The Usefulness of Thyroid Antibodies in the Diagnostic Approach to Autoimmune Thyroid Disease
Abstract
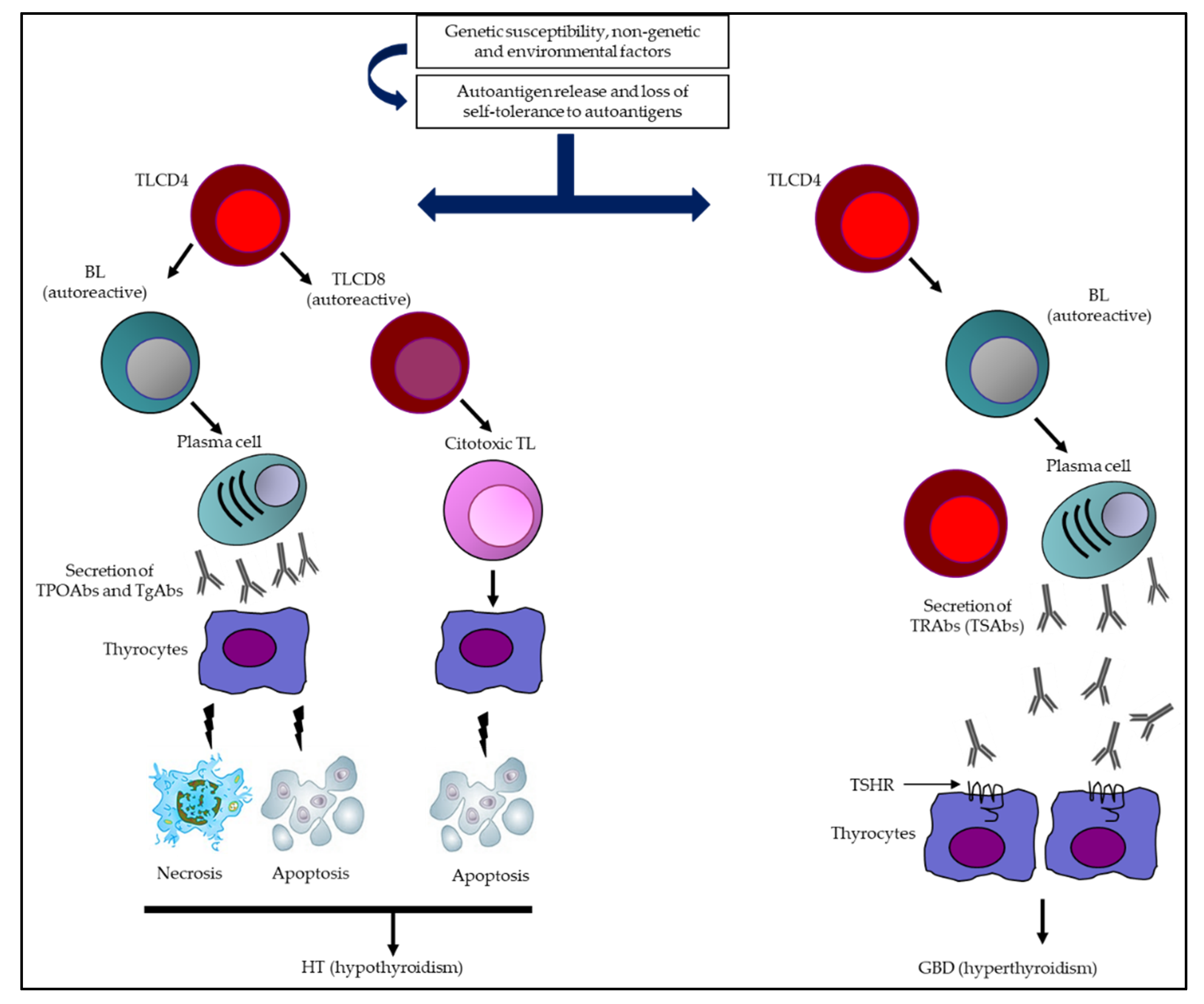
1. Introduction
2. Methods (Search Strategy)
3. Major Thyroid Antigens
3.1. Tg
3.2. TPO
3.3. TSHR
4. Minor Thyroid Antigens
4.1. NIS
4.2. PDN
4.3. Meg
5. Major Thyroid Abs
5.1. TgAbs
5.2. TPOAbs
5.3. TRAbs
5.4. NISAbs and PDNAbs
5.5. MegAbs
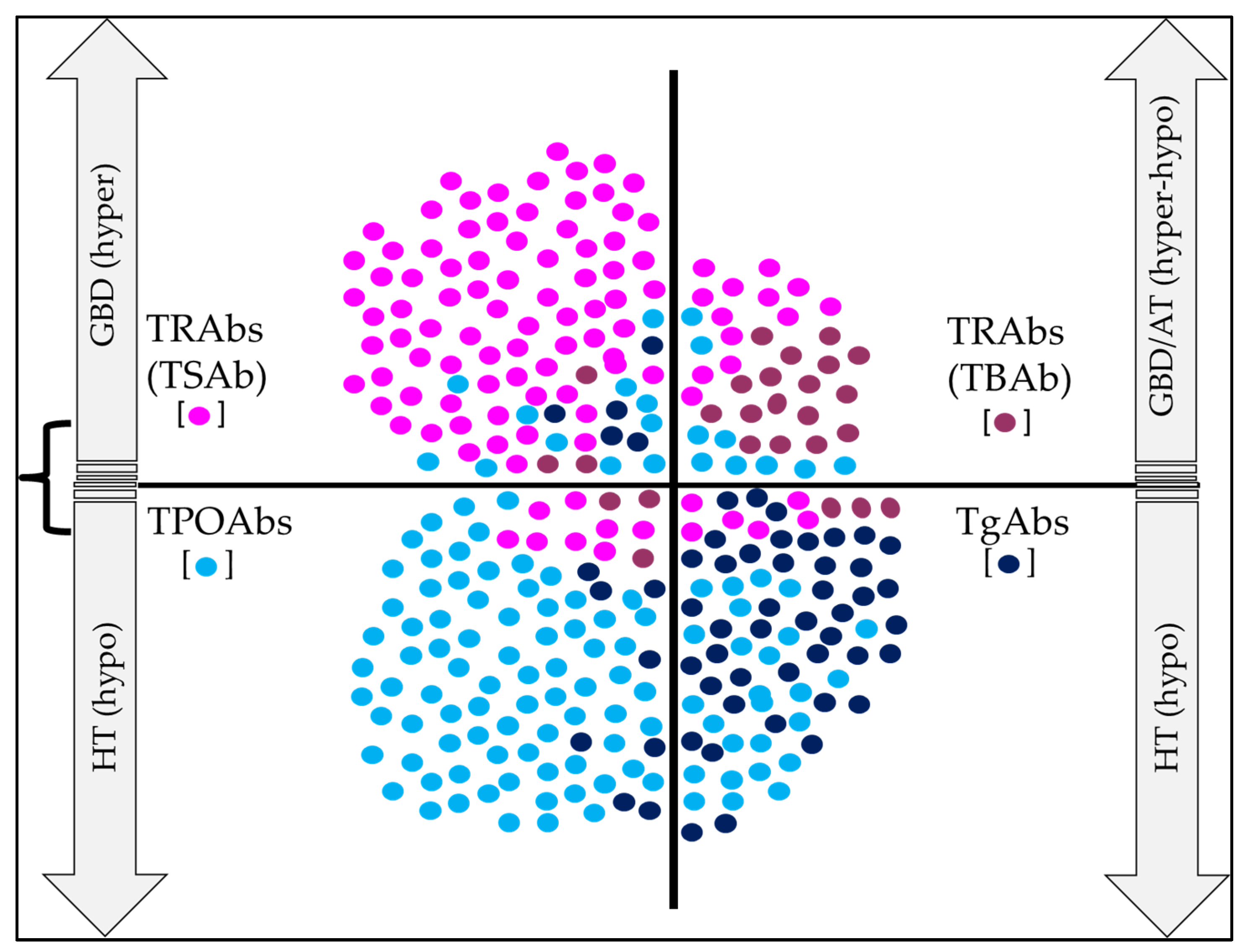
6. Clinical Utility of Thyroid Abs in AITD
6.1. Clinical Utility of TgAbs
6.2. Clinical Utility of TPOAbs
6.3. Clinical Utility of TRAbs
- a.
