Performance Analysis and Soil Quality Indexing for Dalbergia sissoo Roxb. Grown in Marginal and Degraded Land of Eastern Uttar Pradesh, India
Abstract
1. Introduction
Dalbergia sissoo Roxb.: A Plant with Bioenergy Value and Multipurpose Environmental Benefits
2. Materials and Methods
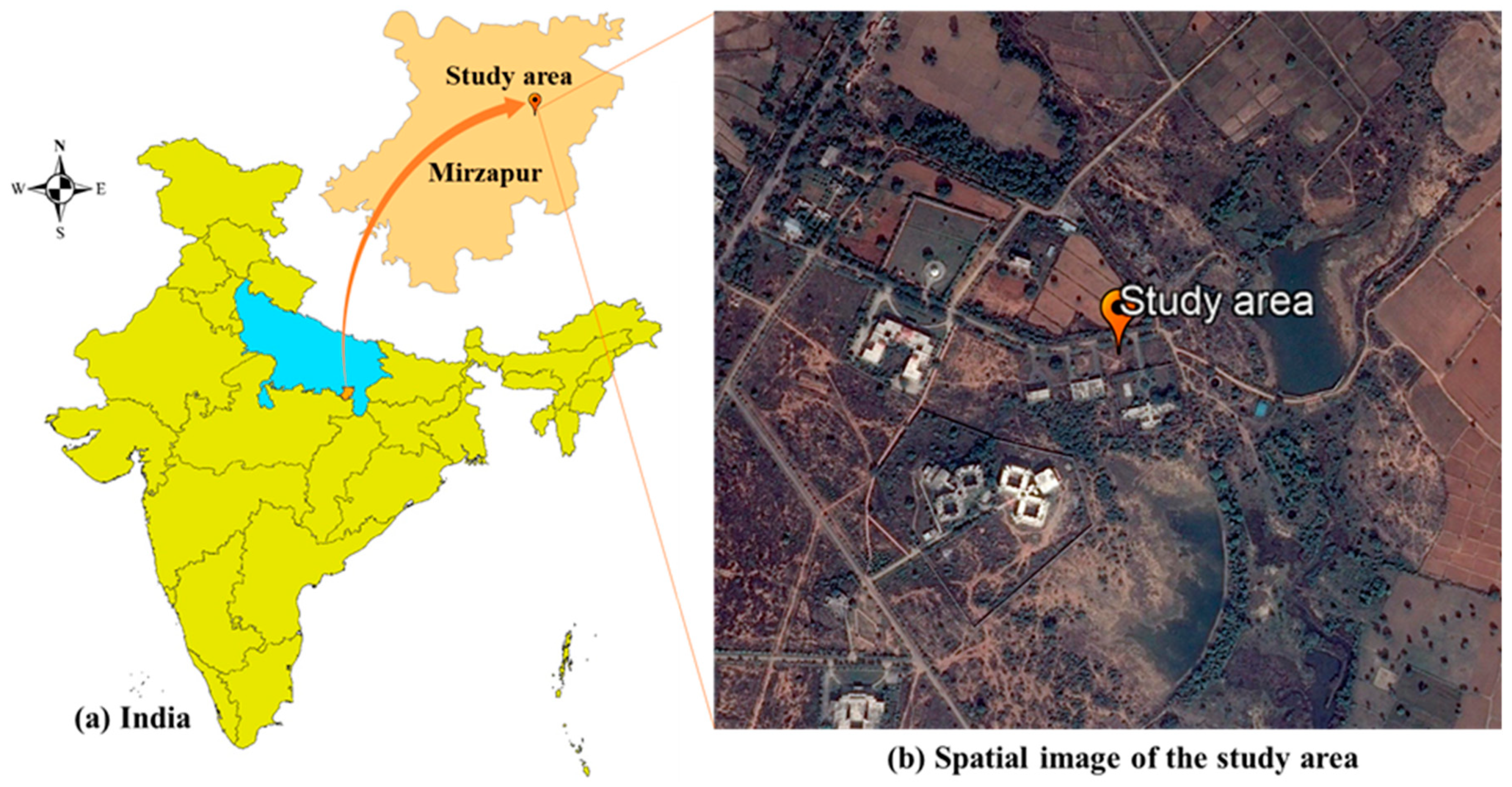
2.1. Study Area and Field Sampling
2.2. Soil Physico-Chemical Parameters
2.3. Soil Biological Parameters
2.4. Soil Quality Index (SQI)
2.5. Data Analyses
3. Results and Discussions
3.1. Soil Physico-Chemical Parameters
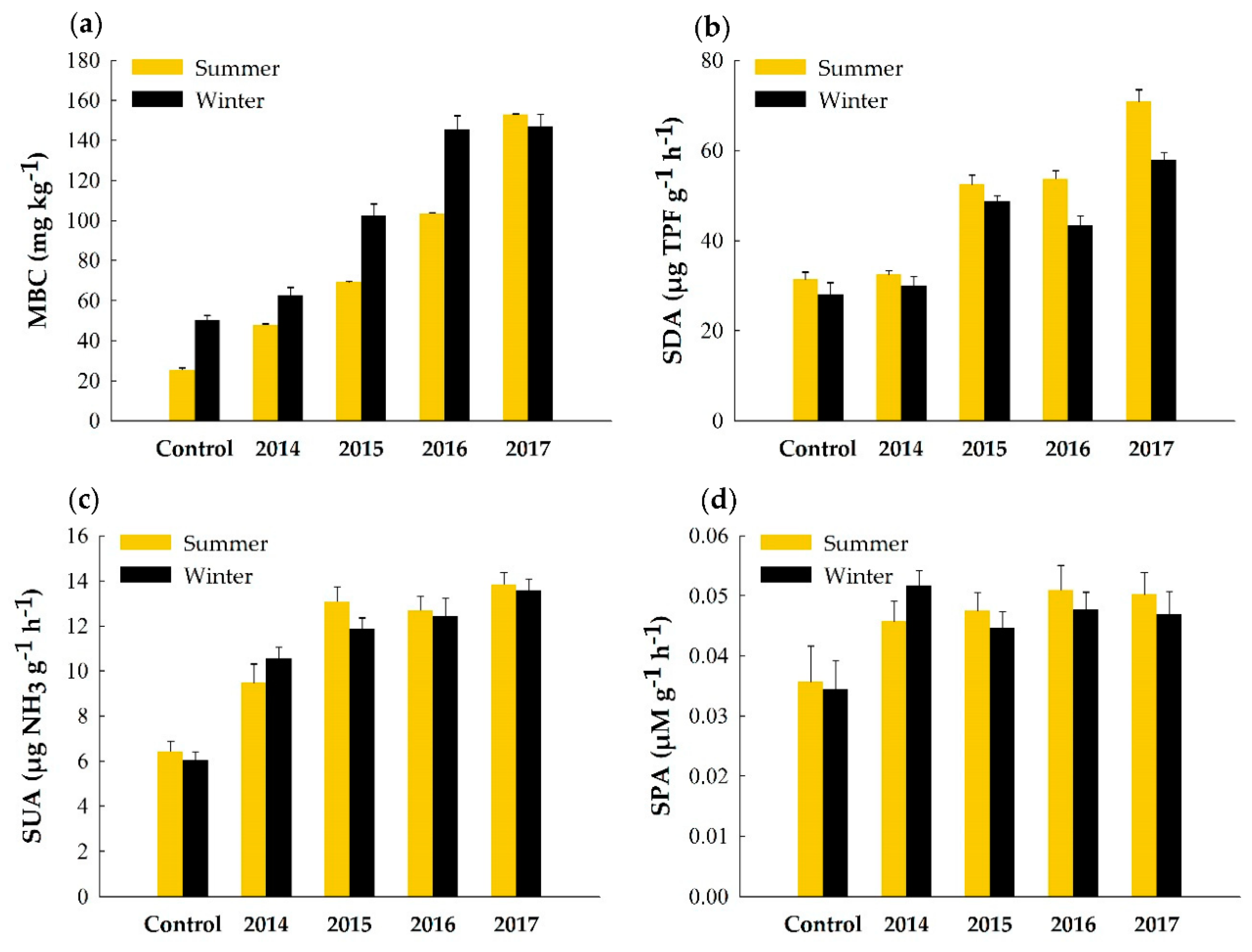
3.2. Soil Biological Parameters
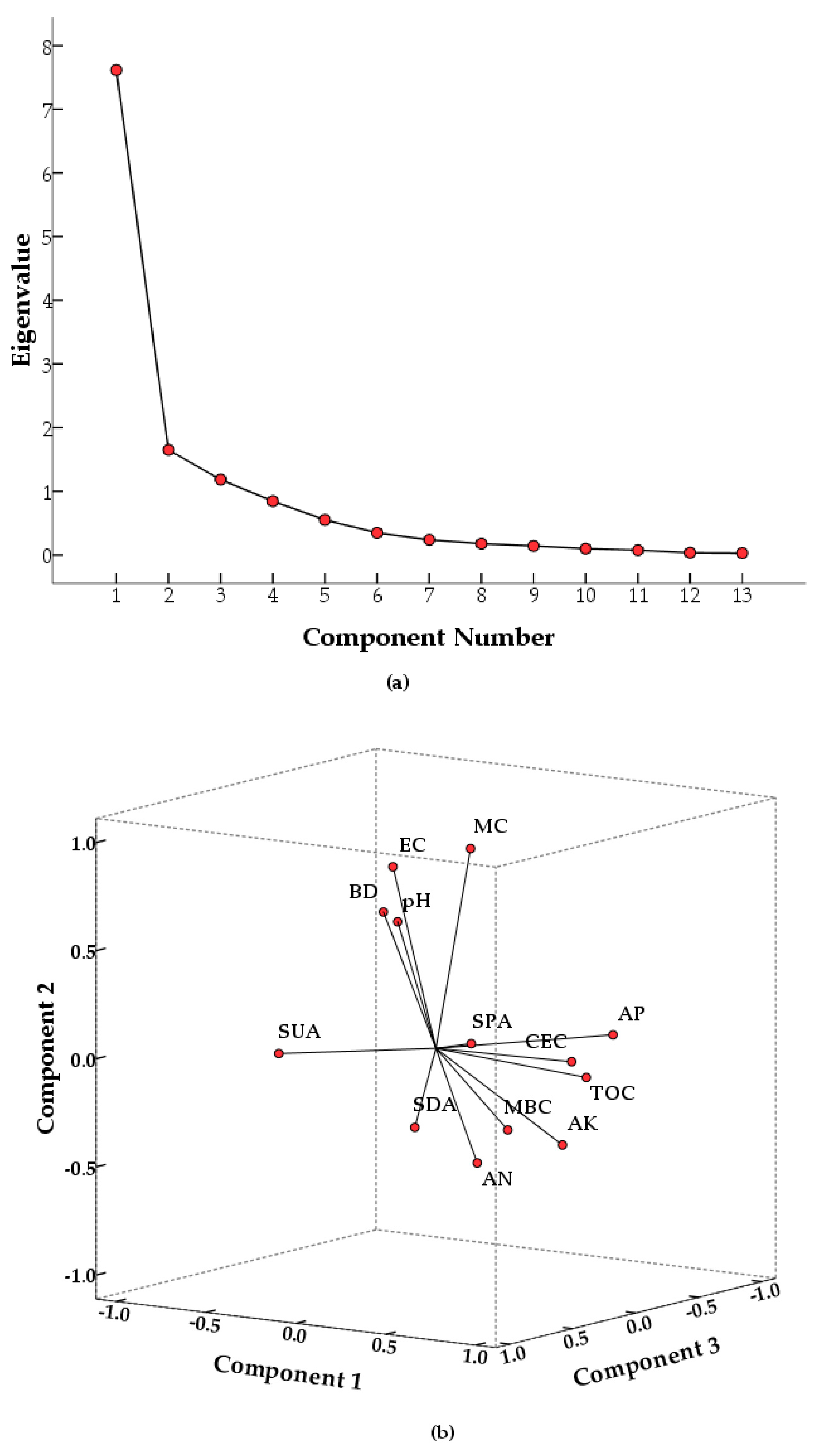
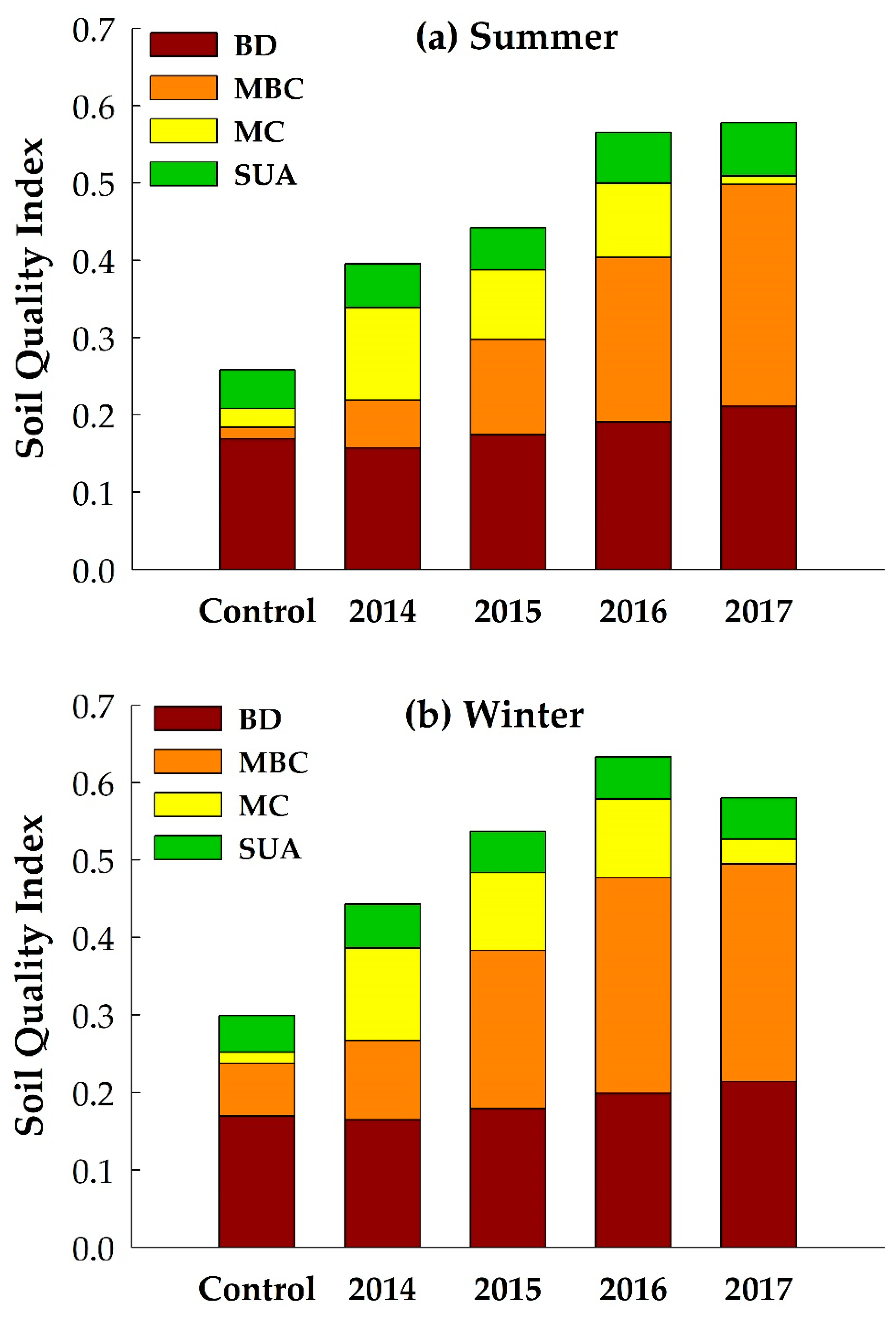
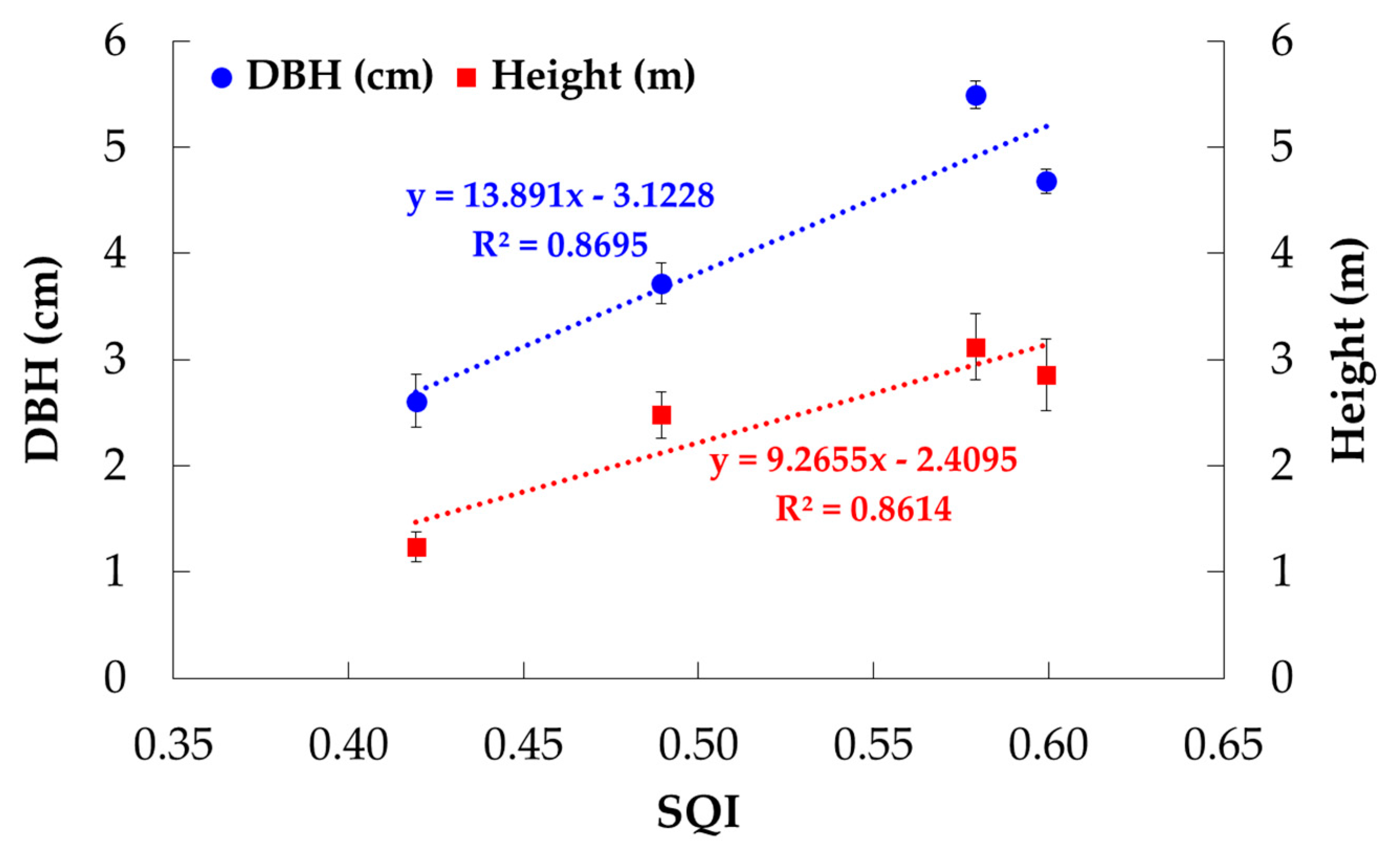
3.3. Developing PCA-Based SQI
4. Conclusions and Future Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bauen, A.; Berndes, G.; Junginger, M.; Londo, M.; Vuille, F. Bioenergy—A Sustainable and Reliable Energy Source, Main Report; IEA Bioenergy: Utrecht, The Netherlands, 2009; pp. 1–108. [Google Scholar]
- Edrisi, S.A.; Abhilash, P.C. Exploring marginal and degraded lands for biomass and bioenergy production: An Indian scenario. Renew. Sustain. Energy Rev. 2016, 54, 1537–1551. [Google Scholar] [CrossRef]
- Kauffman, N.S.; Hayes, D.J. The trade-off between bioenergy and emissions with land constraints. Energy Policy 2013, 54, 300–310. [Google Scholar] [CrossRef]
- Edrisi, S.A. Sustainable Biofuel Production from Marginal and Degraded Lands: An Ecological and Socio-Economic Approach. M.Phil. Thesis, Banaras Hindu University, Varanasi, India, 2013; p. 82. [Google Scholar]
