1. Introduction
Morphine is one of the most medicinally important analgesics and narcotics. Structurally, it is classified as an alkaloid because of the presence of nitrogen. Its structure is similar to that of codeine, thebaine, and heroin. An immunoassay to accurately discriminate between these analogous alkaloids would be highly beneficial. A key factor for such an assay is specificity with high sensitivity, which is totally dependent on the antibody employed [
1,
2]. However, most antibodies against haptens are polyclonal serum antibodies that exhibit significant cross-reactivities with closely related compounds.
After a polyclonal anti-morphine antibody was reported in 1971, a number of studies attempted to establish anti-morphine antibodies with higher specificities [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22]. However, all of these antibodies have shown cross-reactivity among structurally related compounds, particularly with codeine and heroin. For example, Glasel and Sawada reported that their antibody cross-reacted with codeine [
4,
5]. Usagawa
et al. reported that their antibody exhibited cross-reactivity with nalorphine, morphine-6-glucuronide and naltrexone, but not with codeine [
9]. Rahbarizadeh
et al. [
12] established an antibody that had 10% cross-reactivity with heroin and low cross-reactivity with codeine. Dillon
et al. [
14] produced scFv antibody to morphine-3-glucuronide using a pre-immunized phage display library with a morphine-3-glucuronide-BSA conjugate. This scFv antibody showed cross reactivity with morphine (23.9%), codeine (28.4%) and 6-monoacetylmorphine (15.7%). In 2002, isolation of an scFv antibody that recognized morphine was reported using a pre-immune phage library [
15]. However, its specificity to related compounds has not been characterized. Moghaddam
et al. [
22] reported human scFv antibodies which recognized 6-monoacetylmorphine with high affinities of 1–3 × 10
