CEA-4-1BBL: CEACAM5-Targeted 4-1BB Ligand Fusion Proteins for Cis Co-Stimulation with CEA-TCB
Abstract
1. Introduction
2. Materials and Methods
3. Results
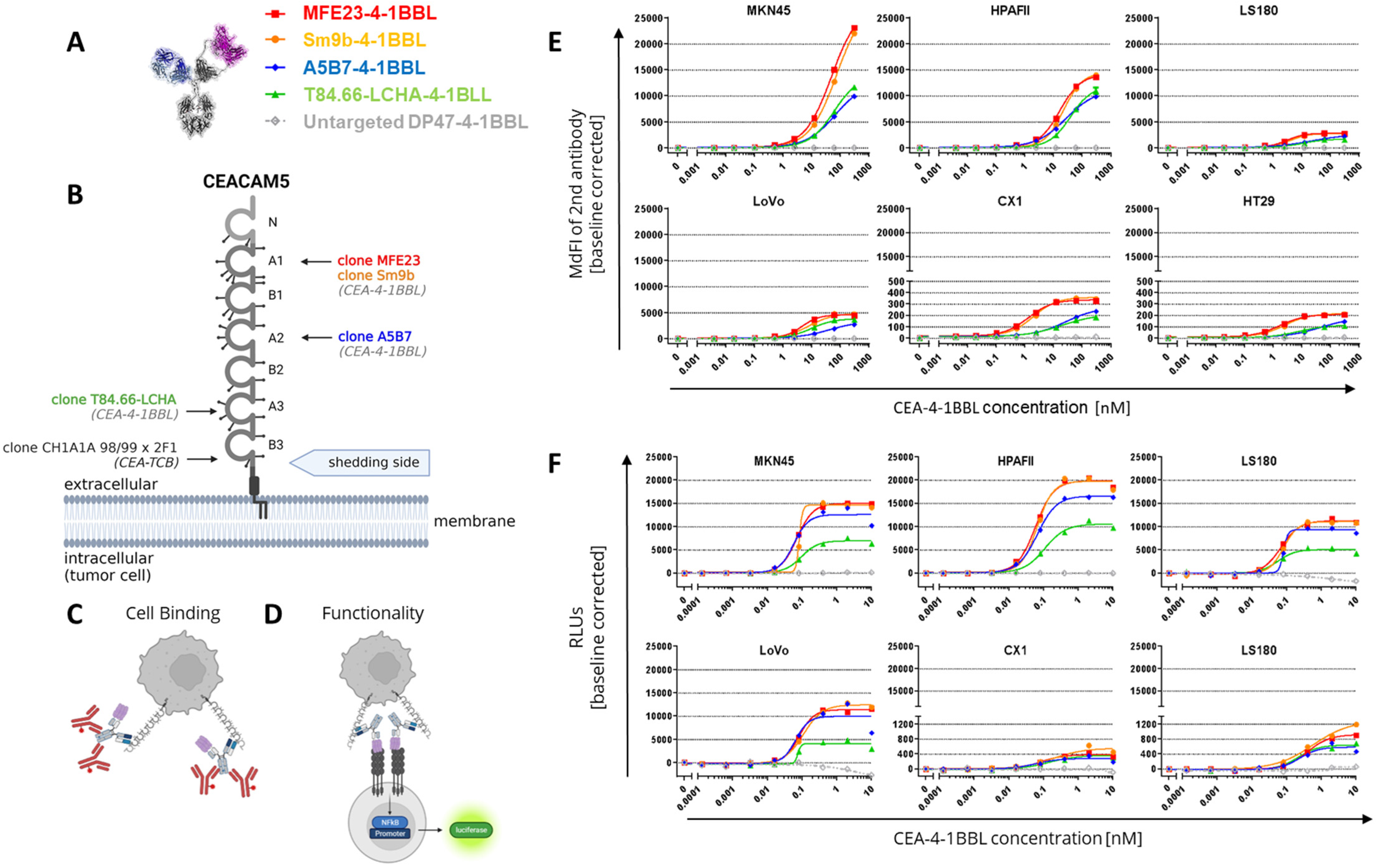
3.1. CEA-4-1BBL Antibody Fusion Protein Design
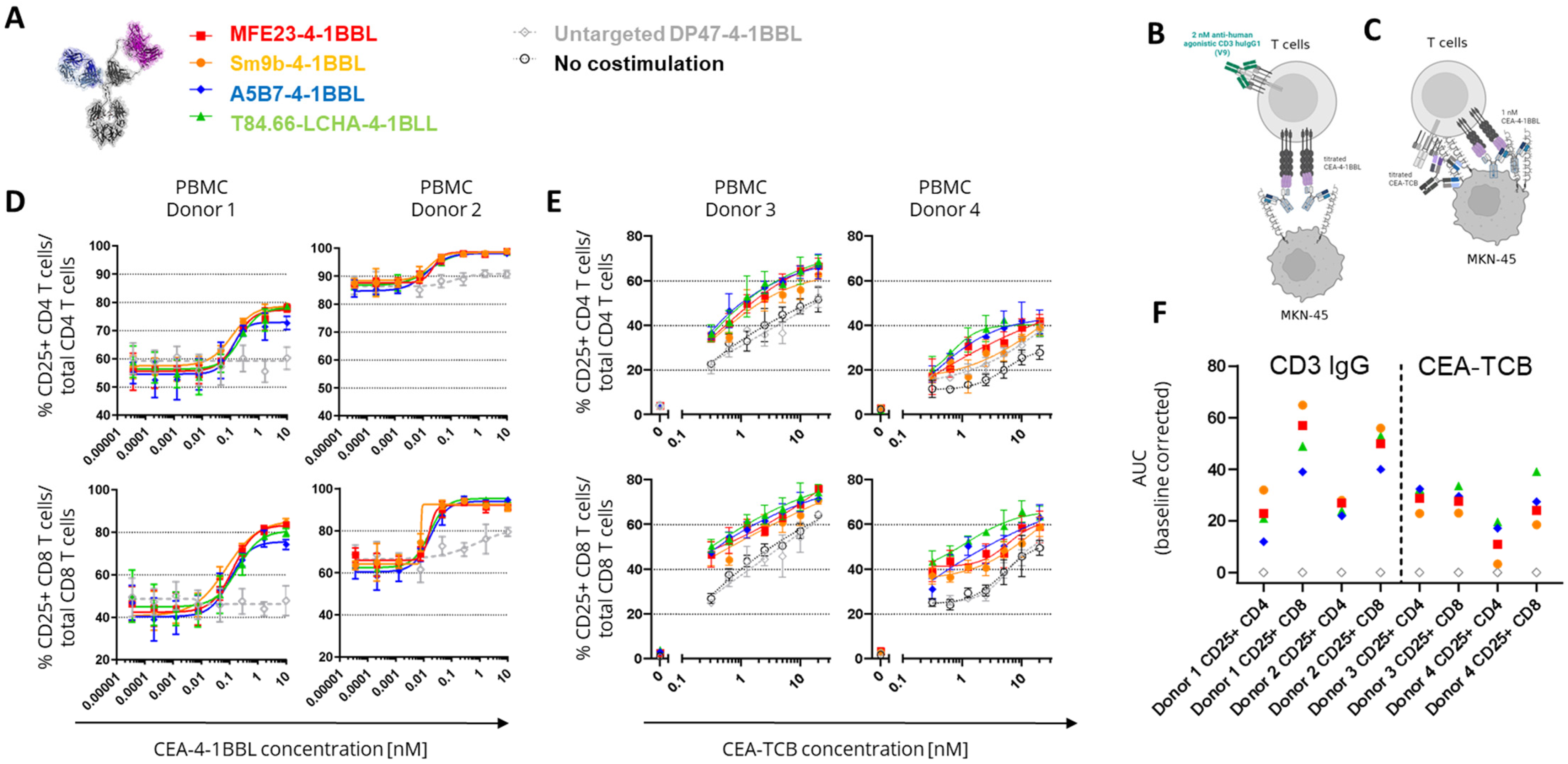
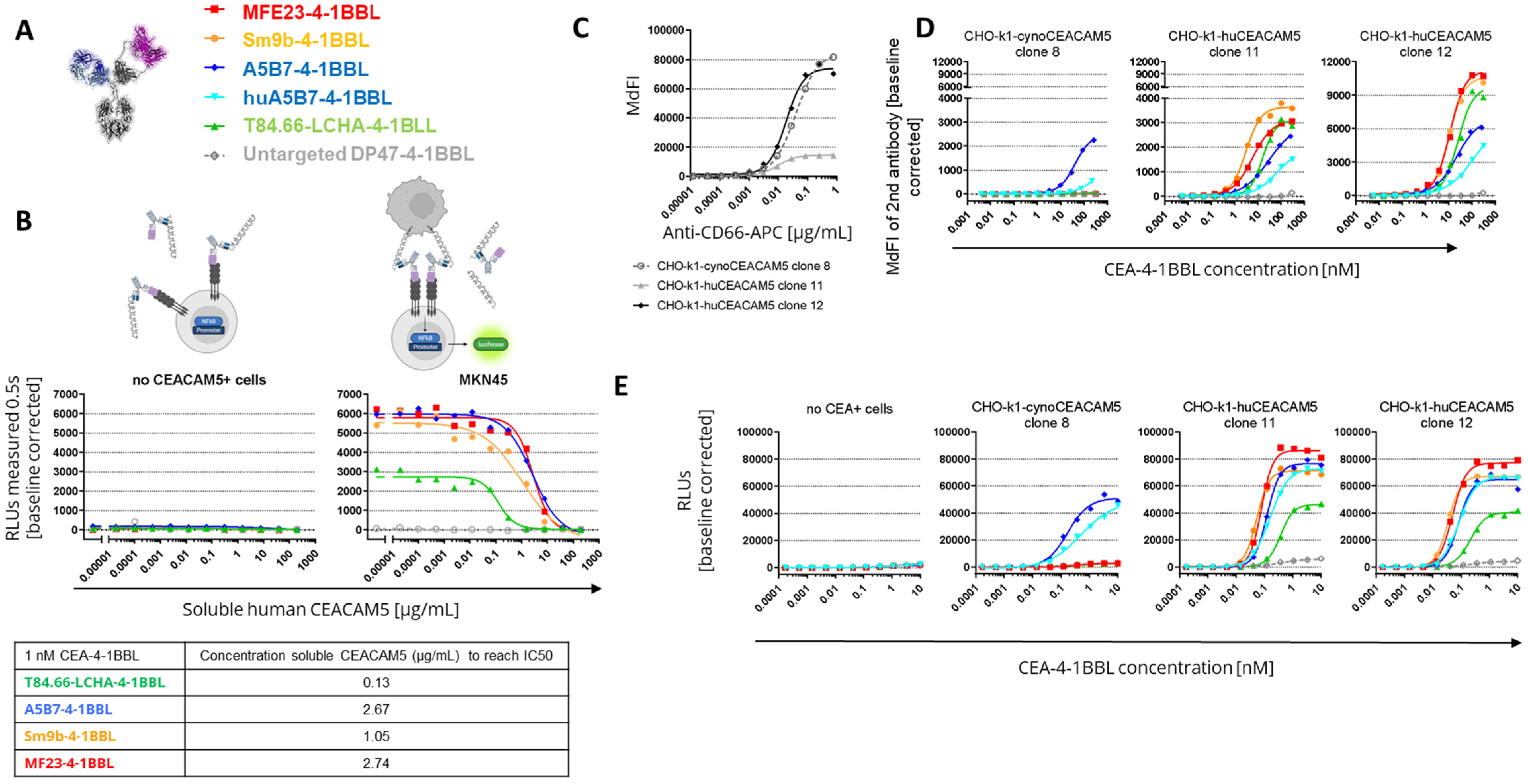
3.2. CEA-4-1BBL In Vitro Characterization
3.3. Cynomolgus Cross-Reactivity
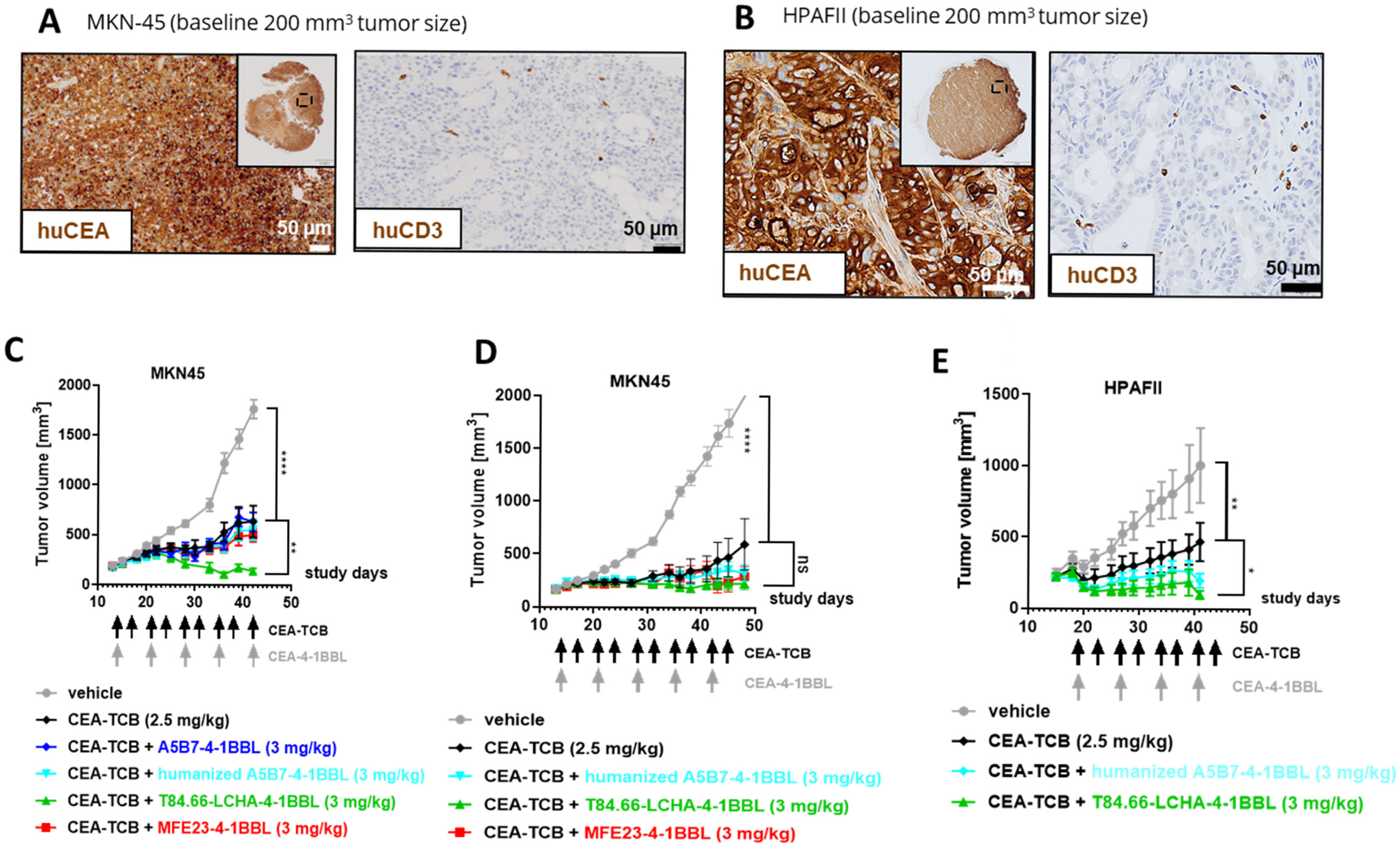
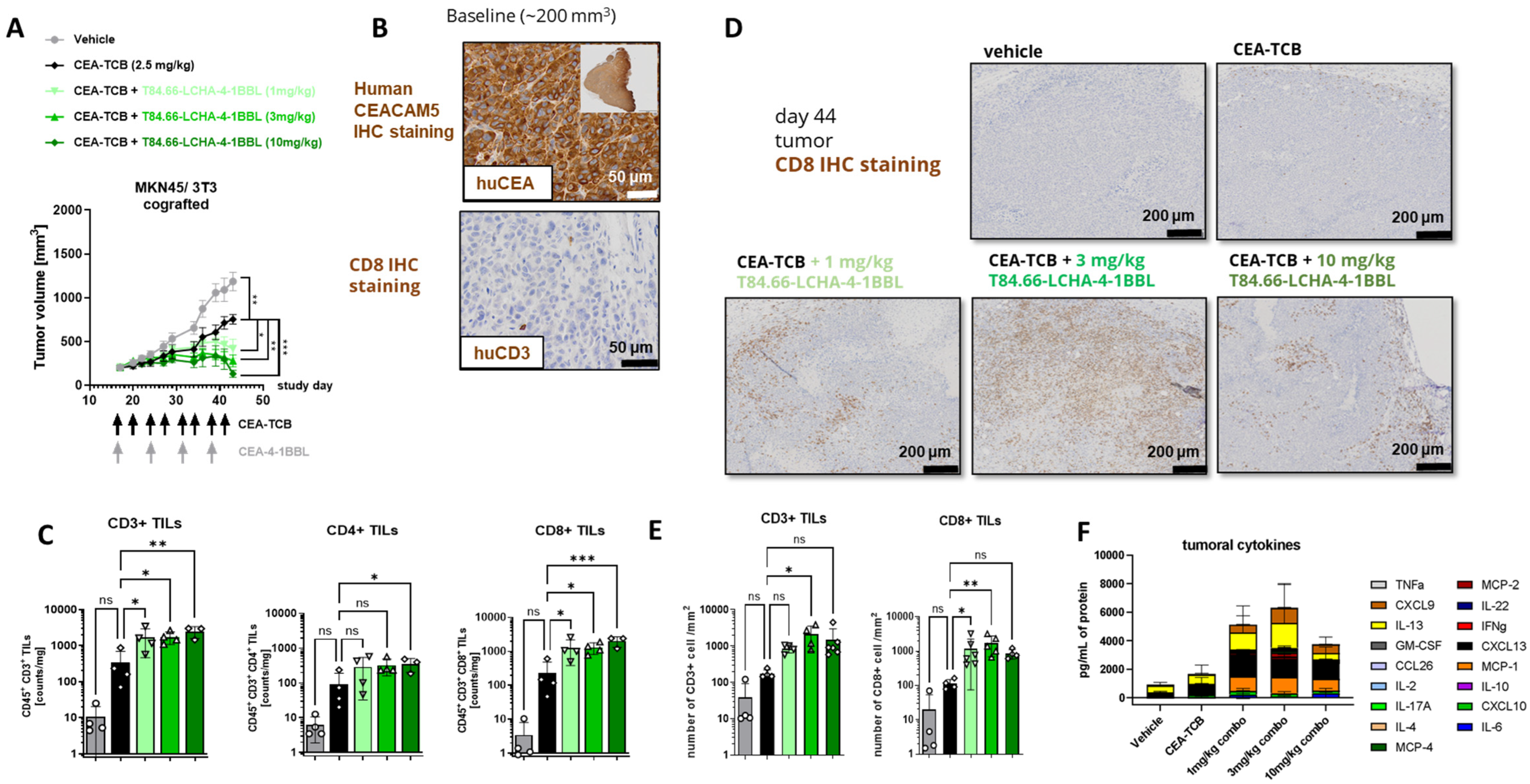
3.4. In Vivo Efficacy Studies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CEA | Carcinoembryonic Antigen 5 |
| CEACAM5 | Carcinoembryonic Antigen Cell Adhesion Molecule 5 |
| FAP | Fibroblast Activation Protein |
| MdFI | Median Fluorescence Intensity |
| PBMC | Peripheral Blood Mononuclear Cell |
| PET | Positron Emission Tomography |
| SPR | Surface Plasmon Resonance |
| TCB | T Cell Bispecific Antibody |
| TCR | T Cell Receptor |
References
- Chan, A.C.; Martyn, G.D.; Carter, P.J. Fifty Years of Monoclonals: The Past, Present and Future of Antibody Therapeutics. Nat. Rev. Immunol. 2025, 25, 745–765. [Google Scholar] [CrossRef]
- Klein, C.; Brinkmann, U.; Reichert, J.M.; Kontermann, R.E. The Present and Future of Bispecific Antibodies for Cancer Therapy. Nat. Rev. Drug Discov. 2024, 23, 301–319. [Google Scholar] [CrossRef]
- Surowka, M.; Klein, C. A Pivotal Decade for Bispecific Antibodies? mAbs 2024, 16, 2321635. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific Antibodies: A Mechanistic Review of the Pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef]
- Arvedson, T.; Bailis, J.M.; Britten, C.D.; Klinger, M.; Nagorsen, D.; Coxon, A.; Egen, J.G.; Martin, F. Targeting Solid Tumors with Bispecific T Cell Engager Immune Therapy. Annu. Rev. Cancer Biol. 2022, 6, 17–34. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Wesche, H. T-Cell-Engaging Antibodies for the Treatment of Solid Tumors: Challenges and Opportunities. Curr. Opin. Oncol. 2022, 34, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Middelburg, J.; Kemper, K.; Engelberts, P.; Labrijn, A.F.; Schuurman, J.; van Hall, T. Overcoming Challenges for CD3-Bispecific Antibody Therapy in Solid Tumors. Cancers 2021, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Leclercq-Cohen, G.; Klein, C.; Sorrentino, A.; Bacac, M. Mechanistic Insights into Resistance Mechanisms to T Cell Engagers. Front. Immunol. 2025, 16, 1583044. [Google Scholar] [CrossRef] [PubMed]
