Transplacental Antibody Transfer: Mechanisms, Pregnancy-Related Disruptions, and Emerging Experimental Models
Abstract
1. Introduction
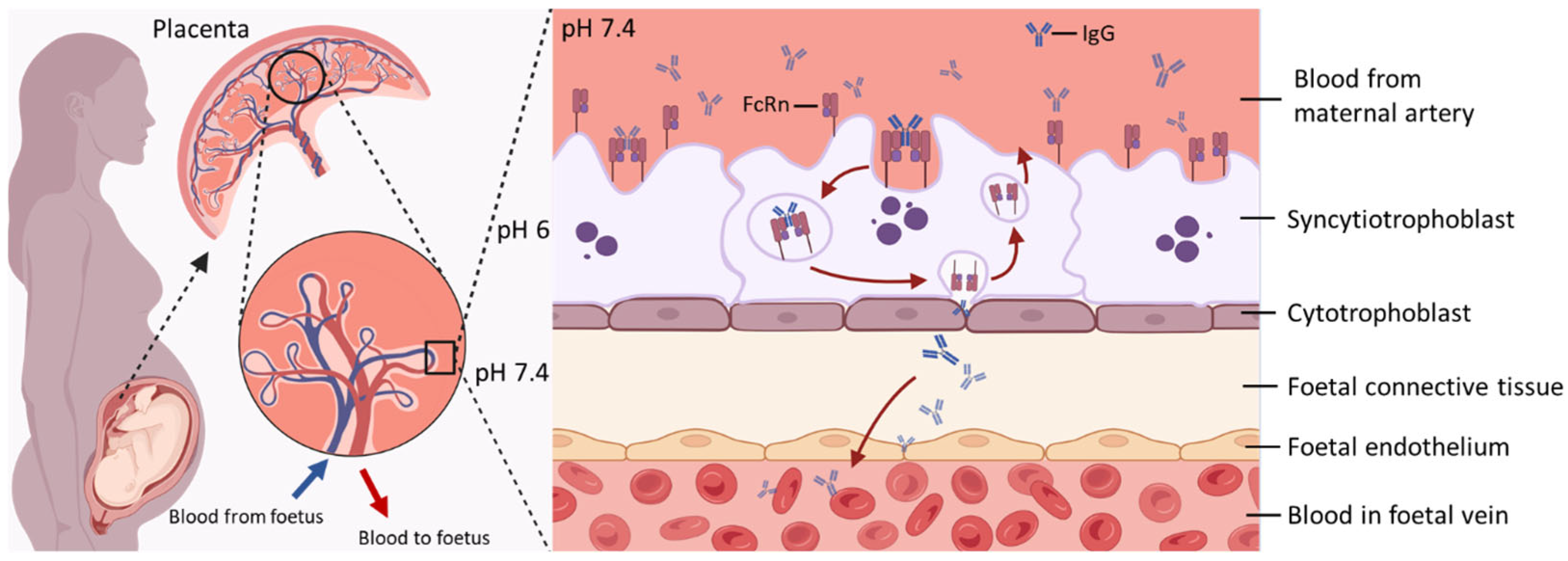
2. Mechanisms of FcRn-Mediated Transport of IgG in the Placenta
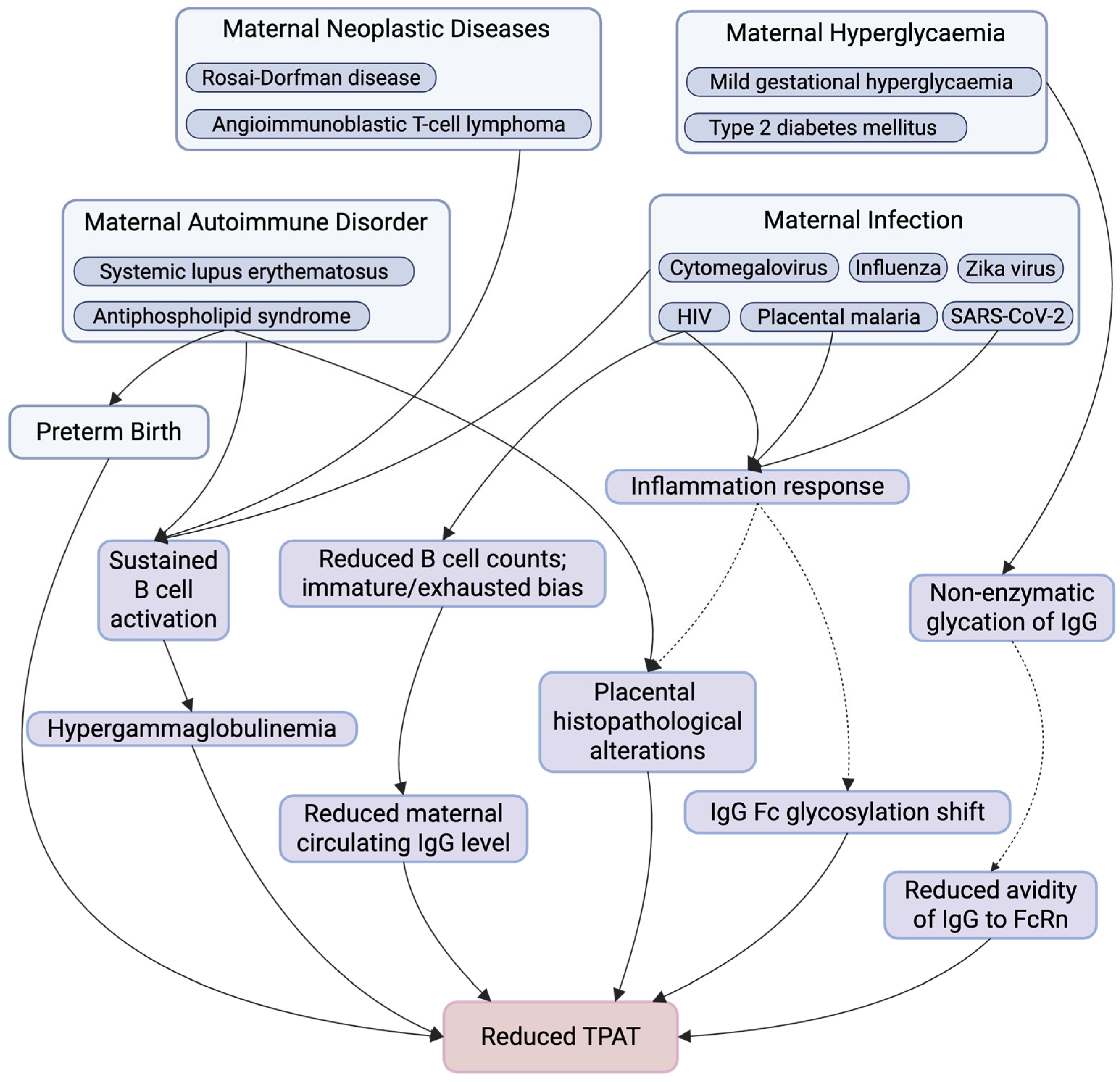
3. Maternal Factors Affecting Antibody Transplacental Transfer
3.1. Kinetics of TPAT During Pregnancy
3.2. Maternal IgG Competition—Hypergammaglobulinemia
3.3. Pathogenic Maternal IgG—Antiphospholipid Syndrome
3.4. Maternal Infection
3.4.1. HIV
3.4.2. Malaria
3.4.3. SARS-CoV-2
3.5. Maternal Hyperglycaemia
3.6. Transplacental Transfer of Bioengineering IgG—Pregnant Women Require Immunotherapies
4. Future Perspectives on Models of Placental Function Available to Study IgG Transplacental Transfer
4.1. Murine Model
4.2. Placenta-Originated Model
4.3. Computational Modelling
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IgG | Immunoglobulin G |
| FcRn | Neonatal Fc receptor |
| TPAT | Transplacental antibody transfer |
| SCT | Syncytiotrophoblast |
| FcγRIIIa | Fcγ receptor III-A |
| C/M ratios | Cord-to-maternal antibody titre ratios |
| RSV | Respiratory syncytial virus |
| PM | Placental malaria |
| APS | Antiphospholipid syndrome |
| β2GPI | β2-glycoprotein I |
| HIV | Human immunodeficiency virus |
| ART | Antiretroviral therapy |
| N-protein | Nucleoprotein |
| DM-2 | Type 2 diabetes mellitus |
| MGH | Mild gestational hyperglycaemia |
| mAb | Monoclonal antibody |
| TNFα | Tumour necrosis factor alpha |
| TNFi | Tumour necrosis factor alpha inhibitor |
References
- Glezen, W.P.; Alpers, M. Maternal immunization. Clin. Infect. Dis. 1999, 28, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Barrau, M.; Roblin, X.; Andromaque, L.; Rozieres, A.; Faure, M.; Paul, S.; Nancey, S. What Should We Know about Drug Levels and Therapeutic Drug Monitoring during Pregnancy and Breastfeeding in Inflammatory Bowel Disease under Biologic Therapy? J. Clin. Med. 2023, 12, 7495. [Google Scholar] [CrossRef]
- Malek, A.; Sager, R.; Kuhn, P.; Nicolaides, K.H.; Schneider, H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am. J. Reprod. Immunol. 1996, 36, 248–255. [Google Scholar] [CrossRef]
- Clements, T.; Rice, T.F.; Vamvakas, G.; Barnett, S.; Barnes, M.; Donaldson, B.; Jones, C.E.; Kampmann, B.; Holder, B. Update on Transplacental Transfer of IgG Subclasses: Impact of Maternal and Fetal Factors. Front. Immunol. 2020, 11, 1920. [Google Scholar] [CrossRef]
- Donald, K.; Petersen, C.; Turvey, S.E.; Finlay, B.B.; Azad, M.B. Secretory IgA: Linking microbes, maternal health, and infant health through human milk. Cell Host Microbe 2022, 30, 650–659. [Google Scholar] [CrossRef]
- Albrecht, M.; Arck, P.C. Vertically Transferred Immunity in Neonates: Mothers, Mechanisms and Mediators. Front. Immunol. 2020, 11, 555. [Google Scholar] [CrossRef]
- Ciobanu, A.M.; Dumitru, A.E.; Gica, N.; Botezatu, R.; Peltecu, G.; Panaitescu, A.M. Benefits and Risks of IgG Transplacental Transfer. Diagnostics 2020, 10, 583. [Google Scholar] [CrossRef]
- Kingdom, J.; Huppertz, B.; Seaward, G.; Kaufmann, P. Development of the placental villous tree and its consequences for fetal growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 92, 35–43. [Google Scholar] [CrossRef]
- DeSesso, J.M.; Williams, A.L.; Ahuja, A.; Bowman, C.J.; Hurtt, M.E. The placenta, transfer of immunoglobulins, and safety assessment of biopharmaceuticals in pregnancy. Crit. Rev. Toxicol. 2012, 42, 185–210. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Jennewein, M.F.; Goldfarb, I.; Dolatshahi, S.; Cosgrove, C.; Noelette, F.J.; Krykbaeva, M.; Das, J.; Sarkar, A.; Gorman, M.J.; Fischinger, S.; et al. Fc Glycan-Mediated Regulation of Placental Antibody Transfer. Cell 2019, 178, 202–215.e14. [Google Scholar] [CrossRef]
- Borghi, S.; Bournazos, S.; Thulin, N.K.; Li, C.; Gajewski, A.; Sherwood, R.W.; Zhang, S.; Harris, E.; Jagannathan, P.; Wang, L.X.; et al. FcRn, but not FcγRs, drives maternal-fetal transplacental transport of human IgG antibodies. Proc. Natl. Acad. Sci. USA 2020, 117, 12943–12951. [Google Scholar] [CrossRef]
- Vaccaro, C.; Bawdon, R.; Wanjie, S.; Ober, R.J.; Ward, E.S. Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc. Natl. Acad. Sci. USA 2006, 103, 18709–18714. [Google Scholar] [CrossRef]
- Firan, M.; Bawdon, R.; Radu, C.; Ober, R.J.; Eaken, D.; Antohe, F.; Ghetie, V.; Ward, E.S. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int. Immunol. 2001, 13, 993–1002. [Google Scholar] [CrossRef]
- Martinez, D.R.; Fong, Y.; Li, S.H.; Yang, F.; Jennewein, M.F.; Weiner, J.A.; Harrell, E.A.; Mangold, J.F.; Goswami, R.; Seage, G.R., 3rd; et al. Fc Characteristics Mediate Selective Placental Transfer of IgG in HIV-Infected Women. Cell 2019, 178, 190–201.e111. [Google Scholar] [CrossRef]
- Einarsdottir, H.K.; Selman, M.H.; Kapur, R.; Scherjon, S.; Koeleman, C.A.; Deelder, A.M.; van der Schoot, C.E.; Vidarsson, G.; Wuhrer, M. Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconj. J. 2013, 30, 147–157. [Google Scholar] [CrossRef]
- Mathiesen, L.; Nielsen, L.K.; Andersen, J.T.; Grevys, A.; Sandlie, I.; Michaelsen, T.E.; Hedegaard, M.; Knudsen, L.E.; Dziegiel, M.H. Maternofetal transplacental transport of recombinant IgG antibodies lacking effector functions. Blood 2013, 122, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Takizawa, T.; Iwaki, J.; Mishima, T.; Ui-Tei, K.; Takeshita, T.; Matsubara, S.; Takizawa, T. Fc gamma receptor IIb participates in maternal IgG trafficking of human placental endothelial cells. Int. J. Mol. Med. 2015, 35, 1273–1289. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, J.J.; Pyzik, M.; Rath, T.; Kozicky, L.K.; Sand, K.M.K.; Gandhi, A.K.; Grevys, A.; Foss, S.; Menzies, S.C.; Glickman, J.N.; et al. FcRn is a CD32a coreceptor that determines susceptibility to IgG immune complex-driven autoimmunity. J. Exp. Med. 2020, 217, e20200359. [Google Scholar] [CrossRef] [PubMed]
- Bergtold, A.; Desai, D.D.; Gavhane, A.; Clynes, R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity 2005, 23, 503–514. [Google Scholar] [CrossRef]
- Ganesan, L.P.; Kim, J.; Wu, Y.; Mohanty, S.; Phillips, G.S.; Birmingham, D.J.; Robinson, J.M.; Anderson, C.L. FcγRIIb on liver sinusoidal endothelium clears small immune complexes. J. Immunol. 2012, 189, 4981–4988. [Google Scholar] [CrossRef]
- Pyzik, M.; Kozicky, L.K.; Gandhi, A.K.; Blumberg, R.S. The therapeutic age of the neonatal Fc receptor. Nat. Rev. Immunol. 2023, 23, 415–432. [Google Scholar] [CrossRef]
