Soil Properties and Biomass Attributes in a Former Gravel Mine Area after Two Decades of Forest Restoration
Abstract
1. Introduction
2. Materials and Methods
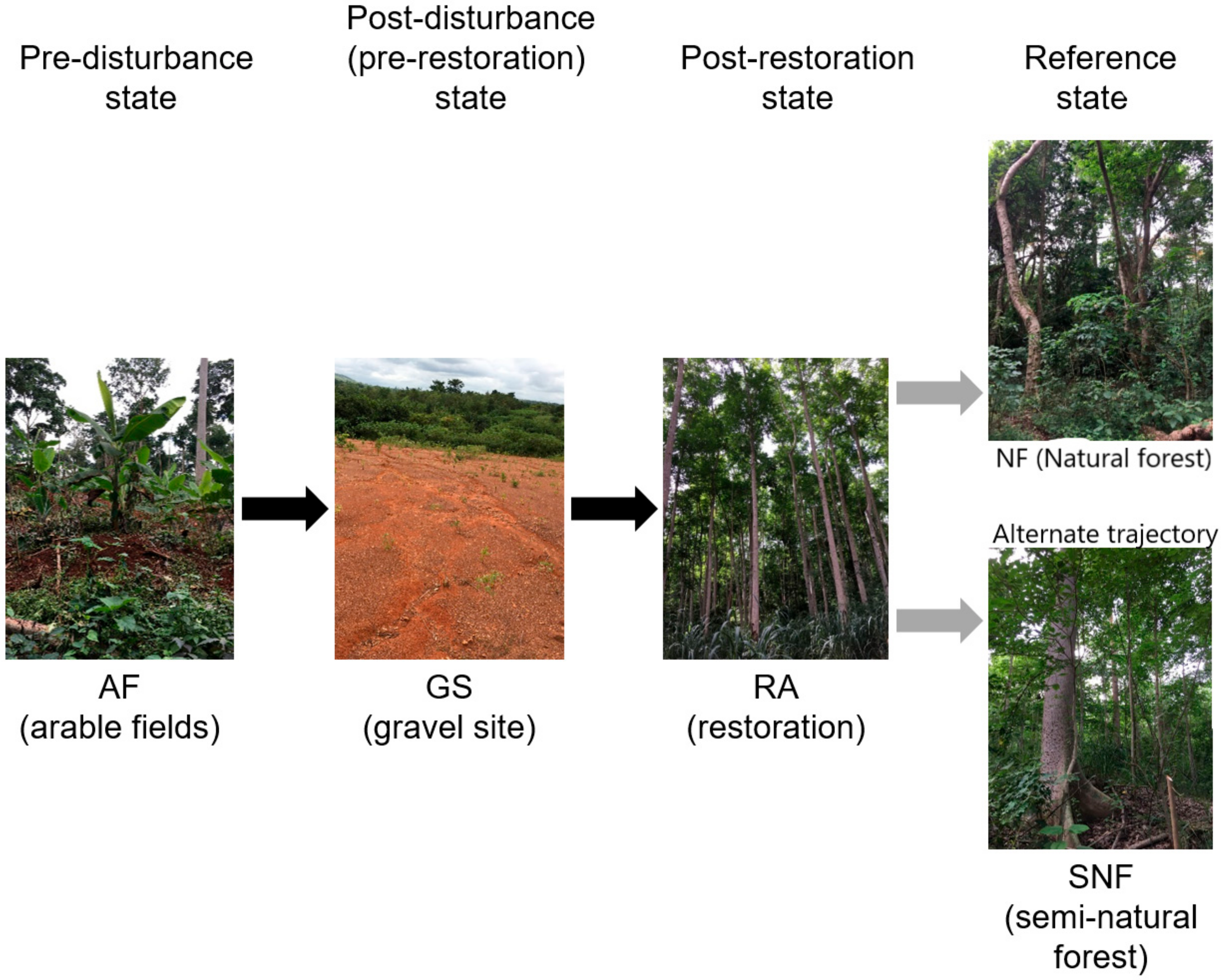
2.1. Study Area
2.2. Sampling Design
2.3. Laboratory Analyses of Soil and Root Samples
2.4. Data Analysis
3. Results
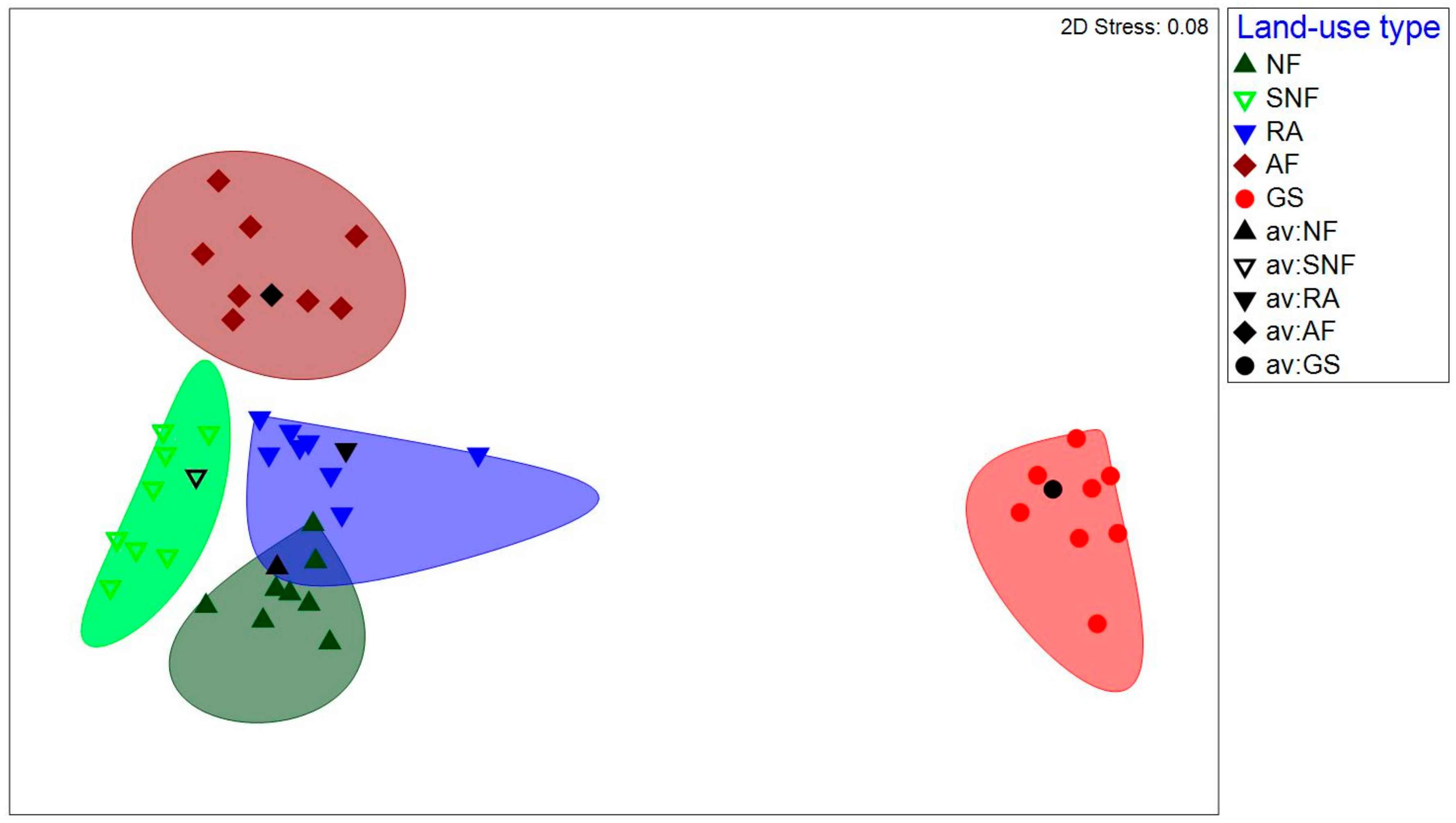
3.1. Physicochemical Soil Properties
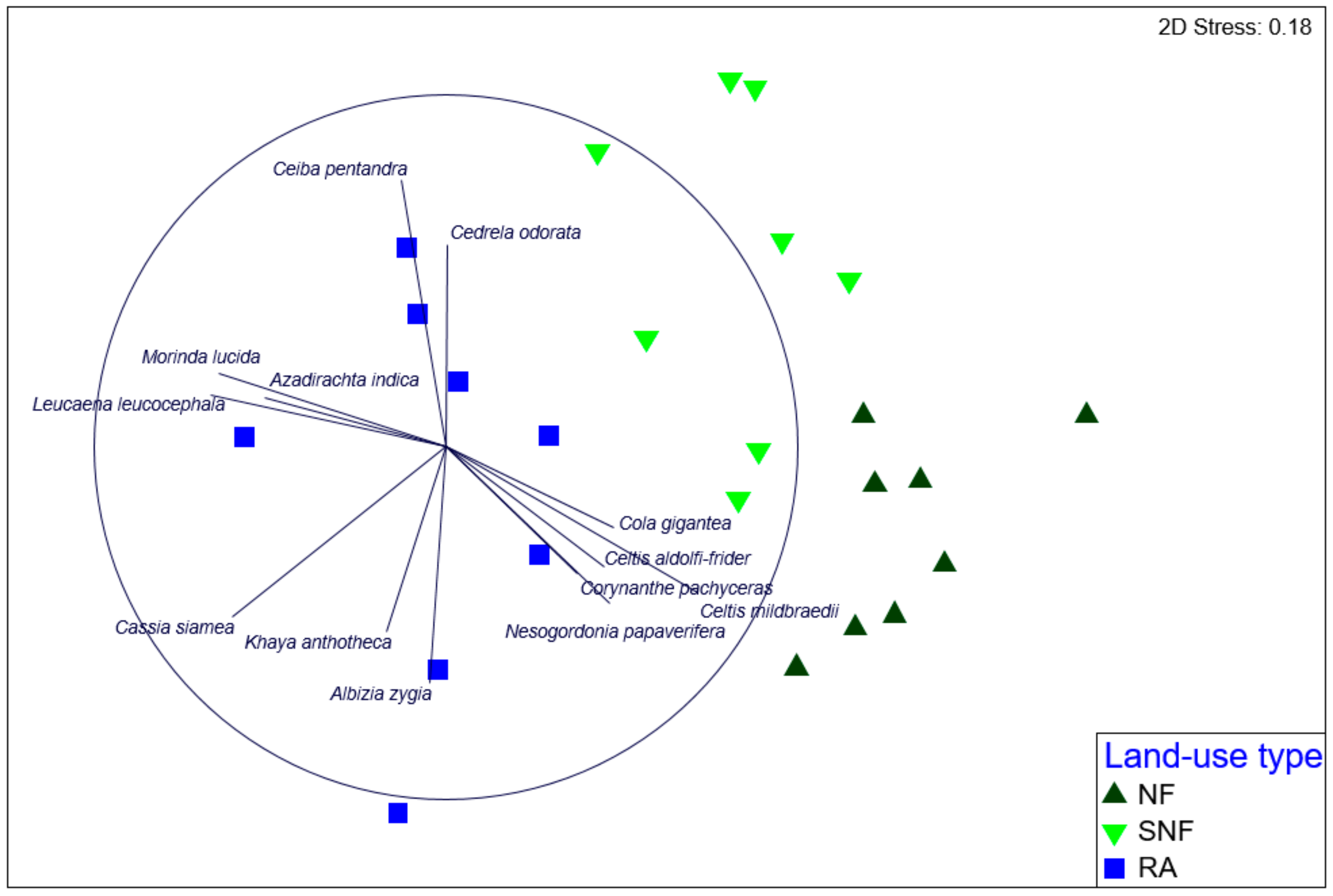
3.2. Species Composition and Abundance
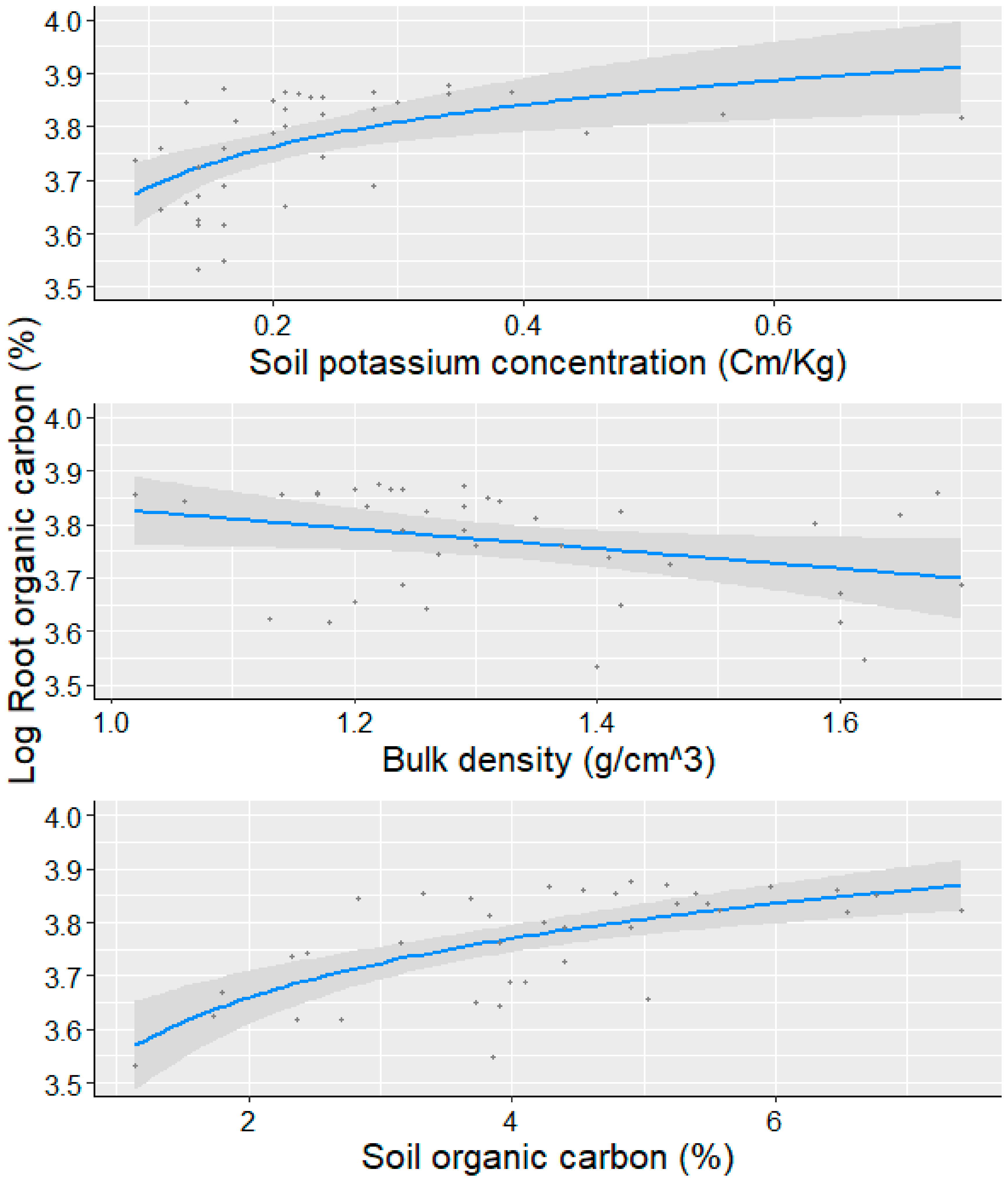
3.3. Root Organic Carbon, Above-Ground Biomass, and Its Relationship to Soil Variables
4. Discussion
4.1. Influence of Restoration Interventions on Soil Attributes
4.2. Carbon Sequestration and Biomass Reclaim
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pellikka, P.K.E.; Heikinheimo, V.; Hietanen, J.; Schäfer, E.; Siljander, M.; Heiskanen, J. Impact of land cover change on aboveground carbon stocks in Afromontane landscape in Kenya. Appl. Geogr. 2018, 94, 178–189. [Google Scholar] [CrossRef]
- Giam, X. Global biodiversity loss from tropical deforestation. Proc. Natl. Acad. Sci. USA 2017, 114, 5775–5777. [Google Scholar] [CrossRef]
- Guo, X.; Meng, M.; Zhang, J.; Chen, H.Y.H. Vegetation change impacts on soil organic carbon chemical composition in subtropical forests. Sci. Rep. 2016, 6, 29607. [Google Scholar] [CrossRef] [PubMed]
- Society for Ecological Restoration International Science & Policy Working Group. The SER International Primer on Ecological Restoration; Society for Ecological Restoration International: Washington, DC, USA, 2004. [Google Scholar]
- Bullock, J.M.; Aronson, J.; Newton, A.C.; Pywell, R.; Benayas, J.M.R. Restoration of ecosystem services and biodiversity: conflicts and opportunities. Trends Ecol. Evol. 2011, 26, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Aradottir, A.L.; Hagen, D.; Halld; Mitchell, R.J.; Raulund-Rasmussen, K.; Svavarsdóttir, K.; Tolvanen, A.; Wilson, S.D. Evaluating the process of ecological restoration. Ecol. Soc. 2016, 21. [Google Scholar] [CrossRef]
- IUCN, WRI. A Guide to the Restoration Opportunities Assessment Methodology (ROAM): Assessing Forest Landscape Restoration Opportunities at the National or Sub-National Level; Working Paper (Road-Test Edition); IUCN: Gland, Switzerland, 2014. [Google Scholar]
- De la Riva, E.G.; Jimenez, M.D.; Mola, I.; Balaguer, L.; Costa-Tenorio, M.; Casado, M.A. Rates of local colonization and extinction reveal different plant community assembly mechanisms on road verges in central Spain. J. Veg. Sci. 2011, 22, 292–302. [Google Scholar] [CrossRef]