- Oostindie, S.C.; Lazar, G.A.; Schuurman, J.; Parren, P.W.H.I. Avidity in Antibody Effector Functions and Biotherapeutic Drug Design. Nat. Rev. Drug Discov. 2022, 21, 715–735. [Google Scholar] [CrossRef]
- Claus, C.; Ferrara-Koller, C.; Klein, C. The Emerging Landscape of Novel 4-1BB (CD137) Agonistic Drugs for Cancer Immunotherapy. mAbs 2023, 15, 2167189. [Google Scholar] [CrossRef]
- Lotze, M.T.; Olejniczak, S.H.; Skokos, D. CD28 Co-Stimulation: Novel Insights and Applications in Cancer Immunotherapy. Nat. Rev. Immunol. 2024, 24, 878–895. [Google Scholar] [CrossRef]
- Olejniczak, S.H.; Lotze, M.T.; Skokos, D. Second Signals for Cancer Immunotherapy. J. Immunother. Cancer 2025, 13, e010530. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Etxeberría, I.; Otano, I.; Melero, I. Twists and Turns to Translating 4-1BB Cancer Immunotherapy. Sci. Transl. Med. 2019, 11, eaax4738. [Google Scholar] [CrossRef]
- Watts, T.H.; Yeung, K.K.M.; Yu, T.; Lee, S.; Eshraghisamani, R. TNF/TNFR Superfamily Members in Costimulation of T Cell Responses-Revisited. Annu. Rev. Immunol. 2025, 43, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Eguren-Santamaría, I.; Sanmamed, M.F.; Molero-Glez, P.; Perez-Gracia, J.L.; Melero, I. Targeting T-Cell Costimulation to the Surface of Tumor Cells. Clin. Cancer Res. 2024, 31, 231–233. [Google Scholar] [CrossRef]
- Piha-Paul, S.; Olwill, S.A.; Hamilton, E.; Tolcher, A.; Pohlmann, P.; Liu, S.V.; Wurzenberger, C.; Hasenkamp, L.-C.; Hansbauer, E.-M.; Shroff, R.; et al. A First-in-Human Study of Cinrebafusp Alfa, a HER2/4-1BB Bispecific Molecule, in Patients with HER2-Positive Advanced Solid Malignancies. Clin. Cancer Res. 2025, 31, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.; Shen, L.; Dayyani, F.; Kratz, J.; Liang, X.; Liu, F.; Wang, Z.; Feller, L.; Girda, E.; Pan, H.; et al. A First-in-Human Study of Givastomig, a CLDN18.2 and 4-1BB Bispecific Antibody, as Monotherapy in Patients with CLDN18.2-Positive Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2025, OF1–OF9. [Google Scholar] [CrossRef]
- Muik, A.; Garralda, E.; Altintas, I.; Gieseke, F.; Geva, R.; Ben-Ami, E.; Maurice-Dror, C.; Calvo, E.; LoRusso, P.M.; Alonso, G.; et al. Preclinical Characterization and Phase I Trial Results of a Bispecific Antibody Targeting PD-L1 and 4-1BB (GEN1046) in Patients with Advanced Refractory Solid Tumors. Cancer Discov. 2022, 12, 1248–1265. [Google Scholar] [CrossRef]
