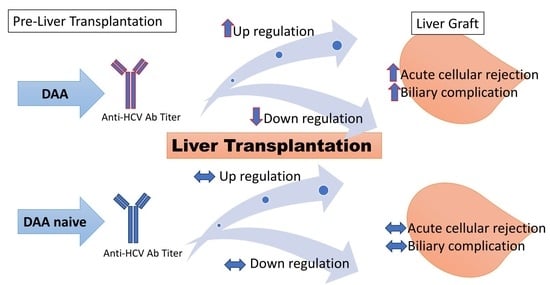
Immune Responses to Anti-Hepatitis C Virus Antibodies during Pre-Liver Transplantation Direct-Acting Antiviral Therapy in Hepatitis C Virus-Infected Recipients Associated with Post-Liver Transplantation Allograft Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Pre-LT DAA Therapy
2.3. Sample
2.4. Detection of Anti-HCV Antibodies
2.5. RT-PCR for HCV-RNA Detection
2.6. Immunosuppression Protocol
2.7. Follow-Up Parameters
2.8. Pathological Interpretation
2.9. Ethics
2.10. Statistics
3. Results
3.1. Patient Characteristics
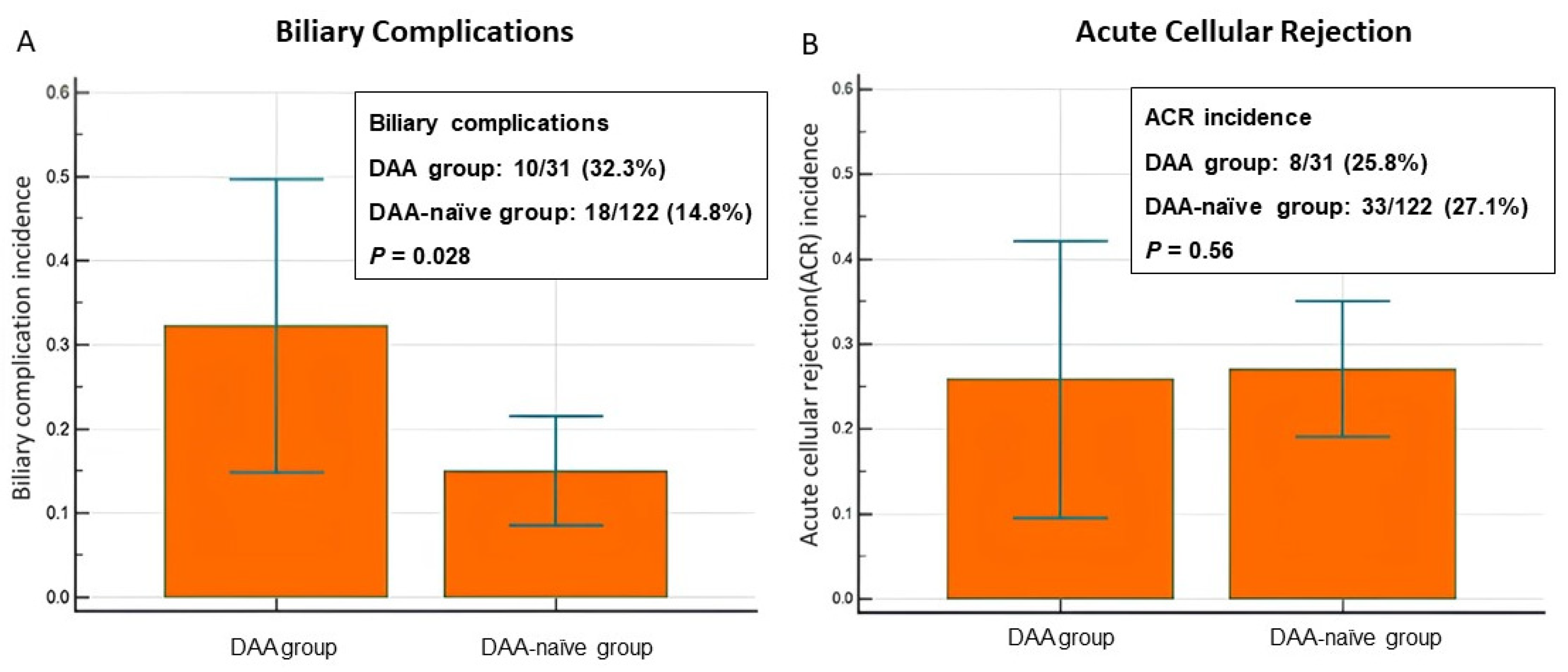
3.2. Incidence of Allograft Injury: BCs and ACR
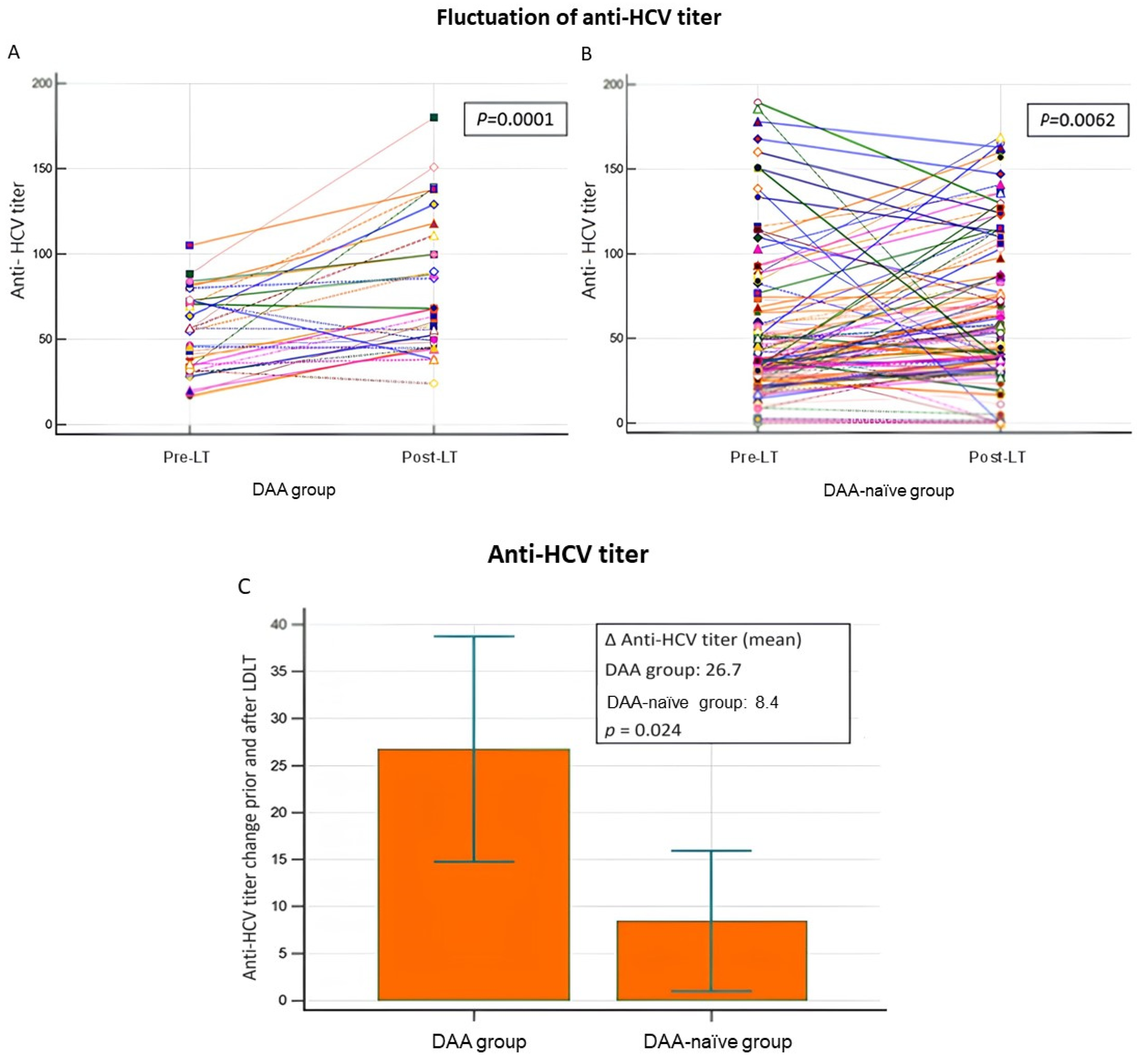
3.3. Fluctuation in Anti-HCV Antibody Titers and Allograft Injury
3.4. Multivariate Logistical Analysis of the Impact of Confounding Factors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crespo, G.; Mariño, Z.; Navasa, M.; Forns, X. Viral hepatitis in liver transplantation. Gastroenterology 2012, 142, 1373–1383.e1. [Google Scholar] [CrossRef] [PubMed]
- Crespo, G.; Trota, N.; Londoño, M.C.; Mauro, E.; Baliellas, C.; Castells, L.; Castellote, J.; Tort, J.; Forns, X.; Navasa, M. The efficacy of direct anti-HCV drugs improves early post-liver transplant survival and induces significant changes in waiting list composition. J. Hepatol. 2018, 69, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Liu, B.; Bhuket, T.; Wong, R.J. Lower likelihood of post-transplant graft failure, death, and retransplantation in the era of direct-acting antivirals. J. Clin. Exp. Hepatol. 2020, 10, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Millson, C.; Considine, A.; Cramp, M.E.; Holt, A.; Hubscher, S.; Hutchinson, J.; Jones, k.; Leithead, J.; Masson, S.; Menon, K.; et al. Adult liver transplantation: A UK clinical guideline—Part 1: Pre-operation. Front. Gastroenterol. 