In Vivo Applications of Single Chain Fv (Variable Domain) (scFv) Fragments
Abstract
:1. Introduction
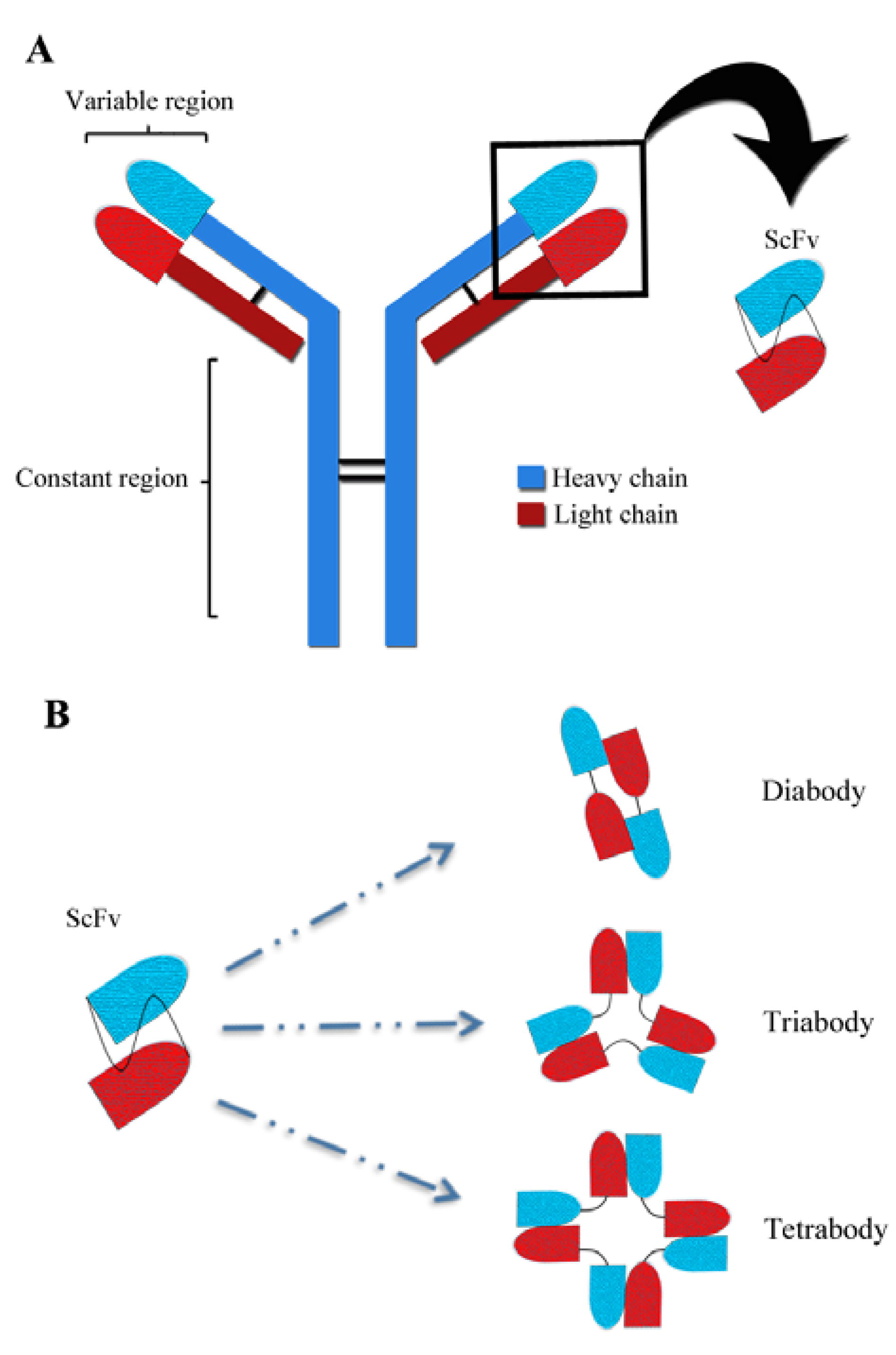
2. In Vivo Delivery
2.1.Viral Mediated Delivery
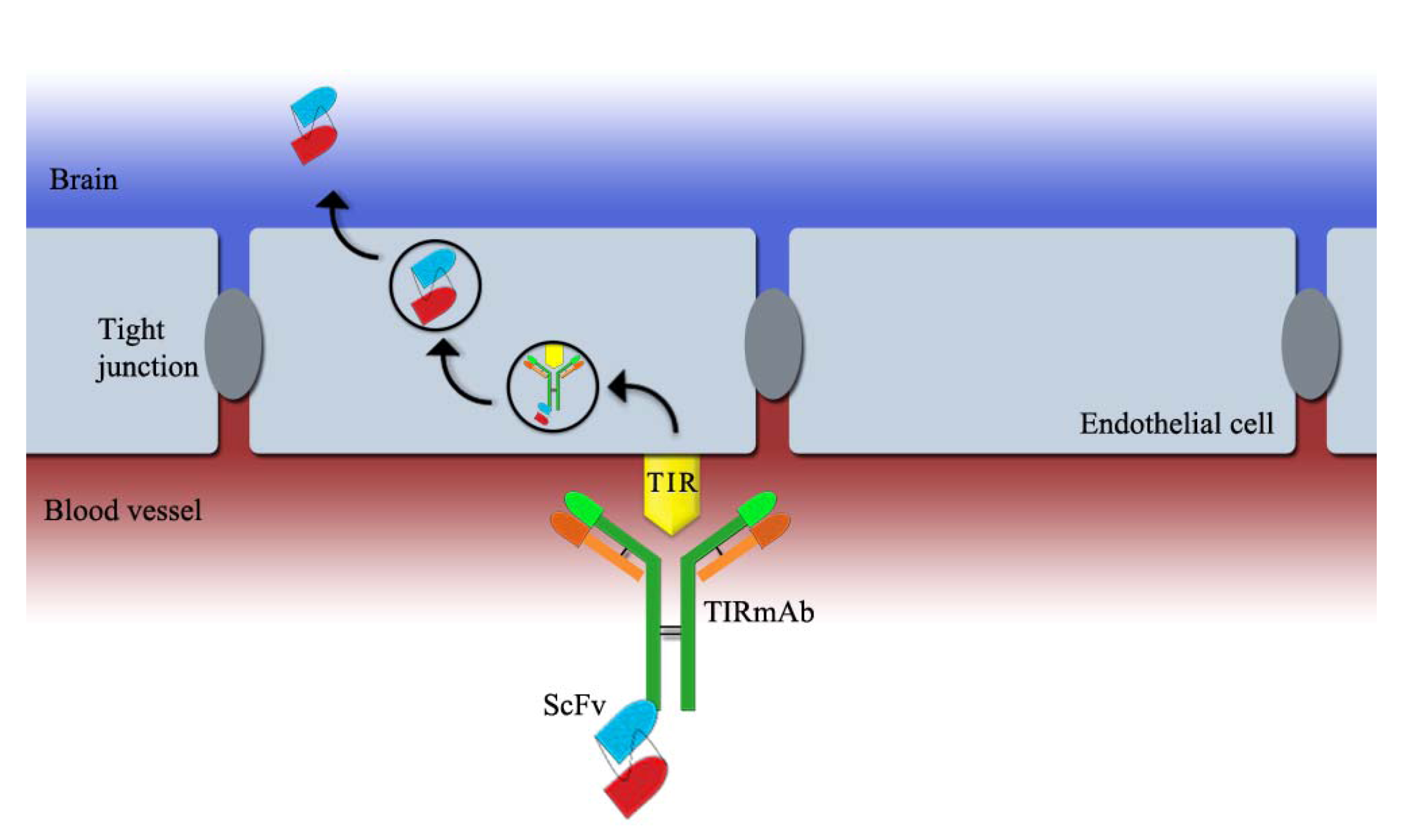
2.2. ScFv Linked to a Blood-Brain Barrier (BBB) Receptor Antibody
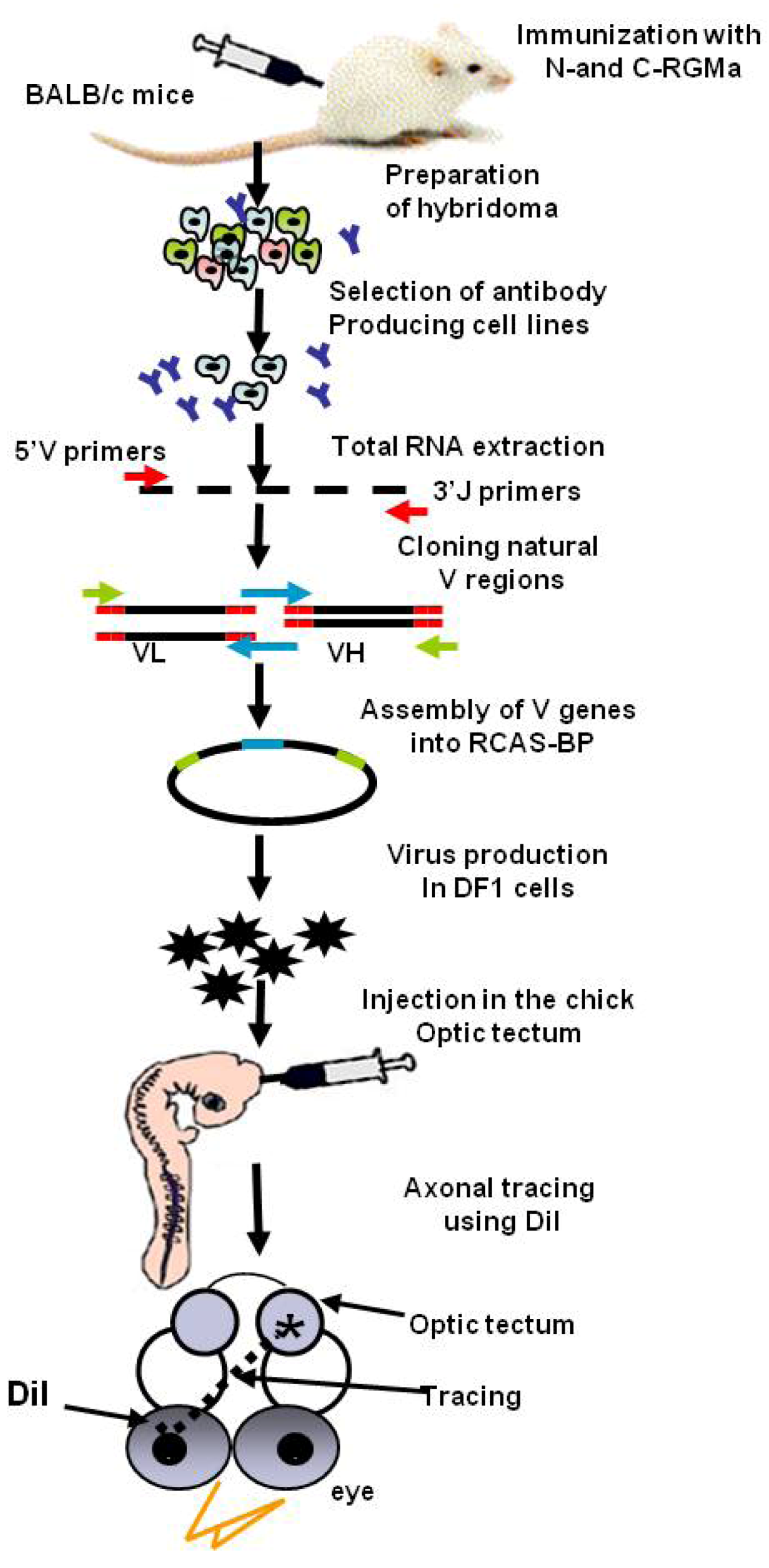
3. In Vivo Application of scFv Fragments
3.1.A Tool to Study Protein Functions
3.2. Cancer Therapy
3.3. Neurodegenerative Diseases
3.3.1. Alzheimer’s Disease (AD)
3.3.2. Huntington’s Disease (HD)
3.4. In Vivo Imaging
3.5. Vehicles to Deliver Drugs/Nanoparticles
4. Conclusions
Acknowledgments
References
- Ward, E.S.; Martinez, C.; Vaccaro, C.; Zhou, J.; Tang, Q.; Ober, R.J. From sorting endosomes to exocytosis: Association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, During recycling. Mol. Biol. Cell 2005, 16, 2028–2038. [Google Scholar] [CrossRef]
- Woof, J.M.; Burton, D.R. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat. Rev. Immunol. 2004, 4, 89–99. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef]
- Wu, A.M.; Senter, P.D. Arming antibodies: Prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005, 23, 1137–1146. [Google Scholar] [CrossRef]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef]
- Porter, R.R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem. J. 1959, 73, 119–126. [Google Scholar]
- Hudson, P.J. Recombinant antibody fragments. Curr. Opin. Biotechnol. 1998, 9, 395–402. [Google Scholar] [CrossRef]
- Little, M.; Kipriyanov, S.M.; le Gall, F.; Moldenhauer, G. Of mice and men: Hybridoma and recombinant antibodies. Immunol. Today 2000, 21, 364–370. [Google Scholar] [CrossRef]
- Skerra, A.; Pluckthun, A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science 1988, 240, 1038–1041. [Google Scholar]
- Hust, M.; Jostock, T.; Menzel, C.; Voedisch, B.; Mohr, A.; Brenneis, M.; Kirsch, M.I.; Meier, D.; Dubel, S. Single chain Fab (scFab) fragment. BMC Biotechnol. 2007, 7, 14. [Google Scholar] [Green Version]
- Edwardraja, S.; Sriram, S.; Govindan, R.; Budisa, N.; Lee, S.G. Enhancing the thermal stability of a single-chain Fv fragment by in vivo global fluorination of the proline residues. Mol. Biosyst. 2011, 7, 258–265. [Google Scholar] [CrossRef]
- Nelson, A.L. Antibody fragments: Hope and hype. MAbs 2010, 2, 77–83. [Google Scholar] [CrossRef]
- Nelson, A.L.; Reichert, J.M. Development trends for therapeutic antibody fragments. Nat. Biotechnol. 2009, 27, 331–337. [Google Scholar] [CrossRef]