- In individuals with HT and hypothyroidism, where there is adequate and stable control with very low doses of levothyroxine, since it is possible in this type of patient that a release of TBAb has occurred, causing a type of transient hypothyroidism, which could have a high recovery rate;
- b.
- In a newborn born to a mother with HT but with the presence of contradictory or bizarre clinical findings;
- c.
- In patients with HT and clinical findings suggestive of thyroid ophthalmopathy;
- d.
- When in individuals with long-standing hypothyroidism, under treatment with stable doses of levothyroxine, a change to the state of hyperthyroidism is noted;
- e.
- When alternating periods of hyperthyroidism and hypothyroidism occur in the same patient.
6.4. Clinical Utility of PDNAbs, NISAbs, MegAbs, and Other Abs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skevaki, C.; Wesemann, D.R. Antibody repertoire and autoimmunity. J. Allergy Clin. Immunol. 2023, 151, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Huffaker, M.F.; Sanda, S.; Chandran, S.; Chung, S.A.; Clair, E.W.S.; Nepom, G.T.; Smilek, D.E. Approaches to Establishing Tolerance in Immune Mediated Diseases. Front. Immunol. 2021, 12, 744804. [Google Scholar] [CrossRef]
- Petersone, L.; Edner, N.M.; Ovcinnikovs, V.; Heuts, F.; Ross, E.M.; Ntavli, E.; Wang, C.J.; Walker, L.S.K. T Cell/B Cell Collaboration and Autoimmunity: An Intimate Relationship. Front. Immunol. 2018, 9, 1941. [Google Scholar] [CrossRef] [PubMed]
- Corneth, O.B.J.; Neys, S.F.H.; Hendriks, R.W. Aberrant B Cell Signaling in Autoimmune Diseases. Cells 2022, 11, 3391. [Google Scholar] [CrossRef] [PubMed]
- Samuels, H.; Malov, M.; Detroja, T.S.; Ben Zaken, K.; Bloch, N.; Gal-Tanamy, M.; Avni, O.; Polis, B.; Samson, A.O. Autoimmune Disease Classification Based on PubMed Text Mining. J. Clin. Med. 2022, 11, 4345. [Google Scholar] [CrossRef]
- Hundt, J.E.; Hoffmann, M.H.; Amber, K.T.; Ludwig, R.J. Editorial: Autoimmune pre-disease. Front. Immunol. 2023, 14, 1159396. [Google Scholar] [CrossRef]
- Chi, X.; Huang, M.; Tu, H.; Zhang, B.; Lin, X.; Xu, H.; Dong, C.; Hu, X. Innate and adaptive immune abnormalities underlying autoimmune diseases: The genetic connections. Sci. China Life Sci. 2023, 66, 1482–1517. [Google Scholar] [CrossRef]
- Mazzone, R.; Zwergel, C.; Artico, M.; Taurone, S.; Ralli, M.; Greco, A.; Mai, A. The emerging role of epigenetics in human autoimmune disorders. Clin. Epigenetics 2019, 11, 34. [Google Scholar] [CrossRef]
- Bogusławska, J.; Godlewska, M.; Gajda, E.; Piekiełko-Witkowska, A. Cellular and molecular basis of thyroid autoimmunity. Eur. Thyroid. J. 2022, 11, e210024. [Google Scholar] [CrossRef]
- McLachlan, S.M.; Rapoport, B. Discoveries in Thyroid Autoimmunity in the Past Century. Thyroid 2023, 33, 278–286. [Google Scholar] [CrossRef]
- Rahimova, R.R. Autoimmune thyroiditis (review of literature). Russ. Clin. Lab. Diagn. 2022, 67, 286–291. [Google Scholar] [CrossRef]
- Stasiak, M.; Lewiński, A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev. Endocr. Metab. Disord. 2021, 22, 1027–1039. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H. Molecular Mechanisms in Autoimmune Thyroid Disease. Cells 2023, 12, 918. [Google Scholar] [CrossRef]
- Daramjav, N.; Takagi, J.; Iwayama, H.; Uchino, K.; Inukai, D.; Otake, K.; Ogawa, T.; Takami, A. Autoimmune Thyroiditis Shifting from Hashimoto’s Thyroiditis to Graves’ Disease. Medicina 2023, 59, 757. [Google Scholar] [CrossRef]
- Wémeau, J.-L.; Klein, M.; Sadoul, J.-L.; Briet, C.; Vélayoudom-Céphise, F.-L. Graves’ disease: Introduction, epidemiology, endogenous and environmental pathogenic factors. Ann. Endocrinol. 2018, 79, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y. Graves’ Disease is a Thyroid Autoimmune Disorder Identified by Excessive Thyroid Hormone Production. Thyroid. Disorders Ther. 2023, 12, 294. [Google Scholar]
- Coscia, F.; Taler-Verčič, A.; Chang, V.T.; Sinn, L.; O’reilly, F.J.; Izoré, T.; Renko, M.; Berger, I.; Rappsilber, J.; Turk, D.; et al. The structure of human thyroglobulin. Nature 2020, 578, 627–630. [Google Scholar] [CrossRef]
- Citterio, C.E.; Rivolta, C.M.; Targovnik, H.M. Structure and genetic variants of thyroglobulin: Pathophysiological implications. Mol. Cell. Endocrinol. 2021, 528, 111227. [Google Scholar] [CrossRef] [PubMed]
- Tosatto, L.; Coscia, F. A glance at post-translational modifications of human thyroglobulin: Potential impact on function and pathogenesis. Eur. Thyroid. J. 2022, 11, e220046. [Google Scholar] [CrossRef]
- Godlewska, M.; Gawel, D.; Buckle, A.M.; Banga, J.P. Thyroid Peroxidase Revisited—What’s New? Horm. Metab. Res. 2019, 51, 765–769. [Google Scholar] [CrossRef]
- Ruf, J.; Carayon, P. Structural and functional aspects of thyroid peroxidase. Arch. Biochem. Biophys. 2006, 445, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, M.; Banga, P.J. Thyroid peroxidase as a dual active site enzyme: Focus on biosynthesis, hormonogenesis and thyroid disorders of autoimmunity and cancer. Biochimie 2019, 160, 34–45. [Google Scholar] [CrossRef]
- Williams, D.E.; Le, S.N.; Godlewska, M.; Hoke, D.E.; Buckle, A.M. Thyroid Peroxidase as an Autoantigen in Hashimoto’s Disease: Structure, Function, and Antigenicity. Horm. Metab. Res. 2018, 50, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Raja, K.; Schweizer, U.; Mugesh, G. Chemistry and Biology in the Biosynthesis and Action of Thyroid Hormones. Angew. Chem. Int. Ed. 2016, 55, 7606–7630. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-D.; Yeh, C.-T. The Molecular Function and Clinical Role of Thyroid Stimulating Hormone Receptor in Cancer Cells. Cells 2020, 9, 1730. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.H.; Rodrigues, D.; Paiva, I. The Mysterious Universe of the TSH Receptor. Front. Endocrinol. 2022, 13, 944715. [Google Scholar] [CrossRef]
- Marín-Sánchez, A.; Álvarez-Sierra, D.; González, O.; Lucas-Martin, A.; Sellés-Sánchez, A.; Rudilla, F.; Enrich, E.; Colobran, R.; Pujol-Borrell, R. Regulation of TSHR Expression in the Thyroid and Thymus May Contribute to TSHR Tolerance Failure in Graves’ Disease Patients via Two Distinct Mechanisms. Front. Immunol. 2019, 10, 1695. [Google Scholar] [CrossRef]
- Kleinau, G.; Vassart, G. TSH Receptor Mutations and Diseases. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Gershengorn, M.C.; Osman, R. Molecular and cellular biology of thyrotropin-releasing hormone receptors. Physiol. Rev. 1996, 76, 175–191. [Google Scholar] [CrossRef]