- Pretelli, G.; Garralda, E. Rewiring Antitumor Immunity: Targeting CLDN18.2 with Conditional 4-1BB Activation. Clin. Cancer Res. 2025, OF1–OF3. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Tanos, T.; Bustamante, M.; Sanmamed, M.F.; Calvo, E.; Moreno, I.; Moreno, V.; Hernandez, T.; Martinez Garcia, M.; Rodriguez-Vida, A.; et al. A First-in-Human Study of the Fibroblast Activation Protein-Targeted, 4-1BB Agonist RO7122290 in Patients with Advanced Solid Tumors. Sci. Transl. Med. 2023, 15, eabp9229. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, S.; Fuks, A.; Jothy, S.; Beauchemin, N.; Shirota, K.; Stanners, C.P. Carcinoembryonic Antigen, a Human Tumor Marker, Functions as an Intercellular Adhesion Molecule. Cell 1989, 57, 327–334. [Google Scholar] [CrossRef]
- Thomas, J.; Klebanov, A.; John, S.; Miller, L.S.; Vegesna, A.; Amdur, R.L.; Bhowmick, K.; Mishra, L. CEACAMS 1, 5, and 6 in Disease and Cancer: Interactions with Pathogens. Genes Cancer 2023, 14, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukarasu, P.; Sukumar, S.; Sathaiah, M.; Mahan, M.; Pragatheeshwar, K.D.; Pingpank, J.F.; Zeh, H.; Bartels, C.J.; Lee, K.K.W.; Bartlett, D.L. C-Stage in Colon Cancer: Implications of Carcinoembryonic Antigen Biomarker in Staging, Prognosis, and Management. J. Natl. Cancer Inst. 2011, 103, 689–697. [Google Scholar] [CrossRef]
- Claus, C.; Ferrara, C.; Xu, W.; Sam, J.; Lang, S.; Uhlenbrock, F.; Albrecht, R.; Herter, S.; Schlenker, R.; Hüsser, T.; et al. Tumor-Targeted 4-1BB Agonists for Combination with T Cell Bispecific Antibodies as off-the-Shelf Therapy. Sci. Transl. Med. 2019, 11, eaav5989. [Google Scholar] [CrossRef] [PubMed]
- Trüb, M.; Uhlenbrock, F.; Claus, C.; Herzig, P.; Thelen, M.; Karanikas, V.; Bacac, M.; Amann, M.; Albrecht, R.; Ferrara-Koller, C.; et al. Fibroblast Activation Protein-Targeted-4-1BB Ligand Agonist Amplifies Effector Functions of Intratumoral T Cells in Human Cancer. J. Immunother. Cancer 2020, 8, e000238. [Google Scholar] [CrossRef] [PubMed]
- Bacac, M.; Colombetti, S.; Herter, S.; Sam, J.; Perro, M.; Chen, S.; Bianchi, R.; Richard, M.; Schoenle, A.; Nicolini, V.; et al. CD20-TCB with Obinutuzumab Pretreatment as Next-Generation Treatment of Hematologic Malignancies. Clin. Cancer Res. 2018, 24, 4785–4797. [Google Scholar] [CrossRef]
