Novel Humanized Anti-HER3 Antibodies: Structural Characterization and Therapeutic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibody Humanization
2.2. Cell Culture
2.3. Transient Transfection and Production of Recombinant Antibodies in HEK293 Cells
2.4. ELISA Analysis
2.5. Epitope Mapping
2.6. HER3-ECD Deglycosylation
2.7. TK-hu A3 Fab Fragments Generation and HER3::TK-hu A3 (Fab) Complex Crystallization
2.8. Data Collection
2.9. Flow Cytometry-Based Binding Assay
2.10. Antibody Binding Affinity
2.11. Antibody Treatments and Immunoblot Analysis
2.12. Colony-Formation Assay
2.13. Proliferation Assay
2.14. In Vivo Study
2.15. Toxicity Study of TK-hu A3-H1L1 in Rats
2.16. Statistical Analysis
3. Results
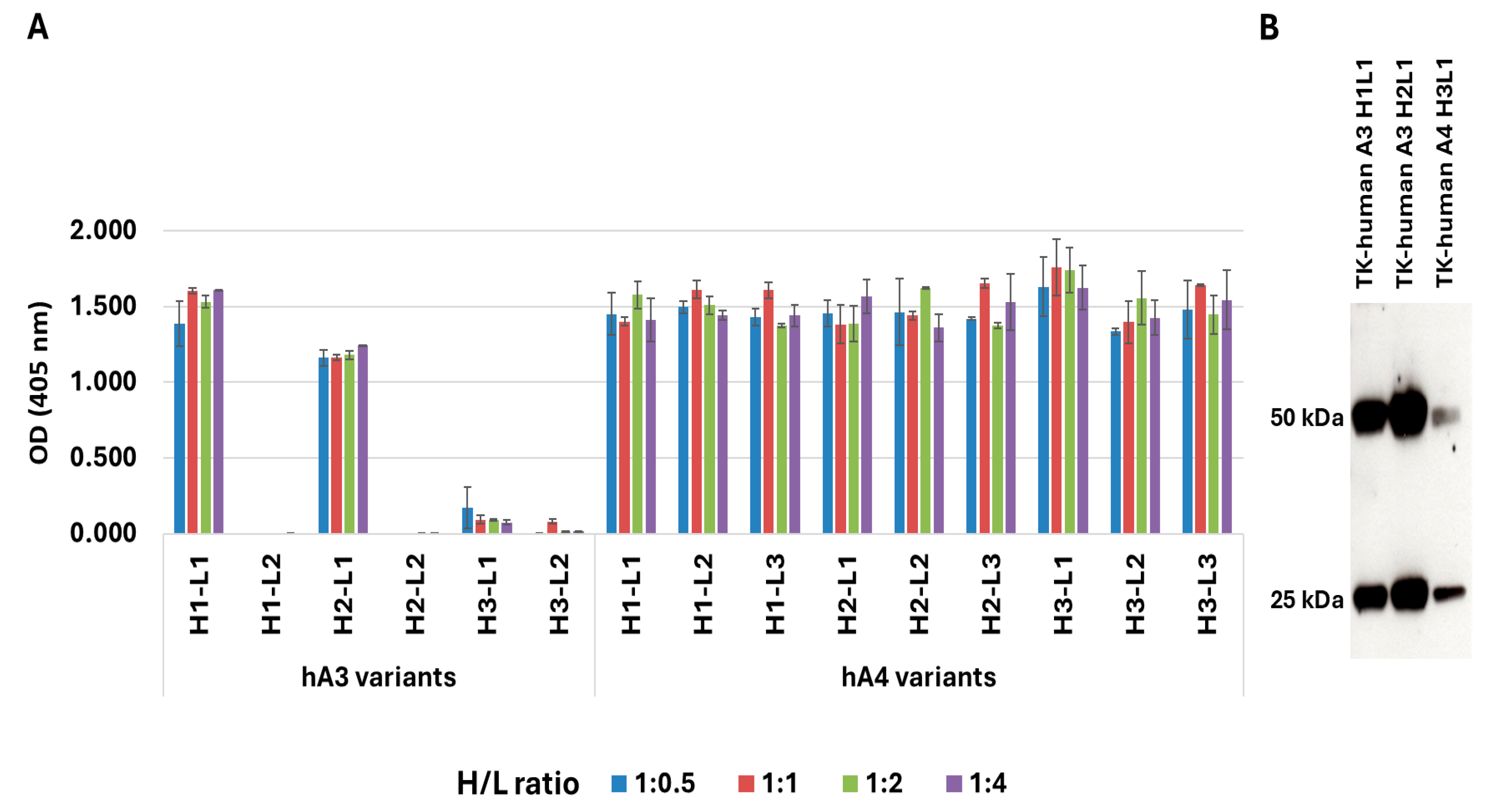
3.1. Antibody Humanization
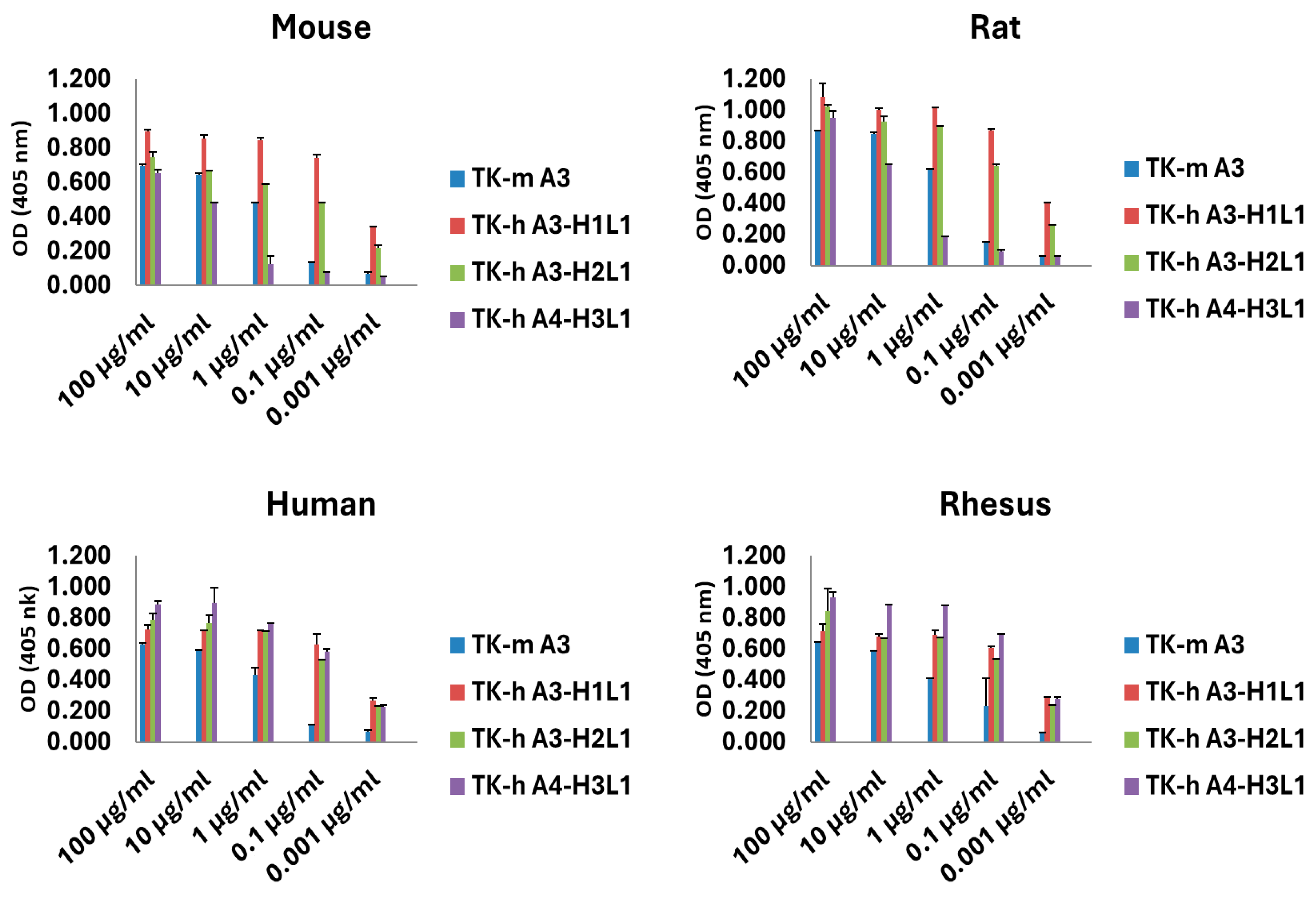
3.2. Species Cross-Reactivity
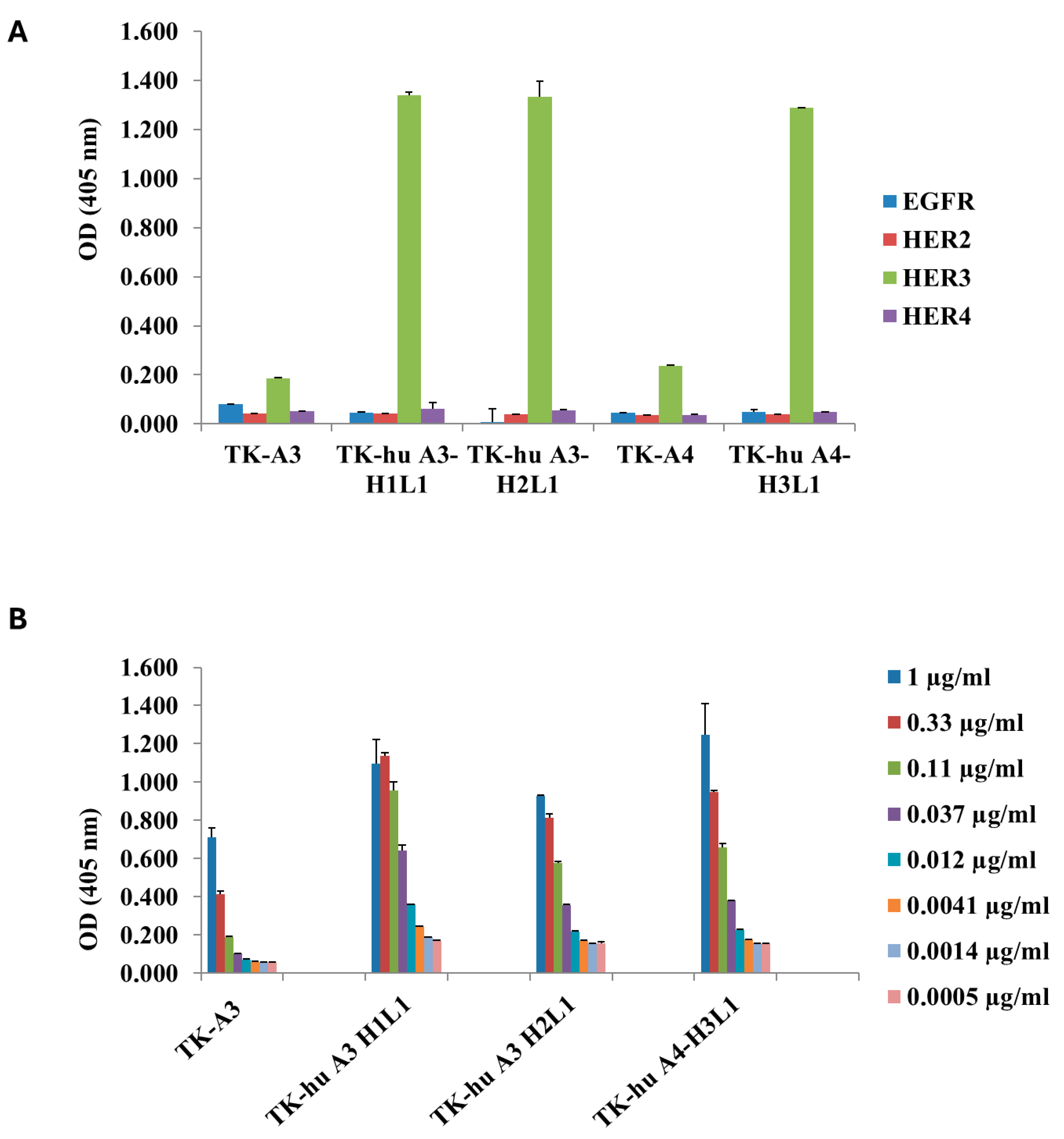
3.3. ErbB Family Receptor Specificity for TK-hu A3 and TK-hu A4
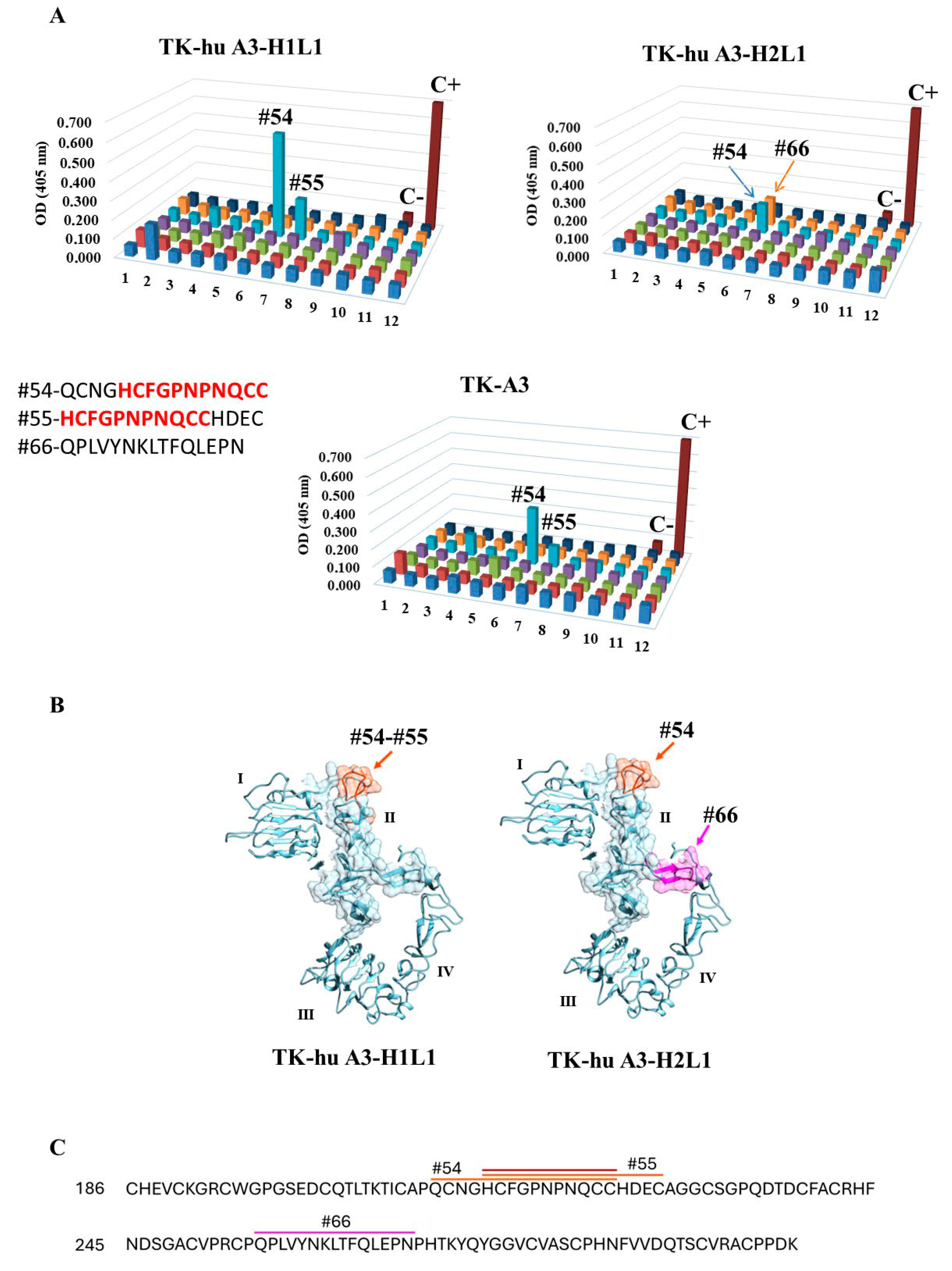
3.4. Epitope Mapping
3.5. Structure Determination and Epitope Characterization
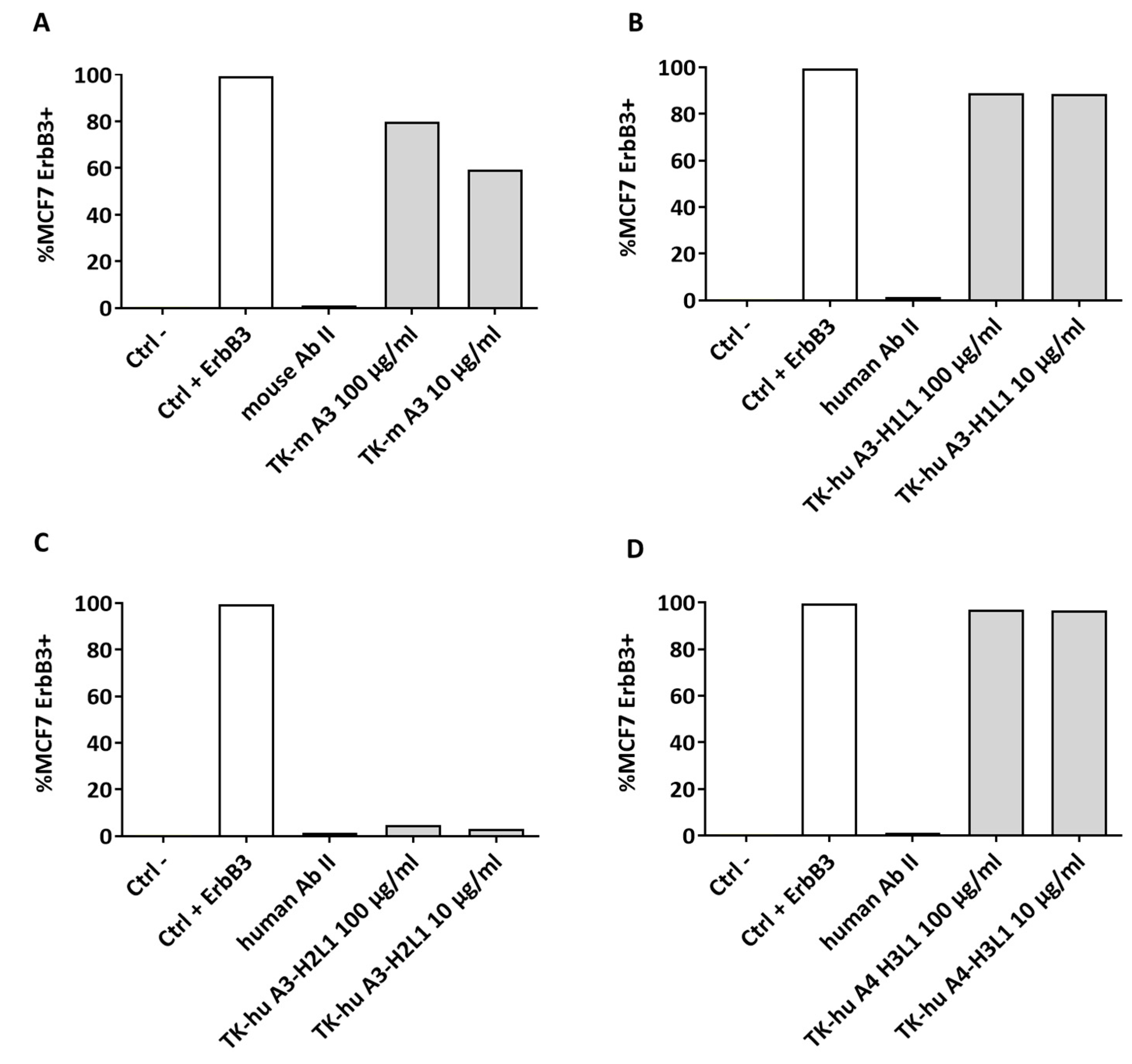
3.6. Native Conformation Binding and Flow Cytometry Analysis
3.7. Binding Affinity
3.8. Mechanism of Action of TK-hu A3 and TK-hu A4 Antibodies
3.9. In Vivo Antitumor Potential of TK-hu A3
