Biodiversity of Diatoms as Indicators of Water Quality and Landscape Sustainable Dynamics in the Zarafshan River, Uzbekistan
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Study Site
2.2. Material
2.3. Methods
3. Results
3.1. Physicochemical Properties of the Zarafshan River Water
3.2. Biological Characteristics of the Zarafshan River
3.3. Bioindicators
3.4. Species Environment Relationships
3.5. Integral Indices of Water Toxicity (WESI) and River Pollution (RPI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Station | 2 | 2 | 3 | 3 | 4 | 4 | 5 | 5 | 6 | 6 | 7 | 7 | 9 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Average | Max. | Average | Max. | Average | Max. | Average | Max. | Average | Max. | Average | Max. | Average | Max. |
| O2 mg L−1 | 8.72 ± 1.16 | 10.36 | 8.7 ± 1.14 | 10.31 | 6.3 ± 2.09 | 9.25 | 6.3 ± 2.09 | 9.25 | 8.415 ± 1.28 | 9.69 | 7.295 ± 2.17 | 9.54 | 6.983 ± 1.69 | 8.44 |
| BOD, mgO L−1 | 0.53 ± 0.30 | 0.95 | 0.8 ± 0.28 | 1.2 | 0.84 ± 0.40 | 1.4 | 0.84 ± 0.40 | 1.4 | 0.86 ± 0.24 | 1.1 | 1.638 ± 0.97 | 2.7 | 2.86 ± 0.99 | 3.8 |
| COD, mgO L−1 | 3.32 ± 1.46 | 5.39 | 3.29 ± 1.48 | 5.39 | 3.3 ± 1.9 | 5.98 | 3.3 ± 1.90 | 5.98 | 5.71 ± 1.76 | 7.47 | 12.438 ± 6.49 | 19.7 | 31.125 ± 6.93 | 40.9 |
| N-NH4, mg L−1 | 0.03 ± 0.04 | 0.08 | 0.04 ± 0.03 | 0.08 | 0.03 ± 0.01 | 0.07 | 0.03 ± 0.03 | 0.07 | 0.1 ± 0.05 | 0.15 | 0.043 ± 0.03 | 0.07 | 0.165 ± 0.08 | 0.25 |
| N-NO2, mg L−1 | 0.86 ± 0.64 | 1.77 | 0.59 ± 0.33 | 1.05 | 0.8 ± 1.65 | 3.14 | 1.65 ± 1.65 | 3.14 | 2.965 ± 1.59 | 4.55 | 0.025 ± 0.01 | 0.035 | 0.12 ± 0.06 | 0.178 |
| N-NO3, mg L−1 | 0.005 ± 0.12 | 0.17 | 0.007 ± 0.01 | 0.028 | 0.007 ± 0.01 | 0.023 | 0.007 ± 0.01 | 0.023 | 0.025 ± 0.01 | 0.037 | 2.688 ± 2.27 | 5.57 | 4.83 ± 3.28 | 8.79 |
| Fe, mg L−1 | 0.01 ± 0.04 | 0.06 | 0.01 ± 0.03 | 0.05 | 0.01 ± 0.01 | 0.03 | 0.01 ± 0.01 | 0.03 | 0.025 ± 0.02 | 0.04 | 0.03 ± 0.03 | 0.07 | 0.09 ± 0.07 | 0.18 |
| Cu, mcg L−1 | 1.60 ± 1.34 | 3.5 | 2 ± 1.14 | 4 | 1.9 ± 1.27 | 3.7 | 1.9 ± 1.27 | 3.7 | 4.65 ± 2.15 | 6.8 | 5.25 ± 3.16 | 8.9 | 6.1 ± 2.10 | 8.2 |
| Zn, mcg L−1 | 2.0 ± 1.34 | 3.9 | 2.1 ± 2.19 | 5.2 | 2.3 ± 2.47 | 5.8 | 2.3 ± 2.47 | 5.8 | 4.35 ± 2.05 | 6.4 | 5.65 ± 3.61 | 9.7 | 4.275 ± 0.62 | 5.5 |
| Phenols, mg L−1 | 0.001 ± 0.0 | 0.005 | 0.002 ± 0.0 | 0.006 | 0.001 ± 0.0 | 0.004 | 0.001 ± 0.0 | 0.004 | 0.006 ± 0.003 | 0.009 | 0.005 ± 0.0 | 0.009 | 0.002 ± 0.0 | 0.005 |
| Oil, mg L−1 | 0 ± 0.007 | 0.01 | 0.01 ± 0.0 | 0.01 | 0.01 ± 0.05 | 0.08 | 0.01 ± 0.05 | 0.08 | 0.03 ± 0.02 | 0.05 | 0.02 ± 0.03 | 0.05 | 0.03 ± 0.05 | 0.09 |
| Detergents, mg L−1 | 0.01 ± 0.01 | 0.02 | 0.01 ± 0.001 | 0.01 | 0 ± 0.01 | 0.01 | 0 ± 0.01 | 0.01 | 0.01 ± 0.01 | 0.02 | 0.015 ± 0.01 | 0.05 | 0.005 ± 0.01 | 0.02 |
| TSS, mg L−1 | 277.3 ± 867.5 | 1504.2 | 249.8 ± 669.5 | 1196.6 | 230.7 ± 654.3 | 1156 | 230.7 ± 654.3 | 1156 | 993.8 ± 629.2 | 1623 | 591 ± 461.2 | 1118 | 1028.1 ± 477.8 | 2316 |
| DDT, mg L−1 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 |
| Alpha-HCH, mcg L−1 | 0.001 ± 0.0 | 0.007 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0.004 | 0 ± 0.0 | 0.004 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 | 0.002 ± 0.0 | 0.004 |
| Gamma-HCH, mcg L−1 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 | 0 ± 0.0 | 0 |
| Cr VI, mcg L−1 | 0.1 ± 0.21 | 0.4 | 0.3 ± 0.18 | 0.05 | 0.4 ± 0.42 | 1 | 0.4 ± 0.42 | 1 | 0.3 ± 0.0 | 0.3 | 0.825 ± 0.60 | 1.5 | 1.275 ± 0.50 | 1.8 |
| F, mg L−1 | 0.17 ± 0.06 | 0.26 | 0.19 ± 0.08 | 0.3 | 0.16 ± 0.06 | 0.25 | 0.16 ± 0.06 | 0.25 | 0.36 ± 0.1 | 0.46 | 0.693 ± 0.24 | 1 | 0.865 ± 0.20 | 1.02 |
| As, mcg L−1 | 0.9 ± 1.48 | 3.00 | 0.5 ± 1.14 | 2.5 | 0.3 ± 1.20 | 2 | 0.3 ± 1.20 | 2 | 1.35 ± 0.85 | 2.2 | 3.125 ± 5.67 | 11 | 0 ± 0.0 | 0 |
| TDS, mg L−1 | 305 ± 42.2 | 364.7 | 300.5 ± 79.1 | 412.3 | 292.6 ± 63.1 | 381.9 | 292.6 ± 63.1 | 381.9 | 515.75 ± 171.4 | 687.1 | 728.8 ± 170.6 | 878.1 | 1710.8 ± 342.6 | 2014.5 |
| Taxa | Upper | Middle | Lower | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Hab | T | OXY | HAL | pH | D | Index S | SAP | AUT-HET | TRO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Achnanthes coarctata (Brébisson ex W.Smith) Grunow 1880 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | B | - | st-str | hl | ind | - | 0.20 | o | ats | ot |
| Achnanthes conspicua var. brevistriata Hustedt 1930 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | eh | - | - | - | - | - | - |
| Achnanthes dispar var angustissima (Jasnitsky) Sheshukova 1950 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Achnanthes gibberula var. interrupta Poretzky and Anisimova 1933 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Achnanthes neoskortzowii Simonsen 1987 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | B | - | - | i | ind | - | - | - | - | - |
| Achnanthes parvula Kützing 1844 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | mh | alf | - | 2.00 | b | ats | me |
| Achnanthes profunda Skvortzov 1937 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Achnanthes striata Skvortzov and K.I. Meyer 1928 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | ind | - | - | - | - | - |
| Achnanthidium exile (Kützing) Heiberg 1863 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | str | i | alb | sx | 1.80 | o | ats | om |
| Achnanthidium minutissimum (Kützing) Czarnecki 1994 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | P-B | eterm | st-str | i | ind | es | 0.95 | b | ate | e |
| Achnanthidium nodosum (Cleve) Tseplik and Chudaev 2020 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | B | - | - | hb | acf | - | 1.00 | o | - | ot |
| Achnanthidium pyrenaicum (Hustedt) H. Kobayasi 1997 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | B | temp | - | mh | alf | es | 1.10 | o | ate | - |
| Actinella punctata F.W. Lewis 1864 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | - | hb | acf | - | 1.00 | o | - | ot |
| Adlafia minuscula (Grunow) Lange-Bertalot 1999 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | P-B. aer | temp | st-str | i | alf | es | 2.80 | b | hce | ot |
| Altana cingens (Skvortzov) Kulikovskiy, Metzeltin and Lange-Bertalot 2012 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | - | - | - | - | - | - |
| Amphipleura pellucida (Kützing) Kützing 1844 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | st | i | alf | sp | 0.80 | b | ate | om |
| Amphora ovalis (Kützing) Kützing 1844 var. ovalis | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | B | temp | st-str | i | alf | sx | 1.50 | b | ate | e |
| Amphora commutata Grunow 1880 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | hl. mh | alf | - | - | - | ats | e |
| Amphora costulata Skvortzov 1937 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | i | - | - | 1.00 | o | - | - |
| Amphora gracilis Ehrenberg 1843 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | - | - | - | - | - | - | - |
| Amphora libyca Ehrenberg 1841 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | temp | st | i | alf | es | 1.50 | o-b | ate | om |
| Amphora mongolica Østrup 1908 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | - | - | - | - | - |
| Amphora ovalis var. gracilis (Ehrenberg) Van Heurck 1885 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | alf | sx | 1.50 | o-b | - | - |
| Amphora pediculus (Kützing) Grunow 1875 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | temp | st | i | alf | es | 1.70 | b | ate | me |
| Amphora proteus var. baikalensis Skvortzov 1937 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Amphora robusta W. Gregory 1857 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | temp | - | eh | - | - | - | - | - | - |
| Amphora subconstricta Levkov 2009 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | alf | - | - | - | - | - |
| Aneumastus tusculus (Ehrenberg) D.G. Mann and A.J. Stickle 1990 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | P-B | - | st | i | alb | - | 0.90 | b | ate | o-e |
| Anomoeoneis costata (Kützing) Hustedt 1959 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | st-str | mh | alf | - | 2.70 | a-o | hne | e |
| Aulacoseira ambigua (Grunow) Simonsen 1979 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P | temp | st-str | i | alf | sp | 1.70 | b-o | ate | om |
| Aulacoseira granulata (Ehrenberg) Simonsen 1979 var. granulata | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | temp | st-str | i | alf | es | 2.00 | b | ate | e |
| Aulacoseira granulata var. angustissima (O.Müller) Simonsen 1979 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P | temp | st-str | i | alf | es | 2.10 | b | ate | e |
| Aulacoseira italica (Ehrenberg) Simonsen 1979 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | cool | st-str | i | ind | es | 1.45 | b | ate | me |
| Bacillaria paxillifera (O.F.Müller) T.Marsson 1901 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | P-B | - | st-str | mh | ind | es | 2.30 | a | ate | e |
| Belonastrum berolinense (Lemmermann) Round and Maidana 2001 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | st-str | hl | alf | - | 2.20 | b | ate | he |
| Berkeleya fragilis Greville 1827 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Brachysira microcephala (Grunow) Compère 1986 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | i | alf | sx | 1.30 | b | ats | om |
| Brachysira serians (Brébisson) Round and D.G.Mann 1981 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | hb | acb | - | 0.20 | o | ats | ot |
| Brebissonia lanceolata (C.Agardh) R.K.Mahoney and Reimer 1986 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | - | hl | alf | - | 2.00 | x-o | - | - |
| Caloneis amphisbaena (Bory) Cleve 1894 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | B | - | st-str | i | alf | - | 2.30 | a | ate | e |
| Caloneis bacillum (Grunow) Cleve 1894 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | alf | es | 1.30 | b | ats | me |
| Caloneis budensis (Grunow) Krammer 1985 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st | hl | alf | - | - | - | - | - |