- Bacac, M.; Fauti, T.; Sam, J.; Colombetti, S.; Weinzierl, T.; Ouaret, D.; Bodmer, W.; Lehmann, S.; Hofer, T.; Hosse, R.J.; et al. A Novel Carcinoembryonic Antigen T-Cell Bispecific Antibody (CEA TCB) for the Treatment of Solid Tumors. Clin. Cancer Res. 2016, 22, 3286–3297. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Perera, R.; Grimm, H.-P.; Sam, J.; Colombetti, S.; Fauti, T.; Fahrni, L.; Schaller, T.; Freimoser-Grundschober, A.; Zielonka, J.; et al. In Vivo Fluorescence Imaging of the Activity of CEA TCB, a Novel T-Cell Bispecific Antibody, Reveals Highly Specific Tumor Targeting and Fast Induction of T-Cell-Mediated Tumor Killing. Clin. Cancer Res. 2016, 22, 4417–4427. [Google Scholar] [CrossRef]
- Griessinger, C.M.; Olafsen, T.; Mascioni, A.; Jiang, Z.K.; Zamilpa, C.; Jia, F.; Torgov, M.; Romero, J.M.; Marchioni, F.; Satpayev, D.; et al. The PET-Tracer 89Zr-Df-IAB22M2C Enables Monitoring of Intratumoral CD8 T-Cell Infiltrates in Tumor-Bearing Humanized Mice after T-Cell Bispecific Antibody Treatment. Cancer Res. 2020, 80, 2903–2913. [Google Scholar] [CrossRef]
- Otano, I.; Azpilikueta, A.; Glez-Vaz, J.; Alvarez, M.; Medina-Echeverz, J.; Cortés-Domínguez, I.; Ortiz-de-Solorzano, C.; Ellmark, P.; Fritzell, S.; Hernandez-Hoyos, G.; et al. CD137 (4-1BB) Costimulation of CD8+ T Cells Is More Potent When Provided in Cis than in Trans with Respect to CD3-TCR Stimulation. Nat. Commun. 2021, 12, 7296. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, L.; Gu, X.; Zhao, J.; Bi, J.; Pan, L. Leveraging T Cell Co-Stimulation for Enhanced Therapeutic Efficacy of Trispecific Antibodies Targeting Prostate Cancer. J. Immunother. Cancer 2025, 13, e010140. [Google Scholar] [CrossRef] [PubMed]
- Kamikawa, T.; Kimura, N.; Ishii, S.; Muraoka, M.; Kodama, T.; Taniguchi, K.; Yoshimoto, M.; Miura-Okuda, M.; Uchikawa, R.; Kato, C.; et al. SAIL66, a next Generation CLDN6-Targeting T-Cell Engager, Demonstrates Potent Antitumor Efficacy through Dual Binding to CD3/CD137. J. Immunother. Cancer 2024, 12, e009563. [Google Scholar] [CrossRef]
- Mikami, H.; Feng, S.; Matsuda, Y.; Ishii, S.; Naoi, S.; Azuma, Y.; Nagano, H.; Asanuma, K.; Kayukawa, Y.; Tsunenari, T.; et al. Engineering CD3/CD137 Dual Specificity into a DLL3-Targeted T-Cell Engager Enhances T-Cell Infiltration and Efficacy against Small-Cell Lung Cancer. Cancer Immunol. Res. 2024, 12, 719–730. [Google Scholar] [CrossRef]
- Carter, P. Bispecific Human IgG by Design. J. Immunol. Methods 2001, 248, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, W.; Regula, J.T.; Bähner, M.; Schanzer, J.; Croasdale, R.; Dürr, H.; Gassner, C.; Georges, G.; Kettenberger, H.; Imhof-Jung, S.; et al. Immunoglobulin Domain Crossover as a Generic Approach for the Production of Bispecific IgG Antibodies. Proc. Natl. Acad. Sci. USA 2011, 108, 11187–11192. [Google Scholar] [CrossRef]