3.10. Toxicology Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RTKs | Receptor tyrosine kinases |
| TKis | Small-molecule tyrosine kinase inhibitors |
| FBS | Fetal Bovine Serum |
| FCS | Fetal calf serum |
| FGFR2 | Fibroblast growth factor receptor 2 |
| PBS | Phosphate-Buffered Saline |
| ATCC | American Type Culture Collection |
| siRNA | Small-interfering RNA |
| AP | Alkaline Phosphatase |
| APC | Allophycocyanin |
| NRG | Neuregulin |
| ASU | Asymmetric unit |
References
- Yarden, Y.; Pines, G. The ERBB network: At last, cancer therapy meets systems biology. Nat. Rev. Cancer 2012, 12, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, C.L.; Engelman, J.A. ERBB Receptors: From Oncogene Discovery to Basic Science to Mechanism-Based Cancer Therapeutics. Cancer Cell 2014, 25, 282–303. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, C.L. ErbB-targeted therapeutic approaches in human cancer. Exp. Cell Res. 2003, 284, 122–130. [Google Scholar] [CrossRef]
- Jura, N.; Shan, Y.; Cao, X.; Shaw, D.E.; Kuriyan, J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc. Natl. Acad. Sci. USA 2009, 106, 21608–21613. [Google Scholar] [CrossRef]
- Arteaga, C.L. HER3 and mutant EGFR meet MET. Nat. Med. 2007, 13, 675–677. [Google Scholar] [CrossRef]
