How Important Are Resistance, Dispersal Ability, Population Density and Mortality in Temporally Dynamic Simulations of Population Connectivity? A Case Study of Tigers in Southeast Asia
Abstract
:1. Introduction
2. Materials and Methods
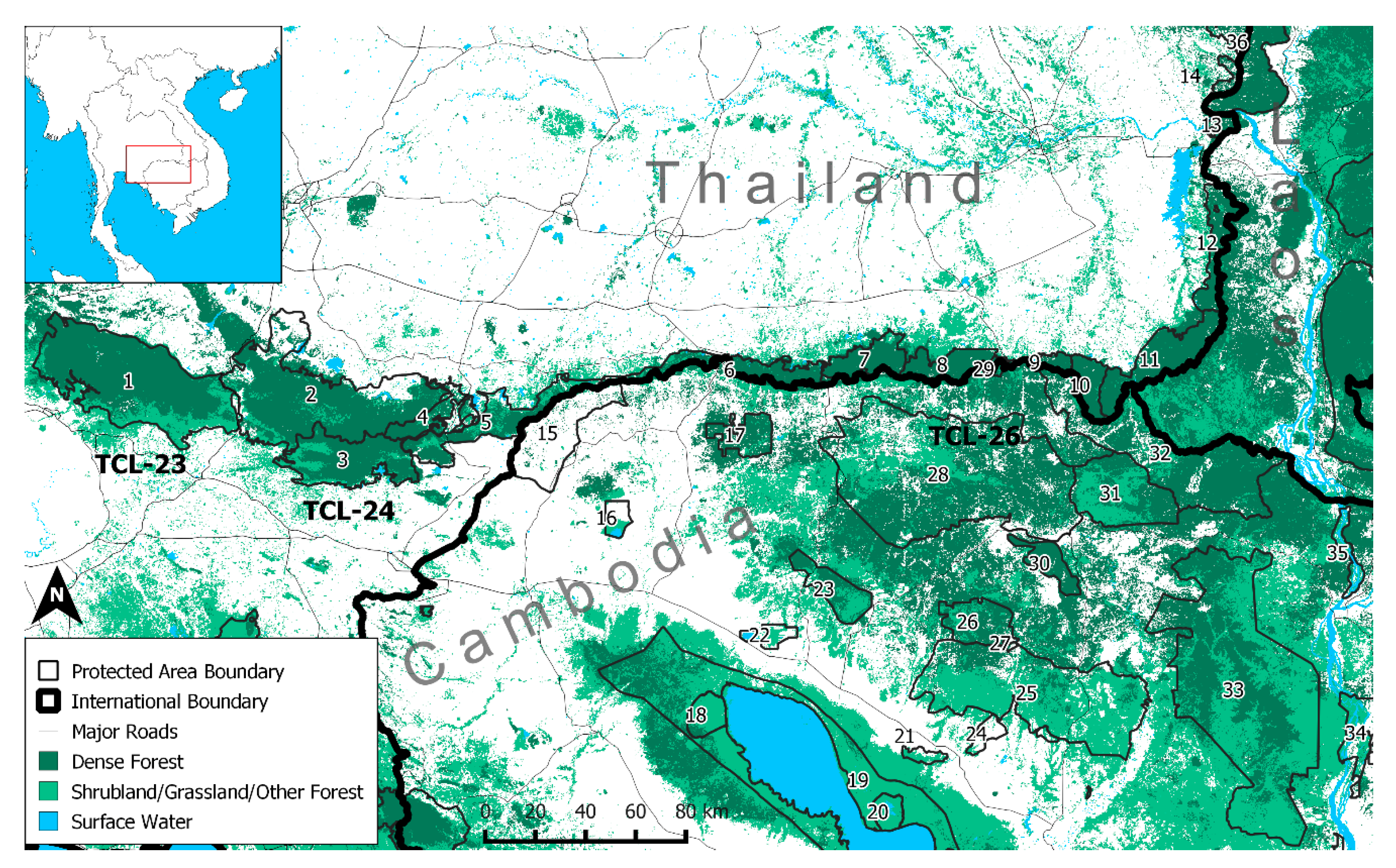
2.1. Study Area
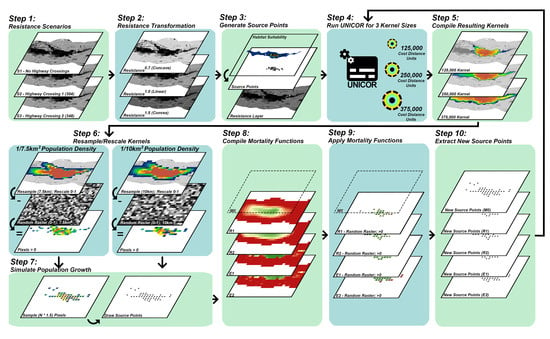
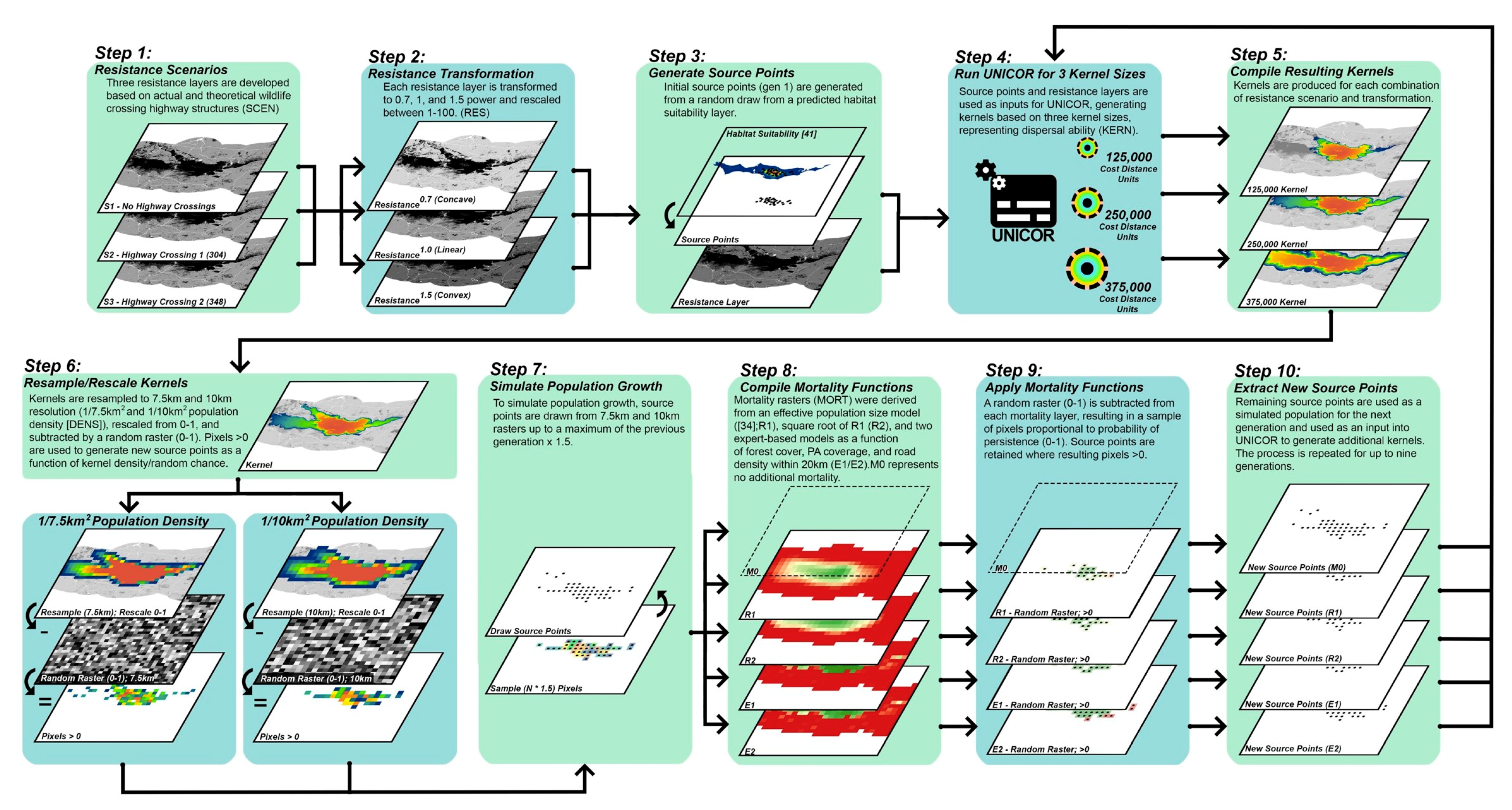
2.2. Landscape Connectivity Simulations
2.2.1. Resistance Surfaces
2.2.2. Population Source Points
2.2.3. Dispersal Ability
2.2.4. Population Density and Growth
2.2.5. Mortality
2.3. Evaluation of Simulated Scenarios
3. Results
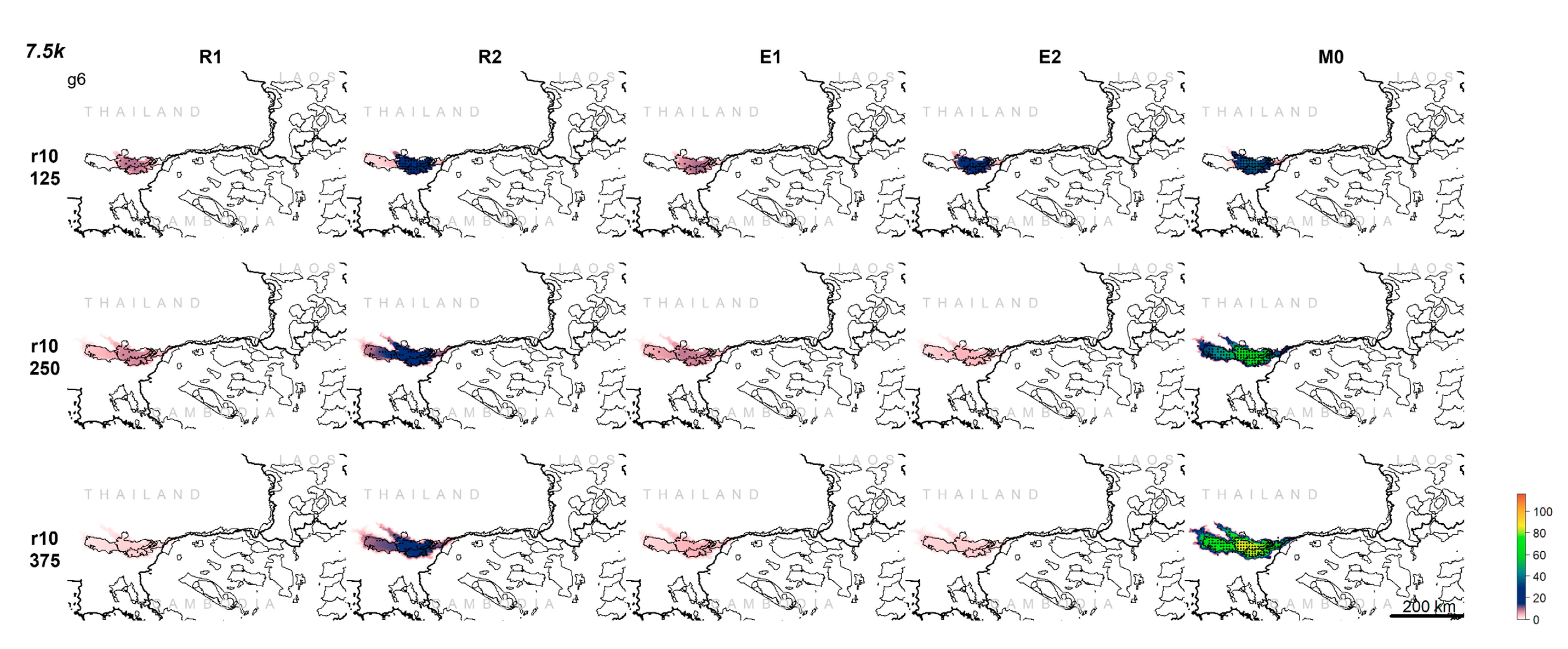
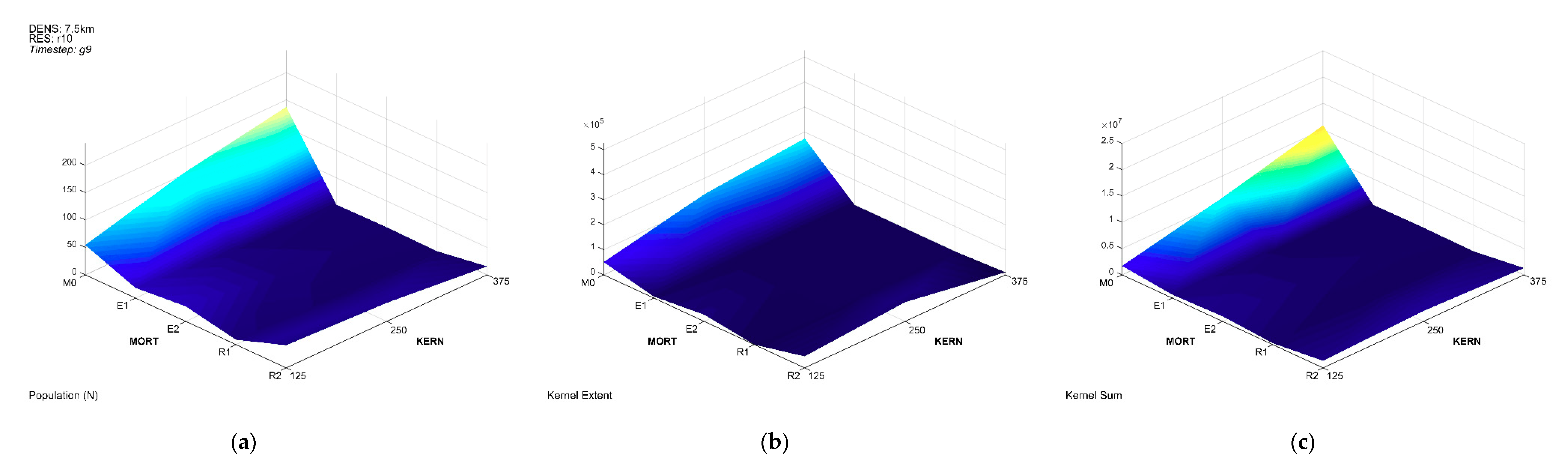
3.1. Population Trajectory and Colonization
3.2. Analysis of Variance
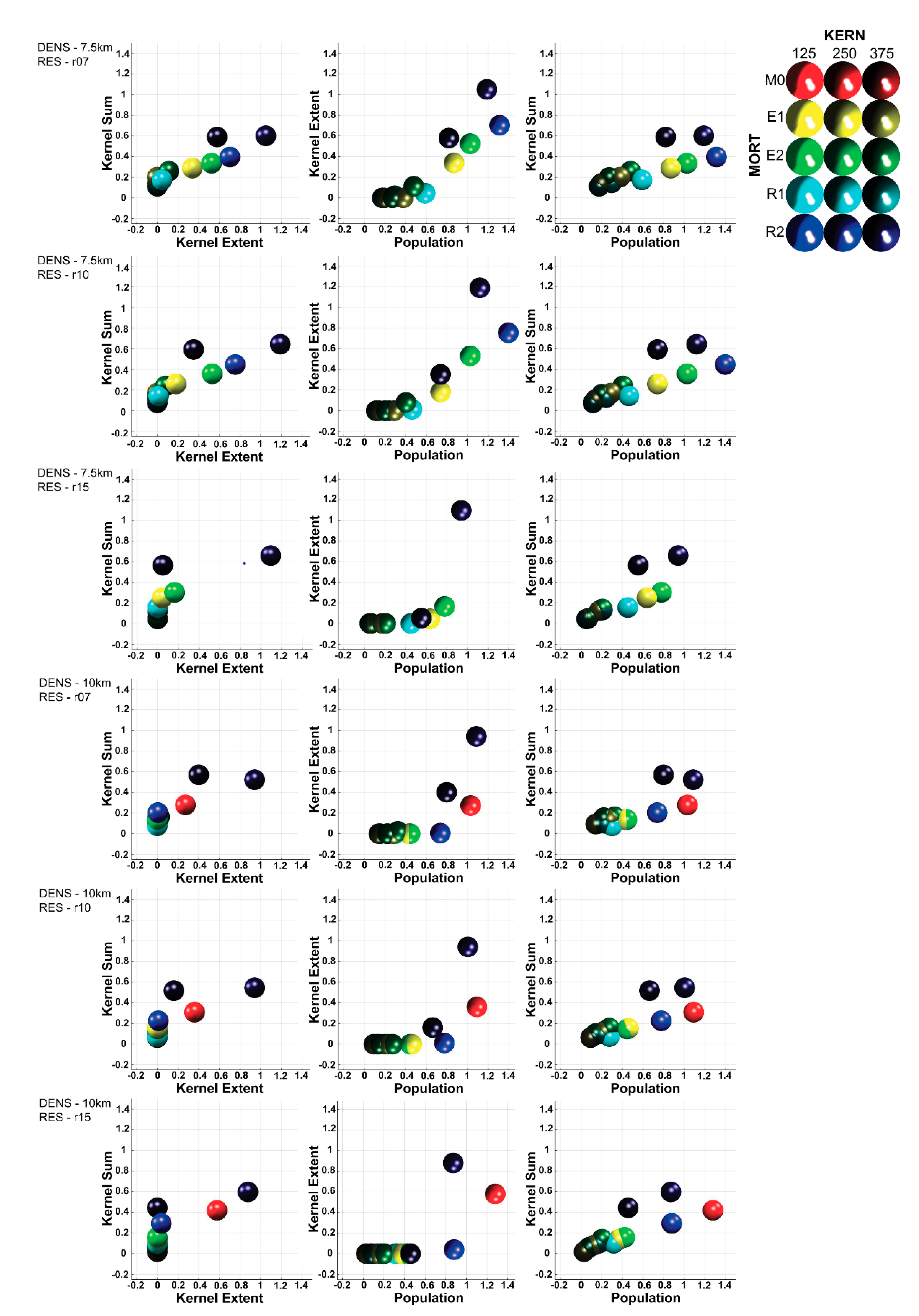
3.3. Multivariate Interactions
3.4. Multivariate Trajectory Analysis
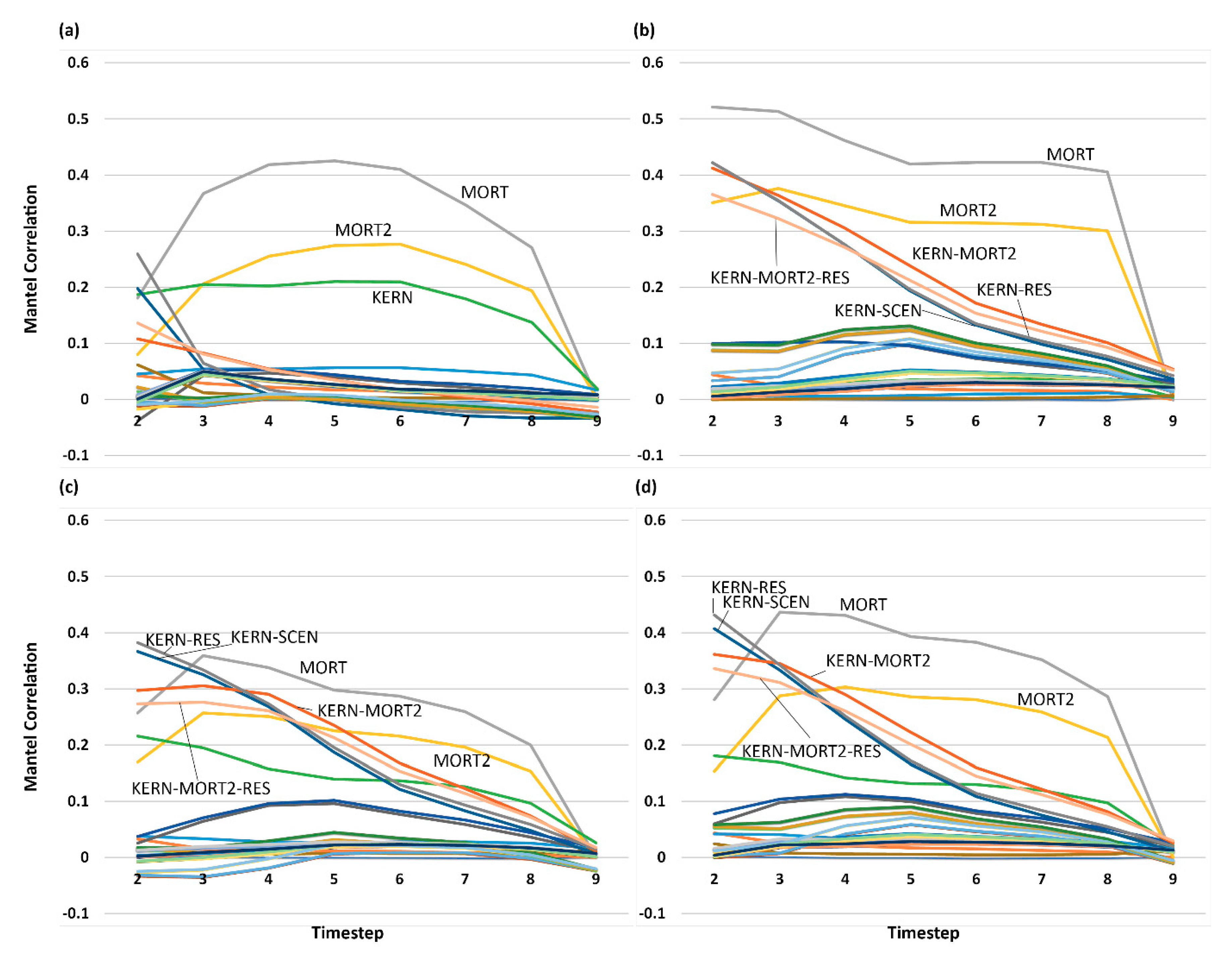
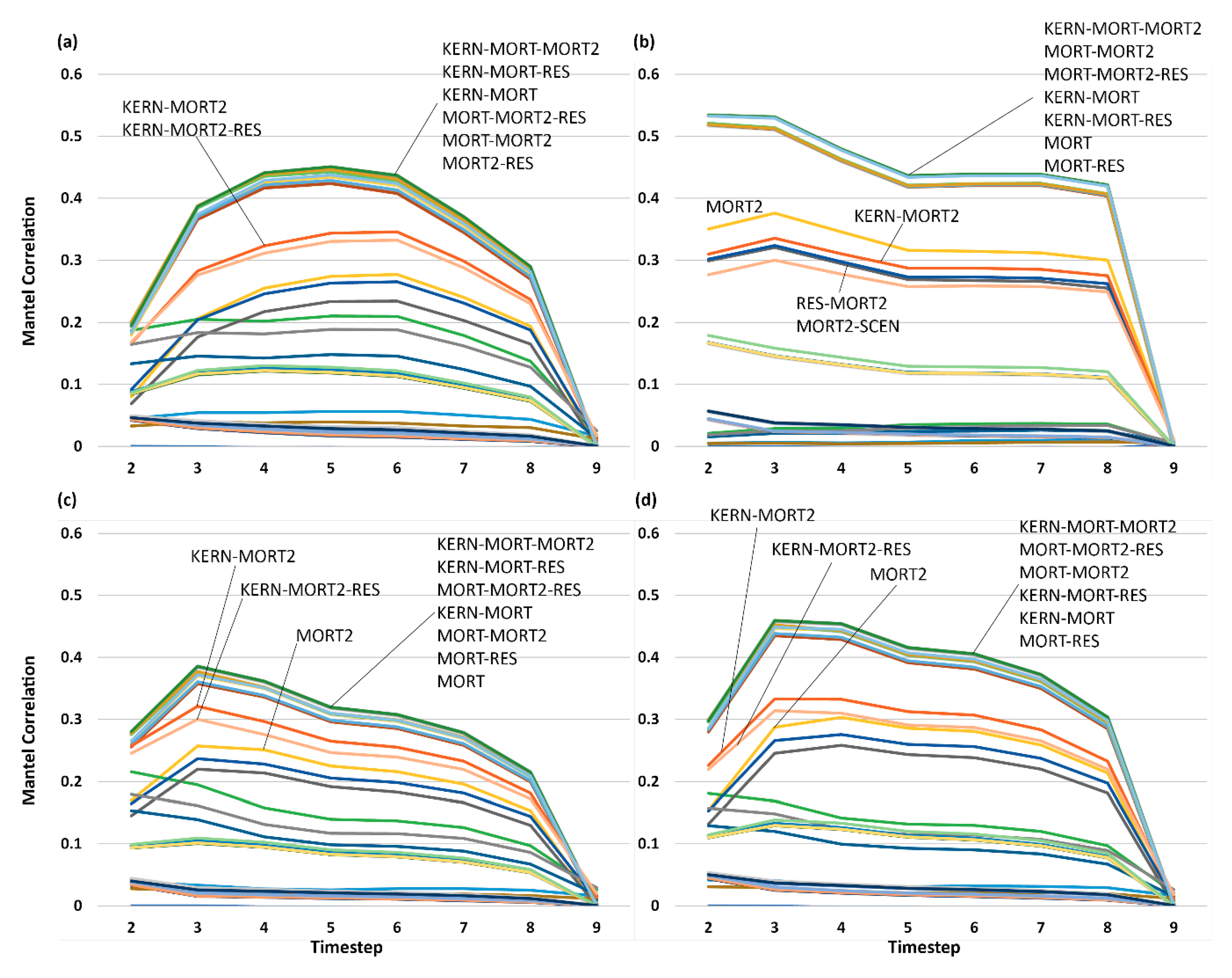
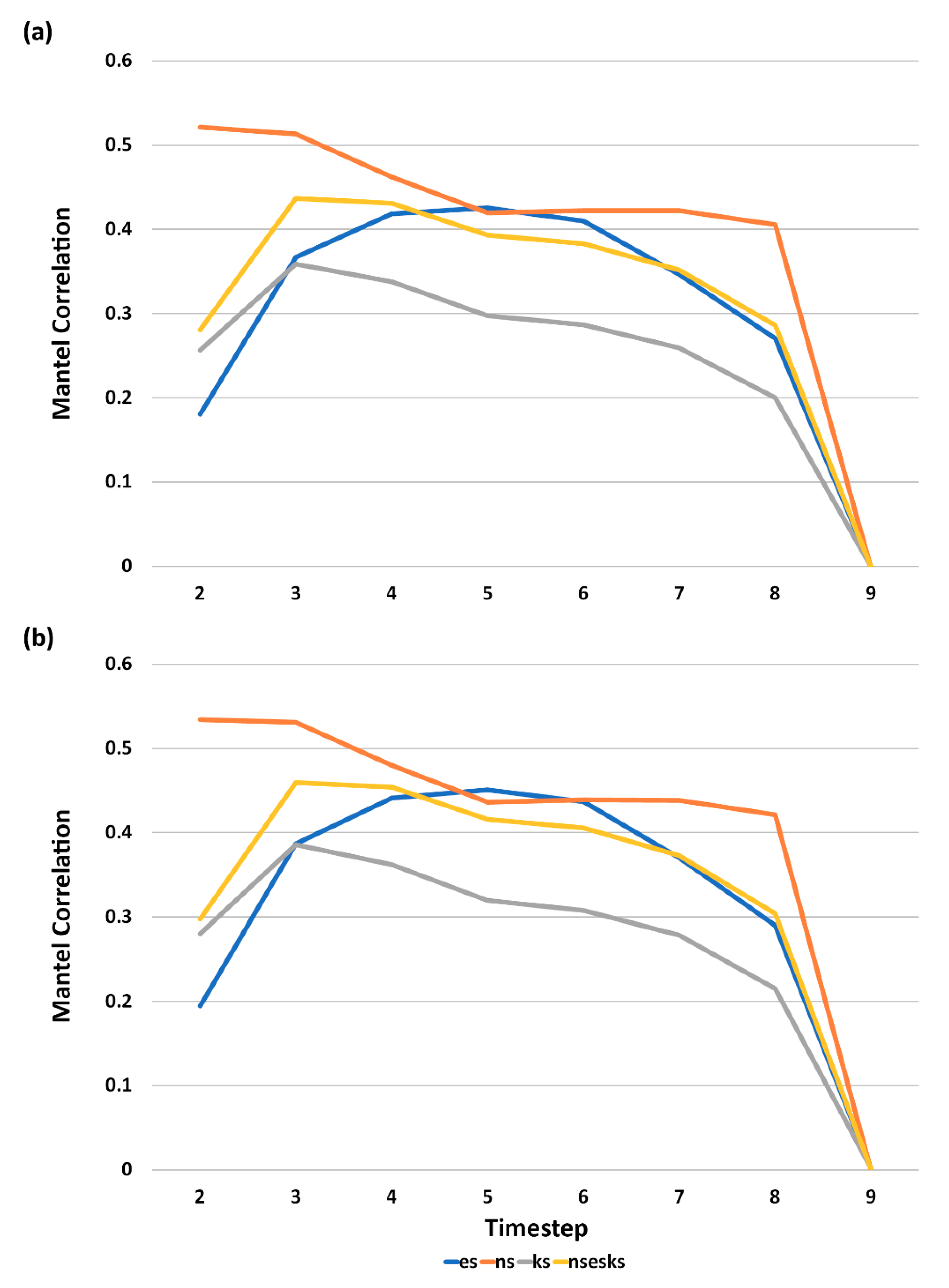
3.5. Analysis of Effect Size and Interactions among Factors Using Mantel Tests
4. Discussion
4.1. Implications for Tiger Management and Conservation
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lotka, A.J. The growth of mixed populations: Two species competing for a common food supply. J. Washingt. Acad. Sci. 1932, 22, 461–469. [Google Scholar]
- Volterra, V. Variations and fluctuations of the number of individuals in animal species living together. ICES J. Mar. Sci. 1928, 3, 3–51. [Google Scholar] [CrossRef]
- Levins, R. Some mathematical problems in biology. In Extinction; The American Mathematical Society: Providence, RI, USA, 1970; pp. 77–107. [Google Scholar]
- Cushman, S.A.; H. McRae, B.; McGarigal, K. Basics of Landscape Ecology: An Introduction to Landscapes and Population Processes for Landscape Geneticists. In Landscape Genetics; Wiley Online Books; Balkenhol, N., Cushman, S.A., Storfer, A.T., Waits, L.P., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 9–34. ISBN 9781118525258. [Google Scholar]