- Tripathi, V.; Edrisi, S.A.; Abhilash, P.C. Towards the coupling of phytoremediation with bioenergy production. Renew. Sustain. Energy Rev. 2015, 57, 1386–1389. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Tripathi, V.; Edrisi, S.A.; Dubey, R.K.; Bakshi, M.; Dubey, P.K.; Singh, H.B.; Ebbs, S.D. Sustainability of crop production from polluted lands. Energy Ecol. Environ. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Powell, J.R.; Singh, H.B.; Singh, B.K. Plant-microbe interactions: Novel applications for exploitation in multipurpose remediation technologies. Trend Biotechnol. 2012, 30, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Keesstra, S.; Mol, G.; de Leeuw, J.; Okx, J.; Molenaar, C.; de Cleen, M.; Visser, S. Soil-Related Sustainable Development Goals: Four Concepts to Make Land Degradation Neutrality and Restoration Work. Land 2018, 7, 133. [Google Scholar] [CrossRef]
- Tripathi, V.; Edrisi, S.A.; Chen, B.; Gupta, V.K.; Vilu, R.; Gathergood, N.; Abhilash, P.C. Biotechnological Advances for Restoring Degraded Land for Sustainable Development. Trend Biotechnol. 2017, 35, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.; Fraceto, L.F.; Abhilash, P.C. Sustainable clean-up technologies for soils contaminated with multiple pollutants: Plant-microbe-pollutant and climate nexus. Ecol. Eng. 2015, 82, 330–335. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Dubey, R.K.; Tripathi, V.; Srivastava, P.; Verma, J.P.; Singh, H.B. Remediation and management of POPs-contaminated soils in a warming climate: Challenges and perspectives. Environ. Sci. Pollut. Res. Int. 2013, 20, 5879–5885. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.; Edrisi, S.A.; O’Donovan, A.; Gupta, V.K.; Abhilash, P.C. Bioremediation for Fueling the Biobased Economy. Trend Biotechnol. 2016, 34, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Tianjiao, F.; Wei, W.; Liding, C.; Keesstra, S.D.; Yang, Y. Effects of land preparation and plantings of vegetation on soil moisture in a hilly loess catchment in China. Land Degrad. Dev. 2018, 29, 1427–1441. [Google Scholar] [CrossRef]
