Abstract
Engineered antibodies are key players in therapy, diagnostics and research. In addition to full size immunoglobulin gamma (IgG) molecules, smaller formats of recombinant antibodies, such as single-chain variable fragments (scFv) and antigen binding fragments (Fab), have emerged as promising alternatives since they possess different advantageous properties. Cell-based production technologies of antibodies and antibody fragments are well-established, allowing researchers to design and manufacture highly specific molecular recognition tools. However, as these technologies are accompanied by the drawbacks of being rather time-consuming and cost-intensive, efficient and powerful cell-free protein synthesis systems have been developed over the last decade as alternatives. So far, prokaryotic cell-free systems have been the focus of interest. Recently, eukaryotic in vitro translation systems have enriched the antibody production pipeline, as these systems are able to mimic the natural pathway of antibody synthesis in eukaryotic cells. This review aims to overview and summarize the advances made in the production of antibodies and antibody fragments in cell-free systems.
1. Introduction
Antibodies are important tools in therapy, diagnostics and research applications. Nowadays, they are the key detection elements for biological targets. In this context, it is estimated that engineered antibodies represent nearly 30% of all biopharmaceutical agents in clinical trials [1]. The growing clinical importance of therapeutic and diagnostic antibodies has led to a tremendous increase in approaches and developed technologies to provide binders with high specificity for biologically relevant targets [2].
The generation of antibodies by hybridoma technology, as well as the synthesis of antibodies and antibody fragments in prokaryotic and mammalian cells are well-established. However, a cell-based production of target proteins is known to be rather time-consuming and cost-intensive, thus resulting in the development of efficient and powerful cell-free protein synthesis systems. Over the last decade, cell-free systems have proven themselves as reliable, flexible and potent protein production platform, providing proteins of varying origins, classes and properties [3,4,5,6,7]. In particular, cell-free systems have been successfully used for the synthesis of antibodies and antibody fragments [8,9,10]. Since this class of molecules is highly in demand, this review aims to overview and summarize the advances made in the production of antibodies and antibody fragments in cell-free systems.
2. Conventional Antibody Production Technologies
The invention of the hybridoma technology in 1975 was a major breakthrough in the field of antibody production. Mouse hybridomas represented the first reliable source of monoclonal antibodies [11]. Due to their murine origin, these molecules were not suitable for therapeutic applications since they caused the generation of human anti-mouse antibodies (HAMA response) leading to severe immune reactions in humans [12]. The implementation of genetic engineering techniques helped to overcome these issues by the generation of chimeric and humanized antibodies [1]. These procedures aimed to reduce the murine content of the antibody in order to decrease immunogenicity.
Besides the classical IgG antibody, recombinant antibody fragments are in the focus of research and development projects, as they are considered to be produced more economically. In addition, smaller antibody formats can easily be modified and manipulated by using genetic engineering techniques [13]. scFv antibody fragments are one of the most popular formats of recombinant antibody fragments. Although they are rather small representatives of recombinant antibody formats (26–28 kDa), they provide the full binding specificity as the antigen binding surface is not altered [14,15]. In comparison to full length antibodies they are less complex, consisting of only the variable domain of the heavy antibody chain (VH) and the variable domain of the light antibody chain (VL) linked by a flexible polypeptide linker.
Currently, the phage display technology is the most commonly used technique for the in vitro selection and evolution of antibody fragments, initially published by Smith in 1985 and further developed by other research groups [16,17,18,19,20]. The basis of this technique is a DNA library which can be generated by polymerase chain reaction (PCR) from human B-lymphocytes, comprising a vast number (up to 1010) of heavy and light chain variable regions that are genetically fused to the phage gene pIII, which is coding for the phage minor coat protein. Antibody fragments are produced as pIII fusion proteins and displayed on the surface of the bacteriophage [16]. Therefore, the synthesized protein (phenotype) is directly linked to its encoding DNA (genotype) encapsulated inside the phage’s capsid. Phages carrying the antibody fragments on their surface are produced in microbial cells, such as Escherichia coli (E. coli), and are selected against a desired antigen in a process called “panning”. Beyond phage display, several other display techniques have been evolved in the past, such as bacterial surface display [21], yeast surface display [22], messenger RNA (mRNA) display [23,24] and ribosome display [25,26], all enabling the linkage of a protein’s genotype to its corresponding phenotype.
In addition to the development of display technologies, various strategies have been developed to produce antibody fragments in soluble form by using microorganisms as hosts. In this context, it was shown that antibody fragments could be successfully assembled in E. coli and secreted into the periplasmic space and into the media by genetic fusion of the antibody fragment to a bacterial secretory signal sequence [27,28]. Later on, bivalent humanized Fab fragments [29] and even full length immunoglobulins were successfully produced in E. coli [30]. Nowadays, several in vivo expression systems are available for the synthesis of antibody fragments using a variety of different hosts including E. coli [31], Saccharomyces cerevisiae [32], Pichia pastoris [33], insect cells [34], algae [35], plants [36] as well as mammalian cells [37] and of course, each of these systems has its own potentials and limitations (reviewed in [13]).
Production of antibodies in mammalian cell cultures is well-established. The majority of recombinant therapeutic proteins (approximately 70%) is currently produced in mammalian cells, with Chinese hamster ovary (CHO) cells as the most widely used expression host [38,39]. In principle, there are two possible ways to express proteins in mammalian cells, transient and stable expression. The robust performance of these modes is enabled by intense development and optimization efforts, including cell line development and engineering—to synthesize the desired protein in sufficient amounts while ensuring low operating costs, selection of clones, medium and feeding strategy development as well as bioreactor optimization—in order to fulfill the requirements for productivity and product quality specifications—and finally up-scaling, process characterization and validation [39,40,41]. As these development and optimization steps are rather extensive and time-consuming, a novel strategy for antibody production has emerged in the very recent past, designated as cell-free protein synthesis.
3. Cell-Free Protein Synthesis
Cell-free protein synthesis, also termed in vitro translation, facilitates the production of a given target protein by utilizing the translational machinery without using the living cell. Thus, protein synthesis is disconnected from cell fate and the protein synthesis reaction itself is not constrained by a cell wall, meaning that reaction conditions are accessible from the outside of the reaction vessel [5,7,42].
Going back to the 1960s, cell-free protein synthesis was originally developed as a tool to elucidate the genetic code [43]. Nowadays, the system has proven its potential to serve as a reliable, versatile and highly flexible protein production platform, enabling the synthesis of difficult-to-express proteins, as for example membrane proteins [7] and even toxic proteins [6,44]. Cell-free protein synthesis has been shown to be scalable from the microliter to liter scale [4]. Thus, cell-free systems have been successfully used for the high-throughput production of protein libraries [3,45] as well as for the high-yield synthesis of selected target proteins [46]. In particular, the use of linear DNA templates contributes to the ease and speed of cell-free translation systems, since no time-consuming cloning steps are required prior to protein production. Cell-free synthesis of a given target protein takes approximately one to two days, whereas cell-based expression, including the cloning procedure and cell-transformation, may take up to two weeks [47].
3.1. Disulfide Bond Formation in Cell-Free Systems
Antibodies are rather complex molecules, consisting of two identical heavy polypeptide chains and two identical light polypeptide chains. Each antibody chain is organized in distinct domains, designated as immunoglobulin domains. In every antibody domain, two β-sheets are connected by a disulfide bond, resulting in the formation of a β-barrel [48,49]. The internal disulfide bonds are known to have a tremendous influence on the folding and functionality of full length antibodies and antibody fragments [49,50]. Thus, cell-free systems need to provide a reaction environment that facilitates the formation of disulfide bonds in de novo synthesized target proteins (Figure 1).
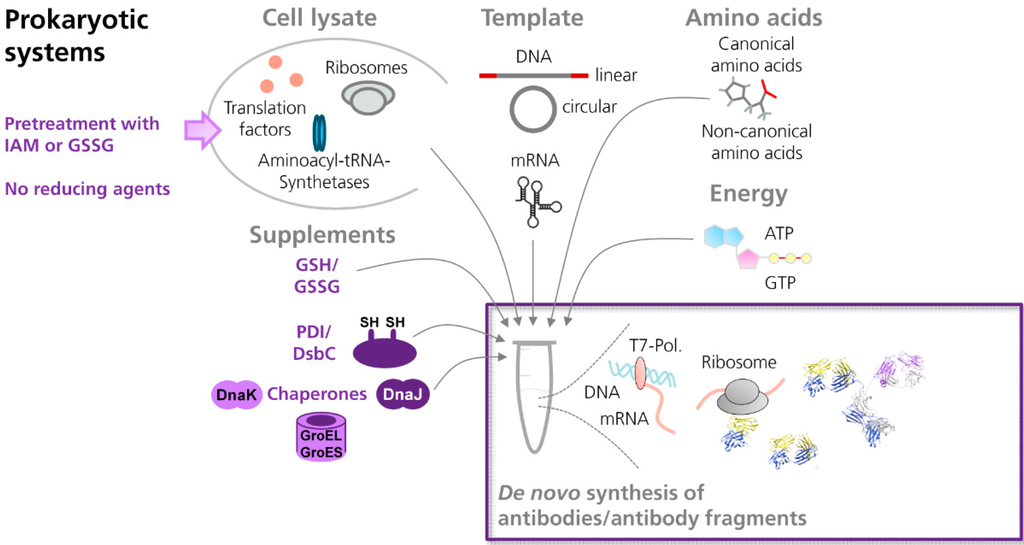
Figure 1.