2020, 11, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; DiMartini, A.; Feng, S.; Brown, R., Jr.; Fallon, M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014, 59, 1144–1165. [Google Scholar] [CrossRef] [PubMed]
- Ghany, M.G.; Morgan, T.R.; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C guidance 2019 update: American association for the study of liver diseases-infectious diseases society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, W.; Amini, A.; Boeras, D.; Falconer, J.; Kelly, H.; Peeling, R.; Varsaneux, O.; Tucker, J.D.; Easterbrook, P. Diagnostic accuracy of tests to detect Hepatitis C antibody: A meta-analysis and review of the literature. BMC Infect. Dis. 2017, 17, 695. [Google Scholar] [CrossRef] [PubMed]
- Bonacci, M.; Lens, S.; Mariño, Z.; Londoño, M.C.; Rodriguez-Tajes, S.; Sánchez-Tapias, J.M.; Ramos-Casals, M.; Hernández-Rodríguez, J.; Forns, X. Long-Term outcomes of patients with HCV-associated cryoglobulinemic vasculitis after virologic cure. Gastroenterology 2018, 155, 311–315.e6. [Google Scholar] [CrossRef]
- Lin, C.H.; Kao, J.H. Acute hepatitis C virus infection: Clinical update to remaining challenges. Clin. Mol. Hepatol. 2023, 29, 623–642. [Google Scholar] [CrossRef]
- Liu, H.Y.; Lin, Y.H.; Lin, P.J.; Tsai, P.C.; Liu, S.F.; Huang, Y.C.; Tsai, J.J.; Huang, C.I.; Yeh, M.L.; Liang, P.C.; et al. Anti-HCV antibody titer highly predicts HCV viremia in patients with hepatitis B virus dual-infection. PLoS ONE 2021, 16, e0254028. [Google Scholar] [CrossRef]
- Law, M. Antibody Responses in Hepatitis C Infection. Cold Spring Harb. Perspect. Med. 2021, 11, a036962. [Google Scholar] [CrossRef] [PubMed]
