Where to Protect? Spatial Ecology and Conservation Prioritization of the Persian Squirrel at the Westernmost Edge of Its Distribution
Abstract
1. Introduction
2. Materials and Methods
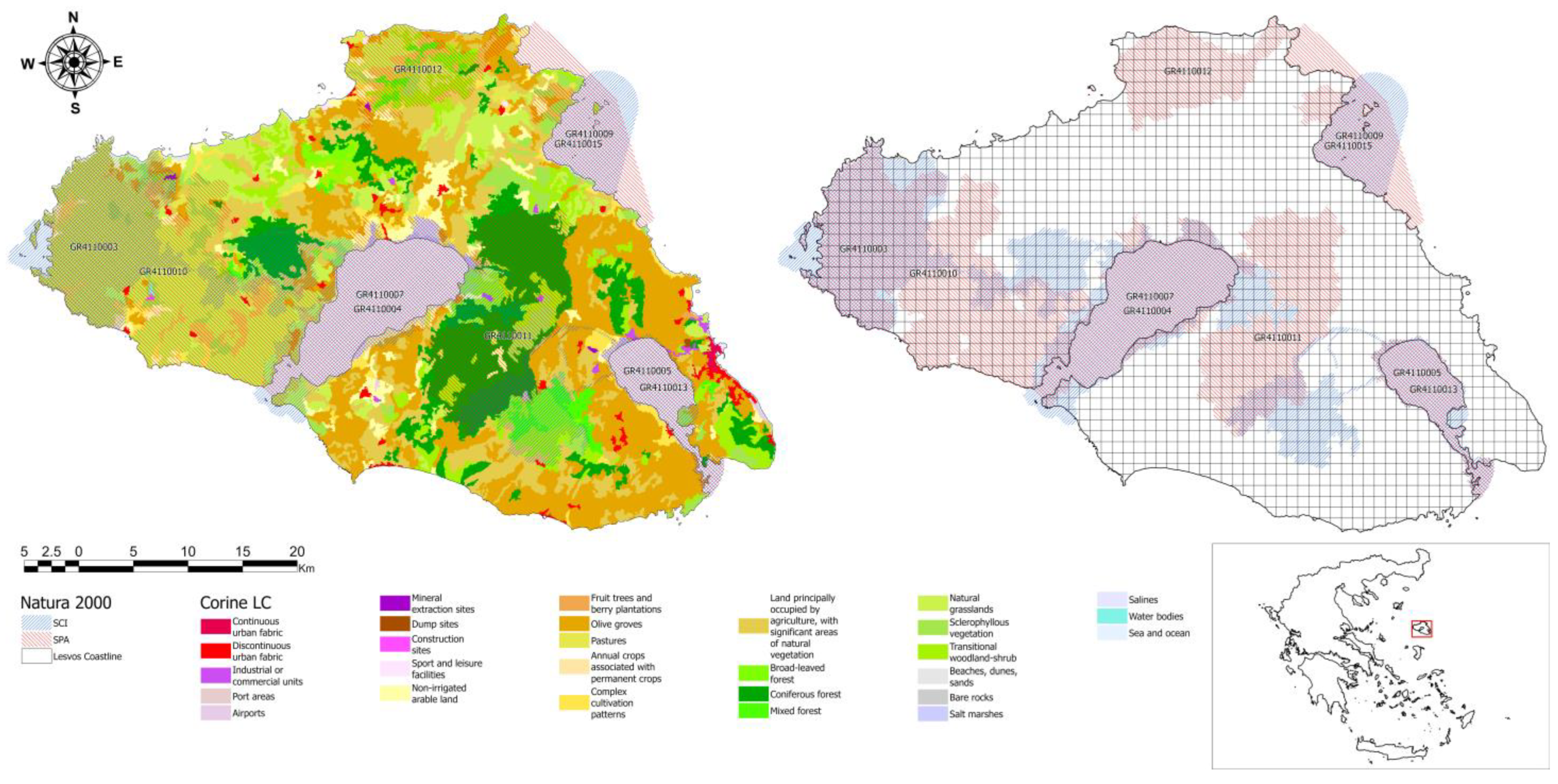
2.1. Study Area
2.2. Field Surveys
2.3. Ecological and Anthropogenic Drivers
2.4. Spatial Analysis
2.5. Statistical Analysis
3. Results
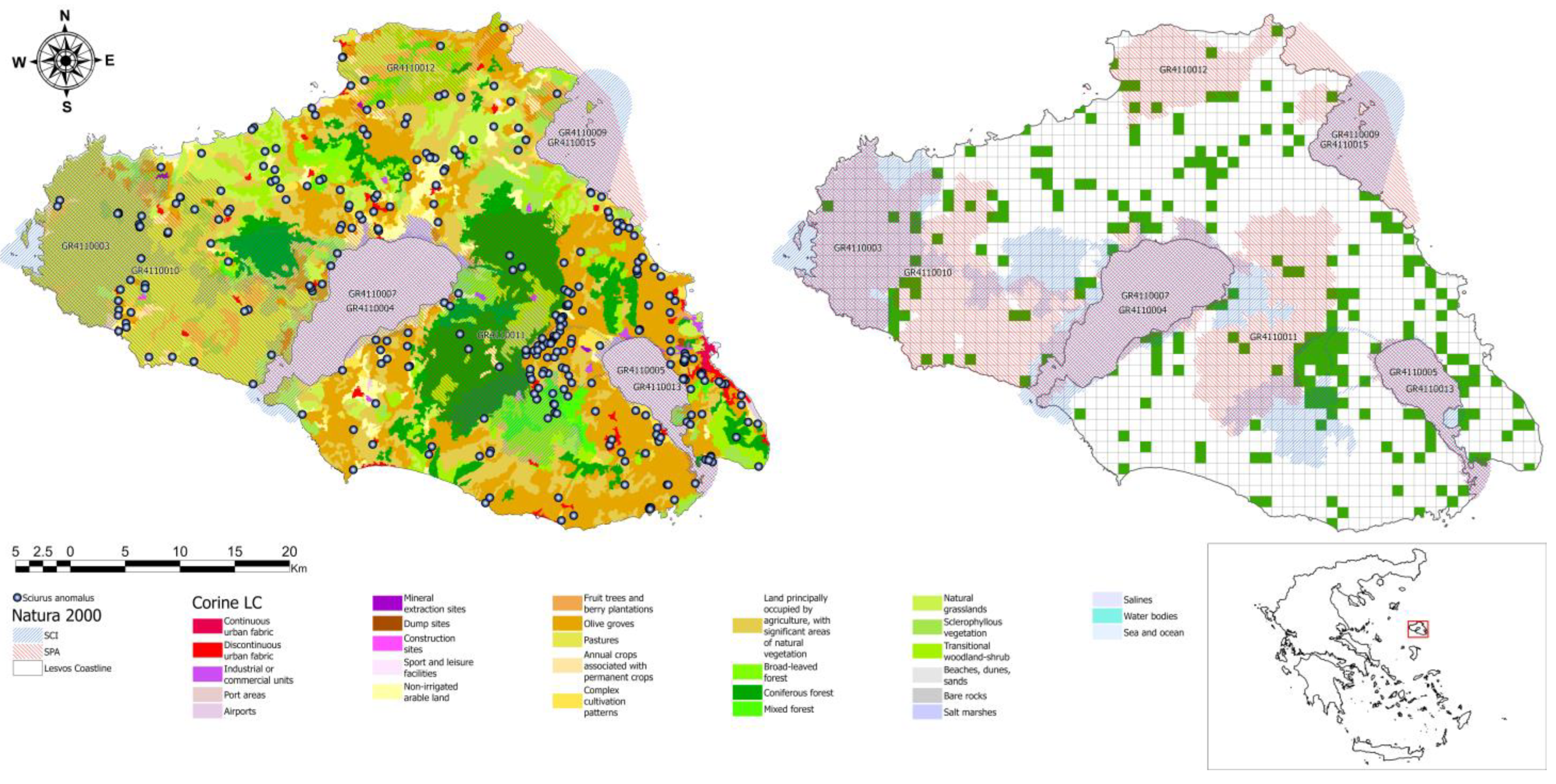
3.1. Spatial Distribution of the Persian Squirrel
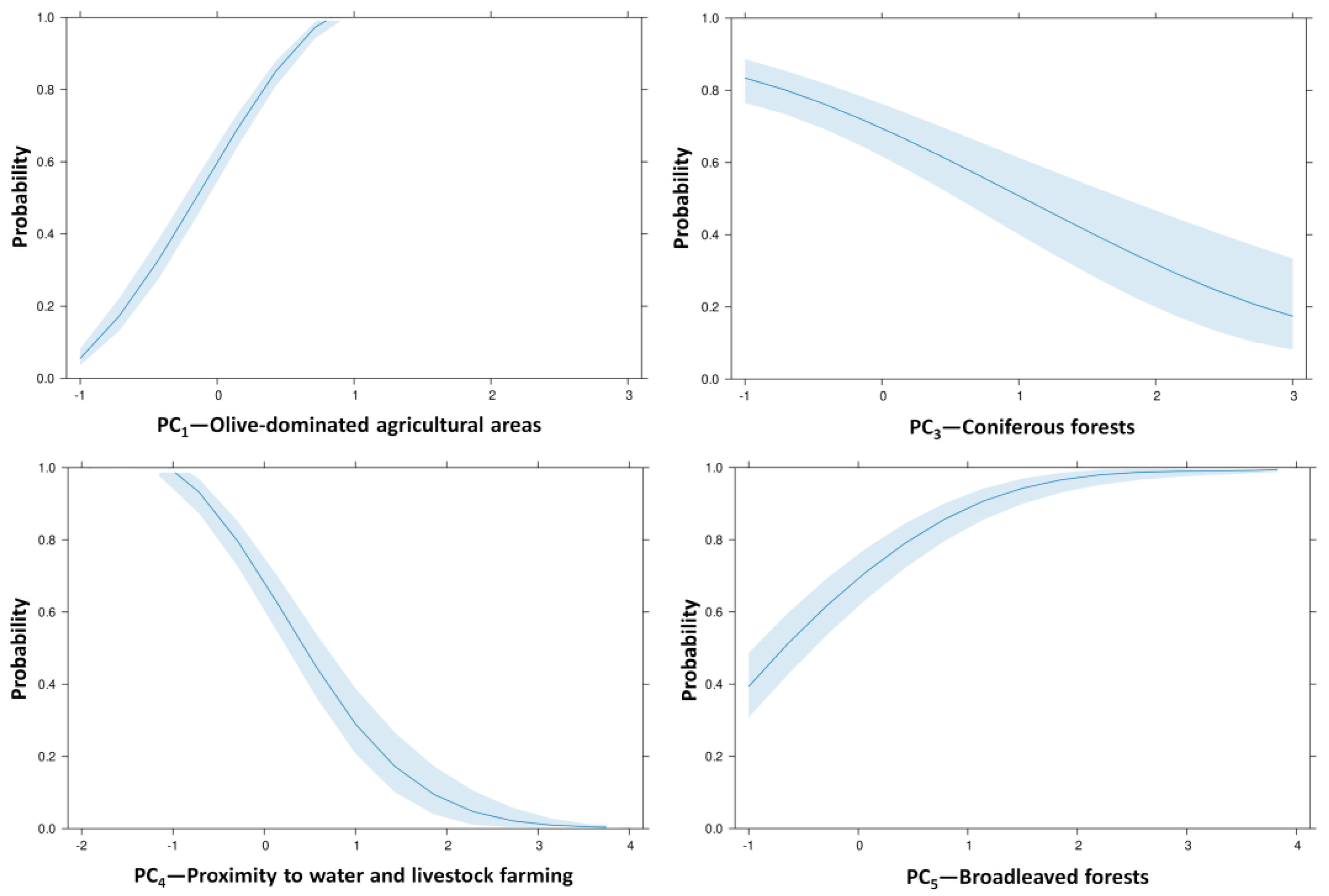
3.2. Principal Components of Ecological and Anthropogenic Drivers
3.3. Factors Influencing the Presence of the Persian Squirrel Across the Island
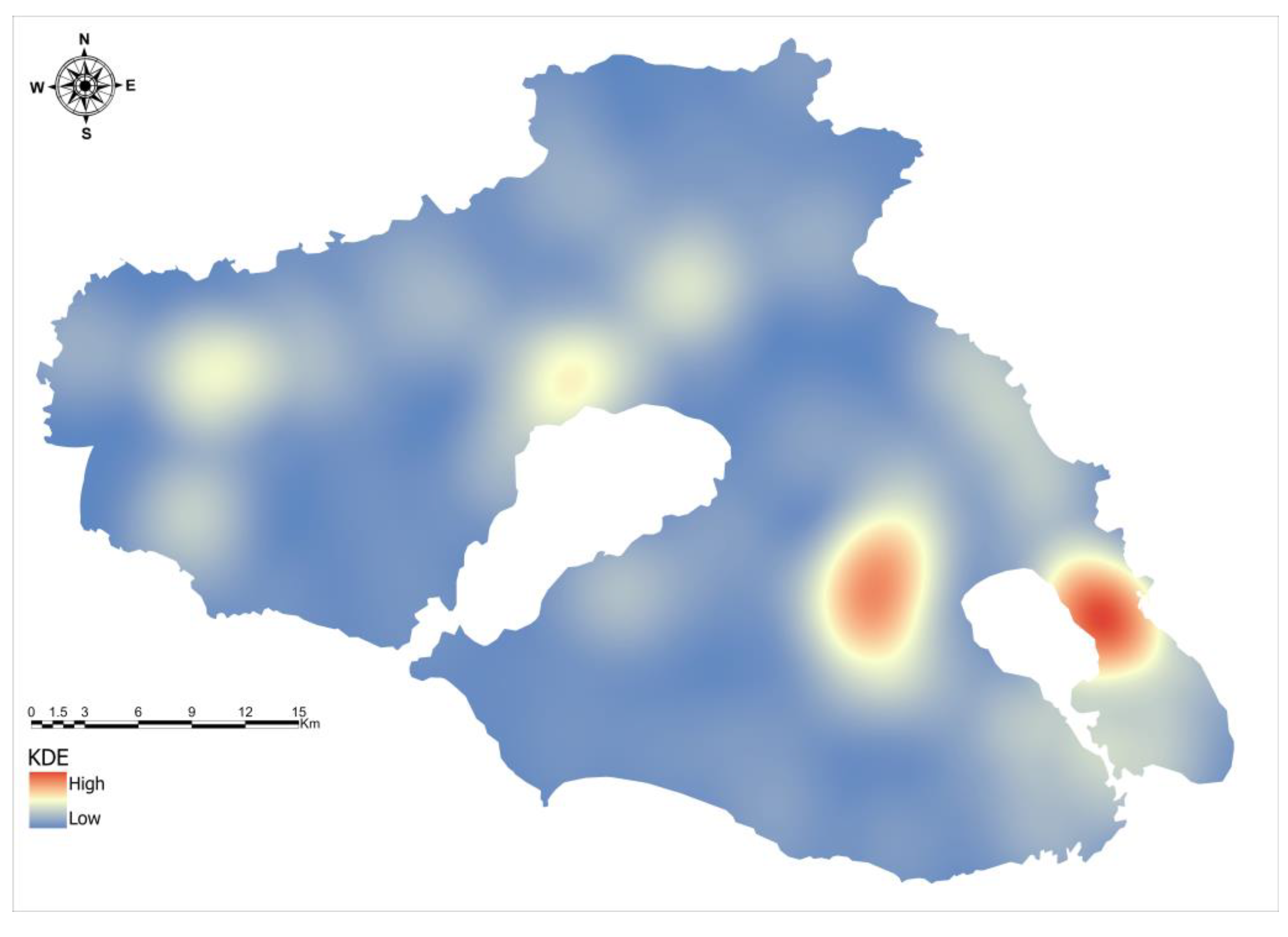
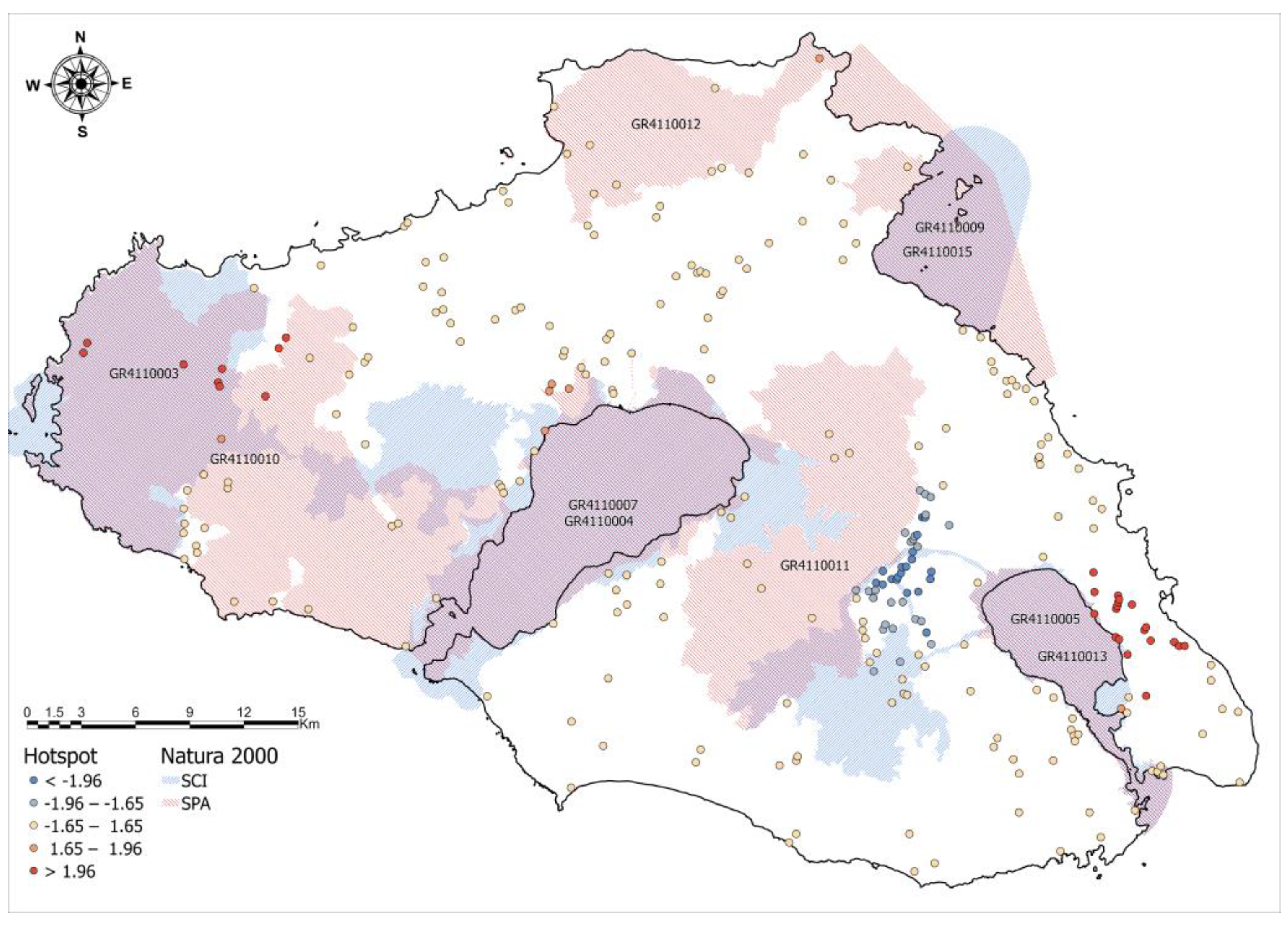
3.4. High-Density Areas, Hotspots, and Spatial Clusters of Sciurus Anomalus
3.5. Spatial Regression Analysis of Hotspot Intensity and Clustering Patterns
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittaker, R.J.; Fernández-Palacios, J.M.; Matthews, T.J.; Borregaard, M.K.; Triantis, K.A. Island biogeography: Taking the long view of nature’s laboratories. Science 2017, 357, eaam8326. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.C.; Kueffer, C. Island Biodiversity in the Anthropocene. Annu. Rev. Environ. Resour. 2019, 44, 31–60. [Google Scholar] [CrossRef]
- Zachos, F.E.; Habel, J.C. Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Springer Science & Business Media: Boston, NY, USA, 2011. [Google Scholar]
- Jolly, C.J.; Webb, J.K.; Phillips, B.L. The perils of paradise: An endangered species conserved on an island loses antipredator behaviours within 13 generations. Biol. Lett. 2018, 14, 20180222. [Google Scholar] [CrossRef] [PubMed]
- Ogawa-Onishi, Y.; Berry, P.M.; Tanaka, N. Assessing the potential impacts of climate change and their conservation implications in Japan: A case study of conifers. Biol. Conserv. 2010, 143, 1728–1736. [Google Scholar] [CrossRef]
- Cronk, Q.C.B. Islands: Stability, diversity, conservation. Biodivers. Conserv. 1997, 6, 477–493. [Google Scholar] [CrossRef]
- Adler, G.H.; Levins, R. The Island Syndrome in Rodent Populations. Q. Rev. Biol. 1994, 69, 473–490. [Google Scholar] [CrossRef]
- Kier, G.; Kreft, H.; Tien, M.L.; Jetz, W.; Ibisch, P.L.; Nowicki, C.; Mutke, J.; Barthlott, W. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. USA 2009, 106, 9322–9327. [Google Scholar] [CrossRef]
- Barreto, E.; Rangel, T.F.; Pellissier, L.; Graham, C.H. Area, isolation and climate explain the diversity of mammals on islands worldwide. Proc. R. Soc. B Biol. Sci. 2021, 288, 20211879. [Google Scholar] [CrossRef]
- Sato, J.J.; Tasaka, Y.; Tasaka, R.; Gunji, K.; Yamamoto, Y.; Takada, Y.; Uematsu, Y.; Sakai, E.; Tateishi, T.; Yamaguchi, Y. Effects of Isolation by Continental Islands in the Seto Inland Sea, Japan, on Genetic Diversity of the Large Japanese Field Mouse, Apodemus speciosus (Rodentia: Muridae), Inferred from the Mitochondrial Dloop Region. Zoolog. Sci. 2017, 34, 112. [Google Scholar] [CrossRef]
- Jones Lennon, M.; Taggart, D.A.; Temple-Smith, P.D.; Eldridge, M.D.B. The impact of isolation and bottlenecks on genetic diversity in the Pearson Island population of the black-footed rock-wallaby (Petrogale lateralis pearsoni; Marsupialia: Macropodidae). Aust. Mammal. 2011, 33, 152. [Google Scholar] [CrossRef]