−7 M but not with free morphine.
To develop antibodies that were more specific to morphine, we tested two strategic methodologies to generate morphine-specific antibodies in this study: (1) immunogen preparation (novel types of morphine derivatives and two different conjugated forms) and (2) antibody preparation technology. To prepare immunogens, protein conjugated morphine-6-hemisuccinate, protein conjugated morphine-3-glucuronid, and protein conjugated
N-carboxypropylnormorphine in which the 3- and 6-positions and piperidine ring nitrogen atoms were used for protein conjugation as in previous studies [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22]. Findlay
et al. [
21] reported that an antiserum against 6-position conjugates recognized structural changes around the piperidine ring nitrogen atom and an antiserum against piperidine ring nitrogen atom conjugates recognized structural changes around the 3- and 6-positions. Thus, to extend the recognition capability of antibodies, we prepared novel types of morphine derivatives (C conjugates) as immunogens, which were attached to carrier proteins via the 2-position. The second method was antibody preparation technology. In antibody preparation technologies, in addition to hybridoma technology, a single chain Fv-displaying phage library has become available, which may make it possible to generate very highly diverse antibody specificity [
23].
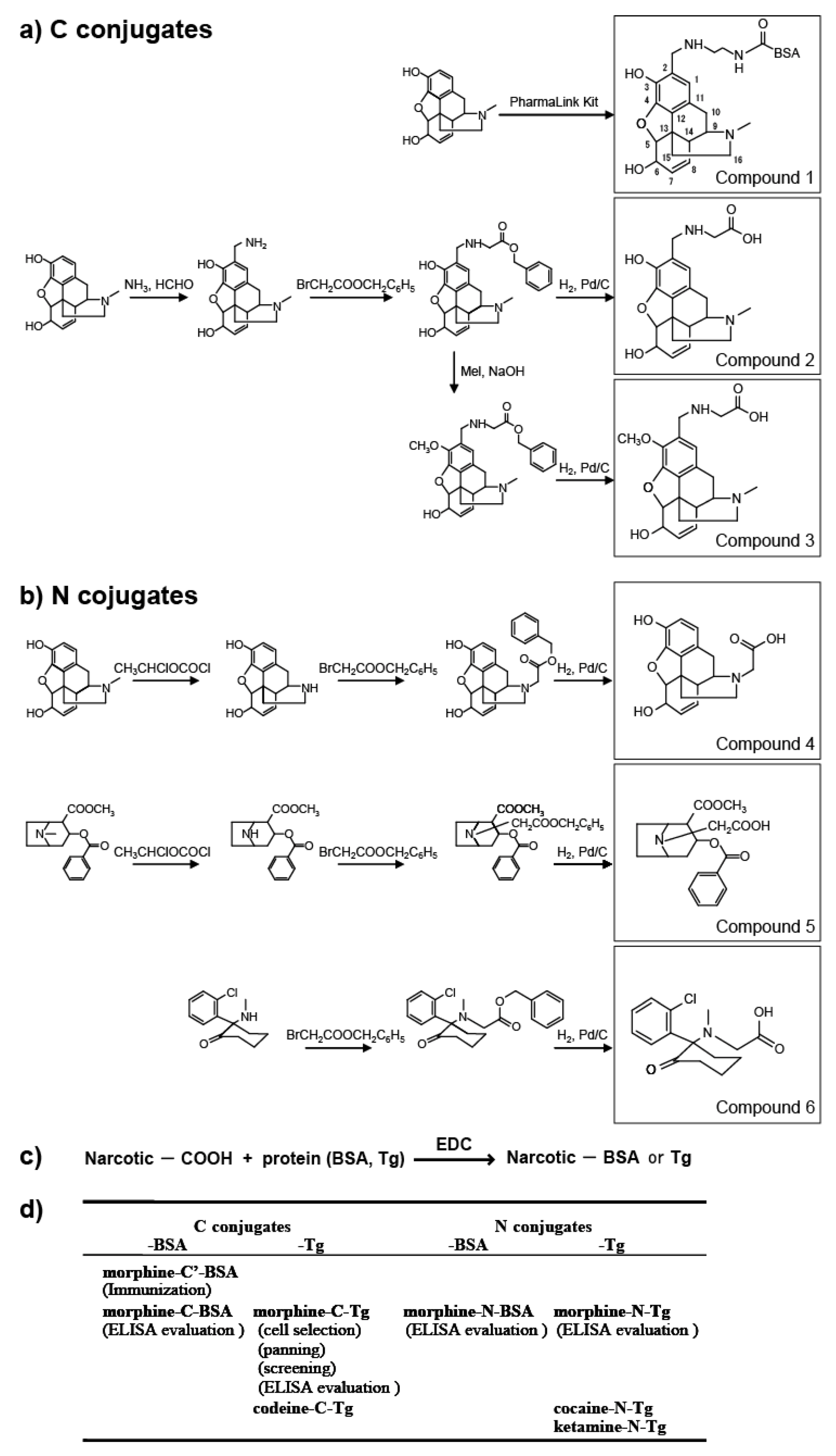
In this study, we prepared eight types of carrier protein-conjugated narcotics as immunogens or panning antigens to assure specificity to the morphine portion (
Figure 1d). These were N conjugates (conjugated through the piperidine ring nitrogen atom of narcotics;
Figure 1) and C conjugates (conjugated through the carbon atom at the 2-position of narcotics;
Figure 1). N compounds
4,
5, and
6 were synthesized and their protein-conjugated forms were abbreviated as morphine-