| Caloneis dubia Krammer 1987 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | P-B | - | st-str | hb | - | - | 1.00 | o | - | ot |
| Caloneis fossilis A.Cleve 1915 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | - | warm | - | eh | - | - | - | - | - | - |
| Caloneis leptosoma (Grunow) Krammer 1985 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | B | - | str | i | ind | - | 1.00 | o | ats | om |
| Caloneis molaris (Grunow) Krammer 1985 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | - | str | i | ind | es | 1.00 | o | - | ot |
| Caloneis silicula (Ehrenberg) Cleve 1894 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | warm | st | i | ind | sp | 1.30 | o | ats | om |
| Campylodiscus echeneis Ehrenberg ex Kützing 1844 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | P | - | st | mh | - | - | - | - | - | - |
| Campylodiscus lacus-baikali Skvortzov 1937 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | - | - | - | - | - |
| Campylodiscus rutilus Skvortzov 1937 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cavinula scutiformis (Grunow) D.G.Mann and A.J.Stickle 1990 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | - | - | - | - | - | - |
| Chamaepinnularia krookii (Grunow) Lange-Bertalot and Krammer 1999 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | aer | i | alf | - | 1.00 | o | ats | om |
| Cocconeis diminuta var. intermedia Kisselev 1932 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cocconeis disculus (Schumann) Cleve 1882 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | - | st-str | i | alf | es | 0.70 | b | ats | me |
| Cocconeis lineata Ehrenberg 1849 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | P-B | temp | st-str | i | alf | sx | 1.20 | b | ate | e |
| Cocconeis neodiminuta Krammer 1990 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | P-B | temp | st-str | i | alf | sx | 0.90 | b | ats | me |
| Cocconeis pediculus Ehrenberg 1838 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | st | hl | alf | - | - | b | ate | e |
| Cocconeis placentula Ehrenberg 1838 var. placentula | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | es | 1.35 | o | ate | me |
| Cocconeis placentula var. euglypta (Ehrenberg) Cleve 1895 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | P-B | temp | st-str | i | alf | sx | 1.30 | b | ate | om |
| Cocconeis placentula var. intermedia (M.Peragallo and Héribaud) Cleve 1895 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | i | alf | - | 1.40 | o-b | ate | ot |
| Cocconeis placentula var. rouxii (Héribaud and Brun) Cleve 1895 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | i | alf | - | 1.40 | o-b | - | - |
| Cocconeis skvortsovii Sheshukova-Poretskaya 1951 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | alb | - | - | - | - | - |
| Cosmioneis pusilla (W.Smith) D.G.Mann and A.J.Stickle 1990 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B. aer | - | st-str | hl | ind | sp | 1.80 | o-a | ats | om |
| Craticula halophila (Grunow) D.G.Mann 1990 var. halophila | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | B | temp | st-str | mh | alf | es | 3.00 | a | ate | e |
| Craticula halophila var. subcapitata (Østrup) Czarnecki 1995 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | B | - | st-str | mh | alf | es | - | - | - | - |
| Craticula simplex (Krasske) Levkov 2016 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | alb | - | - | - | - | - |
| Crenotia thermalis (Rabenhorst) Wojtal 2013 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | eterm | st-str | mh | ind | sx | 0.30 | o | - | om |
| Ctenophora pulchella (Ralfs ex Kützing) D.M.Williams and Round 1986 var. pulchella | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | P-B. Ep | temp | st-str | mh | alf | sx | 2.30 | a | ate | e |
| Ctenophora pulchella var. lacerata (Hustedt) Bukhtiyarova 1995 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | mh | - | - | - | - | hne | - |
| Ctenophora pulchella var. lanceolata (O’Meara) Bukhtiyarova 1995 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | P-B | - | - | I | alf | - | 2.00 | b | hne | - |
| Cyclostephanos dubius (Hustedt) Round 1988 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | P-B | temp | st-str | hl | alf | es | 2.00 | a | ate | e |
| Cyclostephanos mansfeldensis Houk. Kleen and H.Tanaka 2014 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P | - | - | i | ind | - | - | - | - | - |
| Cyclotella choctawhatcheeana Prasad 1990 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | P | - | - | hl | - | - | - | - | - | - |
| Cyclotella comta var. spectabilis A.Cleve 1915 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cyclotella distinguenda Hustedt 1928 var. distinguenda | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | P | - | str | hl | alf | - | 1.30 | o | - | om |
| Cyclotella distinguenda var. unipunctata (Hustedt) Håkansson and J.R.Carter 1990 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | P | - | - | i | ind | - | - | - | - | - |
| Cyclotella melosiroides (Kirchner) Lemmermann 1900 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | P | - | - | i | - | - | - | - | - | - |
| Cyclotella meneghiniana Kützing 1844 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | P-B | temp | st-str | hl | alf | sp | 2.80 | a | hne | e |
| Cyclotella operculata var. mesoleia Grunnow 1878 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P | - | - | i | ind | - | - | - | - | - |
| Cylindrotheca closterium (Ehrenberg) Reimann and J.C.Lewin 1964 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | eh | alf | - | 2.00 | b | hne | - |
| Cymatopleura angulata Greville 1862 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | alf | - | - | - | - | - |
| Cymatopleura elliptica (Brébisson) W.Smith 1851 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | - | 1.40 | b | ate | e |
| Cymbella lanceolata C.Agardh 1830 var. lanceolata | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | B | cool | - | i | ind | sx | - | - | - | - |
| Cymbella affinis Kützing 1844 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | - | - | - | - | - | - |
| Cymbella aspera (Ehrenberg) Cleve 1894 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | alf | - | 1.00 | o | - | ot |
| Cymbella bergii Kisselev 1932 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Cymbella cistula (Ehrenberg) O.Kirchner 1878 var. cistula | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | B | - | - | i | ind | - | - | - | - | - |
| Cymbella cistula var. maculata (Kützing) Van Heurck 1885 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cymbella cymbiformis C.Agardh 1830 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | alf | sx | 2.00 | b | ats | om |
| Cymbella helvetica var. curta Cleve 1894 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | temp | st-str | i | ind | - | 0.60 | b | ats | me |
| Cymbella helvetica var. helvetica Kützing 1844 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Cymbella helvetica var. punctata Hustedt 1922 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | - | - | - | - | - | - |
| Cymbella lacustris f. baicalensis Skvortzov and K.I.Meyer 1928 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cymbella laevis Nägeli 1863 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | oh | alf | - | - | - | - | - |
| Cymbella lanceolata var. notata Wislouch and Poretzky 1924 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | temp | st-str | i | alf | es | 2.50 | b-a | ate | e |
| Cymbella obtusiuscula Kützing 1844 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st | i | alf | - | 1.20 | o | - | om |
| Cymbella parva (W.Smith) Kirchner 1878 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | ind | - | 1.00 | o | - | - |
| Cymbella proschkinae Muzafarov 1965 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Cymbella skvortzovii Skabichevskij 1936 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | st | I | ind | sx | 2.00 | b | ate | m |
| Cymbella stuxbergii (Cleve) Cleve 1894 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | - | - | - | - | - | - | - |
| Cymbella tartuensis Molder 1937 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | B | - | - | i | ind | - | - | - | - | - |
| Cymbella tumida (Brébisson) Van Heurck 1880 var. tumida | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | B | - | - | - | - | - | - | - | - | - |
| Cymbella tumida var. borealis (Grunow) Cleve 1894 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | alf | sx | 2.20 | b | ats | me |
| Cymbella tumidula Grunow 1875 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | - | - | - | - | - | - |
| Cymbella turgidula Grunow 1875 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | P-B | - | str | hb | ind | - | 1.00 | o | ats | ot |
| Cymbopleura amphicephala (Nägeli ex Kützing) Krammer 2003 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | i | ind | - | - | - | - | - |
| Cymbopleura austriaca (Grunow) Krammer 2003 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | ind | - | 1.00 | o | - | ot |
| Cymbopleura lata (Grunow ex Cleve) Krammer 2003 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | i | ind | - | 1.00 | o | - | ot |
| Cymbopleura naviculiformis (Auerswald ex Heiberg) Krammer 2003 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | B | temp | st-str | i | ind | - | - | - | - | - |
| Denticula elegans Kützing 1844 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | oh | alf | - | 2.00 | b | - | - |
| Denticula tenuis Kützing 1844 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | aer | hl | - | es | 1.00 | o | ats | e |
| Denticula tenuis var. crassula (Nägeli ex Kützing) West and G.S.West 1901 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | str | i | ind | sx | 0.30 | o | ats | m |
| Diatoma elongata (Lyngbye) C.Agardh 1824 var. elongata | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | - | - | ind | - | - | a | ats | me |