- Rapoport, B.; McLachlan, S.M. TSH Receptor Cleavage Into Subunits and Shedding of the A-Subunit; A Molecular and Clinical Perspective. Endocr. Rev. 2016, 37, 114–134. [Google Scholar] [CrossRef]
- Helfinger, L.; Tate, C.G. Expression and Purification of the Human Thyroid-Stimulating Hormone Receptor. In Heterologous Expression of Membrane Proteins; Springer: New York, NY, USA, 2022; Volume 2507, pp. 313–325. [Google Scholar] [CrossRef]
- Führer, D. Constitutive TSH receptor activation as a hallmark of thyroid autonomy. Endocrine 2020, 68, 274–278. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E. Glycosylation of thyroid-stimulating hormone receptor. Endokrynol. Polska 2019, 70, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Darrouzet, E.; Lindenthal, S.; Marcellin, D.; Pellequer, J.-L.; Pourcher, T. The sodium/iodide symporter: State of the art of its molecular characterization. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838 Pt B, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Reyna-Neyra, A.; Ferrandino, G.; Amzel, L.M.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 2017, 79, 261–289. [Google Scholar] [CrossRef] [PubMed]
- Riesco-Eizaguirre, G.; Santisteban, P.; De la Vieja, A. The complex regulation of NIS expression and activity in thyroid and extrathyroidal tissues. Endocrine-Related Cancer 2021, 28, T141–T165. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J.; Fletcher, A.; Brookes, K.; Nieto, H.; Alshahrani, M.M.; Mueller, J.W.; Fine, N.H.; Hodson, D.J.; Boelaert, K.; Read, M.; et al. Dimerization of the Sodium/Iodide Symporter. Thyroid 2019, 29, 1485–1498. [Google Scholar] [CrossRef]
- Portulano, C.; Paroder-Belenitsky, M.; Carrasco, N. The Na+/I− Symporter (NIS): Mechanism and Medical Impact. Endocr. Rev. 2014, 35, 106–149. [Google Scholar] [CrossRef]
- Ravera, S.; Nicola, J.P.; Simone, G.S.-D.; Sigworth, F.J.; Karakas, E.; Amzel, L.M.; Bianchet, M.A.; Carrasco, N. Structural insights into the mechanism of the sodium/iodide symporter. Nature 2022, 612, 795–801. [Google Scholar] [CrossRef]
- Rozenfeld, J.; Efrati, E.; Adler, L.; Tal, O.; Carrithers, S.L.; Alper, S.L.; Zelikovic, I. Transcriptional Regulation of the Pendrin Gene. Cell. Physiol. Biochem. 2011, 28, 385–396. [Google Scholar] [CrossRef]
- Dossena, S.; Nofziger, C.; Tamma, G.; Bernardinelli, E.; Vanoni, S.; Nowak, C.; Grabmayer, E.; Kössler, S.; Stephan, S.; Patsch, W.; et al. Molecular and Functional Characterization of Human Pendrin and its Allelic Variants. Cell. Physiol. Biochem. 2011, 28, 451–466. [Google Scholar] [CrossRef]
- Dossena, S.; Bizhanova, A.; Nofziger, C.; Bernardinelli, E.; Ramsauer, J.; Kopp, P.; Paulmichl, M. Identification of Allelic Variants of Pendrin (SLC26A4) with Loss and Gain of Function. Cell. Physiol. Biochem. 2011, 28, 467–476. [Google Scholar] [CrossRef]
- Bizhanova, A.; Kopp, P. Controversies Concerning the Role of Pendrin as an Apical Iodide Transporter in Thyroid Follicular Cells. Cell. Physiol. Biochem. 2011, 28, 485–490. [Google Scholar] [CrossRef]
- Twyffels, L.; Massart, C.; Golstein, P.E.; Raspe, E.; Van Sande, J.; Dumont, J.E.; Beauwens, R.; Kruys, V. Pendrin: The Thyrocyte Apical Membrane Iodide Transporter? Cell. Physiol. Biochem. 2011, 28, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Marino, M.; Zhao, J.; McCluskey, R.T. Megalin (gp330): A Putative Endocytic Receptor for Thyroglobulin (Tg). Endocrinology 1998, 139, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Marinò, M.; Pinchera, A.; McCluskey, R.T.; Chiovato, L. Megalin in Thyroid Physiology and Pathology. Thyroid 2001, 11, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Marinò, M.; Zheng, G.; Chiovato, L.; Pinchera, A.; Brown, D.; Andrews, D.; McCluskey, R.T. Role of Megalin (gp330) in Transcytosis of Thyroglobulin by Thyroid Cells. A novel function in the control of thyroid hormone release. J. Biol. Chem. 2000, 275, 7125–7137. [Google Scholar] [CrossRef]
- Goto, S.; Hosojima, M.; Kabasawa, H.; Saito, A. The endocytosis receptor megalin: From bench to bedside. Int. J. Biochem. Cell Biol. 2023, 157, 106393. [Google Scholar] [CrossRef]
- Lee, J.; Sul, H.J.; Kim, K.-H.; Chang, J.Y.; Shong, M. Primary Cilia Mediate TSH-Regulated Thyroglobulin Endocytic Pathways. Front. Endocrinol. 2021, 12, 700083. [Google Scholar] [CrossRef]
- Doggui, R. Immunoanalytical profile of thyroglobulin antibodies. Ann. Biol. Clin. 2018, 76, 695–704. [Google Scholar] [CrossRef]
- Soh, S.-B.; Aw, T.-C. Laboratory Testing in Thyroid Conditions—Pitfalls and Clinical Utility. Ann. Lab. Med. 2019, 39, 3–14. [Google Scholar] [CrossRef]
- Dwivedi, S.N.; Kalaria, T.; Buch, H. Thyroid autoantibodies. J. Clin. Pathol. 2023, 76, 19–28. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Zhao, K.; Yu, N.; Yu, Y.; Zhang, Y.; Song, Z.; Huang, Y.; Lu, G.; Gao, Y.; et al. Glycosylation of Anti-Thyroglobulin IgG1 and IgG4 Subclasses in Thyroid Diseases. Eur. Thyroid. J. 2021, 10, 114–124. [Google Scholar] [CrossRef]
- Mcintosh, R.; Weetman, A.; Fortunato, R.S.; Ferreira, A.C.; Hecht, F.; Dupuy, C.; Carvalho, D.P.; Bradbury, A.R.; Stang, M.T.; Yim, J.H.; et al. Molecular Analysis of the Antibody Response to Thyroglobulin and Thyroid Peroxidase. Thyroid 1997, 7, 471–487. [Google Scholar] [CrossRef]
- Khan, F.A.; Al-Jameil, N.; Khan, M.F.; Al-Rashid, M.; Tabassum, H. Thyroid dysfunction: An autoimmune aspect. Int. J. Clin. Exp. Med. 2015, 8, 6677–6681. [Google Scholar]
- Bílek, R.; Dvořáková, M.; Grimmichová, T.; Jiskra, J. Iodine, Thyroglobulin and Thyroid Gland. Physiol. Res. 2020, 69 (Suppl. S2), S225–S236. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.A.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, S.M.; Rapoport, B. Thyroid Autoantibodies Display both “Original Antigenic Sin” and Epitope Spreading. Front. Immunol. 2017, 8, 1845. [Google Scholar] [CrossRef]
- Baker, S.; Miguel, R.N.; Thomas, D.; Powell, M.; Furmaniak, J.; Smith, B.R. Cryo-electron microscopy structures of human thyroid peroxidase (TPO) in complex with TPO antibodies. J. Mol. Endocrinol. 2023, 70, e220149. [Google Scholar] [CrossRef] [PubMed]