- Novara, A.; Pulido, M.; Rodrigo-Comino, J.; Di Prima, S.; Smith, P.; Gristina, L.; Gimenez-Morera, A.; Terol, E.; Salesa, D.; Keesstra, S. Long-term organic farming on a citrus plantation results in soil organic carbon recovery. Cuad. Investig. Geográfica 2019. [Google Scholar] [CrossRef]
- Villacís, J.; Casanoves, F.; Hang, S.; Keesstra, S.; Armas, C. Selection of forest species for the rehabilitation of disturbed soils in oil fields in the Ecuadorian Amazon. Sci. Total Environ. 2016, 566, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Hein, U.; Kowarik, I. What are indicators? On the definition of indicators in ecology and environmental planning. Ecol. Indic. 2010, 10, 584–593. [Google Scholar] [CrossRef]
- McBride, A.C.; Dale, V.H.; Baskaran, L.M.; Downing, M.E.; Eaton, L.M.; Efroymson, R.A.; Garten, C.T., Jr.; Kline, K.L.; Jager, H.I.; Mulholland, P.J.; et al. Indicators to support environmental sustainability of bioenergy systems. Ecol. Indic. 2011, 11, 1277–1289. [Google Scholar] [CrossRef]
- De Graff, M.A.; van Groenigen, K.J.; Six, J.; Hungate, B.; van Kessel, C. Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta-analysis. Glob. Chang. Biol. 2006, 12, 2077–2091. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Dubey, R.K. Integrating aboveground-belowground responses to climate change. Curr. Sci. 2014, 106, 1637–1638. [Google Scholar]
- Zambon, I.; Colantoni, A.; Carlucci, M.; Morrow, N.; Sateriano, A.; Salvati, L. Land quality, sustainable development and environmental degradation in agricultural districts: A computational approach based on entropy indexes. Environ. Impact Assess. Rev. 2017, 64, 37–46. [Google Scholar] [CrossRef]