- Luxenburger, H.; Neumann-Haefelin, C.; Thimme, R.; Boettler, T. HCV specific T cell responses during and after chronic HCV infection. Viruses 2018, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Osburn, W.O.; Snider, A.E.; Wells, B.L.; Latanich, R.; Bailey, J.R.; Thomas, D.L.; Cox, A.L.; Ray, S.C. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 2014, 59, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.M.; Zeisel, M.B.; Bläser, E.; Schürmann, P.; Bartosch, B.; Cosset, F.L.; Patel, A.H.; Meisel, H.; Baumert, J.; Viazov, S.; et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc. Natl. Acad. Sci. USA 2007, 104, 6025–6030. [Google Scholar] [CrossRef]
- Ding, M.; He, Y.; Zhang, S.; Guo, W. Recent Advances in costimulatory blockade to induce immune tolerance in liver transplantation. Front. Immunol. 2021, 12, 537079. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, A.; Cantarelli, C.; Riella, L.V.; Fribourg, M.; Cravedi, P. T cell exhaustion in organ transplantation. Transplantation 2022, 106, 489–499. [Google Scholar] [CrossRef]
- Osuch, S.; Metzner, K.J.; Caraballo Cortés, K. Reversal of T Cell Exhaustion in Chronic HCV Infection. Viruses 2020, 12, 799. [Google Scholar] [CrossRef]
- Lin, S.H.; Wu, K.T.; Wang, C.C.; Huang, K.T.; Chen, K.D.; Hsu, L.W.; Eng, H.L.; Chiu, K.W. Liver Graft MicroRNAs Expression in Different Etiology of Acute Jaundice after Living Donor Liver Transplantation. Biology 2022, 11, 1228. [Google Scholar] [CrossRef]
- Lin, S.H.; Wu, K.T.; Wang, C.C.; Huang, K.T.; Chen, K.D.; Lin, C.C.; Hsu, L.W.; Chiu, K.W. HCV RNA in serum and liver samples of patients undergoing living donor liver transplantation. J. Int. Med. Res. 2021, 49, 3000605211034945. [Google Scholar] [CrossRef]
- Lucey, M.R.; Terrault, N.; Ojo, L.; Hay, J.E.; Neuberger, J.; Blumberg, E.; Teperman, L.W. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013, 19, 3–26. [Google Scholar] [CrossRef]