- Frankham, R. Do island populations have less genetic variation than mainland populations? Heredity 1997, 78, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Courchamp, F.; Chapuis, J.; Pascal, M. Mammal invaders on islands: Impact, control and control impact. Biol. Rev. 2003, 78, 347–383. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Genovesi, P.; Jeschke, J.M. Global patterns in threats to vertebrates by biological invasions. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152454. [Google Scholar] [CrossRef]
- Harper, G.A.; Bunbury, N. Invasive rats on tropical islands: Their population biology and impacts on native species. Glob. Ecol. Conserv. 2015, 3, 607–627. [Google Scholar] [CrossRef]
- Helmus, M.R.; Mahler, D.L.; Losos, J.B. Island biogeography of the Anthropocene. Nature 2014, 513, 543–546. [Google Scholar] [CrossRef]
- Marathianou, M.; Kosmas, C.; Gerontidis, S.; Detsis, V. Land-use evolution and degradation in Lesvos (Greece): A historical approach. Land Degrad. Dev. 2000, 11, 63–73. [Google Scholar] [CrossRef]
- Jesse, W.A.M.; Behm, J.E.; Helmus, M.R.; Ellers, J. Human land use promotes the abundance and diversity of exotic species on Caribbean islands. Glob. Chang. Biol. 2018, 24, 4784–4796. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, K.; Taylor, K.J.M.; De Palma, A.; Essl, F.; Dawson, W.; Kreft, H.; Pergl, J.; Pyšek, P.; van Kleunen, M.; Weigelt, P.; et al. Effects of land-use change and related pressures on alien and native subsets of island communities. PLoS ONE 2020, 15, e0227169. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Kouris, A.; Christopoulos, A. Spatiotemporal Patterns and Road Mortality Hotspots of Herpetofauna on a Mediterranean Island. Diversity 2023, 15, 478. [Google Scholar] [CrossRef]
- Dawson, W.; Moser, D.; Van Kleunen, M.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; Winter, M.; Lenzner, B.; Blackburn, T.M.; et al. Global hotspots and correlates of alien species richness across taxonomic groups. Nat. Ecol. Evol. 2017, 1, 0186. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzakis, I.N.; Mannion, A.M.; Sarris, D. Mediterranean island biodiversity and climate change: The last 10,000 years and the future. Biodivers. Conserv. 2016, 25, 2597–2627. [Google Scholar] [CrossRef]
- Sfenthourakis, S.; Triantis, K.A. The Aegean archipelago: A natural laboratory of evolution, ecology and civilisations. J. Biol. Res. 2017, 24, 4. [Google Scholar] [CrossRef]
- Masseti, M. Homeless mammals from the Ionian and Aegean islands. Bonner Zool. Beiträge 2010, 57, 367–373. [Google Scholar]
- Pafilis, P.; Foufopoulos, J.; Poulakakis, N.; Lymberakis, P.; Valakos, E.D. Tail shedding in island lizards [Lacertidae, Reptilia]: Decline of antipredator defenses in relaxed predation environments. Evolution 2009, 63, 1262–1278. [Google Scholar] [CrossRef]