- Regula, J.T.; Imhof-Jung, S.; Mølhøj, M.; Benz, J.; Ehler, A.; Bujotzek, A.; Schaefer, W.; Klein, C. Variable Heavy-Variable Light Domain and Fab-Arm CrossMabs with Charged Residue Exchanges to Enforce Correct Light Chain Assembly. Protein Eng. Des. Sel. 2018, 31, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Schlothauer, T.; Herter, S.; Koller, C.F.; Grau-Richards, S.; Steinhart, V.; Spick, C.; Kubbies, M.; Klein, C.; Umaña, P.; Mössner, E. Novel Human IgG1 and IgG4 Fc-Engineered Antibodies with Completely Abolished Immune Effector Functions. Protein Eng. Des. Sel. 2016, 29, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.; Magana, P.; Nair, S.; Tsenkov, M.; Bertoni, D.; Pidruchna, I.; Lima Afonso, M.Q.; Midlik, A.; Paramval, U.; Žídek, A.; et al. AlphaFold Protein Structure Database and 3D-Beacons: New Data and Capabilities. J. Mol. Biol. 2025, 437, 168967. [Google Scholar] [CrossRef]
- Korotkova, N.; Yang, Y.; Le Trong, I.; Cota, E.; Demeler, B.; Marchant, J.; Thomas, W.E.; Stenkamp, R.E.; Moseley, S.L.; Matthews, S. Binding of Dr Adhesins of Escherichia Coli to Carcinoembryonic Antigen Triggers Receptor Dissociation. Mol. Microbiol. 2008, 67, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Duffieux, F.; Gagnaire, M.; Rapisarda, C.; Bertrand, T.; Rak, A. Structural Insights into Epitope-Paratope Interactions of a Monoclonal Antibody Targeting CEACAM5-Expressing Tumors. Nat. Commun. 2024, 15, 9377. [Google Scholar] [CrossRef]
- Bacac, M.; Klein, C.; Umana, P. CEA TCB: A Novel Head-to-Tail 2:1 T Cell Bispecific Antibody for Treatment of CEA-Positive Solid Tumors. Oncoimmunology 2016, 5, e1203498. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, A.; Migueliz, I.; Garasa, S.; Karanikas, V.; Luri, C.; Cirella, A.; Olivera, I.; Cañamero, M.; Alvarez, M.; Ochoa, M.C.; et al. Three-Dimensional Colon Cancer Organoids Model the Response to CEA-CD3 T-Cell Engagers. Theranostics 2022, 12, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Neumaier, M.; Shively, L.; Chen, F.S.; Gaida, F.J.; Ilgen, C.; Paxton, R.J.; Shively, J.E.; Riggs, A.D. Cloning of the Genes for T84.66, an Antibody That Has a High Specificity and Affinity for Carcinoembryonic Antigen, and Expression of Chimeric Human/Mouse T84.66 Genes in Myeloma and Chinese Hamster Ovary Cells. Cancer Res. 1990, 50, 2128–2134. [Google Scholar]