- Chiu, K.W.; Chen, Y.S.; de Villa, V.H.; Chih-Chi Wang, C.C.; Eng, H.L.; Wang, S.H.; Liu, P.P.; Jawan, B.; Huang, T.L.; Cheng, Y.F.; et al. Characterization of liver enzymes on living related liver transplantation patients with acute rejection. Hepatogastroenterology 2005, 52, 1825–1827. [Google Scholar]
- Torbenson, M.; Washington, K. Pathology of liver disease: Advances in the last 50 years. Hum. Pathol. 2020, 95, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Grassi, A.; Ballardini, G. Post-liver transplant hepatitis C virus recurrence: An unresolved thorny problem. World J. Gastroenterol. 2014, 20, 11095–11115. [Google Scholar] [CrossRef] [PubMed]
- Vranjkovic, A.; Deonarine, F.; Kaka, S.; Angel, J.B.; Cooper, C.L.; Crawley, A.M. Direct-Acting Antiviral Treatment of HCV Infection Does Not Resolve the Dysfunction of Circulating CD8+ T-Cells in Advanced Liver Disease. Front Immunol. 2019, 10, 1926. [Google Scholar] [CrossRef] [PubMed]
- Rehermann, B.; Thimme, R. Insights from antiviral therapy into immune responses to hepatitis B and C virus infection. Gastroenterology 2019, 156, 369–383. [Google Scholar] [CrossRef]
- Heim, M.H.; Thimme, R. Innate and adaptive immune responses in HCV infections. J. Hepatol. 2014, 61, S14–S25. [Google Scholar] [CrossRef]
- Sepulveda-Crespo, D.; Resino, S.; Martinez, I. Hepatitis C virus vaccine design: Focus on the humoral immune response. J. Biomed. Sci. 2020, 27, 78. [Google Scholar] [CrossRef]
- Casey, J.L.; Feld, J.J.; MacParland, S.A. Restoration of HCV-specific immune responses with antiviral therapy: A case for DAA treatment in acute HCV infection. Cells 2019, 8, 317. [Google Scholar] [CrossRef]
- Ito, T.; Botros, M.; Aziz, A.; Guorgui, J.G.; Agopian, V.G.; Farmer, D.G.; Busuttil, R.W.; Kaldas, F.M. Nonanastomotic biliary strictures after liver transplantation. Am. Surg. 2020, 86, 1363–1367. [Google Scholar] [CrossRef]