- Runemark, A.; Brydegaard, M.; Svensson, E.I. Does relaxed predation drive phenotypic divergence among insular populations? J. Evol. Biol. 2014, 27, 1676–1690. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Zotou, S.; Iliou, A.; Christopoulos, A. Subterranean to submarine: Stress-induced locomotor repertoire expansion and aquatic escape in the Anatolian mole rat (Nannospalax xanthodon) under risk of predation. J. Ethol. 2024, 43, 39–43. [Google Scholar] [CrossRef]
- Marshall, K.L.A.; Philpot, K.E.; Damas-Moreira, I.; Stevens, M. Intraspecific Colour Variation among Lizards in Distinct Island Environments Enhances Local Camouflage. PLoS ONE 2015, 10, e0135241. [Google Scholar] [CrossRef]
- Kumar, L.; Tehrany, M.S. Climate change impacts on the threatened terrestrial vertebrates of the Pacific Islands. Sci. Rep. 2017, 7, 5030. [Google Scholar] [CrossRef]
- Leclerc, C.; Villéger, S.; Marino, C.; Bellard, C. Global changes threaten functional and taxonomic diversity of insular species worldwide. Divers. Distrib. 2020, 26, 402–414. [Google Scholar] [CrossRef]
- Robertson, J.M.; Langin, K.M.; Sillett, T.S.; Morrison, S.A.; Ghalambor, C.K.; Funk, W.C. Identifying Evolutionarily Significant Units and Prioritizing Populations for Management on Islands. Monogr. West. N. Am. Nat. 2014, 7, 397–411. [Google Scholar] [CrossRef][Green Version]
- Martín, J.L.; Cardoso, P.; Arechavaleta, M.; Borges, P.A.V.; Faria, B.F.; Abreu, C.; Aguiar, A.F.; Carvalho, J.A.; Costa, A.C.; Cunha, R.T.; et al. Using taxonomically unbiased criteria to prioritize resource allocation for oceanic island species conservation. Biodivers. Conserv. 2010, 19, 1659–1682. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Brooks, T.M.; Butchart, S.H.M.; Chanson, J.; Cox, N.; Hoffmann, M.; Stuart, S.N. Spatially Explicit Trends in the Global Conservation Status of Vertebrates. PLoS ONE 2014, 9, e113934. [Google Scholar] [CrossRef]
- Gibson, L.A.; Cowan, M.A.; Lyons, M.N.; Palmer, R.; Pearson, D.J.; Doughty, P. Island refuges: Conservation significance of the biodiversity patterns resulting from ‘natural’ fragmentation. Biol. Conserv. 2017, 212, 349–356. [Google Scholar] [CrossRef]
- Tulloch, A.I.T.; Sutcliffe, P.; Naujokaitis-Lewis, I.; Tingley, R.; Brotons, L.; Ferraz, K.M.P.M.B.; Possingham, H.; Guisan, A.; Rhodes, J.R. Conservation planners tend to ignore improved accuracy of modelled species distributions to focus on multiple threats and ecological processes. Biol. Conserv. 2016, 199, 157–171. [Google Scholar] [CrossRef]
- Fletcher, R.J.; McCleery, R.A.; Greene, D.U.; Tye, C.A. Integrated models that unite local and regional data reveal larger-scale environmental relationships and improve predictions of species distributions. Landsc. Ecol. 2016, 31, 1369–1382. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Konsola, F.; Bouloutsi, A.-Z.; Douskou, N.-N.; Emmanouilidou, I.; Kordatou, M.-A.; Lekka, A.; Limnioti, M.-E.; Loupou, M.; Papageorgiou, D.; et al. Spatial Distribution Patterns, Environmental Drivers, and Hotspot Dynamics of the European Rabbit on a Mediterranean Island: Implications for Conservation and Management. Biology 2025, 14, 225. [Google Scholar] [CrossRef]