- Espenbetova, M.; Kuzmina, N.; Zubkov, A.; Akhmetova, V.; Zamanbekova, Z.; Krykpaeva, A.; Zhumanbayeva, Z.; Amrenova, K.; Smailova, Z.; Glushkova, N. Epitopes specificity of antibodies to thyroid peroxidase in patients with Graves’ disease, Hashimoto’s thyroiditis and overlap-syndrome. J. Clin. Transl. Endocrinol. 2022, 27, 100293. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, N.; Su, R.; Dai, C.; Zhang, R. Selenium Supplementation May Decrease Thyroid Peroxidase Antibody Titer via Reducing Oxidative Stress in Euthyroid Patients with Autoimmune Thyroiditis. Int. J. Endocrinol. 2020, 2020, 9210572. [Google Scholar] [CrossRef]
- Williams, D.E.; Le, S.N.; Hoke, D.E.; Chandler, P.G.; Gora, M.; Godlewska, M.; Banga, J.P.; Buckle, A.M. Structural Studies of Thyroid Peroxidase Show the Monomer Interacting With Autoantibodies in Thyroid Autoimmune Disease. Endocrinology 2020, 161, bqaa016. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, E.; Amino, N.; Kudo, T.; Ito, M.; Fukata, S.; Nishikawa, M.; Nakamura, H.; Miyauchi, A. Comparison of thyroglobulin and thyroid peroxidase antibodies measured by five different kits in autoimmune thyroid diseases. Endocr. J. 2017, 64, 955–961. [Google Scholar] [CrossRef]
- Wolffenbuttel, B.H.R.; Wouters, H.J.C.M.; Muller Kobold, A.C.; Roozendaal, C.; Klauw, M.M. Comparison of Four Commercially Available Thyroid Peroxidase Autoantibody and Two Thyroglobulin Autoantibody Assays. Available online: https://assets.researchsquare.com/files/rs-1550125/v1/a9384112-5494-4d6c-9c27-3e8e464faebd.pdf?c=1655112553 (accessed on 3 May 2023).
- La’Ulu, S.L.; Slev, P.R.; Roberts, W.L. Performance characteristics of 5 automated thyroglobulin autoantibody and thyroid peroxidase autoantibody assays. Clin. Chim. Acta 2007, 376, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Y.; Shen, Y.; Tian, R.; Sheng, Y.; Que, H. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: A systematic review and meta-analysis. Front. Public Health 2022, 10, 1020709. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, I.B.; Knudsen, N.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Laurberg, P. Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency. Clin. Endocrinol. 2003, 58, 36–42. [Google Scholar] [CrossRef]
- Camargo, R.Y.A.; Tomimori, E.K.; Neves, S.C.; Knobel, M.; Medeiros-Neto, G. Prevalence of chronic autoimmune thyroiditis in the urban area neighboring a petrochemical complex and a control area in Sao Paulo, Brazil. Clinics 2006, 61, 307–312. [Google Scholar] [CrossRef]
- Tammaro, A.; Pigliacelli, F.; Fumarola, A.; Persechino, S. Trends of thyroid function and autoimmunity to 5 years after the introduction of mandatory iodization in Italy. Eur. Ann. Allergy Clin. Immunol. 2016, 48, 77–81. [Google Scholar]
- Ehlers, M.; Allelein, S.; Schott, M. TSH-receptor autoantibodies: Pathophysiology, assay methods, and clinical applications. Minerva Endocrinol. 2018, 43, 323–332. [Google Scholar] [CrossRef]
- Nicolì, F.; Lanzolla, G.; Mantuano, M.; Ionni, I.; Mazzi, B.; Leo, M.; Sframeli, A.; Posarelli, C.; Maglionico, M.N.; Figus, M.; et al. Correlation between serum anti-TSH receptor autoantibodies (TRAbs) and the clinical feature of Graves’ orbitopathy. J. Endocrinol. Investig. 2021, 44, 581–585. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Diana, T.; Olivo, P.D. TSH Receptor Antibodies: Relevance & Utility. Endocr. Pract. 2020, 26, 97–106. [Google Scholar] [CrossRef]
- Shrestha, A.; Adhikari, N.; Devkota, S.; Chowdhury, T.; Shiferaw-Deribe, Z.; Gousy, N.; Adhikari, S. Fluctuating Hyperthyroidism and Hypothyroidism in Graves’ Disease: The Swinging Between Two Clinical Entities. Cureus 2022, 14, e27715. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.D.S.; Oliveira, J.C.; Freitas, C.; de Carvalho, A.C. Thyroid-Stimulatory Antibody as a Predictive Factor for Graves’ Disease Relapse. Cureus 2022, 14, e22190. [Google Scholar] [CrossRef]
- Arshad, I.; Zahra, T.; Vargas-Jerez, J. New-Onset Graves’ Disease in the Background of Hashimoto’s Thyroiditis: Spectrums of the Same Disease With Changing Autoantibodies. Cureus 2022, 14, e28296. [Google Scholar] [CrossRef]
- Kotwal, A.; Stan, M. Thyrotropin Receptor Antibodies—An Overview. Ophthalmic Plast. Reconstr. Surg. 2018, 34 (Suppl. S1), S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Michalek, K.; Morshed, S.A.; Latif, R.; Davies, T.F. TSH receptor autoantibodies. Autoimmun. Rev. 2009, 9, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Kumar, S. Utility of Antibodies in the Diagnoses of Thyroid Diseases: A Review Article. Cureus 2022, 14, e31233. [Google Scholar] [CrossRef]
- Autilio, C.; Morelli, R.; Locantore, P.; Pontecorvi, A.; Zuppi, C.; Carrozza, C. Stimulating TSH receptor autoantibodies immunoassay: Analytical evaluation and clinical performance in Graves’ disease. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2018, 55, 172–177. [Google Scholar] [CrossRef]
- Ortega, J.M.L.; Martínez, P.S.; Acevedo-León, D.; Capell, N.E. Anti-TSH receptor antibodies (TRAb): Comparison of two third generation automated immunoassays broadly used in clinical laboratories and results interpretation. PLoS ONE 2022, 17, e0270890. [Google Scholar] [CrossRef]
- Struja, T.; Jutzi, R.; Imahorn, N.; Kaeslin, M.; Boesiger, F.; Kutz, A.; Mundwiler, E.; Huber, A.; Kraenzlin, M.; Mueller, B.; et al. Comparison of Five TSH-Receptor Antibody Assays in Graves’ disease: Results from an observational pilot study. BMC Endocr. Disord. 2019, 19, 38. [Google Scholar] [CrossRef]
- Allelein, S.; Ehlers, M.; Goretzki, S.; Hermsen, D.; Feldkamp, J.; Haase, M.; Dringenberg, T.; Schmid, C.; Hautzel, H.; Schott, M. Clinical Evaluation of the First Automated Assay for the Detection of Stimulating TSH Receptor Autoantibodies. Horm. Metab. Res. 2016, 48, 795–801. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Long, L.; Zhou, L.; Chen, J.; Li, M.; Gao, Y.; Zhou, X.; Han, X.; Ji, L. Clinical evaluation of an automated TSI bridge immunoassay in the diagnosis of Graves’ disease and its relationship to the degree of hyperthyroidism. BMC Endocr. Disord. 2022, 22, 218. [Google Scholar] [CrossRef]
- Brix, T.H.; Hegedüs, L.; Weetman, A.P.; Kemp, H.E. Pendrin and NIS antibodies are absent in healthy individuals and are rare in autoimmune thyroid disease: Evidence from a Danish twin study. Clin. Endocrinol. 2014, 81, 440–444. [Google Scholar] [CrossRef]
- Eleftheriadou, A.-M.; Mehl, S.; Renko, K.; Kasim, R.H.; Schaefer, J.-A.; Minich, W.B.; Schomburg, L. Re-visiting autoimmunity to sodium-iodide symporter and pendrin in thyroid disease. Eur. J. Endocrinol. 2020, 183, 571–580. [Google Scholar] [CrossRef]
- Available online: https://www.biossusa.com/products/bs-0448r (accessed on 5 May 2023).