- Yazaki, P.J.; Sherman, M.A.; Shively, J.E.; Ikle, D.; Williams, L.E.; Wong, J.Y.C.; Colcher, D.; Wu, A.M.; Raubitschek, A.A. Humanization of the Anti-CEA T84.66 Antibody Based on Crystal Structure Data. Protein Eng. Des. Sel. 2004, 17, 481–489. [Google Scholar] [CrossRef]
- Harwood, P.J.; Britton, D.W.; Southall, P.J.; Boxer, G.M.; Rawlins, G.; Rogers, G.T. Mapping Epitope Characteristics on Carcinoembryonic Antigen. Br. J. Cancer 1986, 54, 75–82. [Google Scholar] [CrossRef]
- Chester, K.A.; Robson, L.; Keep, P.A.; Pedley, R.B.; Boden, J.A.; Boxer, G.M.; Hawkins, R.E.; Begent, R.H. Production and Tumour-Binding Characterization of a Chimeric Anti-CEA Fab Expressed in Escherichia Coli. Int. J. Cancer 1994, 57, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.K.; Corper, A.L.; Wan, T.; Sohi, M.K.; Sutton, B.J.; Thornton, J.D.; Keep, P.A.; Chester, K.A.; Begent, R.H.; Perkins, S.J. Crystal Structure of the Anti-(Carcinoembryonic Antigen) Single-Chain Fv Antibody MFE-23 and a Model for Antigen Binding Based on Intermolecular Contacts. Biochem. J. 2000, 346, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Chester, K.A.; Begent, R.H.; Robson, L.; Keep, P.; Pedley, R.B.; Boden, J.A.; Boxer, G.; Green, A.; Winter, G.; Cochet, O. Phage Libraries for Generation of Clinically Useful Antibodies. Lancet 1994, 343, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Graff, C.P.; Chester, K.; Begent, R.; Wittrup, K.D. Directed Evolution of an Anti-Carcinoembryonic Antigen scFv with a 4-Day Monovalent Dissociation Half-Time at 37 Degrees C. Protein Eng. Des. Sel. 2004, 17, 293–304. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hirakawa, E.; Mori, S.; Hamada, Y.; Kawaguchi, N.; Matsuura, N. Cleavage of Carcinoembryonic Antigen Induces Metastatic Potential in Colorectal Carcinoma. Biochem. Biophys. Res. Commun. 2005, 333, 223–229. [Google Scholar] [CrossRef]
- Conaghan, P.; Ashraf, S.; Tytherleigh, M.; Wilding, J.; Tchilian, E.; Bicknell, D.; Mortensen, N.J.; Bodmer, W. Targeted Killing of Colorectal Cancer Cell Lines by a Humanised IgG1 Monoclonal Antibody That Binds to Membrane-Bound Carcinoembryonic Antigen. Br. J. Cancer 2008, 98, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Wild, N.; Andres, H.; Rollinger, W.; Krause, F.; Dilba, P.; Tacke, M.; Karl, J. A Combination of Serum Markers for the Early Detection of Colorectal Cancer. Clin. Cancer Res. 2010, 16, 6111–6121. [Google Scholar] [CrossRef] [PubMed]