- Maestre, F.T.; Cortina, J.; Vallejo, V.R. Are Ecosystem Composition, Structure, and Functional Status Related to Restoration Success? A Test from Semiarid Mediterranean Steppes. Restor. Ecol. 2006, 14, 258–266. [Google Scholar] [CrossRef]
- Magro, S.; López, M.G.; Casado, M.A.; Jiménez, M.D.; Trigo, D.; Mola, I.; Balaguer, L. Soil functionality at the roadside: Zooming in on a microarthropod community in an anthropogenic soil. Ecol. Eng. 2013, 60, 81–87. [Google Scholar] [CrossRef]
- Jimenez, M.D.; Mola, I.; Casado, M.A.; Balaguer, L.; Ruiz-Capillas, P.; Corona, M.E.P. SOIL Development at the Roadside: A Case Study of a Novel Ecosystem. Land Degrad. Dev. 2011, 24, 564–574. [Google Scholar] [CrossRef]
- Harris, J. Soil Microbial Communities and Restoration Ecology: Facilitators or Followers? Science 2009, 325, 573–574. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M. Soil quality indicators: Critical tools in ecosystem restoration. Curr. Opin. Environ. Sci. Heal. 2018, 5, 47–52. [Google Scholar] [CrossRef]
- Greiner, L.; Keller, A.; Grêt-Regamey, A.; Papritz, A. Soil function assessment: review of methods for quantifying the contributions of soils to ecosystem services. Land Use Policy 2017, 69, 224–237. [Google Scholar] [CrossRef]
- Maharjan, M. Effect of Land Use on Soil Fertility and Carbon Sequestration; Georg-August University of Göttingen: Göttingen, Germany, 2018. [Google Scholar]
- Laban, P.; Metternicht, G.; Davies, J. Soil Biodiversity and Soil Organic Carbon: Keeping Drylands Alive, 1st ed.; IUCN—International Union for Conservation of Nature: Gland, Switzerland, 2008; Available online: https://portals.iucn.org/library/node/47735 (accessed on 10 October 2019).
- Qiu, K.; Xie, Y.; Xu, N.; Pott, R. Ecosystem functions including soil organic carbon, total nitrogen and available potassium are crucial for vegetation recovery. Sci. Rep. 2018, 8, 7607. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.S.; Latawiec, A.E.; Sansevero, J.B.B.; Crouzeilles, R.; Moraes, L.F.D.; Castro, A.; Alves-Pinto, H.N.; Brancalion, P.H.S.; Rodrigues, R.R.; Chazdon, R.L.; et al. Look down-there is a gap-the need to include soil data in Atlantic Forest restoration. Restor. Ecol. 2018, 27, 361–370. [Google Scholar] [CrossRef]
- Peng, X.; Horn, R.; Hallet, P. Soil structure and its functions in ecosystems: Phase matter & scale matter. Soil Tillage Res. 2015, 146, 1–3. [Google Scholar] [CrossRef]
- Shimamoto, C.Y.; Padial, A.A.; Da Rosa, C.M.; Marques, M.C.M. Restoration of ecosystem services in tropical forests: A global meta-analysis. PLoS ONE 2018, 13, e0208523. [Google Scholar] [CrossRef]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Nsiah, P.K.; Schaaf, W. The potentials of biological geotextiles in erosion and sediment control during gold mine reclamation in Ghana. J. Soils Sediments 2018, 19, 1995–2006. [Google Scholar] [CrossRef]
