Development of Eastern Blotting Technique for Analysis of Baicalin Using Anti-Baicalin Monoclonal Antibody
Abstract
:1. Introduction
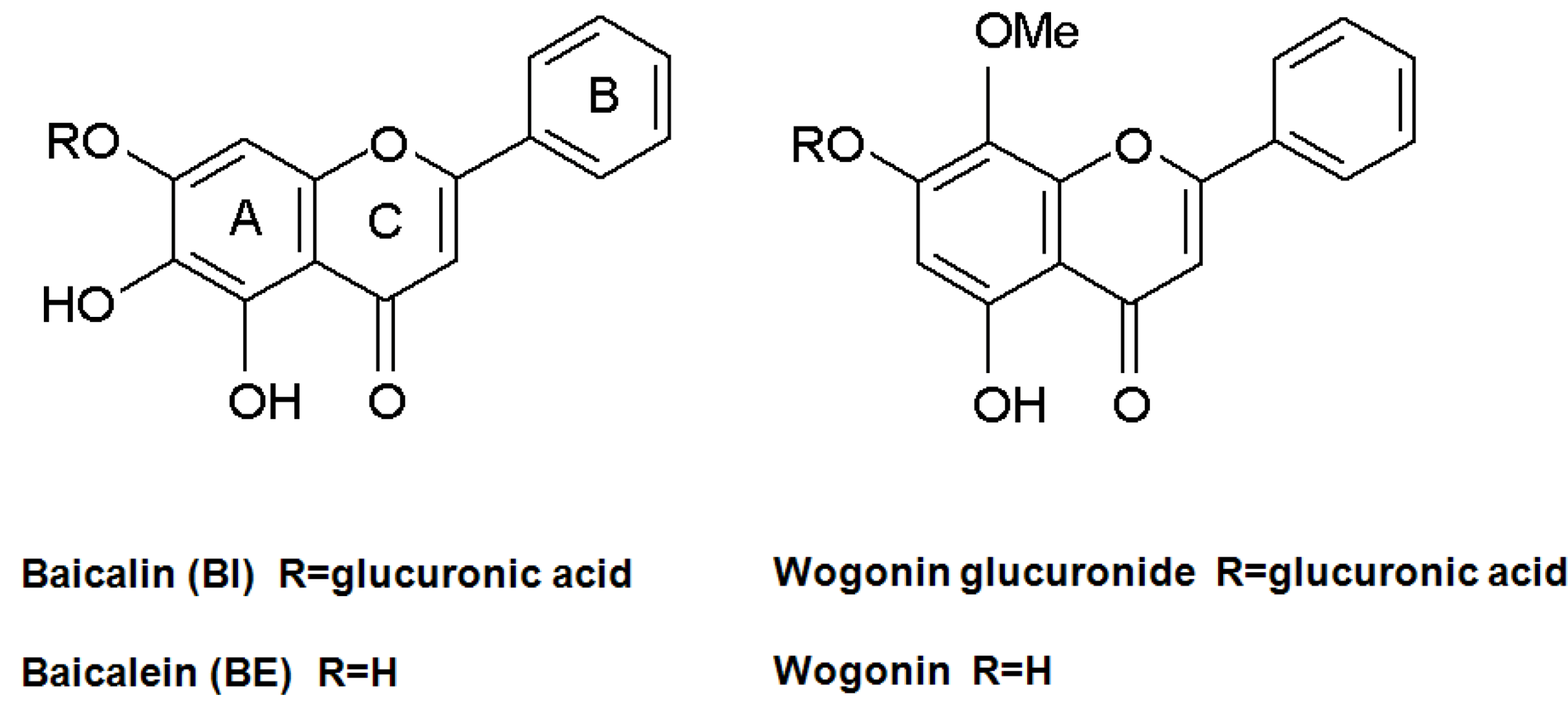
2. Results and Discussion
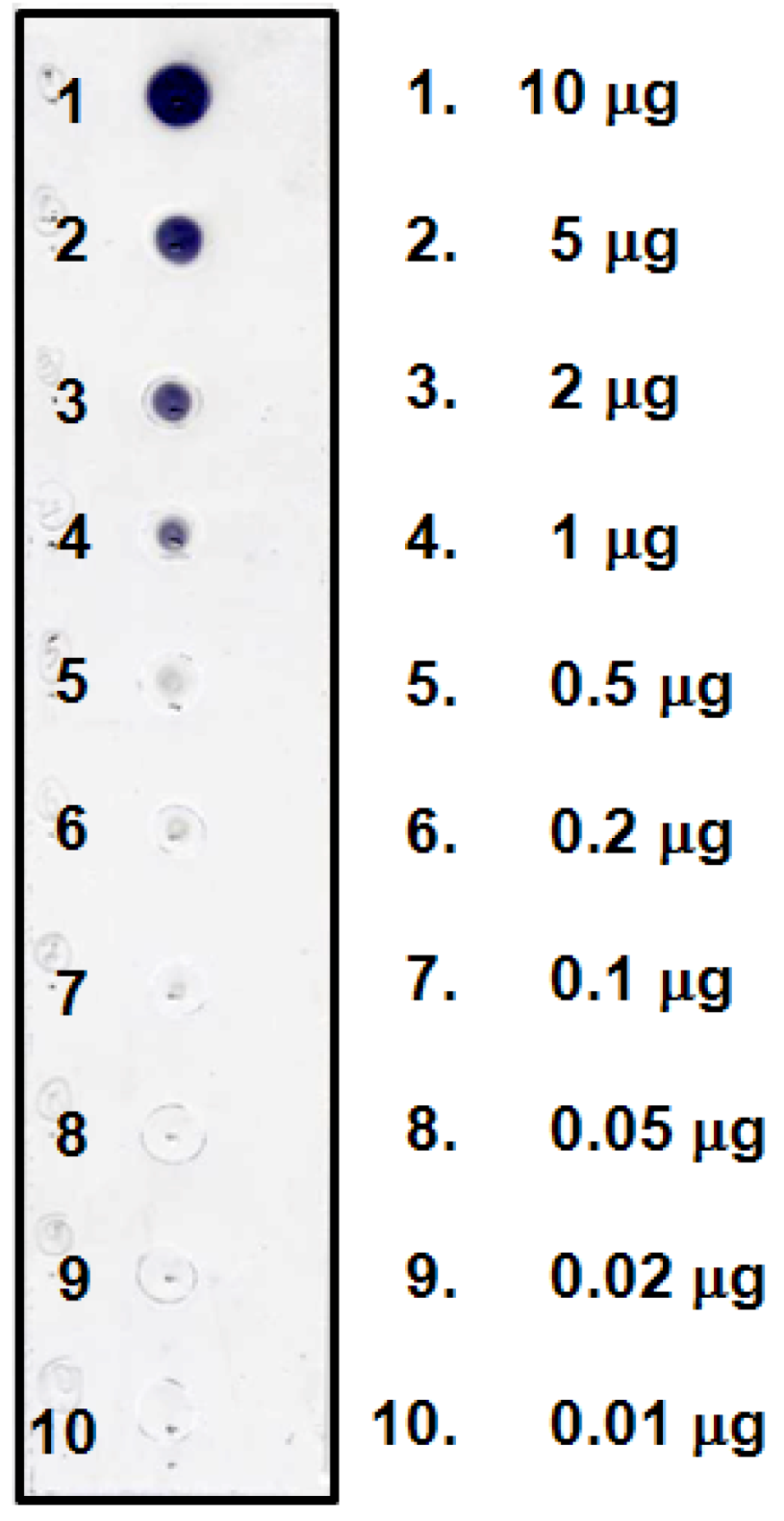
2.1. Dot Blot Assay of BI
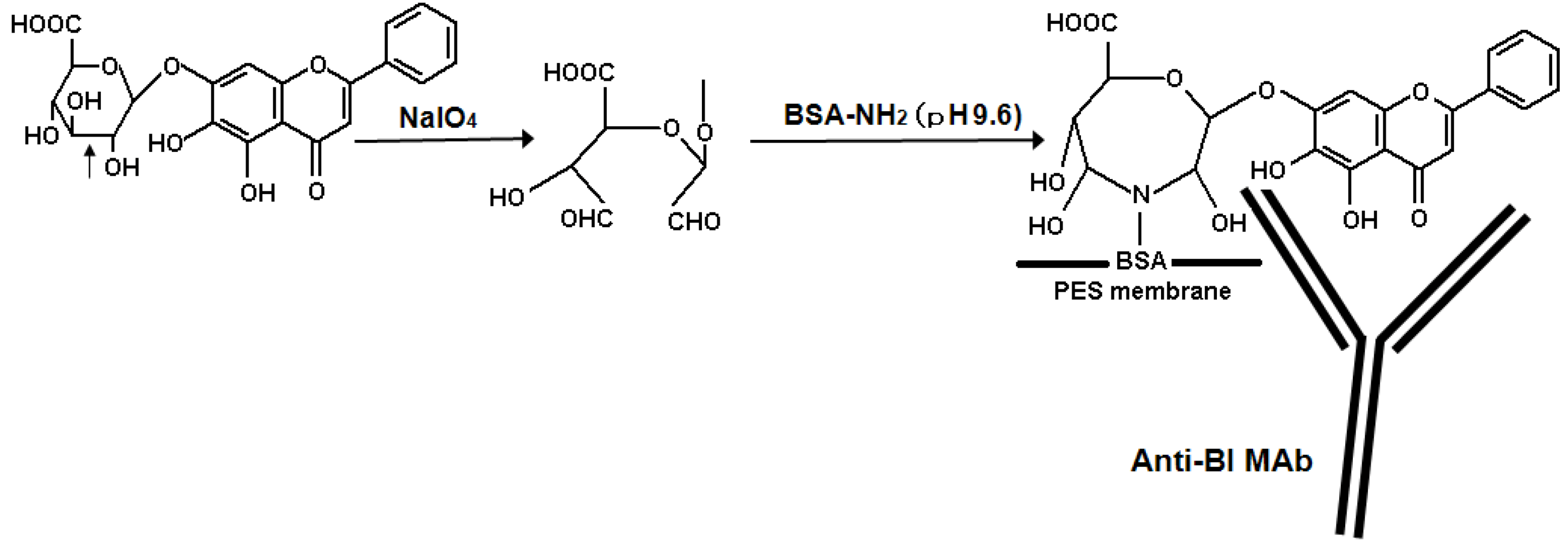
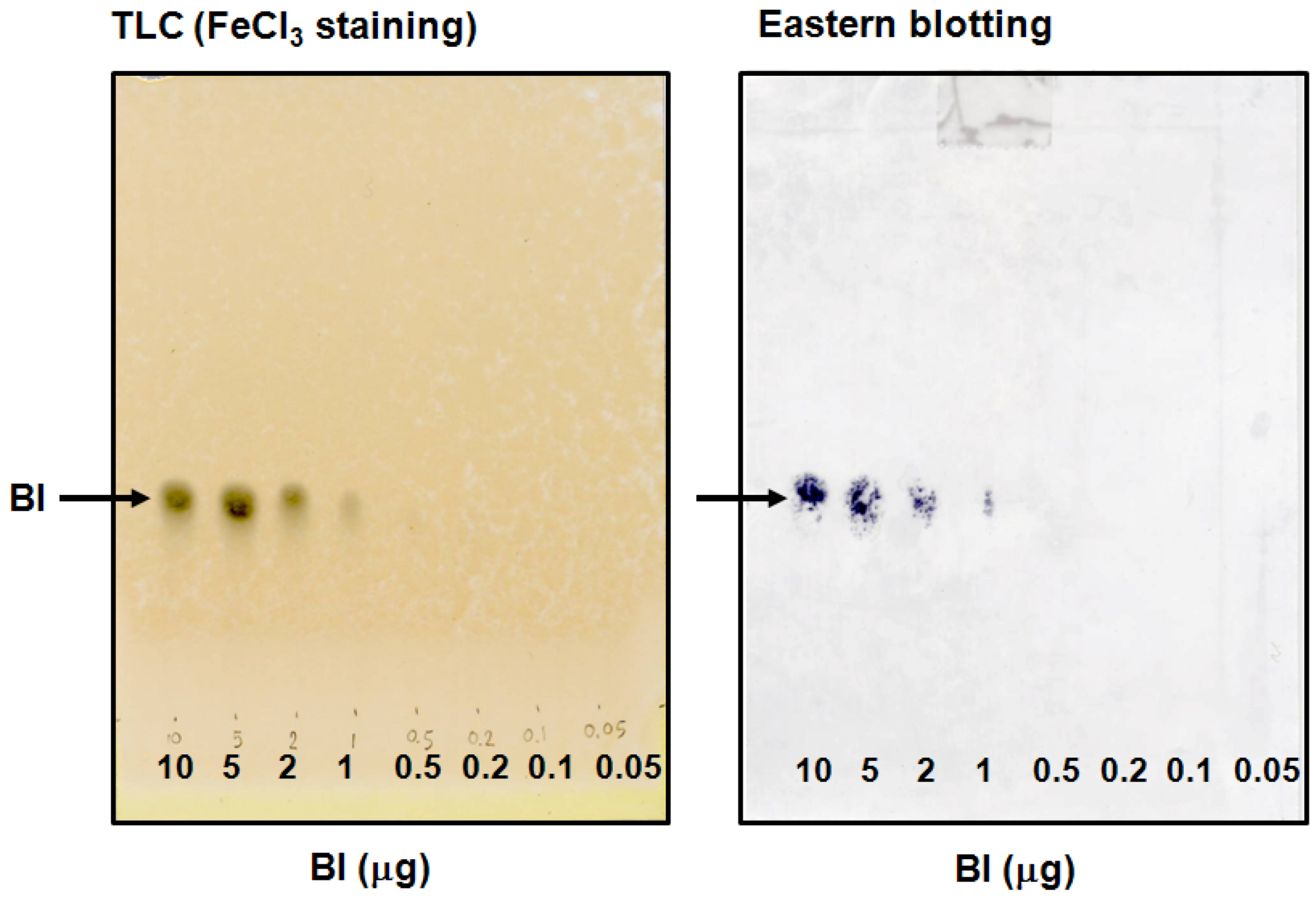
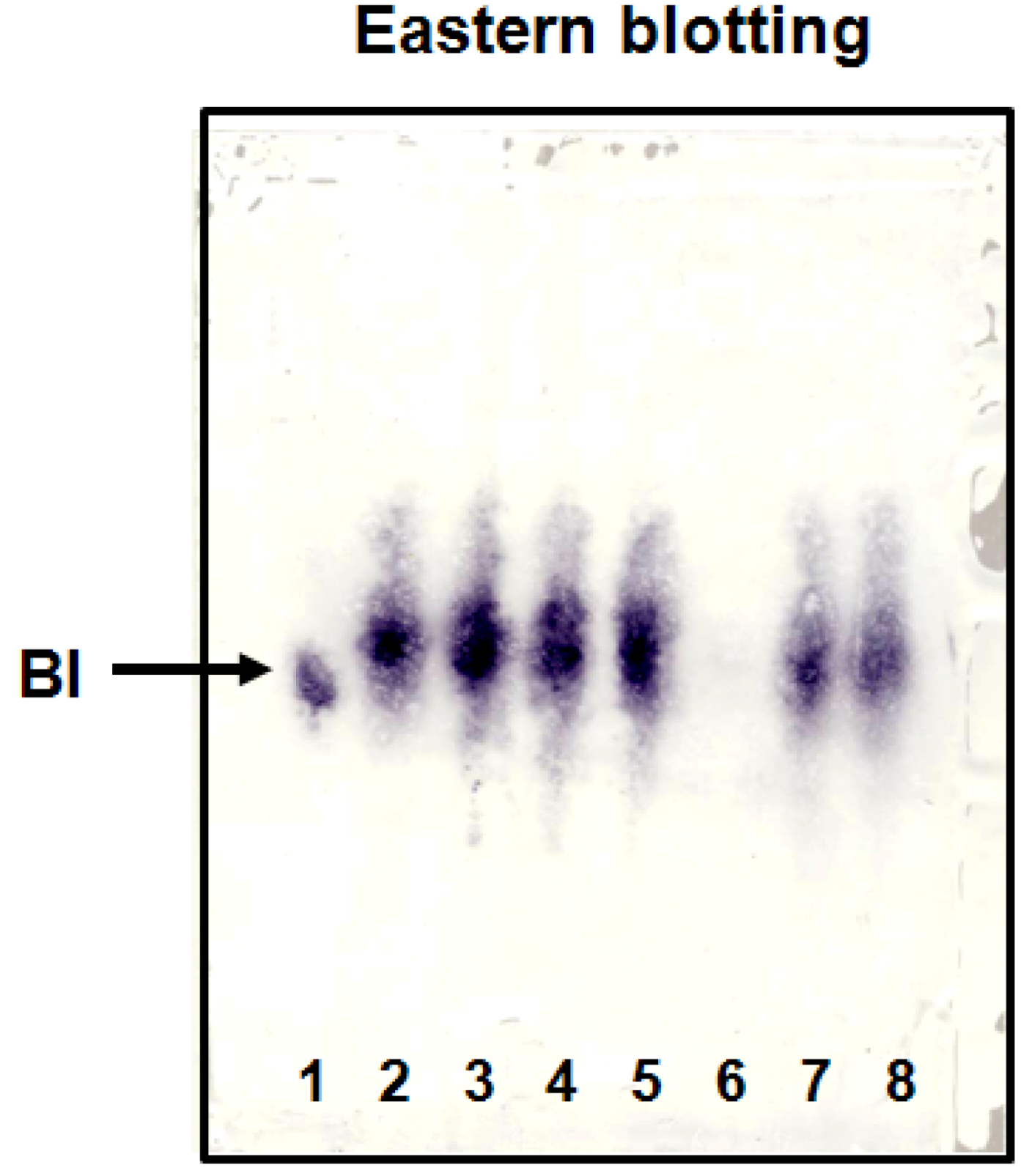
2.2. Eastern Blotting of BI

2.3. Qualitative and Quantitative Analysis of BI Using Eastern Blotting Technique and Competitive ELISA in Crude Extracts of Various S. radix Samples and KMs
| Sample name | BI (μg/mg dry wt.) |
|---|---|
| Scutellariae radix main root | 74.2 ± 7.8 |
| S. radix A | 126.6 ± 17.3 |
| S. radix B | 177.8 ± 6.1 |
| S. radix C | 80.4 ± 10.0 |
| Shakuyakukanzoto | N.D. |
| Shosaikoto | 29.0 ± 4.7 |
| Saikokaryukotsuboreito | 9.2 ± 1.1 |
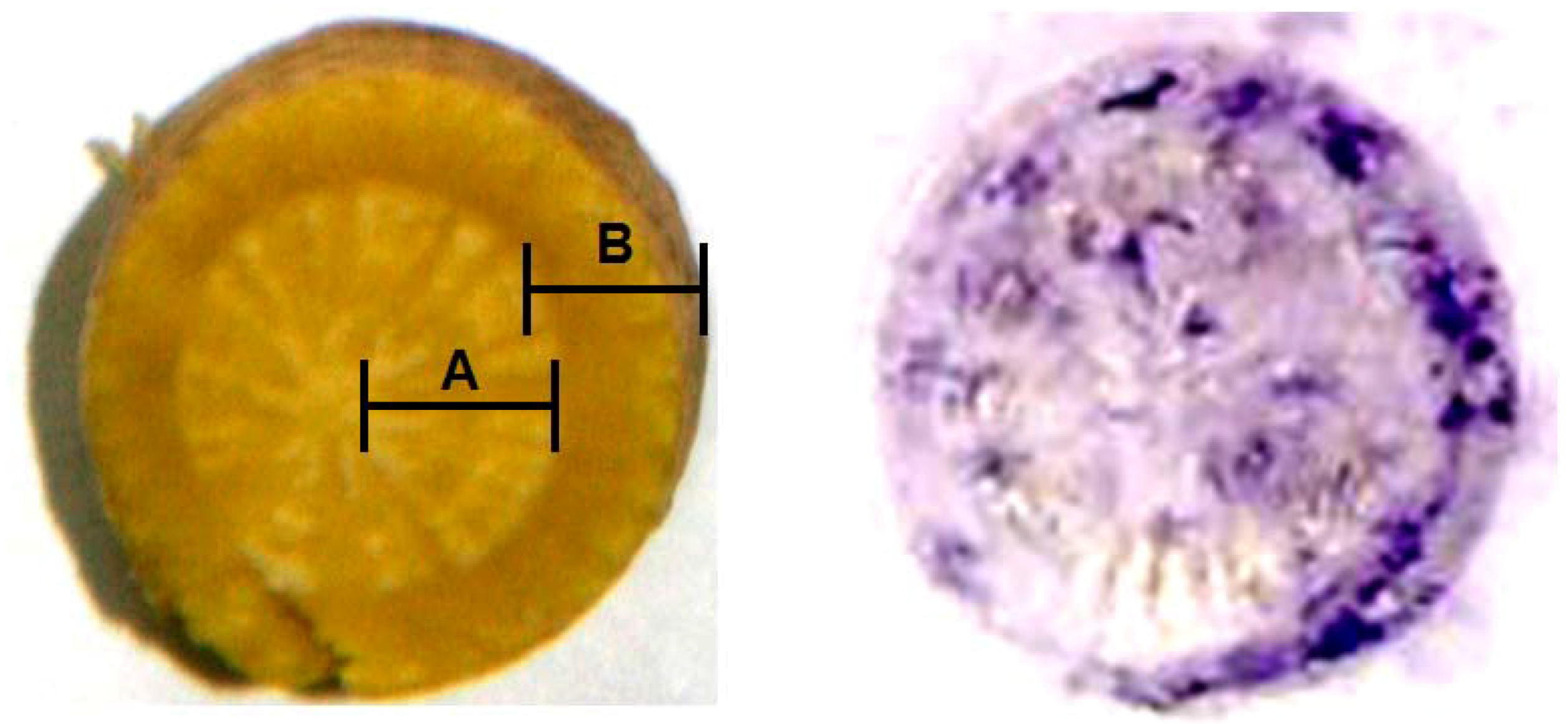
2.4. Validation of Eastern Blotting Technique to Identify the Distribution of BI in Fresh Root of S. Baicalensis
3. Experimental