Scheme showing the cell-free synthesis of antibodies and antibody fragments in prokaryotic in vitro translation systems. In general, cell-extracts prepared from prokaryotic or eukaryotic cells contain the essential components necessary for translation, such as ribosomes, translation factors and enzymes. For in vitro translation, the cell lysate is supplemented with amino acids, ATP and GTP, and an energy regenerating system (e.g., creatine phosphate⁄creatine kinase). Synthesis of the target protein is initiated by the addition of an appropriate template either in form of DNA or mRNA. In order to allow the formation of disulfide bonds in de novo synthesized proteins, prokaryotic cell-free systems (e.g., based on E. coli) can be supplemented with mixtures of reduced (GSH) and oxidized glutathione (GSSG), disulfide bond isomerase C (DsbC), protein disulfide isomerase (PDI) and/or chaperones (e.g., DnaK, DnaJ, GroEL, GroES). In addition, cell extracts can be pretreated with iodoacetamide (IAM) and/or defined mixtures of GSH and GSSG, in order to inactivate endogenous reductases which are present in the cell extract. (NMR structure antibody molecule, source: Protein Data Bank, 1 1IGT, Chimera).
In the last decade, cell-free systems have successfully been used to synthesize active disulfide-bonded proteins [51,52]. In the beginning of this development, two major hurdles had to be overcome: First, the presence of reducing agents, such as dithiothreitol (DTT) which were usually added in order to preserve the activity of the cell lysate during storage and the translation reaction itself and secondly, the lack of compartments with appropriate redox conditions as they are present in living cells (periplasmic space in prokaryotes, endoplasmic reticulum (ER) in eukaroytes) [53].
Depending on the source of the extract, different strategies have been applied to overcome these hurdles: For example, translation systems based on E. coli cell lysates were supplemented with defined mixtures of reduced and oxidized glutathione, in order to establish an appropriate and more oxidizing redox potential [52,54,55,56,57]. Other translation systems focused on the preincubation of the lysate with alkylating reagents, such as iodoacetamide, in order to inactivate endogenous reductases which are present in the cell extract [51,52,57]. Since the addition of iodoacetamide may result in the reduction of the overall translational activity, other strategies such as pretreatment of lysates with an excess of oxidized glutathione have been applied [58]. Furthermore, the addition of exogenous enzymes, such as eukaryotic protein disulfide isomerase (PDI), was shown to significantly improve the functionality of cell-free synthesized scFv fragments [54,55]. Supplementation of cell-free reactions with chaperones, such as DnaK, DnaJ, GroEL and GroES, was reported to be beneficial as well, since they increased the amount of soluble, cell-free synthesized scFv and Fab fragments [54,56].
The most successful E. coli-based cell-free translation systems, enabling the synthesis of functional disulfide-bonded proteins, were developed by applying a combination of different strategies [52]. For instance, the combination of the following modifications was shown to be very effective: First, cell extracts were prepared from a mutant E. coli strain that lacks one of the major endogenous reductases (∆ gor strain, KGK10). Secondly, cell extracts were pretreated with iodoacetamide and defined mixtures of oxidized and reduced glutathione, in order to establish a redox potential suitable for disulfide bond formation. Thirdly, cell-free translation reactions were supplemented with exogenous proteins, such as disulfide bond isomerase C (DsbC) and PDI, enabling the formation and isomerization of disulfide bonds [59,60]. In a different approach, protein synthesis and disulfide bond formation were performed in two separate reactions, starting with the cell-free reaction itself, followed by purification of the target protein and its subsequent oxidation in a second separated reaction compartment [52]. In a very recent report, Groff and colleagues published a potent cell-free translation system based on a chaperone-enriched E. coli cell extract enabling the synthesis of full length IgG molecules in gram per liter quantities. In this study, the authors were able to show the beneficial effects of the disulfide isomerase DsbC and the prolyl isomerase FkpA on antibody folding and assembly [61].
In addition, eukaryotic translation systems have been successfully applied to synthesize active disulfide-bonded proteins. In 1992, the synthesis of active tissue-type plasminogen activator (tPA) was reported by applying a cell-free translation system based on rabbit reticulocyte lysate supplemented with dog pancreas microsomal vesicles and oxidized glutathione [62]. Later on, insect lysates were used to synthesize active disulfide-bonded proteins [63]. Ezure and coworkers demonstrated the synthesis of E. coli alkaline phosphatase (two disulfide bonds) under non-reducing conditions in a soluble and active form. Furthermore, active human lysozyme (four disulfide bonds) was synthesized under non-reducing conditions in the presence of reduced and oxidized glutathione and PDI [63].
Whereas organisms separate protein synthesis and oxidative protein folding in distinct cellular regions –a phenomenon designated as compartmentalization–the beforehand mentioned “cell-free” strategies mainly attempted to accomplish both tasks in one and the same compartment. In contrast, a cell-free system based on lysates prepared from Spodoptera frugiperda 21 cells (Sf21) has been developed comprising both, the capability to synthesize a given target protein and to achieve its translocation into a separate endogenous compartment, where protein folding and posttranslational modifications are facilitated [64,65] (Figure 2). These features are made possible as the cell extract contains translationally active, endogenous microsomal vesicles having their origin in the ER of the insect cells. These vesicles are formed during the lysate preparation procedure, where the native structure of the ER is ripped, followed by its rearrangement and formation of smaller compartments designated as microsomal vesicles [5]. Due to the presence of these vesicles, target proteins can be translocated into the lumen of these vesicles, if they are fused to an appropriate signal sequence [65]. In addition, the de novo synthesis of membrane proteins and their subsequent embedding into the vesicular membrane of the insect vesicles have been reported [66,67,68].
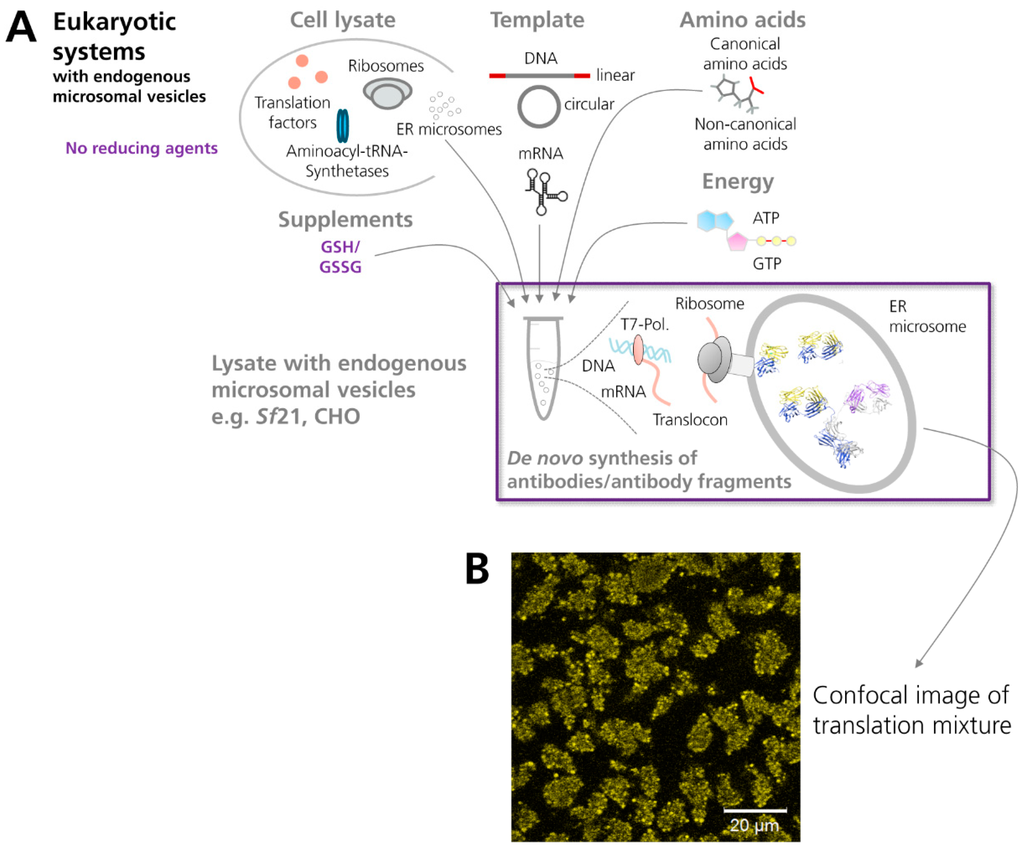
Figure 2.