| Diatoma elongata var. pachycephala (Grunow) Hustedt 1931 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Diatoma moniliformis subsp. ovalis (F.Fricke) Lange-Bertalot. Rumrich and G.Hofmann 1991 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | temp | st-str | i | alf | - | 0.40 | x-o | - | - |
| Diatoma tenuis C.Agardh 1812 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | - | - | - | 1.30 | o | - | om |
| Diatoma vulgaris Bory 1824 var. vulgaris | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | P-B | temp | st-str | hl | alf | - | 2.40 | b-a | - | om |
| Diatoma vulgaris var. brevis Grunow 1862 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | - | 2.40 | b-a | - | - |
| Diatoma vulgaris var. linearis Grunow 1881 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | - | - | sx | 2.20 | b | ate | me |
| Diatomella balfouriana Greville 1855 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | - | - | - | 0.70 | o | ats | m |
| Diploneis smithii (Brébisson) Cleve 1894 var. smithii | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | - | - | - | 1.00 | o | - | ot |
| Diploneis smithii var. pumila (Grunow) Hustedt 1937 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | B | - | - | mh | alf | - | - | - | - | - |
| Diploneis boldtiana Cleve 1891 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | mh | - | - | - | - | - | - |
| Diploneis domblittensis (Grunow) Cleve 1894 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | hl | alb | - | - | - | - | - |
| Diploneis ovalis (Hilse) Cleve 1891 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | oh | - | sx | 0.90 | o | ats | me |
| Diploneis subovalis var. baikalensis Skvortzov | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Discostella stelligera (Cleve and Grunow) Houk and Klee 2004 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | P | temp | st-str | i | ind | - | 2.70 | a-o | - | - |
| Dorofeyukea grimmei (Krasske) Kulikovskiy and Kociolek 2019 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | eh | - | - | - | - | - | - |
| Encyonema ventricosum (C.Agardh) Grunow 1875 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | st-str | i | ind | - | - | - | ate | - |
| Encyonema caespitosum var. ovatum Grunow 1875 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | - | - | - | 1.30 | o | hne | - |
| Encyonema elginense (Krammer) D.G.Mann 1990 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | B | temp | st | i | alf | es | 1.40 | o-b | - | - |
| Encyonema hebridicum Grunow ex Cleve 1891 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | - | acf | - | 1.00 | o | - | ot |
| Encyonema leibleinii (C.Agardh) W.J.Silva. R.Jahn. T.A.V.Ludwig and M.Menezes 2013 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | - | - | - | 1.00 | o | ats | ot |
| Encyonema minutum (Hilse) D.G.Mann 1990 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | - | - | i | alf | - | 0.30 | x | - | - |
| Encyonema perpusillum (A.Cleve) D.G.Mann 1990 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | - | - | - | 1.00 | o | - | - |
| Encyonema ventricosum var. hankensis (Skvortzov) Rodionova and Pomazkina 2014 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Encyonopsis falaisensis (Grunow) Krammer 1997 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Encyonopsis microcephala (Grunow) Krammer 1997 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | - | - | - | 1.00 | o | - | ot |
| Entomoneis alata (Ehrenberg) Ehrenberg 1845 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | P-B | - | st | mh | alf | - | 2.50 | b-a | - | - |
| Entomoneis japonica (Cleve) K.Osada 2002 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Entomoneis ornata (Bailey) Reimer 1975 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | B | - | st-str | i | alf | - | 2.00 | b | hne | - |
| Entomoneis paludosa (W.Smith) Reimer 1975 var. paludosa | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | P-B | - | - | mh | alf | - | - | - | - | - |
| Entomoneis paludosa var. duplex (Donkin) Czarnecki and D.C.Reinke 1982 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Entomoneis paludosa var. subsalina (Cleve) Krammer 1987 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | B | - | - | hl | - | - | 1.20 | o-b | ats | om |
| Eolimna minima (Grunow) Lange-Bertalot. nom. illeg. 1998 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | temp | - | hl | alf | - | - | - | - | - |
| Epithemia adnata (Kützing) Brébisson 1838 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | alb | - | 1.20 | o | - | - |
| Epithemia argus var. angusta Fricke 1904 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | es | 0.70 | o-x | hne | m |
| Epithemia operculata (C.Agardh) Ruck and Nakov 2016 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P | - | st | i | ind | es | 2.00 | b | - | - |
| Epithemia parallela (Grunow) Ruck and Nakov 2016 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | - | - | - | - | 1.30 | o | - | - |
| Epithemia turgida (Ehrenberg) Kützing 1844 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | alf | - | 1.10 | - | - | - |
| Eucocconeis austriaca (Hustedt) Lange-Bertalot 1999 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | alf | - | 0.20 | x | ats | ot |
| Eucocconeis depressa (Cleve) Lange-Bertalot 1999 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | hb | acf | - | 1.00 | o | - | ot |
| Eucocconeis elliptica Saveljewa-Dolgowa 1925 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | sx | 1.00 | o | - | ot |
| Eucocconeis flexella (Kützing) F.Meister 1912 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | str | mh | ind | - | - | - | - | - |
| Eunotia exigua (Brébisson ex Kützing) Rabenhorst 1864 var. exigua | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B. aer | temp | st-str | hb | acb | - | 1.00 | o | - | ot |
| Eunotia exigua var. bidens Hustedt 1930 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | hb | acb | es | 0.45 | x-o | ate | o-e |
| Eunotia glacialis F.Meister 1912 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | str | hb | acf | - | 0.60 | o-x | - | - |
| Eunotia lunaris var. capitata (Grunow) Schönfeldt 1907 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st | i | ind | - | - | - | - | - |
| Eunotia minor (Kützing) Grunow 1881 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | temp | st-str | hb | acf | - | - | - | - | - |
| Eunotia pectinalis (Kützing) Rabenhorst 1864 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | st-str | i | acf | sx | 0.50 | x-o | - | ot |
| Eunotia praerupta Ehrenberg 1843 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | cool | st-str | hb | acf | - | 0.30 | x | - | - |
| Eunotia pseudopectinalis Hustedt 1924 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | cool | str | hb | acf | - | 1.00 | o | - | ot |
| Eunotia robusta Ralfs. nom. illeg. 1861 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | st | hb | acf | - | 1.00 | o | - | ot |
| Eunotia tenella (Grunow) Hustedt 1913 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | hb | acf | - | - | - | - | - |
| Eunotia vanheurckii R.M.Patrick 1958 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | temp | st-str | i | acf | - | 0.50 | x-o | ats | ot |
| Fallacia reichardtii (Grunow) Witkowski. Lange-Bertalot and Metzeltin 2000 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | - | st-str | i | alf | es | 2.70 | a-o | hne | e |
| Fallacia subhamulata (Grunow) D.G.Mann 1990 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | temp | str | i | ind | - | - | - | - | - |
| Fragilaria capucina Desmazières 1830 var. capucina | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | P-B | temp | st-str | i | ind | - | - | - | - | - |
| Fragilaria capucina var. lanceolata Grunow 1881 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | - | - | - | - | - | - | - |
| Fragilaria crotonensis Kitton 1869 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | - | - | - | - | - |
| Fragilaria intermedia (Grunow) Grunow 1881 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Fragilaria septentrionalis (Østrup) Van de Vijver. C.E.Wetzel and Ector 2020 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Fragilaria vaucheriae (Kützing) J.B.Petersen 1938 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | P-B. Ep | temp | st-str | i | alf | - | - | - | - | - |
| Fragilariforma bicapitata (A.Mayer) D.M.Williams and Round 1988 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | P-B | - | st-str | hb | ind | - | - | - | - | - |
| Fragilariforma nitzschioides (Grunow) Lange-Bertalot 2011 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | sx | 1.90 | o-a | ats | me |
| Fragilariforma virescens (Ralfs) D.M.Williams and Round 1988 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | P-B | temp | st-str | hb | ind | - | 1.00 | o | - | ot |
| Frustulia vulgaris (Thwaites) De Toni 1891 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | - | 1.00 | o | - | - |
| Geissleria annulata (Grunow) Lange-Bertalot and Metzeltin 1996 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Geissleria schoenfeldii (Hustedt) Lange-Bertalot and Metzeltin 1996 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | alf | - | - | - | - | m |
| Gogorevia exilis (Kützing) Kulikovskiy and Kociolek 2020 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | eterm | st-str | i | alf | - | - | - | - | - |
| Gomphoneis clevei (Fricke) Gil 1989 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | alf | - | 2.70 | a-o | - | - |
| Gomphonella calcarea (Cleve) R.Jahn and N.Abarca 2019 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | i | alf | - | - | - | - | - |
| Gomphonella olivacea (Hornemann) Rabenhorst 1853 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | alf | - | 2.30 | b | ate | om |
| Gomphonema truncatum Ehrenberg 1832 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | ind | - | 2.00 | b | - | - |
| Gomphonema acuminatum Ehrenberg 1832 var. acuminatum | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | B | temp | st-str | i | ind | - | 0.80 | x-b | - | - |