3.1. Chemicals and Immunochemicals
3.2. Plant Materials and Extraction
3.3. MAb against BI and Its Development of ELISA System for BI
3.4. Dot Blot Assay of BI
3.5. Thin-Layer Chromatography and FeCl3 Staining
3.6. Eastern Blotting of BI
3.7. Immunohistochemical Localization of BI in Fresh Root of S. baicalensis
4. Conclusions
Acknowledgments
References
- Kawashima, K.; Nomura, A.; Makino, T.; Saito, K.; Kano, Y. Pharmacological properties of traditional medicine (XXIX): Effect of Hange-shashin-to and the combinations of its herbal constituents on rat experimental colitis. Biol. Pharm. Bull. 2006, 29, 1599–1603. [Google Scholar]
- Koda, A.; Watanabe, S.; Yanagihara, Y.; Nagai, H.; Sakamoto, K. A comparative study of the anti-allergic effects of disodium baicalein 6-phosphate (BPS) and disodium cromoglycate (DSCG). Jpn. J. Pharmacol. 1977, 27, 31–38. [Google Scholar] [CrossRef]
- Li, B.Q.; Fu, T.; Gong, W.H.; Dunlop, N.; Kung, H.F.; Yan, Y.D.; Kang, J.; Wang, J.M. The flavonoid baicalin exhibits anti-inflammatory activity by binding to chemokines. Immunopharmacology 2000, 49, 295–306. [Google Scholar] [CrossRef]
