Influence and Role of Regulatory B Cells in Organ Transplantation: The State of the Art, Prospects, and Emerging Insights
Abstract
1. Introduction
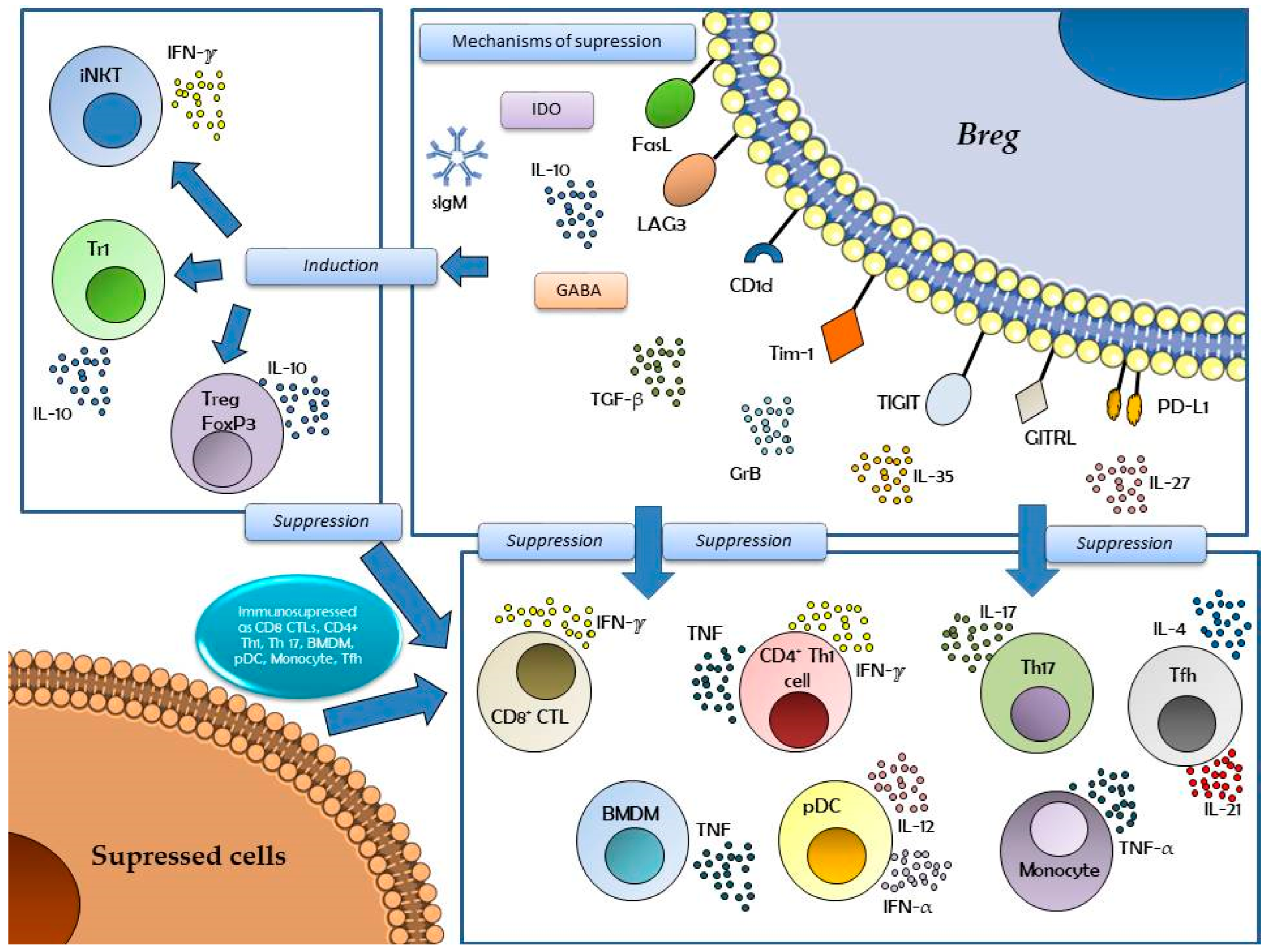
2. Phenotypes of Regulatory B Cells
3. Regulatory B Cells in Several Pathologies
4. Regulatory B Cells in Kidney Transplantation
5. Regulatory B Cells in the Transplantation of Other Solid Organs
6. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABMR | Antibody-mediated rejection |
| AMR | Antibody-mediated rejection |
| APRIL | A Proliferation-Inducing Ligand |
| BAFF | B-cell Activating Factor |
| BCR | B-cell receptor |
| BMDM | Bone Marrow-Derived Macrophage |
| Bregs | Regulatory B cells |
| BR1 | IL-10–producing Regulatory B Cells that promote IgG4 |
| CD | Cluster of Differentiation |
| CF | Flow Cytometry |
| CTL | Cytotoxic T Lymphocyte |
| DC/DCs | Dendritic Cell(s) |
| DSA | Donor-specific antibodies |
| expBregs | Expanded Regulatory B Cells |
| FasL | Fas Ligand |
| FO | Follicular B Cell |
| Foxp3 | Forkhead box P3 (Treg transcription factor) |
| GABA | Gamma-Aminobutyric Acid |
| GITRL | GIT Receptor Ligand (G protein-coupled receptor kinase-interacting protein receptor ligand) |
| GrB | Granzyme B |
| HBV | Hepatitis B Virus |
| HLA | Human Leukocyte Antigens |
| IDO | Indoleamine 2,3-Dioxygenase |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IgD | Immunoglobulin D |
| IgE | Immunoglobulin E |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IL | Interleukin |
| iNKT | Invariant Natural Killer T |
| ITIM | Immunoreceptor Tyrosine-based Inhibition Motif |
| LAG3 | Lymphocyte Activation Gene 3 |
| MZ | Marginal Zone (B Cell) |
| MZP | Marginal Zone Precursor (B Cell) |
| PBMC | Peripheral Blood Mononuclear Cell |
| PC | Plasma Cell |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| pDC | Plasmacytoid Dendritic Cell |
| SLE | Systemic Lupus Erythematosus |
| T2-MZP | Transitional-2 Marginal Zone Precursor |
| TCR | T-cell receptor |
| Tfh | T Follicular Helper (Cell) |
| TGF | Transforming Growth Factor |
| Th | T Helper (Cell) |
| TIGIT | T Cell Immunoreceptor with Ig and ITIM Domains |
| Tim-1 | T-cell Immunoglobulin and Mucin Domain 1 |
| TLR | Toll-like receptors |
| TNF | Tumor Necrosis Factor |
| Tr1 | Type 1 Regulatory T Cell |
| Tregs | Regulatory T Cells |
| US | United States |
References
- Song, Y.; Wang, Y.; Wang, W.; Xie, Y.; Zhang, J.; Liu, J.; Jin, Q.; Wu, W.; Li, H.; Wang, J.; et al. Advancements in noninvasive techniques for transplant rejection: From biomarker detection to molecular imaging. J. Transl. Med. 2025, 23, 147. [Google Scholar] [CrossRef]
- Tamargo, C.L.; Kant, S. Pathophysiology of Rejection in Kidney Transplantation. J. Clin. Med. 2023, 12, 4130. [Google Scholar] [CrossRef]
- Terasaki, P.I. Humoral theory of transplantation. Am. J. Transplant. 2003, 3, 665–673. [Google Scholar] [CrossRef]
- Alasfar, S.; Kodali, L.; Schinstock, C.A. Current Therapies in Kidney Transplant Rejection. J. Clin. Med. 2023, 12, 4927. [Google Scholar] [CrossRef]