- Maynard, J.; Georgiou, G. Antibody engineering. Annu. Rev. Biomed. Eng. 2000, 2, 339–376. [Google Scholar] [CrossRef]
- Bird, R.E.; Hardman, K.D.; Jacobson, J.W.; Johnson, S.; Kaufman, B.M.; Lee, S.M.; Lee, T.; Pope, S.H.; Riordan, G.S.; Whitlow, M. Single-chain antigen-binding proteins. Science 1988, 242, 423–426. [Google Scholar]
- Huston, J.S.; Levinson, D.; Mudgett-Hunter, M.; Tai, M.S.; Novotny, J.; Margolies, M.N.; Ridge, R.J.; Bruccoleri, R.E.; Haber, E.; Crea, R.; et al. Protein engineering of antibody binding sites: Recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl. Acad. Sci. USA 1988, 85, 5879–5883. [Google Scholar] [CrossRef]
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.; Hamid, M. scFv antibody: Principles and clinical application. Dev. Immunol. 2012, 2012, 980250. [Google Scholar]
- Whitlow, M.; Bell, B.A.; Feng, S.L.; Filpula, D.; Hardman, K.D.; Hubert, S.L.; Rollence, M.L.; Wood, J.F.; Schott, M.E.; Milenic, D.E.; et al. An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Eng. 1993, 6, 989–995. [Google Scholar] [CrossRef]
- Alfthan, K.; Takkinen, K.; Sizmann, D.; Soderlund, H.; Teeri, T.T. Properties of a single-chain antibody containing different linker peptides. Protein Eng. 1995, 8, 725–731. [Google Scholar] [CrossRef]
- Baneyx, F. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol. 1999, 10, 411–421. [Google Scholar] [CrossRef]
- Ho, M.; Nagata, S.; Pastan, I. Isolation of anti-CD22 Fv with high affinity by Fv display on human cells. Proc. Natl. Acad. Sci. USA 2006, 103, 9637–9642. [Google Scholar] [CrossRef]
- Galeffi, P.; Lombardi, A.; Pietraforte, I.; Novelli, F.; Di Donato, M.; Sperandei, M.; Tornambe, A.; Fraioli, R.; Martayan, A.; Natali, P.G.; et al. Functional expression of a single-chain antibody to ErbB-2 in plants and cell-free systems. J. Transl. Med. 2006, 4, 39. [Google Scholar] [CrossRef]
- Choo, A.B.; Dunn, R.D.; Broady, K.W.; Raison, R.L. Soluble expression of a functional recombinant cytolytic immunotoxin in insect cells. Protein Expres. Purif. 2002, 24, 338–347. [Google Scholar] [CrossRef]
- Chowdhury, P.S.; Viner, J.L.; Beers, R.; Pastan, I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc. National. Acad. Sci. USA 1998, 95, 669–674. [Google Scholar] [CrossRef]
- Deckert, P.M. Current constructs and targets in clinical development for antibody-based cancer therapy. Curr. Drug Targets 2009, 10, 158–175. [Google Scholar] [CrossRef]