- Cushman, S.A. Pushing the envelope in genetic analysis of species invasion. Mol. Ecol. 2015, 24, 259–262. [Google Scholar] [CrossRef]
- Barros, T.; Carvalho, J.; Fonseca, C.; Cushman, S.A. Assessing the complex relationship between landscape, gene flow, and range expansion of a Mediterranean carnivore. Eur. J. Wildl. Res. 2019, 65, 44. [Google Scholar] [CrossRef]
- Walters, S. Modeling scale-dependent landscape pattern, dispersal, and connectivity from the perspective of the organism. Landsc. Ecol. 2007, 22, 867–881. [Google Scholar] [CrossRef]
- Diffendorfer, J.E. Testing Models of Source-Sink Dynamics and Balanced Dispersal. Oikos 1998, 81, 417–433. [Google Scholar] [CrossRef]
- Cushman, S.A.; Compton, B.W.; McGarigal, K. Habitat Fragmentation Effects Depend on Complex Interactions Between Population Size and Dispersal Ability: Modeling Influences of Roads, Agriculture and Residential Development Across a Range of Life-History Characteristics. In Spatial Complexity, Informatics, and Wildlife Conservation; Cushman, S.A., Huettmann, F., Eds.; Springer: Tokyo, Japan, 2010; pp. 369–385. ISBN 978-4-431-87771-4. [Google Scholar]
- Cushman, S.A.; Landguth, E.L.; Flather, C.H. Evaluating population connectivity for species of conservation concern in the American Great Plains. Biodivers. Conserv. 2013, 22, 2583–2605. [Google Scholar] [CrossRef] [Green Version]
- Kramer-Schadt, S.; Revilla, E.; Wiegand, T.; Breitenmoser, U. Fragmented landscapes, road mortality and patch connectivity: Modelling influences on the dispersal of Eurasian lynx. J. Appl. Ecol. 2004, 41, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Kaszta, Ż.; Cushman, S.A.; Hearn, A.J.; Burnham, D.; Macdonald, E.A.; Goossens, B.; Nathan, S.K.S.S.; Macdonald, D.W. Integrating Sunda clouded leopard (Neofelis diardi) conservation into development and restoration planning in Sabah (Borneo). Biol. Conserv. 2019, 235, 63–76. [Google Scholar] [CrossRef]