| Gomphonema acuminatum var. longiceps (Ehrenberg) N.Abarca and R.Jahn 2020 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | str | i | ind | - | - | - | - | - |
| Gomphonema angustatum (Kützing) Rabenhorst 1864 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | ind | - | 1.00 | o | - | - |
| Gomphonema brebissonii Kützing 1849 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | B | - | st | i | ind | - | - | - | - | m |
| Gomphonema capitatum Ehrenberg 1838 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | temp | st | i | alf | - | 1.20 | o | - | om |
| Gomphonema gracile Ehrenberg 1838 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | B | temp | st-str | i | alf | - | - | - | - | - |
| Gomphonema grunowii R.M.Patrick and Reimer 1975 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | B | temp | - | i | alf | es | 0.80 | x-b | ats | m |
| Gomphonema intricatum Kützing 1844 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | B | - | st-str | i | ind | - | - | - | - | - |
| Gomphonema lagenula Kützing 1844 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | - | - | - | - | b | ate | e |
| Gomphonema lanceolatum var. capitatum Skvortzov 1937 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | ind | - | 1.30 | o | ats | ot |
| Gomphonema micropus Kützing 1844 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | ind | - | 1.10 | - | - | - |
| Gomphonema olivaceum var. minutissimum Hustedt 1930 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | str | i | alf | - | - | - | - | - |
| Gomphonema parvulum (Kützing) Kützing 1849 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | B | temp | st-str | i | ind | - | 0.70 | o-x | ats | ot |
| Gomphonema tergestinum (Grunow) Fricke 1902 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | B | - | str | i | ind | - | 1.00 | o | - | - |
| Gomphonema vibrio var. bohemicum (Reichelt and Fricke) R.Ross 1986 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | hb | ind | es | - | - | - | - |
| Grunowia tabellaria (Grunow) Rabenhorst 1864 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | str | i | ind | - | 3.60 | a-b | ats | me |
| Gyrosigma acuminatum (Kützing) Rabenhorst 1853 var. acuminatum | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | alf | - | - | - | - | - |
| Gyrosigma acuminatum var. gallicum (Grunow) Cleve 1894 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | B | - | st-str | hl | alf | - | - | - | - | - |
| Gyrosigma acuminatum var. lacustre (W.Smith) F.Meister 1912 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | - | - | a | ats | e |
| Gyrosigma attenuatum (Kützing) Rabenhorst 1853 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | - | - | - | - | - |
| Gyrosigma eximium (Thwaites) Boyer 1927 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | hl | alb | - | - | - | - | - |
| Gyrosigma kuetzingii (Grunow) Cleve 1894 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Gyrosigma peisonis (Grunow) Hustedt 1930 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | st-str | mh | alf | sp | 2.00 | b | ate | e |
| Gyrosigma scalproides (Rabenhorst) Cleve 1894 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | B | - | str | i | alf | - | - | - | - | - |
| Halamphora acutiuscula (Kützing) Levkov 2009 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | P-B | warm | - | mh | alf | - | 1.30 | o | - | - |
| Halamphora coffeiformis (C.Agardh) Mereschkowsky 1903 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | mh | alf | - | - | - | - | - |
| Halamphora holsatica (Hustedt) Levkov 2009 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | - | st-str | hl | alf | - | - | - | - | - |
| Halamphora hybrida (Grunow) Levkov 2009 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | mh | - | - | - | - | - | - |
| Halamphora perpusilla (Grunow) Q.M.You and Kociolek 2015 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | mh | alf | - | 1.00 | o | - | - |
| Halamphora subcapitata (Kisselev) Levkov 2009 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | B | temp | str | hl | alf | - | - | - | - | - |
| Halamphora transcaspica (J.B.Petersen) Q.M.You and Kociolek 2015 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Halamphora veneta (Kützing) Levkov 2009 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | B | temp | st-str | hl | alf | - | - | - | - | - |
| Hannaea arcus (Ehrenberg) R.M.Patrick 1966 var. arcus | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | cool | str | i | - | es | 0.30 | x | ats | om |
| Hannaea arcus var. amphioxys (Rabenhorst) R.M.Patrick 1966 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | cool | str | i | alf | sx | 0.30 | x | - | - |
| Hantzschia amphioxys (Ehrenberg) Grunow 1880 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B. aer | temp | st-str | i | ind | - | 3.00 | a | - | me |
| Hantzschia amphioxys f. capitata O.Müller 1909 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | I | ind | es | 1.90 | a | ate | o-e |
| Hantzschia spectabilis (Ehrenberg) Hustedt 1959 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | hl | alf | - | - | - | - | - |
| Hantzschia virgata var. capitellata Hustedt 1930 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | hl | alf | - | - | - | - | - |
| Hantzschia weiprechtii Grunow 1880 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | - | - | hl | - | - | - | - | - | - |
| Haslea crucigera (W.Smith) Simonsen 1974 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | mh | - | - | - | - | - | - |
| Hippodonta linearis (Østrup) Lange-Bertalot, Metzeltin and Witkowski 1996 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | i | alf | - | - | o | ats | - |
| Hippodonta luneburgensis (Grunow) Lange-Bertalot, Metzeltin and A.Witkowski 1996 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | B | - | st-str | hl | ind | es | 2.40 | b-a | - | e |
| Iconella hibernica (Ehrenberg) Ruck and Nakov 2016 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | - | ind | - | - | - | - | - |
| Iconella linearis (W.Smith) Ruck and Nakov 2016 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | - | - | - | 0.55 | x-o | ats | om |
| Iconella nervosa (A.W.F.Schmidt) C.Cocquyt and R.Jahn 2017 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | st-str | i | ind | - | - | - | - | - |
| Iconella splendida (Ehrenberg) Ruck and Nakov 2016 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | P-B | - | st-str | i | alf | - | - | - | - | - |
| Iconella tenera (W.Gregory) Ruck and Nakov 2016 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | str | i | alf | - | 1.10 | o | - | - |
| Kurtkrammeria aequalis (W.Smith) Bahls 2015 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | B | - | - | hb | acf | - | 1.00 | o | - | ot |
| Lacustriella lacustris (W.Gregory) Lange-Bertalot and Kulikovskiy 2012 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | - | - | hb | ind | - | - | - | - | e |
| Lindavia antiqua (W.Smith) Nakov, Guillory, M.L.Julius, E.C.Theriot and A.J.Alverson 2015 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | P-B | temp | - | hb | acf | - | 1.20 | o | - | - |
| Lindavia bodanica (Eulenstein ex Grunow) T.Nakov, Guillory, Julius, Theriot and Alverson 2015 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | P | - | st-str | i | ind | - | - | - | hne | - |
| Lindavia comta (Kützing) T.Nakov et al. 2015 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P | temp | st | i | alf | - | - | - | - | - |
| Luticola kotschyana var. robusta J.Y.Li and Y.Z.Qi | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | i | acf | - | - | - | - | - |
| Luticola cohnii (Hilse) D.G.Mann 1990 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B. aer | - | st-str. aer | i | alf | - | - | - | - | - |
| Luticola mutica (Kützing) D.G.Mann 1990 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B.S | temp | st-str | hl | ind | - | 1.90 | o-a | ats | e |
| Mastogloia braunii Grunow 1863 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | P-B | - | - | mh | alf | - | - | - | - | - |
| Melosira normanii Arnott ex Van Heurck 1882 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Melosira undulata (Ehrenberg) Kützing 1844 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | P-B | - | - | i | ind | - | 2.80 | a-o | - | - |
| Melosira varians C.Agardh 1827 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | P-B | temp | st-str | hl | ind | - | 2.40 | b-a | - | - |
| Meridion circulare (Greville) C.Agardh 1831 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | ind | - | - | - | - | - |
| Meridion constrictum Ralfs 1843 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | hb | ind | - | - | - | - | - |
| Navicula arenaria Donkin 1861 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | B | - | - | hl | - | - | 2.40 | b-a | - | - |
| Navicula bicapitellata Hustedt 1925 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | acf | - | - | - | - | e |
| Navicula capitatoradiata H.Germain ex Gasse 1986 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | mh | alf | - | - | - | - | - |
| Navicula cincta (Ehrenberg) Ralfs 1861 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | B | temp | st-str | hl | alf | - | - | - | - | - |
| Navicula crucicula var. obtusata Grunow 1880 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | mh | - | - | - | - | - | - |
| Navicula cryptocephala Kützing 1844 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | P-B | temp | st-str | i | ind | - | 2.40 | b-a | - | - |
| Navicula exigua var. elliptica Hustedt 1927 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | - | - | - | b | ate | - |
| Navicula exilis Kützing 1844 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | - | - | - | - | - | - | - |
| Navicula fluens Hustedt 1930 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | - | - | - | - | - | - | - |
| Navicula gottlandica Grunow 1880 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | P-B | - | - | hl | alf | es | 2.50 | b-a | ate | e |