- Gould, I.J.; Quinton, J.N.; Weigelt, A.; De Deyn, G.B.; Bardgett, R.D. Plant diversity and root traits benefit physical properties key to soil function in grasslands. Ecol. Lett. 2016, 19, 1140–1149. [Google Scholar] [CrossRef]
- Omoro, L.M.A.; Laiho, R.; Starr, M.; Pellikka, P.K. Relationships between native tree species and soil properties in the indigenous forest fragments of the Eastern Arc Mountains of the Taita Hills, Kenya. For. Stud. China 2011, 13, 198–210. [Google Scholar] [CrossRef]
- De la Riva, E.G.; Prieto, I.; Villar, R. The leaf economic spectrum drives leaf litter decomposition in Mediterranean forests. Plant Soil 2018, 435, 353–366. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment; Main Report: FAO Forestry Paper 163; FAO: Rome, Italy, 2010. [Google Scholar]
- Acheampong, E.O.; MacGregor, C.J.; Sloan, S.; Sayer, J. Deforestation is driven by agricultural expansion in Ghana’s forest reserves. Sci. Afr. 2019, 5. [Google Scholar] [CrossRef]
- Nindel, S. Forest Fire Dynamics and Carbon Stocks in Different Ecological Zones of Ghana; Technische Universität Dresden: Dresden, Germany, 2017. [Google Scholar]
- Addai, G.; Baidoo, P. The effects of forest destruction on the abundance, species richness and diversity of butterflies in the Bosomkese Forest Reserve, Brong Ahafo Region, Ghana. J. Appl. Biosci. 2013, 64, 4763. [Google Scholar] [CrossRef]
- Cash, R.N. A Reference for Restoration: Applying the Reference Site Model to Riparian Restoration Sites in the California Coastal Sage Scrub, Chaparral, and Oak Woodlands Ecoregion; Riparian Restoration: San Dimas, CA, USA, 2013. [Google Scholar]
- Ahirwal, J.; Maiti, S.K. Assessment of carbon sequestration potential of revegetated coal mine overburden dumps: A chronosequence study from dry tropical climate. J. Environ. Manag. 2017, 201, 369–377. [Google Scholar] [CrossRef] [PubMed]
- NGGL Newmont Ghana Gold Limited. Reclamation and Closure Plan Ahafo South Project; Newmont Mining Corporation: Denver, CO, USA, 2015; p. 77. [Google Scholar]
- Hawthorne, W.D.; Gyakari, N. Photo Guide for Forest Tree of Ghana. A Tree Spotters Guide for Identification of Large Trees; Oxford Forestry Institute: Oxford, UK, 2006. [Google Scholar]
- Larjavaara, M.; Muller-Landau, H.C. Measuring tree height: a quantitative comparison of two common field methods in a moist tropical forest. Methods Ecol. Evol. 2013, 4, 793–801. [Google Scholar] [CrossRef]