- Cushman, A.S.; Lewis, S.J.; Landguth, L.E. Why Did the Bear Cross the Road? Comparing the Performance of Multiple Resistance Surfaces and Connectivity Modeling Methods. Diversity 2014, 6, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Elliot, N.; Cushman, S.; Macdonald, D.W.; Loveridge, A. The devil is in the dispersers: The metrics of landscape connectivity change with demography. J. Appl. Ecol. 2014, 51, 1169–1178. [Google Scholar] [CrossRef]
- Kaszta, Ż.; Cushman, S.A.; Htun, S.; Naing, H.; Burnham, D.; Macdonald, D.W. Simulating the impact of Belt and Road initiative and other major developments in Myanmar on an ambassador felid, the clouded leopard, Neofelis nebulosa. Landsc. Ecol. 2020, 35, 727–746. [Google Scholar] [CrossRef] [Green Version]
- Zeller, K.A.; McGarigal, K.; Whiteley, A.R. Estimating landscape resistance to movement: A review. Landsc. Ecol. 2012, 27, 777–797. [Google Scholar] [CrossRef]
- Spear, S.F.; Balkenhol, N.; Fortin, M.J.; McRae, B.H.; Scribner, K. Use of resistance surfaces for landscape genetic studies: Considerations for parameterization and analysis. Mol. Ecol. 2010, 19, 3576–3591. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.A.; Hansen, A.J.; Inman, R.M.; Lawrence, R.L.; Hoegh, A.B. Testing landscape resistance layers and modeling connectivity for wolverines in the western United States. Glob. Ecol. Conserv. 2020, 23, e01125. [Google Scholar] [CrossRef]
- Diniz, M.F.; Cushman, S.A.; Machado, R.B.; De Marco Júnior, P. Landscape connectivity modeling from the perspective of animal dispersal. Landsc. Ecol. 2020, 35, 41–58. [Google Scholar] [CrossRef]
- Cushman, S.A.; Elliot, N.B.; Macdonald, D.W.; Loveridge, A.J. A multi-scale assessment of population connectivity in African lions (Panthera leo) in response to landscape change. Landsc. Ecol. 2016, 31, 1337–1353. [Google Scholar] [CrossRef]
- Cushman, S.A. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Conserv. 2006, 128, 231–240. [Google Scholar] [CrossRef]
- Thatte, P.; Joshi, A.; Vaidyanathan, S.; Landguth, E.; Ramakrishnan, U. Maintaining tiger connectivity and minimizing extinction into the next century: Insights from landscape genetics and spatially-explicit simulations. Biol. Conserv. 2018, 218, 181–191. [Google Scholar] [CrossRef]