- Madejón, P.; Domínguez, M.T.; Díaz, M.J.; Madejón, E. Improving sustainability in the remediation of contaminated soils by the use of compost and energy valorization by Paulownia fortunei. Sci. Total Environ. 2016, 539, 401–409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hebb, C.; Schoderbek, D.; Hernandez-Ramirez, G.; Hewins, D.; Carlyle, C.N.; Bork, E. Soil physical quality varies among contrasting land uses in Northern Prairie regions. Agric. Ecosyst. Environ. 2017, 240, 14–23. [Google Scholar] [CrossRef]
- Li, P.; Shi, K.; Wang, Y.; Kong, D.; Liu, T.; Jiao, J.; Liu, M.; Li, H.; Hu, F. Soil quality assessment of wheat-maize cropping system with different productivities in China: Establishing a minimum data set. Soil Till. Res. 2019, 190, 31–40. [Google Scholar] [CrossRef]
- Juhos, K.; Czigány, S.; Madarász, B.; Ladányi, M. Interpretation of soil quality indicators for land suitability assessment–A multivariate approach for Central European arable soils. Ecol. Ind. 2019, 99, 261–272. [Google Scholar] [CrossRef]
- Jøker, D. Dalbergia sissoo. Seed Leafl. 2002, 65, 2–3. [Google Scholar]
- Goel, V.L.; Singh, B. Growth and productivity potential of Dalbergia sissoo in short rotation coppice system on sodic soil. Ind. J. For. 2008, 31, 491–499. [Google Scholar]
- Tewari, D.N. A Monograph on Dalbergia sissoo Roxb; International Book Distributors: Dehra Dun, India, 1994. [Google Scholar]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestree Database: A tree reference and selection guide version 4.0. 2009. Available online: http://www.worldagroforestry.org (accessed on 7 December 2018).