N-protein (BSA or Tg), cocaine-N-Tg, and ketamine-N-Tg, respectively. A PharmaLink Immunogen kit was used to synthesize Compound
1, its protein-conjugated form was abbreviated morphine-C’-BSA. C compounds
2 and
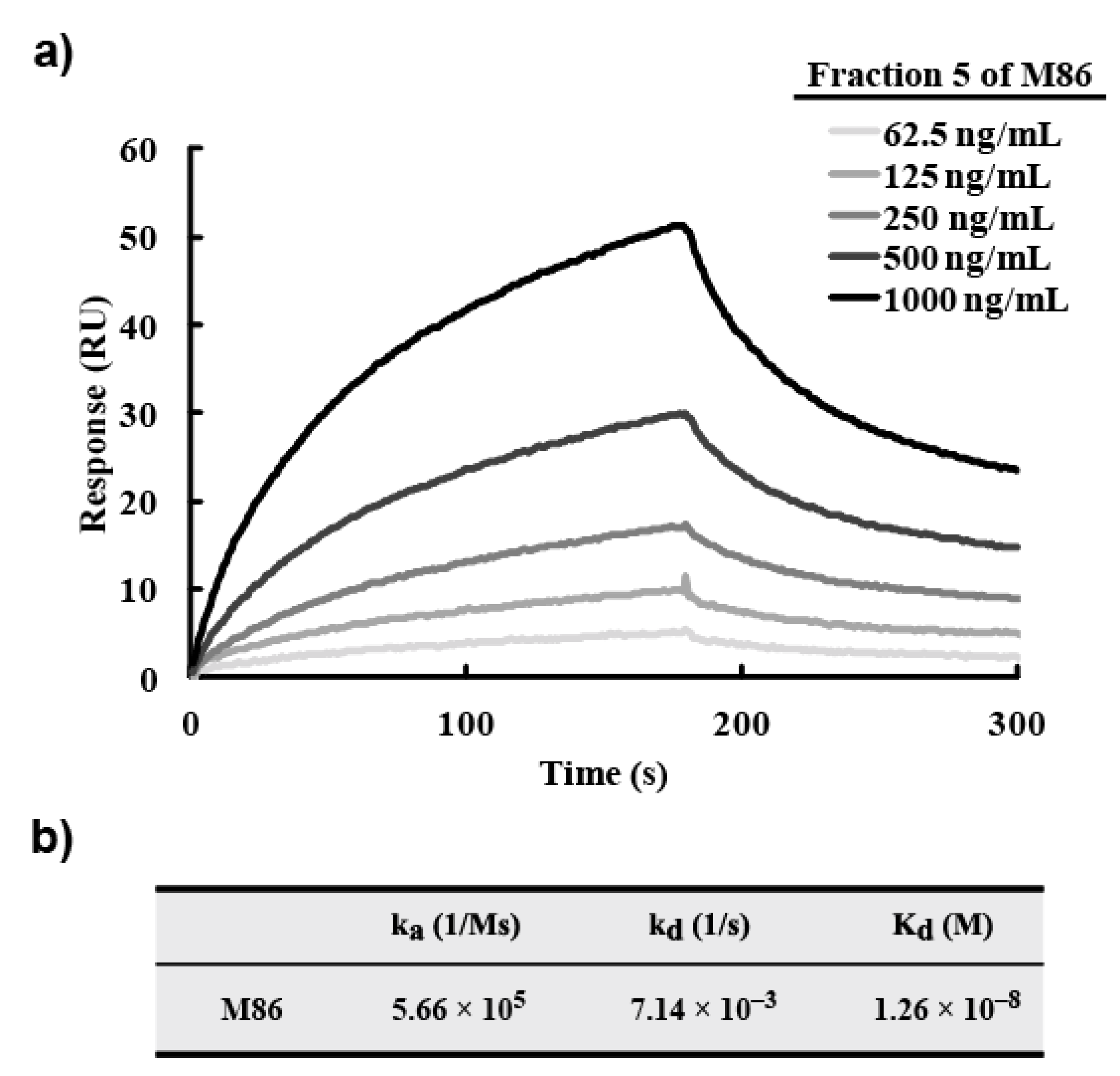
3 were synthesized and their protein-conjugated forms were abbreviated morphine-C-protein (BSA or Tg) and codeine-C-Tg, respectively. The difference between the C’ and C conjugate was in the linkage portion: that of C’ conjugate was carbonylaminoethylaminomethyl and that of C conjugate was carbonylmethylaminomethyl. During biopanning for an scFv-displaying phage clone, we used morphine-C-conjugated thyroglobulin (Tg; morphine-C-Tg). As a result, we isolated a single clone (M86) that binds to morphine-C-conjugated BSA (morphine-C-BSA) and morphine-C-Tg but not to related compounds conjugated to BSA or Tg. This indicated morphine-specificity to the hapten moiety conjugated to a protein carrier. The K
d value of M86 was 1.26 × 10
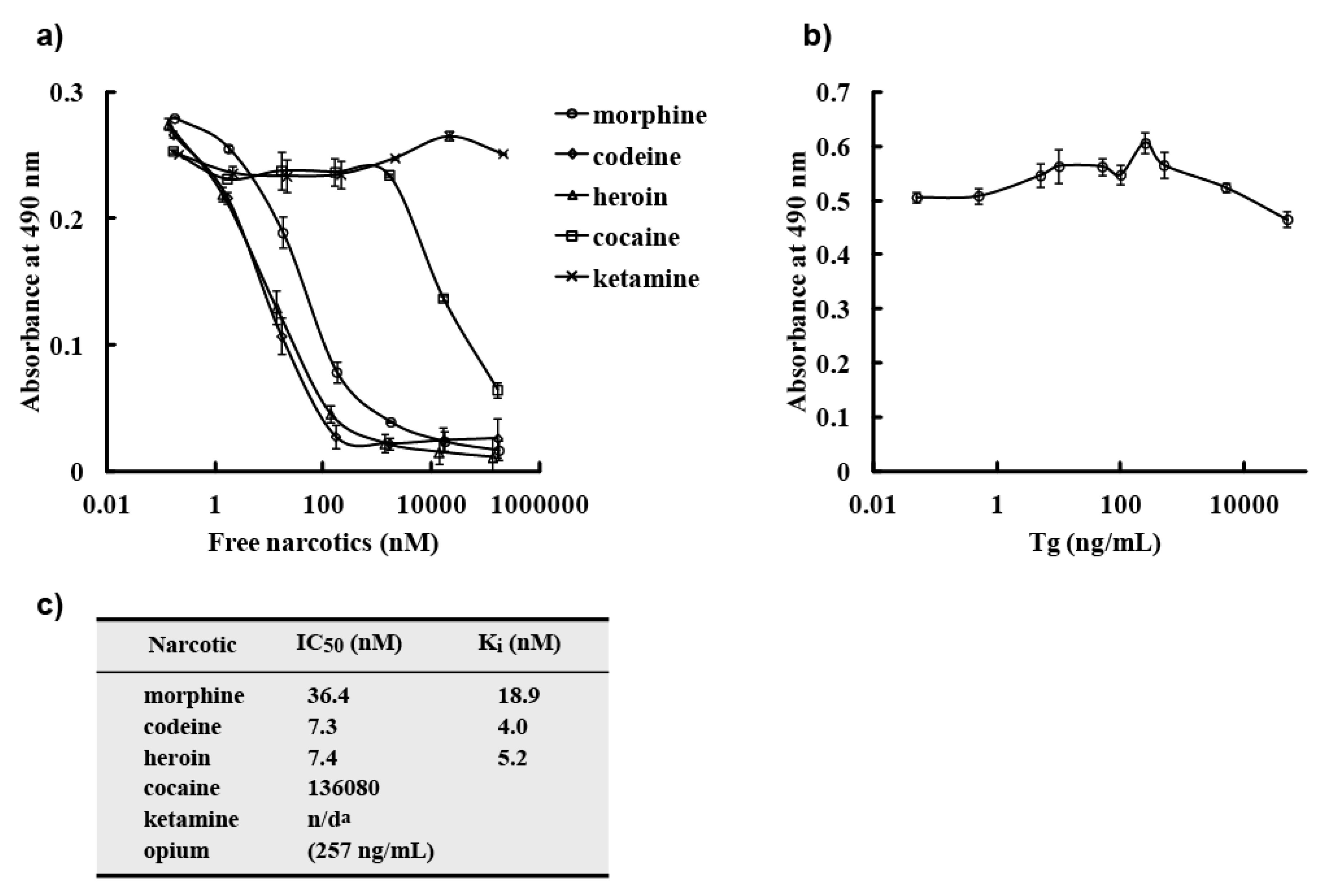
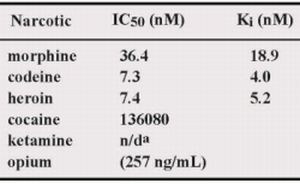
−8 M. However, a competitive ELISA with free morphine, opium, codeine, and heroin showed that M86 bound to not only free morphine but also to opium, free codeine, and heroin. IC
50 values of M86 for opium, morphine, codeine and heroin was 257 ng/mL, 36.4, 7.3 and 7.4 nM, respectively.
Figure 1.
Synthesis of protein (BSA or Tg) conjugated with narcotics. (a) C conjugate derivatives; (b) N conjugate derivatives; (c) Conjugation of compounds 2–6 with proteins; (d) Summary of antigens used in this study. Parentheses indicate the experimental procedure.
Figure 1.
Synthesis of protein (BSA or Tg) conjugated with narcotics. (a) C conjugate derivatives; (b) N conjugate derivatives; (c) Conjugation of compounds 2–6 with proteins; (d) Summary of antigens used in this study. Parentheses indicate the experimental procedure.