- Cheng, K.T. Radioiodinated-anti-TAG-72 covalently linked CC49 divalent single-chain Fv antibody. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information: Bethesda, MD, USA, 2004. [Google Scholar]
- Kobayashi, N.; Odaka, K.; Uehara, T.; Imanaka-Yoshida, K.; Kato, Y.; Oyama, H.; Tadokoro, H.; Akizawa, H.; Tanada, S.; Hiroe, M.; et al. Toward in vivo imaging of heart disease using a radiolabeled single-chain Fv fragment targeting tenascin-C. Anal. Chem. 2011, 83, 9123–9130. [Google Scholar] [CrossRef]
- Robinson, M.K.; Doss, M.; Shaller, C.; Narayanan, D.; Marks, J.D.; Adler, L.P.; Gonzalez Trotter, D.E.; Adams, G.P. Quantitative immuno-positron emission tomography imaging of HER2-positive tumor xenografts with an iodine-124 labeled anti-HER2 diabody. Cancer Res. 2005, 65, 1471–1478. [Google Scholar] [CrossRef]
- Sundaresan, G.; Yazaki, P.J.; Shively, J.E.; Finn, R.D.; Larson, S.M.; Raubitschek, A.A.; Williams, L.E.; Chatziioannou, A.F.; Gambhir, S.S.; Wu, A.M. 124I-labeled engineered anti-CEAcea minibodies and diabodies allow high-contrast, antigen-specific small-animal PETimaging of xenografts in athymic mice. J. Nucl. Med. 2003, 44, 1962–1969. [Google Scholar]
- Chari, R.V. Targeted delivery of chemotherapeutics: Tumor-activated prodrug therapy. Adv. Drug Deliv. Rev. 1998, 31, 89–104. [Google Scholar] [CrossRef]
- Gattenlohner, S.; Jorissen, H.; Huhn, M.; Vincent, A.; Beeson, D.; Tzartos, S.; Mamalaki, A.; Etschmann, B.; Muller-Hermelink, H.K.; Koscielniak, E.; et al. A human recombinant autoantibody-based immunotoxin specific for the fetal acetylcholine receptor inhibits rhabdomyosarcoma growth in vitro and in a murine transplantation model. J. Biomed. Biotechnol. 2010, 2010, 187621. [Google Scholar]
- Tong, Q.; Liu, K.; Lu, X.M.; Shu, X.G.; Wang, G.B. Construction and characterization of a novel fusion protein MG7-scFv/SEB against gastric cancer. J. Biomed. Biotechnol. 2010, 2010, 121094. [Google Scholar]
- Tassew, N.G.; Charish, J.; Chestopalova, L.; Monnier, P.P. Sustained in vivo inhibition of protein domains using single-chain Fv recombinant antibodies and its application to dissect rgma activity on axonal outgrowth. J. Neurosci. 2009, 29, 1126–1131. [Google Scholar] [CrossRef]
- Di Lullo, E.; Haton, C.; Le Poupon, C.; Volovitch, M.; Joliot, A.; Thomas, J.L.; Prochiantz, A. Paracrine Pax6 activity regulates oligodendrocyte precursor cell migration in the chick embryonic neural tube. Development 2011, 138, 4991–5001. [Google Scholar] [CrossRef]
