From Cereal Grains to Immunochemistry—What Role Have Antibodies Played in the History of the Home Pregnancy Test
Abstract
1. Introduction
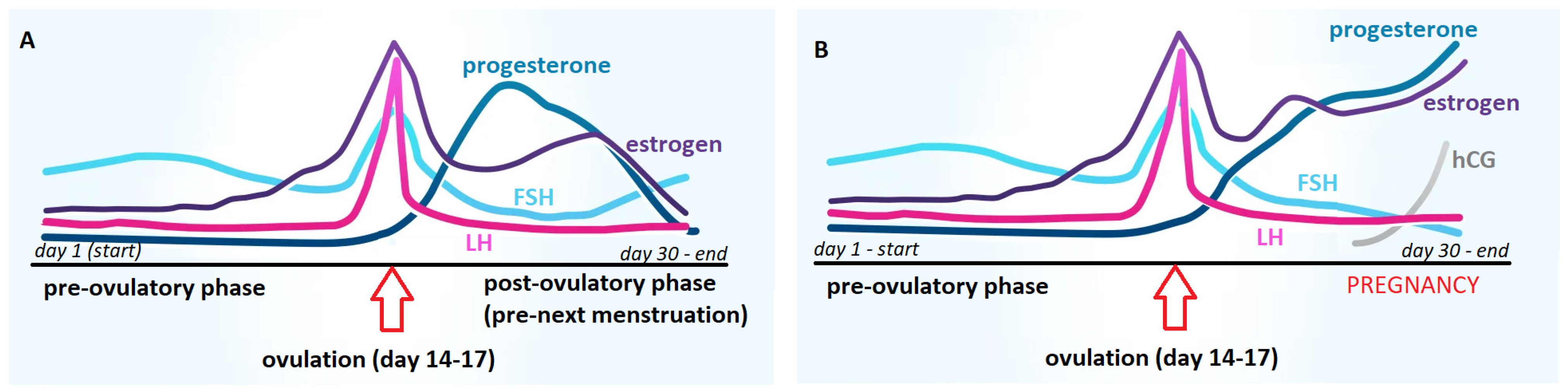
2. Modern In Vitro Pregnancy Test
3. History of Pregnancy Test
3.1. Ancient Egypt
3.2. From Hippocrates to Gallen
3.3. From the Middle Ages through the Seventeenth Century
3.4. Nineteenth Century
3.5. From the 1920s to the 1960s
3.6. 1960s—Agglutination Tests
3.7. 1970s—Time of the Radioimmunoassay
3.8. From 1970 to Today—Timeof the Home Pregnancy Tests
3.9. The Twenty-First Century—The Timeof Digital Pregnancy Tests
4. Summary and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Xu, W.; Wang, B.; Si, X.; Li, S. Methods and Advances in the Design, Testing and Development of In Vitro Diagnostic Instruments. Processes 2023, 11, 403. [Google Scholar] [CrossRef]
- Chard, T. Pregnancy tests: Are view. Hum. Reprod. 1992, 7, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.; Lapthorn, A.J. Standardization of Epitopes for Human Chorionic Gonadotropin (hCG) Immunoassays. Curr. Med. Chem. 2016, 23, 3481–3494. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.A.; Khanlian, S.A.; Cole, L.A. Detection of early pregnancy forms of human chorionic gonadotropin by home pregnancy test devices. Clin. Chem. 2001, 47, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Gnoth, C.; Johnson, S. Strips of Hope: Accuracy of Home Pregnancy Tests and New Developments. Geburtshilfe Frauenheilkd. 2014, 74, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Grenache, D.G. Variable accuracy of home pregnancy tests: Truth in advertising? Clin. Chem. Lab. Med. 2015, 53, 339–341. [Google Scholar] [CrossRef]
- Krause, J.E.; VanHyfte, E.D.; Wulf, J.N.; Hazbun, T.R. Home Urine Pregnancy Tests: Application of Immunology to Patient Care. Indiana Pharmacists Alliance Continuing Pharmacy Education (CPE) 2015, article 5. Available online: https://www.phpr.purdue.edu/directory/jkrause (accessed on 1 August 2023).
- Burstein, J.; Braunstein, G.D. Urine pregnancy tests from antiquity to the present. Early Pregnancy 1995, 1, 288–296. [Google Scholar]
- Available online: https://history.nih.gov/display/history/Pregnancy+Test+Timeline (accessed on 1 July 2023).
- Bayon, H.P. Ancient Pregnancy Tests in Light of Contemporary Knowledge. Proc. R. Soc. Med. 1939, 32, 1527–1538. [Google Scholar] [CrossRef]
- Ghalioungui, P.; Khalil, S.; Ammar, A.R. On an ancient Egyptian method of diagnosing pregnancy and determining foetal sex. Med. Hist. 1963, 7, 241–246. [Google Scholar] [CrossRef]
- Roy, N.K.; Sronivasarao, K.; Devi, N.S. Antenatal determination of foetal sex. Indian J. Med. Res. 1965, 53, 213–220. [Google Scholar]
- Manger, J. Untersuchungen zum Problem der Geschlectsdiagnose aus Schwangerenhamn. Dtsch. Med. Wschr. 1933, 59, 885–887. [Google Scholar] [CrossRef]
- Głodek, A. Pregnancy tests in the past and today. Now. Lek. 2008, 77, 377–381. [Google Scholar]
- Marcus, P. The Evolution of the Urine Pregnancy Test. Female Patient. 2011, 36, 41–43. [Google Scholar]
- Kwon, Y.-I. Development History of Pregnancy Test Technology. Korean J. Clin. Lab. Sci. 2018, 50, 382–390. [Google Scholar] [CrossRef]
- Leavitt, S.A. A Private Little Revolution: The Home Pregnancy Test in American Culture. Bull. Hist. Med. 2006, 80, 317–345. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R. Home and Point-of-Care Pregnancy Tests: A Review of the Technology. Epidemiology 2002, 13, 15–18. [Google Scholar] [CrossRef]
- Available online: https://www.understandinganimalresearch.org.uk/news/animal-research-and-pregnancy-testing-a-history (accessed on 30 August 2023).
- Newton, S. Dead Rabbits and Other Historical Pregnancy Tests. Available online: http://mentalfloss.com/article/18569/dead-rabbits-other-historical-pregnancy-tests (accessed on 2 July 2023).