- Karimian, N.; Op den Dries, S.; Porte, R.J. The origin of biliary strictures after liver transplantation: Is it the amount of epithelial injury or insufficient regeneration that counts? J. Hepatol. 2013, 58, 1065–1067. [Google Scholar] [CrossRef]
- de Vries, Y.; von Meijenfeldt, F.A.; Porte, R.J. Post-transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Op den Dries, S.; Sutton, M.E.; Lisman, T.; Porte, R.J. Protection of bile ducts in liver transplantation: Looking beyond ischemia. Transplantation 2011, 92, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bojadzic, D.; Buchwald, P. Toward small-molecule inhibition of protein-protein interactions: General aspects and recent progress in targeting costimulatory and coinhibitory (immune checkpoint) interactions. Curr. Top. Med. Chem. 2018, 18, 674–699. [Google Scholar] [CrossRef] [PubMed]
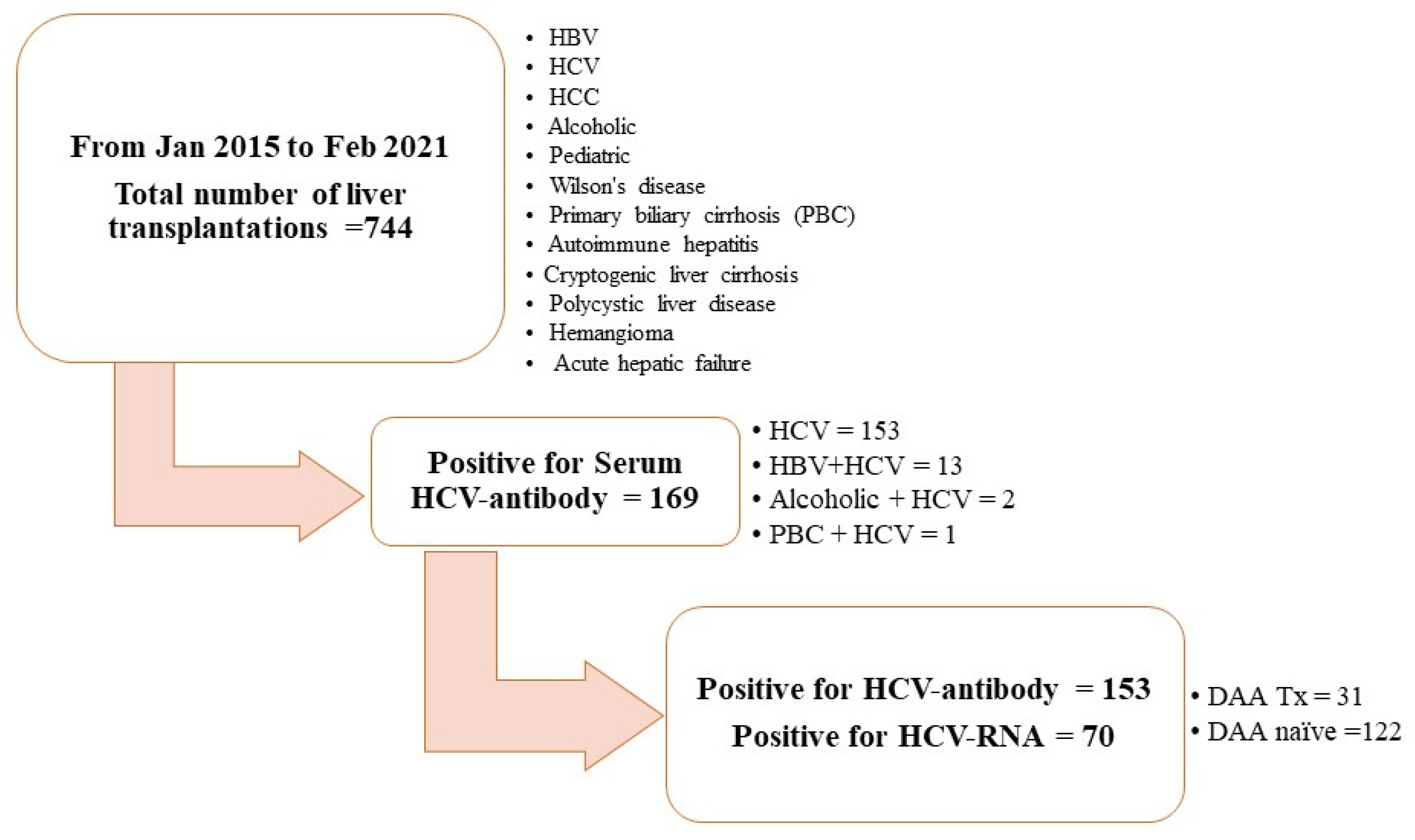
| Variable, n (%)/Median ± SD | All Patients n = 153 | DAA Group n = 31 (20.3) | DAA-Naïve Group n = 122 (79.7) | p-Value |
|---|---|---|---|---|
| Sex, n (%) Male/female | 72 (47.7)/81 (52.3) | 12 (38.7)/19 (61.3) | 60 (49.6)/62 (50.4) | 0.28 |
| Age at transplant (years), mean ± SD | 54.5 | 57.4 ± 7.5 | 57.2 ± 6.6 | 0.85 |
| Follow-up (months), mean ± SD | 43.6 | 33.2 ± 18.6 | 46.1 ± 21.7 | 0.0029 |
| Serum HCV RNA positive, n (%) | ||||
| Pre-transplant | 70 (45.8) | 1 (3.2) | 69 (56.6) | <0.000001 |
| Post-transplant | 61 (39.9) | 0 (0) | 61 (50) | <0.000001 |
| HCV genotype, n (%) 1/2/3/6/undetected | 54 (35.3)/39 (25.5)/2 (1.3)/2 (1.3)/56 (36.6) | 8 (25.8)/10 (32.3)/1 (3.2)/0 (0)/12 (38.7) | 46 (37.7)/29 (23.8)/1 (0.8)/2 (1.6)/44 (36.1) | 0.19 |
| AFP (ng/mL), mean ± SD | ||||
| Pre-transplant | 14.1 ± 70.0 | 11.9 ± 21.4 | 14.7 ± 37.8 | 0.69 |