- Kumar, J.N.; Patel, K.; Kumar, R.N.; Bhoi, R.K. An evaluation of fuelwood properties of some Aravally mountain tree and shrub species of Western India. Biomass Bioenerg. 2011, 35, 411–414. [Google Scholar] [CrossRef]
- Singh, K.; Gautam, N.N.; Singh, B.; Goel, V.L.; Patra, D.D. Screening of environmentally less-hazardous fuelwood species. Ecol. Eng. 2014, 64, 424–429. [Google Scholar] [CrossRef]
- Niranjan, R.; Mohan, V.; Rao, V.M. Effect of Indole Acetic Acid on the synergistic interactions of Bradyrhizobium and Glomus fasciculatum on growth, nodulation, and nitrogen fixation of Dalbergia sissoo Roxb. Arid Land Res. Manag. 2007, 21, 329–342. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, S.D.; Khan, G.H. Rehabilitation of degraded sodic lands during a decade of Dalbergia sissoo plantation in Sultanpur district of Uttar Pradesh, India. Land Degrad. Dev. 2002, 13, 375–386. [Google Scholar] [CrossRef]
- Kanime, N.; Kaushal, R.; Tewari, S.K.; Raverkar, K.P.; Chaturvedi, O.P. Biomass production and carbon sequestration in different tree-based systems of Central Himalayan Tarai region. For. Trees Livelihoods 2013, 22, 38–50. [Google Scholar] [CrossRef]
- Guleria, V.; Vashisht, A.; Gupta, A.; Salven, T.; Thakur, C.; Kumar, P. Carbon stocks and soil properties under nitrogen-fixing trees on degraded site in subtropical Himalayan region. Ind. J. Soil Conserv. 2014, 42, 293–297. [Google Scholar]
- Rawat, J.S.; Banerjee, S.P. The influence of salinity on growth, biomass production and photosynthesis of Eucalyptus camaldulensis Dehnh. and Dalbergia sissoo Roxb. seedlings. Plant. Soil 1998, 205, 163–169. [Google Scholar] [CrossRef]
- Roy, N.; Laskar, R.A.; Ismail, S.K.; Kumari, D.; Ghosh, T.; Begum, N.A. A detailed study on the antioxidant activity of the stem bark of Dalbergia sissoo Roxb., an Indian medicinal plant. Food Chem. 2011, 126, 1115–1121. [Google Scholar] [CrossRef]
- Ecocrop, FAO. Food and Agriculture Organization of the United Nations, “The Crop Environmental Requirements Database”. 2007. Available online: http://ecocrop.fao.org/ (accessed on 7 December 2018).
- Sadiq, M.B.; Hanpithakpong, W.; Tarning, J.; Anal, A.K. Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Ind. Crop. Prod. 2015, 77, 873–882. [Google Scholar] [CrossRef]
- Singh, A.N.; Raghubanshi, A.S.; Singh, J.S. Comparative performance and restoration potential of two Albizia species planted on mine spoil in a dry tropical region, India. Ecol. Eng. 2004, 22, 123–140. [Google Scholar] [CrossRef]
- Kokila, K.; Priyadharshini, S.D.; Sujatha, V. Phytopharmacological properties of Albizia species: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 70–73. [Google Scholar]
- Garg, R.K.; Ranawat, M.P.S.; Vyas, L.N. Studies on the production relations of deciduous forests of semi-arid zone of Rajasthan (India). Forst Wissen Schaft Liches Central Blatt 1972, 91, 357–364. [Google Scholar] [CrossRef]
- Chokchaisiri, R.; Suaisom, C.; Sriphota, S.; Chindaduang, A.; Chuprajob, T.; Suksamrarn, A. Bioactive flavonoids of the flowers of Butea monosperma. Chem. Pharm. Bull. 2009, 57, 428–432. [Google Scholar] [CrossRef]
- Tona, L.; Kambu, K.; Ngimbi, N.; Cimanga, K.; Vlietinck, A.J. Antiamoebic and phytochemical screening of some Congolese medicinal plants. J. Ethnopharmacol. 1998, 61, 57–65. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Zenone, T.; Chen, J. (Eds.) Sustainable Biofuels: An. Ecological Assessment of Future Energy; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2015; p. 365. [Google Scholar]
- Zayed, M.Z.; Sallam, S.M.A.; Shetta, N.D. Review article on Leucaena leucocephala as one of the miracle timber trees. Int. J. Pharm. Pharm. Sci 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Suzaki, Y.; Tanaka, M.; Arihara, S.; Nigam, S.K. Three acylated saponins and a related compound from Pithecellobium dulce. J. Nat. Prod. 1997, 60, 1269–1274. [Google Scholar] [CrossRef]
- Nagmoti, D.M.; Khatri, D.K.; Juvekar, P.R.; Juvekar, A.R. Antioxidant activity free radical-scavenging potential of Pithecellobium dulce Benth seed extracts. Free Rad. Antioxid. 2012, 2, 37–43. [Google Scholar] [CrossRef]
- Bhojvaid, P.P.; Timmer, V.R. Soil dynamics in an age sequence of Prosopis juliflora planted for sodic soil restoration in India. For. Ecol. Manag. 1998, 106, 181–193. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, K.A.; Ahmad, V.U.; Oazi, S. Antibacterial activity of juliflorine isolated from Prosopis juliflora. Planta Medica (Mex.) 1986, 1, 339. [Google Scholar]
- Prabha, D.S.; Dahms, H.U.; Malliga, P. Pharmacological potentials of phenolic compounds from Prosopis spp.-a. J. Coast. Life Med. 2014, 2, 918–924. [Google Scholar]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis. Part 2, 2nd ed.; Page, A.L., Ed.; ASA and SSSA: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Blake, G.R.; Harte, K.H. Bulk density. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant., and Water Analysis: A manual for the West. Asia and North. Africa region; ICARDA: Beirut, Lebanon, 2013. [Google Scholar]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils: Effect of variations in digestion conditions and of organic soil constituents. Soil Sci. 1947, 63, 251–263. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A rapid procedure for the determination of available nitrogen in soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954.