- Available online: https://www.mybiosource.com/polyclonal-nis-human-antibody/sodium-iodide-symporter/2026331 (accessed on 5 May 2023).
- Available online: https://www.arp1.com/anti-nai-symporter-nis-polyclonal-antibody-03-16093.html (accessed on 5 May 2023).
- Available online: https://www.lsbio.com/antibodies/ihc-plus-slc5a5-antibody-nis-antibody-if-immunofluorescence-ihc-wb-western-ls-b15569/697202?trid=247 (accessed on 5 May 2023).
- Available online: https://www.biorbyt.com/nis-antibody-orb11131.html (accessed on 5 May 2023).
- Available online: https://www.genetex.com/Product/Detail/NIS-antibody/GTX37599?utm_source=Biocompare&utm_medium=referral&utm_campaign=Biocompare_GeneTex (accessed on 5 May 2023).
- Available online: https://www.biocompare.com/9776-Antibodies/17754578-SLC26A4-Pendrin-Antibody/?pda=9776|17754578_0_1||1|Pendrin&dfp=true (accessed on 8 May 2023).
- Available online: https://www.biocompare.com/9776-Antibodies/8077560-SLC26A4-antibody/?pda=9776|8077560_0_1||2|Pendrin&dfp=true (accessed on 8 May 2023).
- Available online: https://www.biocompare.com/9776-Antibodies/4920922-SLC26A4-Pendrin-Mouse-anti-Human-Monoclonal-3D2-Antibody/?pda=9776|4920922_0_0||3|Pendrin (accessed on 8 May 2023).
- Available online: https://www.biocompare.com/9776-Antibodies/12145581-Pendrin-Antibody-SLC26A4/?pda=9776|12145581_0_0||4|Pendrin (accessed on 8 May 2023).
- Available online: https://www.biocompare.com/9776-Antibodies/14074098-Immunotag-8482-S26A4-Polyclonal-Antibody/?pda=9776|14074098_0_0||5|Pendrin (accessed on 8 May 2023).
- Seissler, J.; Wagner, S.; Schott, M.; Lettmann, M.; Feldkamp, J.; Scherbaum, W.A.; Morgenthaler, N.G. Low frequency of autoantibodies to the human Na(+)/I(−) symporter in patients with autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 2000, 85, 4630–4634. [Google Scholar] [CrossRef]
- Morris, J.C.; Bergert, E.R.; Bryant, W.P.; Brix, T.H.; Hegedüs, L.; Weetman, A.P.; Kemp, H.E.; López-Fraga, M.; Martínez, T.; Jiménez, A.; et al. Binding of Immunoglobulin G from Patients with Autoimmune Thyroid Disease to Rat Sodium-Iodide Symporter Peptides: Evidence for the Iodide Transporter as an Autoantigen. Thyroid 1997, 7, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Hisatome, I.; Taniguchi, S.; Shirayoshi, Y.; Yamamoto, Y.; Miake, J.; Ohkura, T.; Akama, T.; Igawa, O.; Shigemasa, C.; et al. Pendrin Is a Novel Autoantigen Recognized by Patients with Autoimmune Thyroid Diseases. J. Clin. Endocrinol. Metab. 2009, 94, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, R.A.; Kemp, E.H.; Waterman, E.A.; Watson, P.F.; Endo, T.; Onaya, T.; Weetman, A.P. Detection of Binding and Blocking Autoantibodies to the Human Sodium-Iodide Symporter in Patients with Autoimmune Thyroid Disease*. J. Clin. Endocrinol. Metab. 2000, 85, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Guarneri, F. Homology of pendrin, sodium-iodide symporter and apical iodide transporter. Front. Biosci. Landmark Ed. 2018, 23, 1864–1873. [Google Scholar]
- Ajjan, R.A.; Findlay, C.; Metcalfe, R.A.; Watson, P.F.; Crisp, M.; Ludgate, M.; Weetman, A.P. The Modulation of the Human Sodium Iodide Symporter Activity by Graves’ Disease Sera 1. J. Clin. Endocrinol. Metab. 1998, 83, 1217–1221. [Google Scholar] [CrossRef]
- Eng, P.H.K.; Ho, S.C. Clinical Relevance of the Thyroid Sodium/Iodide Symporter. In Diseases of the Thyroid. Contemporary Endocrinology; Braverman, L.E., Ed.; Humana Press: Totowa, NJ, USA, 2003. [Google Scholar]
- Lisi, S.; Pinchera, A.; McCluskey, R.T.; Willnow, T.E.; Refetoff, S.; Marcocci, C.; Vitti, P.; Menconi, F.; Grasso, L.; Luchetti, F.; et al. Preferential megalin-mediated transcytosis of low-hormonogenic thyroglobulin: A control mechanism for thyroid hormone release. Proc. Natl. Acad. Sci. USA 2003, 100, 14858–14863. [Google Scholar] [CrossRef]
- Wang, Y.M.; Lee, V.W.; Wu, H.; Harris, D.C.; Alexander, S.I. Heymann Nephritis in Lewis Rats. Curr. Protoc. Immunol. 2015, 109, 15.29.1–15.29.6. [Google Scholar] [CrossRef]
- Akiyama, S.; Imai, E.; Maruyama, S. Immunology of membranous nephropathy. F1000Research 2019, 8, 734. [Google Scholar] [CrossRef]
- Marinoò, M.; Chiovato, L.; Friedlander, J.A.; Latrofa, F.; Pinchera, A.; McCluskey, R.T. Serum Antibodies against Megalin (GP330) in Patients with Autoimmune Thyroiditis1. J. Clin. Endocrinol. Metab. 1999, 84, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.biocompare.com/Assay-Kit-Product-Search/?search=megalin (accessed on 10 May 2023).
- Available online: https://www.mybiosource.com/human-elisa-kits/megalin/3804658 (accessed on 10 May 2023).