- Poggio, E.D.; Augustine, J.J.; Arrigain, S.; Brennan, D.C.; Schold, J.D. Long-term kidney transplant graft survival-Making progress when most needed. Am. J. Transplant. 2021, 21, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Veh, J.; Ludwig, C.; Schrezenmeier, H.; Jahrsdörfer, B. Regulatory B Cells-Immunopathological and Prognostic Potential in Humans. Cells 2024, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, M.R.; Watson, C.J.; Plotnek, G.; Bardsley, V.; Chaudhry, A.N.; Bradley, J.A.; Smith, K.G. B-cell-depleting induction therapy and acute cellular rejection. N. Engl. J. Med. 2009, 360, 2683–2685. [Google Scholar] [CrossRef] [PubMed]
- Starling, R.C.; Armstrong, B.; Bridges, N.D.; Eisen, H.; Givertz, M.M.; Kfoury, A.G.; Kobashigawa, J.; Ikle, D.; Morrison, Y.; Pinney, S.; et al. CTOT-11 Study Investigators. Accelerated Allograft Vasculopathy with Rituximab After Cardiac Transplantation. J. Am. Coll. Cardiol. 2019, 74, 36–51. [Google Scholar] [CrossRef]
- Baba, Y.; Matsumoto, M.; Kurosaki, T. Signals controlling the development and activity of regulatory B-lineage cells. Int. Immunol. 2015, 27, 487–493. [Google Scholar] [CrossRef]
- Abebe, E.C.; Dejenie, T.A.; Ayele, T.L.; Baye, N.D.; Teshome, A.A.; Muche, Z.T. The Role of Regulatory B Cells in Health and Diseases: A Systemic Review. J. Inflamm. Res. 2021, 14, 75–84. [Google Scholar] [CrossRef]
- Bradford, H.F.; Mauri, C. Diversity of regulatory B cells: Markers and functions. Eur. J. Immunol. 2024, 54, 2350496. [Google Scholar] [CrossRef]
- Ahsan, N.F.; Lourenço, S.; Psyllou, D.; Long, A.; Shankar, S.; Bashford-Rogers, R. The current understanding of the phenotypic and functional properties of human regulatory B cells (Bregs). Oxf. Open Immunol. 2024, 5, iqae012. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Liao, J.; Yang, Y.; Li, J.; Liu, Z.; Song, S.; Zeng, Y.; Wang, Y. Regulatory B cells, the key regulator to induce immune tolerance in organ transplantation. Front. Immunol. 2025, 16, 1561171. [Google Scholar] [CrossRef]
- Alfaro, R.; Legaz, I.; González-Martínez, G.; Jimenez-Coll, V.; Martínez-Banaclocha, H.; Galián, J.A.; Botella, C.; de la Peña-Moral, J.; Moya-Quiles, M.R.; Campillo, J.A.; et al. Monitoring of B Cell in Kidney Transplantation: Development of a Novel Clusters Analysis and Role of Transitional B Cells in Transplant Outcome. Diagnostics 2021, 11, 641. [Google Scholar] [CrossRef]
- Jansen, K.; Cevhertas, L.; Ma, S.; Satitsuksanoa, P.; Akdis, M.; van de Veen, W.; O’Hehir, R. Regulatory B cells, A to Z. Allergy 2021, 76, 2699–2715. [Google Scholar] [CrossRef]
- Catalán, D.; Mansilla, M.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillón, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef]
- Menon, M.; Hussell, T.; Ali, S. Regulatory B cells in respiratory health and diseases. Immunol. Rev. 2021, 299, 61–73. [Google Scholar] [CrossRef]
- Khan, A.R.; Hams, E.; Floudas, A.; Sparwasser, T.; Weaver, C.T.; Falhon, P.G. PD-L1hi B cells are critical regulators of humoral immunity. Nat. Commun. 2015, 6, 5997. [Google Scholar] [CrossRef]
- Blair, P.A.; Noreña, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef]
- Flores-Borja, F.; Bosma, A.; Ng, D.; Reddy, V.; Ehrenstein, M.R.; Isenberg, D.A.; Mauri, C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl. Med. 2013, 5, 173ra23. [Google Scholar] [CrossRef] [PubMed]
- Duddy, M.; Niino, M.; Adatia, F.; Hebert, S.; Freedman, M.; Atkins, H.; Kim, H.J.; Bar-Or, A. Distinct effector cytokine profiles ofmemory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 2007, 178, 6092–6099. [Google Scholar] [CrossRef]