- Toth, S.J.; Prince, A.L. Estimation of cation-exchange capacity and exchangeable Ca, K, and Na contents of soils by flame photometer techniques. Soil Sci. 1949, 67, 439–446. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Wang, B.; Xue, S.; Liu, G.B.; Zhang, G.H.; Li, G.; Ren, Z.P. Changes in soil nutrient and enzyme activities under different vegetations in the Loess Plateau area, Northwest China. Catena 2012, 92, 186–195. [Google Scholar] [CrossRef]
- Casida, Jr. L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Johnsen, A.R.; Jacobsen, O.S. A quick and sensitive method for the quantification of peroxidase activity of organic surface soil from forests. Soil Biol. Biochem. 2008, 40, 814–821. [Google Scholar] [CrossRef]
- Wander, M.M.; Bollero, G.A. Soil quality assessment of tillage impacts in Illinois. Soil Sci. Soc. Am. J. 1999, 63, 961–971. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Mitchell, J.P. A comparison of soil quality indexing methods for vegetable production systems in Northern California. Agric. Ecosyst. Environ. 2002, 90, 25–45. [Google Scholar] [CrossRef]
- Sharma, K.L.; Mandal, U.K.; Srinivas, K.; Vittal, K.P.R.; Mandal, B.; Grace, J.K.; Ramesh, V. Long-term soil management effects on crop yields and soil quality in a dryland Alfisol. Soil Till. Res. 2005, 83, 246–259. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Alternative soil quality indices for evaluating the effect of intensive cropping, fertilization and manuring for 31 years in the semi-arid soils of India. Environ. Monit. Assess. 2008, 136, 419–435. [Google Scholar] [CrossRef]
- Imaz, M.J.; Virto, I.; Bescansa, P.; Enrique, A.; Fernandez-Ugalde, O.; Karlen, D.L. Soil quality indicator response to tillage and residue management on semi-arid Mediterranean cropland. Soil Till. Res. 2010, 107, 17–25. [Google Scholar] [CrossRef]
- Mandal, U.K.; Warrington, D.N.; Bhardwaj, A.K.; Bar-Tal, A.; Kautsky, L.; Minz, D.; Levy, G.J. Evaluating impact of irrigation water quality on acalcareous clay soil using principal component analysis. Geoderma 2008, 144, 189–197. [Google Scholar] [CrossRef]
- Campos, C.A.; Oleschko, L.K.; Etchevers, B.J.; Hidalgo, M.C. Exploring the effect of changes in land use on soil quality on the eastern slope of the Cofre de Perote Volcano (Mexico). For. Ecol. Manag. 2007, 248, 174–182. [Google Scholar] [CrossRef]
- Bastida, F.; Moreno, J.L.; Hernández, T.; García, C. Microbiological degradation index of soils in a semiarid climate. Soil Biol. Biochem. 2006, 38, 3463–3473. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Purakayastha, T.J.; Patra, A.K.; Singh, D. Soil quality indices for evaluation of long-term land use and soil management practices in semi-arid sub-tropical India. Land Degrad. Dev. 2008, 19, 516–529. [Google Scholar] [CrossRef]
- Sinha, S.; Masto, R.E.; Ram, L.C.; Selvi, V.A.; Srivastava, N.K.; Tripathi, R.C.; George, J. Rhizosphere soil microbial index of tree species in a coal mining ecosystem. Soil Biol. Biochem. 2009, 41, 1824–1832. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maiti, S.K.; Masto, R.E. Use of Reclaimed Mine Soil Index (RMSI) for screening of tree species for reclamation of coal mine degraded land. Ecol. Eng. 2013, 57, 133–142. [Google Scholar] [CrossRef]
- Kaiser, H.F. The application of electronic computers for factor analysis. Educ. Psychol. Meas. 1960, 29, 141–151. [Google Scholar] [CrossRef]
- Barnshiel, R.L.; Hower, J.M. Coal surface mine reclamation in the eastern United States: The revegetation of disturbed lands to hayland/pasture or cropland. Adv. Agron. 1997, 61, 233–275. [Google Scholar]
- Andrews, S.S.; Carroll, C.R. Designing a decision tool for sustainable agroecosystem management: Soil quality assessment of a poultry litter management case study. Ecol. Appl. 2001, 11, 1573–1585. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.B.; Xue, S.; Song, Z.L. Rhizosphere soil microbial activity under different vegetation types on the Loess Plateau, China. Geoderma 2011, 161, 115–125. [Google Scholar] [CrossRef]
- Liu, D.; Fang, S.; Tian, Y.; Dun, X. Variation in rhizosphere soil microbial index of tree species on seasonal flooding land: An in situ rhizobox approach. Appl. Soil Ecol. 2012, 59, 1–11. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maiti, S.K.; Masto, R.E. Development of mine soil quality index (MSQI) for evaluation of reclamation success: A chronosequence study. Ecol. Eng. 2014, 71, 10–20. [Google Scholar] [CrossRef]
- Li, P.; Zhang, T.; Wang, X.; Yu, D. Development of biological soil quality indicator system for subtropical China. Soil Till. Res. 2013, 31, 112–118. [Google Scholar] [CrossRef]
- Das, B.; Chakraborty, D.; Singh, V.K.; Ahmed, M.; Singh, A.K.; Barman, A. evaluating fertilization effects on soil physical properties using a soil quality index in an intensive Rice-Wheat cropping system. Pedosphere 2016, 26, 887–894. [Google Scholar] [CrossRef]
- Xu, C.; Xu, X.; Liu, M.; Yang, J.; Zhang, Y.; Li, Z. Developing pedotransfer functions to estimate the S-index for indicating soil quality. Ecol. Indic. 2017, 83, 338–345. [Google Scholar] [CrossRef]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Biswas, S.; Hazra, G.C.; Purakayastha, T.J.; Saha, N.; Mitran, T.; Roy, S.S.; Nirmalendu, B.; Biswapati, M. Establishment of critical limits of indicators and indices of soil quality in rice-rice cropping systems under different soil orders. Geoderma 2017, 292, 34–48. [Google Scholar] [CrossRef]