- Marshall, M. The Kyesteine Pellicle: An Early Biological Test for Pregnancy. Bull. Hist. Med. 1948, 22, 178–195. [Google Scholar]
- Olszynko-Gryn, J. The dem and for pregnancy testing: The Aschheim-Zondek reaction, diagnostic versatility, and laboratory services in 1930s Britain. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2014, 47, 233–247. [Google Scholar] [CrossRef]
- Hirose, T. Exogenous stimulation of corpus luteum form ationin the rabbit: Influence of extracts of human placenta, decidua, fetus, hydatidmole, and corpus luteum on the rabbit gonad. J. Jpn. Gynecol. Soc. 1920, 16, 1055. [Google Scholar]
- Cole, L.A. The hCG assay or pregnancy test. Clin. Chem. Lab. Med. 2012, 50, 617–630. [Google Scholar] [CrossRef]
- Kirksey, E.; Hannah, D.; Lotterman, C.; Moore, L.J. The Xenopus Pregnancy Test. A Performative Experiment. Environ. Hum. 2016, 8, 37–56. [Google Scholar] [CrossRef]
- Hobson, B.M. Pregnancy diagnosis. J. Reprod. Fert. 1966, 12, 33–48. [Google Scholar] [CrossRef]
- Elkan, E.R. The Xenopus Pregnancy Test. Br. Med. J. 1938, 2, 1253–1256, 1274-2. [Google Scholar] [CrossRef] [PubMed]
- Zondek, B.; Aschheim, S. The Zondek-Ascheim Pregnancy Test. Can. Med. Assoc. J. 1930, 22, 251–253. [Google Scholar]
- Fried, P.H. An Evaluation of the Two-Hour Rat Test for Pregnancy. Am. J. Obstet. Gynecol. 1947, 54, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.D. The Concentration and Detoxification of Human Urine for Biological Pregnancy Diagnosis. Br. J. Exp. Pathol. 1940, 21, 320–324. [Google Scholar]
- Cuyler, W.K. The Friedman Test: Technique and Interpretation. Available online: https://www.ccjm.org/content/ccjom/4/1/61.full.pdf (accessed on 29 June 2023).
- Thomas, B.; Maght, M.D.; Lawrence, M.; Randall, M.D. Friedman’s hormone test for pregnancy. JAMA 1931, 96, 1933–1935. [Google Scholar] [CrossRef]
- Plass, E.D. Edibility of rabbits used for pregnancy tests. JAMA 1943, 122, 194. [Google Scholar] [CrossRef]
- Elek, S.D. Evaluation of the rat ovarian hyperaemia test for pregnancy diagnosis. Br. Med. J. 1955, 2, 600–602. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valle, J.R. Ovarian Hyperemia in the Immature Ratas a Pregnancy Testin Equids. Proc. Soc. Exp. Biol. Med. 1947, 66, 352–354. [Google Scholar] [CrossRef]
- Bunde, C.A. An evaluation of the pregnancy test based on ovarian hyperemia in the immature rat. Am. J. Obstet. Gynecol. 1947, 53, 317–320. [Google Scholar] [CrossRef]
- Greenblatt, R.B.; Clark, S.L.; West, R.M. The male frog (Rana pipiens) compared to the female rat as a pregnancy test animal. Fertil. Steril. 1950, 1, 533–542. [Google Scholar] [CrossRef]
- Riley, G.M.; Smith, M.H.; Brown, P. The rapid rat test for pregnancy; the ovarian hyperemi are sponse as a routine diagnostic procedure. J. Clin. Endocrinol. Meta 1948, 8, 233–243. [Google Scholar] [CrossRef]
- Murrary, F. Minthorn Xenopus laevis pregnancy test. 1948. Available online: https://digitalcommons.unmc.edu/cgi/viewcontent.cgi?article=2553&context=mdtheses (accessed on 29 June 2023).
- Hogben, L. The Hogben Test. Br. Med. J. 1946, 2, 962–963. [Google Scholar] [CrossRef]
- Polack, S.S. The Xenopus pregnancy test. Can. Med. Assoc. J. 1949, 60, 159–161. [Google Scholar]
- Olszynko-Gryn, J. When Pregnancy Tests Were Toads. Available online: https://wellcomehistory.wordpress.com/2013/02/26/when-pregnancy-tests-were-toads/ (accessed on 24 July 2023).
- Mainini, C.G. Pregnancy test using male toad. J. Clin. Endocrinol. Metab. 1947, 7, 653–658. [Google Scholar] [CrossRef]
- Sulman, F.G.; Sulman, E. Pregnancy test with the male toad (Bufo viridis). J. Obstet. Gynaecol. Br. Emp. 1949, 56, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Soucy, L.B. The use of ordinary toads and frogs for pregnancy tests. Am. J. Med. Technol. 1949, 15, 184–196. [Google Scholar] [PubMed]
- Bhaduri, J.L.; Bardhan, N.R. Male Frogs and Toads as Test Animals for Early Pregnancy and Certain Related Conditions. Science 1949, 109, 517–518. [Google Scholar] [CrossRef]
- Vredenburg, V.T.; Felt, S.A.; Morgan, E.C.; McNally, S.V.; Wilson, S.; Green, S.L. Prevalence of Batrachochytrium dendrobatidis in Xenopus collected in Africa (1871–2000) and in California (2001–2010). PLoS ONE 2013, 8, e63791. [Google Scholar] [CrossRef] [PubMed]
- Talwar, G.P.; Gupta, J.C.; Shankar, N.V. Immunological approach es against human chorionic gonadotropin for control of fertility and therapy of advanced—Stage cancer sex pressing hCG/subunits. Am. J. Reprod. Immunol. 2011, 66, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.R. Genesis and evolution of diagnostic and clinical immunology. Clin. Diagn. Lab. Immunol. 1999, 6, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Wide, L.; Gemzell, C.A. An immunological pregnancy test. Acta Endocrinol. 1960, 35, 261–267. [Google Scholar] [CrossRef]
- Politzer, W.M. Pregnancy Diagnosis-Haemagglutination Inhibition Method (Prepuerin) Compared with the Xenopus Laevis test. S. Afr. Med. J. 1963, 37, 905–910. [Google Scholar]
- Drewnowska, I.; Nowakowska-Kosmalska, B. Comparative assessment of the clinical usefulness of serological pregnancy tests. Diag. Lab. 1968, 4, 171–176. [Google Scholar]
- Hutchins, C.J. A sensitive hemagglutination assay of human chorionic gonadotropin in the diagnosis of ectopic pregnancy. Obstet. Gynecol. 1978, 52, 499–502. [Google Scholar] [PubMed]