- Kunii, K.; Davis, L.; Gorenstein, J.; Hatch, H.; Yashiro, M.; Di Bacco, A.; Elbi, C.; Lutterbach, B. FGFR2-amplified gastric cancer cell lines require FGFR2 and HER3 signaling for growth and survival. Cancer Res. 2008, 68, 2340–2348. [Google Scholar] [CrossRef]
- Olayioye, M.A.; Neve, R.M.; Lane, H.A.; Hynes, N.E. New EMBO Members' Review: The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000, 19, 3159–3167. [Google Scholar] [CrossRef]
- Holbro, T.; Beerli, R.R.; Maurer, F.; Koziczak, M.; Barbas, C.F.; Hynes, N.E. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc. Natl. Acad. Sci. USA 2003, 100, 8933–8938. [Google Scholar] [CrossRef]
- Lee, Y.; Ma, J.; Lyu, H.; Huang, J.; Kim, A.; Liu, B. Role of HER3 receptors in cancer therapeutic resistance. Acta Biochim. Biophys. Sin. 2014, 46, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Lee-Hoeflich, S.T.; Crocker, L.; Yao, E.; Pham, T.; Munroe, X.; Hoeflich, K.P.; Sliwkowski, M.X.; Stern, H.M. A central role for HER3 in HER2-amplified breast cancer: Implications for targeted therapy. Cancer Res. 2008, 68, 5878–5887. [Google Scholar] [CrossRef]
- Jaiswal, B.S.; Kljavin, N.M.; Stawiski, E.W.; Chan, E.; Parikh, C.; Durinck, S.; Chaudhuri, S.; Pujara, K.; Guillory, J.; Edgar, K.A.; et al. Oncogenic ERBB3 mutations in human cancers. Cancer Cell. 2013, 23, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Kiavue, N.; Cabel, L.; Melaabi, S.; Bataillon, G.; Callens, C.; Lerebours, F.; Pierga, J.