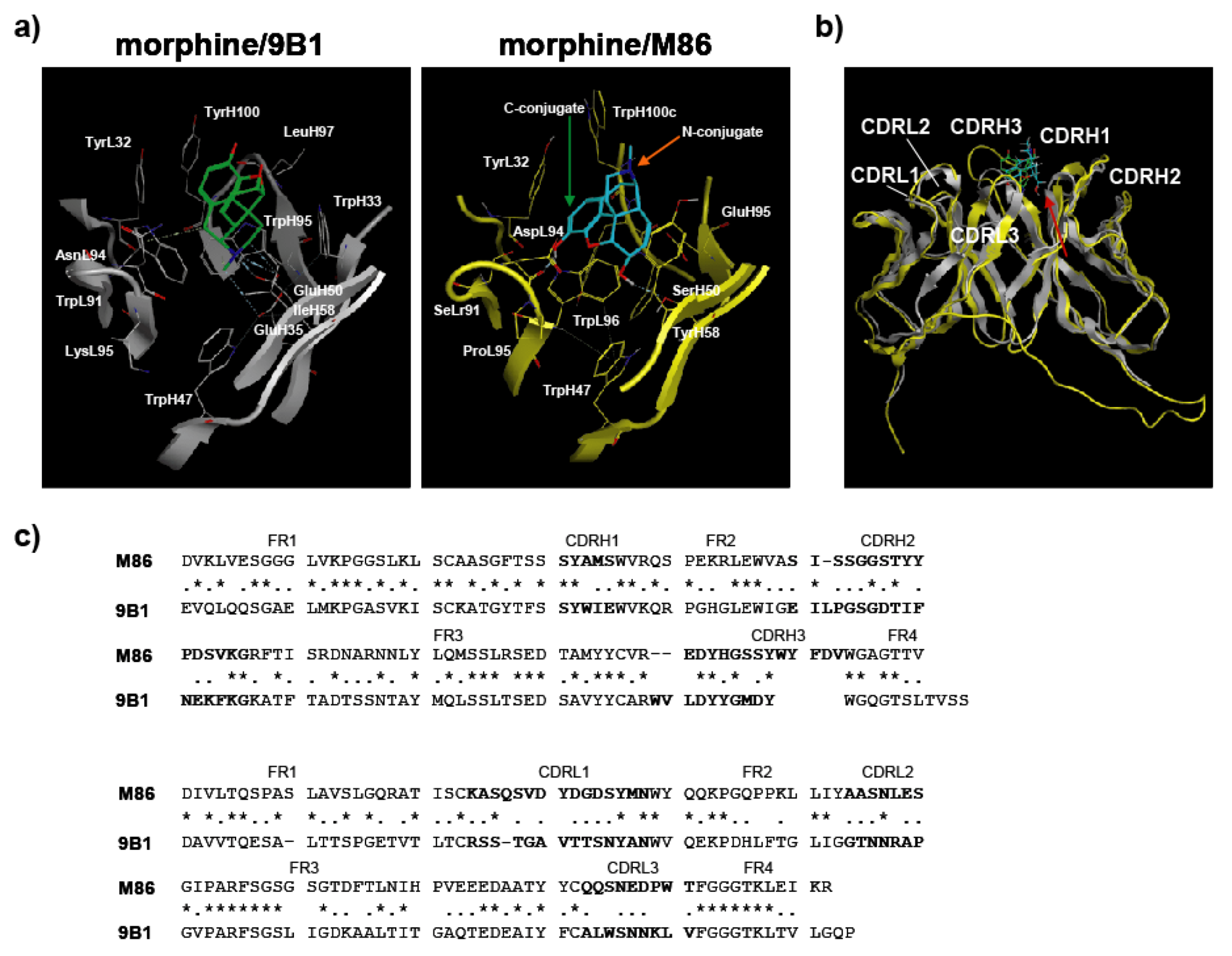
This study presents the results for the development of an scFv antibody, M86, with its detailed characteristics for morphine-binding specificity being defined for the first time. However, this raised a strategic question for using hapten-protein conjugates to establish a free hapten-specific antibody. MOE analysis based on only one X-ray crystallographic structural data of the morphine-reactive antibody 9B1 (PDB ID: IQ0Y, [
20]) suggested the possibility of creating a free morphine-specific antibody; therefore, M86 may be useful for developing analytical probes that can specifically detect morphine, codeine, heroin, and therapeutic reagents for opiate addictions.
3. Experimental Section
3.1. Antigen Synthesis
We prepared (5α,6α)-7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol (morphine), (5α,6α)-7,8-didehydro-4,5-epoxy-3-methoxy-17-methylmorphinan-6-ol (codeine), (5α,6α)-7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol diacetate (heroin), (
RS)-2-(2-chlorophenyl)-2-methylamino-cyclohexan-1-one (ketamine), and methyl (1
R,2
R,3
S,5
S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate (cocaine). All narcotics were handled at the Central Customs Laboratory or Kyushu University with permission for their use. Three different conjugated forms of hapten conjugates (C’ conjugate, C conjugate, and N conjugate) were synthesized as described below (
Figure 1, compounds
1–
6).
3.1.1. C Position-Conjugated Derivatives (Figure 1a, Compounds 1–3)
A Thermo Scientific® PharmaLink Immunogen Kit (77158; Thermo Scientific, Waltham, MA, USA) was used to synthesize compound 1 (morphine-C’-BSA) from morphine by the Mannich reaction according to the manufacturer’s instructions. Morphine (10 µmol) was incubated with SuperCarrier® at room temperature for 24 h. The immunogen was purified by gel filtration. Compounds 2 and 3 were synthesized as described below. After aminomethylation of morphine (30 µmol) by the Mannich reaction, the 2-aminomethylated compound was mixed with bromoacetic acid benzyl ester (30 µmol) to give the benzyloxycarbonylmethylaminomethyl-conjugated intermediate. The benzyloxycarbonyl-methylaminomethyl-conjugated intermediate (ca. 10 µmol) was reduced under hydrogen in the presence of Pd/C to give hydroxycarbonylmethylaminomethyl-conjugated derivative compound 2. The benzyloxycarbonylmethylaminomethyl-conjugated intermediate (ca. 10 µmol) was treated with 10 µmol methyl iodide in the presence of sodium hydride to give the corresponding product, 3-methoxy-2-benzyloxycarbonylmethylaminomethyl (intermediate). This intermediate was reduced in a manner similar to that used for synthesizing compound 2 to give compound 3.
3.1.2. N Position-Conjugated Derivatives (Figure 1b, Compounds 4–6)
1-Chloroethyl chloroformate was used to synthesize normorphine and norcocaine by
N-demethylation of morphine and cocaine, respectively [
26]. Morphine or cocaine (20 µmol) was mixed with four equivalents of 1-chloroethyl chloroformate, and the mixture was refluxed for 15 h under nitrogen to give normorphine or norcocaine depending on the starting compound. Normorphine and norcocaine (10 µmol) were reacted separately with bromoacetic acid benzyl ester following reduction using Pd/C under hydrogen to give compounds
4 and
5, respectively, in a manner similar to compound
2 synthesis. Ketamine (20 µmol) was mixed with bromoacetic acid benzyl ester (22 µmol) to give the
N-benzyloxy-carboxymethyl derivative, which was reduced by hydrogen over a Pd/C catalyst to give compound
6.