- Zanne, A.M.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L. Global Wood Density Database; Dryad: Oakland, CA, USA, 2009. [Google Scholar]
- Chave, J.; Réjou-Méchain, M.; Burquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Vogt, K.A.; Vogt, D.J.; Bloomfield, J. Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant Soil 1998, 200, 71–89. [Google Scholar] [CrossRef]
- Motsara, M.R.; Roy, R.N. Guide to Laboratory Establishment for Plant Nutrient Analysis; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008; p. 204. [Google Scholar]
- Hazelton, P.; Murphy, B. Interpreting soil test results. In What do All the Numbers Mean? CSIRO Publishing: Clayton, Victoria, Australia, 2007. [Google Scholar]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 5th ed.; Pearson: Columbus, OH, USA, 2008; p. 1086. [Google Scholar]
- Bouyoucos, G.J. The Hydrometer As a New Method for the Mechanical Analysis of Soils. Soil Sci. 1927, 23, 343–354. [Google Scholar] [CrossRef]
- Frasier, I.; Noellemeyer, E.; Fernández, R.; Quiroga, A. Direct field method for root biomass quantification in agroecosystems. MethodsX 2016, 3, 513–519. [Google Scholar] [CrossRef]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.C.; Fisher, D.N.; Goodwin, C.E.; Robinson, B.; Hodgson, D.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2009, 1, 3–14. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Claeke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B. Package Vegan; Community Ecology Package; CRAN: Vienna, Austria, 2013; Volume 9, pp. 1–295. [Google Scholar]
- Claeke, K.R.; Gorley, R.N.; Sommerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E: Plymouth, UK, 2014. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Getting Started with PRIMER v7; PRIMER-E Ltd.: Devon, UK, 2015. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed effects models and extensions in ecology with R. Stat. Biol. Health 2009, 32, 574. [Google Scholar]
- Luo, S.; He, Y.; Wang, Z.; Duan, D.; Zhang, J.; Zhang, Y.; Zhu, Y.; Yu, J.; Zhang, S.; Xu, F.; et al. Comparison of the retrieving precision of potato leaf area index derived from several vegetation indices and spectral parameters of the continuum removal method. Eur. J. Remote Sens. 2019, 52, 155–168. [Google Scholar] [CrossRef]