- Mishima, T.; Kurasawa, G.; Ishikawa, G.; Mori, M.; Kawahigashi, Y.; Ishikawa, T.; Luo, S.S.; Takizawa, T.; Goto, T.; Matsubara, S.; et al. Endothelial expression of Fc gamma receptor IIb in the full-term human placenta. Placenta 2007, 28, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, T.; Anderson, C.L.; Robinson, J.M. A novel Fc gamma R-defined, IgG-containing organelle in placental endothelium. J. Immunol. 2005, 175, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, M.F.; Abu-Raya, B.; Jiang, Y.; Alter, G.; Marchant, A. Transfer of maternal immunity and programming of the newborn immune system. Semin. Immunopathol. 2017, 39, 605–613. [Google Scholar] [CrossRef]
- Braibant, M.; Barin, F. The role of neutralizing antibodies in prevention of HIV-1 infection: What can we learn from the mother-to-child transmission context? Retrovirology 2013, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Van Savage, J.; Decker, M.D.; Edwards, K.M.; Sell, S.H.; Karzon, D.T. Natural history of pertussis antibody in the infant and effect on vaccine response. J. Infect. Dis. 1990, 161, 487–492. [Google Scholar] [CrossRef]
- Fouda, G.G.; Martinez, D.R.; Swamy, G.K.; Permar, S.R. The Impact of IgG transplacental transfer on early life immunity. Immunohorizons 2018, 2, 14–25. [Google Scholar] [CrossRef]
- Medoro, A.K.; Puopolo, K.M. Transplacental Antibodies: Role of Maternal Vaccines and Immunity. Clin. Perinatol. 2025, 52, 101–113. [Google Scholar] [CrossRef]
- Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat. Rev. Immunol. 2022, 22, 277–282. [Google Scholar] [CrossRef]
- Langel, S.N.; Blasi, M.; Permar, S.R. Maternal immune protection against infectious diseases. Cell Host Microbe 2022, 30, 660–674. [Google Scholar] [CrossRef]
- Dekkers, G.; Treffers, L.; Plomp, R.; Bentlage, A.E.H.; de Boer, M.; Koeleman, C.A.M.; Lissenberg-Thunnissen, S.N.; Visser, R.; Brouwer, M.; Mok, J.Y.; et al. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front. Immunol. 2017, 8, 877. [Google Scholar] [CrossRef]
- Martinez, D.R.; Fouda, G.G.; Peng, X.; Ackerman, M.E.; Permar, S.R. Noncanonical placental Fc receptors: What is their role in modulating transplacental transfer of maternal IgG? PLoS Pathog. 2018, 14, e1007161. [Google Scholar] [CrossRef]
- Sun, Y.; Estevez, A.; Schlothauer, T.; Wecksler, A.T. Antigen physiochemical properties allosterically effect the IgG Fc-region and Fc neonatal receptor affinity. mAbs 2020, 12, 1802135. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Oganesyan, V.; Damschroder, M.M.; Cook, K.E.; Li, Q.; Gao, C.; Wu, H.; Dall’Acqua, W.F. Structural insights into neonatal Fc receptor-based recycling mechanisms. J. Biol. Chem. 2014, 289, 7812–7824. [Google Scholar] [CrossRef]
- Martin, W.L.; West, A.P., Jr.; Gan, L.; Bjorkman, P.J. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: Mechanism of pH-dependent binding. Mol. Cell 2001, 7, 867–877. [Google Scholar] [CrossRef]
- Raghavan, M.; Bonagura, V.R.; Morrison, S.L.; Bjorkman, P.J. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry 1995, 34, 14649–14657. [Google Scholar] [CrossRef]
- Kim, J.K.; Firan, M.; Radu, C.G.; Kim, C.H.; Ghetie, V.; Ward, E.S. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur. J. Immunol. 1999, 29, 2819–2825. [Google Scholar] [CrossRef]
- Suzuki, T.; Ishii-Watabe, A.; Tada, M.; Kobayashi, T.; Kanayasu-Toyoda, T.; Kawanishi, T.; Yamaguchi, T. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: A comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J. Immunol. 2010, 184, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Vidarsson, G.; Cragg, M.S. Effect of posttranslational modifications and subclass on IgG activity: From immunity to immunotherapy. Nat. Immunol. 2023, 24, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti-Ciarlet, A.; Wang, W.; Lownes, R.; Pristatsky, P.; Fang, Y.; McKelvey, T.; Li, Y.; Li, Y.; Drummond, J.; Prueksaritanont, T.; et al. Impact of methionine oxidation on the binding of human IgG1 to Fc Rn and Fc gamma receptors. Mol. Immunol. 2009, 46, 1878–1882. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef]
- Lu, J.; Chu, J.; Zou, Z.; Hamacher, N.B.; Rixon, M.W.; Sun, P.D. Structure of FcγRI in complex with Fc reveals the importance of glycan recognition for high-affinity IgG binding. Proc. Natl. Acad. Sci. USA 2015, 112, 833–838. [Google Scholar] [CrossRef]
- Subedi, G.P.; Barb, A.W. The Structural Role of Antibody N-Glycosylation in Receptor Interactions. Structure 2015, 23, 1573–1583. [Google Scholar] [CrossRef]
- Jensen, P.F.; Larraillet, V.; Schlothauer, T.; Kettenberger, H.; Hilger, M.; Rand, K.D. Investigating the interaction between the neonatal Fc receptor and monoclonal antibody variants by hydrogen/deuterium exchange mass spectrometry. Mol. Cell. Proteom. 2015, 14, 148–161. [Google Scholar] [CrossRef]
- Houde, D.; Peng, Y.; Berkowitz, S.A.; Engen, J.R. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol. Cell. Proteom. 2010, 9, 1716–1728. [Google Scholar] [CrossRef]
- Anumula, K.R. Quantitative glycan profiling of normal human plasma derived immunoglobulin and its fragments Fab and Fc. J. Immunol. Methods 2012, 382, 167–176. [Google Scholar] [CrossRef]
- Holland, M.; Yagi, H.; Takahashi, N.; Kato, K.; Savage, C.O.; Goodall, D.M.; Jefferis, R. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim. Biophys. Acta 2006, 1760, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Malan Borel, I.; Gentile, T.; Angelucci, J.; Pividori, J.; Guala, M.C.; Binaghi, R.A.; Margni, R.A. IgG asymmetric molecules with antipaternal activity isolated from sera and placenta of pregnant human. J. Reprod. Immunol. 1991, 20, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Volkov, M.; Brinkhaus, M.; van Schie, K.A.; Bondt, A.; Kissel, T.; van der Kooi, E.J.; Bentlage, A.E.H.; Koeleman, C.A.M.; de Taeye, S.W.; Derksen, N.I.; et al. IgG Fab Glycans Hinder FcRn-Mediated Placental Transport. J. Immunol. 2023, 210, 158–167. [Google Scholar] [CrossRef]
- Jefferis, R.; Lefranc, M.P. Human immunoglobulin allotypes: Possible implications for immunogenicity. mAbs 2009, 1, 332–338. [Google Scholar] [CrossRef]
- Wang, W.; Vlasak, J.; Li, Y.; Pristatsky, P.; Fang, Y.; Pittman, T.; Roman, J.; Wang, Y.; Prueksaritanont, T.; Ionescu, R. Impact of methionine oxidation in human IgG1 Fc on serum half-life of monoclonal antibodies. Mol. Immunol. 2011, 48, 860–866. [Google Scholar] [CrossRef]
- Wang, W.; Lu, P.; Fang, Y.; Hamuro, L.; Pittman, T.; Carr, B.; Hochman, J.; Prueksaritanont, T. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab. Dispos. 2011, 39, 1469–1477. [Google Scholar] [CrossRef]