- Iwata, Y.; Matsushita, T.; Horikawa, M.; DiLillo, D.J.; Yanaba, K.; Venturi, G.M.; Szabolcs, P.M.; Bernstein, S.H.; Magro, C.M.; Williams, A.D.; et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011, 117, 530–541. [Google Scholar] [CrossRef]
- Matsumoto, M.; Baba, A.; Yokota, T.; Nishikawa, H.; Ohkawa, Y.; Kayama, H.; Kallies, A.; Nutt, S.L.; Sakaguchi, S.; Takeda, K.; et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 2014, 41, 1040–1051. [Google Scholar] [CrossRef]
- Rojas, O.L.; Pröbstel, A.-K.; Porfilio, E.A.; Wang, A.A.; Charabati, M.; Sun, T.; Lee, D.S.; Galicia, G.; Ramaglia, V.; Ward, L.A.; et al. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell 2019, 176, 610–624. [Google Scholar] [CrossRef]
- Pröbstel, A.K.; Zhou, X.; Baumann, R.; Wischnewski, S.; Kutza, M.; Rojas, O.L.; Sellrie, K.; Bischof, A.; Kim, K.; Ramesh, A.; et al. Gut microbiota–specific IGA+ B cells traffic to the CNS in active multiple sclerosis. Sci. Immunol. 2020, 5, eabc7191. [Google Scholar] [CrossRef]
- Fehres, C.M.; van Uden, N.O.; Yeremenko, N.G.; Fernandez, L.; Salinas, G.F.; van Duivenvoorde, L.M.; Huard, B.; Morel, J.; Spits, H.; Hahne, M.; et al. APRIL induces a novel subset of IGA+ regulatory B cells that suppress inflammation via expression of IL-10 and PD-L1. Front Immunol. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Lindner, S.; Dahlke, K.; Sontheimer, K.; Hagn, M.; Kaltenmeier, C.; Barth, T.F.; Beyer, T.; Reister, F.; Fabricius, D.; Lotfi, R.; et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate t cells. Cancer Res. 2013, 73, 2468–2479. [Google Scholar] [CrossRef]
- Bosma, A.; Abdel-Gadir, A.; Isenberg, D.A.; Jury, E.C.; Mauri, C. Lipid antigen presentation by CD1d + B cells is essential for the maintenance of invariant natural killer T cells. Immunity 2012, 36, 477–490. [Google Scholar] [CrossRef]
- Huo, J.L.; Wang, Y.T.; Fu, W.J.; Lu, N.; Liu, Z.S. The promising immune checkpoint LAG-3 in cancer immunotherapy: Frombasic research to clinical application. Front. Immunol. 2022, 13, 956090. [Google Scholar] [CrossRef]
- Zacca, E.R.; Onofrio, L.I.; Acosta, C.D.V.; Ferrero, P.V.; Alonso, S.M.; Ramello, M.C.; Mussano, E.; Onetti, L.; Cadile, I.I.; Stancich, M.I.; et al. PD-L1+ regulatory B cells are significantly decreased in rheumatoid arthritis patients and increase after successful treatment. Front. Immunol. 2018, 9, 2241. [Google Scholar] [CrossRef]
- Tousif, S.; Wang, Y.; Jackson, J.; Hough, K.P.; Strenkowski, J.G.; Athar, M.; Thannickal, V.J.; McCusker, R.H.; Ponnazhagan, S.; Deshane, J.S. Indoleamine 2, 3-dioxygenase promotes aryl hydrocarbon receptor-dependent differentiation of regulatory B cells in lung cancer. Front. Immunol. 2021, 12, 747780. [Google Scholar] [CrossRef]
- Iglesia, M.D.; Parker, J.S.; Hoadley, K.A.; Serody, J.S.; Perou, C.M.; Vincent, B.G. Genomic analysis of immune cell infiltrates across 11 tumor types. J. Natl. Cancer Inst. 2016, 108, djw144. [Google Scholar] [CrossRef]
- Woo, J.R.; A Liss, M.; Muldong, M.T.; Palazzi, K.; Strasner, A.; Ammirante, M.; Varki, N.; Shabaik, A.; Howell, S.; Kane, C.J.; et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J. Transl. Med. 2014, 12, 30. [Google Scholar] [CrossRef]