-Y.; Bidard, F.-C. ERBB3 mutations in cancer: Biological aspects, prevalence and therapeutics. Oncogene 2019, 39, 487–502. [Google Scholar] [CrossRef]
- Laskin, J.; Liu, S.; Tolba, K.; Heining, C.; Schlenk, R.; Cheema, P.; Cadranel, J.; Jones, M.; Drilon, A.; Cseh, A.; et al. NRG1 fusion-driven tumors: Biology, detection, and the therapeutic role of afatinib and other ErbB-targeting agents. Ann. Oncol. 2020, 31, 1693–1703. [Google Scholar] [CrossRef]
- Zhong, H.; He, J.; Yu, J.; Li, X.; Mei, Y.; Hao, L.; Wu, X. Mig6 not only inhibits EGFR and HER2 but also targets HER3 and HER4 in a differential specificity: Implications for targeted esophageal cancer therapy. Biochimie 2021, 190, 132–142. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Huang, S.; Kruser, T.J.; Nechrebecki, M.M.; Armstrong, E.A.; Benavente, S.; Gondi, V.; Hsu, K.-T.; Harari, P.M. Mechanisms of acquired resistance to cetuximab: Role of HER (ErbB) family members. Oncogene 2008, 27, 3944–3956. [Google Scholar] [CrossRef]