Scheme showing the cell-free synthesis of antibodies and antibody fragments in vesicle-containing eukaryotic in vitro translation systems. (A) The eukaryotic cell extract depicted in the scheme (e.g., based on lysates from Sf21 or CHO cells) comprises endogenous microsomal vesicles. These vesicles enable a co-translational translocation of target proteins into their lumen and subsequent posttranslational modifications, such as the formation of disulfide bonds, which is one of the hallmarks of antibody domains. (B) Confocal image of cell-free synthesized scFv-eYFP fusion molecules which have been N-terminally fused to the melittin signal sequence. The signal sequence induces translocation of de novo synthesized molecules into the endogenous microsomal vesicles present in the insect (Sf21) cell-free translation system. (NMR structure antibody molecule, source: Protein Data Bank, 1 1IGT, Chimera).
3.2. Production of Antibodies in Cell-Free Systems
3.2.1. Prokaryotic Cell-Free Systems
At the current state, prokaryotic cell extracts are the predominant source of recombinant antibody formats and even full length antibodies (Figure 3). In 1997, Ryabova and coworkers published the first comprehensive report on the cell-free synthesis of scFv antibody fragments in an E. coli-based cell-free translation system [54]. Ryabova and colleagues analyzed the effect of different supplements on the synthesis of functional scFv antibody fragments [14] derived from an anti-hemagglutinin antibody [69]. Here it was shown that the addition of PDI increased the yield of functional scFv antibody fragments three-fold in comparison to a control reaction without the addition of PDI. Moreover, it was shown that the molecular chaperones DnaK and DnaJ increased the amount of soluble protein, but not the amount of functional protein. Finally, the combination of different supplements (reduced and oxidized glutathione, PDI and chaperones) was shown to be most successful, where nearly 50% of total scFv antibody fragments produced (approximately 8 µg/mL in 15 min) were recovered as active antigen-binding entities. This study laid the foundation for the in vitro synthesis of scFv antibody fragments in E. coli-based cell-free translation systems, subsequently enabling the in vitro selection and evolution of proteins by ribosome display [26]. Later on, Merk and colleagues succeeded in the synthesis of two anti-lysozyme scFv antibody fragments by using a similar cell-free translation system supplemented with reduced and oxidized glutathione, PDI and chaperones [55]. In 2006, two other research groups reported the cell-free synthesis of scFv antibody fragments in E. coli-based cell-free translation systems [70,71].
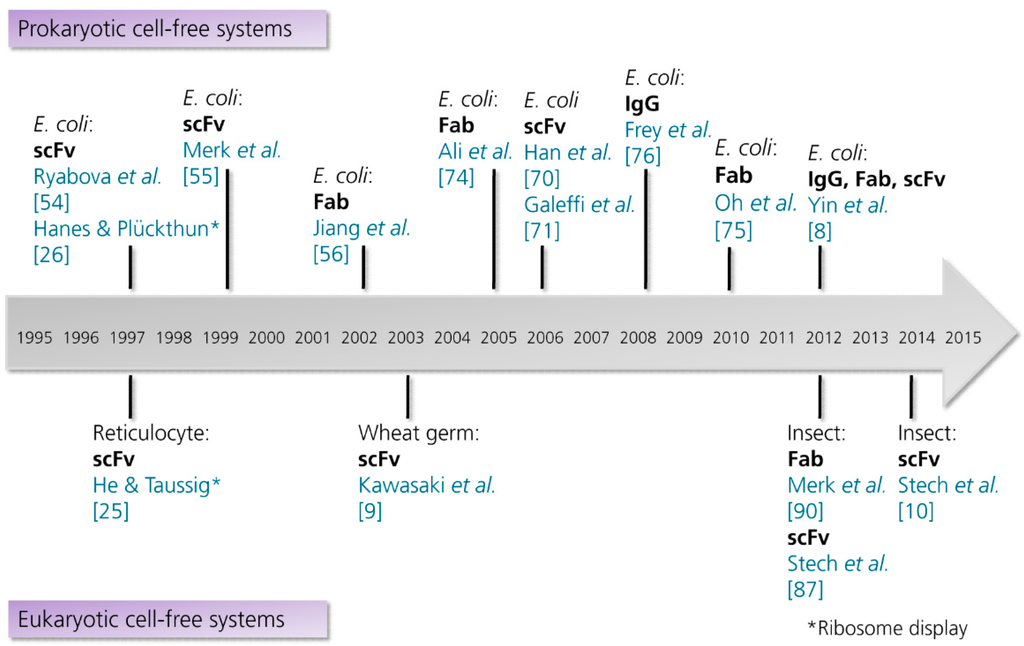
Figure 3.
Timeline showing the milestones in the cell-free synthesis of antibody fragments and antibodies. Abbreviations: E. coli, Escherichia coli; Fab, antigen binding fragment; IgG, immunoglobulin G; scFv, single-chain variable fragment. References are listed in brackets. (This list is not intended to be exhaustive. Examples for the synthesis of antibody-protein and antibody-toxin fusions are excluded).
Jiang and coworkers demonstrated for the first time the cell-free synthesis of functional Fab fragments by using a coupled E. coli transcription/translation system [56]. The Fab fragment used as model protein, was derived from the catalytic antibody 6D9 which has been developed by Miyashita and colleagues [72,73]. Interestingly, the antibody 6D9 binds to a phosphonate transition-state analogue and catalyzes the hydrolysis of a non-bioactive chloramphenicol monoester derivative to chloramphenicol. Heavy and light chains of 6D9 Fab fragments were simultaneously synthesized in the coupled cell-free translation system. As already shown for the synthesis of scFv antibody fragments, the addition of reduced and oxidized glutathione, PDI and molecular chaperones increased the solubility and the antigen-binding activity of target proteins [56]. The drawback of comparatively low protein yields reported by Jiang and coworkers was subsequently solved by the same group. Endogenous serine proteases were identified to be the cause of target protein degradation. As a solution for this problem, serine protease-deficient mutants of the E. coli strain (BW25113) were constructed and used for cell-free protein synthesis. Usage of these modified cell extracts resulted in increased yields of cell-free synthesized anti-HSA (human serum albumin) scFv and 6D9 Fab fragment [74]. Later on, another research group succeeded in synthesizing Fab fragments specific for the Botulinum neurotoxin serotype B (BoNT/B) [75]. Fab fragments were produced in a redox-optimized cell-free translation system based on GroEL/ES-enriched E. coli S30 cell extracts. The cell extract used had to be pretreated with an excess of oxidized glutathione, in order to exhaust its reducing capability while maintaining its translational activity. Redox optimization was conducted by the addition of glutathione and DsbC. By using this supplement combination, the yields of cell-free synthesized and soluble Fab fragments were increased to 30 µg of purified and functional Fab from 1 mL reaction mixture [75].
The next milestone regarding the cell-free synthesis of antibodies was achieved by Frey et al. in 2008 [76]. Frey and colleagues succeeded to produce functional full length IgG (mouse monoclonal antibody MAK33) in an E. coli-based cell-free translation system supplemented with PDI, DsbC and the ER-specific chaperones Grp94 and BiP (~500 ng/mL active antibody). This major breakthrough was subsequently succeeded by Yin and colleagues who managed to synthesize the IgG1 antibody trastuzumab in yields of several hundred micrograms per milliliter reaction volume [8]. The IgG1 antibody as well as scFv and Fab fragments that bind to human IL-23 and IL-13α1R were produced in a scalable transcription/translation system based on E. coli cell extracts. Remarkably, the cell-free system they used was shown to be scalable from the microliter to liter range. These experiments have shown for the first time that Fab fragments and full length antibodies can be synthesized in standard bioreactors in large scale (up to 5 L) and high production yields (~800 µg/mL scFv, ~250 µg/mL Fab, ~400 µg/mL IgG). Subsequently, the developed cell-free translation system was used for the in vitro display of antibody Fab fragments by using ribosome display [77]. In 2014, protein yields of cell-free synthesized trastuzumab full length antibody were further increased to gram per liter quantities by using a chaperone-enriched extract prepared from an engineered E. coli strain [61].
Besides the formation of disulfide bonds, the correct assembly of multi domain proteins can be challenging in both cell-free and cell-based protein synthesis systems. As nucleic acid templates may be translated at different rates—due to their codon usage and/or mRNA secondary structures—an empiric optimization of antibody heavy to light chain ratios may be necessary. Taking advantage of the open nature of cell-free reactions, the total amount as well as the ratio of antibody heavy to light chain encoding DNA templates can be varied systematically, in order to optimize the correct assembly of antibody polypeptide chains as successfully demonstrated by Yin et al. and Groff et al. [8,61].
In addition, eukaryotic cell-free translation systems have been used to synthesize functional disulfide-bonded proteins including scFv and Fab antibody fragments. The intention behind these studies was to provide a more suitable environment for the folding of complex molecules. At the current state, eukaryotic cell-free translation systems are less productive compared to E. coli but they provide a better basis for the synthesis of soluble full length proteins [78,79], complex proteins and proteins which require posttranslational modifications [80]. In addition, proteins synthesized in E. coli bear the potential to be contaminated with endotoxins. Thus, laborious purification steps are usually required prior to the utilization of these valuable target proteins in cell-based assays [81] or even in diagnostics and therapeutics.