- Ou, Z.; Wang, Y.; Liu, L.; Li, L.; Yeh, S.; Qi, L.; Chang, C. Tumor microenvironment B cells increase bladder cancer metastasis via modulation of the IL-8/androgen receptor (AR)/MMPs signals. Oncotarget 2015, 6, 26065–26078. [Google Scholar] [CrossRef]
- Mohammed, Z.M.A.; Going, J.J.; Edwards, J.; Elsberger, B.; Mcmillan, D.C. The relationship between lymphocyte subsets and clinicopathological determinants of survival in patients with primary operable invasive ductal breast cancer. Br. J. Cancer 2013, 109, 1676–1684. [Google Scholar] [CrossRef]
- Xiao, X.; Lao, X.M.; Chen, M.M.; Liu, R.X.; Wei, Y.; Ouyang, F.Z.; Chen, D.P.; Zhao, X.-Y.; Zhao, Q.; Li, X.-F.; et al. PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov. 2016, 6, 546–559. [Google Scholar] [CrossRef]
- Mirlekar, B.; Michaud, D.; Lee, S.J.; Kren, N.P.; Harris, C.; Greene, K.; Goldman, E.C.; Gupta, G.P.; Fields, R.C.; Hawkins, W.G.; et al. B cell-derived IL35 drives STAT3-dependent CD8+ T-cell exclusion in pancreatic cancer. Cancer Immunol. Res. 2020, 8, 292–308. [Google Scholar] [CrossRef]
- Mirlekar, B.; Michaud, D.; Searcy, R.; Greene, K.; Pylayeva-Gupta, Y. IL35 hinders endogenous antitumor T-cell immunity and responsiveness to immunotherapy in pancreatic cancer. Cancer Immunol. Res. 2018, 6, 1014–1024. [Google Scholar] [CrossRef]
- Olkhanud, P.B.; Damdinsuren, B.; Bodogai, M.; Gress, R.E.; Sen, R.; Wejksza, K.; Malchinkhuu, E.; Wersto, R.P.; Biragyn, A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T regulatory cells. Cancer Res. 2011, 71, 3505–3515. [Google Scholar] [CrossRef]
- Zhang, Y.; Morgan, R.; Chen, C.; Cai, Y.; Clark, E.; Khan, W.N.; Shin, S.U.; Cho, H.-M.; Al Bayati, A.; Pimentel, A.; et al. Mammary-tumor-educated B cells acquire lap/TGF-β and PDl1 expression and suppress antitumor immune responses. Int. Immunol. 2016, 28, 423–433. [Google Scholar] [CrossRef]
- Shankar, S.; Stolp, J.; Juvet, S.C.; Beckett, J.; Macklin, P.S.; Issa, F.; Hester, J.; Wood, K.J. Ex vivo-expanded human CD19+TIM-1+ regulatory B cells suppress immune responses in vivo and are dependent upon the TIM-1/STAT3 axis. Nat. Commun. 2022, 13, 3121. [Google Scholar] [CrossRef]
- Caka, C.; Sonmez, G.; Cagdas, D. Decoding T and B cell dynamics in inborn errors of immunity: Insights into immune dysfunction. Expert Rev. Clin. Immunol. 2025, 21, 1251–1267. [Google Scholar] [CrossRef]
- Luthers, C.R.; Mittelhauser, A.; Colamartino, A.; Wu, X.; Cirigliano, S.; Long, J.D.; Sanchez, J.M.; Romero, Z.; Kohn, D.B. Hematopoietic stem cell gene therapy for the treatment of X-linked agammaglobulinemia. Mol. Ther. Methods Clin. Dev. 2025, 33, 101555. [Google Scholar] [CrossRef]
- Kader, H.A.; Sabih Ur Rehman, S.; Saraswathiamma, D.; Shiek, S.S.; Bencomo-Hernández, A.A.; Moorakkan, U.R.; Haider, M.T.; Munawar, N.; Iratni, R.; Serfling, E.; et al. NFATc1 deficiency in B cells ameliorates atopic dermatitis. Sci. Rep. 2025, 15, 25170. [Google Scholar] [CrossRef]
- Clement, R.L.; Dilollo, J.; Rodríguez-López, E.M.; Guerrier, C.M.; Hill, D.A. IFNgamma Signaling Impairs Regulatory B Cell Function Resulting in Worse Control of Esophageal Food Allergy. Allergy 2025, 80, 2824–2836. [Google Scholar] [CrossRef]