3.1.3. Protein Conjugation (Figure 1c)
An aqueous solution of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) was used to activate the hydroxycarbonyl side chain-linked narcotic (compounds 2–6; ca. 10 µmol) for coupling. The solution was added to 10 mg of thyroglobulin (Tg) in 2 mL of 50 mM phosphate buffer (pH 7.5), stirred at 4 °C for 15 h, and cleaned up by gel filtration. The same protocol was used to couple compounds 2 and 4 to bovine serum albumin (BSA).
3.2. Immunization
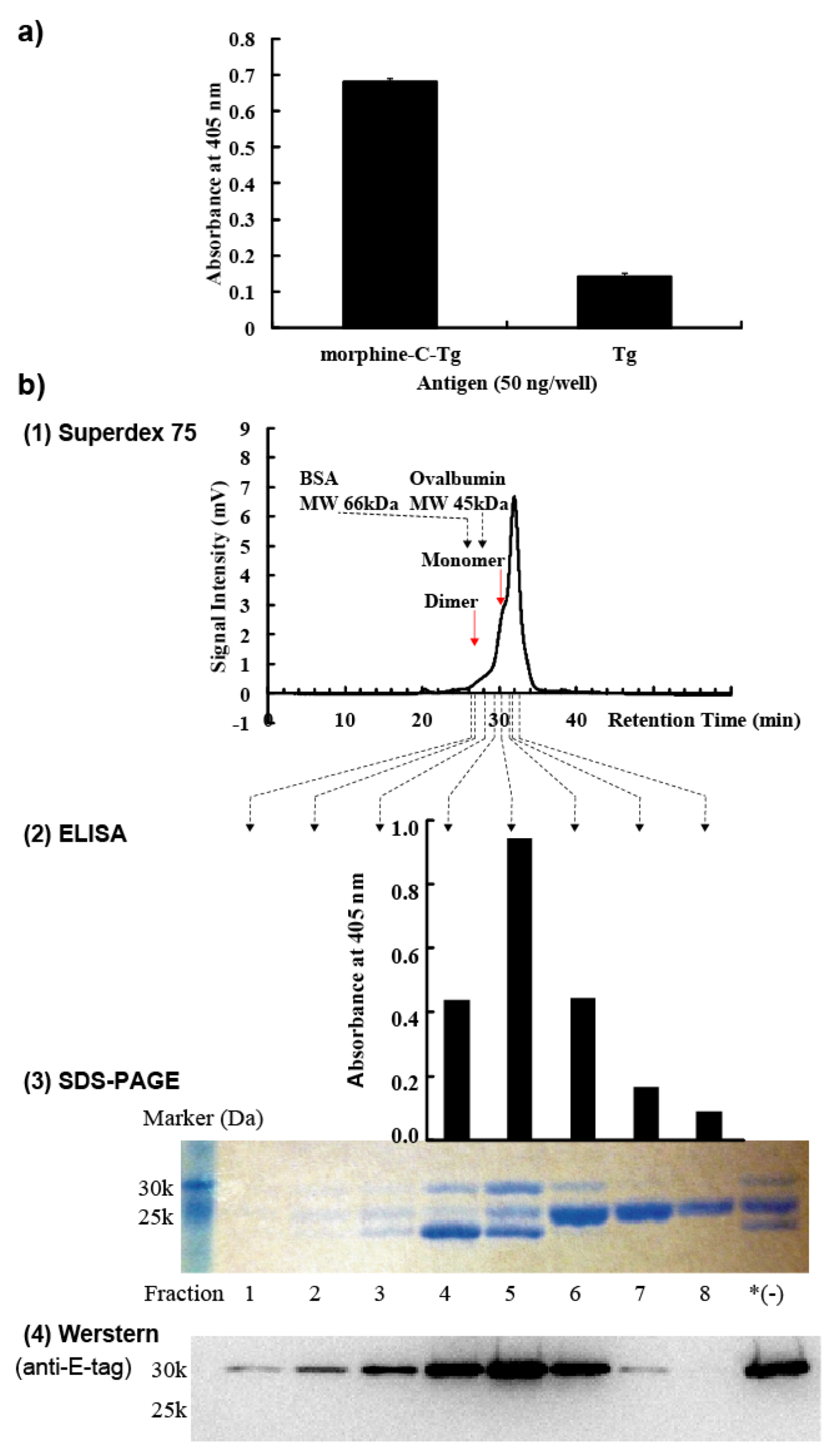
We used 7-week-old female BALB/c mice (n = 3; Charles River Laboratories Japan, Inc., Yokohama, Japan). Morphine-C′-BSA with complete Freund’s adjuvant (263810; Difco Laboratories, Detroit, MI, USA) was intraperitoneally administered at a dose of 25 µg/100 µL morphine-C’-BSA/phosphate buffered saline (PBS). Subsequently, each mouse was booster immunized on days 14, 28, 42, and 56 with the same amount of morphine-C′-BSA together with incomplete Freund’s adjuvant (263910; Difco Laboratories, Detroit, MI, USA). Blood was collected through the orbital vein of the mouse eye and its serum antibody titer was determined by ELISA. Three days after the fifth immunization, using spleen cells derived from mice hyperimmunized with morphine-C′-BSA, morphine moiety-reactive lymphocytes were enriched with morphine-C-Tg-coated on plastic plates. All animal experiments were performed under the laboratory guidelines for Animal Experiments of Kagoshima University.