| Navicula gregaria Donkin 1861 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | temp | st-str | i | alf | - | - | - | - | - |
| Navicula johncarteri D.M.Williams 2001 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | st-str | i | alf | - | 1.50 | o-b | ats | om |
| Navicula karelica var. baicalensis Skvortzov and K.I.Meyer | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Navicula kolbei Meister 1932 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Navicula lacustris var. paulseniana (J.B.Petersen) Zabelina 1951 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | - | - | 1.00 | o | - | - |
| Navicula lanceolata Ehrenberg 1838 var. lanceolata | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Navicula lanceolata var. tenella Cleve | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | - | es | 2.00 | b | - | om |
| Navicula lanceolata var. tenuirostris Skvortzov 1937 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | alf | - | 1.00 | o | - | - |
| Navicula laterostrata Hustedt 1925 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | P-B | - | str | i | alf | - | 1.10 | o | - | - |
| Navicula libonensis Schoeman 1970 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | - | i | alf | - | - | - | - | - |
| Navicula meniscus Schumann 1867 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | temp | - | hl | alf | - | 1.40 | o-b | - | - |
| Navicula minima Grunow 1880 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | hl | alf | - | 1.00 | o | hce | e |
| Navicula oblonga (Kützing) Kützing 1844 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | B | - | st-str | i | alf | sx | 1.50 | o-b | ate | om |
| Navicula peregrina (Ehrenberg) Kützing 1844 var. peregrina | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | - | mh | alf | - | 1.00 | o | - | om |
| Navicula peregrina var. lanceolata Skvortzov 1929 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | alf | - | - | - | - | - |
| Navicula peregrina var. minuta Skvortzov 1929 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | mh | - | es | - | - | - | - |
| Navicula placentula f. minuta J.B.Petersen 1946 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | i | - | - | - | - | - | - |
| Navicula radiosa Kützing 1844 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | temp | st-str | i | ind | sx | - | - | - | - |
| Navicula rhynchocephala Kützing 1844 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | hl | alf | - | 1.30 | o | - | - |
| Navicula rostellata Kützing 1844 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | B | - | st-str | i | alf | - | 0.70 | o-x | ate | ot |
| Navicula rotaeana (Rabenhorst) Grunow 1880 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | - | st | i | ind | - | - | - | - | - |
| Navicula salinicola Hustedt 1939 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | mh | - | - | - | - | - | - |
| Navicula slesvicensis Grunow 1880 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | - | st-str | hl | alf | - | - | - | - | - |
| Navicula tripunctata (O.F.Müller) Bory 1822 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | es | - | - | - | e |
| Navicula viridula (Kützing) Ehrenberg 1836 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | hl | alf | - | - | - | - | - |
| Navicymbula pusilla (Grunow) Krammer 2003 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | B | - | - | mh | alf | - | - | - | - | - |
| Neidium bisulcatum (Lagerstedt) Cleve 1894 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | i | ind | - | 1.00 | o | - | - |
| Neidium iridis (Ehrenberg) Cleve 1894 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | hb | ind | - | - | - | - | - |
| Neidium kozlowii Mereschkovsky 1906 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | - | - | - | - | - |
| Neidium lanceolata Skvortzov 1937 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Neidium punctulatum Hustedt | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | - | - | - | 0.80 | x-b | - | - |
| Nitzschia frustulum var. asiatica Hustedt 1922 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | hl | - | - | 2.40 | b-a | - | e |
| Nitzschia acicularis (Kützing) W.Smith 1853 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | P-B | temp | st | i | alf | es | 1.40 | o-b | ats | om |
| Nitzschia angularis W.Smith 1853 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | B | - | - | eh | - | - | 2.00 | b | hne | - |
| Nitzschia angustata var. curta Grunow 1881 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | P-B | - | - | i | ind | - | - | - | - | - |
| Nitzschia commutata Grunow 1880 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | st-str | mh | alf | - | - | - | - | - |
| Nitzschia dissipata (Kützing) Rabenhorst 1860 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | B | temp | st-str | i | alf | sx | 1.40 | o-b | - | - |
| Nitzschia distans W.Gregory 1857 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | eh | - | - | 3.60 | a-b | - | e |
| Nitzschia dubia W.Smith 1853 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | hl | alf | - | 1.00 | o | - | - |
| Nitzschia gracilis Hantzsch 1860 var. gracilis | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | ind | - | - | - | - | - |
| Nitzschia gracilis var. minor Skabichevskij 1950 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | - | - | - | - | - |
| Nitzschia gradifera Hustedt 1922 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | hl | - | es | 0.50 | x-o | ats | m |
| Nitzschia heufleriana Grunow 1862 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | P-B | - | str | i | alf | - | - | - | ats | - |
| Nitzschia holsatica Hustedt 1924 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | - | i | ind | - | - | - | - | - |
| Nitzschia inconspicua Grunow 1862 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | hl | alf | - | - | - | - | - |
| Nitzschia incurva Grunow 1878 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | mh | - | - | - | - | - | - |
| Nitzschia intermedia Hantzsch ex Cleve and Grunow 1880 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | P-B | temp | - | i | ind | - | - | - | - | - |
| Nitzschia lanceolata var. minor (Grunow) H.Peragallo and M.Peragallo 1900 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | hl | - | - | - | - | - | - |
| Nitzschia lanceolata W.Smith 1853 var. lanceolata | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | hl | alf | - | - | - | - | - |
| Nitzschia linearis W.Smith 1853 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | alf | - | - | - | - | - |
| Nitzschia lorenziana var. subtilis Grunow 1880 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | B | - | - | mh | - | - | - | - | - | - |
| Nitzschia microcephala Grunow 1880 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | - | - | - | - | - |
| Nitzschia palea (Kützing) W.Smith 1856 var. palea | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | ind | - | 2.00 | b | - | - |
| Nitzschia palea var. capitata Wislouch and Poretsky 1924 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | - | - | - | - | - |
| Nitzschia palea var. debilis (Kützing) Grunow 1880 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | temp | - | i | ind | - | - | - | - | - |
| Nitzschia paleacea (Grunow) Grunow 1881 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | es | 2.00 | b | ate | o-e |
| Nitzschia pamirensis Hustedt 1922 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Nitzschia pusilla Grunow 1862 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B. S | temp | st-str | i | alf | - | 1.00 | o | - | - |
| Nitzschia recta Hantzsch ex Rabenhorst 1862 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | B | temp | st-str | i | alf | - | 1.00 | o | - | - |
| Nitzschia regula Hustedt 1922 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | - | - | - | - | - | - | 1.40 | o-b | - | - |
| Nitzschia scalpelliformis Grunow 1880 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | mh | alf | - | - | - | - | - |
| Nitzschia sigma (Kützing) W.Smith 1853 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | temp | st-str | mh | alf | - | - | - | - | - |
| Nitzschia sigmoidea (Nitzsch) W.Smith 1853 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | st-str | i | alf | - | - | - | - | - |
| Nitzschia sinuata (Thwaites) Grunow 1880 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | i | alf | - | 1.90 | o-a | ate | e |
| Nitzschia sublinearis Hustedt 1930 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | P-B | - | - | i | alf | - | - | - | - | - |
| Nitzschia subtilis (Kützing) Grunow 1880 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | i | - | - | - | - | - | - |
| Nitzschia telezkoensis Sheshukova 1950 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Nitzschia thermalis (Ehrenberg) Auerswald 1861 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | - | i | ind | es | - | - | - | - |
| Nitzschia tryblionella Hantzsch 1860 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | i | ind | - | - | - | - | - |
| Nitzschia tubicola Grunow 1880 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | temp | - | mh | ind | es | 2.80 | a-o | hce | e |
| Nitzschia vermicularis (Kützing) Hantzsch 1860 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | P-B | temp | st-str | i | alf | - | - | - | - | - |
| Nitzschia vitrea G.Norman 1861 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | temp | st | mh | alf | - | 2.70 | a-o | ats | e |
| Nupela neogracillima Kulikovskiy and Lange-Bertalot 2009 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | - | - | i | ind | - | - | - | - | ot |
| Odontidium anceps (Ehrenberg) Ralfs 1861 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | P-B | cool | st-str | hb | ind | - | - | - | - | - |
| Odontidium elongatum var. actinastroides (Krieger) R.M.Patrick 1939 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | st-str | hl | alf | sx | 0.40 | x-o | ats | ot |