- Steffen, T.; Ajendra, J.; Koschel, M.; Palmen, A.; Wegner, H.; Risch, F.; Bach, L.; Ritter, M.; Hübner, M.P.; Baumjohann, D. Escalation of Germinal Center Responses in Chronic Litomosoides sigmodontis Filarial Infection. Eur. J. Immunol. 2025, 55, e202451400. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Mu, Q.; Xiong, H.; Liu, M.; Yang, F.; Zhou, L.; Zhou, B. Regulatory B cells in parasitic infections: Roles and therapeutic potential. Parasitol. Res. 2025, 124, 5. [Google Scholar] [CrossRef]
- Sanaei, M.J.; Nahid-Samiei, M.; Abadi, M.S.S.; Arjmand, M.H.; Ferns, G.A.; Bashash, D.; Rahimian, G.; Bagheri, N. New insights into regulatory B cells biology in viral, bacterial, and parasitic infections. Infect. Genet. Evol. 2021, 89, 104753. [Google Scholar] [CrossRef] [PubMed]
- Maerz, J.K.; Trostel, C.; Lange, A.; Parusel, R.; Michaelis, L.; Schäfer, A.; Yao, H.; Löw, H.C.; Frick, J.S. Bacterial Immunogenicity Is Critical for the Induction of Regulatory B Cells in Suppressing Inflammatory Immune Responses. Front. Immunol. 2020, 10, 3093. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, L.S.; Wu, S.D.; Wang, S.Q.; Li, L.; She, W.M.; Li, J.; Wang, J.Y.; Jiang, W. IL-10-producing regulatory B-cells suppressed effector T-cells but enhanced regulatory T-cells in chronic HBV infection. Clin. Sci. 2016, 130, 907–919. [Google Scholar] [CrossRef]
- He, Y.; Qian, H.; Liu, Y.; Duan, L.; Li, Y.; Shi, G. The roles of regulatory B cells in cancer. J. Immunol. Res. 2014, 2014, 215471. [Google Scholar] [CrossRef]
- Han, J.; Sun, L.; Fan, X.; Wang, Z.; Cheng, Y.; Zhu, J.; Jin, T. Role of regulatory b cells in neuroimmunologic disorders. J. Neurosci. Res. 2016, 94, 693–701. [Google Scholar] [CrossRef]
- Zheremyan, E.A.; Ustiugova, A.S.; Karamushka, N.M.; Uvarova, A.N.; Stasevich, E.M.; Bogolyubova, A.V.; Kuprash, D.V.; Korneev, K.V. Breg-Mediated Immunoregulation in the Skin. Int. J. Mol. Sci. 2024, 25, 583. [Google Scholar] [CrossRef]
- Lee, D.; Jo, M.G.; Min, K.Y.; Choi, M.Y.; Kim, Y.M.; Kim, H.S.; Choi, W.S. IL-10+ regulatory B cells mitigate atopic dermatitis by suppressing eosinophil activation. Sci. Rep. 2024, 14, 18164. [Google Scholar] [CrossRef]
- Guzel, H.G.; Yilmaz, V.T.; Koksoy, S.; Kocak, H.; Kisaoglu, A.; Soylu, M.; Akkaya, B.; Demiryilmaz, I.; Aydinli, B.; Suleymanlar, G. Regulatory B Cells Profile in Kidney Transplant Recipients with Chronic-Active Antibody-Mediated Rejection. Transplant. Proc. 2023, 55, 1140–1146. [Google Scholar] [CrossRef]
- Mai, H.L.; Degauque, N.; Lorent, M.; Rimbert, M.; Renaudin, K.; Danger, R.; Kerleau, C.; Tilly, G.; Vivet, A.; Le Bot, S.; et al. Kidney allograft rejection is associated with an imbalance of B cells, regulatory T cells and differentiated CD28-CD8+ T cells: Analysis of a cohort of 1095 graft biopsies. Front. Immunol. 2023, 14, 1151127. [Google Scholar] [CrossRef]
- Altulea, D.; van den Born, J.; Bijma, T.; Bonasia, C.; Inrueangsri, N.; Lammerts, R.; Berger, S.; Heeringa, P.; Sanders, J.S. Comprehensive Phenotyping and Cytokine Production of Circulating B Cells Associate Resting Memory B Cells with Early Antibody-mediated Rejection in Kidney Transplant Recipients. Transplant. Direct. 2025, 11, e1775. [Google Scholar] [CrossRef] [PubMed]