- Wang, S.E.; Xiang, B.; Guix, M.; Olivares, M.G.; Parker, J.; Chung, C.H.; Pandiella, A.; Arteaga, C.L. Transforming Growth Factor β Engages TACE and ErbB3 To Activate Phosphatidylinositol-3 Kinase/Akt in ErbB2-Overexpressing Breast Cancer and Desensitizes Cells to Trastuzumab. Mol. Cell. Biol. 2008, 28, 5605–5620. [Google Scholar] [CrossRef]
- Garrett, J.T.; Olivares, M.G.; Rinehart, C.; Granja-Ingram, N.D.; Sánchez, V.; Chakrabarty, A.; Dave, B.; Cook, R.S.; Pao, W.; McKinely, E.; et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc. Natl. Acad. Sci. USA 2011, 108, 5021–5026. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Sánchez, V.; Kuba, M.G.; Rinehart, C.; Arteaga, C.L. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc. Natl. Acad. Sci. USA 2011, 109, 2718–2723. [Google Scholar] [CrossRef]
- Chandarlapaty, S.; Sawai, A.; Scaltriti, M.; Rodrik-Outmezguine, V.; Grbovic-Huezo, O.; Serra, V.; Majumder, P.K.; Baselga, J.; Rosen, N. AKT Inhibition Relieves Feedback Suppression of Receptor Tyrosine Kinase Expression and Activity. Cancer Cell 2011, 19, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, A.; Hu, Z.; Li, J.; Lu, J. ERBB3 targeting: A promising approach to overcoming cancer therapeutic resistance. Cancer Lett. 2024, 599, 217146. [Google Scholar] [CrossRef] [PubMed]
- STARANISO Anisotropy & Bayesian Estimation Server. Available online: https://staraniso.globalphasing.org/cgi-bin/staraniso.cgi (accessed on 2 July 2025).