- Grueber, C.E.; Nakagawa, S.; Laws, R.J.; Jamieson, I.G. Multimodel inference in ecology and evolution: challenges and solutions. J. Evol. Biol. 2011, 24, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.D.; Ward, B.A.; Williams, R.G.; Follows, M.J. The impact of fine-scale turbulence on phytoplankton community structure. Limnol. Oceanogr. Fluids Environ. 2014, 4, 34–49. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed effects models. Methods Ecol. Evol. 2012, 4, 133–142. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Wang, J.; Zhao, F.; Yang, J.; Li, X. Mining Site Reclamation Planning Based on Land Suitability Analysis and Ecosystem Services Evaluation: A Case Study in Liaoning Province, China. Sustainability 2017, 9, 890. [Google Scholar] [CrossRef]
- Dutta, R.K.; Agrawal, M. Effect of tree plantations on the soil characteristics and microbial activity of coal mine spoil land. Trop. Ecol. 2002, 43, 315–324. [Google Scholar]
- Borišev, M.; Pajević, S.; Nikolić, S.; Pilipović, A.; Arsenov, D.; Župunski, M. Mine Site Restoration Using Silvicultural Approach. In Bio-Geotechnologies for Mine Site Rehabilitation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–130. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128129869000075 (accessed on 10 October 2019).
- Moreno-de las Heras, M. Development of soil physical structure and biological functionality in mining spoils affected by soil erosion in a Mediterranean-Continental environment. Geoderma 2009, 149, 249–256. [Google Scholar] [CrossRef]
- Nurtjahya, E.; Setiada, D.; Guhardja, E.M.; Setiadi, Y. Revegetation of tin-mined land using various local tree species in Bangka island, Indonesia. J. Am. Soc. Min. Reclam. 2008, 1, 14–19. [Google Scholar]
- Chaer, G.M.; Resende, A.S.; Campello, E.F.C.; De Faria, S.M.; Boddey, R.M. Nitrogen-fixing legume tree species for the reclamation of severely degraded lands in Brazil. Tree Physiol. 2011, 31, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Bekunda, M. Nitrogen Fixation in Leucaena Leucocephala and Effects of Pruning on Cereal Yield; No. IAEA-TECDOC-1053; International Atomic Energy Agency: Vienna, Autstria, 1998. [Google Scholar]
- Vanlauwe, B.; Akinnifesi, F.K.; Tossah, B.K.; Lyasse, O.; Sanginga, N.; Merckx, R. Root distribution of Senna siamea grown on a series of derived-savanna-zone soils in Togo, West Africa. Agrofor. Syst. 2002, 54, 1–12. [Google Scholar] [CrossRef]
- Zipper, C.E.; Burger, J.A.; Skousen, J.G.; Angel, P.N.; Barton, C.D.; Davis, V.; Franklin, J. Restoring Forests and Associated Ecosystem Services on Appalachian Coal Surface Mines. Environ. Manag. 2011, 47, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Buta, M.; Blaga, G.; Paulette, L.; Păcurar, I.; Roșca, S.; Borsai, O.; Grecu, F.; Sînziana, P.E.; Negrușier, C. Soil Reclamation of Abandoned Mine Lands by Revegetation in Northwestern Part of Transylvania: A 40-Year Retrospective Study. Sustainability 2019, 11, 3393. [Google Scholar] [CrossRef]