- Rakshit, A.; Sarkar, B.; Abhilash, P.C. (Eds.) Soil Amendments for Sustainability: Challenges and Perspectives; CRC Press: Boca Raton, FL, USA, 2018; p. 404. [Google Scholar]

| S. No. | Tree Species | Adaptive Traits | Invasive Nature/Negative Traits | Usability of Wood/Timber | Major Phytochemicals/Pharmaceutical Products | Medicinal Values | Additional Benefits | References |
|---|---|---|---|---|---|---|---|---|
| 1. | Dalbergia sissoo | Tolerant under saline, sodic, acidic, arid soils, heavy metal and POPs contaminated soils, control soil erosion, also suitable for the restoration of mine spoils | Little risk in Australia and Florida but easy to manage | Furniture, used in the interiors and floorings, high calorific value, excellent fuelwood, sporting goods, tobacco pipes, etc. | Tannins, terpenes, saponins, alkaloids, chalcone, isoflavone, flavone, biochanin A, rotenoid, dehydroamorphigenin, etc. | Cure of reactive oxygen species (ROS) induced diseases, emesis, ulcers, leukoderma, dysentery, stomach troubles and skin diseases | Fodder and fibre production, livestock management, regaining ecosystem services, alkaloids from woods are good source of insecticides | [26,35,36] |
| 2. | Acacia nilotica | Luxurious growth in sodic, saline and arid soils | Water intensive, reduce the quality of pasture and compete with native plants | Fuelwood, charcoal, timber wood | Saponins, tannins and phenols, alkaloids, glycosides, anthraquinones, flavonoids, proteins, phenols, anthocyanins, magniferin, myricetin, taxifolin, vitexin, etc. | Barks used in cough, nerve stimulation, diarrhoea, dysentery and leprosy, bruised leaves can treat ulcers | Leaves and fruits has fodder value, shade and shelter tree, | [37,38] |
| 3. | Albizia lebbeck | Can grow well in riverine belts, saline or sodic lands | Potential invader of natural and semi-natural areas | Used in interior moulding, parquet, furniture, panelling, turnery and general construction | Julibroside, budmunchiamines, quercitrin and isoquercitrin, etc. | Leaves and seeds are used for eye diseases, and bark to treat boils. Saponin from pods and roots has spermicidal activity, antitumor, antiplatelets aggregation and bactericidal activities | Fodder for camels, buffalo and cattle, nitrogen-rich leaves are used as mulch and green manure, has ornamental importance, bark is used locally in India for tanning fishing nets. | [39,40] |
| 4. | Butea monosperma | Adapted to sodic, saline, marginal and other degraded lands | - | used for utensils, low fuel wood value, charcoal, | dihydrochalcone, dihydromonospermoside, chalcones, butein, monospermoside and isoliquiritigenin, etc. | Flowers are used in liver disorders, seeds are anthelminthic, astringent gum from stem has application in diarrhoea, ethanolic extract of leaves can enhance blood insulin level | Seeds exhibit bactericidal and fungicidal activities, young leaves are good fodder, eaten mainly by buffaloes, coarse fibres from the inner bark used for cordage, caulking the seams of boats and making paper, red exudate is obtained from the bark used as dye and tannins | [41,42] |
| 5. | Cassia siamea | Adapted to diverse soil conditions including aluminium mine tailings. | - | Fuelwood, charcoal, high timber value, often used for poles, posts, bridges, mine poles and beams | Flavonoids, tannins, terpenes, saponins, alkaloids, anthraquinones, etc. | Used against intestinal worms, heartwood as laxative and scabies can be cured from its decoction | Ornamental importance due to yellow flowers, leaves used as green manure, used in mulching, acts as a host plant for Santalum spp. | [43,44] |
| 6. | Leucaena leucocephala | Potential to grow under saline, sodic or acidic soils, slower down surface run-off | High risk of invasiveness in the disturbed sites even in the agricultural land where it has been planted as a shade tree. | Excellent firewood and charcoal, also used as sawn timber, mine props, furniture and parquet flooring, | Diterpene, triterpene, palmitic acid, fatty acid ester, terpene alcohol, linolenic acid ester, dicarboxylic acid, etc. | Antimicrobial, antibacterial, antioxidant, anti-inflammatory, diuretic, antihistaminic, nematicide, pesticide, anti-androgenic, hypocholesterolemic and hepatoprotective | Red, brown and black dyes from pods, leaves and bark respectively, paper, pulp, rayon and particleboard production, used in green manuring, mulching, weed control, etc. | [44,45] |
| 7. | Pithecellobium dulce | Potential to grow in nutrient poor soil, including saline and arid soils | - | Low calorific value thus not attractive as fuelwood, it is often used in drums, and matchsticks | Triterpenoids, glycosides, saponins and flavonoids, pitheduloside, oleanane glycosides, arabinose, d-glucose, l-rhamnose, and d-xylose, 1, 1-Diphenyl-2-picrylhydrazyl (DPPH), etc. | Used against diarrhoea, pulverised seeds are ingested for internal ulcers, root bark may be used to cure dysentery | Bark exudes gum and resins, popular as an ornamental, useful shelter belts, Seeds contain a greenish oil used in soaps, used as tannin, its flower supports apiculture, fodder for livestock | [44,46,47] |