- Moqanaki, E.M.; Cushman, S.A. All roads lead to Iran: Predicting landscape connectivity of the last stronghold for the critically endangered Asiatic cheetah. Anim. Conserv. 2017, 20, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Kanagaraj, R.; Wiegand, T.; Kramer-Schadt, S.; Goyal, S.P. Using individual-based movement models to assess inter-patch connectivity for large carnivores in fragmented landscapes. Biol. Conserv. 2013, 167, 298–309. [Google Scholar] [CrossRef]
- Carmona, P.; Franco, D. Impact of Dispersal on the Total Population Size, Constancy and Persistence of Two-patch Spatially-separated Populations. Math. Model. Nat. Phenom. 2015, 10, 45–55. [Google Scholar] [CrossRef]
- Cushman, S.A.; McGarigal, K. Multivariate Landscape Trajectory Analysis. In Temporal Dimensions of Landscape Ecology: Wildlife Responses to Variable Resources; Bissonette, J.A., Storch, I., Eds.; Springer US: Boston, MA, USA, 2007; pp. 119–140. ISBN 978-0-387-45447-4. [Google Scholar]
- Ash, E.; Kaszta, Ż.; Noochdumrong, A.; Redford, T.; Chanteap, P.; Hallam, C.; Jaroensuk, B.; Raksat, S.; Srinoppawan, K.; Macdonald, D.W.; et al. Opportunity for Thailand’s Forgotten Tigers: Assessment of Indochinese tiger Panthera tigris corbetti and prey from camera-trap surveys in Eastern Thailand. Oryx 2020, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Vasudev, D.; Nichols, J.D.; Ramakrishnan, U.; Ramesh, K.; Vaidyanathan, S. Assessing Landscape Connectivity for Tigers and Prey Species: Concepts and Practice. In Methods for Monitoring Tiger and Prey Populations; Karanth, K.U., Nichols, J.D., Eds.; Springer: Singapore, 2017; pp. 255–288. ISBN 978-981-10-5436-5. [Google Scholar]
- Thapa, K.; Wikramanayake, E.; Malla, S.; Acharya, K.P.; Lamichhane, B.R.; Subedi, N.; Pokharel, C.P.; Thapa, G.J.; Dhakal, M.; Bista, A.; et al. Tigers in the Terai: Strong evidence for meta-population dynamics contributing to tiger recovery and conservation in the Terai Arc Landscape. PLoS ONE 2017, 12, e0177548. [Google Scholar] [CrossRef]