3.3. Single-Chain Library Construction
A phage library was constructed according to Burmester
et al. [
27]. Total RNA was purified from the morphine moiety-reactive lymphocytes, which were enriched from mice spleen cells using morphine-C-Tg-coated on plastic plates. VH and VL genes were amplified from cDNA that was prepared as previously described [
27]. The primers used for amplifying VH and VL genes and overlapping regions were according to the previously described primer set [
27] except that the integration order of DNA segments was changed from the original sequence, 5′-
SfiI site-VL-linker-VH-
SfiI site-3′ to 5′
-SfiI site-VH-linker-VL-
SpeI site-3′. In addition, no FLAG was used. For VH gene amplification, 76 separate PCR amplifications were performed using 19 different VH forward primers (HF) and four different VH back primers (HB). For Vκ genes, 51 separate PCR amplifications were performed using 17 different Vκ forward primers (LF) and three different Vκ back primers (LB). For Vλ genes, one PCR amplification was performed using a Vλ forward primer (LFλ) and a Vλ back primer (LBλ). The scFv genes were generated by assembly of VH and VL genes through a (Gly
4Ser)
3 linker sequence by PCR with an scFv forward primer and an scFv back primer. The scFv genes were digested with
SfiI (R0123S; New England Biolabs Inc., Ipswich, MA, USA) and
SpeI (R0133L; New England Biolabs Inc.), agarose gel purified, and ligated into the phagemid vector pCANTAB/5E. The ligation products were electroporated into
Escherichia coli TG1 DUOs Phage Display Electrocompetent Cells (60502-2; Lucigen Corporation, Middleton, WI, USA). Library diversity was defined as the number of recombinant single ampicillin-resistant clones obtained from consecutive ligations and transformations of the plasmid into competent cells prior to any amplification procedures for the genes. Clones were selected at random, and the insert plasmid DNA was sequenced and analyzed with an Applied Biosystems 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
3.4. ScFv-displaying Phage Preparation
ScFv-displaying phage preparation was as described by Hashiguchi
et al. [
28].
E. coli TG1 cells harboring the constructed phagemids were shaken in a 2YT medium supplemented with 100 μg/mL ampicillin and 2% glucose (2YTAG medium) at 37 °C until the
A600 reached approximately 0.5, and the M13KO7 helper phage [final, ca. 10
9 colony-forming units (cfu)/mL] were added. After 30 min incubation at 37 °C, the culture was shaken for 30 min at 37 °C. After centrifugation, the cells were suspended in 2YT supplemented with 100 μg/mL ampicillin and 25 μg/mL kanamycin. The culture was then shaken overnight at 30 °C. Phage particles were purified by precipitation with 0.2 volume of 12.5% (w/v) polyethylene glycol (PEG) 6000 and 2.48 M sodium chloride. The scFv-displaying phages were resuspended in PBS.
3.5. Biopanning
The library was subjected to three rounds of panning as described by Hashiguchi
et al. [
28]. An immunotube (Maxisorp; Nunc, Roskilde, Denmark) was coated with 1 µg of morphine-C-Tg. After blocking with 1% BlockAce (UK-B40; DS Pharma Biomedical, Osaka, Japan), 10
12 cfu phage particles in 1 ml of 1% BSA plus 1% Tg that were pre-adsorbed to blocking solution were added to the immunotube and incubated for 1 h. The immunotube was washed 10 times with PBS/0.1% Tween 20 (PBST) followed by five times with PBS. Bound phages were eluted by adding 1 ml of 0.1 M glycine–HCl (pH 2.2). Eluted phages were amplified by infection in fresh
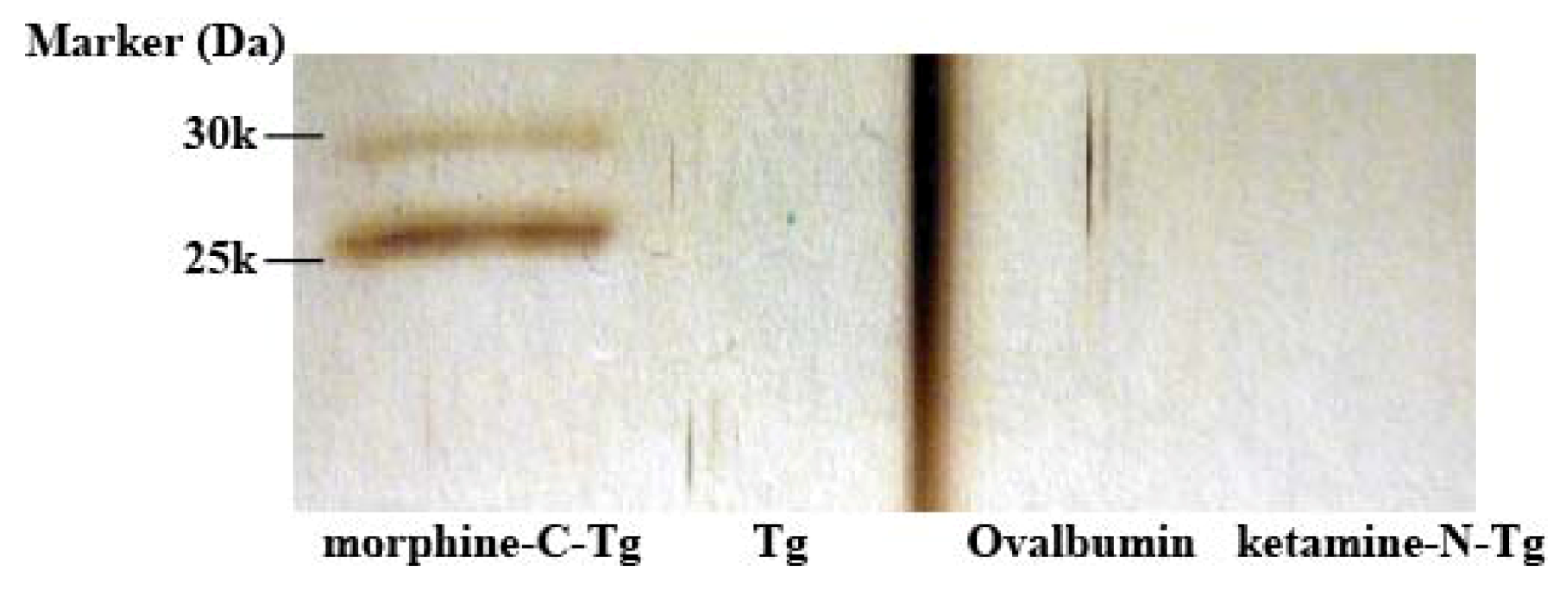
E. coli TG1 cells, and the phages were rescued as described earlier. To recover morphine-binding component, 1 µg/0.5 mL of affinity-purified M86 with HisTrap HP column was incubated for 2 h in an immunotube coated with 2 µg/0.5 mL of morphine-C-Tg, Tg, Ovalbumin or ketamine-N-Tg. Blocking was performed with 1% BlockAce, and washing was performed 5 times with PBST followed by once with PBS. Elution was in a manner similar to that for phage panning, except that 0.5 mL of 0.1 M glycine–HCl (pH 2.2) was added.