- Louis, K.; Fadakar, P.; Macedo, C.; Yamada, M.; Lucas, M.; Gu, X.; Zeevi, A.; Randhawa, P.; Lefaucheur, C.; Metes, D. Concomitant loss of regulatory T and B cells is a distinguishing immune feature of antibody-mediated rejection in kidney transplantation. Kidney Int. 2022, 101, 1003–1016. [Google Scholar] [CrossRef]
- Perez-Payá, I.; Garcia, S.G.; Clos-Sansalvador, M.; Sanroque-Muñoz, M.; Font-Morón, M.; Rodríguez-Martínez, P.; Vila-Santandreu, A.; Bover, J.; Borràs, F.E.; Cañas, L.; et al. Molecular screening of transitional B cells as a prognostic marker of improved graft outcome and reduced rejection risk in kidney transplant. Front. Immunol. 2024, 15, 1433832. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Shahi, A.; Afzali, S.; Keshtkar, A.A.; Farashi-Bonab, S.; Soleymanian, T.; Ansaripour, B.; Amirzargar, A.A. Transitional immature regulatory B cells and regulatory cytokines can discriminate chronic antibody-mediated rejection from stable graft function. Int. Immunopharmacol. 2020, 86, 106750. [Google Scholar] [CrossRef]
- Shabir, S.; Girdlestone, J.; Briggs, D.; Kaul, B.; Smith, H.; Daga, S.; Chand, S.; Jham, S.; Navarrete, C.; Harper, L.; et al. Transitional B lymphocytes are associated with protection from kidney allograft rejection: A prospective study. Am. J. Transplant. 2015, 15, 1384–1391. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, F.; Zhang, K.; Wang, S.; Zhang, H.; Yang, X.; Shang, W.; Wang, J.; Wang, Z.; Pang, X.; et al. Elevated Circulating IL-10 Producing Breg, but Not Regulatory B Cell Levels, Restrain Antibody-Mediated Rejection After Kidney Transplantation. Front. Immunol. 2021, 11, 627496. [Google Scholar] [CrossRef]
- Guinn, M.T.; Szuter, E.S.; Yokose, T.; Ge, J.; Rosales, I.A.; Chetal, K.; Sadreyev, R.I.; Cuenca, A.G.; Kreisel, D.; Sage, P.T.; et al. Intragraft B cell differentiation during the development of tolerance to kidney allografts is associated with a regulatory B cell signature revealed by single cell transcriptomics. Am. J. Transplant. 2023, 23, 1319–1330. [Google Scholar] [CrossRef]
- Süsal, C.; Alvarez, C.M.; Benning, L.; Daniel, V.; Zeier, M.; Schaier, M.; Morath, C.; Speer, C. The balance between memory and regulatory cell populations in kidney transplant recipients with operational tolerance. Clin. Exp. Immunol. 2024, 216, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhan, F.; Zhang, H.; Gu, J.; Mu, X.; Gao, J.; Rao, J.; Ji, G.; Ni, X.; Lu, L.; et al. The proportion of CD19+CD24hiCD27+ regulatory B cells predicts the occurrence of acute allograft rejection in liver transplantation. Ann. Transl. Med. 2019, 7, 465. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Du, G.; Chen, W.; Bao, P.; Li, B.; Lu, Q.; Shi, B. The advantage of Sirolimus in amplifying regulatory B cells and regulatory T cells in liver transplant patients. Eur. J. Pharmacol. 2020, 869, 172872. [Google Scholar] [CrossRef]
- Piloni, D.; Morosini, M.; Magni, S.; Balderacchi, A.; Inghilleri, S.; Cova, E.; Oggionni, T.; Frangipane, V.; Pandolfi, L.; Scudeller, L.; et al. Peripheral CD19+CD24highCD38high B-regulatory cells in lung transplant recipients. Transpl. Immunol. 2019, 57, 101245. [Google Scholar] [CrossRef]