- Rasphone, A.; Kéry, M.; Kamler, J.F.; Macdonald, D.W. Documenting the demise of tiger and leopard, and the status of other carnivores and prey, in Lao PDR’s most prized protected area: Nam Et–Phou Louey. Glob. Ecol. Conserv. 2019, 20, e00766. [Google Scholar] [CrossRef]
- Goodrich, J.M.; Lynam, A.; Miquelle, D.G.; Wibisono, H.T.; Kawanishi, K.; Pattanavibool, A.; Htun, S.; Tempa, T.; Karki, J.; Jhala, Y.V.; et al. Panthera tigris. IUCN Red List Threat. Species 2015: e.T15955A50659951. 2015. Available online: https://www.iucnredlist.org/species/15955/50659951 (accessed on 26 October 2020).
- Gray, T.N.E.; Crouthers, R.; Ramesh, K.; Vattakaven, J.; Borah, J.; Pasha, M.K.S.; Lim, T.; Phan, C.; Singh, R.; Long, B.; et al. A framework for assessing readiness for tiger Panthera tigris reintroduction: A case study from eastern Cambodia. Biodivers. Conserv. 2017, 26, 2383–2399. [Google Scholar] [CrossRef]
- Sanderson, E.; Forrest, J.; Loucks, C.; Ginsberg, J.; Dinerstein, E.; Seidensticker, J.; Leimgruber, P.; Songer, M.; Heydlauff, A.; O’Brien, T.; et al. Setting Priorities for the Conservation and Recovery of Wild Tigers: 2005–2015—The Technical Assessment; WCS, WWF, Smithsonian, and NFWF-STF: New York, NY, USA; Washington, DC, USA, 2006. [Google Scholar]
- Reddy, P.A.; Cushman, S.A.; Srivastava, A.; Sarkar, M.S.; Shivaji, S. Tiger abundance and gene flow in Central India are driven by disparate combinations of topography and land cover. Divers. Distrib. 2017, 23, 863–874. [Google Scholar] [CrossRef]
- UNEP-WCMC; IUCN Protected Planet. The World Database on Protected Areas (WDPA). Cambridge, UK. Available online: www.protectedplanet.net (accessed on 15 July 2019).
- SERVIR-Mekong. SERVIR–Mekong Regional Land Cover Monitoring System (RLCMS); SERVIR-Mekong/USAID/NASA/ADPC/ICIMOD; Asian Disaster Preparedness Center (ADPC): Bangkok, Thailand, 2018. [Google Scholar]