- Curigliano, G.; Spitaleri, G.; Dettori, M.; Locatelli, M.; Scarano, E.; Goldhirsch, A. Vaccine immunotherapy in breast cancer treatment: Promising, but still early. Expert Rev. Anticancer Ther. 2007, 7, 1225–1241. [Google Scholar] [CrossRef]
- Bard, F.; Cannon, C.; Barbour, R.; Burke, R.L.; Games, D.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000, 6, 916–919. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Fellouse, F.A. Synthetic therapeutic antibodies. Nat. Chem. Biol. 2006, 2, 682–688. [Google Scholar] [CrossRef]
- Bradbury, A.R.; Sidhu, S.; Dubel, S.; McCafferty, J. Beyond natural antibodies: The power of in vitro display technologies. Nat. Biotechnol. 2011, 29, 245–254. [Google Scholar]
- Butler, D.C.; McLear, J.A.; Messer, A. Engineered antibody therapies to counteract mutant huntingtin and related toxic intracellular proteins. Prog. Neurobiol. 2012, 97, 190–204. [Google Scholar] [CrossRef]
- Zuber, C.; Mitteregger, G.; Schuhmann, N.; Rey, C.; Knackmuss, S.; Rupprecht, W.; Reusch, U.; Pace, C.; Little, M.; Kretzschmar, H.A.; et al. Delivery of single-chain antibodies (scFvs) directed against the 37/67 kDa laminin receptor into mice via recombinant adeno-associated viral vectors for prion disease gene therapy. J. Gen. Virol. 2008, 89, 2055–2061. [Google Scholar] [CrossRef]
- Daya, S.; Berns, K.I. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 2008, 21, 583–593. [Google Scholar] [CrossRef]
- Han, T.; Abdel-Motal, U.M.; Chang, D.K.; Sui, J.; Muvaffak, A.; Campbell, J.; Zhu, Q.; Kupper, T.S.; Marasco, W.A. Human anti-CCR4 minibody gene transfer for the treatment of cutaneous t-cell lymphoma. PLoS One 2012, 7, e44455. [Google Scholar] [CrossRef]
- Hughes, S.H.; Greenhouse, J.J.; Petropoulos, C.J.; Sutrave, P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J. Virol. 1987, 61, 3004–3012. [Google Scholar]
- Atwal, J.K.; Chen, Y.; Chiu, C.; Mortensen, D.L.; Meilandt, W.J.; Liu, Y.; Heise, C.E.; Hoyte, K.; Luk, W.; Lu, Y.; et al. A therapeutic antibody targeting BACE1 inhibits amyloid-beta production in vivo. Sci. Transl. Med. 2011, 3, 84ra43. [Google Scholar] [CrossRef]
- Hock, C.; Konietzko, U.; Papassotiropoulos, A.; Wollmer, A.; Streffer, J.; von Rotz, R.C.; Davey, G.; Moritz, E.; Nitsch, R.M. Generation of antibodies specific for beta-amyloid by vaccination of patients with Alzheimer disease. Nat. Med. 2002, 8, 1270–1275. [Google Scholar] [CrossRef]