- La Marca, W.; Elith, J.; Firth, R.S.C.; Murphy, B.P.; Regan, T.J.; Woinarski, J.C.Z.; Nicholson, E. The influence of data source and species distribution modelling method on spatial conservation priorities. Divers. Distrib. 2019, 25, 1060–1073. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Kouris, A.D.; Zannetos, S.P.; Selimas, I.; Kontos, T.D.; Christopoulos, A.; Dimitrakopoulos, P.G.; Akriotis, T. Invasion Patterns of the Coypu, Myocastor coypus, in Western Central Greece: New Records Reveal Expanding Range, Emerging Hotspots, and Habitat Preferences. Land 2025, 14, 365. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ulrich, W. Statistical challenges in null model analysis. Oikos 2012, 121, 171–180. [Google Scholar] [CrossRef]
- Abedin, I.; Singha, H.; Kang, H.-E.; Kim, H.-W.; Kundu, S. Forecasting Suitable Habitats of the Clouded Leopard (Neofelis nebulosa) in Asia: Insights into the Present and Future Climate Projections Within and Beyond Extant Boundaries. Biology 2024, 13, 902. [Google Scholar] [CrossRef] [PubMed]
- de Knegt, H.J.; van Langevelde, F.; Coughenour, M.B.; Skidmore, A.K.; de Boer, W.F.; Heitkönig, I.M.A.; Knox, N.M.; Slotow, R.; van der Waal, C.; Prins, H.H.T. Spatial autocorrelation and the scaling of species–environment relationships. Ecology 2010, 91, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.; Fortin, M.-J. Connectivity. In Spatial Ecology and Conservation Modeling; Springer International Publishing: Cham, Switzerland, 2018; pp. 321–367. [Google Scholar]
- Gavish, L.; Gurnell, J. Sciurus anomalus Güldenstaedt, 1785. In The Atlas of the European Mammals; Mitchell-Jones, A.J., Amori, G., Bogdanowicz, W., Krystufek, B., Reijnders, P.J.H., Spitzenberger, F., Stubbe, M., Thissen, J.B.M., Vohralik, V., Zima, J., Eds.; Academic Press: London, UK, 1999; pp. 176–177. [Google Scholar]
- Ondrias, J.C. The taxonomy and geographical distribution of the rodents of Greece. Saugetierkd. Mitteilungen 1966, 14, 1–134. [Google Scholar]
- Koprowski, J.L.; Gavish, L.; Doumas, S.L. Sciurus anomalus (Rodentia: Sciuridae). Mamm. Species 2016, 48, 48–58. [Google Scholar] [CrossRef]
- European Topic Centre on Biological Diversity Article 17 Web Tool on Biogeographical Assessment of Conservation Status of Species and Habitat Under Article 17 of the Habitats Directive. Available online: https://nature-art17.eionet.europa.eu/article17/species/summary/?period=5&group=Mammals&subject=Sciurus+anomalus®ion= (accessed on 15 January 2025).
- Angert, A.L.; Bontrager, M.G.; Ågren, J. What Do We Really Know About Adaptation at Range Edges? Annu. Rev. Ecol. Evol. Syst. 2020, 51, 341–361. [Google Scholar] [CrossRef]
- Hardie, D.C.; Hutchings, J.A. Evolutionary ecology at the extremes of species’ ranges. Environ. Rev. 2010, 18, 1–20. [Google Scholar] [CrossRef]
- De Kort, H.; Prunier, J.G.; Ducatez, S.; Honnay, O.; Baguette, M.; Stevens, V.M.; Blanchet, S. Life history, climate and biogeography interactively affect worldwide genetic diversity of plant and animal populations. Nat. Commun. 2021, 12, 516. [Google Scholar] [CrossRef]
- Eckert, C.G.; Samis, K.E.; Lougheed, S.C. Genetic variation across species’ geographical ranges: The central–marginal hypothesis and beyond. Mol. Ecol. 2008, 17, 1170–1188. [Google Scholar] [CrossRef]
