub, D.; Corvaia, N.; Van Dorsselaer, A.; Beck, A.; Cianferani, S. Innovative native MS methodologies for antibody drug conjugate characterization: High resolution native MS and IM-MS for average DAR and DAR distribution assessment. Anal. Chem. 2014, 86, 10674–10683. [Google Scholar] [CrossRef] [PubMed]
- Ezan, E.; Dubois, M.; Becher, F. Bioanalysis of recombinant proteins and antibodies by mass spectrometry. Analyst 2009, 134, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Hengel, S.M.; Sanderson, R.; Valliere-Douglass, J.; Nicholas, N.; Leiske, C.; Alley, S.C. Measurement of in vivo drug load distribution of cysteine-linked antibody-drug conjugates using microscale liquid chromatography mass spectrometry. Anal. Chem. 2014, 86, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Rago, B.; Clark, T.; King, L.; Zhang, J.; Tumey, L.N.; Li, F.; Barletta, F.; Wei, C.; Leal, M.; Hansel, S.; et al. Calculated conjugated payload from immunoassay and LC-MS intact protein analysis measurements of antibody-drug conjugate. Bioanalysis 2016, 8, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, R.J.; Hering, M.A.; James, S.F.; Sun, M.M.; Doronina, S.O.; Siadak, A.W.; Senter, P.D.; Wahl, A.F. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin. Cancer Res. 2005, 11, 843–852. [Google Scholar] [PubMed]
- Henry, M.D.; Wen, S.; Silva, M.D.; Chandra, S.; Milton, M.; Worland, P.J. A prostate-specific membrane antigen-targeted monoclonal antibody-chemotherapeutic conjugate designed for the treatment of prostate cancer. Cancer Res. 2004, 64, 7995–8001. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.V.; Audette, C.A.; Ye, Y.; Xie, H.; Ruberti, M.F.; Phinney, S.J.; Leece, B.A.; Chittenden, T.; Blattler, W.A.; Goldmacher, V.S. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006, 66, 3214–3221. [Google Scholar] [CrossRef] [PubMed]
- Sievers, E.L.; Appelbaum, F.R.; Spielberger, R.T.; Forman, S.J.; Flowers, D.; Smith, F.O.; Shannon-Dorcy, K.; Berger, M.S.; Bernstein, I.D. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: A phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood 1999, 93, 3678–3684. [Google Scholar] [PubMed]
- Iwamoto, N.; Hamada, A.; Shimada, T. Antibody drug quantitation in coexistence with anti-drug antibodies on nSMOL bioanalysis. Anal. Biochem. 2018, 540–541, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Budhraja, R.H.; Shah, M.A.; Suthar, M.; Yadav, A.; Shah, S.P.; Kale, P.; Asvadi, P.; Valan Arasu, M.; Al-Dhabi, N.A.; Park, C.G.; et al. LC-MS/MS Validation Analysis of Trastuzumab Using dSIL Approach for Evaluating Pharmacokinetics. Molecules 2016, 21, 1464. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, N.; Yokoyama, K.; Takanashi, M.; Yonezawa, A.; Matsubara, K.; Shimada, T. Application of nSMOL coupled with LC-MS bioanalysis for monitoring the Fc-fusion biopharmaceuticals Etanercept and Abatacept in human serum. Pharmacol. Res. Perspect. 2018, 6, e00422. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, H.; Liu, A.; Kozhich, A.; Rangan, V.; Myler, H.; Luo, L.; Wong, R.; Sun, H.; Wang, B.; et al. Antibody-drug conjugate bioanalysis using LB-LC-MS/MS hybrid assays: strategies, methodology and correlation to ligand-binding assays. Bioanalysis 2016, 8, 1383–1401. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, C.; Locuson, C.; Chen, S.; Qian, M.G. A Two-Step Immunocapture LC/MS/MS Assay for Plasma Stability and Payload Migration Assessment of Cysteine-Maleimide-Based Antibody Drug Conjugates. Anal. Chem. 2018, 90, 5989–5994. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Lee, A.; Garofolo, F.; Kaur, S.; Duggan, J.; Evans, C.; Palandra, J.; Donato, L.D.; Xu, K.; Bauer, R.; et al. 2016 White Paper on recent issues in bioanalysis: Focus on biomarker assay validation (BAV): (Part 2-Hybrid LBA/LCMS and input from regulatory agencies). Bioanalysis 2016, 8, 2457–2474. [Google Scholar] [CrossRef] [PubMed]
- Neubert, H.; Song, A.; Lee, A.; Wei, C.; Duggan, J.; Xu, K.; Woolf, E.; Evans, C.; Palandra, J.; Laterza, O.; et al. 2017 White Paper: Rise of hybrid LBA/LCMS immunogenicity assays (Part 2: Hybrid LBA/LCMS biotherapeutics, biomarkers & immunogenicity assays and regulatory agencies’ inputs). Bioanalysis 2017, 9, 1895–1912. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, G.; Zambito, F.C.; Zhang, Y.J.; DeSilva, B.S.; Kozhich, A.T.; Shen, J.X. A multiplexed immunocapture liquid chromatography tandem mass spectrometry assay for the simultaneous measurement of myostatin and GDF-11 in rat serum using an automated sample preparation platform. Anal. Chim. Acta 2017, 979, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Spellman, D.S.; Song, Y.; Choi, B.; Hatcher, N.G.; Tomazela, D.; Beaumont, M.; Tabrizifard, M.; Prabhavalkar, D.; Seghezzi, W.; et al. Generic automated method for liquid chromatography-multiple reaction monitoring mass spectrometry based monoclonal antibody quantitation for preclinical pharmacokinetic studies. Anal. Chem. 2014, 86, 8776–8784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tomazela, D.; Vasicek, L.A.; Spellman, D.S.; Beaumont, M.; Shyong, B.; Kenny, J.; Fauty, S.; Fillgrove, K.; Harrelson, J.; et al. Automated DBS microsampling, microscale automation and microflow LC-MS for therapeutic protein PK. Bioanalysis 2016, 8, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Fuerstenau, S.D.; Benner, W.H.; Thomas, J.J.; Brugidou, C.; Bothner, B.; Siuzdak, G. Mass Spectrometry of an Intact Virus The authors gratefully acknowledge Jennifer Boydston for her helpful comments and suggestions. G.S. is grateful for support from the NIH (GM55775). The work at LBL was supported by the Director, Office of Energy Research, Office of Health and Environmental Research, Human Genome Program, U.S. Department of Energy under contract number DE-AC03-76SF00098. Angew. Chem. Int. Ed. Engl. 2001, 40, 9822. [Google Scholar] [PubMed]
- Siuzdak, G.; Bothner, B.; Yeager, M.; Brugidou, C.; Fauquet, C.M.; Hoey, K.; Chang, C.M. Mass spectrometry and viral analysis. Chem. Biol. 1996, 3, 45–48. [Google Scholar] [CrossRef]
- Orbitrap LC-MS. Available online: https://www.thermofisher.com/us/en/home/industrial/mass-spectrometry/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-systems/orbitrap-lc-ms.html (accessed on 4 October 2018).
- McClure, R.A.; Williams, J.D. Impact of Mass Spectrometry-Based Technologies and Strategies on Chemoproteomics as a Tool for Drug Discovery. ACS Med. Chem. Lett. 2018, 9, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Wagner-Rousset, E.; Ayoub, D.; Van Dorsselaer, A.; Sanglier-Cianferani, S. Characterization of therapeutic antibodies and related products. Anal. Chem. 2013, 85, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Wakankar, A.; Chen, Y.; Gokarn, Y.; Jacobson, F.S. Analytical methods for physicochemical characterization of antibody drug conjugates. mAbs 2011, 3, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Burton, L.; Moore, I. LC-HRMS quantitation of intact antibody drug conjugate trastuzumab emtansine from rat plasma. Bioanalysis 2018, 10, 851–862. [Google Scholar] [CrossRef] [PubMed]
| Assay | Advantage | Challenges |
|---|---|---|
| LBA | Sensitive quantitative analysis for large molecules | DAR insensitive; does not provide measurement of the DAR or the overall drug load |
| Low equipment cost | Typically not sensitive to biotransformation | |
| High throughput | Specificity and selectivity is determined only by the capture and detection antibodies | |
| Easy to implement | Lack of structural/sequence information of the ADCs | |
| Potential cross-reactivity between antibodies in a multiplexed immunoassay | ||
| Limited multiplexing capability | ||
| Time-consuming to develop highly selective and specific antibodies | ||
| Hybrid LBA-LC-MS | Sensitive quantitative analysis for complex biotherapeutics such as ADCs | Relatively higher equipment cost compared to LBA assays |
| DAR sensitive--able to measure DAR/drug load | Complexity of instrument operation and data interpretation | |
| Specificity and selectivity achieved using antibody capture, chromatographic separation and characteristic fragmentation of surrogate peptides | Lower throughput due to additional steps such as proteolytic digestion and chromatographic separation requiring samples to be injected one at a time | |
| Able to provide ADC analyte structure information | Relatively low sensitivity for intact ADC analysis | |
| Can be sensitive to biotransformation | Reliance on surrogate analytes for quantification | |
| Could be highly multiplexed; many analytes can be analyzed at a time in a single LC-MS analysis |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mou, S.; Huang, Y.; Rosenbaum, A.I. ADME Considerations and Bioanalytical Strategies for Pharmacokinetic Assessments of Antibody-Drug Conjugates. Antibodies 2018, 7, 41. https://doi.org/10.3390/antib7040041
Mou S, Huang Y, Rosenbaum AI. ADME Considerations and Bioanalytical Strategies for Pharmacokinetic Assessments of Antibody-Drug Conjugates. Antibodies. 2018; 7(4):41. https://doi.org/10.3390/antib7040041
Chicago/Turabian StyleMou, Si, Yue Huang, and Anton I. Rosenbaum. 2018. "ADME Considerations and Bioanalytical Strategies for Pharmacokinetic Assessments of Antibody-Drug Conjugates" Antibodies 7, no. 4: 41. https://doi.org/10.3390/antib7040041
APA StyleMou, S., Huang, Y., & Rosenbaum, A. I. (2018). ADME Considerations and Bioanalytical Strategies for Pharmacokinetic Assessments of Antibody-Drug Conjugates. Antibodies, 7(4), 41. https://doi.org/10.3390/antib7040041