| Post-transplant | 3.8 ± 7.3 | 4.2 ± 5.1 | 3.7 ± 3.2 | 0.52 |
| Liver donor, n (%) Living donor/deceased donor | 136 (88.9)/17 (11.1) | 27 (87.1)/4 (12.9) | 109 (87.3)/13 (10.7) | 0.75 |
| HCC diagnosed at LT, n (%) Absent/present | 80 (52.3)/73 (47.7) | 19 (61.3)/12 (38.7) | 61 (50)/61 (50) | 0.057 |
| MELD score, mean ± SD | 18.0 ± 18.7 | 17.6 ± 9.6 | 18.2 ± 9.4 | 0.75 |
| Liver explant pathology | ||||
| Viable tumor identified, n (%) | 60 (39.2) | 18 (58.1) | 42 (34.4) | 0.11 |
| Largest tumor (cm), mean ± SD | 2.9 ± 3.0 | 2.6 ± 1.1 | 2.9 ± 1.6 | 0.39 |
| Number of lesions, mean | 2.5 | 2.3 ± 1.4 | 2.6 ± 1.8 | 0.52 |
| Lymphovascular invasion, n (%) | 16 (10.5) | 3 (15.0) | 13 (22.8) | 0.47 |
| Post-LT complications, n (%) | ||||
| Biliary complication | 28 (18.3) | 10 (32.3) | 18 (14.8) | 0.028 |
| Acute cellular rejection | 41 (26.8) | 8 (25.8) | 33 (27) | 0.56 |
| Post-LT de novo HCC/recurrence, n (%) | 6 (3.9)/0 | 0 (0)/0 (0) | 6 (4.9)/0 (0) | 0.35 |
| Category | Anti-HCV (+) n = 153 (%) | p-Value | |
|---|---|---|---|
| DAA Group n = 31 (20.3) | DAA-Naïve Group n = 122 (79.7) | ||
| Positive BC | 10 (32.3) | 18 (14.8) | <0.05 |
| Negative BC | 21 (67.7) | 104 (85.2) | |
| Positive ACR | 8 (25.8) | 33 (27.1) | >0.05 |
| Negative ACR | 23 (74.2) | 89 (73.0) | |
| Variable/Median ± SD | BCs (+) (n = 28) | BCs (−) (n = 125) | p-Value | ACR (+) (n = 41) | ACR (−) (n = 112) | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 58.0 ± 5.9 | 57.0 ± 6.9 | 0.49 | 59.6 ± 5.3 | 56.3 ± 7.0 | 0.0065 |
| Sex (M/F) | 14/14 | 59/66 | 0.84 | 20/21 | 53/59 | 1.00 |
| Pre-LT DAA use (Yes/no) | 10/18 | 21/104 | 0.036 | 8/33 | 23/89 | 1.00 |
| Graft warm ischemic time (min) | 35.4 ± 4.9 | 37.9 ± 6.7 | 0.16 | 36.3 ± 4.6 | 37.8 ± 7.0 | 0.33 |