3.6. Soluble ScFv Preparation
Soluble scFv was prepared by infecting phage clones in
E. coli HB2151 and then incubating with 1 mM IPTG for 12 h at 25 °C as described by Hashiguchi [
28]. Cell pellets were collected by centrifugation, and resuspended in 1 mM EDTA/PBS. After incubation on ice for 30 min, periplasmic fraction was collected by centrifugation. A HisTrap HP column (17-0404-03; GE Healthcare, Princeton, NJ, USA) was used to purify these periplasmic scFvs according to the manufacturer’s recommendations. Eluted fractions were dialyzed against PBS. To prepare the monomeric M86 scFv fraction, affinity-purified M86 with a HisTrap HP column was fractionated to 100 µL by Superdex 75 10/300 GL (17-5174-01; GE Healthcare), which was developed with PBS at a flow rate of 0.4 ml/min. BSA and ovalbumin were used as molecular size markers.
3.7. Western Blotting
Western blotting analysis was performed as described by Hashiguchi [
28]. Fractionated scFvs were subjected to 15% SDS-PAGE under reducing conditions and the gel was blotted onto a PVDF membrane (Millipore, MA, USA). Detection was performed using an anti-E-tag polyclonal antibody in combination with HRP-conjugated goat poly anti-rabbit IgG and visualized by Chemi-Lumi One (Nacalai Tesque Inc., Kyoto, Japan) and LAS-1000 (Fuji Film, Tokyo, Japan).
3.8. ELISA and Competitive ELISA
ELISA was performed as described previously [
28]. A Nunc-Immuno plate (Maxisorp, Nunc, Roskilde, Denmark) was coated with BSA- (or Tg)-conjugated narcotic antigens (50 ng in 50 µL/well) or control protein. Each well was blocked with 1% BlockAce. For scFv-displayed phage detection, 50 µL of diluted PEG-precipitated phage clones (dilution of 1:50/well) was added and incubated for 2 h. To detect bound phage clones, the clones were incubated with an anti-M13 monoclonal antibody (27-9420-01; GE Healthcare) at a dilution of 1:1,000 as a primary antibody for 1 h and with alkaline phosphatase (AP)-conjugated goat poly anti-mouse IgG (H + L) (115-055-003; Jackson ImmunoResearch, West Grove, PA, USA) at a dilution of 1:1000 as a secondary antibody for 30 min. A microplate reader (EnSpire 2300; PerkinElmer, Waltham, MA, USA) was used to measure absorbance at 405 nm after incubation with 50 µl of a
p-nitrophenyl phosphate/10% diethanol amine solution. For soluble scFv detection, diluted scFvs was incubated for 2 h, followed by incubation with an anti-E-tag polyclonal antibody (PC-229; Kamiya Biomedical Company, Seattle, WA, USA) at a dilution of 1:1,000 as a primary antibody for 1 h and horseradish peroxidase (HRP)-conjugated goat poly anti-rabbit IgG (H + L) (31462; Thermo Scientific, Waltham, MA, USA) at a dilution of 1:1,000 as a secondary antibody for 30 min. After incubation with a SigmaFast OPD tablet set (P9187-50SET; Sigma–Aldrich Corporation, Munich, Germany), absorbance was measured at 490 nm. Detection by MOP-7 (murine anti-morphine IgG: sc-69864; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was in a manner similar to that for soluble scFv detection, except that diluted MOP-7 (1:5,000) was added to each well in place of scFv and HRP-conjugated goat poly anti-mouse IgG (sc-2031; Santa Cruz Biotechnology) at a dilution of 1:2,500 was used as a secondary antibody. Competitive ELISA was done in a manner similar to that for soluble scFv detection, except that pre-mixed solutions of scFv and free narcotic were added to each well in place of scFv.