| Odontidium hyemale (Roth) Kützing 1844 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | P-B | cool | st-str | hb | ind | - | - | - | - | - |
| Odontidium mesodon (Kützing) Kützing 1849 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | B | cool | st-str | hb | ind | - | 0.90 | x-b | - | - |
| Pantocsekiella kuetzingiana (Thwaites) K.T.Kiss and E.Ács 2016 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | P-B | temp | st | I | ind | - | - | - | - | - |
| Pantocsekiella ocellata (Pantocsek) K.T.Kiss and Ács 2016 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | cool | st-str | hl | alf | - | 0.90 | x-b | - | ot |
| Pantocsekiella rossii (H.Håkansson) K.T.Kiss and E.Ács 2016 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | P | temp | st | i | alf | - | 1.00 | o | - | ot |
| Paralia scabrosa (Østrup) Moiseyeva 1986 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | - | - | i | ind | - | 3.00 | a | - | - |
| Paraplaconeis placentula (Ehrenberg) Kulikovskiy and Lange-Bertalot 2012 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | alf | - | 2.00 | b | - | ot |
| Peroniopsis heribaudii (J.Brun and M.Peragallo) Hustedt 1952 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | B | - | - | i | acf | - | 2.00 | b | - | - |
| Pinnularia angulosa Krammer 2000 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | ind | - | - | - | ats | - |
| Pinnularia bogotensis (Grunow) Cleve 1895 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | i | acf | - | - | - | - | - |
| Pinnularia borealis Ehrenberg 1843 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | B. aer | - | st-str.aer | i | ind | - | 1.00 | o | - | ot |
| Pinnularia brauniana (Grunow) Studnicka 1888 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | - | st-str | i | acf | - | - | - | - | - |
| Pinnularia brebissonii (Kützing) Rabenhorst 1864 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | B | temp | st-str | i | ind | - | 1.00 | o | - | - |
| Pinnularia intermedia (Lagerstedt) Cleve 1895 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | cool | st-str | i | ind | - | 1.00 | o | - | - |
| Pinnularia isostauron (Ehrenberg) Cleve 1895 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | - | - | o | ats | om |
| Pinnularia karelica var. baicalensis Skvortzov and K.I.Meyer 1928 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Pinnularia microstauron (Ehrenberg) Cleve 1891 var. microstauron | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | P-B | temp | st-str | i | ind | - | 0.30 | x | ats | ot |
| Pinnularia microstauron var. diminuta Shirshov 1935 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | - | - | 1.00 | o | - | - |
| Pinnularia oriunda Krammer 1992 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | - | - | i | neu | - | 1.00 | o | ats | ot |
| Pinnularia paragracillima Kulikovskiy, Lange-Bertalot and Witkowski 2010 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | str | i | ind | - | 1.00 | o | - | om |
| Pinnularia pectinalis var. rostrata Skvortzov 1937 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Pinnularia subborealis Hustedt 1922 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | - | - | 0.20 | x | ats | ot |
| Pinnularia subcapitata W.Gregory 1856 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | acf | - | 0.60 | o-x | - | - |
| Pinnularia sudetica Hilse 1861 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | B | - | - | hb | neu | - | 1.00 | o | - | - |
| Placoneis exigua (W.Gregory) Mereschkovsky 1903 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | str | i | ind | es | 1.40 | o-b | - | - |
| Placoneis dicephala (Ehrenberg) Mereschkowsky 1903 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | es | 2.00 | b | ate | me |
| Placoneis elginensis (W.Gregory) E.J.Cox 1988 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | st-str | i | alf | - | - | - | - | - |
| Placoneis placentula var. lanceolata (Grunow) Aboal 2003 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | alf | - | - | - | - | - |
| Planothidium grimmei (Krasske) I.W.Bishop and Spaulding 2018 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | - | - | - | - | 1.00 | o | ats | om |
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot 1999 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | warm | - | i | alf | sx | 1.60 | b-o | ate | e |
| Planothidium rostratoholarcticum Lange-Bertalot and Bak 2015 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | i | alf | - | 1.60 | b-o | - | om |
| Pleurosira laevis (Ehrenberg) Compère 1982 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | P-B | eterm | - | eh | alf | - | - | - | - | - |
| Prestauroneis protracta (Grunow) Kulikovskiy and Glushchenko 2016 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | P-B | - | st-str | mh | ind | - | 0.40 | x-o | ate | e |
| Psammothidium marginulatum (Grunow) Bukhtiyarova and Round 1996 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | temp | st-str | hb | acf | sx | 0.20 | x | ats | ot |
| Pseudostaurosira brevistriata var. capitata (Héribaud) N.A.Andresen. Stoermer. and R.G.Kreis. Jr. 2000 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | - | i | alf | es | 1.20 | o | ats | o-e |
| Rhoicosphenia abbreviata (C.Agardh) Lange-Bertalot 1980 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | B | temp | st-str | i | alf | es | 1.90 | o-a | ate | me |
| Rhopalodia gibba (Ehrenberg) O.Müller 1895 var. gibba | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | es | 1.40 | x-o | ate | om |
| Rhopalodia gibba var. mongolica (Østrup) Proshkina-Lavrenko 1950 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | - | - | - | - | - | - | - | - | - | - |
| Rhopalodia gibberula var. producta (Grunow) O.Müller 1900 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | str | hl | alf | - | - | - | - | - |
| Sellaphora americana (Ehrenberg) D.G.Mann 1989 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | str | i | alf | - | 1.50 | o-b | ats | ot |
| Sellaphora bacillum (Ehrenberg) D.G.Mann 2018 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | st-str | i | alf | sx | 1.50 | o-b | ats | me |
| Sellaphora hustedtii (Krasske) Lange-Bertalot and Werum 2004 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | str | i | ind | sx | 0.30 | x | - | - |
| Sellaphora lambda (Cleve) Metzeltin and Lange-Bertalot 1998 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | B | - | - | i | - | - | - | - | - | - |
| Sellaphora mutata (Krasske) Lange-Bertalot 1996 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | st-str | hl | ind | es | 1.90 | b | hne | om |
| Sellaphora parapupula Lange-Bertalot 1996 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st | i | ind | - | 1.00 | o | ate | m |
| Sellaphora pupula (Kützing) Mereschkovsky 1902 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | eterm | st-str | hl | ind | sx | 1.90 | o-a | ate | me |
| Sellaphora rostrata (Hustedt) J.R.Johansen 2004 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | temp | - | hl | ind | - | 1.90 | o-a | hne | - |
| Sellaphora seminulum (Grunow) D.G.Mann 1989 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | ind | sp | 2.50 | b-a | hne | e |
| Sellaphora verecunda (Hustedt) C.E.Wetzel, L.Ector, B.Van de Vijver, Compère and D.G.Mann 2015 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Sellaphora wummensis J.R.Johansen 2004 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | hl | ind | es | 1.90 | o-a | hne | me |
| Stauroneis anceps Ehrenberg 1843 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | P-B | temp | st-str | i | ind | sx | 1.30 | o | ats | om |
| Stauroneis parvula (Grunow) Cleve. nom. illeg. 1894 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | B | - | st | mh | ind | - | - | - | - | - |
| Stauroneis smithii Grunow 1860 var. smithii | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | st-str | i | alf | - | 1.00 | o | - | om |
| Stauroneis smithii var. karelica Wislouch and Kolbe 1917 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | B | cool | - | i | - | - | 1.00 | o | - | ot |
| Staurophora wislouchii (Poretzsky and Anisimova) D.G.Mann 1990 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | mh | - | - | - | - | - | - |
| Staurosira construens var. triundulata (Reichelt) Bukhtiyarova 1995 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | - | 1.00 | o | - | - |
| Staurosira dubia Grunow. nom. inval. 1879 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P | - | - | i | alf | sp | 1.30 | o | ate | me |
| Staurosira leptostauron (Ehrenberg) Kulikovskiy and Genkal 2011 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | - | 1.30 | o | - | - |
| Staurosira subsalina (Hustedt) Lange-Bertalot 2004 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | P-B | - | st-str | hl | alf | - | - | - | - | - |
| Staurosira venter (Ehrenberg) Cleve and J.D.Möller 1879 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | - | 1.30 | o | - | ot |
| Staurosirella martyi (Héribaud) Morales and Manoylov 2006 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | - | - | es | 2.70 | a-o | - | - |
| Staurosirella pinnata (Ehrenberg) D.M.Williams and Round 1988 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | hl | alf | es | 1.10 | o | ats | om |
| Staurosirella rhomboides (Grunow) E.A.Morales and K.M.Manoylov 2010 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | B | - | st | hb | alf | - | 1.00 | o | - | - |
| Stenopterobia intermedia (F.W.Lewis) Van Heurck ex Hanna 1933 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | B | - | - | hb | acf | - | 1.00 | o | - | ot |
| Stephanodiscus minutulus (Kützing) Cleve and Möller 1882 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | P | temp | - | i | alb | - | - | - | - | - |
| Surirella conifera var. punctata Skvortsov | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Surirella angusta Kützing 1844 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | - | i | ind | - | - | - | - | - |