- Boado, R.J.; Zhou, Q.H.; Lu, J.Z.; Hui, E.K.; Pardridge, W.M. Pharmacokinetics and brain uptake of a genetically engineered bifunctional fusion antibody targeting the mouse transferrin receptor. Mol. Pharm. 2010, 7, 237–244. [Google Scholar]
- Boado, R.J.; Lu, J.Z.; Hui, E.K.; Pardridge, W.M. IgG-single chain Fv fusion protein therapeutic for Alzheimer’s disease: Expression in CHO cells and pharmacokinetics and brain delivery in the rhesus monkey. Biotechnol. Bioeng. 2010, 105, 627–635. [Google Scholar] [CrossRef]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci .Transl. Med. 2011, 3, 84ra44. [Google Scholar] [CrossRef]
- Skrlj, N.; Drevensek, G.; Hudoklin, S.; Romih, R.; Curin Serbec, V.; Dolinar, M. Recombinant single-chain antibody with the Trojan peptide penetratin positioned in the linker region enables cargo transfer across the blood-brain barrier. Appl. Biochem. Biotechnol. 2013, 169, 159–169. [Google Scholar] [CrossRef]
- Hogrefe, R.I.; Lebedev, A.V.; Zon, G.; Pirollo, K.F.; Rait, A.; Zhou, Q.; Yu, W.; Chang, E.H. Chemically modified short interfering hybrids (siHYBRIDS): Nanoimmunoliposome delivery in vitro and in vivo for RNAi of HER-2. Nucleos. Nucleot. Nucl. 2006, 25, 889–907. [Google Scholar] [CrossRef]
- Ferris, R.L.; Jaffee, E.M.; Ferrone, S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: Clinical response, cellular immunity, and immunoescape. J. Clin. Oncol. 2010, 28, 4390–4399. [Google Scholar] [CrossRef]
- Wan, L.; Zhu, S.; Zhu, J.; Yang, H.; Li, S.; Li, Y.; Cheng, J.; Lu, X. Production and characterization of a CD25-specific scFv-Fc antibody secreted from Pichia pastoris. Appl. Microbiol. Biotechnol. 2012, in press. [Google Scholar]
- Yang, K.; Basu, A.; Wang, M.; Chintala, R.; Hsieh, M.C.; Liu, S.; Hua, J.; Zhang, Z.; Zhou, J.; Li, M.; et al. Tailoring structure-function and pharmacokinetic properties of single-chain Fv proteins by site-specific PEGylation. Protein Eng. 2003, 16, 761–770. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Onda, M.; Nagata, S.; Lee, B.; Kreitman, R.J.; Pastan, I. Site-specific chemical modification with polyethylene glycol of recombinant immunotoxin anti-TAC(Fv)-PE38 (LMB-2) improves antitumor activity and reduces animal toxicity and immunogenicity. Proc. Natl. Acad. Sci. USA 2000, 97, 8548–8553. [Google Scholar] [CrossRef]