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Esfandiari, N.H.; Papaleontiou, M. Biochemical Testing in Thyroid Disorders. Endocrinol. Metab. Clin. N. Am. 2017, 46, 631–648. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Agredo-Delgado, V.; Vargas-Sierra, H.D.; Pinzón-Fernández, M.V. Prevalence of Functional Alterations and the Effects of Thyroid Autoimmunity on the Levels of TSH in an Urban Population of Colombia: A Population-Based Study. Endocr. Metab. Immune Disord. Drug Targets 2022, 23, 857–866. [Google Scholar] [CrossRef]
- Czarnocka, B. Thyroperoxidase, thyroglobulin, Na+/I- symporter, pendrin in thyroid autoimmunity. Front. Biosci. 2011, 16, 783–802. [Google Scholar] [CrossRef]
- Hadj-Kacem, H.; Rebuffat, S.; Mnif-Féki, M.; Belguith-Maalej, S.; Ayadi, H.; Péraldi-Roux, S. Autoimmune thyroid diseases: Genetic susceptibility of thyroid-specific genes and thyroid autoantigens contributions. Int. J. Immunogenetics 2009, 36, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Baloch, Z.; Carayon, P.; Conte-Devolx, B.; Demers, L.M.; Feldt-Rasmussen, U.; Henry, J.-F.; LiVosli, V.A.; Niccoli-Sire, P.; John, R.; Ruf, J.; et al. Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease. Thyroid 2003, 13, 3–126. [Google Scholar] [CrossRef] [PubMed]
- Demers, L.M.; Spencer, C.A. Laboratory medicine practice guidelines: Laboratory support for the diagnosis and monitoring of thyroid disease. Clin. Endocrinol. 2003, 58, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Dhillon-Smith, R.; Coomarasamy, A. TPO antibody positivity and adverse pregnancy outcomes. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101433. [Google Scholar] [CrossRef]
- Furmaniak, J.; Sanders, J.; Sanders, P.; Miller-Gallacher, J.; Ryder, M.M.; Smith, B.R. Practical applications of studies on the TSH receptor and TSH receptor autoantibodies. Endocrine 2020, 68, 261–264. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Bartalena, L.; Hegedüs, L.; Leenhardt, L.; Poppe, K.; Pearce, S.H. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur. Thyroid. J. 2018, 7, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, R.; de Jong, M.C.; Kit, J.L.W.; Sek, K.; Nam, T.Q.; Thang, T.V.; Khue, N.T.; Aye, T.T.; Tun, P.M.; Cole, T.; et al. 2021 Asia-Pacific Graves’ Disease Consortium Survey of Clinical Practice Patterns in the Management of Graves’ Disease. Endocrine 2023, 79, 135–142. [Google Scholar] [CrossRef]
- Hoang, T.D.; Stocker, D.J.; Chou, E.L.; Burch, H.B. 2022 Update on Clinical Management of Graves Disease and Thyroid Eye Disease. Endocrinol. Metab. Clin. N. Am. 2022, 51, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Tanda, M.L. Current concepts regarding Graves’ orbitopathy. J. Intern. Med. 2022, 292, 692–716. [Google Scholar] [CrossRef] [PubMed]
- Fatourechi, V. Pretibial Myxedema: Pathophysiology and treatment options. Am. J. Clin. Dermatol. 2005, 6, 295–309. [Google Scholar] [CrossRef]
- Trohman, R.G.; Sharma, P.S.; McAninch, E.; Bianco, A. Amiodarone and thyroid physiology, pathophysiology, diagnosis and management. Trends Cardiovasc. Med. 2019, 29, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Medić, F.; Bakula, M.; Alfirević, M.; Bakula, M.; Mucić, K.; Marić, N. Amiodarone and Thyroid Dysfunction. Acta Clin. Croat. 2022, 61, 327–341. [Google Scholar] [CrossRef]
- Martinez Quintero, B.; Yazbeck, C.; Sweeney, L.B. Thyroiditis: Evaluation and Treatment. Am. Fam. Physician 2021, 104, 609–617. [Google Scholar]
- Struja, T.; Fehlberg, H.; Kutz, A.; Guebelin, L.; Degen, C.; Mueller, B.; Schuetz, P. Can we predict relapse in Graves’ disease? Results from a systematic review and meta-analysis. Eur. J. Endocrinol. 2017, 176, 87–97. [Google Scholar] [CrossRef]
- Mooij, C.F.; Cheetham, T.D.; Verburg, F.A.; Eckstein, A.; Pearce, S.H.; Léger, J.; van Trotsenburg, A.S.P. 2022 European Thyroid Association Guideline for the management of pediatric Graves’ disease. Eur. Thyroid. J. 2022, 11, e210073. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, C.; Sztal-Mazer, S.; Topliss, D.J. How to manage Graves’ disease in women of childbearing potential. Clin. Endocrinol. 2023, 98, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Pyrżak, B.; Rumińska, M.; Witkowska-Sędek, E.; Kucharska, A. Follow-Up of Thyroid Function in Children With Neonatal Hyperthyroidism. Front. Endocrinol. 2022, 13, 877119. [Google Scholar] [CrossRef] [PubMed]
- Orgiazzi, J. Anti–Tsh Receptor Antibodies in Clinical Practice. Endocrinol. Metab. Clin. N. Am. 2000, 29, 339–355. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, S.M.; Cheetham, T.D.; Verburg, F.A.; Eckstein, C.H.; Diana, T.; Li, Y.; Olivo, P.D.; Lackner, K.J.; Kim, H.; Kanitz, M.; et al. Thyrotropin-Blocking Autoantibodies. Thyroid 2013, 23, 14–24. [Google Scholar] [CrossRef]
- Diana, T.; Olivo, P.D.; Kahaly, G.J. Thyrotropin Receptor Blocking Antibodies. Horm. Metab. Res. 2018, 50, 853–862. [Google Scholar] [CrossRef]
- Napolitano, G.; Bucci, I.; Di Dalmazi, G.; Giuliani, C. Non-Conventional Clinical Uses of TSH Receptor Antibodies: The Case of Chronic Autoimmune Thyroiditis. Front. Endocrinol. 2021, 12, 769084. [Google Scholar] [CrossRef]
- Benvenga, S.; Bartolone, L.; Squadrito, S.; Trimarchi, F. Thyroid Hormone Autoantibodies Elicited by Diagnostic Fine Needle Biopsy 1. J. Clin. Endocrinol. Metab. 1997, 82, 4217–4223. [Google Scholar] [CrossRef]
- Benvenga, S.; Burek, C.L.; Talor, M.; Rose, N.R.; Trimarchi, F. Heterogeneity of the thyroglobulin epitopes associated with circulating thyroid hormone autoantibodies in Hashimoto’s thyroiditis and non-autoimmune thyroid diseases. J. Endocrinol. Investig. 2002, 25, 977–982. [Google Scholar] [CrossRef]
- Sakata, S.; Nakamura, S.; Miura, K. Autoantibodies against thyroid hormones or iodothyronines. Ann. Intern. Med. 1985, 103, 579–589. [Google Scholar] [CrossRef]
- Ni, J.; Long, Y.; Zhang, L.; Yang, Q.; Kou, C.; Li, S.; Li, J.; Zhang, H. High prevalence of thyroid hormone autoantibody and low rate of thyroid hormone detection interference. J. Clin. Lab. Anal. 2022, 36, e24124. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Galletti, M.; Mandolfino, M.G.; Aragona, P.; Bartolone, S.; Giorgianni, G.; Alesci, D.; Trimarchi, F.; Benvenga, S. Thyroid hormone autoantibodies in primary Sjögren syndrome and rheumatoid arthritis are more prevalent than in autoimmune thyroid disease, becoming progressively more frequent in these diseases. J. Endocrinol. Investig. 2002, 25, 447–454. [Google Scholar] [CrossRef]