- Schlothauer, T.; Rueger, P.; Stracke, J.O.; Hertenberger, H.; Fingas, F.; Kling, L.; Emrich, T.; Drabner, G.; Seeber, S.; Auer, J.; et al. Analytical FcRn affinity chromatography for functional characterization of monoclonal antibodies. mAbs 2013, 5, 576–586. [Google Scholar] [CrossRef]
- Liu, X.; Ye, L.; Christianson, G.J.; Yang, J.Q.; Roopenian, D.C.; Zhu, X. NF-kappaB signaling regulates functional expression of the MHC class I-related neonatal Fc receptor for IgG via intronic binding sequences. J. Immunol. 2007, 179, 2999–3011. [Google Scholar] [CrossRef]
- Qian, S.; Li, C.; Liu, X.; Jia, X.; Xiao, Y.; Li, Z. Activation of the JNK/MAPK Signaling Pathway by TGF-β1 Enhances Neonatal Fc Receptor Expression and IgG Transcytosis. Microorganisms 2021, 9, 879. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, F.; Qian, S.; Bi, D.; He, Q.; Jin, H.; Luo, R.; Li, S.; Meng, X.; Li, Z. TGEV infection up-regulates FcRn expression via activation of NF-κB signaling. Sci. Rep. 2016, 6, 32154. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; Saron, W.A.A.; Lim, T.; Jahan, N.; St John, A.L. Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci. Adv. 2019, 5, eaav3208. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, L.; Bai, Y.; Mojidi, H.; Simister, N.E.; Zhu, X. Activation of the JAK/STAT-1 signaling pathway by IFN-gamma can down-regulate functional expression of the MHC class I-related neonatal Fc receptor for IgG. J. Immunol. 2008, 181, 449–463. [Google Scholar] [CrossRef]
- Lozano, N.A.; Lozano, A.; Marini, V.; Saranz, R.J.; Blumberg, R.S.; Baker, K.; Agresta, M.F.; Ponzio, M.F. Expression of FcRn receptor in placental tissue and its relationship with IgG levels in term and preterm newborns. Am. J. Reprod. Immunol. 2018, 80, e12972. [Google Scholar] [CrossRef]
- Okoko, B.J.; Wesumperuma, L.H.; Ota, M.O.; Pinder, M.; Banya, W.; Gomez, S.F.; McAdam, K.P.; Hart, A.C. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J. Infect. Dis. 2001, 184, 627–632. [Google Scholar] [CrossRef]
- Schlaudecker, E.P.; Ambroggio, L.; McNeal, M.M.; Finkelman, F.D.; Way, S.S. Declining responsiveness to influenza vaccination with progression of human pregnancy. Vaccine 2018, 36, 4734–4741. [Google Scholar] [CrossRef]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 2012, 985646. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Holder, B.; Jones, C.E. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front. Immunol. 2017, 8, 1294. [Google Scholar] [CrossRef]
- Jones, C.E.; Naidoo, S.; De Beer, C.; Esser, M.; Kampmann, B.; Hesseling, A.C. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 2011, 305, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef]
- van den Berg, J.P.; Westerbeek, E.A.; Smits, G.P.; van der Klis, F.R.; Berbers, G.A.; van Elburg, R.M. Lower transplacental antibody transport for measles, mumps, rubella and varicella zoster in very preterm infants. PLoS ONE 2014, 9, e94714. [Google Scholar] [CrossRef]
- Atwell, J.E.; Thumar, B.; Formica, M.A.; Robinson, L.J.; Walsh, E.E.; King, C.L.; Karron, R.A. Hypergammaglobulinemia and Impaired Transplacental Transfer of Respiratory Syncytial Virus Antibody in Papua New Guinea. Pediatr. Infect. Dis. J. 2019, 38, e199–e202. [Google Scholar] [CrossRef] [PubMed]
- Otero, S.; Miller, E.S.; Sunderraj, A.; Shanes, E.D.; Sakowicz, A.; Goldstein, J.A.; Mithal, L.B. Maternal Antibody Response and Transplacental Transfer Following Severe Acute Respiratory Syndrome Coronavirus 2 Infection or Vaccination in Pregnancy. Clin. Infect. Dis. 2023, 76, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Taylor, P.N.; Gillespie, K.M.; Oram, R.A.; Dayan, C.M. Maternal type 1 diabetes and relative protection against offspring transmission. Lancet Diabetes Endocrinol. 2023, 11, 755–767. [Google Scholar] [CrossRef]
- Jasset, O.J.; Lopez Zapana, P.A.; Bahadir, Z.; Shook, L.; Dennis, M.; Gilbert, E.; Liu, Z.A.; Yinger, R.V.; Bald, C.; Bradford, C.G.; et al. Enhanced placental antibody transfer efficiency with longer interval between maternal respiratory syncytial virus vaccination and birth. Am. J. Obstet. Gynecol. 2024, 232, 554.e1–554.e15. [Google Scholar] [CrossRef]
- Jasset, M.O.J.; Andrea Lopez Zapana, P.; Bahadir, Z.; Shook, L.; Dennis, M.M.; Gilbert, M.E.; Liu, M.Z.A.; Yinger, M.R.V.; Bald, M.C.; Bradford, M.C.G.; et al. Longer interval between maternal RSV vaccination and birth increases placental transfer efficiency. medRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Kachikis, A.B.; Rumfelt, K.; Pike, M.; Sosa, M.; Stolarczuk, J.E.; Cho, H.; Eckert, L.O.; Martin, E.T.; Englund, J.A. Transfer of Respiratory Syncytial Virus Prefusion F Protein Antibody in Low Birthweight Infants. Open Forum Infect. Dis. 2024, 11, ofae314. [Google Scholar] [CrossRef]
- Jauniaux, E.; Jurkovic, D.; Gulbis, B.; Liesnard, C.; Lees, C.; Campbell, S. Materno-fetal immunoglobulin transfer and passive immunity during the first trimester of human pregnancy. Hum. Reprod. 1995, 10, 3297–3300. [Google Scholar] [CrossRef]
- Silveira Lessa, A.L.; Krebs, V.L.; Brasil, T.B.; Pontes, G.N.; Carneiro-Sampaio, M.; Palmeira, P. Preterm and term neonates transplacentally acquire IgG antibodies specific to LPS from Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 2011, 62, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Simister, N.E. Placental transport of immunoglobulin G. Vaccine 2003, 21, 3365–3369. [Google Scholar] [CrossRef] [PubMed]
- Kohler, P.F.; Farr, R.S. Elevation of cord over maternal IgG immunoglobulin: Evidence for an active placental IgG transport. Nature 1966, 210, 1070–1071. [Google Scholar] [CrossRef]
- van den Berg, J.P.; Westerbeek, E.A.; Berbers, G.A.; van Gageldonk, P.G.; van der Klis, F.R.; van Elburg, R.M. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr. Infect. Dis. J. 2010, 29, 801–805. [Google Scholar] [CrossRef]
- Eberhardt, C.S.; Blanchard-Rohner, G.; Lemaître, B.; Boukrid, M.; Combescure, C.; Othenin-Girard, V.; Chilin, A.; Petre, J.; de Tejada, B.M.; Siegrist, C.A. Maternal Immunization Earlier in Pregnancy Maximizes Antibody Transfer and Expected Infant Seropositivity Against Pertussis. Clin. Infect. Dis. 2016, 62, 829–836. [Google Scholar] [CrossRef]
- Calvert, A.; Jones, C.E. Placental transfer of antibody and its relationship to vaccination in pregnancy. Curr. Opin. Infect. Dis. 2017, 30, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Calvert, A.; Amirthalingam, G.; Andrews, N.; Basude, S.; Coleman, M.; Cuthbertson, H.; England, A.; Greening, V.; Hallis, B.; Johnstone, E.; et al. Optimising the timing of whooping cough immunisation in mums (OpTIMUM) through investigating pertussis vaccination in pregnancy: An open-label, equivalence, randomised controlled trial. Lancet Microbe 2023, 4, e300–e308. [Google Scholar] [CrossRef]