- Santomauro, A.G.; Sciarra, J.J. Comparative evaluation of a hemagglutination inhibition test and a latex agglutination inhibition test for hCG. Obstet. Gynecol. 1967, 29, 520–525. [Google Scholar]
- DaSilva, L.S. Report on a simple test for pregnancy (Ortho Gravindex Pregnancy Test). Singap. Med. J. 1964, 4, 111–114. [Google Scholar]
- Gibbsan, C.C.J. An evaluation of current trends in pregnancy testing. SAJOG 1965, 76, 73–74. [Google Scholar]
- Bahl, O.P. Human chorionic gonadotropin. I. Purification and physicochemical properties. J. Biol. Chem. 1969, 244, 567–574. [Google Scholar] [CrossRef]
- Swaminathan, N.; Bahl, O.P. Dissociation and recombination of the subunits of human chorionic gonadotropin. Biochem. Biophys. Res. Commun. 1970, 40, 422–427. [Google Scholar] [CrossRef]
- Merz, W.E.; Hilgenfeldt, U.; Brossmer, R.; Rehberger, G. Amino acid and carbohydrate composition of human chorionic gonadotropin fractions obtained by isoelectric focusing. Hoppe Seylers Z. Physiol. Chem. 1974, 355, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Morgan, F.J.; Birken, S.; Canfield, R.E. The amino acid sequence of human chorionic gonadotropin. The α-subunit and the β-subunit. J. Biol. Chem. 1975, 250, 5247–5258. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.M.; Kardana, A.; Lustbader, J.W.; Cole, L.A. Carbohydrate and Peptide structure of the α- and β-subunits of human chorionic gonadotropin from normal and aberrant pregnancy and choriocarcinoma. Endocrine 1997, 7, 15–32. [Google Scholar] [CrossRef]
- Kobata, A.; Takeuchi, M. Structure pathology and function of the N-linked sugar chains of hCG. Biochim. Biophys. Acta 1999, 1455, 315–326. [Google Scholar] [CrossRef]
- Midgley, A.R., Jr. Radioimmunoassay: A method for human chorionic gonadotropin and human luteinizing hormone. Endocrinology 1966, 79, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Vaitukaitis, J.L.; Braunstein, G.D.; Ross, G.T. A radioimmunoassay which specifically measures human chorionic gonadotropinin the presence of human luteinizing hormone. Am. J. Obstet. Gynecol. 1972, 113, 751–758. [Google Scholar] [CrossRef]
- Pastorfide, B.; Goldstein, D.P.; Kosasa, T.S. The use of a radioimmunoassay specific for human chorionic gonadotropin in patients with molar pregnancy and gestational trophoblastic disease. Am. J. Obstet. Gynecol. 1974, 120, 1025–1028. [Google Scholar] [CrossRef]
- Kosasa, T.S.; Levesque, L.A.; Goldstein, D.P.; Taymor, M.L. Clinical use of a solid-phase radioimmunoassay specific for human chorionic gonadotropin. Am. J. Obstet. Gynecol. 1974, 119, 784–791. [Google Scholar] [CrossRef]
- Chartier, M.; Roger, M.; Barrat, J.; Michelon, B. Measurement of plasma human chorionic gonadotropin (hCG) and beta-hCG activities in the late luteal phase: Evidence of the occurrence of spontaneous menstrual abortions in infertile women. Fertil. Steril. 1979, 31, 134–137. [Google Scholar] [CrossRef]
- Boyko, W.L.; Barrett, B. Detection and quantitation of the beta-subunit of human chorionic gonadotropin in serum by radioimmunoassay. Fertil. Steril. 1980, 33, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Saxena, B.B.; Hasan, S.H.; Haour, F.; Schmidt-Gollwitzer, M. Radioreceptor assay of human chorionic gonadotropin: Detection of early pregnancy. Science 1974, 184, 793–795. [Google Scholar] [CrossRef]
- Shimizu, S.Y.; Present, W.A.; Sevier, E.D.; Wang, R.; Saunders, R.L. Chorigonadotropin measured by use of monoclonal antibodies in a two-site immunoradiometricassay. Clin. Chem. 1982, 28, 546–547. [Google Scholar] [CrossRef]
- World Health Organization. Developments in radioimmunoassay and related procedures. In Proceedings of an International Symposium on Radioimmunoassay and Related Procedures: Perspectives in Developing Countries Organized by the International Atomic Energy Agency in cooperation with the World Health Organization and Held in Vienna, Vienna, Austria, 26–30 August 1991. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/23/058/23058638.pdf (accessed on 25 July 2023).
- Lacy, A.; O’Kennedy, R. Immunoassays, Techniques—Radioimmunoassays. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 335–344. ISBN 9780123693976. [Google Scholar] [CrossRef]
- Atmar, R.L. Immunological Detection and Characterization. In Viral Infections of Humans: Epidemiology and Control; Springer: Berlin/Heidelberg, Germany, 2014; pp. 47–62. [Google Scholar] [CrossRef]
- Koivunen, M.E.; Krogsrud, R.L. Principles of Immunochemical Techniques Used in Clinical Laboratories. Lab. Med. 2006, 37, 490–497. [Google Scholar] [CrossRef]
- Wada, H.G.; Danisch, R.J.; Baxter, S.R.; Federici, M.M.; Fraser, R.C.; Brownmiller, L.J.; Lankford, J.C. Enzyme immunoassay of the glycoprotein tropic hormones—Choriogonadotropin, lutropin, thyrotropin—With solid–phase monoclonal antibody for the alpha-subunit and enzyme-coupled monoclonal antibody specific for the beta-subunit. Clin. Chem. 1982, 28, 1862–1866. [Google Scholar] [CrossRef]
- Layne, L.L. The Home Pregnancy Test: A Feminist Technology? WSQ: Women’s Stud. Q. 2009, 37, 61–79. [Google Scholar] [CrossRef]
- Available online: https://sitn.hms.harvard.edu/flash/2018/pee-pregnant-history-science-urine-based-pregnancy-tests/ (accessed on 8 August 2023).