3.2.2. Eukaryotic Cell-Free Systems
The very first eukaryotic in vitro translation systems used for the synthesis of antibodies were prepared from rabbit [82] and rat [83,84] lymph nodes. Due to the high amount of plasma cells in such tissues, the cell extract contained an extraordinarily high amount of ER microsomal vesicles. Although cell-free reactions were not charged with a defined DNA or mRNA template, the authors realized the advantages of using a cell extract which contains a high amount of microsomes, representing the natural place of antibody synthesis, folding and posttranslational modifications.
Later on, additional sources of eukaryotic lysates have been applied to synthesize antibodies in vitro (for an overview see Figure 3). One example was published in 1993 by Nicholls and colleagues. In this study, rabbit reticulocyte lysate was used as a source to synthesize scFv-toxin fusion proteins [85]. Besides E. coli cell extracts, rabbit reticulocyte lysates were subsequently used as basic translation system for the in vitro evolution of antibody fragments by using ribosome display [25] (for a comparison of E. coli and rabbit reticulocyte ribosome display systems see [86]). Nowadays, ribosome [25,26] and mRNA display [23,24] are the most widely used in vitro display techniques, both enabling the linkage of a protein’s genotype to its corresponding phenotype.
The first scFv antibody fragment produced in a cell-free system based on wheat germ was published by Kawasaki et al. in 2003 [9]. The aim of this study was to search for translation conditions which enable the production of disulfide-containing proteins in a functionally active state by using the highly productive eukaryotic wheat germ extract. Initially, a scFv antibody fragment binding to Salmonella O-antigen was used as a model protein. Optimal reaction conditions were found when using a DTT-deficient wheat germ extract in combination with the addition of PDI, resulting in the synthesis of 13 µg of active scFv in a 1 mL batch reaction [9].
In the same year, a novel eukaryotic translation system based on cultured Sf21 cells was developed [64]. In contrast to other eukaryotic cell-free translation systems, the insect cell extract used in this study contains endogenous microsomal vesicles. If target proteins are fused to an appropriate signal sequence, de novo synthesized polypeptide chains can be translocated into the lumen of the microsomal vesicles [65]. Due to this translocation step, two separate compartments for protein synthesis and oxidative protein folding are provided, thus mimicking the conditions present in living cells.
In 2012 and 2014, two successive studies have shown the synthesis of functional scFv antibody fragments by using the vesicle-containing insect cell-free translation system [10,87]. In comparison to other cell-free systems which have been successfully applied for the synthesis of disulfide-bonded target proteins, only moderate modifications were necessary in order to optimize the insect cell-free translation system: The abandonment of the reducing agent DTT, the addition of glutathione and the signal peptide-induced translocation of target proteins were found to be crucial factors. In contrast to already published cell-free translation systems, neither a pretreatment of the lysate with iodoacetamide nor the addition of exogenous enzymes and chaperones were necessary in order to synthesize functional target proteins. Starting with one antibody fragment model, which binds to fluorescein [88,89], it was shown that functional scFv molecules could be synthesized based on PCR-amplified DNA templates [87]. Fusion of antibody molecules to the signal sequence of honeybee melittin induced translocation and enrichment of antibody fragments in microsomal vesicles. For most scFv antibody fragments tested in these studies, signal peptide-induced translocation was shown to have a positive effect on protein functionality [10,87]. Likewise, this positive effect was also shown for the synthesis of Fab fragments by using a comparable insect cell-free translation system [90]. Based on the described results, it may be speculated that the enzymes which are involved in the formation and reshuffling of disulfide bonds in vivo [91], such as ER-resident membrane-associated and luminal proteins (e.g., ER Oxidoreductin 1 (Ero1) and PDI), as well as folding helpers (e.g., binding immunoglobulin protein, BiP) which originate from the lumen of the ER, are maintained inside the vesicles in an active state.
3.2.3. Cell-Free Systems as a Source of Modified Antibodies and Antibody Fragments
3.2.3.1. scFv Fusion Proteins
The generation of protein conjugates, e.g., proteins equipped with functional groups such as fluorescent molecules and affinity tags, or proteins linked to drugs and other proteins, is of special interest for many biophysical, biotechnological and biomedical applications [92,93]. For instance, there is a great interest in the generation of potent antibody-drug conjugates, which facilitate the targeting of cytotoxic payloads to cancer cells, ideally leading to reduced side effects and an enlarged therapeutic window of the chemotherapeutic reagent. In addition, antibodies linked to cytokines or immunostimulatory peptides can be used as vaccines as demonstrated by Kanter et al. in 2007 [94]. In this study, the authors demonstrated the cell-free production of two scFv fusion proteins that were successfully applied as vaccines against a murine B-cell lymphoma. Two vaccines were tested in this study, (1) a tumor-derived scFv fused to granulocyte-macrophage colony-stimulating factor (GM-CSF) and (2) the scFv linked to a peptide derived from interleukin-1β. Both cell-free synthesized vaccines were chemically linked to a foreign protein (keyhole limpet hemocyanin) in order to increase the immunogenicity of the fusion constructs. The vaccines were able to induce tumor-specific humoral immune responses in mice with efficiencies comparable to the conventional immunoglobulin produced in mammalian cells. Subsequently, a similar scFv construct was successfully fused to the tetanus toxin fragment C (TTFrC) [95]. Upon production in the E. coli-based cell-free translation system, this scFv fusion protein elicited anti-tumor humoral responses as well as protection of mice from tumor challenge. These initial results already demonstrated the tremendous potential of cell-free systems to accelerate the production of personalized vaccines. In the future, personalized vaccines may be produced on the basis of PCR products which are directly amplified from the patient´s own immunoglobin variable regions [95].
3.2.3.2. Labeling of Antibodies and Antibody Fragments with Non-canonical Amino Acids
Cell-free methods are a convenient tool to modify the protein of interest and to equip de novo synthesized proteins with extended and beneficial functions. By employing cell-free protein synthesis, target proteins can be labeled co-translationally with one or multiple non-canonical amino acids [96,97]. While in vivo methods run into constraints when a certain modified amino acid appears to exhibit toxic side effects or certain amino acids even cannot enter the cell, cell-free systems provide an efficient alternative, as they represent open systems which are not limited by the potential cytotoxicity of amino acid analogues [98]. Artificial posttranslational modification technologies use the naturally occurring amino acid side chains of defined amino acids (preferably cysteine and lysine) for the chemical conjugation with a certain payload. Since the label is introduced statistically at multiple sites into the protein, thereby resulting in a heterogeneous mixture of labeled proteins, negative effects on protein solubility and functionality cannot be excluded [99]. These drawbacks have led to the development of site-specific labeling techniques, facilitating the introduction of a non-canonical amino acid into a polypeptide chain at one defined position [98,100]. Common methods for co-translational and site-specific protein labeling are either based on pre-charged suppressor tRNAs [101] or orthogonal suppressor tRNA/synthetase pairs which are added to the cell-free reaction; both relying either on the suppression of stop codons [102,103] or on the use of 4-base codons [104,105].
Conjugation by azide-alkyne click chemistry has been successfully applied to generate scFv-fusion proteins. In 2009, Patel et al. demonstrated the cell-free synthesis of antibody fragments site-specifically labeled with Gaussia princeps luciferase [106]. In this study, scFv molecules and luciferase were synthesized and labeled separately from each other. While the tumor idiotype scFv was site-specifically labeled with p-azido-L-phenylalanine by means of an orthogonal tRNA/synthetase pair, the reporter protein luciferase was modified by the global replacement of methionine residues with homopropargylglycine. Subsequently, labeled scFv and luciferase molecules were conjugated by using Cu(I) catalyzed click chemistry [106].
In addition to the generation of scFv-fusion molecules, site-specific labeling techniques can be used to link antibodies to small molecules, such as fluorescent dyes or toxins (reviewed in [107]). In this context, an E. coli-based cell-free translation system has been shown to enable the synthesis of antibody-drug conjugates through the site-specific incorporation of an optimized non-canonical amino acid (p-azidomethyl-L-phenylalanine) into an IgG molecule, followed by its subsequent conjugation to a tumor-specific drug (DBCO-PEG-monomethyl auristatin) [108].
In another approach, an E. coli-based cell-free translation system was used for the generation of 11C-labeled scFv molecules, which should be applied to positron emission tomography (PET) [109]. In this study, the labeling of scFv molecules was achieved by simply replacing the canonical amino acid methionine with the non-canonical amino acid L-[11C] methionine. A similar approach can be applied to label scFv antibody fragments with fluorescent dyes. tRNAs pre-charged with non-canonical amino acids are an easy-to-handle alternative in comparison to orthogonal tRNA/synthetase pairs. In a recent report, scFv antibody fragments were co-translationally labeled by adding the pre-charged suppressor tRNA Bodipy-TMR-lysine to the cell-free reaction based on insect lysate [10]. By using this residue-specific labeling approach, the fluorescent amino acid is incorporated at multiple sites in the protein. Based on its anticodon, the pre-charged tRNA decodes the TTC codon, resulting in a competition of endogenous Phe-tRNAGAA with pre-charged tRNAs. This leads to the incorporation of the fluorescent amino acid in a statistical and residue-specific manner. In addition, site-directed protein labeling was demonstrated by using DNA templates bearing the amber stop codon TAG at the 3'-end of the open reading frame encoding the scFv fragment. Due to the incorporation of fluorescent amino acids, de novo synthesized target proteins could be directly analyzed via their intrinsic fluorescence, thereby neglecting the need for radioactive labeling and additional occupational safety measures that are connected to the work in an isotope lab.