- Li, B.Q.; Fu, T.; Yan, Y.D.; Baylor, N.W.; Ruscetti, F.W.; Kung, H.F. Inhibition of HIV infection by baicalin-a flavonoid compound purified from Chinese herbal medicine. Cell Mol. Biol. Res. 1993, 39, 119–124. [Google Scholar]
- Wu, J.A.; Attele, A.S.; Zhang, L.; Yuan, C.S. Anti-HIV activity of medicinal herbs: usage and potential development. Am. J. Chin. Med. 2001, 29, 69–81. [Google Scholar] [CrossRef]
- Konoshima, T.; Kokumai, M.; Kozuka, M.; Iinuma, M.; Mizuno, M.; Tanaka, T.; Tokuda, H.; Nishino, H.; Iwashima, A. Studies on inhibitors of skin tumor promotion. XI. Inhibitory effects of flavonoids from Scutellaria baicalensis on Epstein-Barr virus activation and their anti-tumor-promoting activities. Chem. Pharm. Bull. 1992, 40, 531–533. [Google Scholar] [CrossRef]
- Shieh, D.E.; Liu, L.T.; Lin, C.C. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000, 20, 2861–2865. [Google Scholar]
- Morimoto, S.; Tateishi, N.; Matsuda, T.; Tanaka, H.; Taura, F.; Furuya, N.; Matsuyama, N.; Shoyama, Y. Novel hydrogen peroxide metabolism in suspension cells of Scutellaria baicalensis Georgi. J. Biol. Chem. 1998, 273, 12606–12611. [Google Scholar]
- Morimoto, S.; Tateishi, N.; Inuyama, M.; Taura, F.; Tanaka, H.; Shoyama, Y. Identification and molecular characterization of novel peroxidase with structural protein-like properties. J. Biol. Chem. 1999, 274, 26192–26198. [Google Scholar]
- Lin, M.C.; Tsai, M.; Wen, K.C. Supercritical fluid extraction of flavonoids from Scutellariae radix. J. Chromatogr. A 1999, 830, 387–395. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, D.; Ding, Y.; Wang, R.; Wang, X.; Du, L. A chromatographic method for baicalin quantification in rat thalamus. Biomed. Chromatogr. 2005, 19, 494–497. [Google Scholar] [CrossRef]
- Kotani, A.; Kojima, S.; Hakamata, H.; Kusu, F. HPLC with electrochemical detection to examine the pharmacokinetics of baicalin and baicalein in rat plasma after oral administration of a Kampo medicine. Anal. Biochem. 2006, 350, 99–104. [Google Scholar]