- Olkhanud, P.B.; Baatar, D.; Bodogai, M.; Hakim, F.; Gress, R.; Anderson, R.L.; Deng, J.; Xu, M.; Briest, S.; Biragyn, A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009, 69, 5996–6004. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Liu, W.; Onda, M.; Lee, B.; Kreitman, R.J.; Hassan, R.; Xiang, L.; Pastan, I. Recombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopes. Proc. Natl. Acad. Sci. USA 2012, 109, 11782–11787. [Google Scholar]
- Kreitman, R.J.; Tallman, M.S.; Robak, T.; Coutre, S.; Wilson, W.H.; Stetler-Stevenson, M.; Fitzgerald, D.J.; Lechleider, R.; Pastan, I. Phase i trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J. Clin. Oncol. 2012, 30, 1822–1828. [Google Scholar] [CrossRef]
- Hassan, R.; Bullock, S.; Premkumar, A.; Kreitman, R.J.; Kindler, H.; Willingham, M.C.; Pastan, I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus i.V. Infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin. Cancer Res. 2007, 13, 5144–5149. [Google Scholar] [CrossRef]
- Soliman, H. Immunotherapy strategies in the treatment of breast cancer. Cancer Control 2013, 20, 17–21. [Google Scholar]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef]
- Grill, J.D.; Cummings, J.L. Current therapeutic targets for the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2010, 10, 711–728. [Google Scholar] [CrossRef]
- Check, E. Nerve inflammation halts trial for Alzheimer’s drug. Nature 2002, 415, 462. [Google Scholar] [CrossRef]
- Orgogozo, J.M.; Gilman, S.; Dartigues, J.F.; Laurent, B.; Puel, M.; Kirby, L.C.; Jouanny, P.; Dubois, B.; Eisner, L.; Flitman, S.; et al. Subacute meningoencephalitis in a subset of patients with AD after abeta42 immunization. Neurology 2003, 61, 46–54. [Google Scholar] [CrossRef]
- Robert, R.; Wark, K.L. Engineered antibody approaches for Alzheimer’s disease immunotherapy. Arch. Biochem. Biophys. 2012, 526, 132–138. [Google Scholar] [CrossRef]
- Wang, Y.J.; Gao, C.Y.; Yang, M.; Liu, X.H.; Sun, Y.; Pollard, A.; Dong, X.Y.; Wu, X.B.; Zhong, J.H.; Zhou, H.D.; et al. Intramuscular delivery of a single chain antibody gene prevents brain abeta deposition and cognitive impairment in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2010, 24, 1281–1293. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pollard, A.; Zhong, J.H.; Dong, X.Y.; Wu, X.B.; Zhou, H.D.; Zhou, X.F. Intramuscular delivery of a single chain antibody gene reduces brain abeta burden in a mouse model of Alzheimer's disease. Neurobiol. Aging 2009, 30, 364–376. [Google Scholar] [CrossRef]