- McCoy, A.J. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr. Sect. D Struct. Biol. 2006, 63, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Agirre, J.; Atanasova, M.; Bagdonas, H.; Ballard, C.B.; Baslé, A.; Beilsten-Edmands, J.; Borges, R.J.; Brown, D.G.; Burgos-Mármol, J.J.; Berrisford, J.M.; et al. The CCP4 suite: Integrative software for macromolecular crystallography. Acta Crystallogr. Sect. D Struct. Biol. 2023, 79, 449–461. [Google Scholar] [CrossRef]
- Mirschberger, C.; Schiller, C.B.; Schräml, M.; Dimoudis, N.; Friess, T.; Gerdes, C.A.; Reiff, U.; Lifke, V.; Hoelzlwimmer, G.; Kolm, I.; et al. RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation. Cancer Res. 2013, 73, 5183–5194. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2019, 66, 2. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.W. EGFR family: Structure physiology signalling and therapeutic targets†. Growth Factors 2008, 26, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Aurisicchio, L.; Marra, E.; Luberto, L.; Carlomosti, F.; De Vitis, C.; Noto, A.; Gunes, Z.; Roscilli, G.; Mesiti, G.; Mancini, R.; et al. Novel anti-ErbB3 monoclonal antibodies show therapeutic efficacy in xenografted and spontaneous mouse tumors. J. Cell. Physiol. 2011, 227, 3381–3388. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of Macromolecular Assemblies from Crystalline State. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- D’acunto, E.; Muzi, A.; Marchese, S.; Donnici, L.; Chiarini, V.; Bucci, F.; Pavoni, E.; Ferrara, F.F.; Cappelletti, M.; Arriga, R.; et al. Isolation and Characterization of Neutralizing Monoclonal Antibodies from a Large Panel of Murine Antibodies against RBD of the SARS-CoV-2 Spike Protein. Antibodies 2024, 13, 5. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Baselga, J.; Swain, S.M. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef]
- Bang, I.; Hattori, T.; Leloup, N.; Corrado, A.; Nyamaa, A.; Koide, A.; Geles, K.; Buck, E.; Koide, S. Selective targeting of oncogenic hotspot mutations of the HER2 extracellular domain. Nat. Chem. Biol. 2024, 21, 706–715. [Google Scholar] [CrossRef]
- Garrett, J.T.; Tendler, S.; Feroz, W.; Kilroy, M.K.; Yu, H. Emerging importance of HER3 in tumorigenesis and cancer therapy. Nat. Rev. Clin. Oncol. 2025, 22, 348–370. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Okamoto, I.; Yonesaka, K.; Okamoto, K.; Shibata, K.; Shinkai, Y.; Sakamoto, H.; Kitano, M.; Tamura, T.; Nishio, K.; et al. The anti-HER3 antibody patritumab abrogates cetuximab resistance mediated by heregulin in colorectal cancer cells. Oncotarget 2014, 5, 11847–11856. [Google Scholar] [CrossRef]
- Engelman, J.A.; Settleman, J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr. Opin. Genet. Dev. 2008, 18, 73–79. [Google Scholar] [CrossRef]
- Morgillo, F.; Della Corte, C.M.; Fasano, M.; Ciardiello, F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open 2016, 1, e000060. [Google Scholar] [CrossRef]