- Bergantini, L.; d’Alessandro, M.; De Vita, E.; Perillo, F.; Fossi, A.; Luzzi, L.; Paladini, P.; Perrone, A.; Rottoli, P.; Sestini, P.; et al. Regulatory and Effector Cell Disequilibrium in Patients with Acute Cellular Rejection and Chronic Lung Allograft Dysfunction after Lung Transplantation: Comparison of Peripheral and Alveolar Distribution. Cells 2021, 10, 780. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Yue, R.; Chen, X.; Liu, A.; Xu, H.; Teng, P.; Wang, Z.; Zou, Y.; Xu, X.; et al. Gut microbes enlarged the protective effect of transplanted regulatory B cells on rejection of cardiac allografts. J. Heart Lung Transplant. 2021, 40, 1502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Mei, F.; Wu, C.; Xu, H.; Liu, Z.; Cui, Y. Protective effect of trichostatin A on CD19+CD5+CD1dhigh regulatory B cells in heart transplantation. Mol. Med. Rep. 2021, 23, 339. [Google Scholar] [CrossRef]
- Lee, K.M.; Stott, R.T.; Zhao, G.; SooHoo, J.; Xiong, W.; Lian, M.M.; Fitzgerald, L.; Shi, S.; Akrawi, E.; Lei, J.; et al. TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur. J. Immunol. 2014, 44, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Yang, M.; Wei, L.; Wei, L.; Deng, S.; Zhao, G. Identification of the Subsets of IL-10-Producing Regulatory B Cells in the Course of Tolerance Induction and Maintenance in Islet Allotransplantation. Transplant. Proc. 2018, 50, 3900–3905. [Google Scholar] [CrossRef] [PubMed]
- Morath, C.; Schaier, M.; Ibrahim, E.; Wang, L.; Kleist, C.; Opelz, G.; Süsal, C.; Ponath, G.; Aly, M.; Alvarez, C.M.; et al. Induction of Long-Lasting Regulatory B Lymphocytes by Modified Immune Cells in Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2023, 34, 160–174. [Google Scholar] [CrossRef] [PubMed]
| Cellular Subtypes | Common Immunophenotype by Flow Cytometry (FC) | Possible Proposed and Reported Functions | References |
|---|---|---|---|
| Transitional | CD19+CD24hiCD38hi | Inhibition of CD4+ T cell proliferation. Inhibition of CD4+ T cell IFN-γ and TNF-α production. Induction of Treg differentiation. Suppression of monocytes and pDCs IFN-α production. | [11,13,16] |
| B10 cells | CD24hiCD27+CD48+ | Suppression of effector CD4+ T cells’ proliferation. Suppression of TNF-α production in monocytes and DCs. Propensity for co-expression with other cytokines such as TGF-β and GrB. | [11,13,16] |
| Plasmablasts | CD19+CD24hiCD27intCD138 hiBlimp1+ | Production of IL-10 and TGF-β cytokines. Suppression of DCs and effector CD4+ T cells. Suppression of CD4+ T cell IFN-γ and TNF-α production. | [11,13,16,17] |
| Memory | CD19+CD24+CD27+IgM+ | Suppression of CD4+ T cell pro-inflammatory cytokine production. Failure to suppress Th1 responses in particular diseases. | [11,13,16,17,18] |
| Marginal zone B cells | CD19+CD21hiCD23− | Production of IL-10 cytokine and induction of Treg cells. Suppression of effector CD4+ and CD8+ T cells. | [11,13,17,18] |
| BR1 cells | CD19+CD25hiCD71hiCD73lo IL-10+ | Production of IL-10 cytokine and promotion of IgG4 production. Suppression of inflammatory responses as Th2/Th17. Induction of Foxp3+ Treg cells and expansion. | [11,16] |
| GrB+ B cells | CD19+CD38+CD1d+IgM+CD147+ CD307bhiCD258hiCD72hiCD21loPD-1hi | Production of granzyme B. Degradation of TCR ζ chain. Inhibition of CD4+ T cell proliferation and Th1 and Th17 responses. | [11,16,18] |
| CD9+ B cells | CD19+CD9+ | Production of IL-10 cytokine Suppression of Th2 and Th17 inflammation | [11,16] |