- Duncan, S.I.; Crespi, E.J.; Mattheus, N.M.; Rissler, L.J. History matters more when explaining genetic diversity within the context of the core–periphery hypothesis. Mol. Ecol. 2015, 24, 4323–4336. [Google Scholar] [CrossRef]
- Yigit, N.; Kryštufek, B.; Sozen, M.; Bukhnikashvili, A.; Shenbrot, G. Sciurus anomalus (errata version published in 2017). In The IUCN Red List of Threatened Species 2016; E.T20000A115154256; IUCN Global Species Programme Red List Unit: Cambridge, UK, 2016. [Google Scholar]
- Zevgolis, Y.G.; Sazeides, C.I.; Bintsi-Frantzi, E.; Kouris, A.D.; Christopoulos, A. Ecological implications of deep pruning: A case report on Persian squirrel nesting in a centennial olive grove on the island of Lesvos, Greece. Hist. Nat. Bulg. 2024, 46, 89–97. [Google Scholar] [CrossRef]
- Legakis, A.; Maragou, P. The Red Data Book of Threatened Animals of Greece; Hellenic Zoological Society: Athens, Greece, 2009. [Google Scholar]
- Youlatos, D.; Rammou, D.L. Sciurus anomalus. The Greek Red List of Threatened Species. Available online: https://redlist.necca.gov.gr/ (accessed on 5 December 2024).
- Zevgolis, Y.G.; Christopoulos, A.; Kalargalis, I.I.; Zannetos, S.P.; Botetzagias, I.; Dimitrakopoulos, P.G. An (Un)Expected Threat for a Regionally Near-Threatened Species: A Predation Case of a Persian Squirrel on an Insular Ecosystem. Animals 2022, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Aidek, A.; Amr, Z.S.; Abu Baker, M. Distribution and conservation of Sciurus anomalus in Syria (Rodentia: Sciuridae). Lynx New Ser. 2022, 52, 5–13. [Google Scholar] [CrossRef]
- Aghbolaghi, A.M.; Keyghobadi, N.; Azarakhsh, Z.; Dadizadeh, M.; Aghbolaghi, A.S.; Zamani, N. An evaluation of isolation by distance and isolation by resistance on genetic structure of the Persian squirrel (Sciurus anomalus) in the Zagros forests of Iran. Ecol. Evol. 2023, 13, e10225. [Google Scholar] [CrossRef]
- Zannetos, S.P.; Zevgolis, Y.G.; Christopoulos, A.; Akriotis, T. Assessing habitat suitability of the Persian Squirrel in the island of Lesvos, Greece. Hystrix Ital. J. Mammal. in review. 2022. [Google Scholar]
- Zevgolis, Y.G.; Kamatsos, E.; Akriotis, T.; Dimitrakopoulos, P.G.; Troumbis, A.Y. Estimating Productivity, Detecting Biotic Disturbances, and Assessing the Health State of Traditional Olive Groves, Using Nondestructive Phenotypic Techniques. Sustainability 2021, 14, 391. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Sazeides, C.I.; Zannetos, S.P.; Grammenou, V.; Fyllas, N.M.; Akriotis, T.; Dimitrakopoulos, P.G.; Troumbis, A.Y. Investigating the effect of resin collection and detecting fungal infection in resin-tapped and non-tapped pine trees, using minimally invasive and non-invasive diagnostics. For. Ecol. Manag. 2022, 524, 120498. [Google Scholar] [CrossRef]
- Christopoulos, A.; Zevgolis, Y.G. A New Invasion of the Common Slider on a Mediterranean Island (Lesvos, Greece): A Potential Threat to Native Terrapin Populations? Diversity 2022, 14, 1018. [Google Scholar] [CrossRef]
- HNMS Climatic Data for Selected Stations in Greece. Available online: https://www.emy.gr/en/climatic-data?tab=statistics-tab (accessed on 15 December 2024).