- Immink, M.M.; Bekker, M.N.; de Melker, H.E.; den Hartog, G.; Rots, N.Y.; van Gageldonk, P.G.M.; Groenendaal, F.; Sanders, E.A.M.; van der Maas, N.A.T. Maternal Pertussis Immunization and Immunoglobulin G Levels in Early- to Late-Term and Preterm Infants. JAMA Netw. Open 2024, 7, e2424608. [Google Scholar] [CrossRef]
- Gomme, J.; Wanlapakorn, N.; Ha, H.T.T.; Leuridan, E.; Herzog, S.A.; Maertens, K. The Impact of Timing of Pertussis Vaccination During Pregnancy on Infant Antibody Levels at Birth: A Multi-Country Analysis. Front. Immunol. 2022, 13, 913922. [Google Scholar] [CrossRef]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Pérez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, L.; Tang, S.; Shi, Y. Efficacy and safety of respiratory syncytial virus vaccination during pregnancy to prevent lower respiratory tract illness in newborns and infants: A systematic review and meta-analysis of randomized controlled trials. Front. Pediatr. 2023, 11, 1260740. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.C.; Marlow, R.; Cunningham, S.; Drysdale, S.B.; Groves, H.E.; Hunt, S.; Iskander, D.; Liu, X.; Lyttle, M.D.; Mpamhanga, C.D.; et al. Bivalent prefusion F vaccination in pregnancy and respiratory syncytial virus hospitalisation in infants in the UK: Results of a multicentre, test-negative, case-control study. Lancet Child Adolesc. Health 2025, 9, 655–662. [Google Scholar] [CrossRef]
- Atyeo, C.G.; Shook, L.L.; Brigida, S.; De Guzman, R.M.; Demidkin, S.; Muir, C.; Akinwunmi, B.; Baez, A.M.; Sheehan, M.L.; McSweeney, E.; et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat. Commun. 2022, 13, 3571. [Google Scholar] [CrossRef]
- Baroncelli, S.; Galluzzo, C.M.; Orlando, S.; Mphwere, R.; Kavalo, T.; Luhanga, R.; Amici, R.; Floridia, M.; Andreotti, M.; Ciccacci, F.; et al. Immunoglobulin G passive transfer from mothers to infants: Total IgG, IgG subclasses and specific antipneumococcal IgG in 6-week Malawian infants exposed or unexposed to HIV. BMC Infect. Dis. 2022, 22, 342. [Google Scholar] [CrossRef]
- Gao, C.; Chen, Q.; Hao, X.; Wang, Q. Immunomodulation of Antibody Glycosylation through the Placental Transfer. Int. J. Mol. Sci. 2023, 24, 16772. [Google Scholar] [CrossRef]
- Qi, T.; Cao, Y. In Translation: FcRn across the Therapeutic Spectrum. Int. J. Mol. Sci. 2021, 22, 3048. [Google Scholar] [CrossRef]
- Hartter, H.K.; Oyedele, O.I.; Dietz, K.; Kreis, S.; Hoffman, J.P.; Muller, C.P. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr. Infect. Dis. J. 2000, 19, 635–641. [Google Scholar] [CrossRef]
- Stach, S.C.; Brizot, M.L.; Liao, A.W.; Palmeira, P.; Francisco, R.P.; Carneiro-Sampaio, M.M.; Zugaib, M. Placental transfer of IgG antibodies specific to Klebsiella and Pseudomonas LPS and to group B Streptococcus in twin pregnancies. Scand. J. Immunol. 2015, 81, 135–141. [Google Scholar] [CrossRef]
- Kardava, L.; Moir, S. B-cell abnormalities in HIV-1 infection: Roles for IgG3 and T-bet. Curr. Opin. HIV AIDS 2019, 14, 240–245. [Google Scholar] [CrossRef]
- Stögerer, T.; Silva-Barrios, S.; Carmona-Pérez, L.; Swaminathan, S.; Mai, L.T.; Leroux, L.P.; Jaramillo, M.; Descoteaux, A.; Stäger, S. Leishmania donovani Exploits Tunneling Nanotubes for Dissemination and Propagation of B Cell Activation. Microbiol. Spectr. 2023, 11, e0509622. [Google Scholar] [CrossRef] [PubMed]
- Greczmiel, U.; Kräutler, N.J.; Borsa, M.; Pedrioli, A.; Bartsch, I.; Richter, K.; Agnellini, P.; Bedenikovic, G.; Oxenius, A. LCMV-specific CD4 T cell dependent polyclonal B-cell activation upon persistent viral infection is short lived and extrafollicular. Eur. J. Immunol. 2020, 50, 396–403. [Google Scholar] [CrossRef]
- Gregorek, H.; Jankowska, I.; Dzierzanowska-Fangrat, K.; Teisseyre, J.; Sawicka, A.; Kasztelewicz, B.; Pawłowska, J. Long-term monitoring of Epstein-Barr virus DNA load and humoral parameter abnormalities in pediatric liver transplant recipients before development of malignancy. Pediatr. Transplant. 2010, 14, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, Q.; Xu, S.; Yin, T.; Li, F. Successful treatment of refractory retroperitoneal Epstein-Barr virus-positive diffuse large B-cell lymphoma with secondary hemophagocytic syndrome by sequential combination regimen of PD-1 blockade and chimeric antigen receptor T cells: A case report. Anticancer Drugs 2022, 33, e769–e775. [Google Scholar] [CrossRef]
- Atwell, J.E.; Thumar, B.; Robinson, L.J.; Tobby, R.; Yambo, P.; Ome-Kaius, M.; Siba, P.M.; Unger, H.W.; Rogerson, S.J.; King, C.L.; et al. Impact of Placental Malaria and Hypergammaglobulinemia on Transplacental Transfer of Respiratory Syncytial Virus Antibody in Papua New Guinea. J. Infect. Dis. 2016, 213, 423–431. [Google Scholar] [CrossRef]
- Pou, C.; Nkulikiyimfura, D.; Henckel, E.; Olin, A.; Lakshmikanth, T.; Mikes, J.; Wang, J.; Chen, Y.; Bernhardsson, A.K.; Gustafsson, A.; et al. The repertoire of maternal anti-viral antibodies in human newborns. Nat. Med. 2019, 25, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Henrie, R.; Cherniawsky, H.; Marcon, K.; Zhao, E.J.; Marinkovic, A.; Pourshahnazari, P.; Parkin, S.; Chen, L.Y.C. Inflammatory diseases in hematology: A review. Am. J. Physiol. Cell Physiol. 2022, 323, C1121–C1136. [Google Scholar] [CrossRef]
- Coler, C.; King-Nakaoka, E.; Every, E.; Chima, S.; Vong, A.; Del Rosario, B.; VanAbel, R.; Adams Waldorf, K.M. Impact of Infections During Pregnancy on Transplacental Antibody Transfer. Vaccines 2024, 12, 1199. [Google Scholar] [CrossRef]
- De Milito, A.; Nilsson, A.; Titanji, K.; Thorstensson, R.; Reizenstein, E.; Narita, M.; Grutzmeier, S.; Sönnerborg, A.; Chiodi, F. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 2004, 103, 2180–2186. [Google Scholar] [CrossRef]
- Mok, M.Y. The immunological basis of B-cell therapy in systemic lupus erythematosus. Int. J. Rheum. Dis. 2010, 13, 3–11. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Anunciación-Llunell, A.; Marques-Soares, J.; Pardos-Gea, J.; Miró-Mur, F. Pathogenesis, Diagnosis and Management of Obstetric Antiphospholipid Syndrome: A Comprehensive Review. J. Clin. Med. 2022, 11, 675. [Google Scholar] [CrossRef]
- Dzirba, D.; Glinko, M.; Skoczyńska, M.; Gruszecka, K.; Trzeszcz, M.; Benedyczak, A.; Szmyrka, M. Placental Pathology in Obstetric Antiphospholipid Syndrome Beyond Thrombosis: A Case Report and Literature Review. J. Clin. Med. 2025, 14, 5172. [Google Scholar] [CrossRef]