| Graft cold ischemic time (min) | 71.1 ± 96.9 | 51.9 ± 64.6 | 0.33 | 34.3 ± 10.5 | 62.8 ± 81.4 | 0.09 |
| Post-LT day 30 | ||||||
| AST (U/L) | 67.9 ± 78.2 | 63.5 ± 211.5 | 0.91 | 53.6 ± 40.8 | 68.2 ± 224.5 | 0.69 |
| ALT (U/L) | 84.5 ± 96.0 | 53.6 ± 98.6 | 0.13 | 70.7 ± 58.3 | 55.2 ± 109.4 | 0.40 |
| Total bilirubin (mg/dL) | 0.92 ± 0.75 | 1.53 ± 7.14 | 0.65 | 0.97 ± 1.3 | 1.58 ± 7.5 | 0.61 |
| Albumin (g/dL) | 4.13 ± 0.47 | 4.11 ± 0.48 | 0.84 | 4.0 ± 0.59 | 4.1 ± 0.42 | 0.21 |
| INR | 1.00 ± 0.16 | 1.08 ± 0.34 | 0.30 | 1.03 ± 0.2 | 1.07 ± 0.35 | 0.50 |
| Category | DAA Group n = 31 (%) | DAA-Naïve Group n = 122 (%) | ||
|---|---|---|---|---|
| Anti-HCV Ab titer | 28.05 ± 33.96 a | 10.50 ± 39.93 a’ | ||
| Upregulation n = 25 (80.7) | Downregulation n = 6 (19.3) | Upregulation n = 85 (69.7) | Downregulation n = 37 (30.3) | |
| 37.58 ± 30.18 b | −11.66 ± 14.02 c | 27.97 ± 25.45 b’ | −29.62 ± 38.37 c’ | |
| Category | BCs n = 28 (%) | ACR n = 41 (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Anti-HCV Ab titer (S/CO ratio) | Upregulation n = 19 (67.9) | Downregulation n = 9 (32.1) | Upregulation n = 29 (70.7) | Downregulation n = 12 (29.3) | ||||
| 25.13 ± 25.39 a | −20.39 ± 27.08 a’ | 18.71 ± 18.81 b | −23.18 ± 23.55 b’ | |||||
| DAA (+) n = 9 (47.4) | DAA (−) n = 10 (52.6) | DAA (+) n = 1 (11.1) | DAA (−) n = 8 (88.9) | DAA (+) n = 7 (24.1) | DAA (−) n = 22 (75.9) | DAA (+) n = 1 (8.3) | DAA (−) n = 11 (91.7) | |
| 34.87 ± 24.41 c | 16.36 ± 24.08 c’ | −2.55 | −22.63 ± 28.05 | 34.07 ± 27.44 d | 13.82 ± 11.34 d’ | −23.14 | −23.18 ± 24.71 | |
| Category n (%) | DAA Group 31 (20.2) | DAA-Naïve Group 122 (79.7) | p-Value |
|---|---|---|---|
| ∆Anti-HCV Ab titer following LT, mean ± SD | 28.05 ± 33.96 | 10.50 ± 39.93 | 0.013 |
| Post-LT positive fluctuation in anti-HCV titer, mean ± SD | 37.58 ± 30.18 | 27.97 ± 25.45 | 0.714 |