- Hecht-markou, P. Beschreibung, geographische Verbreitung, Biotope und Ortswechsel des Sciurus anomalus Gueldenstadt, 1785 auf der Insel Lesbos (Griechenland). Ann. Musei Goulandris 1994, 9, 429–444. [Google Scholar]
- Abi-Said, M.R.; El Khoury, J.; Makhlouf, H.; Amr, Z.S. Ecology of the Persian Squirrel, Sciurus anomalus, in Horsh Ehden Nature Reserve, Lebanon. Vertebr. Zool. 2014, 64, 127–135. [Google Scholar] [CrossRef]
- IUCN SSC. Position Statement on research involving species at risk of extinction. IUCN Species Survival Commission (SSC). 1989. Available online: https://iucn.org/resources (accessed on 15 January 2025).
- Rima, P.C.; Cagnin, M.; Aloise, G.; Preatoni, D.; Wauters, L.A. Scale-dependent environmental variables affecting red squirrel (Sciurus vulgaris meridionalis) distribution. Ital. J. Zool. 2010, 77, 92–101. [Google Scholar] [CrossRef]
- Wauters, L.; Dhondt, A.A. Spacing behaviour of red squirrels, Sciurus vulgaris: Variation between habitats and the sexes. Anim. Behav. 1992, 43, 297–311. [Google Scholar] [CrossRef]
- Copernicus Land Monitoring Service. CORINE Land Cover Product User Manual, Version 1.0; Copernicus Publications: Göttingen, Germany, 2021; Volume 1.0. [Google Scholar]
- Copernicus Land Monitoring Service, Small Woody Features 2018 and Small Woody Features Changes 2015–2018. Available online: https://land.copernicus.eu/en/products/high-resolution-layer-small-woody-features (accessed on 15 January 2025).
- Copernicus Land Monitoring Service, High Resolution Vegetation Phenology and Productivity (HRVPP), Seasonal Trajectories, Vegetation Phenology and Productivity Parameters 2021. Available online: https://www.copernicus.eu/en/access-data/copernicus-services-catalogue/high-resolution-vegetation-phenology-and-productivity (accessed on 15 January 2025).
- Copernicus Land Monitoring Service. HRL Forest 2018 Product User Manual. 2021. Available online: https://land.copernicus.eu/user-corner/technical-library/forest-2018-user-manual.pdf (accessed on 15 January 2025).
- OPEKEPE Greek Payment Authority of Common Agricultural Policy (C.A.P.) Aid Schemes. Available online: https://www.opekepe.gr/en/ (accessed on 29 June 2014).
- Kouris, A.D.; Christopoulos, A.; Vlachopoulos, K.; Christopoulou, A.; Dimitrakopoulos, P.G.; Zevgolis, Y.G. Spatiotemporal Patterns of Reptile and Amphibian Road Fatalities in a Natura 2000 Area: A 12-Year Monitoring of the Lake Karla Mediterranean Wetland. Animals 2024, 14, 708. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, F.; Guin, C.; Zhu, H. A spatio-temporal kernel density estimation framework for predictive crime hotspot mapping and evaluation. Appl. Geogr. 2018, 99, 89–97. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Anselin, L. Local Indicators of Spatial Association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Nagelkerke, N.J.D. A Note on a General Definition of the Coefficient of Determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; Wiley: New York, NY, USA, 2000; ISBN 9780471356325. [Google Scholar]
- Anselin, L. An Introduction to Spatial Data Science with GeoDa; Chapman and Hall/CRC: Boca Raton, FL, USA, 2024; ISBN 9781003274919. [Google Scholar]
- Anselin, L. Spatial Econometrics: Methods and Models; Studies in Operational Regional Science; Springer: Dordrecht, The Netherlands, 1988; Volume 4, ISBN 978-90-481-8311-1. [Google Scholar]
- Lichstein, J.W.; Simons, T.R.; Shriner, S.A.; Franzreb, K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002, 72, 445–463. [Google Scholar] [CrossRef]
- Ver Hoef, J.M.; Peterson, E.E.; Hooten, M.B.; Hanks, E.M.; Fortin, M. Spatial autoregressive models for statistical inference from ecological data. Ecol. Monogr. 2018, 88, 36–59. [Google Scholar] [CrossRef]
- Fotheringham, A.; Rogerson, P. The SAGE Handbook of Spatial Analysis; SAGE Publications, Ltd.: London, UK, 2009; ISBN 9781412910828. [Google Scholar]
- Anselin, L.; Syabri, I.; Kho, Y. GeoDa: An Introduction to Spatial Data Analysis. In Handbook of Applied Spatial Analysis; Springer: Berlin/Heidelberg, Germany, 2010; pp. 73–89. [Google Scholar]
- Dormann, C.F.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Kissling, W.D.; et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Engle, R.F. A general approach to lagrange multiplier model diagnostics. J. Econom. 1982, 20, 83–104. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Mullu, D. A review on the effect of habitat fragmentation on ecosystem. J. Nat. Sci. Res. 2016, 6, 1–15. [Google Scholar]
- Couch, L.; Arslan, A.; Duszynski, D.W. Redescription and New Host Record of Eimeria serbica from the Caucasian Tree Squirrel, Sciurus anomalus, from Turkey. J. Parasitol. 2005, 91, 1399–1400. [Google Scholar] [CrossRef]
- Gavish, L. Preliminary observations on the behavior and ecology of free-living populations of the subspecies Sciurus anomalus syriacus (golden squirrel) on mount Hermon, Israel. Isr. J. Zool. 1993, 39, 275–280. [Google Scholar] [CrossRef]
- Albayrak, I.; Arslan, A. Contribution to the taxonomical and biological characteristics Sciurus anomalus in Turkey (Mammalia: Rodentia). Turkish J. Zool. 2006, 30, 111–116. [Google Scholar]
- Amr, Z.; Eid, E.; Qarqaz, M.; Baker, M.A. The Status and Distribution of the Persian Squirrel, Sciurus anomalus (Mammalia: Rodentia: Sciuridae), in Dibbeen Nature Reserve, Jordan. Zool. Abhandlungen 2006, 55, 199–207. [Google Scholar]
- Khalili, F.; Sadeghi, M.; Malekian, M. Habitat suitability modelling of Persian squirrel (Sciurus anomalus) in Zagros forests, western Iran. J. Wildl. Biodivers. 2018, 2, 56–64. [Google Scholar] [CrossRef]