| Surirella capronii var. hankensis Skvortzov 1929 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Surirella didyma var. minor Skvortzov 1937 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | B | - | - | i | alf | - | - | - | - | - |
| Surirella grunowii Kulikovskiy, Lange-Bertalot and Witkovski 2010 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | B | - | - | i | ind | - | - | - | - | - |
| Surirella librile (Ehrenberg) Ehrenberg 1845 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | - | - | - | hne | - |
| Surirella minuta Brébisson ex Kützing. nom. illeg. 1849 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | temp | st-str | i | alf | - | - | - | - | - |
| Surirella ovalis Brébisson 1838 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | P-B | - | st-str | mh | alf | es | 1.70 | b-o | - | - |
| Surirella quadricornis Jasnitsky 1936 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Surirella salina W.Smith 1851 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | B | - | st-str | i | ind | es | 1.20 | o | ats | ot |
| Surirella turgida var. skvortzowii (K.I.Meyer) Kisselev 1950 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | - | - | - | - | - | - | - | - | - |
| Synedra famelica Kützing 1844 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | P-B | - | str | i | alf | - | 1.50 | o-b | - | ot |
| Synedra goulardii Brébisson ex Cleve and Grunow 1880 var. goulardii | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | - | - | i | ind | - | - | - | - | - |
| Synedra goulardii var. telezkoensis Poretzky ex Proshkina-Lavrenko 1950 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | es | - | - | - | - |
| Synedra actinastroides (Lemmermann) Lemmermann 1900 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | - | - | - | - | - | - | - | - | - | - |
| Tabellaria fenestrata (Lyngbye) Kützing 1844 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | P-B | - | st-str | i | ind | - | 1.90 | o-a | - | - |
| Tabellaria flocculosa (Roth) Kützing 1844 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | P-B | eterm | st-str | i | acf | - | 3.00 | a | - | - |
| Tabularia parva (Kützing) D.M.Williams and Round 1986 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - | mh | alf | - | - | - | - | - |
| Tabularia tabulata (C.Agardh) Snoeijs 1992 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | B | - | - | mh | alf | - | - | - | - | - |
| Tryblionella angustata W.Smith 1853 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | st | i | alf | sx | 1.50 | o-b | ats | e |
| Tryblionella apiculata W.Gregory 1857 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | mh | alf | es | 2.70 | a-o | ate | e |
| Tryblionella debilis Arnott ex O’Meara 1873 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | P-B | temp | st-str. aer | i | alf | es | 2.60 | a-o | ate | e |
| Tryblionella hungarica (Grunow) Frenguelli 1942 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | st-str | mh | alf | sp | 2.90 | a | ate | e |
| Tryblionella levidensis W.Smith 1856 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | st-str | hl | alf | sp | 2.60 | a-o | ate | e |
| Tryblionella victoriae Grunow 1862 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | B | - | st-str | hl | alf | sp | 2.60 | a-o | ate | e |
| Ulnaria oxyrhynchus (Kützing) Aboal 2003 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | P-B | - | st-str | i | alf | es | 2.40 | b-a | ats | e |
| Ulnaria ulna (Nitzsch) Compère 2001 var. ulna | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | P-B | temp | st-str | i | alf | es | 2.40 | b-a | ate | e |
| Ulnaria aequalis (Kützing) D.M.Williams and Van de Vijver 2021 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | - | i | alf | sp | 2.00 | b | - | om |
| Ulnaria amphirhynchus (Ehrenberg) Compère and Bukhtiyarova 2006 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | P-B | - | - | i | alf | es | 2.00 | b | hne | om |
| Ulnaria capitata (Ehrenberg) Compère 2001 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | - | st-str | i | alf | es | 2.00 | b | ats | e |
| Ulnaria danica (Kützing) Compère and Bukhtiyarova 2006 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | P-B | temp | - | i | alf | es | 1.70 | b-o | hne | om |
| Ulnaria delicatissima var. angustissima (Grunow) Aboal and P.C.Silva 2004 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | P-B | - | - | i | alf | es | 1.70 | b-o | - | om |
| Ulnaria ulna var. spathulifera (Grunow) Aboal 2003 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | B | - | st-str | i | alf | - | 1.70 | b-o | ats | e |
| Variable | St.1 | St.2 | St.3 | St.4 | St.5 | St.6 | St.7 | St.8 | St.9 | St.10 | St.11 | St.12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Habitat | ||||||||||||
| B | 59 | 38 | 23 | 7 | 40 | 26 | 44 | 8 | 43 | 22 | 52 | 64 |
| P-B | 43 | 28 | 13 | 5 | 18 | 31 | 27 | 13 | 30 | 11 | 26 | 18 |
| P | 1 | 2 | 1 | 0 | 3 | 2 | 1 | 3 | 4 | 0 | 7 | 5 |
| Temperature | ||||||||||||
| cool | 3 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 4 | 2 | 4 | 2 |
| temp | 48 | 29 | 9 | 8 | 17 | 20 | 27 | 12 | 27 | 16 | 21 | 26 |
| eterm | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 1 | 3 | 1 | 3 | 0 |
| warm | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| Oxygen | ||||||||||||
| aer | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| str | 6 | 5 | 4 | 0 | 3 | 1 | 8 | 1 | 5 | 1 | 3 | 11 |
| st-str | 55 | 30 | 13 | 8 | 29 | 28 | 39 | 13 | 40 | 22 | 40 | 28 |
| st | 8 | 7 | 1 | 0 | 5 | 5 | 3 | 3 | 5 | 1 | 8 | 7 |
| Salinity | ||||||||||||
| eh | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 1 | 4 |
| mh | 10 | 9 | 2 | 0 | 4 | 6 | 9 | 5 | 9 | 4 | 9 | 8 |
| hl | 17 | 7 | 3 | 2 | 9 | 9 | 11 | 1 | 9 | 7 | 13 | 10 |
| i | 65 | 42 | 25 | 9 | 40 | 37 | 47 | 16 | 48 | 18 | 51 | 51 |
| hb | 5 | 5 | 4 | 1 | 3 | 3 | 6 | 1 | 8 | 4 | 7 | 9 |
| pH | ||||||||||||
| acb | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| acf | 4 | 3 | 1 | 0 | 2 | 2 | 5 | 0 | 2 | 2 | 6 | 9 |
| ind | 30 | 18 | 14 | 4 | 16 | 17 | 24 | 7 | 28 | 15 | 33 | 25 |
| alf | 57 | 36 | 14 | 6 | 31 | 30 | 32 | 15 | 38 | 15 | 33 | 35 |
| alb | 1 | 2 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 2 |
| Watanabe | ||||||||||||
| sx | 11 | 5 | 4 | 1 | 5 | 4 | 5 | 4 | 8 | 3 | 8 | 9 |
| es | 22 | 13 | 4 | 2 | 7 | 7 | 11 | 5 | 13 | 9 | 8 | 10 |
| sp | 5 | 1 | 0 | 0 | 5 | 3 | 3 | 0 | 1 | 1 | 1 | 2 |
| Autotrophy-Heterotrophy | ||||||||||||
| ats | 9 | 6 | 7 | 1 | 11 | 11 | 13 | 3 | 9 | 6 | 13 | 14 |
| ate | 20 | 12 | 3 | 1 | 12 | 5 | 11 | 6 | 11 | 5 | 9 | 14 |
| hne | 7 | 3 | 0 | 1 | 4 | 3 | 2 | 2 | 4 | 1 | 4 | 7 |
| hce | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| Trophy | ||||||||||||
| ot | 10 | 6 | 2 | 0 | 9 | 8 | 6 | 1 | 9 | 6 | 13 | 15 |
| om | 10 | 4 | 4 | 1 | 7 | 10 | 7 | 3 | 7 | 2 | 8 | 10 |
| m | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 0 | 1 | 0 | 1 | 3 |
| me | 7 | 6 | 1 | 1 | 4 | 3 | 1 | 1 | 3 | 1 | 4 | 3 |
| e | 18 | 9 | 2 | 1 | 9 | 5 | 12 | 3 | 8 | 6 | 12 | 11 |
| o-e | 1 | 3 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 0 |
| he | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Class of Water Quality | ||||||||||||
| Class 1 | 4 | 1 | 1 | 0 | 4 | 2 | 5 | 2 | 1 | 1 | 4 | 3 |
| Class 2 | 34 | 24 | 8 | 3 | 21 | 17 | 22 | 7 | 26 | 13 | 22 | 38 |
| Class 3 | 30 | 13 | 6 | 4 | 10 | 2 | 10 | 5 | 13 | 6 | 16 | 15 |
| Class 4 | 4 | 1 | 1 | 0 | 3 | 4 | 3 | 2 | 6 | 3 | 6 | 3 |
| Class 5 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
References
- Muzaffarov, A.M. Algal Flora of the Central Asian Water Bodies; Uzbekistan Academy of Science: Tashkent, Uzbekistan, 1965; 570p. (In Russian) [Google Scholar]
- Shultz, V.L. Rivers of Central Asia; LIMIZ: Leningrad, Russia, 1965; 606p. (In Russian) [Google Scholar]
- Curtean-Bănăduc, A.; Burcea, A.; Mihuţ, C.-M.; Bănăduc, D. The Benthic Trophic Corner Stone Compartment in POPs Transfer from Abiotic Environment to Higher Trophic Levels—Trichoptera and Ephemeroptera Pre-Alert Indicator Role. Water 2021, 13, 1778. [Google Scholar] [CrossRef]
- Cianfaglione, K.; Pedrotti, F. Italy in the Danube Geography: Territory, Landscape, Environment, Vegetation, Fauna, Culture, Human Management and Outlooks for the Future. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Geobotany Studies (Basics, Methods and Case Studies); Bănăduc, D., Curtean-Bănăduc, A., Pedrotti, F., Cianfaglione, K., Akeroyd, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 87–118. [Google Scholar]
- Kholmatov, A.P. Analytical Review “State and Prospects of Integrated Water Resources Management in the Zeravshan River Basin”. “Promoting Integrated Water Resources Management and Transboundary Dialogue in Central Asia”, EU-UNDP Project (2009–2012); Ministry of Melioration and Water Resources of the Republic of Tajikistan. United Nations Development Program: Dushanbe, Tajikistan, 2010; 95p. (In Russian)
- Snigirova, A.; Bogatova, Y.; Barinova, S. Assessment of River-Sea Interaction in the Danube Nearshore Area (Ukraine) by Bioindicators and Statistical Mapping. Land 2021, 10, 310. [Google Scholar] [CrossRef]
- Curtean–Bănăduc, A.; Bănăduc, D.; Bucşa, C. Watersheds Management (Transylvania/Romania): Implications, risks, solutions, Strategies to enhance environmental Security in transition countries. In NATO Science for Peace and Security Series C-Environmental Security; Springer: Berlin/Heidelberg, Germany, 2007; pp. 225–238. [Google Scholar]
- Tsoi, W.Y.; Hadwen, W.L.; Sheldon, F. Identifying diatom indicator species of nutrient enrichment: An in situ nutrient enrichment experiment in subtropical upland streams. Ecol. Indic. 2020, 119, 106744. [Google Scholar] [CrossRef]
- Lavoie, I.; Campeau, S.; Darchambeau, F.; Cabana, G.; Dillon, P.J. Are diatoms good integrators of temporal variability in stream water quality? Freshw. Biol. 2007, 53, 827–841. [Google Scholar] [CrossRef]
- Dixit, S.; Smol, J.P.; Kingston, J.C.; Charles, D. Diatoms–Powerful Indicators of Environmental-Change. Environ. Sci. Technol. 1992, 26, 22–33. [Google Scholar] [CrossRef]