- Rodney, M.B. Do rapid immunochemical pregnancy tests work? Clues to clinical interpretation. J. Natl. Med. Assoc. 1966, 58, 359–360. [Google Scholar]
- Advertisement for e.p.t, Vogue, March 1978. Available online: https://history.nih.gov/display/history/Pregnancy+Test+Popular+Culture+-+Advertisements (accessed on 8 August 2023).
- Available online: https://beachpackagingdesign.com/boxvox/packaging-in-the-news-meg-cranes-pregnancy-test (accessed on 8 August 2023).
- Advertisement for ACU-TEST, Mademoiselle, December 1978. Available online: http://history.nih.gov/exhibits/thinblueline/culture1.html (accessed on 8 August 2023).
- Directions and Technical Information on UCG-TEST. 1970. Available online: https://history.nih.gov/display/history/Pregnancy+Test+Timeline?preview=/8882331/8882334/wampole_1970_2_popup.jpg (accessed on 8 August 2023).
- Available online: https://patentimages.storage.googleapis.com/4d/bc/da/778944754b72e6/US3579306.pdf (accessed on 8 August 2023).
- Available online: https://uomustansiriyah.edu.iq/media/lectures/6/6_2022_11_07!12_03_01_AM.pdf (accessed on 8 August 2023).
- Meri, S.; von Bonsdorff, C.H. Complement Fixation Test. In Encyclopedia of Immunology, 2nd ed.; Delves, P.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 617–619. [Google Scholar] [CrossRef]
- Nesargikar, P.N.; Spiller, B.; Chavez, R. The complement system: History, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. 2012, 2, 103–111. [Google Scholar] [CrossRef]
- Loraine, J.A. Assays of human chorionic gonadotrophin in relation to clinical practice. J. Reprod. Fertil. 1966, 12, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tyler, E.T.; Nakabayashi, N.T.; Gotlib, M.H. An immunologic test for pregnancy. Fertil. Steril. 1964, 15, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.L. Comparative study of immunological tests for pregnancy diagnosis. J. Clin. Pathol. 1969, 22, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://clickamericana.com/eras/1970s/early-home-pregnancy-tests-1978-1985 (accessed on 27 April 2023).
- Waters, K. Structure, Mechanism, and Principle of Immunochromatography for Antigen Detection. J. Forensic. Biomech. 2022, 13, 398. [Google Scholar]
- Baker, A.N.; Congdon, T.R.; Richards, S.J.; Georgiou, P.G.; Walker, M.; Dedola, S.; Field, R.A.; Gibson, M.I. End-Functionalized Poly(vinylpyrrolidone) for Ligand Display in Lateral Flow Device Test Lines. ACS Polym. Au. 2022, 2, 69–79. [Google Scholar] [CrossRef]
- Sturgeon, C.; Butler, S.A.; Gould, F.; Johnson, S.; Rowlands, S.; Stenman, U.H.; Grenache, D.G. Recommendations for validation testing of home pregnancy tests (HPTs) in Europe. Clin. Chem. Lab. Med. 2021, 59, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.A.; Khanlian, S.A.; Sutton, J.M.; Davies, S.; Rayburn, W.F. Accuracy of home pregnancy tests at the time of missed menses. Am. J. Obstet. Gynecol. 2004, 190, 100–105. [Google Scholar] [CrossRef]
- Tomlinson, C.; Marshall, J.; Ellis, J. Comparison of accuracy and certainty of results of six home pregnancy tests available over-the-counter. Curr. Med. Res. Opin. 2008, 24, 1645–1649. [Google Scholar] [CrossRef]
- Johnson, S.; Cushion, M.; Bond, S.; Godbert, S.; Pike, J. Comparison of analytical sensitivity and women’s interpretation of home pregnancy tests. Clin. Chem. Lab. Med. 2015, 53, 391–402. [Google Scholar] [CrossRef]
- Available online: https://www.clearblue.com/pregnancy-tests/early-detection (accessed on 8 August 2023).
- Available online: https://www.firstresponse.com/en (accessed on 8 August 2023).
- Annan, J.J.; Gudi, A.; Bhide, P.; Shah, A.; Homburg, R. Biochemical pregnancy during assisted conception: A little bit pregnant. J. Clin. Med. Res. 2013, 5, 269–274. [Google Scholar] [CrossRef]
- Ross, E. Provisionally pregnant: Uncertainty and interpretive work in accounts of home pregnancy testing. Health 2018, 22, 87–105. [Google Scholar] [CrossRef]
- Pike, J.; Godbert, S.; Johnson, S. Comparison of volunteers’ experience of using, and accuracy of reading, different types of home pregnancy test formats. Expert Opin. Med. Diagn. 2013, 7, 435–441. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/K060128.pdf (accessed on 9 August 2023).
- Available online: https://patentimages.storage.googleapis.com/56/37/ac/e2af04b5596cd8/US20140273028A1.pdf (accessed on 9 August 2023).
- Johnson, S.R.; Godbert, S.; Perry, P.; Parsons, P.; Roberts, L.; Buchanan, P.; Larsen, J.; Alonzo, T.A.; Zinaman, M. Accuracy of a home-based device for giving an early estimate of pregnancy duration compared with reference methods. Fertil. Steril. 2013, 100, 1635–1641.e1. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://uk.clearblue.com/sites/default/files/hcp_cb9_professional_brochure.pdf (accessed on 9 August 2023).
- Available online: https://mmrstrategy.com/case_study/an-unclear-case-of-false-advertising/ (accessed on 9 August 2023).
- Available online: https://www.firstresponse.com/en-CA/Products/Pregnancy/~/media/21FDD3B1871044259EB3CF56EC4A5689.ashx (accessed on 9 August 2023).
- Available online: https://natalist.com/blogs/learn/digital-vs-regular-what-s-the-best-home-pregnancy-test-1 (accessed on 9 August 2023).