- Asadi, F.; Rostami, A.; Asadian, P.; Pourkabir, M. Serum biochemistry and hematology values and hemoglobin electrophoresis in Persian squirrels (Sciurus anomalus). Vet. Clin. Pathol. 2007, 36, 188–191. [Google Scholar] [CrossRef]
- Sadeghinezhad, J.; Tootian, Z.; Akbari, G.H.; Chiocchetti, R. The Topography and Gross Anatomy of the Abdominal Gastrointestinal Tract of the Persian Squirrel (Sciurus anomalus). Int. J. Morphol. 2014, 30, 524–530. [Google Scholar] [CrossRef]
- Juškaitis, R.; Balčiauskas, L.; Baltrūnaitė, L.; Augutė, V. Dormouse (Gliridae) populations on the northern periphery of their distributional ranges: A review. Folia Zool. 2015, 64, 302–309. [Google Scholar] [CrossRef]
- Lawton, J.H. Range, population abundance and conservation. Trends Ecol. Evol. 1993, 8, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Sagarin, R.D.; Gaines, S.D.; Gaylord, B. Moving beyond assumptions to understand abundance distributions across the ranges of species. Trends Ecol. Evol. 2006, 21, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Roy Nielsen, C.L.; Wakamiya, S.M.; Nielsen, C.K. Viability and patch occupancy of a swamp rabbit metapopulation at the northern edge of its distribution. Biol. Conserv. 2008, 141, 1043–1054. [Google Scholar] [CrossRef]
- Reif, J.; Št’astný, K.; Bejček, V. Contrasting Effects of Climatic and Habitat Changes on Birds with Northern Range Limits in Central Europe as Revealed by an Analysis of Breeding Bird Distribution in the Czech Republic. Acta Ornithol. 2010, 45, 83–90. [Google Scholar] [CrossRef]
- Avila-Flores, R.; Boyce, M.S.; Boutin, S. Habitat Selection by Prairie Dogs in a Disturbed Landscape at the Edge of Their Geographic Range. J. Wildl. Manag. 2010, 74, 945–953. [Google Scholar] [CrossRef]
- Pafilis, P.; Foufopoulos, J.; Sagonas, K.; Runemark, A.; Svensson, E.; Valakos, E.D. Reproductive biology of insular reptiles: Marine subsidies modulate expression of the Island syndrome. Copeia 2011, 2011, 545–552. [Google Scholar] [CrossRef]
- Novosolov, M.; Raia, P.; Meiri, S. The island syndrome in lizards. Glob. Ecol. Biogeogr. 2013, 22, 184–191. [Google Scholar] [CrossRef]
- Siliceo, I.; Díaz, J.A. A comparative study of clutch size, range size, and the conservation status of island vs. mainland lacertid lizards. Biol. Conserv. 2010, 143, 2601–2608. [Google Scholar] [CrossRef]
- Jones, N.; Malesios, C.; Ioannidou, E.; Kanakaraki, R.; Kazoli, F.; Dimitrakopoulos, P.G. Understanding perceptions of the social impacts of protected areas: Evidence from three NATURA 2000 sites in Greece. Environ. Impact Assess. Rev. 2018, 73, 80–89. [Google Scholar] [CrossRef]
- Gallardo, B.; Aldridge, D.C.; González-Moreno, P.; Pergl, J.; Pizarro, M.; Pyšek, P.; Thuiller, W.; Yesson, C.; Vilà, M. Protected areas offer refuge from invasive species spreading under climate change. Glob. Chang. Biol. 2017, 23, 5331–5343. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulou, K.; Dimitrakopoulos, P.G.; Brooks, T.M.; Kelaidi, G.; Paragamian, K.; Kati, V.; Oikonomou, A.; Vavylis, D.; Trigas, P.; Lymberakis, P.; et al. The Natura 2000 network and the ranges of threatened species in Greece. Biodivers. Conserv. 2021, 30, 945–961. [Google Scholar] [CrossRef]
- Kleining, B. Natura 2000—A Coherent Nature Conservation Network? Springer Nature: Cham, Switzerland, 2024; ISBN 978-3-031-56889-3. [Google Scholar]
- Kryštufek, B.; Vohralík, V. Mammals of Turkey and Cyprus: Rodentia I: Sciuridae, Dipodidae, Gliridae, Arvicolinae; Zgodovinsko Društvo za Južno Primorsko: Koper, Slovenia, 2005; Volume 88, ISBN 1297-9562. [Google Scholar]
- Enukidze, G.P. On the biology of the Transcaucasian squirrel. Soobshcheniia Akad. Nauk Gruz. 1960, 17, 101–123. [Google Scholar]
- Di Pierro, E.; Bertolino, S.; Martinoli, A.; Preatoni, D.; Tosi, G.; Wauters, L.A. Estimating offspring production using capture-mark-recapture and genetic methods in red squirrels. Ecol. Res. 2010, 25, 395–402. [Google Scholar] [CrossRef]
- Kizos, T.; Dalaka, A.; Petanidou, T. Farmers’ attitudes and landscape change: Evidence from the abandonment of terraced cultivations on Lesvos, Greece. Agric. Human Values 2010, 27, 199–212. [Google Scholar] [CrossRef]
- International Olive Council. Production Techniques in Olive Growing; International Olive Council: Madrid, Spain, 2007; ISBN 9788493166366. [Google Scholar]
- López-Bernal, Á.; Alcántara, E.; Testi, L.; Villalobos, F.J. Spatial sap flow and xylem anatomical characteristics in olive trees under different irrigation regimes. Tree Physiol. 2010, 30, 1536–1544. [Google Scholar] [CrossRef]
- Stavrianakis, G.; Sentas, E.; Stattegger, S.R.; Tscheulin, T.; Kizos, T. Effect of olive grove’s understorey management on arthropod diversity. Agroecol. Sustain. Food Syst. 2024, 48, 1115–1138. [Google Scholar] [CrossRef]
- Remm, J.; Lõhmus, A. Tree cavities in forests—The broad distribution pattern of a keystone structure for biodiversity. For. Ecol. Manag. 2011, 262, 579–585. [Google Scholar] [CrossRef]
- Grüebler, M.U.; Schaller, S.; Keil, H.; Naef-Daenzer, B. The occurrence of cavities in fruit trees: Effects of tree age and management on biodiversity in traditional European orchards. Biodivers. Conserv. 2013, 22, 3233–3246. [Google Scholar] [CrossRef]
- Cockle, K.L.; Martin, K.; Wesołowski, T. Woodpeckers, decay, and the future of cavity-nesting vertebrate communities worldwide. Front. Ecol. Environ. 2011, 9, 377–382. [Google Scholar] [CrossRef]
- Rothwell, R. Nest Sites of Red Squirrels (Tamiasciurus hudsonicus) in the Laramie Range of Southeastern Wyoming. J. Mammal. 1979, 60, 404–405. [Google Scholar] [CrossRef]
- Halloran, M.E.; Bekoff, M. Nesting Behaviour of Abert Squirrels (Sciurus aberti). Ethology 1994, 97, 236–248. [Google Scholar] [CrossRef]
- Edelman, A.J.; Koprowski, J.L. Selection of drey sites by Abert’s squirrels in an introduced population. J. Mammal. 2005, 86, 1220–1226. [Google Scholar] [CrossRef]