- Larney, F.J.; Angers, D.A. The role of organic amendments in soil reclamation: A review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Albert, K.M.; Mensah, A.K. Role of revegetation in restoring fertility of degraded mined soils in Ghana: A review. Int. J. Biodivers. Conserv. 2015, 7, 57–80. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, H.; Song, M.; Guan, D. The dynamics of carbon accumulation in Eucalyptus and Acacia plantations in the Pearl River delta region. Ann. For. Sci. 2018, 75, 40. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and Associated Fungi Drive Long-Term Carbon Sequestration in Boreal Forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Dong, J.; Kaufmann, R.K.; Myneni, R.; Tucker, C.J.; E Kauppi, P.; Liski, J.; Buermann, W.; Alexeyev, V.; Hughes, M.K. Remote sensing estimates of boreal and temperate forest woody biomass: carbon pools, sources, and sinks. Remote Sens. Environ. 2003, 84, 393–410. [Google Scholar] [CrossRef]
- Meli, P.; Holl, K.D.; Benayas, J.M.R.; Jones, H.P.; Jones, P.C.; Montoya, D.; Mateos, D.M. A global review of past land use, climate, and active vs. passive restoration effects on forest recovery. PLoS ONE 2017, 12, e0171368. [Google Scholar] [CrossRef]
- Srinivas, K.; Sundarapandian, S. Biomass and carbon stocks of trees in tropical dry forest of East Godavari region, Andhra Pradesh, India. Geol. Ecol. Landsc. 2018, 3, 114–122. [Google Scholar] [CrossRef]
- Gandhi, D.S.; Sundarapandian, S. Large-scale carbon stock assessment of woody vegetation in tropical dry deciduous forest of Sathanur reserve forest, Eastern Ghats, India. Environ. Monit. Assess 2017, 189, 604. [Google Scholar] [CrossRef] [PubMed]
- Lung, M.; Espira, A. The influence of stand variables and human use on biomass and carbon stocks of a transitional African forest: Implications for forest carbon projects. For. Ecol. Manag. 2015, 351, 36–46. [Google Scholar] [CrossRef]
- Padmakumar, B.; Sreekanth, N.; Shanthiprabha, V.; Paul, J.; Sreedharan, K.; Augustine, T.; Jayasooryan, K.; Rameshan, M.; Mohan, M.; Ramasamy, E.; et al. Tree biomass and carbon density estimation in the tropical dry forest of Southern Western Ghats, India. iFor.—Biogeosci. For. 2018, 11, 534–541. [Google Scholar] [CrossRef]
- Liu, F.; Gao, C.; Chen, M.; Li, K. Above- and below-ground biomass relationships of Leucaena leucocephala (Lam.) de Wit in different plant stands. PLoS ONE 2018, 13, e0207059. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Y.-J.; Wu, Y.; Hu, R.; Zhu, J.; Tao, J.; Zhang, T. Relationships between aboveground biomass and plant cover at two spatial scales and their determinants in northern Tibetan grasslands. Ecol. Evol. 2017, 7, 7954–7964. [Google Scholar] [CrossRef]