4. Discussion
In the last decade, remarkable progress has been made in the production of functional scFv, Fab fragments and IgG molecules in cell-free systems [8,54,56]. The general strategy for the adaptation of cell-free systems−in order to allow the formation of disulfide bonds in de novo synthesized proteins−included the optimization of redox conditions (supplementation with glutathione), the addition of exogenous enzymes which promote disulfide isomerization as well as the addition of folding helpers. In living organisms, the biochemical processes of protein translation and oxidative folding occur in separated compartments, each providing optimal reaction conditions (periplasmic space in bacteria; ER in eukaryotes). However, in contrast to living cells conventional cell-free systems were developed in order to accomplish both tasks in the same compartment. The hurdles connected to this endeavor have been circumvented by utilizing structures of the ER in a cell-free system, in order to mimic the conditions present in living cells. Furthermore, two separate and specialized compartments are provided for protein synthesis and oxidative protein folding. Moreover, by providing a separate compartment for protein folding, synthesis and folding of complex eukaryotic proteins was expected to be improved.
The results that were presented by Stech et al. suggest that other eukaryotic and vesicle-containing lysates can potentially serve as valuable antibody production platforms. Recently, cell-free translation systems based on CHO and human cell extracts (K562 cells) were introduced [110,111]. Comparable to the insect cell-free translation system, these lysates contain endogenous microsomal vesicles, enabling the N-linked glycosylation of de novo synthesized target proteins and the embedding of membrane proteins into microsomal membranes. These results indicate that−upon redox optimization−these systems could enable the synthesis of functional antibody fragments and antibodies. CHO cells are the most widely used expression host for therapeutic proteins, with the majority of marketed antibodies being manufactured in this system [38]. As this system is well-established, numerous therapeutic proteins produced in CHO cells have been approved by the FDA [112]. Therefore, one can assume that a redox-optimized CHO cell-free system, capable of producing highly functional antibodies, would be of great interest for the scientific and industrial community. For instance, cell-free systems could be applied to pre-screen diverse antibody constructs for their binding capabilities in a very early stage of the experimental process. Characterization of these antibodies prior to tedious and time-consuming cloning procedures and transient or stable expression of antibodies in CHO cells may dramatically speed-up the whole procedure of antibody engineering and production.
The ability of synthesizing glycosylated proteins is another issue that contributes to the usage of eukaryotic cell-free systems as potent antibody production platform. Glycosylation is a common posttranslational modification in antibodies. IgG1 molecules carry a single N-linked glycan located in the Fc region in each of their two heavy polypeptide chains. The presence or absence of glycosylation and the pattern of glycosylation influences antibody stability and function significantly, e.g., by affecting receptor binding and effector function [113]. Therefore, glycoengineering is often an essential prerequisite to provide antibodies with the desired therapeutic efficacy [114]. Eukaryotic cell-free systems based on mammalian cell lines initially bring along their own glycosylation machinery, which might provide unique engineering possibilities for antibodies. Alternatively, attempts have been made to engineer prokaryotic cell-free systems that are able to synthesize glycosylated proteins [115].
Regarding the protein yields, in vitro translation systems prepared from E. coli and wheat germ are most productive, reaching protein yields up to several milligrams per milliliter of reaction depending on the reaction format and protein of interest [116,117]. Until now, eukaryotic cell-free translation systems based on insect cell extracts, human cell extracts and lysates from CHO cells could not reach such high production yields, although significant improvements have been made in recent years [68,110,111]. Nevertheless, for the future we anticipate an increase in productivity as more advanced reaction formats [68] can be combined with an improved template design [111].
In contrast to E. coli-based systems, proteins that are synthesized in eukaryotic cell-free systems can potentially be applied in cell-based assays even without prior purification [6]. Of course, if the protein of interest shall be used as a mammalian injectable, purification is preferred (e.g., by applying a tag-based purification strategy), since the endogenous lysate proteins most probably lead to the induction of immune responses, an instance, which may not be acceptable from the regulatory point of view [118]. Nevertheless, it was shown that endogenous proteins in Sf9 cells were required to induce autoantibody production against metastatic melanomas in mice, thus facilitating an “adjuvant effect” [119].
The incorporation of non-canonical amino acids can be used to equip different proteins with well-defined reactive side chains for their subsequent conjugation by click chemistry [106,120,121]. This strategy could also be beneficial for the generation of bispecific antibody fragments by fusing two scFv molecules with different specificities to each other. For this reason, one scFv candidate could be labeled with a non-canonical amino acid bearing a single exposed azide group (p-azido-L-phenylalanine), another scFv candidate is labeled with a non-canonical amino acid containing an alkyne group. Upon separation of free non-canonical amino acids from target proteins, the two labeled scFv molecules could be conjugated by click chemistry. In addition, this approach could be broadened to generate scFv-toxin and antibody-toxin conjugates [108]. Besides the use of labeling techniques, bispecific antibodies can be produced by applying the so-called “knob-into-hole” technology. To promote heterodimerization of two heavy chains with different specificities, CH3 domains are engineered to bear either a “knob” or a “hole” in each antibody heavy chain by amino acid replacement [122]. Recently, this technology has been applied in an E. coli-based cell-free translation system creating bispecific antibodies in different designs (e.g., two-armed heterodimeric scFv-knobs-into-holes; one-armed assymetric BiTE knobs-into-holes with tandem scFv) [123].
While in vitro display methods have tremendously influenced the discovery of new binders [124], production of specific antibodies required for diagnostic and/or therapeutic applications is nowadays mainly facilitated by using mammalian cell cultures [38,39]. This review aimed to summarize the rapid and remarkable progress made in the field of cell-free antibody expression. From the first comprehensive report on the cell-free synthesis of scFv antibody fragments [54] to the synthesis of full length IgG molecules [76] no more than 11 years have passed (Figure 3). According to this fast development, we anticipate for the future that cell-free systems might give solutions where cell-based production technologies still have their limitations.
Acknowledgments
The authors would like to thank Rita Sachse (Fraunhofer IZI-BB, Potsdam-Golm, Germany) for the careful revision of this manuscript. This research is supported by the German Ministry of Education and Research (BMBF, No. 0315942 and No. 0312039) and the German Research Foundation (DFG Priority Programme 1623).