- Oberst, M.D.; Fuhrmann, S.; Mulgrew, K.; Amann, M.; Cheng, L.; Lutterbuese, P.; Richman, L.; Coats, S.; Baeuerle, P.A.; Hammond, S.A. CEA/CD3 Bispecific Antibody MEDI-565/AMG 211 Activation of T Cells and Subsequent Killing of Human Tumors Is Independent of Mutations Commonly Found in Colorectal Adenocarcinomas. mAbs 2014, 6, 1571–1584. [Google Scholar] [CrossRef]
- Segal, N.H.; Melero, I.; Moreno, V.; Steeghs, N.; Marabelle, A.; Rohrberg, K.; Rodriguez-Ruiz, M.E.; Eder, J.P.; Eng, C.; Manji, G.A.; et al. CEA-CD3 Bispecific Antibody Cibisatamab with or without Atezolizumab in Patients with CEA-Positive Solid Tumours: Results of Two Multi-Institutional Phase 1 Trials. Nat. Commun. 2024, 15, 4091. [Google Scholar] [CrossRef]
- Kopetz, S.; Boni, V.; Kato, K.; Raghav, K.P.S.; Vieito, M.; Pallis, A.; Habermehl, C.; Siddiqui, A.; Courlet, P.; Sloot, W.; et al. Precemtabart Tocentecan, an Anti-CEACAM5 Antibody-Drug Conjugate, in Metastatic Colorectal Cancer: A Phase 1 Trial. Nat. Med. 2025, 31, 3504–3513. [Google Scholar] [CrossRef] [PubMed]
- Stern, N.; Markel, G.; Arnon, T.I.; Gruda, R.; Wong, H.; Gray-Owen, S.D.; Mandelboim, O. Carcinoembryonic Antigen (CEA) Inhibits NK Killing via Interaction with CEA-Related Cell Adhesion Molecule 1. J. Immunol. 2005, 174, 6692–6701. [Google Scholar] [CrossRef]
- Roda, G.; Jianyu, X.; Park, M.S.; DeMarte, L.; Hovhannisyan, Z.; Couri, R.; Stanners, C.P.; Yeretssian, G.; Mayer, L. Characterizing CEACAM5 Interaction with CD8α and CD1d in Intestinal Homeostasis. Mucosal Immunol. 2014, 7, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Tsang, Y.; Yang, Y. Identification of CEACAM5 as a Stemness-Related Inhibitory Immune Checkpoint in Pancreatic Cancer. BMC Cancer 2022, 22, 1291. [Google Scholar] [CrossRef] [PubMed]
- Bluemel, C.; Hausmann, S.; Fluhr, P.; Sriskandarajah, M.; Stallcup, W.B.; Baeuerle, P.A.; Kufer, P. Epitope Distance to the Target Cell Membrane and Antigen Size Determine the Potency of T Cell-Mediated Lysis by BiTE Antibodies Specific for a Large Melanoma Surface Antigen. Cancer Immunol. Immunother. 2010, 59, 1197–1209. [Google Scholar] [CrossRef]