3.9. DNA Sequencing
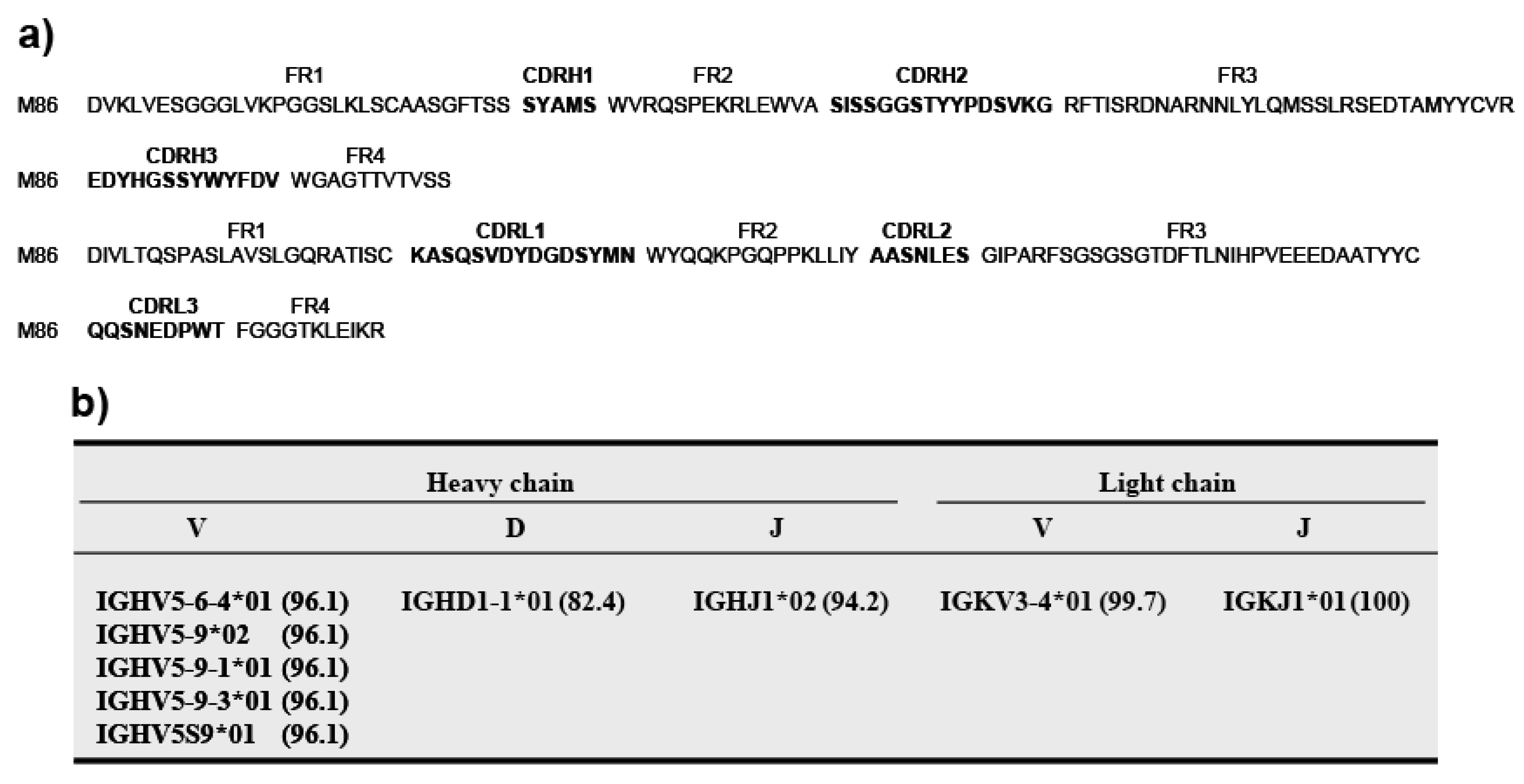
Applied Biosystems 3130xl Genetic Analyzer and BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) was used to identify the nucleotide sequences of the scFv genes. Genetyx ver. 8. software (Genetyx Corporation, Tokyo, Japan) was used to analyze nucleotide sequences. Amino acid sequences deduced from the nucleotide data were aligned and presented in the format described by Kabat and Wu [
24]. The scFv nucleotide sequences were analyzed by searching the IMGT/V-QUEST database. The complementary determining regions (CDR1–CDR3) and flanking regions (FR1–FR4) were deduced according to Kabat and Wu [
24].
3.10. Surface Plasmon Resonance Analysis
A BIAcore X100 system (GE Healthcare) was used according to the manufacturer’s instructions. Morphine-C-Tg (20 µg/mL of 10 mM sodium acetate buffer, pH 4.0) was immobilized onto a CM5 sensor chip according to the amine-coupling protocol provided by the manufacturer, and unreacted sites were masked with 1 M ethanolamine–HCl (pH 8.5). The association reaction was initiated by injecting varying concentrations of scFv solutions. Analyte injection was at a flow rate of 30 µL/min. The dissociation reaction was begun by washing with HBS–EP buffer (0.01 M Hepes, 0.15 M NaCl, 3 mM EDTA, 0.005% v/v surfactant P20, pH 7.4). At the end of a cycle, the sensor chip surface was regenerated with a 0.1 M glycine–HCl buffer (pH 2.5). BIAcore system software (BIAcore X100 evaluation software) was used to calculate the association (ka, M−1s−1) and dissociation (kd, s−1) constants. Kd (M) was calculated according to the 1:1 binding model.
3.11. Computer Simulations
Molecular Operating Environment (MOE) software (Chemical Computing Group Inc., Montreal, Canada) [
29] was used for all molecular modeling studies. The X-ray crystallographic structure of the anti-morphine antibody 9B1 (PDB ID: 1Q0Y) [
20] was obtained from a protein data bank as a template for homology modeling of M86. The MMFF94s force field and the generalized Born salvation model were used for the minimization step. After homology modeling, M86 was prepared for docking studies in which (i) hydrogen atoms were added to the structure with standard geometry; (ii) the structure was minimized using a MMFF94s force field; (iii) MOE Alpha Site Finder was used for active site searches within M86, and dummy atoms were created from the obtained alpha spheres; and (iv) the obtained M86 structure was then used in the ASEDock program (Ryoka Systems Inc., Tokyo, Japan) [
30] with a narcotics library in which the conformational analysis data for morphine, codeine, and heroin were determined by the MOE program. The complexes were evaluated by Udock scores, which provided the affinities between the ligands and M86.
4. Conclusions
In this study, we showed that M86 could bind to morphine-conjugated BSA or Tg but not to other related compounds conjugated to BSA or Tg. This indicated high specificity to morphine but not to codeine. SPR analysis performed using a morphine-C-Tg-coupled CM5 sensor chip showed that its Kd was 1.26 × 10−8 M. However, competitive ELISA for M86–morphine-C-Tg in the absence or presence of varying concentrations of morphine and related compounds showed binding activity with IC50 values of 257 ng/mL, 36.4, 7.3, and 7.4 nM for opium, morphine, codeine, and heroin, respectively. Thus, from a phage library constructed using mice hyperimmunized with morphine-conjugated BSA we isolated an scFv clone (M86), which reacted with free morphine, codeine, and heroin.
This study suggested limitations in the protocols of conventional animal immunization procedures and screening systems using hapten-conjugated proteins, particularly for generating small antigen-specific antibodies. However, our study results suggest that antibody engineering in combination with computer simulations is promising for developing a free morphine-specific antibody.
Thus, we established the first scFv, M86, which is a possible free morphine-specific antibody. M86 may be useful for not only detecting illegal drugs but also for developing therapeutic reagents for opiate addictions.