Author Contributions
M.S. and S.K. contributed equally to the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hudson, P.; Souriau, C. Engineered antibodies. Nat. Med. 2003, 9, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Hagemeyer, C.E.; von zur Muhlen, C.; von Elverfeldt, D.; Peter, K. Single-chain antibodies as diagnostic tools and therapeutic agents. Thromb. Haemost. 2009, 101, 1012–1019. [Google Scholar] [PubMed]
- Goshima, N.; Kawamura, Y.; Fukumoto, A.; Miura, A.; Honma, R.; Satoh, R.; Wakamatsu, A.; Yamamoto, J.; Kimura, K.; Nishikawa, T.; et al. Human protein factory for converting the transcriptome into an in vitro-expressed proteome. Nat. Methods 2008, 5, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Zawada, J.F.; Yin, G.; Steiner, A.R.; Yang, J.; Naresh, A.; Roy, S.M.; Gold, D.S.; Heinsohn, H.G.; Murray, C.J. Microscale to manufacturing scale-up of cell-free cytokine production—A new approach for shortening protein production development timelines. Biotechnol. Bioeng. 2011, 108, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Stech, M.; Brödel, A.K.; Quast, R.B.; Sachse, R.; Kubick, S. Cell-free systems: Functional modules for synthetic and chemical biology. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 67–102. [Google Scholar]
- Orth, J.H.C.; Schorcha, B.; Boundy, S.; Ffrench-Constant, R.; Kubick, S.; Aktories, K. Cell-free synthesis and characterization of a novel cytotoxic pierisin-like protein from the cabbage butterfly Pieris rapae. Toxicon 2011, 57, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Katzen, F.; Peterson, T.C.; Kudlicki, W. Membrane protein expression: No cells required. Trends Biotechnol. 2009, 27, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Garces, E.D.; Yang, J.; Zhang, J.; Tran, C.; Steiner, A.R.; Roos, C.; Bajad, S.; Hudak, S.; Penta, K.; et al. Aglycosylated antibodies and antibody fragments produced in a scalable in vitro transcription-translation system. mAbs 2012, 4, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Gouda, M.D.; Sawasaki, T.; Takai, K.; Endo, Y. Efficient synthesis of a disulfide-containing protein through a batch cell-free system from wheat germ. Eur. J. Biochem. 2003, 270, 4780–4786. [Google Scholar] [CrossRef] [PubMed]
- Stech, M.; Hust, M.; Schulze, C.; Dübel, S.; Kubick, S. Cell-free eukaryotic systems for the production, engineering and modification of scFv antibody fragments. Eng. Life Sci. 2014, 14, 387–398. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Presta, L.G. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv. Drug Deliver. Rev. 2006, 58, 640–656. [Google Scholar] [CrossRef]
- Weisser, N.E.; Hall, J.C. Applications of single-chain variable fragment antibodies in therapeutics and diagnostics. Biotechnol. Adv. 2009, 27, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Huston, J.S.; Levinson, D.; Mudgett-Hunter, M.; Tai, M.S.; Novotný, J.; Margolies, M.N.; Ridge, R.J.; Bruccoleri, R.E.; Haber, E.; Crea, R. Protein engineering of antibody binding sites: Recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl. Acad. Sci. USA 1988, 85, 5879–5883. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.E.; Hardman, K.D.; Jacobson, J.W.; Johnson, S.; Kaufman, B.M.; Lee, S.M.; Lee, T.; Pope, S.H.; Riordan, G.S.; Whitlow, M. Single-chain antigen-binding proteins. Science 1988, 242, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Mccafferty, J.; Griffiths, A.D.; Winter, G.; Chiswell, D.J. Phage antibodies: Filamentous phage displaying antibody variable domain. Nature 1990, 348, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Smith, G. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Barbas, C.F., 3rd; Kang, A.S.; Lerner, R.A.; Benkovic, S.J. Assembly of combinatorial antibody libraries on phages surfaces: The gene III site. Proc. Natl. Acad. Sci. USA 1991, 88, 7987–7982. [Google Scholar] [CrossRef]
- Barbas, C.; Lerner, R. Combinatorial immunoglobulin libraries on the surface of phage (Phabs): Rapid selection of antigen-specific Fabs. Methods 1991, 2, 119–124. [Google Scholar] [CrossRef]
- Hust, M.; Dübel, S. Mating antibody phage display to proteomics. Trends Biotechnol. 2004, 22, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.A.; Campbell, R.; Iverson, B.L.; Georgiou, G. Production and fluorescence-activated cell sorting of Escherichia coli expressing a functional antibody fragment on the external surface. Proc. Natl. Acad. Sci. USA 1993, 90, 10444–10448. [Google Scholar] [CrossRef] [PubMed]
- Boder, E.T.; Wittrup, K.D. Yeast surface display for screening combinatorial polypeptide libraries. Nat. Biotechnol. 1997, 15, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Szostak, J. RNA-peptide fusions for the in vitro selection of peptides and proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 12297–12302. [Google Scholar] [CrossRef]
- Nemoto, N.; Miyamoto-Sato, E.; Husimi, Y.; Yanagawa, H. In vitro virus: Bonding of mRNA bearing puromycin at the 3'-terminal end to the C-terminal end of its encoded protein on the ribosome in vitro. FEBS Lett. 1997, 414, 405–408. [Google Scholar] [CrossRef]
- He, M.; Taussig, M.J. Antibody-ribosome-mRNA (ARM) complexes as efficient selection particles for in vitro display and evolution of antibody combining sites. Nucleic Acids Res. 1997, 25, 5132–5134. [Google Scholar] [CrossRef] [PubMed]
- Hanes, J.; Plückthun, A. In vitro selection and evolution of functional proteins by using ribosome display. Proc. Natl. Acad. Sci. USA 1997, 94, 4937–4942. [Google Scholar]
- Better, M.; Chang, C.P.; Robinson, R.R.; Horwitz, A.H. Escherichia coli secretion of an active chimeric antibody fragment. Science 1988, 240, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Skerra, A.; Plückthun, A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science 1988, 240, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Kelley, R.F.; Rodrigues, M.L.; Snedecor, B.; Covarrubias, M.; Velligan, M.D.; Wong, W.L.T.; Rowland, A.M.; Kotts, C.E.; Carver, M.E.; et al. High Level Escherichia coli Expression and Production of a Bivalent Humanized Antibody Fragment. Nat. Biotechnol. 1992, 10, 163–167. [Google Scholar] [CrossRef]
- Simmons, L.C.; Reilly, D.; Klimowski, L.; Shantha Raju, T.; Meng, G.; Sims, P.; Hong, K.; Shields, R.L.; Damicof, L.A.; Rancatoreg, P.; et al. Expression of full-length immunoglobulins in Escherichia coli: rapid and efficient production of aglycosylated antibodies. J. Immunol. Methods 2002, 263, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Rojas, G.; Mitchell, J.N.; Vincent, K.J.; Wu, J.; McCafferty, J.; Schofield, D.J. A simple vector system to improve performance and utilization of recombinant antibodies. BMC Biotechnol. 2006, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Hackel, B.; Huang, D.; Bubolz, J.C.; Wang, X.X.; Shusta, E.V. Production of soluble and active transferrin receptor-targeting single-chain antibody using Saccharomyces cerevisiae. Pharm. Res. 2006, 23, 790–797. [Google Scholar] [CrossRef]
- Ren, F.; Li, B.-C.; Zhang, N.-N.; Cao, M.; Dan, W.-B.; Zhang, S.-Q. Expression, purification and characterization of anti-BAFF antibody secreted from the yeast Pichia pastoris. Biotechnol. Lett. 2008, 30, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Bruenke, J.; Fischer, B.; Barbin, K.; Schreiter, K.; Wachter, Y.; Mahr, K.; Titgemeyer, F.; Niederweis, M.; Peipp, M.; Zunino, S.J.; et al. A recombinant bispecific single-chain Fv antibody against HLA class II and FcγRIII (CD16) triggers effective lysis of lymphoma cells. Br. J. Haematol. 2004, 125, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, S.P.; Franklin, S.E.; Lerner, R.A. Expression and assembly of a fully active antibody in algae. Proc. Natl. Acad. Sci. USA 2003, 100, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Makvandi-Nejad, S.; McLean, M.D.; Hirama, T.; Almquist, K.C.; MacKenzie, C.R.; Hall, J.C. Transgenic tobacco plants expressing a dimeric single-chain variable fragment (scfv) antibody against Salmonella enterica serotype Paratyphi B. Transgenic. Res. 2005, 14, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Natsume, A.; Wakitani, M.; Yamane-Ohnuki, N.; Shoji-Hosaka, E.; Niwa, R.; Uchida, K.; Satoh, M.; Shitara, K. Fucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded bispecific antibody comprising of two single-chain antibodies linked to the antibody constant region. J. Biochem. 2006, 140, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; James, D. Production of recombinant monoclonal antibodies in mammalian cells. In Cell Engineering; Al-Rubeai, M., Ed.; Springer: New York, NY, USA, 2009; Volume 6. [Google Scholar]
- Li, F.; Shen, A.; Amanullah, A. Cell culture processes for monoclonal antibody production. Pharm. Sci. 2013, 1–38. [Google Scholar]
- Wurm, F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004, 22, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.R.; Racher, A.J. Antibody production. Adv. Drug Deliver. Rev. 2006, 58, 671–685. [Google Scholar] [CrossRef]
- Pedersen, A.; Hellberg, K.; Enberg, J.; Karlsson, B.G. Rational improvement of cell-free protein synthesis. New Biotechnol. 2010, 28, 218–224. [Google Scholar] [CrossRef]
- Nirenberg, M.; Matthaei, J. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc. Natl. Acad. Sci. USA 1961, 47, 1588–1602. [Google Scholar] [CrossRef] [PubMed]