- Vita, R.; Santaguida, M.G.; Virili, C.; Segni, M.; Galletti, M.; Mandolfino, M.; Di Bari, F.; Centanni, M.; Benvenga, S. Serum Thyroid Hormone Antibodies Are Frequent in Patients with Polyglandular Autoimmune Syndrome Type 3, Particularly in Those Who Require Thyroxine Treatment. Front. Endocrinol. 2017, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, K.; Brabant, S.; Prie, D.; Piketty, M.-L. Hormone Immunoassay Interference: A 2021 Update. Ann. Lab. Med. 2022, 42, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Doniach, D.; Roitt, I.M. Clinical Aspects of Immunology; Gell, P.G.H., Coombs, R.A., Lachmann, P.J., Eds.; Blackwell Science: Oxford, UK, 1975; p. 1355. [Google Scholar]
- Ochi, Y. Possible participation of colloid antigen 2 and abhormone (IgG with hormone activity) for the etiology of Graves’ disease. Med. Hypotheses 2019, 127, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Waliszewska-Prosół, M.; Ejma, M. Hashimoto Encephalopathy—Still More Questions than Answers. Cells 2022, 11, 2873. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Mukherjee, A.; Chakravarty, A. Hashimoto’s Encephalopathy: Case Series and Literature Review. Curr. Neurol. Neurosci. Rep. 2023, 23, 167–175. [Google Scholar] [CrossRef]
| Molecular Characteristics and Prevalence | Antigen | |||||
|---|---|---|---|---|---|---|
| Tg | TSHR | TPO | PDN | NIS | Meg | |
| Protein | Iodinated glycoprotein | G-protein-coupled receptor | Hemoprotein enzyme | Hydrophobic transmembrane glycoprotein | Membrane glycoprotein | Integral membrane protein |
| Amino acids | 2748 | 743 | 933 | 780 | 643 | 4655 |
| Molecular weight (kDa) | 660 | 85 | 105–110 | 86 | 70–90 | 600 |
| Thyroid concentration | ++++/++++ | ++/++++ | +++/++++ | +/++++ | +/++++ | +/++++ |
| Epitope localization | Predominantly central region and C-terminus | Predominantly A-subunit | Predominantly myeloperoxidase-like domain and, to lesser extent, complement control protein-like domain | Apical membrane of thyrocytes | Predominantly extramembranous Regions | Apical surface of thyrocytes |
| Immunogenicity | +++++/+++++ | ++++/+++++ | +++++/+++++ | +++/+++++ | ++/+++++ | +/+++++ |
| Chromosomal location | 8q24 | 14q31 | 2p25 | 7q22-31 | 19p12-13.2 | 2q24-q31 |
| Prevalence of antibody in the general population (%) | TgAbs (5–20) | TRAbs (0–3) | TPOAbs (8–30) | Unknown | Unknown | Unknown |
| Prevalence of antibody in autoimmune thyroiditis—HT—(%) | TgAbs (80–90) | TRAbs (10–20) | TPOAbs (90–100) | PDNAbs (variable, 9–98%) | NISAbs (7–8) | MegAbs (~50) |
| Prevalence of antibody in GBD (%) | TgAbs (30–60) | TRAbs (90–95) | TPOAbs (80) | PDNAbs (variable, 10–75%) | NISAbs (10–12) | MegAbs (~50) |
| Principle [kit] | CLIA [Architect (Abbott Diagnostics, USA)] | ECLIA [ECLusys (Roche Diagnostics, Germany)] | EIA [AIA-Pack (Tosoh Bioscience, Japan)] | CLEIA [Lumipulse G (Fujirebio Inc., Japan)] | CLEIA [Immulite 2000 (Siemens Healthcare Diagnostics, USA)] |
|---|---|---|---|---|---|
| Procedure | Two-step sandwich | One-step competitive | Two-step sandwich | Two-step sandwich | Two-step sandwich |
| TgAbs | |||||
| Assay components | Human Tg-coated Microparticles. Acridinium-labeled anti-human IgG (mouse monoclonal) | Biotinylated human Tg. Ruthenylated anti- TgAb (mouse monoclonal) | Human Tg-coated Microparticles. ALP-labeled antihuman IgG (mouse monoclonal | Human Tg-coated Microparticles. ALP-labeled antihuman IgG (mouse monoclonal). 3-(2′-spiroadamantane)-4-methoxy-4-(3″- phosphoryloxy)phenyl-1,2-dioxetane disodium salt | Human Tg-coated Microparticles. ALP-labeled antihuman IgG (mouse monoclonal). 3-(2′-spiroadamantane)-4-methoxy-4-(3″- phosphoryloxy)phenyl-1,2-dioxetane disodium salt |
| Cut off (IU/mL) | 4.11 | 28 | 13.6 | 12.2 | 40 |
| TPOAbs | |||||
| Assay components | Human TPO-coated microparticles. Acridinium-labeled anti-human IgG (mouse monoclonal) | Biotinylated human TPO. Ruthenylated anti- TgAb (goat polyclonal) | Human TPO-coated Microparticles. ALP-labeled antihuman IgG (mouse monoclonal | Human TPO-coated microparticles ALP-labeled antihuman IgG (mouse monoclonal). 3-(2′-spiroadamantane)-4-methoxy-4-(3″- phosphoryloxy)phenyl-1,2-dioxetane disodium salt | Human TPO-coated microparticles ALP-labeled antihuman IgG (mouse monoclonal). 3-(2′-spiroadamantane)-4-methoxy-4-(3″- phosphoryloxy)phenyl-1,2-dioxetane disodium salt |
| Cut off (IU/mL) | 5.6 | 16 | 3.2 | 5.1 | 35 |
| Prevalence of TgAbs and TPOAbs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Principle [kit] | CLIA [Architect (Abbott Diagnostics, USA)] | ECLIA [ECLusys (Roche Diagnostics, Germany)] | EIA [AIA-Pack (Tosoh Bioscience, Japan)] | CLEIA [Lumipulse G (Fujirebio Inc., Japan)] | CLEIA [Immulite 2000 (Siemens Healthcare Diagnostics, USA)] | |||||
| Abs | TgAbs | TPOAbs | TgAbs | TPOAbs | TgAbs | TPOAbs | TgAbs | TPOAbs | TgAbs | TPOAbs |
| Hashimoto’s thyroiditis | ++++ * | ++ | +++ * | ++ | +++ * | ++ | ++++ * | ++ | ++ | ++ |
| GBD | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Painless thyroiditis | ++ * | + | ++ * | + | ++ * | + | ++ * | + | + | + |
| Healthy controls | + ** | +/− | + | +/− | + | +/− | + ** | +/− | +/− | +/− |
| Prevalence of TgAbs (alone) or TPOAbs (alone) | ||||||||||
| Abs | TgAbs | TPOAbs | TgAbs | TPOAbs | TgAbs | TPOAbs | TgAbs | TPOAbs | TgAbs | TPOAbs |
| Hashimoto’s thyroiditis | + * | − | + * | +/− | + * | +/− | + * | − | + | ++ |
| GBD | + | + | +/− | + | +/− | + | + | + | +/− | ++ ** |
| Painless thyroiditis | ++ * | + | ++ * | +/− | ++ * | +/− | ++ * | − | + | +/− |
| Healthy controls | + ** | +/− | +/− | +/− | + | +/− | + ** | +/− | +/− | +/− |
| TRAbs | ||||
|---|---|---|---|---|
| Immunoassays | ELISA | |||
| EliA Anti-TSHR (Thermo Fisher Scientific, Germany) | Elecsys (COBAS, Roche, USA) | BRAHMS TRAK Human KRYPTOR (Thermo Fisher Scientific, Germany) | IMMULITE 2000 TSI (Siemens, Healthineers, Germany) | ELISA RSR TRAb Fast (RSR Limited, United Kingdom) |
| Sensitivity: 96.6% | Sensitivity: 100% | Sensitivity: >98% | Sensitivity: 98.3% | Sensitivity: 85% |
| Specificity: 99.4% | Specificity: 95.3% | Specificity: almost 100% | Specificity: 97% | Specificity: 100% |