- Fukuchi, K.; Tahara, K.; Kim, H.D.; Maxwell, J.A.; Lewis, T.L.; Accavitti-Loper, M.A.; Kim, H.; Ponnazhagan, S.; Lalonde, R. Anti-abeta single-chain antibody delivery via adeno-associated virus for treatment of Alzheimer’s disease. Neurobiol. Dis. 2006, 23, 502–511. [Google Scholar] [CrossRef]
- Ryan, D.A.; Mastrangelo, M.A.; Narrow, W.C.; Sullivan, M.A.; Federoff, H.J.; Bowers, W.J. Abeta-directed single-chain antibody delivery via a serotype-1 AAV vector improves learning behavior and pathology in Alzheimer’s disease mice. Mol. Ther. 2010, 18, 1471–1481. [Google Scholar] [CrossRef]
- Yang, J.; Pattanayak, A.; Song, M.; Kou, J.; Taguchi, H.; Paul, S.; Ponnazhagan, S.; Lalonde, R.; Fukuchi, K. Muscle-directed anti-abeta single-chain antibody delivery via AAV1 reduces cerebral abeta load in an Alzheimer’s disease mouse model. J. Mol. Neurosci. 2013, 49, 277–288. [Google Scholar] [CrossRef]
- Cattepoel, S.; Hanenberg, M.; Kulic, L.; Nitsch, R.M. Chronic intranasal treatment with an anti-abeta(30–42) scFv antibody ameliorates amyloid pathology in a transgenic mouse model of Alzheimer’s disease. PLoS One 2011, 6, e18296. [Google Scholar]
- Crook, Z.R.; Housman, D. Huntington’s disease: Can mice lead the way to treatment? Neuron 2011, 69, 423–435. [Google Scholar] [CrossRef]
- Miller, T.W.; Zhou, C.; Gines, S.; MacDonald, M.E.; Mazarakis, N.D.; Bates, G.P.; Huston, J.S.; Messer, A. A human single-chain v intrabody preferentially targets amino-terminal Huntingtin's fragments in striatal models of Huntington's disease. Neurobiol. Dis. 2005, 19, 47–56. [Google Scholar] [CrossRef]
- Butler, D.C.; Messer, A. Bifunctional anti-huntingtin proteasome-directed intrabodies mediate efficient degradation of mutant huntingtin exon 1 protein fragments. PLoS One 2011, 6, e29199. [Google Scholar] [CrossRef]
- Snyder-Keller, A.; McLear, J.A.; Hathorn, T.; Messer, A. Early or late-stage anti-N-terminal huntingtin intrabody gene therapy reduces pathological features in B6.HDR6/1 mice. J. Neuropathol. Exp. Neurol. 2010, 69, 1078–1085. [Google Scholar] [CrossRef]
- Wang, C.E.; Zhou, H.; McGuire, J.R.; Cerullo, V.; Lee, B.; Li, S.H.; Li, X.J. Suppression of neuropil aggregates and neurological symptoms by an intracellular antibody implicates the cytoplasmic toxicity of mutant huntingtin. J. Cell Biol. 2008, 181, 803–816. [Google Scholar] [CrossRef]
- Zdobnova, T.A.; Stremovskiy, O.A.; Lebedenko, E.N.; Deyev, S.M. Self-assembling complexes of quantum dots and scFv antibodies for cancer cell targeting and imaging. PLoS One 2012, 7, e48248. [Google Scholar]