- Krupa, E.G.; Barinova, S.S.; Romanova, S.M. Ecological Mapping in Assessing the Impact of Environmental Factors on the Aquatic Ecosystem of the Arys River Basin, South Kazakhstan. Diversity 2019, 11, 239. [Google Scholar] [CrossRef]
- Muzaffarov, A.M.; Musaev, K.Y. Materials for the knowledge of the flora of algae in water bodies of the upper reaches of the river Zarafshan. In Algae of Reservoirs of Uzbekistan; Publishing House “Fan”: Tashkent, Uzbekistan, 1969; pp. 3–31. (In Russian) [Google Scholar]
- Polyansky, V.I. On the flora of algae in Samarkand. In Proceedings of the Botanical Institute of the USSR Academy of Sciences; USSR: Saint Petersburg, Russia, 1950; Volume 2, pp. 137–147. (In Russian) [Google Scholar]
- Ergashev, A.E. Patterns of Development and Distribution of Algal Flora in Artificial Reservoirs of Central Asia; Fan: Tashkent, Uzbekistan, 1976; 360p. (In Russian) [Google Scholar]
- Toshpulatov, Y.S. Algal Flora of the Zarafshan River’s Middle Course and It’s Role in Assessing Ecological-Sanitary State of Water. Ph.D. Dissertation, Institute of Botany of Uzbek Academy of Sciences, Tashkent, Uzbekistan, 2018; 111p. (In Russian). [Google Scholar]
- Mamanazarova, K.S. Seasonal development of indicator-system algae of the bottom river basin Zeravshan (Rep. Uzbekistan). Algologia 2014, 24, 67–74. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Toshpulatov, Y.S. Taxonomic and floristic analysis of the middle stream algoflora of the Zarafshan river. Bull. Gulistan State Univ. Gulistan 2015, 3, 45–51. (In Uzbek) [Google Scholar]
- Toshpolatov, Y.S.; Olimzhonova, K.O. Algal Flora of the Middle Reaches of the Zarafshan River, Its Significance in Assessing the Ecological and Sanitary State of Waters; Navroz: Tashkent, Uzbekistan, 2015; 128p. (In Uzbek) [Google Scholar]
- Barinova, S.; Mamanazarova, K. Diatom Algae-Indicators of Water Quality in the Lower Zarafshan River, Uzbekistan. Water 2021, 13, 358. [Google Scholar] [CrossRef]
- Dustov, B.S.; Tashpulatov, Y.S. Taxonomic Analysis and Ecological Features of the Algal Flora of the Water Bodies of the West Zarafshan Range. Am. J. Plant Sci. 2023, 14, 542–551. [Google Scholar] [CrossRef]
- Kulmatov, R.; Opp, C.; Groll, M.; Kulmatova, D. Assessment of Water Quality of the Trans-Boundary Zarafshan River in the Territory of Uzbekistan. J. Water Resour. Prot. 2013, 5, 17–26. [Google Scholar] [CrossRef][Green Version]
- Shultz, V.L.; Mashrapov, R. Hydrography of Central Asia; Teacher: Tashkent, Uzbekistan, 1969; 360p. (In Russian) [Google Scholar]
- Zarafshon davalt Kurikhonasi (Zarafshan Natural Reserve). Available online: https://qomus.info/encyclopedia/cat-z/zarafshon-davlat-qoriqxonasi-uz/ (accessed on 12 August 2024). (In Uzbek).
- Yearbook: Surface Water Quality in the Territory of UzHYDROMET Activity for 2009–2015; Uzhydromet: Tashkent, Uzbekistan, 2015. (In Russian)
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication, National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 18 August 2023).
- Korde, N.V. Methods of biological study of bottom sediments of lakes (field work and biological analyses). In Life of Fresh Waters of the USSR, Volume 4; Pavlovsky, E.N., Zhadin, V.I., Eds.; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia, 1956; pp. 383–413. (In Russian) [Google Scholar]
- Sládeček, V. Diatoms as indicators of organic pollution. Acta Hydroch. Hydrobiol. 1986, 14, 555–566. [Google Scholar] [CrossRef]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Prospects; Publishing House of Haifa University: Haifa, Israel, 2019; 367p. (In Russian) [Google Scholar]
- Parmar, T.K.; Rawtani, D.; Agrawal, Y.K. Bioindicators: The natural indicator of environmental pollution. Front. Life Sci. 2016, 9, 110–118. [Google Scholar] [CrossRef]
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 79–89. [Google Scholar] [CrossRef]
- Barinova, S. On the Classification of Water Quality from an Ecological Point of View. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555581. [Google Scholar] [CrossRef]
- McAleece, N.; Gage, J.D.G.; Lambshead, P.J.D.; Paterson, G.L.J. BioDiversity Professional Statistics Analysis Software; Jointly Developed by the Scottish Association for Marine Science and the Natural History Museum London; Scottish Association for Marine Science: Oban, UK; Natural History Museum London: London, UK, 1997; Available online: https://www.sams.ac.uk/science/outputs/ (accessed on 10 July 2024).
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.A.; Ly, A.; Gronau, F.Q.; Smira, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Novakovsky, A.B. Abilities and base principles of program module “GRAPHS”. Sci. Rep. Komi Sci. Cent. Ural. Div. Russ. Acad. Sci. 2004, 27, 28. (In Russian) [Google Scholar]
- Sumita, M. A numerical water quality assessment of rivers in Hokuriku District using epilithic diatom assemblage in river bed as a biological indicator. (II) The values of RPID in surveyed rivers. Diatom Jpn. J. Diatomol. 1986, 2, 9–18. [Google Scholar]
- Watanabe, T.; Asai, K.; Houki, A. Numerical estimation of organic pollution of flowing water by using the epilithic diatom assemblage—Diatom Assemblage Index (DAIpo). Sci. Total Environ. 1986, 55, 209–218. [Google Scholar] [CrossRef]
- Khuram, I.; Ahmad, N.; Jan, S.; Barinova, S. Freshwater green algal biofouling of boats in the Kabul River, Pakistan. Oceanol. Hydrobiol. Stud. 2014, 43, 329–336. [Google Scholar] [CrossRef]
- Moore, J.W. Toxic Substances in Water. In The Changing Environment; Springer Series on Environmental Management; Springer: New York, NY, USA, 1986; pp. 90–111. [Google Scholar] [CrossRef]
- Behrooz, R.D.; Esmaili-Sari, A.; Chakraborty, P. Distribution and ECo-Toxicological Risk Assessment of Legacy Persistent Organic Pollutants in Surface Water of Talar, Babolrood and Haraz Rivers. Water 2020, 12, 3104. [Google Scholar] [CrossRef]
- Pastorino, P.; Bertoli, M.; Squadrone, S.; Brizio, P.; Piazza, G.; Noser, A.G.O.; Prearo, M.; Abete, M.C.; Pizzul, E. Detection of trace elements in freshwater macrobenthic invertebrates of different functional feeding guilds: A case study in Northeast Italy. Ecohydrol. Hydrobiol. 2019, 19, 428–440. [Google Scholar] [CrossRef]
- Barhoumi, B.; Beldean-Galea, M.S.; Al-Rawabdeh, A.M.; Roba, C.; Martonos, I.M.; Bălc, R.; Kahlaoui, M.; Touil, S.; Tedetti, M.; Driss, M.R.; et al. Occurrence, distribution and ecological risk of trace metals and organic pollutants in surface sediments from a Southeastern European river (Someşu Mic River, Romania). Sci. Total Environ. 2019, 660, 660–676. [Google Scholar] [CrossRef]
- Aljanabi, Z.Z.; Al-Obaidy, A.-H.M.J.; Hassan, F.M. A Novel Water Quality Index for Iraqi Surface Water. Baghdad Sci. J. 2023, 20 (Suppl. S6), 2395–2413. [Google Scholar] [CrossRef]
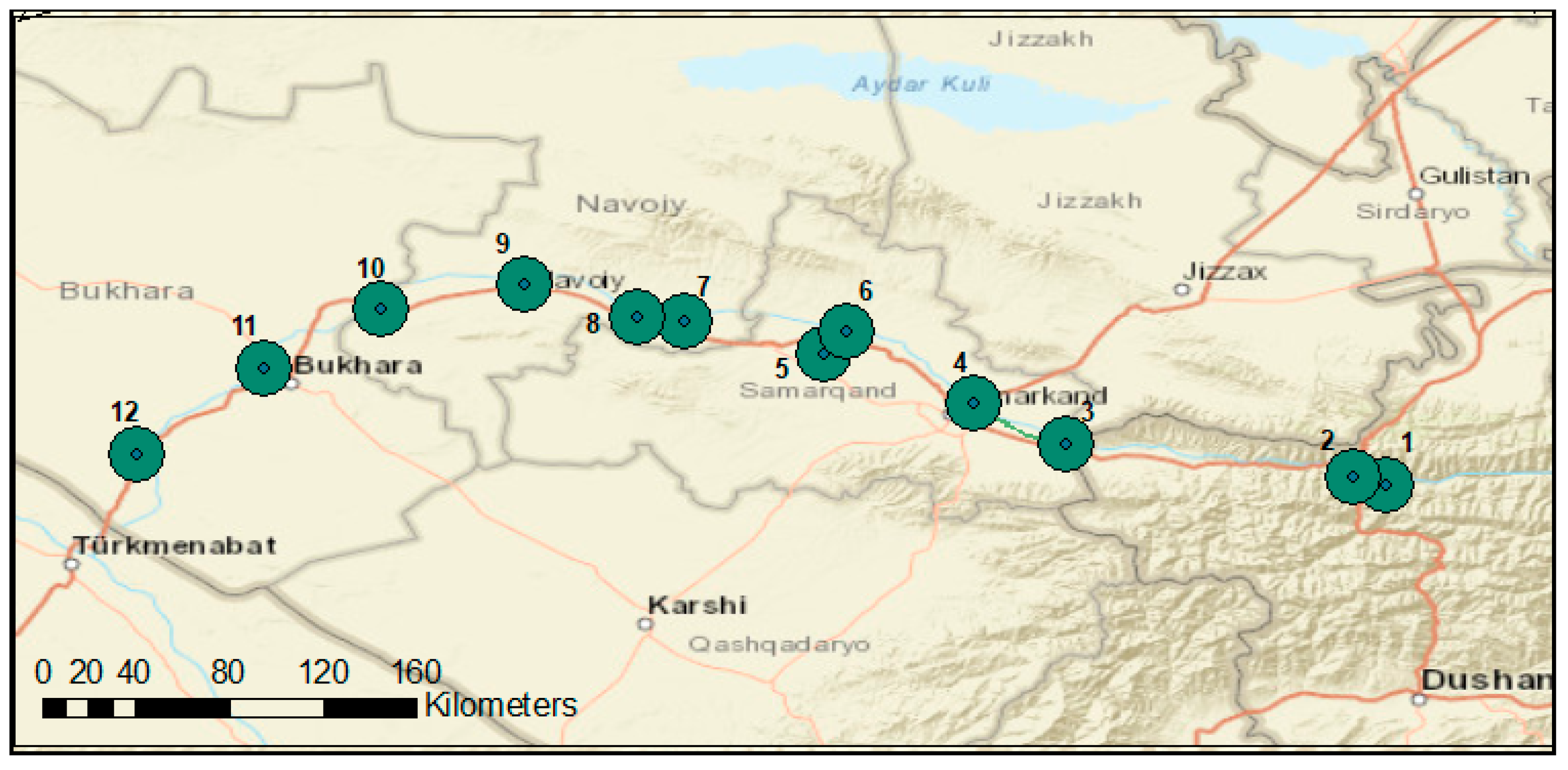

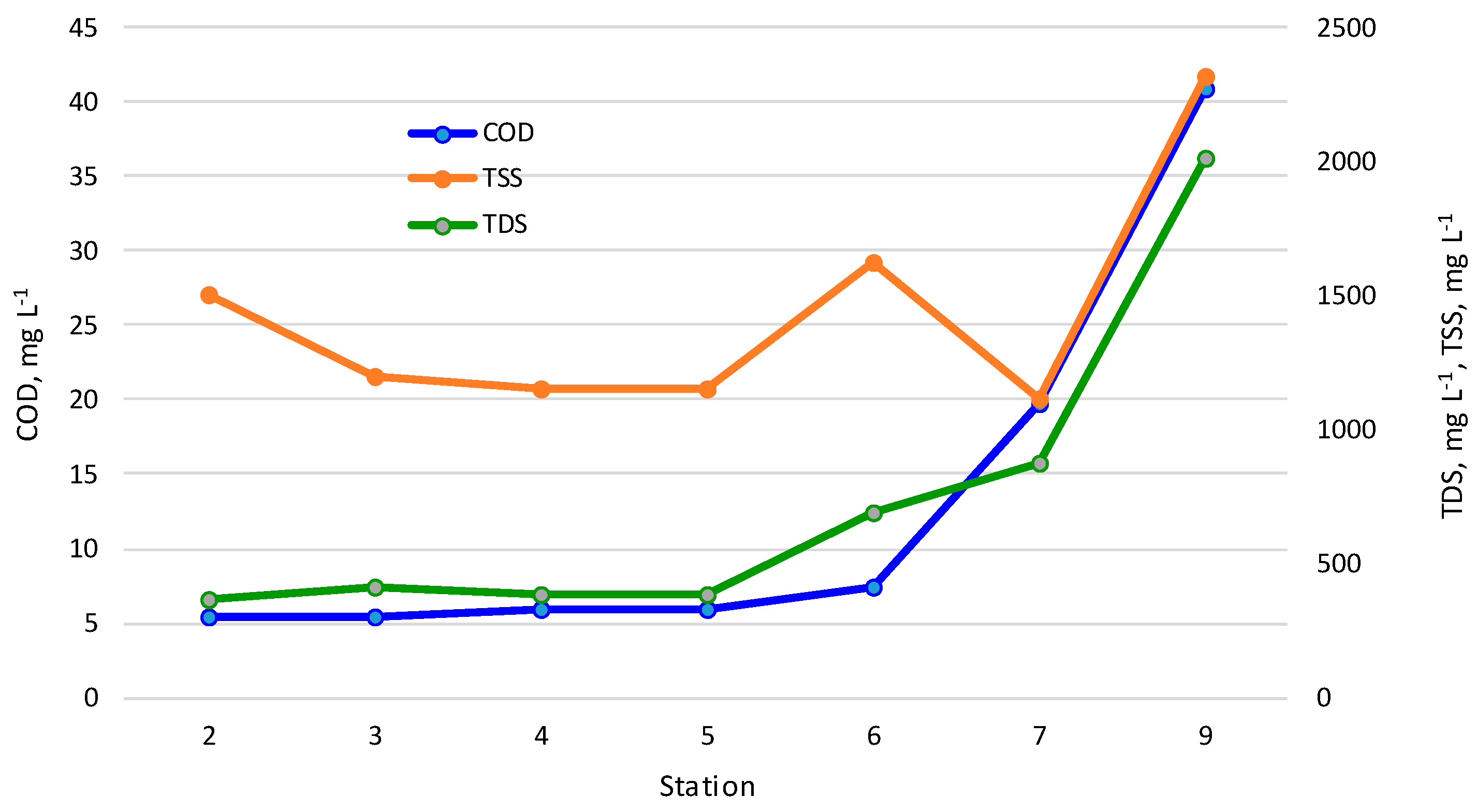
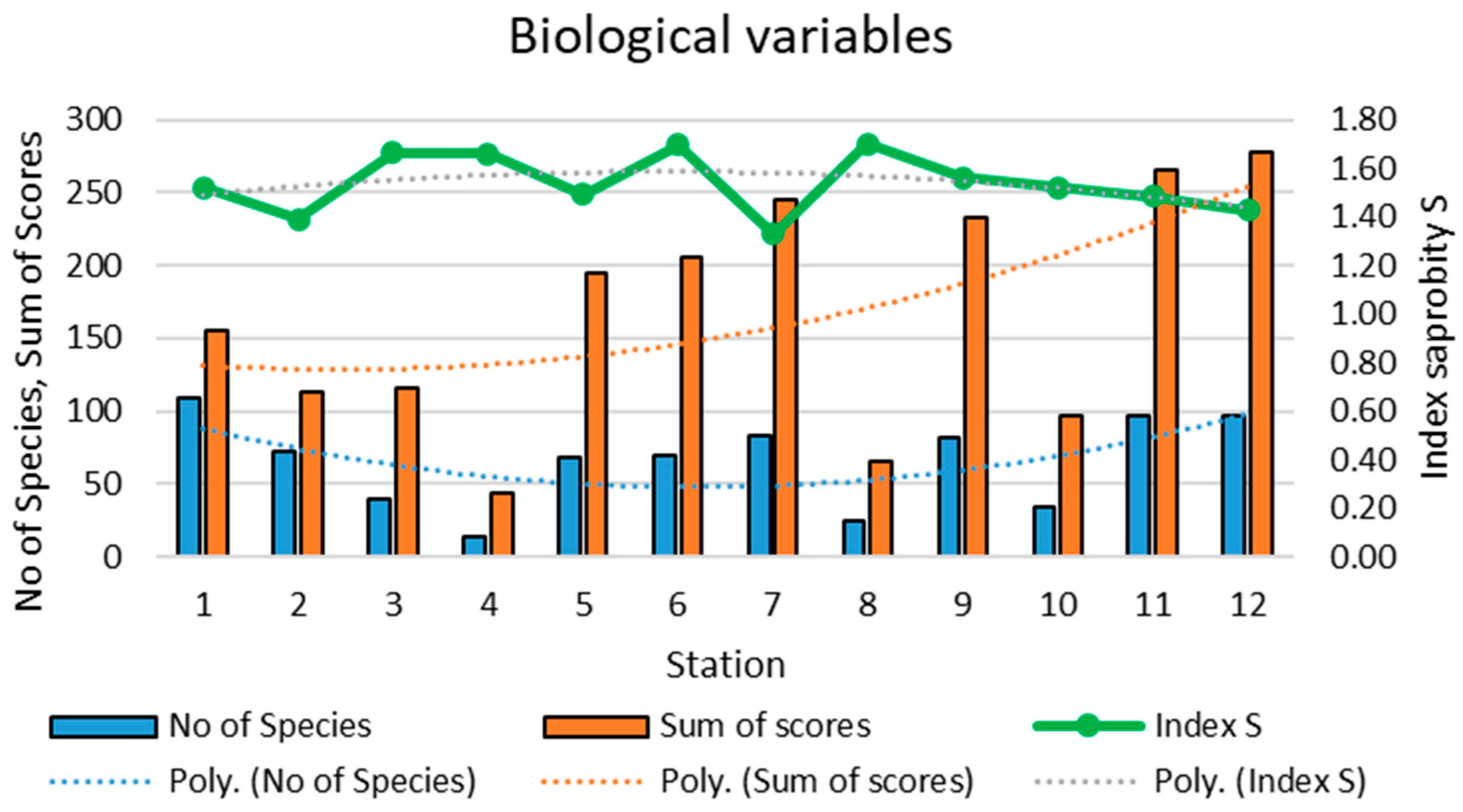
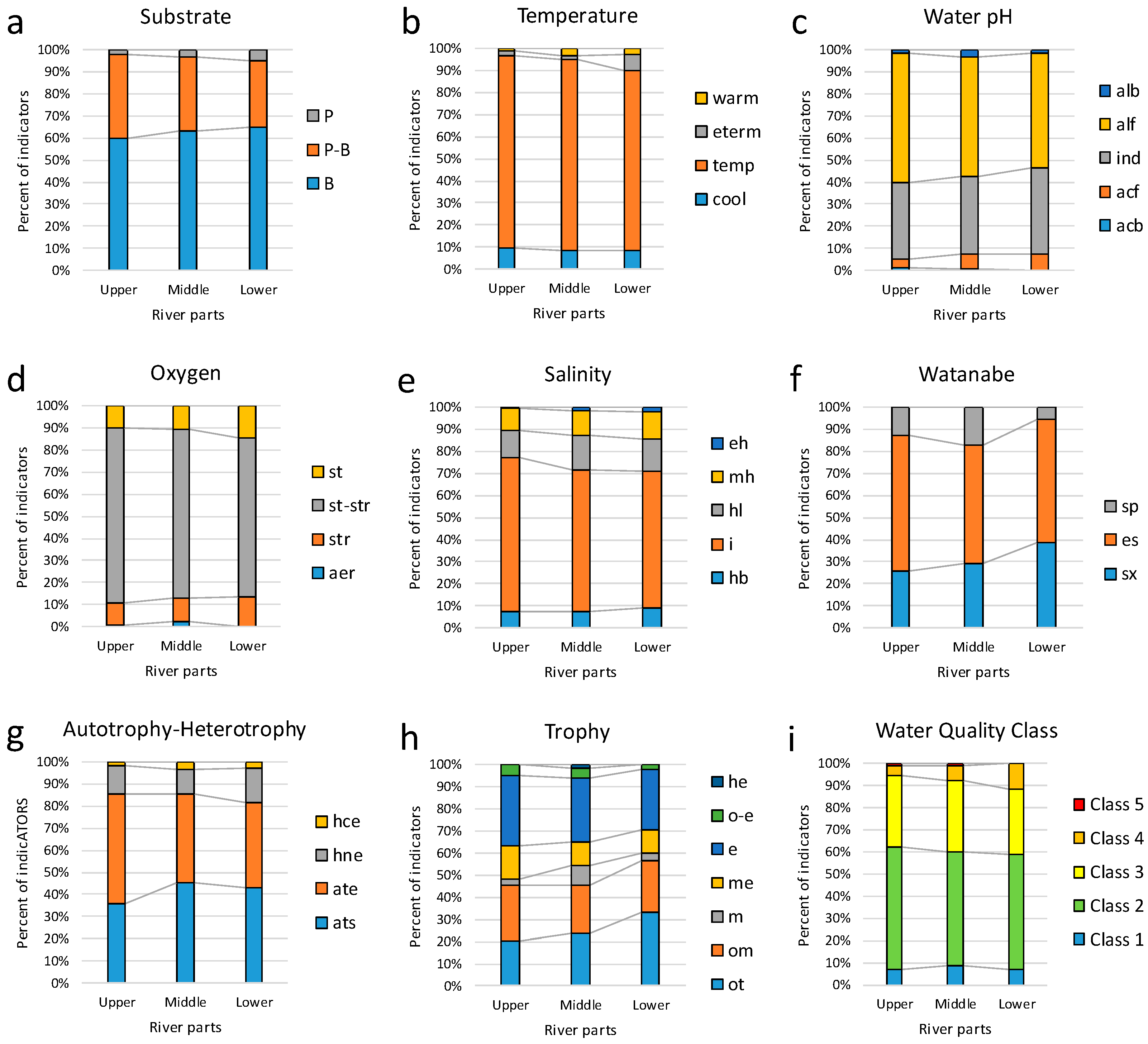

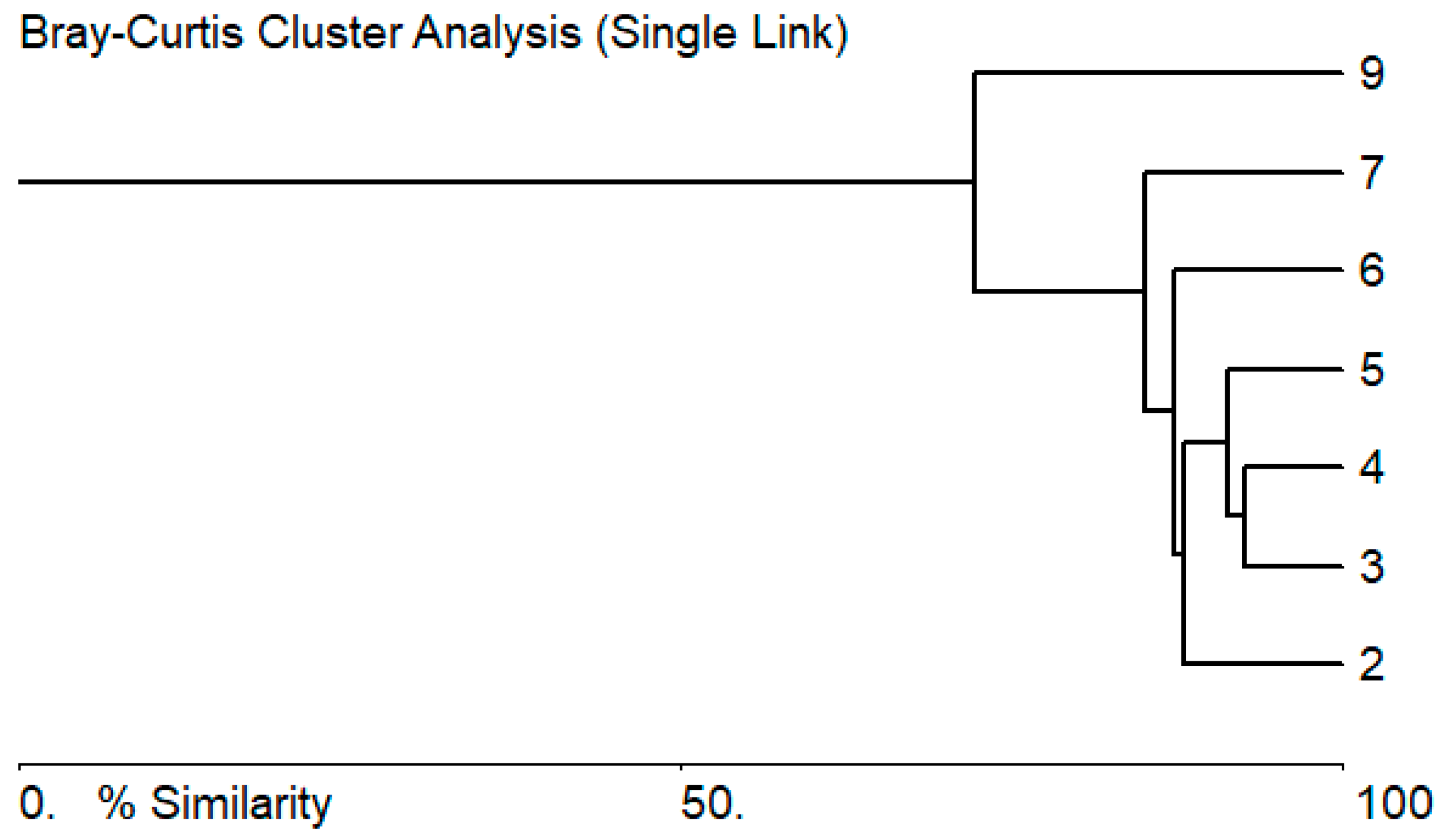

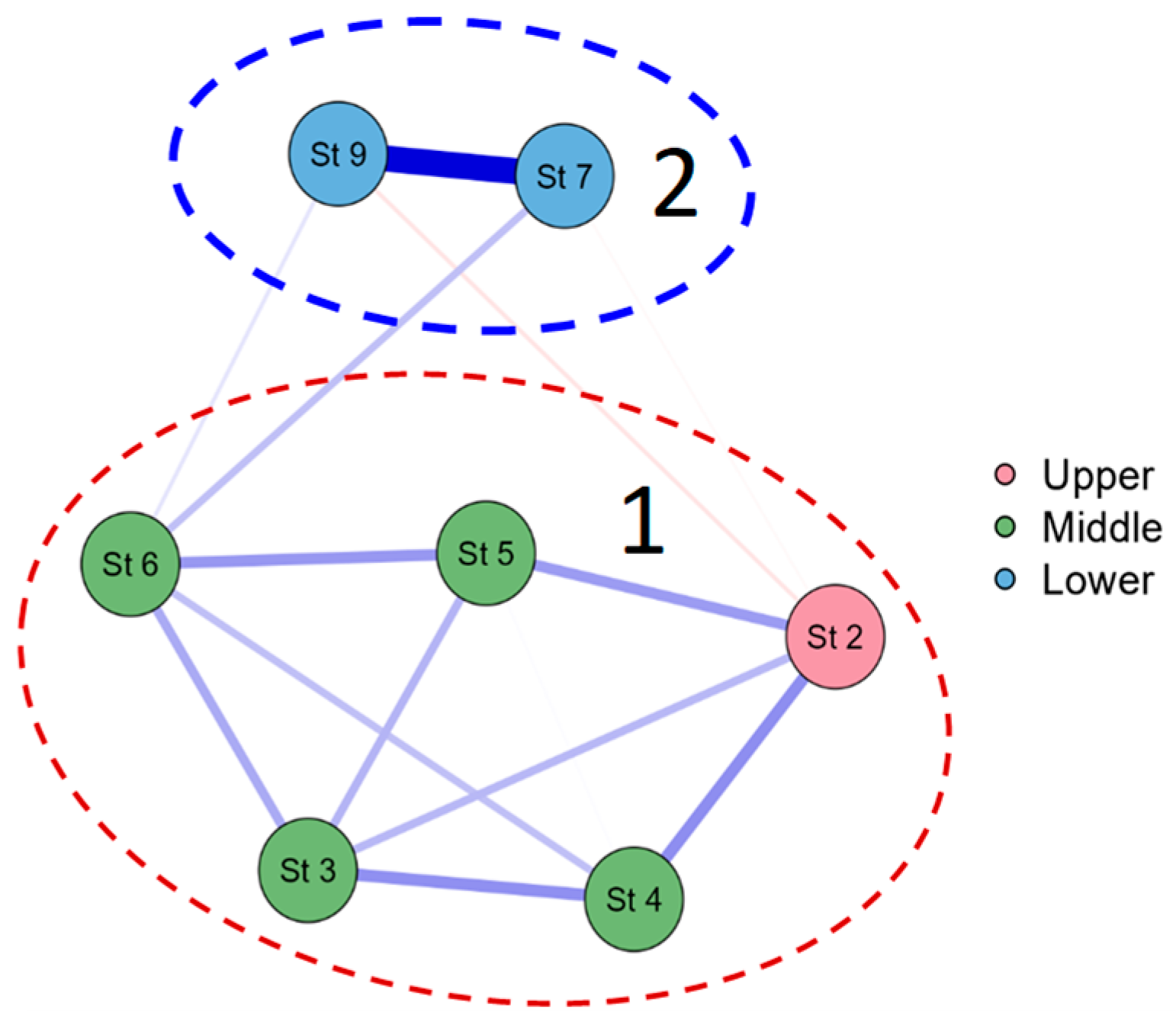


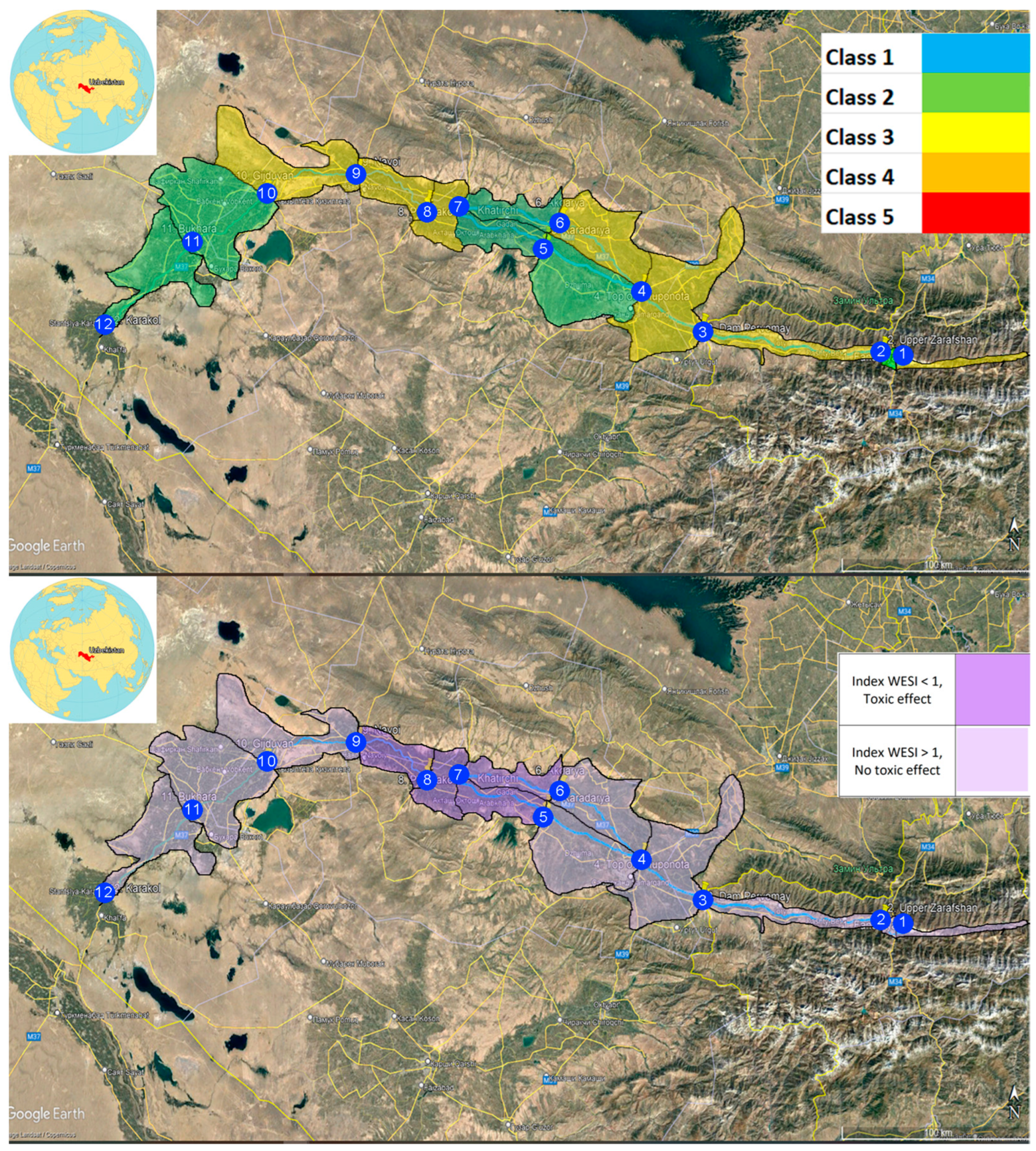
| Site | No. | River Part | Code | North | East | Altitude, m a.s.l. | Distance, km |
|---|---|---|---|---|---|---|---|
| Fandarya | 1 | Upper | Fan | 39°23′2.91″ | 68°38′32.02″ | 1455 | 0 |
| Upper Zeravschan | 2 | Upper | Up-Zer | 39°25′1.48″ | 68°30′56.40″ | 1403 | 21 |
| Pervomayskaya plotina | 3 | Middle | P-mays | 39°32′16.87″ | 67°24′36.43″ | 880 | 188 |
| Upper Choponata | 4 | Middle | Up-Chop | 39°41′40.29″ | 67° 3′14.25″ | 702 | 38 |
| Akdarya | 5 | Middle | Akda | 39°53′20.18″ | 66°28′30.56″ | 522 | 62 |
| Karadarya | 6 | Middle | Kara | 39°58′26.02″ | 66°33′46.88″ | 523 | 55.5 |
| Khatirchi | 7 | Lower | Khat | 40°0′47.40″ | 65°56′34.06″ | 427 | 52.3 |
| Pakhtakor | 8 | Lower | Pakh | 40°1′59.89″ | 65°45′42.50″ | 396 | 32.11 |
| Navoi | 9 | Lower | Navo | 40°9′32.56″ | 65°19′47.08″ | 330 | 34.35 |
| Gizhduvon | 10 | Lower | Gizh | 40°3′48.40″ | 64°46′26.15″ | 277 | 54.74 |
| Bukhara | 11 | Lower | Bukh | 39°49′57.97″ | 64°19′38.60″ | 214 | 48.47 |
| Karakul | 12 | Lower | Kara | 39°29′53.98″ | 63°49′58.58″ | 197 | 63.12 |
| Variable | RPI Cross 5 Average | RPI Cross 6 Average |
|---|---|---|
| km | 479.7 | 469.1 |
| O2 mg L−1 | 7.40 | 7.77 |
| BOD, mgO L−1 | 1.10 | 1.11 |
| COD, mgO L−1 | 7.54 | 8.01 |
| N-NH4, mg L−1 | 0.05 | 0.06 |
| N-NO2, mg L−1 | 0.55 | 0.90 |
| N-NO3, mg L−1 | 0.95 | 0.96 |
| Fe, mg L−1 | 0.02 | 0.02 |
| Cu, mcg L−1 | 2.84 | 3.30 |
| Zn, mcg L−1 | 2.96 | 3.29 |
| Phenols, mg L−1 | 0.002 | 0.003 |
| Oil, mg L−1 | 0.01 | 0.02 |
| Detergents, mg L−1 | 0.00 | 0.01 |
| TSS, mg L−1 | 380.3 | 507.0 |
| DDT, mg L−1 | 0.0 | 0.0 |
| Alpha-HCH, mcg L−1 | 0.0004 | 0.0004 |
| Gamma-HCH, mcg L−1 | 0.0 | 0.0 |
| Cr VI, mcg L−1 | 0.48 | 0.46 |
| F, mg L−1 | 0.33 | 0.36 |
| As, mcg L−1 | 0.92 | 1.09 |
| TDS, mg L−1 | 505.7 | 545.8 |
| No of Species | 58.48 | 58.65 |
| Sum of scores | 154.03 | 155.07 |
| Index S | 1.53 | 1.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamanazarova, K.; Alimjanova, K.; Barinova, S. Biodiversity of Diatoms as Indicators of Water Quality and Landscape Sustainable Dynamics in the Zarafshan River, Uzbekistan. Land 2024, 13, 1809. https://doi.org/10.3390/land13111809
Mamanazarova K, Alimjanova K, Barinova S. Biodiversity of Diatoms as Indicators of Water Quality and Landscape Sustainable Dynamics in the Zarafshan River, Uzbekistan. Land. 2024; 13(11):1809. https://doi.org/10.3390/land13111809
Chicago/Turabian StyleMamanazarova, Karomat, Kholiskhon Alimjanova, and Sophia Barinova. 2024. "Biodiversity of Diatoms as Indicators of Water Quality and Landscape Sustainable Dynamics in the Zarafshan River, Uzbekistan" Land 13, no. 11: 1809. https://doi.org/10.3390/land13111809
APA StyleMamanazarova, K., Alimjanova, K., & Barinova, S. (2024). Biodiversity of Diatoms as Indicators of Water Quality and Landscape Sustainable Dynamics in the Zarafshan River, Uzbekistan. Land, 13(11), 1809. https://doi.org/10.3390/land13111809