- Arteaga, C.L. Epidermal growth factor receptor dependence in human tumors: More than just expression. Oncologist 2002, 7, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Konig, M.F.; Pardoll, D.M.; Bettegowda, C.; Papadopoulos, N.; Wright, K.M.; Gabelli, S.B.; Ho, M.; van Elsas, A.; Zhou, S. Cancer therapy with antibodies. Nat. Rev. Cancer 2024, 24, 399–426. [Google Scholar] [CrossRef] [PubMed]
- Garrett, J.T.; Arteaga, C.L. Resistance to HER2-directed antibodies and tyrosine kinase inhibitors: Mechanisms and clinical implications. Cancer Biol. Ther. 2011, 11, 793–800. [Google Scholar] [CrossRef]
- Geng, W.; Thomas, H.; Chen, Z.; Yan, Z.; Zhang, P.; Zhang, M.; Huang, W.; Ren, X.; Wang, Z.; Ding, K.; et al. Mechanisms of acquired resistance to HER2-Positive breast cancer therapies induced by HER3: A comprehensive review. Eur. J. Pharmacol. 2024, 977, 176725. [Google Scholar] [CrossRef]
- Chen, Q.; Jia, G.; Zhang, X.; Ma, W. Targeting HER3 to overcome EGFR TKI resistance in NSCLC. Front. Immunol. 2024, 14, 1332057. [Google Scholar] [CrossRef]
- Haikala, H.M.; Jänne, P.A. Thirty Years of HER3: From Basic Biology to Therapeutic Interventions. Clin. Cancer Res. 2021, 27, 3528–3539. [Google Scholar] [CrossRef]
- Krop, I.E.; Masuda, N.; Mukohara, T.; Takahashi, S.; Nakayama, T.; Inoue, K.; Iwata, H.; Yamamoto, Y.; Alvarez, R.H.; Toyama, T.; et al. Patritumab Deruxtecan (HER3-DXd), a Human Epidermal Growth Factor Receptor 3–Directed Antibody-Drug Conjugate, in Patients With Previously Treated Human Epidermal Growth Factor Receptor 3–Expressing Metastatic Breast Cancer: A Multicenter, Phase I/II Trial. J. Clin. Oncol. 2023, 41, 5550–5560. [Google Scholar] [CrossRef]
- Li, X.; Yao, J.; Qu, C.; Luo, L.; Li, B.; Zhang, Y.; Zhu, Z.; Qiu, Y.; Hua, H. DB-1310, an ADC comprised of a novel anti-HER3 antibody conjugated to a DNA topoisomerase I inhibitor, is highly effective for the treatment of HER3-positive solid tumors. J. Transl. Med. 2024, 22, 362. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Koyama, K.; Kamai, Y.; Hirotani, K.; Ogitani, Y.; Zembutsu, A.; Abe, M.; Kaneda, Y.; Maeda, N.; Shiose, Y.; et al. A Novel HER3-Targeting Antibody-Drug Conjugate, U3-1402, Exhibits Potent Therapeutic Efficacy through the Delivery of Cytotoxic Payload by Efficient Internalization. Clin. Cancer Res. 2019, 25, 7151–7161. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.E.; Lyu, H.; Liu, B. HER3-targeted therapeutic antibodies and antibody–drug conjugates in non-small cell lung cancer refractory to EGFR-tyrosine kinase inhibitors. Chin. Med. J. Pulm. Crit. Care Med. 2023, 1, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Odintsov, I.; Espinosa-Cotton, M.; Khodos, I.; Sisso, W.J.; Mattar, M.S.; Lui, A.J.; Vojnic, M.; Shameem, S.H.; Chauhan, T.; et al. Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements. Cancer Discov. 2022, 12, 1233–1247. [Google Scholar] [CrossRef] [PubMed]
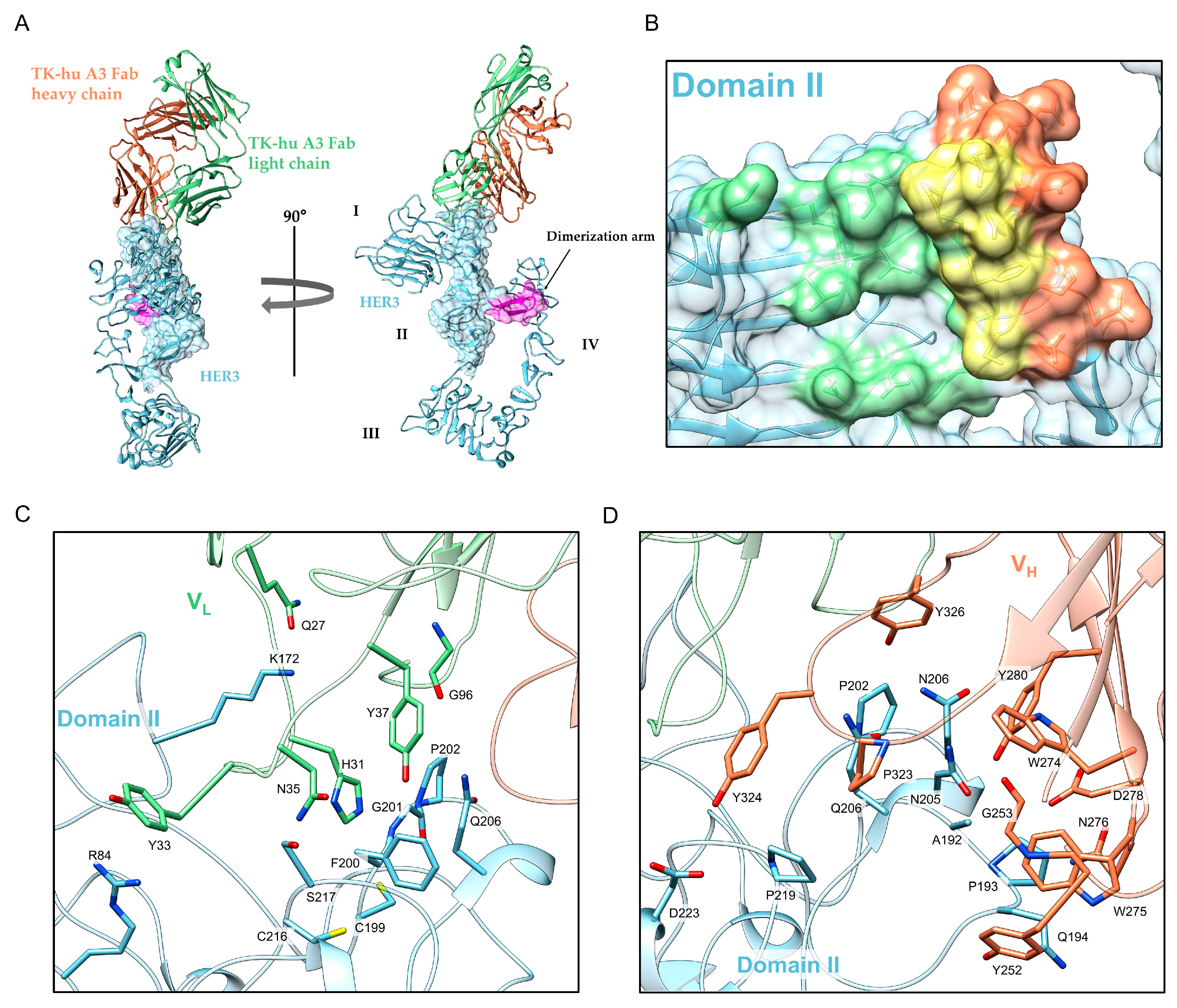