- OpenStreetMap OpenStreetMap. Available online: www.openstreetmap.org (accessed on 10 June 2019).
- Landguth, E.L.; Hand, B.K.; Glassy, J.; Cushman, S.A.; Sawaya, M.A. UNICOR: A species connectivity and corridor network simulator. Ecography 2012, 35, 9–14. [Google Scholar] [CrossRef]
- Graves, T.; Chandler, R.B.; Royle, J.A.; Beier, P.; Kendall, K.C. Estimating landscape resistance to dispersal. Landsc. Ecol. 2014, 29, 1201–1211. [Google Scholar] [CrossRef]
- Compton, B.W.; McGarigal, K.; Cushman, S.A.; Gamble, L.R. A resistant-kernel model of connectivity for amphibians that breed in vernal pools. Conserv. Biol. 2007, 21, 788–799. [Google Scholar] [CrossRef]
- Ash, E.; Macdonald, D.W.; Cushman, S.A.; Noochdumrong, A.; Redford, T.; Kaszta, Ż. Optimization of spatial scale, but not functional shape, affects the performance of habitat suitability models: A case study of tigers (Panthera tigris) in Thailand. Landsc. Ecol. in press.
- Krishnamurthy, R.; Cushman, S.A.; Sarkar, M.S.; Malviya, M.; Naveen, M.; Johnson, J.A.; Sen, S. Multi-scale prediction of landscape resistance for tiger dispersal in central India. Landsc. Ecol. 2016, 31, 1355–1368. [Google Scholar] [CrossRef]
- Karanth, K.U. Estimating tiger Panthera tigris populations from camera-trap data using capture-recapture models. Biol. Conserv. 1995, 71, 333–338. [Google Scholar] [CrossRef]
- Karanth, K.U.; Nichols, J.D. Estimation of tiger densities in India using photographic captures and recaptures. Ecology 1998, 79, 2852–2862. [Google Scholar] [CrossRef]
- Ash, E.; Hallam, C.; Chanteap, P.; Kaszta, Ż.; Macdonald, D.W.; Rojanachinda, W.; Redford, T.; Harihar, A. Estimating the density of a globally important tiger (Panthera tigris) population: Using simulations to evaluate survey design in Eastern Thailand. Biol. Conserv. 2020, 241, 108349. [Google Scholar] [CrossRef]
- Lynam, A.; Nowell, K. Panthera tigris ssp. corbetti. IUCN Red List Threat. Species 2011: e.T136853A4346984. 2011. Available online: https://www.iucnredlist.org/species/136853/4346984 (accessed on 26 October 2020).