- Bechlars, S.; Wüstenhagen, D.A.; Drägert, K.; Dieckmann, R.; Strauch, E.; Kubick, S. Cell-free synthesis of functional thermostable direct hemolysins of Vibrio parahaemolyticus. Toxicon 2013, 76, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.D.; Tawfik, D.S. Directed evolution of an extremely fast phosphotriesterase by in vitro compartmentalization. EMBO J. 2003, 22, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Klammt, C.; Koglin, A.; Löhr, F.; Schneider, B.; Dötsch, V.; Bernhard, F. Preparative scale cell-free expression systems: New tools for the large scale preparation of integral membrane proteins for functional and structural studies. Methods 2007, 41, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.D.; Gan, R.; Hodgman, C.E.; Jewett, M.C. Cell-free protein synthesis: Applications come of age. Biotechnol. Adv. 2012, 30, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.; Padlan, E.; Sheriff, S. Antibody-antigen complexes. Annu. Rev. Biochem. 1990, 59, 439–473. [Google Scholar] [CrossRef] [PubMed]
- Glockshuber, R.; Schmidt, T.; Plückthun, A. The disulfide bonds in antibody variable domains: Effects on stability, folding in vitro, and functional expression in Escherichia coli. Biochemistry 1992, 31, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Hamaguchi, K. The role of the intrachain disulfide bond in the conformation and stability of the constant fragment of the immunoglobulin light chain. J. Biochem. 1979, 86, 1433–1441. [Google Scholar] [PubMed]
- Kim, D.-M.; Swartz, J.R. Efficient production of a bioactive, multiple disulfide-bonded protein using modified extracts of Escherichia coli. Biotechnol. Bioeng. 2004, 85, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Bundy, B.C.; Swartz, J.R. Efficient disulfide bond formation in virus-like particles. J. Biotechnol. 2011, 154, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Katzen, F.; Chang, G.; Kudlicki, W. The past, present and future of cell-free protein synthesis. Trends Biotechnol. 2005, 23, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ryabova, L.A.; Desplancq, D.; Spirin, A.S.; Plückthun, A. Functional antibody production using cell-free translation: Effects of protein disulfide isomerase and chaperones. Nat. Biotechnol. 1997, 15, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Merk, H.; Stiege, W.; Tsumoto, K.; Kumagai, I.; Erdmann, V.A. Cell-free expression of two single-chain monoclonal antibodies against lysozyme: Effect of domain arrangement on the expression. J. Biochem. (Tokyo) 1999, 125, 328–333. [Google Scholar] [CrossRef]
- Jiang, X.; Ookubo, Y.; Fujii, I.; Nakano, H.; Yamane, T. Expression of Fab fragment of catalytic antibody 6D9 in an Escherichia coli in vitro coupled transcription/translation system. FEBS Lett. 2002, 514, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Swartz, J.R. Enhancing multiple disulfide bonded protein folding in a cell-free system. Biotechnol. Bioeng. 2004, 86, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.-S.; Kim, D.-M.; Kim, T.-W.; Park, C.-G.; Choi, C.-Y. Providing an oxidizing environment for the cell-free expression of disulfide-containing proteins by exhausting the reducing activity of Escherichia coli S30 extract. Biotechnol. Progr. 2006, 22, 1225–1228. [Google Scholar] [CrossRef]
- Goerke, A.R.; Swartz, J.R. Development of cell-free protein synthesis platforms for disulfide bonded proteins. Biotechnol. Bioeng. 2008, 99, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Knapp, K.G.; Goerke, A.R.; Swartz, J.R. Cell-free synthesis of proteins that require disulfide bonds using glucose as an energy source. Biotechnol. Bioeng. 2007, 97, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Armstrong, S.; Rivers, P.J.; Zhang, J.; Yang, J.; Green, E.; Rozzelle, J.; Liang, S.; Kittle, J.D.; Steiner, A.R.; et al. Engineering toward a bacterial "endoplasmic reticulum" for the rapid expression of immunoglobulin proteins. mAbs 2014, 6, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Bulleid, N.J.; Bassel-Duby, R.S.; Freedman, R.B.; Sambrook, J.F.; Gething, M.J. Cell-free synthesis of enzymically active tissue-type plasminogen activator. Protein folding determines the extent of N-linked glycosylation. Biochem. J. 1992, 286, 275–280. [Google Scholar] [PubMed]
- Ezure, T.; Suzuki, T.; Shikata, M.; Ito, M.; Ando, E.; Nishimura, O.; Tsunasawa, S. Expression of proteins containing disulfide bonds in an insect cell-free system and confirmation of their arrangements by MALDI-TOF MS. Proteomics 2007, 24, 4424–4434. [Google Scholar] [CrossRef]
- Kubick, S.; Schacherl, J.; Fleischer-Notter, H.; Royall, E.; Roberts, L.O.; Stiege, W. In vitro translation in an insect-based cell-free system. In Cell-Free Protein Expression; Swartz, J.R., Ed.; Springer: Berlin, Germany, 2003; pp. 209–217. [Google Scholar]
- Kubick, S.; Gerrits, M.; Merk, H.; Stiege, W.; Erdmann, V.A. In vitro synthesis of posttranslationally modified membrane proteins. In "Membrane Protein Crystallization" Current Topics in Membranes; DeLucas, L., Ed.; Elsevier: Burlington, Canada, 2009; Chapter 2; pp. 25–49. [Google Scholar]
- Sachse, R.; Wüstenhagen, D.; Šamalíková, M.; Gerrits, M.; Bier, F.F.; Kubick, S. Synthesis of membrane proteins in eukaryotic cell-free systems. Eng. Life Sci. 2013, 13, 39–48. [Google Scholar] [CrossRef]
- Dondapati, S.K.; Kreir, M.; Quast, R.B.; Wüstenhagen, D.A.; Brüggemann, A.; Fertig, N.; Kubick, S. Membrane assembly of the functional KcsA potassium channel in a vesicle-based eukaryotic cell-free translation system. Biosens. Bioelectron. 2014, 59, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Stech, M.; Quast, R.B.; Sachse, R.; Schulze, C.; Wüstenhagen, D.A.; Kubick, S. A continuous-exchange cell-free protein synthesis system based on extracts from cultured insect cells. PLoS One 2014, 9, e96635. [Google Scholar] [CrossRef]
- Schulze-Gahmen, U.; Rini, J.M.; Wilson, I.A. Detailed Analysis of the Free and Bound Conformations of an Antibody: X-ray Structures of Fab 17/9 and Three Different Fab-peptide Complexes. J. Mol. Biol. 1993, 234, 1098–1118. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Haun, Y.; Deng, J.; Gao, F.; Pan, B.; Cui, D. Expression of Single-Chain Fv Gene Specific for gamma-Seminoprotein by RTS and Its Biological Activity Identification. Biotechnol. Prog. 2006, 22, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Galeffi, P.; Lombardi, A.; Pietraforte, I.; Novelli, F.; Donato, M.D.; Sperandei, M.; Tornambé, A.; Fraioli, R.; Martayan, A.; Natali, P.G.; et al. Functional expression of a single-chain antibody to ErbB-2 in plants and cell-free systems. J. Transl. Med. 2006, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Hara, T.; Tanimura, R.; Fukuyama, S.; Cagnon, C.; Kohara, A.; Fujii, I. Site-directed mutagenesis of active site contact residues in a hydrolytic abzyme: Evidence for an essential histidine involved in transition state stabilization. J. Mol. Biol. 1997, 267, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Karaki, Y.; Kikuchi, M.; Fujii, I. Prodrug activation via catalytic antibodies. Proc. Natl. Acad. Sci. USA 1993, 90, 5337–5340. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Suzuki, H.; Fukuba, T.; Jiang, X.; Nakano, H.; Yamane, T. Improvements in the cell-free production of functional antibodies using cell extract from protease-deficient Escherichia coli mutant. J. Biosci. Bioeng. 2005, 99, 181–186. [Google Scholar] [CrossRef]
- Oh, I.-S.; Lee, J.-C.; Lee, M.-S.; Chung, J.-H.; Kim, D.-M. Cell-free production of functional antibody fragments. Bioproc. Biosyst. Eng. 2010, 33, 127–132. [Google Scholar] [CrossRef]
- Frey, S.; Haslbeck, M.; Hainzl, O.; Buchner, J. Synthesis and characterization of a functional intact IgG in a prokaryotic cell-free expression system. Biol. Chem. 2008, 389, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.L.; Matsumoto, M.L.; Yin, G.; Cai, Q.; Fung, J.J.; Stephenson, H.; Gill, A.; You, M.; Lin, S.-H.; Wang, W.D.; et al. In vitro Fab display: A cell-free system for IgG discovery. Protein Eng. Des. Sel. 2014, 27, 97–109. [Google Scholar] [CrossRef]
- Hillebrecht, J.R.; Chong, S. A comparative study of protein synthesis in in vitro systems: From the prokaryotic reconstituted to the eukaryotic extract-based. BMC Biotechnol. 2008, 8, 1790–1793. [Google Scholar] [CrossRef]
- Langlais, C.; Guilleaume, B.; Wermke, N.; Scheuermann, T.; Ebert, L.; LaBaer, J.; Korn, B. A systematic approach for testing expression of human full-length proteins in cell-free expression systems. BMC Biotechnol. 2007, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tarui, H.; Murata, M.; Tani, I.; Imanishi, S.; Nishikawa, S.; Hara, T. Establishment and characterization of translation/glycosylation in insect cell (Spodoptera frugiperda 21) extract prepared with high pressure treatment. Appl. Microbiol. Biotechnol. 2001, 55, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.H.; Huang, C.-J.; Newton, B.S.; Ritter, G.; Old, L.J.; Batt, C.A. Factors affecting endotoxin removal from recombinant therapeutic proteins by anion exchange chromatography. Protein Exp. Purif. 2009, 64, 76–81. [Google Scholar] [CrossRef]