| HER3::Tk-hu A3 (Fab) | |
|---|---|
| Data Collection | |
| Space Group | P21 |
| Unit-cell dimension (Å) | a = 98.03; b = 98.81; c = 270.38 α = γ = 90.00°; β = 99.21° |
| Resolution range (Å) | 96.76–2.55 (2.94–2.55) |
| Number of observations | 540,804 (26,627) |
| Unique reflections | 82,868 (4143) |
| Completeness (%) (spherical) | 49.9 (7.2) |
| Completeness (%) (ellipsoidal) | 87.6 (63.7) |
| Redundancy | 6.5 (6.4) |
| Ι/σ (I) | 12.3 (1.9) |
| Rmerge (%) a | 12.0 (102.9) |
| CC1/2 | 0.999 (0.649) |
| Wilson B-value (Å2) | 58.0 |
| Refinement | |
| Rwork b/Rfree c (%) | 21.1/26.0 |
| Average B, all atoms (Å2) | 71.0 |
| r.m.s.d. bond (Å) | 0.007 |
| r.m.s.d. angles (°) | 1.130 |
| Ramachandran (%) Favored/allowed/outliers | 94/6/0 |
| Total number of atoms | 31,872 |
| Water molecules | 105 |
| Hydrogen Bonds | Hydrophobic Interactions | ||
|---|---|---|---|
| HER3-ECD | TK-hu A3 Fab | HER3-ECD | TK-hu A3 Fab |
| K172 | Q27/F | R84 | Y33/F |
| A192 | Y280/I | ||
| P193 | D278/I | ||
| Q194 | N276/I, W275/I, Y252/I | ||
| C199 | H31/F | ||
| F200 | Y37/F, N35/F | ||
| G201 | H31/F | ||
| P202 | G96/F, Y280/I | ||
| N203 | W274/I, Y326/I | ||
| N205 | G253/I, W274/I | ||
| Q206 | Y37/F, P323/I | ||
| C216, S217 | N35/F | ||
| P219 | Y234/I | ||
| D223 | Y324/I | ||
| Antibody | kon (1/Ms) | koff (1/s) | KD (M) |
|---|---|---|---|
| TK-A3 | 3.83 × 104 | 8.17 × 10−5 | 2.13 × 10−9 |
| TK-A4 | * NA | * NA | * NA |
| TK-hu A3 | 6.24 × 104 | 1.13 × 10−4 | 1.81 × 10−9 |
| TK-hu A4 | 3.39 × 105 | 1.64 × 10−3 | 4.84 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzi, A.; Arriga, R.; Bulfaro, G.; Fata, F.; Costanzo, A.; Chiarini, V.; Cappelletti, M.; Ferrara, F.F.; Bucci, F.; Montemiglio, L.C.; et al. Novel Humanized Anti-HER3 Antibodies: Structural Characterization and Therapeutic Activity. Antibodies 2025, 14, 84. https://doi.org/10.3390/antib14040084
Muzi A, Arriga R, Bulfaro G, Fata F, Costanzo A, Chiarini V, Cappelletti M, Ferrara FF, Bucci F, Montemiglio LC, et al. Novel Humanized Anti-HER3 Antibodies: Structural Characterization and Therapeutic Activity. Antibodies. 2025; 14(4):84. https://doi.org/10.3390/antib14040084
Chicago/Turabian StyleMuzi, Alessia, Roberto Arriga, Giovanni Bulfaro, Francesca Fata, Antonella Costanzo, Valerio Chiarini, Manuela Cappelletti, Fabiana Fosca Ferrara, Federica Bucci, Linda Celeste Montemiglio, and et al. 2025. "Novel Humanized Anti-HER3 Antibodies: Structural Characterization and Therapeutic Activity" Antibodies 14, no. 4: 84. https://doi.org/10.3390/antib14040084
APA StyleMuzi, A., Arriga, R., Bulfaro, G., Fata, F., Costanzo, A., Chiarini, V., Cappelletti, M., Ferrara, F. F., Bucci, F., Montemiglio, L. C., Savino, C., Marra, E., Ciliberto, G., Aurisicchio, L., Vallone, B., & Roscilli, G. (2025). Novel Humanized Anti-HER3 Antibodies: Structural Characterization and Therapeutic Activity. Antibodies, 14(4), 84. https://doi.org/10.3390/antib14040084