- Du, H.; Liu, L.; Su, L.; Zeng, F.; Wang, K.; Peng, W.; Zhang, H.; Song, T. Seasonal Changes and Vertical Distribution of Fine Root Biomass During Vegetation Restoration in a Karst Area, Southwest China. Front. Plant Sci. 2019, 9. [Google Scholar] [CrossRef]
- Sun, T.; Hobbie, S.E.; Berg, B.; Zhang, H.; Wang, Q.; Wang, Z.; Hättenschwiler, S. Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. Proc. Natl. Acad. Sci. USA 2018, 115, 10392–10397. [Google Scholar] [CrossRef]
- Manaye, A.; Negash, M.; Alebachew, M. Effect of degraded land rehabilitation on carbon stocks and biodiversity in semi-arid region of Northern Ethiopia. For. Sci. Technol. 2019, 15, 70–79. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, W.; Wang, Y.; Wan, H.; Chen, D.; Tang, Z.; Tang, X.; Zhou, G.; Xie, Z.; et al. Plant diversity enhances productivity and soil carbon storage. Proc. Natl. Acad. Sci. USA 2018, 115, 4027–4032. [Google Scholar] [CrossRef]
- Meng, S.; Jia, Q.; Zhou, G.; Zhou, H.; Liu, Q.; Yu, J. Fine Root Biomass and Its Relationship with Aboveground Traits of Larix gmelinii Trees in Northeastern China. Forest 2018, 9, 35. [Google Scholar] [CrossRef]
- Sollins, P. Factors influencing species composition in tropical lowland rain forest: does soil matter? Ecology 1998, 79, 23–30. [Google Scholar] [CrossRef]
- Jeon, I.; Nam, K. Change in the site density and surface acidity of clay minerals by acid or alkali spills and its effect on pH buffering capacity. Sci. Rep. 2019, 9, 9878. [Google Scholar] [CrossRef] [PubMed]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Pandey, S.; Pandey, A. Plant roots and carbon sequestration. Curr. Sci. 2006, 91, 85–890. [Google Scholar]
- De Deyn, G.B.; Shiel, R.S.; Ostle, N.; McNamara, N.P.; Oakley, S.; Young, I.; Freeman, C.; Fenner, N.; Quirk, H.; Bardgett, R.D. Additional carbon sequestration benefits of grassland diversity restoration. J. Appl. Ecol. 2010, 48, 600–608. [Google Scholar] [CrossRef]
- Zhao, Y.; Krzic, M.; Bulmer, C.E.; Schmidt, M.G.; Simard, S.W. Relative bulk density as a measure of compaction and its influence on tree height. Can. J. For. Res. 2010, 40, 1724–1735. [Google Scholar] [CrossRef]
- Haile, W.; Boke, S. Response of Irish Potato (Solanum tuberosum) to the Application of Potassium at Acidic Soils of Chencha, Southern Ethiopia. Int. J. Agric. Biol. 2011, 13, 595–598. [Google Scholar]
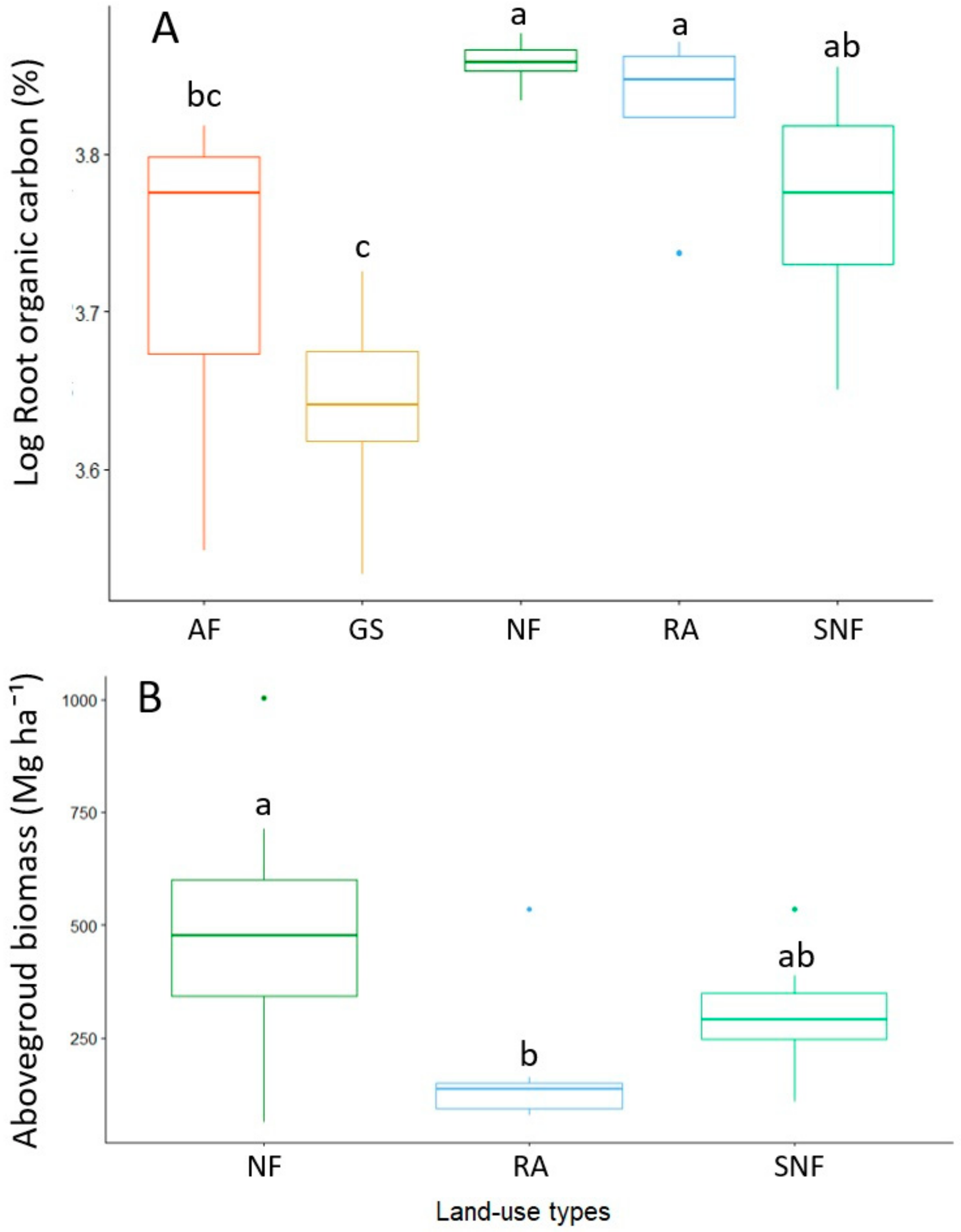
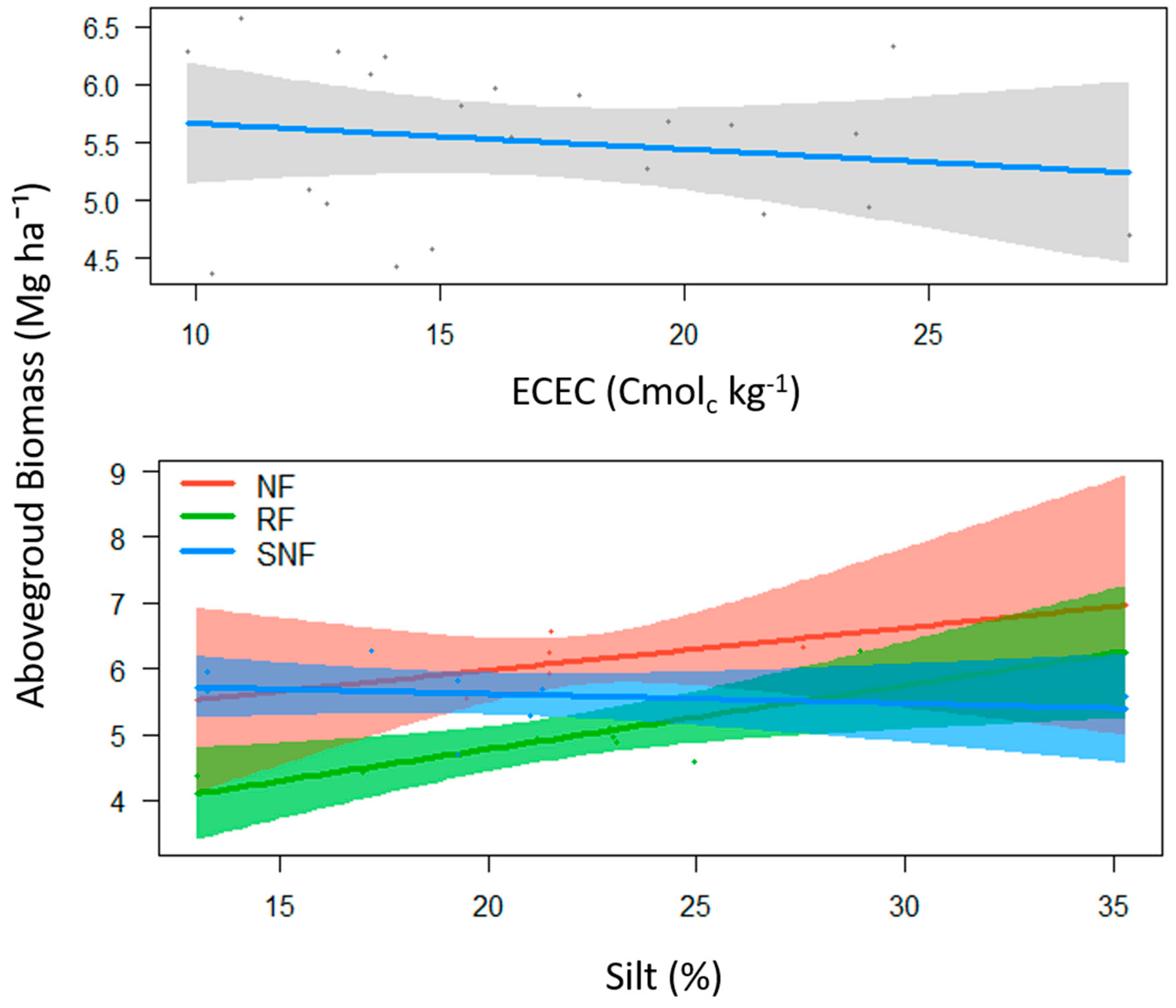