- Abrahams, V.M. Mechanisms of antiphospholipid antibody-associated pregnancy complications. Thromb. Res. 2009, 124, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Truglia, S.; Riitano, G.; Mancuso, S.; Recalchi, S.; Rapino, L.; Garufi, C.; Manganelli, V.; Garofalo, T.; Misasi, R.; Alessandri, C.; et al. Antibody profiles in the mosaic of ‘seronegative’ APS syndrome. Clin. Exp. Immunol. 2024, 218, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Riitano, G.; Capozzi, A.; Recalchi, S.; Augusto, M.; Conti, F.; Misasi, R.; Garofalo, T.; Sorice, M.; Manganelli, V. Role of Lipid Rafts on LRP8 Signaling Triggered by Anti-β2-GPI Antibodies in Endothelial Cells. Biomedicines 2023, 11, 3135. [Google Scholar] [CrossRef]
- Marani, M.; Katul, G.G.; Pan, W.K.; Parolari, A.J. Intensity and frequency of extreme novel epidemics. Proc. Natl. Acad. Sci. USA 2021, 118, e2105482118, Correction in Proc. Natl. Acad. Sci. USA 2023, 120, e2302169120. https://doi.org/10.1073/pnas.2302169120. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Malaspina, A.; Ho, J.; Wang, W.; Dipoto, A.C.; O’Shea, M.A.; Roby, G.; Mican, J.M.; Kottilil, S.; Chun, T.W.; et al. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J. Infect. Dis. 2008, 197, 572–579. [Google Scholar] [CrossRef]
- Ackerman, M.E.; Dugast, A.S.; McAndrew, E.G.; Tsoukas, S.; Licht, A.F.; Irvine, D.J.; Alter, G. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b. J. Virol. 2013, 87, 5468–5476. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.A.; Sharma, S.; Remmel, C.A.L.; Holder, B.; Jones, C.E.; Marchant, A.; Ackerman, M.E. HIV-Associated Alterations of the Biophysical Features of Maternal Antibodies Correlate With Their Reduced Transfer Across the Placenta. J. Infect. Dis. 2022, 226, 1441–1450. [Google Scholar] [CrossRef]
- Crocker, I.P.; Tanner, O.M.; Myers, J.E.; Bulmer, J.N.; Walraven, G.; Baker, P.N. Syncytiotrophoblast degradation and the pathophysiology of the malaria-infected placenta. Placenta 2004, 25, 273–282. [Google Scholar] [CrossRef]
- Elphinstone, R.E.; Weckman, A.M.; McDonald, C.R.; Tran, V.; Zhong, K.; Madanitsa, M.; Kalilani-Phiri, L.; Khairallah, C.; Taylor, S.M.; Meshnick, S.R.; et al. Early malaria infection, dysregulation of angiogenesis, metabolism and inflammation across pregnancy, and risk of preterm birth in Malawi: A cohort study. PLoS Med. 2019, 16, e1002914. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.L.; Nyengaard, J.R.; Larsen, L.G.; Nielsen, K.; Bygbjerg, I.C.; Msemo, O.A.; Lusingu, J.P.A.; Minja, D.T.R.; Theander, T.G.; Schmiegelow, C. Malaria in Early Pregnancy and the Development of the Placental Vasculature. J. Infect. Dis. 2019, 220, 1425–1434. [Google Scholar] [CrossRef]
- Chaikitgosiyakul, S.; Rijken, M.J.; Muehlenbachs, A.; Lee, S.J.; Chaisri, U.; Viriyavejakul, P.; Turner, G.D.; Pongponratn, E.; Nosten, F.; McGready, R. A morphometric and histological study of placental malaria shows significant changes to villous architecture in both Plasmodium falciparum and Plasmodium vivax infection. Malar. J. 2014, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.; Pullen, K.M.; Bordt, E.A.; Fischinger, S.; Burke, J.; Michell, A.; Slein, M.D.; Loos, C.; Shook, L.L.; Boatin, A.A.; et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 2021, 184, 628–642.e610. [Google Scholar] [CrossRef]
- Lucot-Royer, L.; Nallet, C.; Vouga, M.; Puyraveau, M.; Mauny, F.; Marty-Quinternet, S.; Bertholdt, C.; Bory, J.P.; Devalland, C.; Canaguier, M.; et al. Analysis of the transplacental transmission of SARS CoV-2 virus and antibody transfer according to the gestational age at maternal infection. Sci. Rep. 2024, 14, 3458. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Smolen, K.K.; Willems, F.; Kollmann, T.R.; Marchant, A. Transfer of Maternal Antimicrobial Immunity to HIV-Exposed Uninfected Newborns. Front. Immunol. 2016, 7, 338. [Google Scholar] [CrossRef]
- Atwell, J.E.; Lutz, C.S.; Sparrow, E.G.; Feikin, D.R. Biological factors that may impair transplacental transfer of RSV antibodies: Implications for maternal immunization policy and research priorities for low- and middle-income countries. Vaccine 2022, 40, 4361–4370. [Google Scholar] [CrossRef]
- Ackerman, M.E.; Crispin, M.; Yu, X.; Baruah, K.; Boesch, A.W.; Harvey, D.J.; Dugast, A.S.; Heizen, E.L.; Ercan, A.; Choi, I.; et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Investig. 2013, 123, 2183–2192. [Google Scholar] [CrossRef]
- Kollmann, T.R.; Marchant, A.; Way, S.S. Vaccination strategies to enhance immunity in neonates. Science 2020, 368, 612–615. [Google Scholar] [CrossRef]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.J.; Kumar, A.; Giorgi, E.E.; Eudailey, J.; LaBranche, C.C.; Martinez, D.R.; Fouda, G.G.; Moreau, Y.; Thomas, A.; Montefiori, D.; et al. Vertical HIV-1 Transmission in the Setting of Maternal Broad and Potent Antibody Responses. J. Virol. 2022, 96, e0023122. [Google Scholar] [CrossRef]
- Dauby, N.; Gagneux-Brunon, A.; Martin, C.; Mussi-Pinhata, M.M.; Goetghebuer, T. Maternal immunization in women living with HIV. Aids 2024, 38, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Dangor, Z.; Nunes, M.C.; Kwatra, G.; Lala, S.G.; Madhi, S.A. Vaccination of HIV-infected pregnant women: Implications for protection of their young infants. Trop. Dis. Travel. Med. Vaccines 2017, 3, 1. [Google Scholar] [CrossRef]
- Taton, M.; Willems, F.; Widomski, C.; Georges, D.; Martin, C.; Jiang, Y.; Renard, K.; Konopnicki, D.; Cogan, A.; Necsoi, C.; et al. HIV-related immune activation attenuates polyfunctional IgG and memory B-cell responses to Tdap immunization during pregnancy. eBioMedicine 2024, 104, 105179. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Fauci, A.S. B-cell exhaustion in HIV infection: The role of immune activation. Curr. Opin. HIV AIDS 2014, 9, 472–477. [Google Scholar] [CrossRef]
- Golay, J.; Andrea, A.E.; Cattaneo, I. Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Front. Immunol. 2022, 13, 929895. [Google Scholar] [CrossRef]
- Zakama, A.K.; Ozarslan, N.; Gaw, S.L. Placental Malaria. Curr. Trop. Med. Rep. 2020, 7, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ogolla, S.; Daud, I.I.; Asito, A.S.; Sumba, O.P.; Ouma, C.; Vulule, J.; Middeldorp, J.M.; Dent, A.E.; Mehta, S.; Rochford, R. Reduced Transplacental Transfer of a Subset of Epstein-Barr Virus-Specific Antibodies to Neonates of Mothers Infected with Plasmodium falciparum Malaria during Pregnancy. Clin. Vaccine Immunol. 2015, 22, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Seyed Hosseini, E.; Riahi Kashani, N.; Nikzad, H.; Azadbakht, J.; Hassani Bafrani, H.; Haddad Kashani, H. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology 2020, 551, 1–9. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef]