| PD-L1hi B cells | CD19+PD-L1hi | Suppression of circulating Tfh cells | [11,19] |
| CD5+CD1d+ cells | CD19+CD5+CD1dhi | Production of IL-10 cytokine Suppression of Th17 response | [11,16] |
| General Processes | Pathologies | References | |
|---|---|---|---|
| Autoimmune diseases | Systemic lupus erythematosus Multiple sclerosis Rheumatoid arthritis | Neuromyelitis optica Myasthenia gravis Type 1 diabetes | [19,20,21,22,23,24,25,26,27,28,29,30,31] |
| Cancers and hematological diseases | Lung cancer Acute lymphocytic leukemia Chronic lymphocytic leukemia Hodgkin lymphoma Non-Hodgkin lymphoma B cell lymphoma Waldenström’s macroglobulinemia Multiple myeloma | Renal carcinoma Prostate cancer Breast cancer Bladder cancer Hepatoma Pancreatic cancer Breast cancer Tumorigenesis and metastasis | [32,33,34,35,36,37,38,39,40,41] |
| Immunodeficiencies | Inborn errors of immunity Common variable Immunodeficiency Agammaglobulinemia | Hypogammaglobulinemia Hyper-IgE syndrome Selective IgA deficiency | [42,43,44] |
| Allergy | Atopic dermatitis Food allergy | Altered IgE responses Asthma and rhinitis | [45,46] |
| Viral and bacterial infections | HBV COVID-19 | Mycobacterium spp. Helicobacter pylori | [47,48,49,50,51] |
| Parasitic infections | Filarial infection, Leishmania Baberia, Echinococcus Helminths | Plasmodium, Schistosoma Trypanosoma, Toxoplasma | [47,48,49] |
| Transplant responses and rejection | Acute antibody-mediated rejection Chronic humoral rejection Desensitization procedures Graft survival and protection Renal and pancreatic islet cell transplants | Heart and lung transplant Liver transplant Intestine transplants Operational tolerance vs. non-tolerance | [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-González, M.; Llorente, S.; Galián, J.A.; Botella, C.; González-López, R.; Alegría, M.J.; Hita, A.; Moya-Quiles, M.R.; Martinez-Banaclocha, H.; Muro-Pérez, M.; et al. Influence and Role of Regulatory B Cells in Organ Transplantation: The State of the Art, Prospects, and Emerging Insights. Antibodies 2025, 14, 95. https://doi.org/10.3390/antib14040095
Fernández-González M, Llorente S, Galián JA, Botella C, González-López R, Alegría MJ, Hita A, Moya-Quiles MR, Martinez-Banaclocha H, Muro-Pérez M, et al. Influence and Role of Regulatory B Cells in Organ Transplantation: The State of the Art, Prospects, and Emerging Insights. Antibodies. 2025; 14(4):95. https://doi.org/10.3390/antib14040095
Chicago/Turabian StyleFernández-González, Marina, Santiago Llorente, José Antonio Galián, Carmen Botella, Rosana González-López, María José Alegría, Alicia Hita, María Rosa Moya-Quiles, Helios Martinez-Banaclocha, Manuel Muro-Pérez, and et al. 2025. "Influence and Role of Regulatory B Cells in Organ Transplantation: The State of the Art, Prospects, and Emerging Insights" Antibodies 14, no. 4: 95. https://doi.org/10.3390/antib14040095
APA StyleFernández-González, M., Llorente, S., Galián, J. A., Botella, C., González-López, R., Alegría, M. J., Hita, A., Moya-Quiles, M. R., Martinez-Banaclocha, H., Muro-Pérez, M., Muro, J., Minguela, A., Legaz, I., & Muro, M. (2025). Influence and Role of Regulatory B Cells in Organ Transplantation: The State of the Art, Prospects, and Emerging Insights. Antibodies, 14(4), 95. https://doi.org/10.3390/antib14040095