| The lower and upper limit of detection are 1.5 and 80 IU/L, respectively. Intra-assay and inter-assay variance at 3.2 U/L (positive cut-off >3.3 IU/L, negative cut-off <2.9 IU/L) is 10.6% and 11.4%, respectively. | The range is 0.8–40 IU/L. The limit of quantification is the lowest analyte concentration that can be reproducibly measured with an intermediate precision CV of ≤20% | The range is 0.27–20 IU/L (cut-off 1.8 U/L). Intra-assay variance for the range of 1.2 to 2.0 U/L is <7.0%. Inter-assay variance for the range of 1.0 to 2.0 U/L is <18% | The range is 0.10–40 IU/L (cut-off 0.55 IU/L). Intra-assay and inter-assay variance at 0.69 IU/L is 4.1% and 5.1%, respectively | The range is 1–40 IU/L (positive cut-off ≥1.0 IU/L, lower detection limit at 2 SD 0.16 IU/L). Intra-assay and inter-assay variance at 2.0 and 4.6 IU/L is reported 7.2% and 3.3%, respectively |
| Principle/Kit [Ref] | Bioss Inc.’s NIS Polyclonal Antibody; USA [87] | Polyclonal Antibody to NIS, MyBioSource.com; USA [88] | Anti-NIS Polyclonal Antibody, American Research Products Inc., USA [89] | SLC5A5/NIS Monoclonal Antibody, LifeSpan BioSciences; USA [90] | NIS Antibody, Biorbyt; United Kingdom [91] | Anti-NIS Antibody, GeneTex; USA [92] |
|---|---|---|---|---|---|---|
| Clonality | Rabbit Polyclonal antibody | Rabbit Polyclonal antibody | Polyclonal antibody | Mouse monoclonal antibody | Human Polyclonal antibody | Rabbit Polyclonal antibody |
| Isotype | IgG | IgG | IgG | IgG1 | IgG | IgG |
| Immunogen | KLH conjugated peptide, mouse NIS | Rabbit polyclonal antibody raised against NIS | Synthetic peptide from the C-terminus of rat NIS | Synthetic peptide corresponding to aa37–54 of human NIS. | KLH conjugated synthetic peptide derived from mouse NIS | KLH conjugated synthetic peptide derived between 535–608 amino acids of human NIS |
| Purity | Protein A purified | Affinity Chromatography | Immunogen affinity purified | Affinity purified | Affinity purified by Protein A | Protein A purified |
| Reactivity | Human, Rat, Pig | Human | Human, Porcine, Rat | Human | Human | Human, rat |
| Applications | WB, ELISA, IHC-P, IHC-F, ICC, IF | WB, ICC, IHC-P, EIA | IHC | IHC, IHC-P, WB | WB | WB, IHC-P, IHC-Fr, IHC |
| Principle/Kit [Ref] | Polyclonal Rabbit Anti-Human SLC26A4/Pendrin Antibody, LifeSpan BioSciences; USA [93] | SLC26A4 Antibody, MyBioSource.com; USA [94] | SLC26A4/Pendrin Monoclonal Antibody, LifeSpan BioSciences; USA [95] | Pendrin Antibody/SLC26A4, NSJ Bioreagents; USA [96] | Immunotag™ S26A4 Polyclonal Antibody, G Biosciences; USA [97] |
|---|---|---|---|---|---|
| Clonality | IgG Polyclonal | Rabbit Polyclonal | Monoclonal | Polyclonal | Polyclonal |
| Isotype | IgG, epitope: aa287-336 | IgG | IgG2a kappa | IgG | Primary antibody |
| Immunogen | Synthetic peptide located between aa287-336 of human SLC26A4 (O43511, NP_000432). | Amino acids ELNDRFRHKIPVPIPIE VIVTIIATAISYGANLE KNYNAGIVKSIPRGFL | SLC26A4 (NP_000432, aa 674–754). A partial recombinant protein with GST tag. MW of the GST tag alone is 26 KDa. | Amino acids RSLRVIVKEFQRIDVNVYFASLQDYVIEKLEQ | Synthesized peptide derived from part region of human protein |
| Purity | Immunoaffinity purified | Affinity purified | Purified from ascites by Protein A | Antigen affinity purified | Affinity purified |
| Reactivity | Human | Human, mouse | Human | Human | Human |
| Applications | IHC, IHC-P, WB | WB | ELISA | WB | WB, ELISA |
| Antibody | Participants without AITD (Controls, %) | GBD (%) | HT (%) | Ratio of Prevalence of Positivity between Subjects with AITD and Participants without AITD |
|---|---|---|---|---|
| Studies with NISAbs | 0 | 84 | 15 | 4.6 |
| 0–10 | 0–63 | 0–25.9 | 2.7 | |
| 0.6–3 | 5.6–10.7 | 6.9–20.8 | 1.5 | |
| 0 | 22 | 24 | 1.95 | |
| – | 38 | 27.5 | 1.75 | |
| 0 | 20 | 14 | 0.4 | |
| 1.8 | 12.3 | 7.5 | 0.8 | |
| Studies with PDNAbs | 0.0 | 74 | 97.5 | 1.75 |
| 0.0 | 9.9 | 7.6 | 4.89 | |
| 0 | 13 | 8 | 0.4 | |
| 5.0 | 11.0 | 4.7 | 0.8 |
| Principle/Kit (Ref) | LRP2/Megalin Antibody, LifeSpan BioSciences; USA | Anti-Lrp2/Megalin Rabbit Monoclonal Antibody, BosterBio; USA | Rabbit Anti-Megalin/LRP2 Antibody, MyBioSource.com; USA | Human Anti-Human Megalin, Bio-Rad; USA | Mouse Anti-Human LRP2/Megalin Clone CD7D5 mAb from Cell Sciences; USA |
|---|---|---|---|---|---|
| Clonality | Polyclonal | Monoclonal | Polyclonal | Monoclonal | Monoclonal |
| Isotype | IgG, epitope: aa287-336 | Rabbit IgG | IgG | Fab fragment | IgG1 |
| Immunogen | Amino acids 4446–4655 of human LRP2 (NP_004516.2). FHYRRTGSLLPALPKL PSLSSLVKPSENGNGV TFRSGADLNMDIGVS GFGPETAIDRSMAMSE DFVMEMGKQPIIFENP MYSARDSAVKVVQPI QVTVSENVDNKNYGS PINPSEIVPETNPTSPA ADGTQVTKWNLFKRK SKQTTNFENPIYAQME NEQKESVAATPPPSPS LPAKPKPPSRRDPTPT YSATEDTFKDTANLVKEDSEV | A synthesized peptide derived from human Lrp2/Megalin | A synthetic peptide corresponding to the center region of the mouse LRP2/Megalin | Human Megalin (aa sequence 1024–1224)–N1 fusion protein | Purified Human Megalin |
| Purity | Affinity purified | Affinity-chromatography | Protein A and antigen affinity | Affinity purified | Protein G Chromatography |
| Reactivity | Mouse, Rat, Human | Human, Mouse, Rat | Mouse | Human | Human |
| Applications | IHC, IHC-P, WB | WB | IHC-P | WB | IF, IHC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Uricoechea, H.; Nogueira, J.P.; Pinzón-Fernández, M.V.; Schwarzstein, D. The Usefulness of Thyroid Antibodies in the Diagnostic Approach to Autoimmune Thyroid Disease. Antibodies 2023, 12, 48. https://doi.org/10.3390/antib12030048
Vargas-Uricoechea H, Nogueira JP, Pinzón-Fernández MV, Schwarzstein D. The Usefulness of Thyroid Antibodies in the Diagnostic Approach to Autoimmune Thyroid Disease. Antibodies. 2023; 12(3):48. https://doi.org/10.3390/antib12030048
Chicago/Turabian StyleVargas-Uricoechea, Hernando, Juan Patricio Nogueira, María V. Pinzón-Fernández, and Diego Schwarzstein. 2023. "The Usefulness of Thyroid Antibodies in the Diagnostic Approach to Autoimmune Thyroid Disease" Antibodies 12, no. 3: 48. https://doi.org/10.3390/antib12030048
APA StyleVargas-Uricoechea, H., Nogueira, J. P., Pinzón-Fernández, M. V., & Schwarzstein, D. (2023). The Usefulness of Thyroid Antibodies in the Diagnostic Approach to Autoimmune Thyroid Disease. Antibodies, 12(3), 48. https://doi.org/10.3390/antib12030048