- Mourad, M.; Jacob, T.; Sadovsky, E.; Bejerano, S.; Simone, G.S.; Bagalkot, T.R.; Zucker, J.; Yin, M.T.; Chang, J.Y.; Liu, L.; et al. Placental response to maternal SARS-CoV-2 infection. Sci. Rep. 2021, 11, 14390. [Google Scholar] [CrossRef]
- Gee, S.; Chandiramani, M.; Seow, J.; Pollock, E.; Modestini, C.; Das, A.; Tree, T.; Doores, K.J.; Tribe, R.M.; Gibbons, D.L. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat. Immunol. 2021, 22, 1490–1502. [Google Scholar] [CrossRef]
- Gao, L.; Ren, J.; Xu, L.; Ke, X.; Xiong, L.; Tian, X.; Fan, C.; Yan, H.; Yuan, J. Placental pathology of the third trimester pregnant women from COVID-19. Diagn. Pathol. 2021, 16, 8. [Google Scholar] [CrossRef]
- Chucri, T.M.; Monteiro, J.M.; Lima, A.R.; Salvadori, M.L.; Kfoury, J.R., Jr.; Miglino, M.A. A review of immune transfer by the placenta. J. Reprod. Immunol. 2010, 87, 14–20. [Google Scholar] [CrossRef]
- Partey, F.D.; Obiri, D.; Bonney, E.Y.; Pobee, A.N.A.; Damptey, I.K.; Ennuson, K.; Akwetea-Foli, J.; Nuokpem, F.Y.; Courtin, D.; Kusi, K.A.; et al. Efficient transplacental transfer of SARS-CoV-2 antibodies between naturally exposed mothers and infants in Accra, Ghana. Sci. Rep. 2024, 14, 10772. [Google Scholar] [CrossRef] [PubMed]
- Trinité, B.; Tarrés-Freixas, F.; Rodon, J.; Pradenas, E.; Urrea, V.; Marfil, S.; Rodríguez de la Concepción, M.L.; Ávila-Nieto, C.; Aguilar-Gurrieri, C.; Barajas, A.; et al. SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity. Sci. Rep. 2021, 11, 2608. [Google Scholar] [CrossRef]
- Song, D.; Prahl, M.; Gaw, S.L.; Narasimhan, S.R.; Rai, D.S.; Huang, A.; Flores, C.V.; Lin, C.Y.; Jigmeddagva, U.; Wu, A.; et al. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: Prospective cohort study. BMJ Open 2021, 11, e053036. [Google Scholar] [CrossRef] [PubMed]
- Beharier, O.; Plitman Mayo, R.; Raz, T.; Nahum Sacks, K.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e150319, Correction in J. Clin. Investig. 2021, 131, e154834. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Fuglsang, J.; Kampmann, U.; Knorr, S.; Ovesen, P. Hyperglycemia in Pregnancy and Women’s Health in the 21st Century. Int. J. Environ. Res. Public Health 2022, 19, 16827. [Google Scholar] [CrossRef]
- de Souza, E.G.; Hara, C.C.; Fagundes, D.L.; de Queiroz, A.A.; Morceli, G.; Calderon, I.M.; França, E.L.; Honorio-França, A.C. Maternal-Foetal Diabetes Modifies Neonatal Fc Receptor Expression on Human Leucocytes. Scand. J. Immunol. 2016, 84, 237–244. [Google Scholar] [CrossRef]
- Calderon, I.M.; Damasceno, D.C.; Amorin, R.L.; Costa, R.A.; Brasil, M.A.; Rudge, M.V. Morphometric study of placental villi and vessels in women with mild hyperglycemia or gestational or overt diabetes. Diabetes Res. Clin. Pract. 2007, 78, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Wong, I.; Moller, A.; Giachini, F.R.; Lima, V.V.; Toledo, F.; Stojanova, J.; Sobrevia, L.; San Martín, S. Placental structure in gestational diabetes mellitus. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165535. [Google Scholar] [CrossRef]
- Hammes, H.P.; Kiefel, V.; Laube, H.; Federlin, K. Impaired agglutination of IgM resulting from non-enzymatic glycation in diabetes mellitus. Diabetes Res. Clin. Pract. 1990, 9, 37–42. [Google Scholar] [CrossRef]
- Perween, S.; Abidi, M.; Faiz Faizy, A.; Moinuddin. Biophysical changes in methylglyoxal modified fibrinogen and its role in the immunopathology of type 2 diabetes mellitus. Int. J. Biol. Macromol. 2022, 202, 199–214. [Google Scholar] [CrossRef]
- Goetze, A.M.; Liu, Y.D.; Arroll, T.; Chu, L.; Flynn, G.C. Rates and impact of human antibody glycation in vivo. Glycobiology 2012, 22, 221–234. [Google Scholar] [CrossRef]
- Štambuk, T.; Kifer, D.; Smirčić-Duvnjak, L.; Vučić Lovrenčić, M.; Gornik, O. Associations between plasma protein, IgG and IgA N-glycosylation and metabolic health markers in pregnancy and gestational diabetes. PLoS ONE 2023, 18, e0284838. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Shepard, H.M.; Phillips, G.L.; Thanos, C.D.; Feldmann, M. Developments in therapy with monoclonal antibodies and related proteins. Clin. Med. 2017, 17, 220–232. [Google Scholar] [CrossRef]
- Pham-Huy, A.; Top, K.A.; Constantinescu, C.; Seow, C.H.; El-Chaâr, D. The use and impact of monoclonal antibody biologics during pregnancy. Can. Med Assoc. J. 2021, 193, E1129–E1136. [Google Scholar] [CrossRef]
- Reusch, J.; Andersen, J.T.; Rant, U.; Schlothauer, T. Insight into the avidity-affinity relationship of the bivalent, pH-dependent interaction between IgG and FcRn. mAbs 2024, 16, 2361585. [Google Scholar] [CrossRef]
- Suzuki, T.; Hashii, N.; Tada, M.; Ishii-Watabe, A. The influence of antibody engineering on Fc conformation and Fc receptor binding properties: Analysis of FcRn-binding engineered antibodies and an Fc fusion protein. mAbs 2021, 13, 1923366. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Jo, M.; Kyung, M.; Lee, W.; Ko, W.H.; Na, J.H.; Chun, Y.S.; Ko, B.J.; Jung, S.T. Engineering FcRn binding kinetics dramatically extends antibody serum half-life and enhances therapeutic potential. J. Biol. Eng. 2025, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, U.; Long, M.D.; Kane, S.V.; Roy, A.; Dubinsky, M.C.; Sands, B.E.; Cohen, R.D.; Chambers, C.D.; Sandborn, W.J. Pregnancy and Neonatal Outcomes After Fetal Exposure to Biologics and Thiopurines Among Women With Inflammatory Bowel Disease. Gastroenterology 2021, 160, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.; Burchett, S.; Odejide, O.; Cataltepe, S. Septic Episodes in a Premature Infant After In Utero Exposure to Rituximab. Pediatrics 2017, 140, e20162819. [Google Scholar] [CrossRef]
- Chakravarty, E.F.; Murray, E.R.; Kelman, A.; Farmer, P. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011, 117, 1499–1506. [Google Scholar] [CrossRef]
- Mandal, P.K.; Dolai, T.K.; Bagchi, B.; Ghosh, M.K.; Bose, S.; Bhattacharyya, M. B cell suppression in newborn following treatment of pregnant diffuse large B-cell lymphoma patient with rituximab containing regimen. Indian J. Pediatr. 2014, 81, 1092–1094. [Google Scholar] [CrossRef]