- Li, J.; Stagg, N.J.; Johnston, J.; Harris, M.J.; Menzies, S.A.; DiCara, D.; Clark, V.; Hristopoulos, M.; Cook, R.; Slaga, D.; et al. Membrane-Proximal Epitope Facilitates Efficient T Cell Synapse Formation by Anti-FcRH5/CD3 and Is a Requirement for Myeloma Cell Killing. Cancer Cell 2017, 31, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, F.; Wang, C.; Narula, J.; Pascua, E.; Ni, I.; Ding, S.; Deng, X.; Chu, M.L.-H.; Pham, A.; et al. One Size Does Not Fit All: Navigating the Multi-Dimensional Space to Optimize T-Cell Engaging Protein Therapeutics. mAbs 2021, 13, 1871171. [Google Scholar] [CrossRef] [PubMed]
| Anti-CEACAM5 Fab | Human CEACAM5 NABA Construct | ka (1/Ms) | kd (1/s) | KD (M) | SD |
|---|---|---|---|---|---|
| MFE23 | huCEACAM1(A2,B1)-huCEACAM5(N, A1) avi His | 9.2 × 104 | 1.1 × 10−3 | 1.2 × 10−8 | 1.3 × 10−9 |
| Sm9b | huCEACAM1(A2,B1)-huCEACAM5(N, A1) avi His | 8.1 × 104 | 9.0 × 10−4 | 1.1 × 10−8 | 1.3 × 10−9 |
| A5B7 | huCEACAM1(N,A2)-huCEACAM5(A2,B2) avi His | 3.6 × 105 | 3.1 × 10−4 | 8.6 × 10−10 | 5 × 10−11 |
| huA5B7 | huCEACAM1(N,A2)-huCEACAM5(A2,B2) avi His | 1.9 × 105 | 4.6 × 10−4 | 2.5 × 10−9 | 5.5 × 10−11 |
| T84.66-LCHA | huCEACAM1(N,A2)-huCEACAM5(A3,B3) avi His | 8.9 × 105 | 1.1 × 10−4 | 1.2 × 10−10 | 3.0 × 10−11 |
| CH1A1A 98/99 × 2F1 | huCEACAM1(N,A2)-huCEACAM5(A3,B3) avi His | 9.0 × 105 | 8.1 × 10−3 | 9.1 × 10−9 | 7.9 × 10−10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claus, C.; Ferrara-Koller, C.; Sam, J.; Lang, S.; Albrecht, R.; Buser, R.B.; Bommer, E.; Sala, G.L.; Nicolini, V.G.; Colombetti, S.; et al. CEA-4-1BBL: CEACAM5-Targeted 4-1BB Ligand Fusion Proteins for Cis Co-Stimulation with CEA-TCB. Antibodies 2025, 14, 96. https://doi.org/10.3390/antib14040096
Claus C, Ferrara-Koller C, Sam J, Lang S, Albrecht R, Buser RB, Bommer E, Sala GL, Nicolini VG, Colombetti S, et al. CEA-4-1BBL: CEACAM5-Targeted 4-1BB Ligand Fusion Proteins for Cis Co-Stimulation with CEA-TCB. Antibodies. 2025; 14(4):96. https://doi.org/10.3390/antib14040096
Chicago/Turabian StyleClaus, Christina, Claudia Ferrara-Koller, Johannes Sam, Sabine Lang, Rosmarie Albrecht, Regula B. Buser, Esther Bommer, Grégory La Sala, Valeria G. Nicolini, Sara Colombetti, and et al. 2025. "CEA-4-1BBL: CEACAM5-Targeted 4-1BB Ligand Fusion Proteins for Cis Co-Stimulation with CEA-TCB" Antibodies 14, no. 4: 96. https://doi.org/10.3390/antib14040096
APA StyleClaus, C., Ferrara-Koller, C., Sam, J., Lang, S., Albrecht, R., Buser, R. B., Bommer, E., Sala, G. L., Nicolini, V. G., Colombetti, S., Bacac, M., Umaña, P., & Klein, C. (2025). CEA-4-1BBL: CEACAM5-Targeted 4-1BB Ligand Fusion Proteins for Cis Co-Stimulation with CEA-TCB. Antibodies, 14(4), 96. https://doi.org/10.3390/antib14040096