- Joshi, A.R.; Dinerstein, E.; Wikramanayake, E.; Anderson, M.L.; Olson, D.; Jones, B.S.; Seidensticker, J.; Lumpkin, S.; Hansen, M.C.; Sizer, N.C.; et al. Tracking changes and preventing loss in critical tiger habitat. Sci. Adv. 2016, 2, e1501675. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, M.S.; Ramesh, K.; Johnson, J.A.; Sen, S.; Nigam, P.; Gupta, S.K.; Murthy, R.S.; Saha, G.K. Movement and home range characteristics of reintroduced tiger (Panthera tigris) population in Panna Tiger Reserve, central India. Eur. J. Wildl. Res. 2016, 62, 537–547. [Google Scholar] [CrossRef]
- Sunquist, M. What is a Tiger? Ecology and Behavior. In Tigers of the World, 2nd ed.; Tilson, R., Nyhus, P., Eds.; Elsevier: New York, NY, USA, 2010; pp. 19–34. [Google Scholar]
- Smith, J.L.D.; McDougal, C. The Contribution of Variance in Lifetime Reproduction to Effective Population Size in Tigers. Conserv. Biol. 1991, 5, 484–490. [Google Scholar] [CrossRef]
- Karanth, K.U.; Stith, B.M. Prey depletion as a critical determinant of tiger population viability. In Riding the Tiger: Tiger Conservation in Human-Dominated Landscapes; Seidensticker, J., Jackson, P., Christie, S., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 100–113. ISBN 0521648351. [Google Scholar]
- Linkie, M.; Chapron, G.; Martyr, D.J.; Holden, J.; Leader-Williams, N. Assessing the viability of tiger subpopulations in a fragmented landscape. J. Appl. Ecol. 2006, 43, 576–586. [Google Scholar] [CrossRef]
- Walston, J.; Robinson, J.G.; Bennett, E.L.; Breitenmoser, U.; da Fonseca, G.A.B.; Goodrich, J.; Gumal, M.; Hunter, L.; Johnson, A.; Ullas Karanth, K.; et al. Bringing the tiger back from the brink-the six percent solution. PloS Biol. 2010, 8, e1000485. [Google Scholar] [CrossRef] [Green Version]
- Sunarto, S.; Kelly, M.J.; Parakkasi, K.; Klenzendorf, S.; Septayuda, E.; Kurniawan, H. Tigers need cover: Multi-scale occupancy study of the big cat in Sumatran forest and plantation landscapes. PLoS ONE 2012, 7, e30859. [Google Scholar] [CrossRef] [Green Version]
- Kafley, H.; Gompper, M.E.; Sharma, M.; Lamichane, B.R.; Maharjan, R. Tigers (Panthera tigris) respond to fine spatial-scale habitat factors: Occupancy-based habitat association of tigers in Chitwan National Park, Nepal. Wildl. Res. 2016, 43, 398. [Google Scholar] [CrossRef]
- Smith, J.L.D. The Role of Dispersal in Structuring the Chitwan Tiger Population. Behaviour 1993, 124, 165–195. [Google Scholar] [CrossRef]
- Goodrich, J.M.; Kerley, L.L.; Smirnov, E.N.; Miquelle, D.G.; McDonald, L.; Quigley, H.B.; Hornocker, M.G.; McDonald, T. Survival rates and causes of mortality of Amur tigers on and near the Sikhote-Alin Biosphere Zapovednik. J. Zool. 2008, 276, 323–329. [Google Scholar] [CrossRef]
- Mantel, N. The Detection of Disease Clustering and a Generalized Regression Approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Carroll, C.; Miquelle, D.G. Spatial viability analysis of Amur tiger Panthera tigris altaica in the Russian Far East: The role of protected areas and landscape matrix in population persistence. J. Appl. Ecol. 2006, 43, 1056–1068. [Google Scholar] [CrossRef]
- Thapa, T.B. Human caused mortality in the leopard (Panthera pardus) population of Nepal. J. Inst. Sci. Technol. 2014, 19, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Naude, V.N.; Balme, G.A.; O’Riain, J.; Hunter, L.T.B.; Fattebert, J.; Dickerson, T.; Bishop, J.M. Unsustainable anthropogenic mortality disrupts natal dispersal and promotes inbreeding in leopards. Ecol. Evol. 2020, 10, 3605–3619. [Google Scholar] [CrossRef]
- Puyravaud, J.P.; Cushman, S.A.; Davidar, P.; Madappa, D. Predicting landscape connectivity for the Asian elephant in its largest remaining subpopulation. Anim. Conserv. 2017, 20, 225–234. [Google Scholar] [CrossRef]
- Bonte, D.; Van Dyck, H.; Bullock, J.M.; Coulon, A.; Delgado, M.; Gibbs, M.; Lehouck, V.; Matthysen, E.; Mustin, K.; Saastamoinen, M.; et al. Costs of dispersal. Biol. Rev. 2012, 87, 290–312. [Google Scholar] [CrossRef]
- Carr, L.W.; Fahrig, L. Effect of Road Traffic on Two Amphibian Species of Differing Vagility. Conserv. Biol. 2001, 15, 1071–1078. [Google Scholar] [CrossRef]
- Carr, L.W.; Fahrig, L.; Pope, S.E. Impacts of Landscape Transformation by Roads. In Applying Landscape Ecology in Biological Conservation; Gutzwiller, K.J., Ed.; Springer: New York, NY, USA, 2002; pp. 225–243. ISBN 978-1-4613-0059-5. [Google Scholar]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar] [CrossRef]
- Gibbs, J.P. Amphibian Movements in Response to Forest Edges, Roads, and Streambeds in Southern New England. J. Wildl. Manag. 1998, 62, 584–589. [Google Scholar] [CrossRef]
- Goodwin, B.J.; Fahrig, L. How does landscape structure influence landscape connectivity? Oikos 2002, 99, 552–570. [Google Scholar] [CrossRef] [Green Version]
- Jackson, N.D.; Fahrig, L. Relative effects of road mortality and decreased connectivity on population genetic diversity. Biol. Conserv. 2011, 144, 3143–3148. [Google Scholar] [CrossRef]
- Kerley, L.I.; Goodrich, J.M.; Miquelle, D.G.; Smirnov, E.N.; Quigley, H.B.; Hornocker, M.G. Effects of Roads and Human Disturbance on Amur Tigers. Conserv. Biol. 2002, 16, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Coffin, A.W. From roadkill to road ecology: A review of the ecological effects of roads. J. Transp. Geogr. 2007, 15, 396–406. [Google Scholar] [CrossRef]
- Trombulak, S.C.; Frissell, C.A. Review of Ecological Effects of Roads on Terrestrial and Aquatic Communities. Conserv. Biol. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Carroll, C.; Noss, R.F.; Paquet, P.C. Carnivores as focal species for conservation planning in the Rocky Mountain region. Ecol. Appl. 2001, 11, 961–980. [Google Scholar] [CrossRef]
- Schwab, A.C.; Zandbergen, P.A. Vehicle-related mortality and road crossing behavior of the Florida panther. Appl. Geogr. 2011, 31, 859–870. [Google Scholar] [CrossRef]