- Chaparro, M.; Verreth, A.; Lobaton, T.; Gravito-Soares, E.; Julsgaard, M.; Savarino, E.; Magro, F.; Biron, A.I.; Lopez-Serrano, P.; Casanova, M.J.; et al. Long-Term Safety of In Utero Exposure to Anti-TNFα Drugs for the Treatment of Inflammatory Bowel Disease: Results from the Multicenter European TEDDY Study. Am. J. Gastroenterol. 2018, 113, 396–403. [Google Scholar] [CrossRef]
- Komaki, F.; Komaki, Y.; Micic, D.; Ido, A.; Sakuraba, A. Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-α use in females with immune mediated diseases; a systematic review and meta-analysis. J. Autoimmun. 2017, 76, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Bröms, G.; Kieler, H.; Ekbom, A.; Gissler, M.; Hellgren, K.; Lahesmaa-Korpinen, A.M.; Pedersen, L.; Schmitt-Egenolf, M.; Sørensen, H.T.; Granath, F. Anti-TNF treatment during pregnancy and birth outcomes: A population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol. Drug Saf. 2020, 29, 316–327. [Google Scholar] [CrossRef]
- Ward, E.S.; Ober, R.J. Targeting FcRn to Generate Antibody-Based Therapeutics. Trends Pharmacol. Sci. 2018, 39, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Chaparro, M. Vaccines in Children Exposed to Biological Agents In Utero and/or During Breastfeeding: Are They Effective and Safe? J. Crohns Colitis 2023, 17, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Damotte, V.; Gelfand, J.M.; Bevan, C.; Cree, B.A.C.; Do, L.; Green, A.J.; Hauser, S.L.; Bove, R. Rituximab before and during pregnancy: A systematic review, and a case series in MS and NMOSD. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e453. [Google Scholar] [CrossRef]
- Dernoncourt, A.; Liabeuf, S.; Bennis, Y.; Masmoudi, K.; Bodeau, S.; Laville, S.; Hurtel-Lemaire, A.S.; Gras-Champel, V.; Batteux, B. Fetal and Neonatal Adverse Drug Reactions Associated with Biologics Taken During Pregnancy by Women with Autoimmune Diseases: Insights from an Analysis of the World Health Organization Pharmacovigilance Database (VigiBase®). BioDrugs 2023, 37, 73–87. [Google Scholar] [CrossRef]
- Goulden, B.; Chua, N.; Parker, E.; Giles, I. P202 Administering live vaccines to infants exposed to biologic and targeted synthetic DMARDs in-utero for maternal treatment of rheumatic disease: A systematic review of the literature. Rheumatology 2021, 60, keab247.197. [Google Scholar] [CrossRef]
- Ghalandari, N.; Dolhain, R.; Hazes, J.M.W.; van Puijenbroek, E.P.; Kapur, M.; Crijns, H. Intrauterine Exposure to Biologics in Inflammatory Autoimmune Diseases: A Systematic Review. Drugs 2020, 80, 1699–1722. [Google Scholar] [CrossRef]
- Han, L.; Hou, Z.; Li, J.; Wang, Y.; Li, H. Biologic therapy for immune-mediated inflammatory diseases during pregnancy and lactation: Efficacy, safety, and challenges. Precis. Medicat. 2025, 2, 100029. [Google Scholar] [CrossRef]
- Götestam Skorpen, C.; Hoeltzenbein, M.; Tincani, A.; Fischer-Betz, R.; Elefant, E.; Chambers, C.; da Silva, J.; Nelson-Piercy, C.; Cetin, I.; Costedoat-Chalumeau, N.; et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann. Rheum. Dis. 2016, 75, 795–810. [Google Scholar] [CrossRef]
- Lowe, D.E.; Robbins, J.R.; Bakardjiev, A.I. Animal and Human Tissue Models of Vertical Listeria monocytogenes Transmission and Implications for Other Pregnancy-Associated Infections. Infect. Immun. 2018, 86, e00801-17. [Google Scholar] [CrossRef]
- Hemberger, M.; Hanna, C.W.; Dean, W. Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 2020, 21, 27–43. [Google Scholar] [CrossRef]
- Warang, P.; Singh, G.; Moshir, M.; Binazon, O.; Laghlali, G.; Chang, L.A.; Wouters, H.; Vanhoenacker, P.; Notebaert, M.; Elhemdaoui, N.; et al. Impact of FcRn antagonism on vaccine-induced protective immune responses against viral challenge in COVID-19 and influenza mouse vaccination models. Hum. Vaccin. Immunother. 2025, 21, 2470542. [Google Scholar] [CrossRef]
- Proetzel, G.; Roopenian, D.C. Humanized FcRn mouse models for evaluating pharmacokinetics of human IgG antibodies. Methods 2014, 65, 148–153. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Christianson, G.J.; Sproule, T.J.; Brown, A.C.; Akilesh, S.; Jung, N.; Petkova, S.; Avanessian, L.; Choi, E.Y.; Shaffer, D.J.; et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J. Immunol. 2003, 170, 3528–3533. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mohanty, S.; Ganesan, L.P.; Hua, K.; Jarjoura, D.; Hayton, W.L.; Robinson, J.M.; Anderson, C.L. FcRn in the yolk sac endoderm of mouse is required for IgG transport to fetus. J. Immunol. 2009, 182, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, I.; Rothe, A.; Grill, M.; Fuchs, R. Apical to basolateral transcytosis and apical recycling of immunoglobulin G in trophoblast-derived BeWo cells: Effects of low temperature, nocodazole, and cytochalasin D. Exp. Cell Res. 2001, 269, 322–331. [Google Scholar] [CrossRef]
- Ellinger, I.; Schwab, M.; Stefanescu, A.; Hunziker, W.; Fuchs, R. IgG transport across trophoblast-derived BeWo cells: A model system to study IgG transport in the placenta. Eur. J. Immunol. 1999, 29, 733–744. [Google Scholar] [CrossRef]
- Mathiesen, L.; Mørck, T.A.; Zuri, G.; Andersen, M.H.; Pehrson, C.; Frederiksen, M.; Mose, T.; Rytting, E.; Poulsen, M.S.; Nielsen, J.K.; et al. Modelling of human transplacental transport as performed in Copenhagen, Denmark. Basic. Clin. Pharmacol. Toxicol. 2014, 115, 93–100. [Google Scholar] [CrossRef]
- Hori, T.; Okae, H.; Shibata, S.; Kobayashi, N.; Kobayashi, E.H.; Oike, A.; Sekiya, A.; Arima, T.; Kaji, H. Trophoblast stem cell-based organoid models of the human placental barrier. Nat. Commun. 2024, 15, 962. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Armstrong-Fisher, S.; Kopotsha, T.; Smith, B.; Baker, T.; Kevorkian, L.; Nesbitt, A. Certolizumab pegol does not bind the neonatal Fc receptor (FcRn): Consequences for FcRn-mediated in vitro transcytosis and ex vivo human placental transfer. J. Reprod. Immunol. 2016, 116, 7–12. [Google Scholar] [CrossRef]
- Viall, C.A.; Chen, Q.; Liu, B.; Hickey, A.; Snowise, S.; Salmon, J.E.; Stone, P.R.; Chamley, L.W. Antiphospholipid antibodies internalised by human syncytiotrophoblast cause aberrant cell death and the release of necrotic trophoblast debris. J. Autoimmun. 2013, 47, 45–57. [Google Scholar] [CrossRef]
- Roy, S.; Nanovskaya, T.; Patrikeeva, S.; Cochran, E.; Parge, V.; Guess, J.; Schaeck, J.; Choudhury, A.; Ahmed, M.; Ling, L.E. M281, an anti-FcRn antibody, inhibits IgG transfer in a human ex vivo placental perfusion model. Am. J. Obstet. Gynecol. 2019, 220, 498.e491–498.e499. [Google Scholar] [CrossRef]