- Xu, W.; Liu, L.; Brown, N.J.; Christian, S.; Hornby, D. Quantum dot-conjugated anti-GRP78 scFv inhibits cancer growth in mice. Molecules 2012, 17, 796–808. [Google Scholar] [CrossRef]
- Lee, A.S. GRP78 induction in cancer: Therapeutic and prognostic implications. Cancer Res. 2007, 67, 3496–3499. [Google Scholar] [CrossRef]
- Vigor, K.L.; Kyrtatos, P.G.; Minogue, S.; Al-Jamal, K.T.; Kogelberg, H.; Tolner, B.; Kostarelos, K.; Begent, R.H.; Pankhurst, Q.A.; Lythgoe, M.F.; et al. Nanoparticles functionalized with recombinant single chain Fv antibody fragments (scFv) for the magnetic resonance imaging of cancer cells. Biomaterials 2010, 31, 1307–1315. [Google Scholar] [CrossRef]
- Olafsen, T.; Wu, A.M. Antibody vectors for imaging. Semin. Nucl. Med. 2010, 40, 167–181. [Google Scholar] [CrossRef]
- Kumar, P.; Ban, H.S.; Kim, S.S.; Wu, H.; Pearson, T.; Greiner, D.L.; Laouar, A.; Yao, J.; Haridas, V.; Habiro, K.; et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell 2008, 134, 577–586. [Google Scholar] [CrossRef]
- Pasche, N.; Wulhfard, S.; Pretto, F.; Carugati, E.; Neri, D. The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel. Clin. Cancer Res. 2012, 18, 4092–4103. [Google Scholar] [CrossRef]
- Choi, S.; Lee, J.; Kumar, P.; Lee, K.Y.; Lee, S.K. Single chain variable fragment CD7 antibody conjugated PLGA/HDAC inhibitor immuno-nanoparticles: Developing human T cell-specific nano-technology for delivery of therapeutic drugs targeting latent HIV. J. Control Release 2011, 152 (Suppl 1), e9–e10. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Monnier, P.P.; Vigouroux, R.J.; Tassew, N.G. In Vivo Applications of Single Chain Fv (Variable Domain) (scFv) Fragments. Antibodies 2013, 2, 193-208. https://doi.org/10.3390/antib2020193
Monnier PP, Vigouroux RJ, Tassew NG. In Vivo Applications of Single Chain Fv (Variable Domain) (scFv) Fragments. Antibodies. 2013; 2(2):193-208. https://doi.org/10.3390/antib2020193
Chicago/Turabian StyleMonnier, Philippe P., Robin J. Vigouroux, and Nardos G. Tassew. 2013. "In Vivo Applications of Single Chain Fv (Variable Domain) (scFv) Fragments" Antibodies 2, no. 2: 193-208. https://doi.org/10.3390/antib2020193
APA StyleMonnier, P. P., Vigouroux, R. J., & Tassew, N. G. (2013). In Vivo Applications of Single Chain Fv (Variable Domain) (scFv) Fragments. Antibodies, 2(2), 193-208. https://doi.org/10.3390/antib2020193