- Zhang, H.; Tian, K.; Tang, J.; Qi, S.; Chen, H.; Chen, X.; Hu, Z. Analysis of baicalein, baicalin and wogonin in Scutellariae radix and its preparation by microemulsion electrokinetic chromatography with 1-butyl-3-methylimizolium tetrafluoborate ionic liquid as additive. J. Chromatogr. A 2006, 1129, 304–307. [Google Scholar] [CrossRef]
- Weiler, E.W.; Kruger, H.; Zenk, M.H. Radioimmunoassay for the determination of the steroidal alkaloid solasodine and related compounds in living plants and herbarium specimens. Planta Med. 1980, 39, 112–124. [Google Scholar] [CrossRef]
- Kido, K.; Morinaga, O.; Shoyama, Y.; Tanaka, H. Quick analysis of baicalin in Scutellariae radix by enzyme-linked immunosorbent assay using a monoclonal antibody. Talanta 2008, 77, 346–350. [Google Scholar] [CrossRef]
- Paudel, M.K.; Putalun, W.; Sritularak, B.; Morinaga, O.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Development of a combined technique using a rapid one-step immunochromatographic assay and indirect competitive ELISA for the rapid detection of baicalin. Anal. Chim. Acta. 2011, 701, 189–193. [Google Scholar] [CrossRef]