- Braunstein, G.D. The long gestation of the modern home pregnancy test. Clin. Chem. 2014, 60, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.E.; Yeh, P.T.; Gholbzouri, K.; Narasimhan, M. Self-testing for pregnancy: A systematic review and meta-analysis. BMJ Open 2022, 12, e054120. [Google Scholar] [CrossRef] [PubMed]





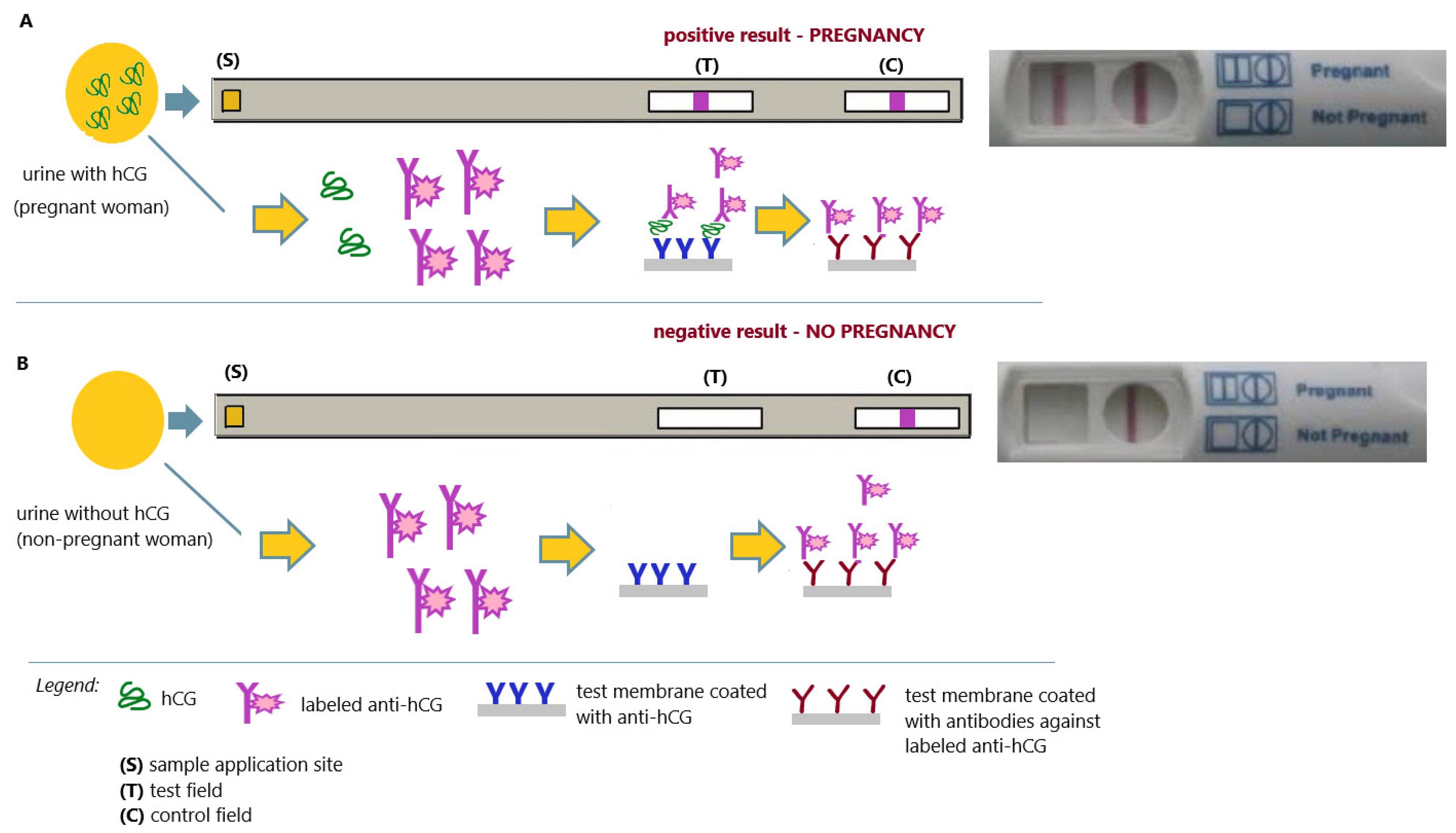
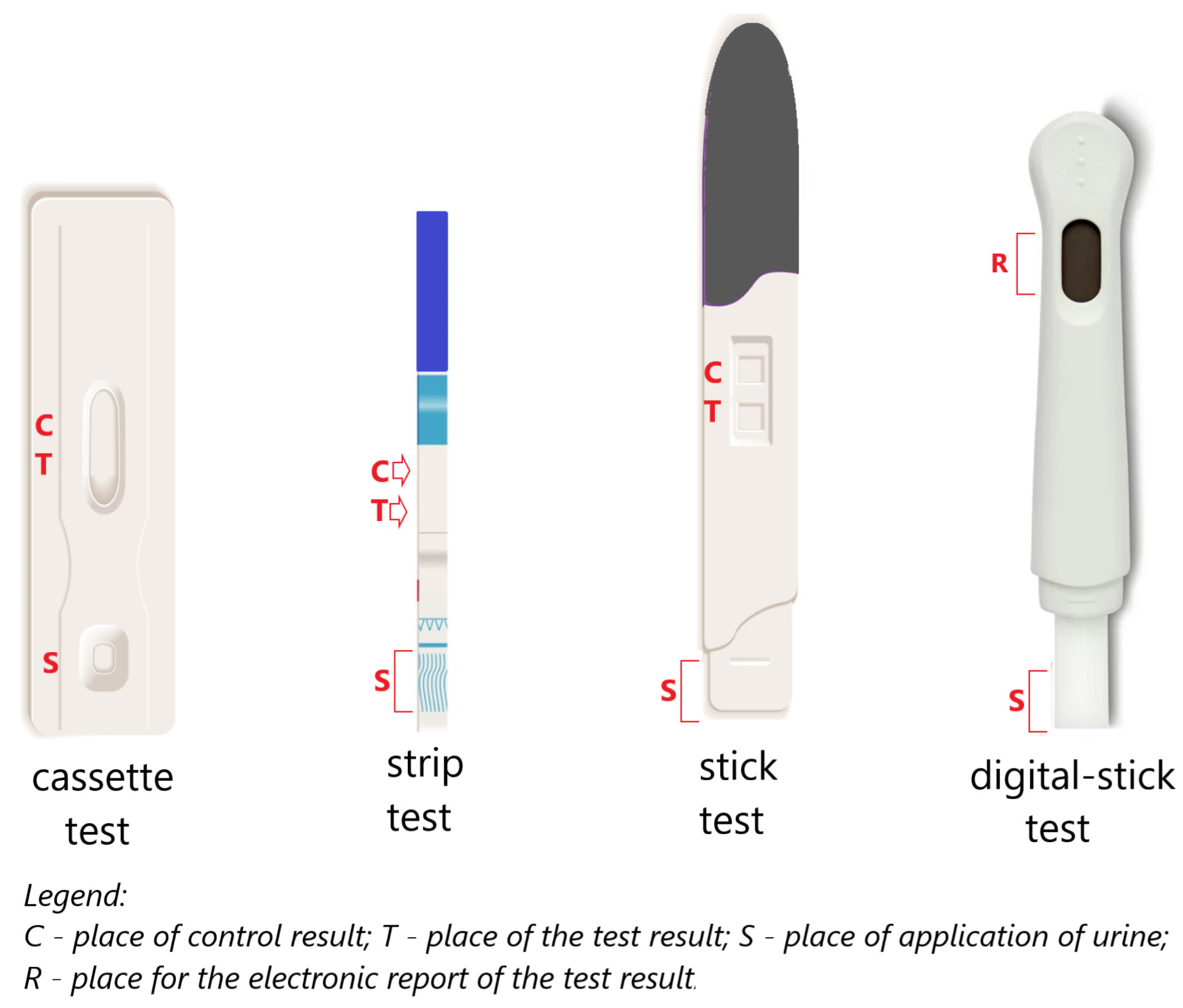
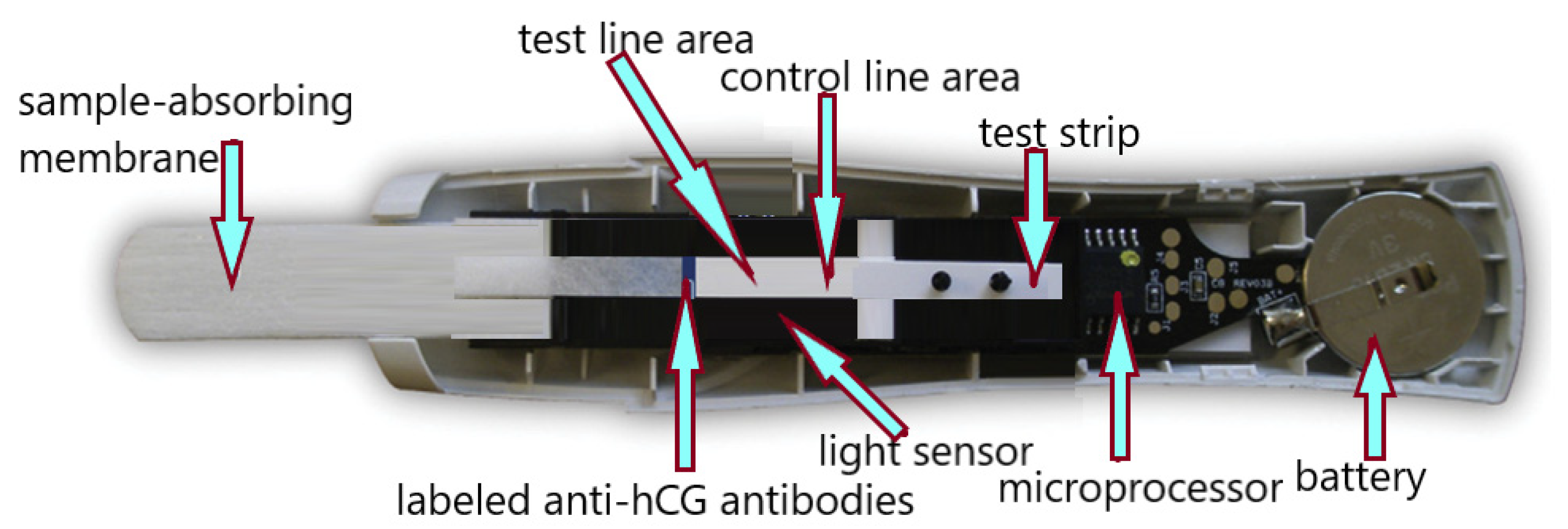


| A Significant History Point | Test Type and/or Test Technique | Interpretation |
|---|---|---|
| Ancient Egypt | cereal grains watered with woman’s urine (sprout test) | if the watered grains sprout—the woman is pregnant |
| From Hippocrates to Gallen | cereal grains watered with woman’s urine (sprout test) | if the watered grains sprout—the woman is pregnant |
| onion test | an onion was inserted into the woman’s vagina and left there overnight—if in the morning a woman’s breath smelled of onions, she was not pregnant | |
| drinking milk (milk test) | if a woman felt sick after drinking milk, she was pregnant | |