- Dusza, H.M.; van Boxel, J.; van Duursen, M.B.M.; Forsberg, M.M.; Legler, J.; Vähäkangas, K.H. Experimental human placental models for studying uptake, transport and toxicity of micro- and nanoplastics. Sci. Total Environ. 2023, 860, 160403. [Google Scholar] [CrossRef]
- Myllynen, P.; Vähäkangas, K. Placental transfer and metabolism: An overview of the experimental models utilizing human placental tissue. Toxicol. In Vitro 2013, 27, 507–512. [Google Scholar] [CrossRef]
- Wessel, R.E.; Dolatshahi, S. Quantitative mechanistic model reveals key determinants of placental IgG transfer and informs prenatal immunization strategies. PLoS Comput. Biol. 2023, 19, e1011109. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, F.; Wang, H.; Wang, L.; Yuan, J.; Qin, J. Placental Barrier-on-a-Chip: Modeling Placental Inflammatory Responses to Bacterial Infection. ACS Biomater. Sci. Eng. 2018, 4, 3356–3363. [Google Scholar] [CrossRef]
- Lee, J.S.; Romero, R.; Han, Y.M.; Kim, H.C.; Kim, C.J.; Hong, J.S.; Huh, D. Placenta-on-a-chip: A novel platform to study the biology of the human placenta. J. Matern. Fetal Neonatal Med. 2016, 29, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, J.I.; Bollini, M.; Cavasotto, C.N. A Machine Learning Model to Predict Drug Transfer Across the Human Placenta Barrier. Front. Chem. 2021, 9, 714678. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, N.M.; Armstrong-Fisher, S.S.; Andersen, J.T.; van der Schoot, C.E.; Porter, C.; Page, K.R.; Falconer, D.; de Haas, M.; Williamson, L.M.; Clark, M.R.; et al. Human IgG lacking effector functions demonstrate lower FcRn-binding and reduced transplacental transport. Mol. Immunol. 2018, 95, 1–9. [Google Scholar] [CrossRef]
- Sand, K.M.K.; Gruber, M.M.; Sandlie, I.; Mathiesen, L.; Andersen, J.T.; Wadsack, C. Contribution of the ex vivo placental perfusion model in understanding transplacental immunoglobulin G transfer. Placenta 2022, 127, 77–87. [Google Scholar] [CrossRef]
- Wang, J.; Han, T.; Zhu, X. Role of maternal-fetal immune tolerance in the establishment and maintenance of pregnancy. Chin. Med. J. 2024, 137, 1399–1406. [Google Scholar] [CrossRef]
- Zalevsky, J.; Chamberlain, A.K.; Horton, H.M.; Karki, S.; Leung, I.W.; Sproule, T.J.; Lazar, G.A.; Roopenian, D.C.; Desjarlais, J.R. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 2010, 28, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Peter, H.H.; Ochs, H.D.; Cunningham-Rundles, C.; Vinh, D.C.; Kiessling, P.; Greve, B.; Jolles, S. Targeting FcRn for immunomodulation: Benefits, risks, and practical considerations. J. Allergy Clin. Immunol. 2020, 146, 479–491.e475. [Google Scholar] [CrossRef] [PubMed]
- Wessel, R.E.; Dolatshahi, S. Regulators of placental antibody transfer through a modeling lens. Nat. Immunol. 2024, 25, 2024–2036. [Google Scholar] [CrossRef] [PubMed]
| Infection | Maternal Infection-Associated Alterations | References |
|---|---|---|
| HIV | Alters maternal B cell subsets, reduces maternal IgG levels and induces IgG glycosylation shift compromising IgG binding to FcRn | [112,120,121,122] |
| Malaria | Induces local placental inflammation and leads to histopathological alterations in placental structure | [114,115,116,117] |
| SARS-CoV-2 | TPAT efficiency is positively correlated with the interval between maternal infection and delivery | [119] |
| Influenza | Impairs maternal antibody quality with potential downstream effects on TPAT efficiency | [123] |
| Zika virus | Causes placental infection and barrier disruption based on mechanistic studies in mice | [124] |
| Name | Species | Model Type | Target of Interest | Properties | References |
|---|---|---|---|---|---|
| Transgenic mice deficient in FcRn | Mice | In vivo | FcRn function | FcRn−/− foetuses showed negligible IgG concentrations. | [177] |
| Fc receptor humanized mice | Mice | In vivo | FcγR or FcRn function | Deficient in mouse Fc receptors and expressing human Fc receptors as a transgene. | [12,53,176] |
| Choriocarcinoma- derived cell line BeWo | Human | In vitro | SCT layer | Cells that can be cultured to form polarized, confluent monolayers with tight junctions. | [178,179,180] |
| Trophoblast organoids | Human | In vitro | SCT layer | Human trophoblast stem cells form spherical organoids with a single outer layer of SCT cells that display a barrier function. | [181] |
| Placental transfer model | Human | Ex vivo | The binding affinity of antitumour monoclonal antibodies to FcRn/FcγR | Cannulated and re-perfused term placental cotyledon. | [182] |
| First-trimester placental villous explants | Human | In vitro | The internalization of antiphospholipid antibodies by SCT cells | Placental explants of 10–30 mg wet weight were cultured in culture media supplemented with different antibodies. | [183] |
| Recombinant IgG antibodies lacking effector functions | Human | In vitro | The strategy to control the transplacental transfer of pathogenic maternal IgG | Human IgG3 antibodies are devoid of FcgR and C1q binding, cellular cytotoxicity and complement activation but retain FcRn binding. | [17] |
| Engineered IgG1 antibody | Human | Ex vivo | The activity of the engineered human IgG1 in both human and murine systems. | IgG1 mutants (H433K and N434F) transferred across human placenta more efficiently than wild-type IgG1. | [13] |
| M281, a fully human, aglycosylated monoclonal IgG1 anti-FcRn antibody | Human | Ex vivo | Blockage of FcRn for inhibition of transfer of pathogenic IgG antibodies | M281 significantly decreases the transfer rate of adalimumab across dually perfused human placental lobule. | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Li, Q.; Huang, Z.; Saeed, Z.; Greer, O.; Harker, J.A.; Shah, N.M. Transplacental Antibody Transfer: Mechanisms, Pregnancy-Related Disruptions, and Emerging Experimental Models. Antibodies 2026, 15, 14. https://doi.org/10.3390/antib15010014
Li Q, Huang Z, Saeed Z, Greer O, Harker JA, Shah NM. Transplacental Antibody Transfer: Mechanisms, Pregnancy-Related Disruptions, and Emerging Experimental Models. Antibodies. 2026; 15(1):14. https://doi.org/10.3390/antib15010014
Chicago/Turabian StyleLi, Qiqi, Zhengyuan Huang, Zainab Saeed, Orene Greer, James A. Harker, and Nishel M. Shah. 2026. "Transplacental Antibody Transfer: Mechanisms, Pregnancy-Related Disruptions, and Emerging Experimental Models" Antibodies 15, no. 1: 14. https://doi.org/10.3390/antib15010014
APA StyleLi, Q., Huang, Z., Saeed, Z., Greer, O., Harker, J. A., & Shah, N. M. (2026). Transplacental Antibody Transfer: Mechanisms, Pregnancy-Related Disruptions, and Emerging Experimental Models. Antibodies, 15(1), 14. https://doi.org/10.3390/antib15010014