- Tanaka, H.; Putalun, W.; Tsuzaki, C.; Shoyama, Y. A simple determination of steroidal alkaloid glycosides by thin-layer chromatography immunostaining using monoclonal antibody against solamargine. FEBS Lett. 1997, 404, 279–282. [Google Scholar] [CrossRef]
- Taki, T.; Kasama, T.; Handa, S.; Ishikawa, D. A simple and quantitative purification of glycosphingolipids and phospholipids by thin-layer chromatography blotting. Anal. Biochem. 1994, 223, 232–238. [Google Scholar]
- Shan, S.; Tanaka, H.; Shoyama, Y. Enzyme-linked immunosorbent assay for glycyrrhizin using anti-glycyrrhizin monoclonal antibody and an eastern blotting technique for glucuronides of glycyrrhetic acid. Anal. Chem. 2001, 73, 5784–5790. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morinaga, O.; Mukae, R.; Uto, T.; Pariyawongsakul, Y.; Putalun, W.; Tanaka, H.; Shoyama, Y. Development of Eastern Blotting Technique for Analysis of Baicalin Using Anti-Baicalin Monoclonal Antibody. Antibodies 2012, 1, 284-293. https://doi.org/10.3390/antib1030284
Morinaga O, Mukae R, Uto T, Pariyawongsakul Y, Putalun W, Tanaka H, Shoyama Y. Development of Eastern Blotting Technique for Analysis of Baicalin Using Anti-Baicalin Monoclonal Antibody. Antibodies. 2012; 1(3):284-293. https://doi.org/10.3390/antib1030284
Chicago/Turabian StyleMorinaga, Osamu, Ryo Mukae, Takuhiro Uto, Yothawathorn Pariyawongsakul, Waraporn Putalun, Hiroyuki Tanaka, and Yukihiro Shoyama. 2012. "Development of Eastern Blotting Technique for Analysis of Baicalin Using Anti-Baicalin Monoclonal Antibody" Antibodies 1, no. 3: 284-293. https://doi.org/10.3390/antib1030284
APA StyleMorinaga, O., Mukae, R., Uto, T., Pariyawongsakul, Y., Putalun, W., Tanaka, H., & Shoyama, Y. (2012). Development of Eastern Blotting Technique for Analysis of Baicalin Using Anti-Baicalin Monoclonal Antibody. Antibodies, 1(3), 284-293. https://doi.org/10.3390/antib1030284