| From the Middle Ages through the Seventeenth Century | visual analysis of a woman’s urine | when the urine is clear, light lemon, turning to whitish, with a foamy surface—the woman is pregnant |
| urine/milk flotation test | milk floats on the surface of a pregnant woman’s urine | |
| urine/needle test | a needle inserted into a vial of pregnant woman’s urine turns rust-red or black | |
| wine/urine mixing test | precipitation—the woman is pregnant | |
| sweet drink test before bedtime | navel pain in the morning meant pregnancy | |
| ribbon/urine odor test | if the smell of a ribbon dipped in a woman’s urine made a woman vomit or feel sick, she was pregnant | |
| ribbon-urinary-flame test | if the smell of a ribbon dipped in a woman’s urine and burned in a flame makes a woman sick, the woman is pregnant | |
| Nineteenth Century | urine film formation test “Kyesteine pellicle” | if a sticky film forms on the surface of the urine—the woman is pregnant |
| From the 1920s to the 1960s | Aschheim–Zondek test (mouse/rat test) (laboratory) | a woman’s urine was injected into male rats or female mice—if the rat was sexually aroused and the mice were ovulating, the woman whose urine was injected into the animal was pregnant |
| Friedman test (rabbit test) (laboratory) | a woman’s urine was injected into a female rabbit—if the rabbit ovulated, the woman whose urine was injected into the animal was pregnant | |
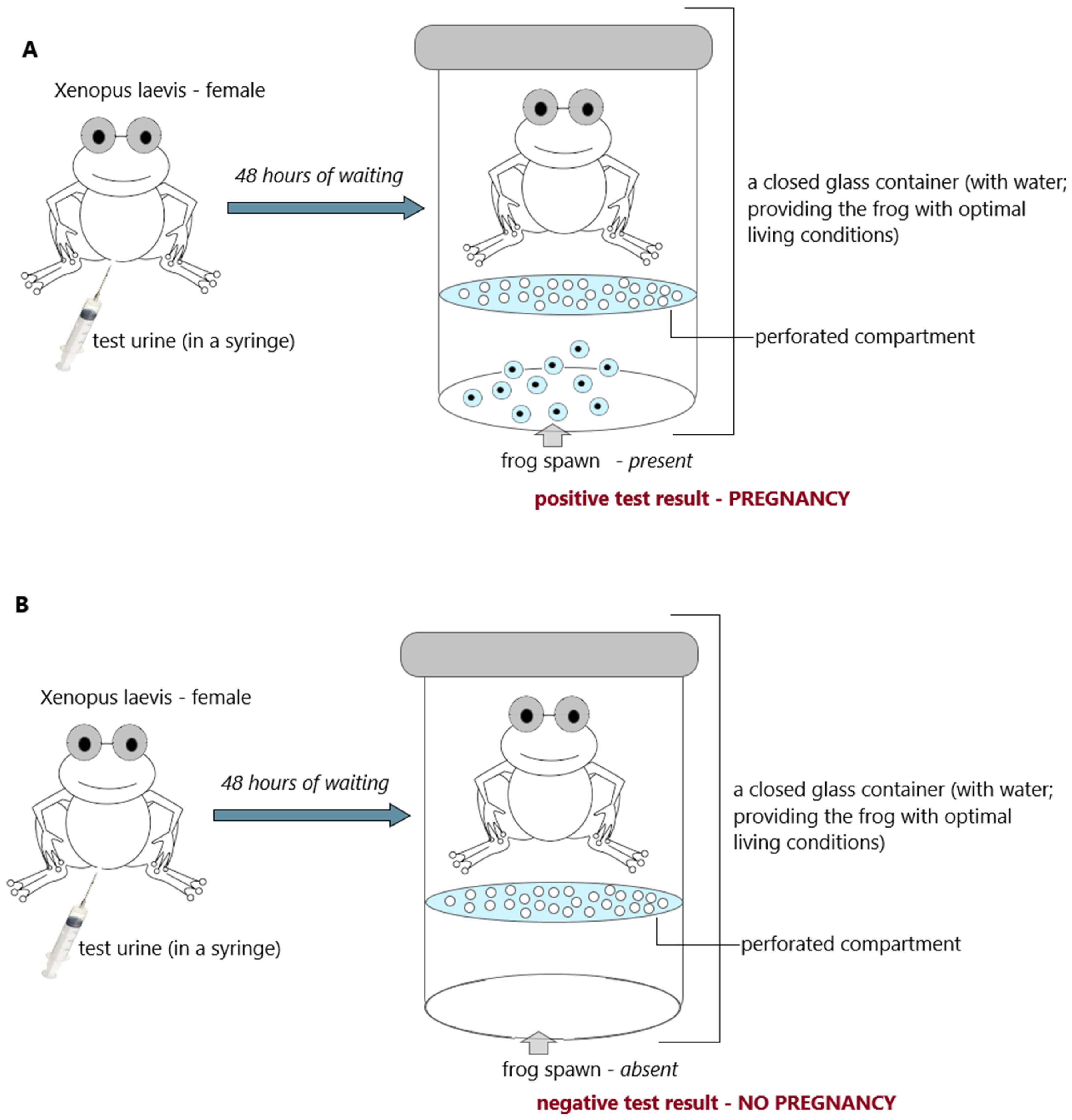
| Hogben test (frog test) (laboratory) | a female Xenopus laevis frog stimulated by an injection of pregnant woman’s urine produces a spawn | |
| Galli Maininitest (toad test) (laboratory) | injecting a pregnant woman’s urine into the toad’s (Bufoarenarum Hensel) lymphatic sac stimulates the animal to produce sperm | |
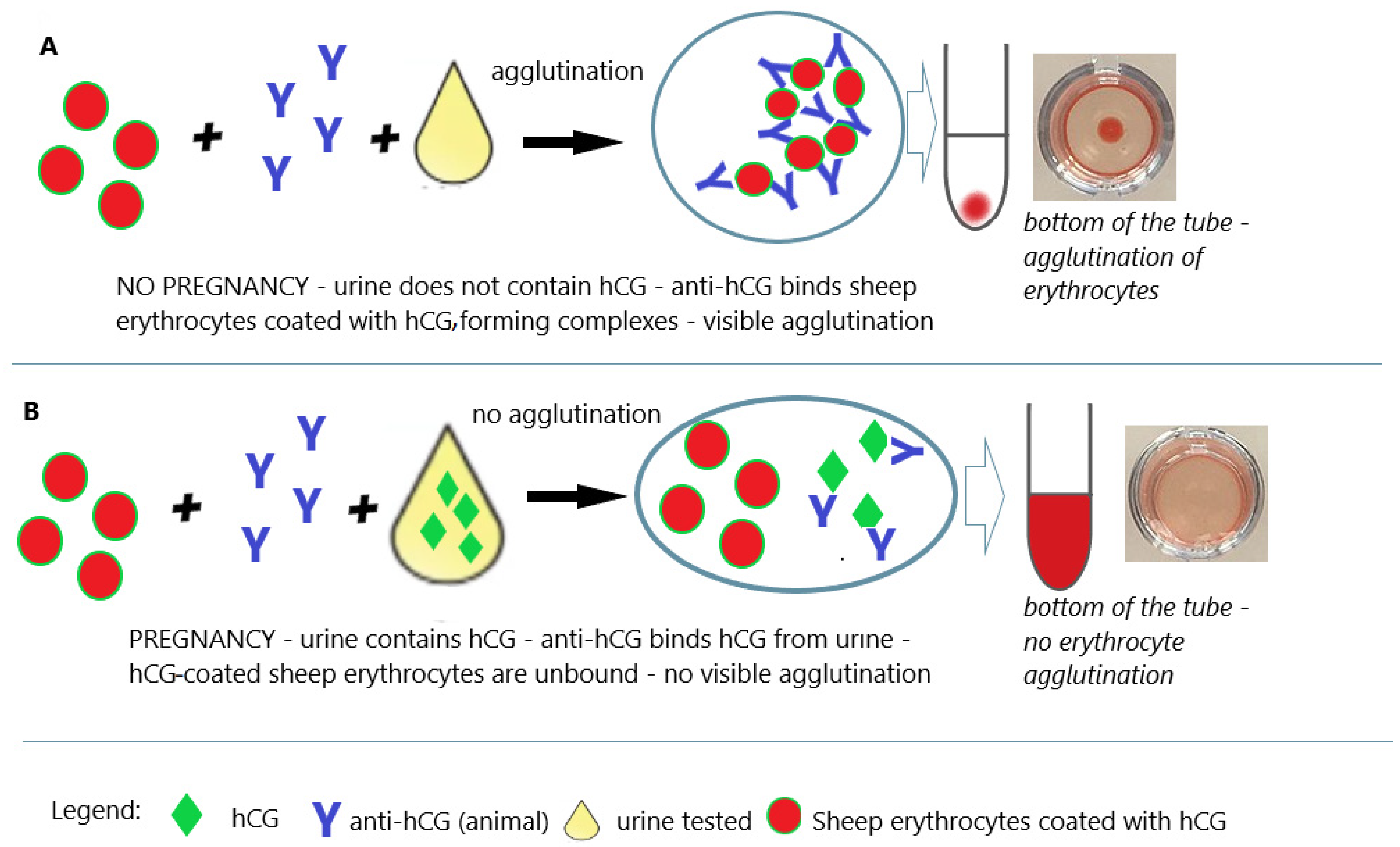
| The 1960s | First immunological tests | hemagglutination inhibition test (Wide–Gemzell test) |
| latex agglutination inhibition test (slide test) | ||
| complement fixation test | ||
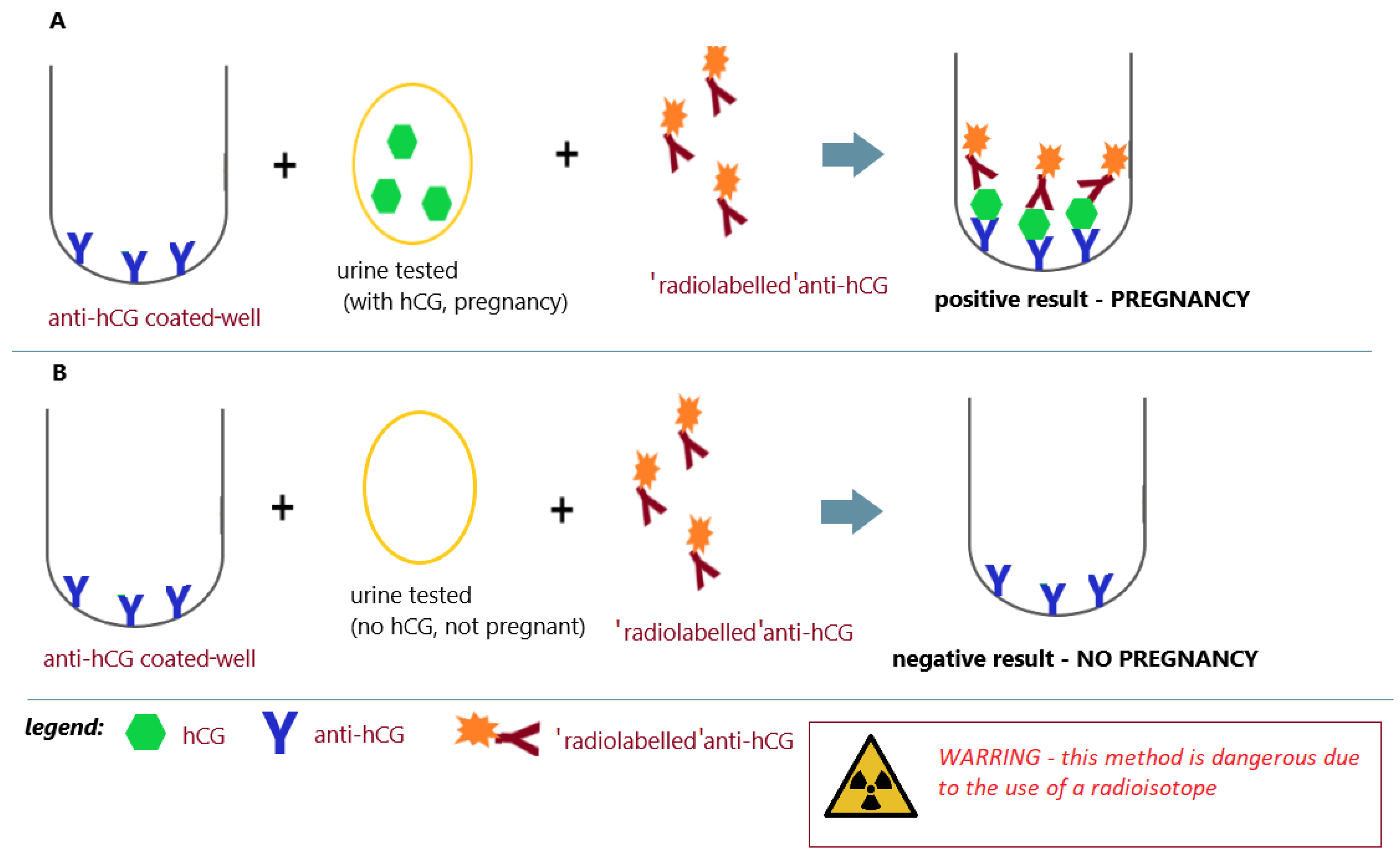
| The 1970s | radioimmunoassay (laboratory) | immunological tests using radiation-generating tracers |
| From the 1970s/1980s to the present | immunochemical tests (laboratory) | enzyme immunoassays using colored or fluorescent markers |
| immunochromatographic tests using colored or fluorescent markers | ||
| home pregnancy tests (based on immunological techniques) | complement fixation test | |
| strip or cassette immunochromatographic tests with colorimetric detection or detection based on biosensors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis, K. From Cereal Grains to Immunochemistry—What Role Have Antibodies Played in the History of the Home Pregnancy Test. Antibodies 2023, 12, 56. https://doi.org/10.3390/antib12030056
Lis K. From Cereal Grains to Immunochemistry—What Role Have Antibodies Played in the History of the Home Pregnancy Test. Antibodies. 2023; 12(3):56. https://doi.org/10.3390/antib12030056
Chicago/Turabian StyleLis, Kinga. 2023. "From Cereal Grains to Immunochemistry—What Role Have Antibodies Played in the History of the Home Pregnancy Test" Antibodies 12, no. 3: 56. https://doi.org/10.3390/antib12030056
APA StyleLis, K. (2023). From Cereal Grains to Immunochemistry—What Role Have Antibodies Played in the History of the Home Pregnancy Test. Antibodies, 12(3), 56. https://doi.org/10.3390/antib12030056