| Properties | NF | SNF | RA | AF | GS | Significance Levels |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | ||
| Sand (%) | 62.14 ± 2.42 | 67.40 ± 2.32 | 61.62 ± 2.61 | 62.79 ± 1.66 | 61.49 ± 3.51 | 0.464 |
| Clay (%) | 15.13 ± 2.76 | 12.62 ± 2.70 | 16.63 ± 3.30 | 20.90 ± 3.12 | 21.17 ± 4.85 | 0.343 |
| Silt (%) | 22.74 ± 1.60 | 19.98 ± 2.45 | 21.75 ± 1.72 | 16.32 ± 1.82 | 17.35 ± 1.88 | 0.106 |
| BD (gcm−3) | 1.17 ± 0.03c | 1.28 ± 0.03b | 1.36 ± 0.05ab | 1.50 ± 0.06a | 1.41 ± 0.08ab | 0.001 |
| pH | 5.71 ± 0.18b | 6.44 ± 0.19a | 5.69 ± 0.14b | 6.19 ± 0.12a | 4.51 ± 0.10c | 0.001 |
| N (%) | 0.29 ± 0.02a | 0.19 ± 0.01b | 0.22 ± 0.03ab | 0.21 ± 0.01b | 0.10 ± 0.01c | 0.001 |
| P (mgkg-1) | 6.01 ± 0.52b | 9.33 ± 2.93b | 8.44 ± 2.29b | 31.37 ± 12.17a | 2.83 ± 0.30c | 0.009 |
| K (Cmolckg−1) | 0.29 ± 0.02 | 0.22 ± 0.02 | 0.23 ± 0.05 | 0.26 ± 0.08 | 0.14 ± 0.00 | 0.195 |
| Na (%) | 0.18 ± 0.02a | 0.14 ± 0.00a | 0.17 ± 0.01a | 0.18 ± 0.02a | 0.11 ± 0.01b | 0.007 |
| Ca(Cmolckg−1) | 10.85 ± 1.66a | 12.91 ± 1.63a | 9.48 ± 0.99a | 11.09 ± 1.12a | 5.48 ± 0.37b | 0.003 |
| Mg(Cmolckg−1) | 3.43 ± 0.53a | 5.33 ± 1.06a | 4.24 ± 0.83a | 2.58 ± 0.69ab | 1.69 ± 0.17b | 0.011 |
| SOM (%) | 7.98 ± 0.53a | 7.04 ± 0.63a | 8.80 ± 1.12a | 8.50 ± 0.76a | 4.55 ± 0.79b | 0.004 |
| ECEC Cmolkg−1) | 15.45 ± 1.49a | 19.23 ± 2.03a | 15.32 ± 1.69a | 14.81 ± 1.45ab | 8.92 ± 0.34b | 0.004 |
| Base Saturation (%) | 95.19 ± 0.88a | 95.98 ± 1.38a | 92.26 ± 0.85b | 94.95 ± 0.65a | 82.83 ± 2.06c | 0.001 |
| C/N | 16.68 ± 1.84c | 21.53 ±1.69b | 24.08 ± 3.27b | 21.25 ± 1.69b | 32.98 ± 7.21a | 0.048 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damptey, F.G.; Birkhofer, K.; Nsiah, P.K.; de la Riva, E.G. Soil Properties and Biomass Attributes in a Former Gravel Mine Area after Two Decades of Forest Restoration. Land 2020, 9, 209. https://doi.org/10.3390/land9060209
Damptey FG, Birkhofer K, Nsiah PK, de la Riva EG. Soil Properties and Biomass Attributes in a Former Gravel Mine Area after Two Decades of Forest Restoration. Land. 2020; 9(6):209. https://doi.org/10.3390/land9060209
Chicago/Turabian StyleDamptey, Frederick Gyasi, Klaus Birkhofer, Paul Kofi Nsiah, and Enrique G. de la Riva. 2020. "Soil Properties and Biomass Attributes in a Former Gravel Mine Area after Two Decades of Forest Restoration" Land 9, no. 6: 209. https://doi.org/10.3390/land9060209
APA StyleDamptey, F. G., Birkhofer, K., Nsiah, P. K., & de la Riva, E. G. (2020). Soil Properties and Biomass Attributes in a Former Gravel Mine Area after Two Decades of Forest Restoration. Land, 9(6), 209. https://doi.org/10.3390/land9060209