- Keeley, A.T.H.; Beier, P.; Gagnon, J.W. Estimating landscape resistance from habitat suitability: Effects of data source and nonlinearities. Landsc. Ecol. 2016, 31, 2151–2162. [Google Scholar] [CrossRef]
- Zeller, K.A.; Vickers, T.W.; Ernest, H.B.; Boyce, W.M. Multi-level, multi-scale resource selection functions and resistance surfaces for conservation planning: Pumas as a case study. PLoS ONE 2017, 12, e0179570. [Google Scholar] [CrossRef]
- Winiarski, K.J.; Peterman, W.E.; Whiteley, A.R.; McGarigal, K. Multiscale resistant kernel surfaces derived from inferred gene flow: An application with vernal pool breeding salamanders. Mol. Ecol. Resour. 2020, 20, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Matthysen, E. Density-dependent dispersal in birds and mammals. Ecography 2005, 28, 403–416. [Google Scholar] [CrossRef]
- Gurung, B.; Smith, J.L.D.; McDougal, C.; Karki, J.B. Tiger Human Conflicts: Investigating Ecological and Sociological Issues of Tiger Conservation in the Buffer Zone of Chitwan National Park, Nepal; WWF-Nepal Program: Kathmandu, Nepal, 2006. [Google Scholar]
- Gour, D.S.; Bhagavatula, J.; Bhavanishankar, M.; Reddy, P.A.; Gupta, J.A.; Sarkar, M.S.; Hussain, S.M.; Harika, S.; Gulia, R.; Shivaji, S. Philopatry and Dispersal Patterns in Tiger (Panthera tigris). PLoS ONE 2013, 8, e66956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Qureshi, Q.; Sankar, K.; Krausman, P.R.; Goyal, S.P. Use of camera traps to determine dispersal of tigers in semi-arid landscape, western India. J. Arid Environ. 2013, 98, 105–108. [Google Scholar] [CrossRef]
- Sunquist, M.; Karanth, U.K.; Sunquist, F. Ecology, behaviour and resilience of the tiger and its conservation needs. In Riding the Tiger: Tiger Conservation in Human-Dominated Landscapes; Seidensticker, J., Jackson, P., Christie, S., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 5–18. [Google Scholar]
- Lohani, S.; Dilts, T.E.; Weisberg, P.J.; Null, S.E.; Hogan, Z.S. Rapidly Accelerating Deforestation in Cambodia’s Mekong River Basin: A Comparative Analysis of Spatial Patterns and Drivers. Water 2020, 12, 2191. [Google Scholar] [CrossRef]
- Rostro-García, S.; Kamler, J.F.; Ash, E.; Clements, G.R.; Gibson, L.; Lynam, A.J.; McEwing, R.; Naing, H.; Paglia, S. Endangered leopards: Range collapse of the Indochinese leopard (Panthera pardus delacouri) in Southeast Asia. Biol. Conserv. 2016, 201, 293–300. [Google Scholar] [CrossRef]
- Karanth, K.U.; Nichols, J.D.; Kumar, N.S.; Link, W.A.; Hines, J.E. Tigers and their prey: Predicting carnivore densities from prey abundance. Proc. Natl. Acad. Sci. USA 2004, 101, 4854–4858. [Google Scholar] [CrossRef] [Green Version]
- Clements, G.R.; Lynam, A.J.; Gaveau, D.; Yap, W.L.; Lhota, S.; Goosem, M.; Laurance, S.; Laurance, W.F. Where and how are roads endangering mammals in Southeast Asia’s forests? PLoS ONE 2014, 9, e115376. [Google Scholar] [CrossRef] [Green Version]
- González-Gallina, A.; Hidalgo-Mihart, M.G.; Castelazo-Calva, V. Conservation implications for jaguars and other neotropical mammals using highway underpasses. PLoS ONE 2018, 13, e0206614. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.T.; Barrueto, M.; Clevenger, A.P. Road mitigation is a demographic filter for grizzly bears. Wildl. Soc. Bull. 2017, 41, 712–719. [Google Scholar] [CrossRef]
- Gloyne, C.C.; Clevenger, A.P. Cougar Puma concolor use of wildlife crossing structures on the Trans-Canada highway in Banff National Park, Alberta. Wildl. Biol. 2001, 7, 117–124. [Google Scholar] [CrossRef]
- Clevenger, A.P.; Waltho, N. Factors Influencing the Effectiveness of Wildlife Underpasses in Banff National Park, Alberta, Canada. Conserv. Biol. 2000, 14, 47–56. [Google Scholar] [CrossRef]
- Caldwell, M.R.; Klip, J.M.K. Wildlife Interactions within Highway Underpasses. J. Wildl. Manag. 2020, 84, 227–236. [Google Scholar] [CrossRef]
- Ash, E.; Kaszta, Ż.; Redford, T.; Noochdumrong, A.; Macdonald, D.W. Environmental factors, human presence, and prey interact to explain patterns of tiger presence in Eastern Thailand. Anim. Conserv. 2020. [Google Scholar] [CrossRef]
| Factor | Parameter | (a) | (b) | |||||
|---|---|---|---|---|---|---|---|---|
| N > 30 | N = 30 | N = 1 to 30 | N = 0 | Khao Yai NP | Cambodia | Laos | ||
| SCEN | S1 | 233 | 7 | 500 | 160 | 455 | 79 | 16 |
| S2 | 238 | 11 | 508 | 143 | 498 | 76 | 9 | |
| S3 | 235 | 11 | 499 | 155 | 472 | 88 | 13 | |
| DENS | 10 km | 268 | 18 | 812 | 252 | 641 | 99 | 18 |
| 7.5 km | 438 | 11 | 695 | 206 | 784 | 144 | 20 | |
| MORT | M0 | 507 | 5 | 28 | - | 474 | 235 | 38 |
| E1 | 4 | 3 | 363 | 170 | 169 | 1 | - | |
| E2 | 21 | 5 | 393 | 121 | 220 | 1 | - | |
| R1 | - | - | 373 | 167 | 124 | - | - | |
| R2 | 174 | 16 | 350 | - | 438 | 6 | - | |
| RES | r07 | 243 | 17 | 555 | 85 | 438 | 64 | - |
| r10 | 240 | 5 | 508 | 147 | 473 | 69 | 1 | |
| r15 | 223 | 7 | 444 | 226 | 514 | 110 | 37 | |
| KERN | 125 kcu | 264 | 17 | 608 | 11 | 371 | 12 | - |
| 250 kcu | 256 | 10 | 525 | 109 | 609 | 92 | - | |
| 375 kcu | 186 | 2 | 374 | 338 | 445 | 139 | 38 | |
| All | 706 | 29 | 1507 | 458 | 1425 | 243 | 38 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ash, E.; Cushman, S.A.; Macdonald, D.W.; Redford, T.; Kaszta, Ż. How Important Are Resistance, Dispersal Ability, Population Density and Mortality in Temporally Dynamic Simulations of Population Connectivity? A Case Study of Tigers in Southeast Asia. Land 2020, 9, 415. https://doi.org/10.3390/land9110415
Ash E, Cushman SA, Macdonald DW, Redford T, Kaszta Ż. How Important Are Resistance, Dispersal Ability, Population Density and Mortality in Temporally Dynamic Simulations of Population Connectivity? A Case Study of Tigers in Southeast Asia. Land. 2020; 9(11):415. https://doi.org/10.3390/land9110415
Chicago/Turabian StyleAsh, Eric, Samuel A. Cushman, David W. Macdonald, Tim Redford, and Żaneta Kaszta. 2020. "How Important Are Resistance, Dispersal Ability, Population Density and Mortality in Temporally Dynamic Simulations of Population Connectivity? A Case Study of Tigers in Southeast Asia" Land 9, no. 11: 415. https://doi.org/10.3390/land9110415