| Post-LT negative fluctuation in anti-HCV titer, mean ± SD | −11.66 ± 14.02 | −29.62 ± 38.37 | 0.43 |
| Post-LT positive fluctuation in anti-HCV titer, n (%) | 6 (19.3) | 37 (30.3) | 0.27 |
| Post-LT negative fluctuation in anti-HCV titer, n (%) | 25 (80.7) | 85 (69.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-H.; Wu, K.-T.; Wang, C.-C.; Huang, K.-T.; Hsu, L.-W.; Eng, H.-L.; Chiu, K.-W. Immune Responses to Anti-Hepatitis C Virus Antibodies during Pre-Liver Transplantation Direct-Acting Antiviral Therapy in Hepatitis C Virus-Infected Recipients Associated with Post-Liver Transplantation Allograft Injury. Antibodies 2024, 13, 7. https://doi.org/10.3390/antib13010007
Lin S-H, Wu K-T, Wang C-C, Huang K-T, Hsu L-W, Eng H-L, Chiu K-W. Immune Responses to Anti-Hepatitis C Virus Antibodies during Pre-Liver Transplantation Direct-Acting Antiviral Therapy in Hepatitis C Virus-Infected Recipients Associated with Post-Liver Transplantation Allograft Injury. Antibodies. 2024; 13(1):7. https://doi.org/10.3390/antib13010007
Chicago/Turabian StyleLin, Shu-Hsien, Kun-Ta Wu, Chih-Chi Wang, Kuang-Tzu Huang, Li-Wen Hsu, Hock-Liew Eng, and King-Wah Chiu. 2024. "Immune Responses to Anti-Hepatitis C Virus Antibodies during Pre-Liver Transplantation Direct-Acting Antiviral Therapy in Hepatitis C Virus-Infected Recipients Associated with Post-Liver Transplantation Allograft Injury" Antibodies 13, no. 1: 7. https://doi.org/10.3390/antib13010007
APA StyleLin, S.-H., Wu, K.-T., Wang, C.-C., Huang, K.-T., Hsu, L.-W., Eng, H.-L., & Chiu, K.-W. (2024). Immune Responses to Anti-Hepatitis C Virus Antibodies during Pre-Liver Transplantation Direct-Acting Antiviral Therapy in Hepatitis C Virus-Infected Recipients Associated with Post-Liver Transplantation Allograft Injury. Antibodies, 13(1), 7. https://doi.org/10.3390/antib13010007