| 8. | Prosopis juliflora | Adapted to many types of the soils including alkaline, saline, sodic soils, tolerant to drought and even seasonal waterlogged conditions, soils contaminated with specific metals, heavy metals or organic contaminants | Highly invasive in nature, invade various grassland ecosystems, savannas, pastures and even abandoned and agricultural land | Good firewood and excellent charcoal, seasoned wood is used for fence posts, furniture, crafts and corrals | alkaloids, flavonoids, terpenoids, saponins and phenolic compounds, juliflorine, juliprosinene, sceojuliprosopinol, juliprosine, L-manopyranoside, julifloravizole, mesquitol, catechin, quercetin, etc. | syrup prepared from ground pods provide weight gain and enhanced motor development, enhanced lactation, expectorants, helpful in digestive disturbances and skin lesions | Low quality tannin or dyestuff, phenol-formaldehyde polymeric resins, pods serves as for making alcoholic products | [48,49,50] |
| Geographical Characteristics * | Meteorological Characteristics | |||
|---|---|---|---|---|
| Total Geographic Area (km2) | Mean Elevation (m) | Climate Pattern * | Mean Annual Temp. (°C) ± SD # | Mean Annual Precipitation (mm) # |
| 4521 | 80 | Warm and tropical dry | 23.25 ± 5.25 | 975 |
| Summer Season | |||||||||
| Soil Samples | BD (g cm−3) | MC (%) | pH (1:4 w/v) | EC (ds m−1) | TOC (%) | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) | CEC (cmol kg−1) |
| Control (unplanted) | 1.48 ± 0.03 d | 2.33 ± 1.33 a | 7.46 ± 0.08 b | 0.23 ± 0.01 c | 0.36 ± 0.01 a | 66.40 ± 7.36 a | 4.56 ± 0.03 a | 21.87 ± 2.02 b | 12.92 ± 0.05 a |
| 2014 | 1.56 ± 0.01 e | 8.16 ± 1.70 b | 7.91 ± 0.06 c | 0.29 ± 0.009 e | 0.35 ± 0.35 a | 70.46 ± 3.57 a | 4.75 ± 0.17 a | 18.04 ± 2.67 a | 13.21 ± 0.10 b |
| 2015 | 1.45 ± 0.02 c | 5.82 ± 1.90 b | 7.62 ± 0.06 d | 0.27 ± 0.004 d | 0.38 ± 0.38 b | 89.00 ± 4.71 b | 5.11 ± 0.18 b | 28.14 ± 1.73 c | 14.64 ± 0.11 d |
| 2016 | 1.34 ± 0.01 b | 5.99 ± 1.37 b | 7.49 ± 0.05 b | 0.19 ± 0.002 b | 0.43 ± 0.43 c | 121.67 ± 6.73 c | 5.41 ± 0.33 b | 32.69 ± 1.01 d | 16.30 ± 0.20 e |
| 2017 | 1.23 ± 0.01 a | 1.61 ± 0.53 a | 7.24 ± 0.08 a | 0.18 ± 0.004 a | 0.49 ± 0.49 d | 194.11 ± 7.38 d | 5.36 ± 0.18 b | 35.40 ± 0.82 d | 14.40 ± 0.03 c |
| Winter Season | |||||||||
| Control (unplanted) | 1.47 ± 0.04 d | 1.88 ± 0.17 a | 7.31 ± 0.04 a,b | 0.19 ± 0.01 a | 0.41 ± 0.03 a | 67.74 ± 4.81 a | 5.19 ± 0.16 a | 25.84 ± 3.05 a | 13.61 ± 0.03 a |
| 2014 | 1.51 ± 0.01 d | 7.78 ± 1.00 c | 7.84 ± 0.18 c | 0.27 ± 0.02 b | 0.44 ± 0.44 a,b | 85.45 ± 7.36 b | 5.39 ± 0.37 a | 23.45 ± 0.48 a | 14.66 ± 0.05 b |
| 2015 | 1.41 ± 0.03 c | 6.13 ± 0.60 b | 7.43 ± 0.20 b | 0.26 ± 0.01 b | 0.46 ± 0.46 b | 104.36 ± 4.36 c | 6.32 ± 1.43 a | 31.75 ± 1.75 b | 14.77 ± 0.07 b |
| 2016 | 1.30 ± 0.01 b | 6.12 ± 0.28 b | 7.32 ± 0.12 a,b | 0.18 ± 0.01 a | 0.51 ± 0.57 c | 140.42 ± 4.97 d | 6.09 ± 1.07 a | 38.32 ± 1.80 c | 15.75 ± 0.03 c |
| 2017 | 1.21 ± 0.01 a | 2.79 ± 0.62 a | 7.18 ± 0.05 a | 0.17 ± 0.01 a | 0.57 ± 0.51 d | 212.46 ± 4.76 e | 6.14 ± 0.65 a | 41.16 ± 0.90 c | 16.37 ± 0.12 d |
| Principal Components | PC-1 | PC-2 | PC-3 |
|---|---|---|---|
| Eigen values a | 7.616 | 1.650 | 1.184 |
| Variation (%) | 58.584 | 12.690 | 9.111 |
| Cumulative variation (%) | 58.584 | 71.274 | 80.386 |
| Eigenvectors b | |||
| Bulk density (BD) | −0.960 * | 0.189 | −0.035 |
| Moisture content (MC) | −0.288 | 0.870 * | −0.011 |
| pH (1:4; w/v) | −0.812 | 0.217 | −0.039 |
| EC (1:4; w/v) | −0.696 | 0.567 | 0.097 |
| Total organic carbon (TOC) | 0.779 | 0.248 | −0.294 |
| Available N (AN) | 0.932 | −0.089 | 0.094 |
| Available P (AP) | 0.486 | 0.302 | −0.472 |
| Available K (AK) | 0.941 | −0.009 | −0.215 |
| Cation exchange capacity (CEC) | 0.781 | 0.333 | −0.216 |
| Microbial biomass carbon (MBC) | 0.951 * | 0.086 | 0.023 |
| Soil dehydrogenase activity (SDA) | 0.855 | 0.065 | 0.351 |
| Soil urease activity (SUA) | 0.399 | 0.225 | 0.782 * |
| Soil peroxidase activity (SPA) | 0.682 | 0.393 | 0.159 |
| BD | AN | AK | MBC | |
|---|---|---|---|---|
| BD | 1 | |||
| AN | −0.930 ** | 1 | ||
| AK | −0.903 ** | 0.843 ** | 1 | |
| MBC | −0.913 ** | 0.908 ** | 0.901 ** | 1 |
| Parameter | BD | MBC | MC | SUA |
|---|---|---|---|---|
| Curve type | Less is better | More is better | More is better | More is better |
| Mean (x0) | 1.39 | 90.58 | 4.86 | 44.92 |
| Slope (b) | 2.50 | −2.50 | −2.50 | −2.50 |
| R2 | 0.998 | 0.986 | 0.975 | 0.980 |
| F significance | 0.000 | 0.000 | 0.000 | 0.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edrisi, S.A.; Tripathi, V.; Abhilash, P.C. Performance Analysis and Soil Quality Indexing for Dalbergia sissoo Roxb. Grown in Marginal and Degraded Land of Eastern Uttar Pradesh, India. Land 2019, 8, 63. https://doi.org/10.3390/land8040063
Edrisi SA, Tripathi V, Abhilash PC. Performance Analysis and Soil Quality Indexing for Dalbergia sissoo Roxb. Grown in Marginal and Degraded Land of Eastern Uttar Pradesh, India. Land. 2019; 8(4):63. https://doi.org/10.3390/land8040063
Chicago/Turabian StyleEdrisi, Sheikh Adil, Vishal Tripathi, and Purushothaman Chirakkuzhyil Abhilash. 2019. "Performance Analysis and Soil Quality Indexing for Dalbergia sissoo Roxb. Grown in Marginal and Degraded Land of Eastern Uttar Pradesh, India" Land 8, no. 4: 63. https://doi.org/10.3390/land8040063
APA StyleEdrisi, S. A., Tripathi, V., & Abhilash, P. C. (2019). Performance Analysis and Soil Quality Indexing for Dalbergia sissoo Roxb. Grown in Marginal and Degraded Land of Eastern Uttar Pradesh, India. Land, 8(4), 63. https://doi.org/10.3390/land8040063