- Gusdon, J.P.J.; Stavitsky, A.B.; Armentrout, S.A. Synthesis of gamma G antibody and immunoglobulin on polyribosomes in a cell-free system. Proc. Natl. Acad. Sci. USA 1967, 58, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, P. Studies on cell-free synthesis of rat immunoglobulins, I. A cell-free system for protein synthesis prepared from lymph-node microsomal vesicles. Proc. Natl. Acad. Sci. USA 1967, 58, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, P.; Lisowska-Bernstein, B.; Lamm, M.E.; Benacerraf, B. Studies on cell-free synthesis of rat immunoglobulins, II. Synthesis of immunoglobulin and of antibody to the dinitrophenyl hapten. Proc. Natl. Acad. Sci. USA 1967, 58, 2422–2429. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P.J.; Johnson, V.G.; Andrew, S.M.; Hoogenboom, H.R.; Raus, J.C.; Youle, R.J. Characterization of single-chain antibody (sFv)-toxin fusion proteins produced in vitro in rabbit reticulocyte lysate. J. Biol. Chem. 1993, 268, 5302–5308. [Google Scholar] [PubMed]
- Hanes, J.; Jermutus, L.; Schaffitze, C.; Plückthun, A. Comparison of Escherichia coli and rabbit reticulocyte ribosome display systems. FEBS Lett. 1999, 450, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Stech, M.; Merk, H.; Schenk, J.A.; Stöcklein, W.F.M.; Wüstenhagen, D.A.; Micheel, B.; Duschl, C.; Bier, F.F.; Kubick, S. Production of functional antibody fragments in a vesicle-based eukaryotic cell-free translation system. J. Biotechnol. 2012, 164, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Schenk, J.A.; Sellrie, F.; Böttger, V.; Menning, A.; Stöcklein, W.F.M.; Micheel, B. Generation and application of a fluorescein-specific single chain antibody. Biochimie 2007, 89, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Micheel, B.; Janstcheff, P.; Böttger, V.; Scharte, G.; Kaiser, G.; Stolley, P.; Karawajew, L. The production and radioimmunoassay application of monoclonal antibodies to fluorescein isothiocyanate (FITC). J. Immunol. Methods 1988, 111, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Merk, H.; Gless, C.; Maertens, B.; Gerrits, M.; Stiege, W. Cell-free synthesis of functional and endotoxin-free antibody Fab fragments by translocation into microsomes. BioTechniques 2012, 53, 153–160. [Google Scholar] [PubMed]
- Tu, T.; Weissman, J. Oxidative protein folding in eukaryotes mechanisms and consequences. J. Cell Biol. 2004, 164, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Kapanidis, A.N.; Weiss, S. Fluorescent probes and bioconjugation chemistries for single-molecule fluorescence analysis of biomolecules. J. Chem. Phys. 2002, 117, 10953–10964. [Google Scholar] [CrossRef]
- Elvira, C.; Gallardo, A.; Roman, J.; Cifuentes, A. Covalent polymer-drug conjugates. Molecules 2005, 10, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Kanter, G.; Yang, J.; Voloshin, A.; Levy, S.; Swartz, J.R.; Levy, R. Cell-free production of scFv fusion proteins: An efficient approach for personalized lymphoma vaccines. Blood 2007, 109, 3393–3399. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.G.; Ng, P.P.; Levy, S.; Levy, R.; Swartz, J.R. Escherichia coli-based production of a tumor idiotype antibody fragment - tetanus toxin fragment C fusion protein vaccine for B cell lymphoma. Protein Exp. Purif. 2011, 75, 15–20. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kuruma, Y.; Ying, B.-W.; Umekage, S.; Ueda, T. Cell-free translation systems for protein engineering. FEBS J. 2006, 273, 4133–4140. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, M.; Strey, J.; Claußnitzer, I.; von Groll, U.; Schäfer, F.; Rimmele, M.; Stiege, W. Cell-Free synthesis of defined protein conjugates by site-directed cotranslational labeling. In Cell-free Protein Expression; Kudlicki, T., Katzen, F., Bennett, R., Eds.; Landes Bioscience: Austin, TX, USA, 2007. [Google Scholar]
- Budisa, N. Prolegomena to future experimental efforts on genetic code engineering by expanding its amino acid repertoire. Angew. Chem. Int. Ed. 2004, 43, 6426–6463. [Google Scholar] [CrossRef]
- Service, R.F. Unnatural amino acid could prove boon for protein therapeutics. Science 2005, 308, 44. [Google Scholar] [PubMed]
- Mendel, D.; Cornish, V.; Schultz, P. Site-directed mutagenesis with an expanded genetic code. Annu. Rev. Biophys. Biomol. Struct. 1995, 24, 435–462. [Google Scholar] [CrossRef] [PubMed]
- Kurzchalia, T.V.; Wiedmann, M.; Breter, H.; Zimmermann, W.; Bauschke, E.; Rapoport, T.A. tRNA-mediated labelling of proteins with biotin. A nonradioactive method for the detection of cell-free translation products. Eur. J. Biochem. 1988, 172, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Schultz, P.G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 2010, 79, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Quast, R.B.; Claussnitzer, I.; Merk, H.; Kubick, S.; Gerrits, M. Synthesis and site-directed fluorescence labeling of azido proteins using eukaryotic cell-free orthogonal translation systems. Anal. Biochem. 2014, 451, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Schmied, W.H.; Chin, J.W. Reprogramming the genetic code: From triplet to quadruplet codes. Angew. Chem. Int. Ed. 2012, 51, 2288–2297. [Google Scholar] [CrossRef]
- Sisido, M.; Ninomiya, K.; Ohtsuki, T.; Hohsaka, T. Four-base codon/anticodon strategy and non-enzymatic aminoacylation for protein engineering with non-natural amino acids. Methods 2005, 36, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.G.; Ng, P.P.; Kuo, C.-C.; Levy, S.; Levy, R.; Swartz, J.R. Cell-free production of Gaussia princeps luciferase—antibody fragment bioconjugates for ex vivo detection of tumor cells. Biochem. Biophys. Res. Commun. 2009, 390, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Hallam, T.; Smider, V. Unnatural amino acids in novel antibody conjugates. Future Med. Chem. 2014, 6, 1309–1324. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, E.S.; Heibeck, T.H.; Gill, A.; Li, X.; Murray, C.J.; Madlansacay, M.R.; Tran, C.; Uter, N.T.; Yin, G.; Rivers, P.J.; et al. Production of site-specific antibody-drug conjugates using optimized non-natural amino acids in a cell-free expression system. Bioconjug. Chem. 2014, 25, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Furumoto, S.; Higuchi, K.; Yokoyama, J.; Zhang, M.-R.; Yanai, K.; Iwata, R.; Kigawa, T. Rapid biochemical synthesis of 11C-labeled single chain variable fragment antibody for immuno-PET by cell-free protein synthesis. Bioorg. Med. Chem. 2012, 20, 6579–6582. [Google Scholar] [CrossRef] [PubMed]
- Brödel, A.K.; Sonnabend, A.; Kubick, S. Cell-free protein expression based on extracts from CHO cells. Biotechnol. Bioeng. 2013, 111, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Brödel, A.K.; Sonnabend, A.; Roberts, L.O.; Stech, M.; Wüstenhagen, D.A.; Kubick, S. IRES-mediated translation of membrane proteins and glycoproteins in eukaryotic cell-free systems. PLoS One 2013, 8, e82234. [Google Scholar] [CrossRef]
- Ghaderi, D.; Zhang, M.; Hurtado-Ziola, N.; Varki, A. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol. Genet. Eng. Rev. 2012, 28, 147–175. [Google Scholar] [PubMed]
- Shade, K.-T.C.; Anthony, R.M. Antibody Glycosylation and Inflammation. Antibodies 2013, 2, 92–414. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Guarino, C.; DeLisa, M. A prokaryote-based cell-free translation system that efficiently synthesizes glycoproteins. Glycobiology 2012, 22, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Kigawa, T.; Yabuki, T.; Yoshida, Y.; Tsutsui, M.; Ito, Y.; Shibata, T.; Yokoyama, S. Cell-free production and stable-isotope labeling of milligram quantities of proteins. FEBS Lett. 1999, 442, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Madin, K.; Sawasaki, T.; Ogasawara, T.; Endo, Y. A highly efficient and robust protein synthesis system prepared from wheat embryos: Plants apparently contain a suicide system directed at ribosomes. Proc. Natl. Acad. Sci. USA 2000, 97, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Ramya, R.; Subramanian, B.M.; Sivakumar, V.; Senthilkumar, R.L.; Rao, K.R.S.S.; Srinivasan, V.A. Expression and solubilization of insect cell-based rabies virus glycoprotein and assessment of its immunogenicity and protective efficacy in mice. Clin. Vaccine Immunol. 2011, 18, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Naftzger, C.; Takechi, Y.; Kohda, H.; Hara, I.; Vijayasaradhi, S.; Houghton, A.N. Immune response to a differentiation antigen induced by altered antigen: A study of tumor rejection and autoimmunity. Proc. Natl. Acad. Sci. USA 1996, 93, 14809–14814. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Nguyen, D.P.; Lusic, H.; Neumann, H.; Kapadnis, P.B.; Deiters, A.; Chin, J.W. Genetic encoding and labeling of aliphatic azides and alkynes in recombinant proteins via a pyrrolysyl-tRNA synthetase/tRNACUA pair and click chemistry. J. Am. Chem. Soc. 2009, 131, 8720–8721. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, J.B.B.; Presta, L.G.; Carter, P. "Knobs-into-holes" engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996, 9, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lee, J.; Tran, C.; Heibeck, T.H.; Wang, W.D.; Yang, J.; Stafford, R.L.; Steiner, A.R.; Sato, A.K.; Hallam, T.J.; et al. Production of bispecific antibodies in "Knobs-into-Holes" using a cell-free expression system. mAbs 2014. [Google Scholar] [CrossRef]
- Murray, C.J.; Baliga, R. Cell-free translation of peptides and proteins: From high throughput screening to clinical production. Curr. Opin. Chem. Biol. 2013, 17, 420–426. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).