- Douglass, N.J.; Reinert, H.K. The utilization of fallen logs as runways by small mammals. Proc. Pennsylvania Acad. Sci. 1982, 56, 162–164. [Google Scholar]
- Honda, S.; Saito, M. Use of fallen dead trees by Japanese squirrels within cedar plantations in northeastern Japan. iForest—Biogeosci. For. 2023, 16, 262–267. [Google Scholar] [CrossRef]
- Smith, A.A.; Mannan, W. Distinguishing Characteristics of Mount Graham Red Squirrel Midden Sites. J. Wildl. Manag. 1994, 58, 437–445. [Google Scholar] [CrossRef]
- Carey, A.B.; Harrington, C.A. Small mammals in young forests: Implications for management for sustainability. For. Ecol. Manag. 2001, 154, 289–309. [Google Scholar] [CrossRef]
- Palaiologou, P.; Kalabokidis, K.; Ager, A.A.; Day, M.A. Development of comprehensive fuel management strategies for reducing wildfire risk in Greece. Forests 2020, 11, 789. [Google Scholar] [CrossRef]
- Wauters, L.A.; Dhondt, A.A. The use of red squirrel Sciurus vulgaris dreys to estimate population density. J. Zool. 1988, 214, 179–187. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Kotselis, C.; Kouris, A.D.; Christopoulos, A. A New Aspect of Predator–Prey Dynamics: The Case of a Livestock Guardian Dog Predating upon an Invasive Coypu in Lake Kerkini, Greece. Conservation 2024, 4, 609–616. [Google Scholar] [CrossRef]
- Doherty, T.S.; Dickman, C.R.; Glen, A.S.; Newsome, T.M.; Nimmo, D.G.; Ritchie, E.G.; Vanak, A.T.; Wirsing, A.J. The global impacts of domestic dogs on threatened vertebrates. Biol. Conserv. 2017, 210, 56–59. [Google Scholar] [CrossRef]
- Chen, Y.-C. A tutorial on kernel density estimation and recent advances. Biostat. Epidemiol. 2017, 1, 161–187. [Google Scholar] [CrossRef]
- Sussman, A.L.; Gardner, B.; Adams, E.M.; Salas, L.; Kenow, K.P.; Luukkonen, D.R.; Monfils, M.J.; Mueller, W.P.; Williams, K.A.; Leduc-Lapierre, M.; et al. A comparative analysis of common methods to identify waterbird hotspots. Methods Ecol. Evol. 2019, 10, 1454–1468. [Google Scholar] [CrossRef]
- Nelson, T.A.; Boots, B. Detecting spatial hot spots in landscape ecology. Ecography 2008, 31, 556–566. [Google Scholar] [CrossRef]


| Principal Components | Variable | Principal Components Loadings | ||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | ||
| PC1—Olive-dominated agricultural areas | Olive groves (actively cultivated) | 0.920 | - | - | - | - |
| Total cultivated land | 0.908 | - | - | - | - | |
| Olive cover density | 0.655 | - | - | - | - | |
| Vegetation productivity | 0.624 | - | - | - | - | |
| PC2—Chestnut forests | Chestnut plantations | - | 0.977 | - | - | - |
| Chestnut cover density | - | 0.976 | - | - | - | |
| PC3—Coniferous forests | Coniferous cover density | - | - | 0.913 | - | - |
| Coniferous forests | - | - | 0.863 | - | - | |
| PC4—Proximity to water and livestock farming | Proximity to livestock facilities | - | - | - | 0.863 | - |
| Proximity to water | - | - | - | 0.801 | - | |
| PC5—Broadleaved forests | Broadleaf cover density | - | - | - | - | 0.871 |
| Broadleaved forests | - | - | - | - | 0.777 | |
| Predictor | Β | S.E. | Wald’s χ2 | df | p-Value |
|---|---|---|---|---|---|
| PC1—Olive-dominated agricultural areas | 3.660 | 0.305 | 143.938 | 1 | <0.0001 |
| PC3—Coniferous forests | −0.793 | 0.136 | 34.131 | 1 | <0.0001 |
| PC4—Proximity to water and livestock farming | −1.720 | 0.184 | 87.076 | 1 | <0.0001 |
| PC5—Broadleaved forests | 1.255 | 0.128 | 96.486 | 1 | <0.0001 |
| Constant | 0.823 | 0.175 | 22.102 | 1 | <0.0001 |
| Variable | B | S.E. | z-Value | p-Value |
|---|---|---|---|---|
| (a) SLM—Hotspot Intensity | ||||
| Spatial autoregressive parameter (Rho) | 0.410 | 0.028 | 14.268 | <0.0001 |
| Constant | −0.291 | 0.052 | −5.593 | <0.0001 |
| PC1—Olive-dominated agricultural areas | 2.567 | 0.217 | 11.780 | <0.0001 |
| PC2—Chestnut forests | 0.306 | 0.037 | 8.079 | <0.0001 |
| PC3—Coniferous forests | −0.602 | 0.063 | −9.539 | <0.0001 |
| PC5—Broadleaved forests | 0.462 | 0.043 | 10.65 | <0.0001 |
| (b) SEM—Clustering Patterns | ||||
| Spatial error parameter (Lamda) | 0.188 | 0.041 | 4.517 | <0.0001 |
| PC1—Olive-dominated agricultural areas | 0.419 | 0.040 | 10.350 | <0.0001 |
| PC5—Broadleaved forests | 0.460 | 0.044 | 10.389 | <0.0001 |
| Constant | −0.008 | 0.059 | −0.140 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zevgolis, Y.G.; Kouris, A.D.; Christopoulos, A.; Leros, M.; Loupou, M.; Rammou, D.-L.; Youlatos, D.; Troumbis, A.Y. Where to Protect? Spatial Ecology and Conservation Prioritization of the Persian Squirrel at the Westernmost Edge of Its Distribution. Land 2025, 14, 876. https://doi.org/10.3390/land14040876
Zevgolis YG, Kouris AD, Christopoulos A, Leros M, Loupou M, Rammou D-L, Youlatos D, Troumbis AY. Where to Protect? Spatial Ecology and Conservation Prioritization of the Persian Squirrel at the Westernmost Edge of Its Distribution. Land. 2025; 14(4):876. https://doi.org/10.3390/land14040876
Chicago/Turabian StyleZevgolis, Yiannis G., Alexandros D. Kouris, Apostolos Christopoulos, Marios Leros, Maria Loupou, Dimitra-Lida Rammou, Dionisios Youlatos, and Andreas Y. Troumbis. 2025. "Where to Protect? Spatial Ecology and Conservation Prioritization of the Persian Squirrel at the Westernmost Edge of Its Distribution" Land 14, no. 4: 876. https://doi.org/10.3390/land14040876
APA StyleZevgolis, Y. G., Kouris, A. D., Christopoulos, A., Leros, M., Loupou, M., Rammou, D.-L., Youlatos, D., & Troumbis, A. Y. (2025). Where to Protect? Spatial Ecology and Conservation Prioritization of the Persian Squirrel at the Westernmost Edge of Its Distribution. Land, 14(4), 876. https://doi.org/10.3390/land14040876










